Abstract
With the rising prevalence of both diabetes mellitus (DM) and peripheral arterial disease (PAD), the aim of this project was to examine the association between dietary intake and lifestyle on the risk of developing PAD among individuals with DM. The Malmö Diet and Cancer study was a prospective cohort study with baseline examinations carried out between 1991 and 1996 in Malmö, Sweden (n = 30,446). Individuals with prevalent PAD and cardiovascular disease (prior stroke or myocardial infarction) were excluded from the study, resulting in a total study population of 1112 patients with prevalent DM. The diagnosis of incident PAD was validated and confirmed in 98% of patients. Of the 1112 individuals, 136 (12.2%) were diagnosed with PAD during a median follow up of 19.7 years (interquartile range 12.9–22.4). Kaplan–Meier analysis showed that men with DM more often developed incident PAD compared with women (cumulative incidences 15.5% and 8.9%, respectively, p = 0.012). In Cox multivariable regression analysis, smoking (hazard ratio of 1.96, 95% confidence interval of 1.28–3.00) was associated with increased risk of PAD, and there was a trend that a higher intake of fish and shellfish (hazard ratio per additional gram per week of 0.99, 95% confidence interval of 0.99–1.00; p = 0.051) was associated with a decreased risk of PAD. In conclusion, the present study demonstrated a trend towards a protective effect of higher intake of fish and shellfish upon incident symptomatic PAD among individuals with DM.
Keywords: diabetes mellitus, diet, fish and shellfish, incident peripheral arterial disease
Introduction
In 2018, more than 435,000 individuals had been diagnosed with diabetes mellitus (DM) in Sweden, out of whom 89% had type 2 DM.1 In 2015, 30.3 million Americans were estimated to have DM, with 23.8% being unaware of their diagnosis.2 Peripheral arterial disease (PAD) defined as atherosclerotic occlusions of lower- or upper-extremity arteries is reported to affect approximately 8.5 million Americans aged ⩾40 years.3,4 Between 2000 and 2010 the prevalence of PAD increased with 23% worldwide due to a growing and aging global population, an increased number of patients with diabetes, and smoking. By 2010, nearly two thirds of patients with prevalent PAD resided in low- and middle-income countries.5 The proportion of generalized atherosclerosis is higher among patients with PAD compared with patients with cardiovascular or cerebrovascular disease.5 Furthermore, symptomatic PAD is associated with a high rate of silent myocardial infarction, 29%.5 According to previous epidemiological studies, patients with PAD experience higher cardiovascular mortality than patients with coronary heart or cerebrovascular disease.6 However, patients with PAD in a primary-care setting received less intensive treatment for hypertension and hyperlipidemia, and were prescribed antiplatelet medication less frequently compared with patients with cardiovascular disease.7 Therefore, primary prevention of PAD calls for increased attention due to the growing global burden of the disease.
Among individuals with DM, symptomatic PAD is about twice as common compared with individuals without DM.8 Previous studies have shown that insulin resistance is associated with a higher risk of developing PAD among individuals >65 years.9 It is well known that a healthy diet reduces the risk of atherosclerosis.10 A Mediterranean diet has been shown to reduce the risk of death from all causes including death due to cardiovascular disease.11 However, not many previous studies have focused on dietary components and its effects on the development of PAD in a high-risk group, such as individuals with DM. The Malmö Diet and Cancer study (MDCS) was a large prospective cohort with a long duration of follow up, thus offering a unique opportunity to study the association between dietary components and the risk of developing PAD among individuals with DM.
Therefore, the main aim of this longitudinal cohort study was to investigate how different dietary components and lifestyle affect the development of PAD among individuals with DM.
Method
Study sample and data collection
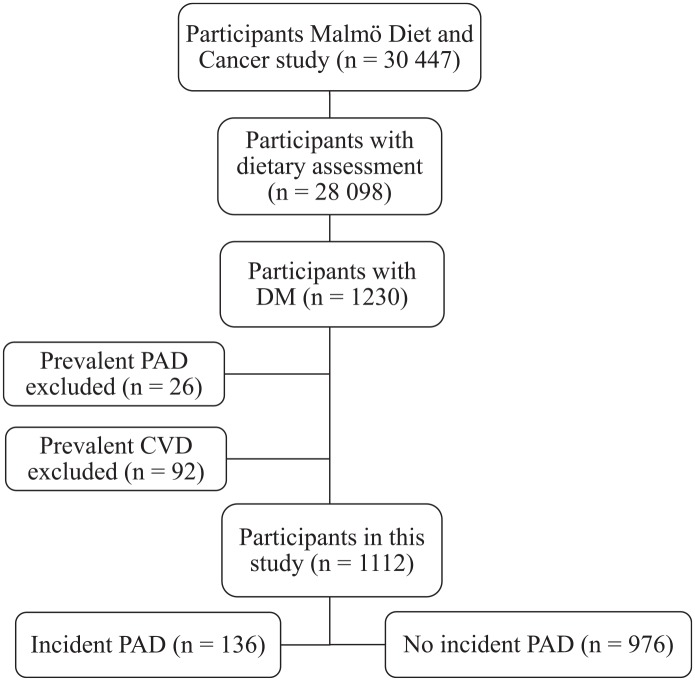
The MDCS with baseline examinations carried out between 1991 and 1996 was a prospective cohort study. The study included 30,446 middle-aged individuals residing in Malmö, Sweden.12 A total of 28,098 individuals participated in diet assessment, anthropometric measurements, and answered a comprehensive questionnaire. Among these, 1230 participants had prevalent DM. Among those, individuals with prevalent PAD or other forms of cardiovascular disease (prior stroke or myocardial infarction) were excluded in the present study, resulting in a total study population of 1112 (Figure 1).
Figure 1.
Descriptive flow diagram of study participants, dietary data and exclusions.
DM, diabetes mellitus; CVD, cardiovascular disease (prior stroke or myocardial infarction); PAD, peripheral arterial disease.
Informed consent was obtained from the study participants and the Regional Ethical Review Board in Lund, Sweden, gave ethical approval to the study (Dnr LU 51/90). All research was performed in accordance with relevant ethical guidelines.
Definitions
Using the civic registration number of each individual, the age and sex of each could be determined. DM was defined as fasting blood glucose >6.0 mmol/l, use of antidiabetic drugs or self-reported physician’s diagnosis. Body mass index (BMI) was calculated using weight divided by height2, expressed in kg/m2. Hypertension was defined as use of antihypertensive drugs or blood pressure ⩾140/90 mmHg. Smoking was defined as former or current smoking.
Diet variables
Dietary habits were collected at baseline through a combination of a 7-day food diary, a 168-item food frequency questionnaire and a 1-hour interview, where detailed information on cooking practises, portion sizes and recipes of the food recorded in the diary was gathered during the interview.13 Average daily food intake (g/day) was calculated by combining the information from the food diary and the questionnaire. The summary variable whole grains (servings/day) includes all high-fiber bread and cereals and the summary variable refined grains (servings/day) contains all low-fiber bread and cereals. Total energy intake (kcal/day, including alcohol and fiber), and fiber intake (g/day) was estimated by combining the intake from foods and supplements with the food composition database. Fish and shellfish intake was expressed in g/week, and 250 g/week corresponded to two servings per week.14
Lifestyle
Lifestyle variables were evaluated through a self-administered questionnaire. Based on the highest educational level attained, the study participants were divided into three categories, that is, <9 years, elementary (9–10 years) ± upper secondary school (11–13 years), and university level. Leisure-time physical activity level was defined as metabolic equivalent of task (MET) hours per week based on the intensity level and the time spent on 17 different activities and was divided into three groups. Alcohol consumption was divided into three groups based on the participant’s reported intake in the 7-day food diary.
Endpoint ascertainment
The personal number of individuals from the MDCS was used to identify the first registered diagnosis of PAD in the Swedish national registers. The included registers were the Inpatient and Outpatient Register and the Cause of Death Register. In both registers, diagnoses are coded using a revised Swedish version of the International Classification of Disease, version 8, 9, and 10.
Validation of PAD diagnosis during follow up
A total of 100 patients with the diagnosis of PAD were randomly selected. Using patient record data, the validation showed that PAD could be confirmed in 98% of the cases, symptomatic in 97%, and only misdiagnosed in 2%.15
Statistics
The baseline characteristics for age, sex, BMI, diet and lifestyle variables were expressed as median and interquartile range for continuous variables and as total count and percentage for the categorical variables. Differences in proportions were analyzed with Pearson Chi-square test or Kendall tau-b test. The Mann–Whitney U test and Student’s t test were used to test differences in continuous variables. Correlations between fish and shellfish consumption and potential risk factors for PAD were assessed by Spearman’s or Pearson correlation coefficients and p values. Variables differing (p < 0.1) between incident PAD and not, in a univariable analysis, were further entered as covariates in a Cox multivariate regression analysis adjusting for age, sex, hypertension and smoking. Differences were expressed in hazard ratios (HR) with 95% confidence interval (CI). The cumulative incidence of PAD was described according to the Kaplan–Meier method with life tables, and the difference between sex was analyzed with a log-rank test. A p value <0.05 was considered significant. Statistical analyses were performed using IBM SPSS Statistics 25 (SPSS, Chicago, IL, USA).
Results
Baseline characteristics of diabetic patients with and without incident symptomatic PAD
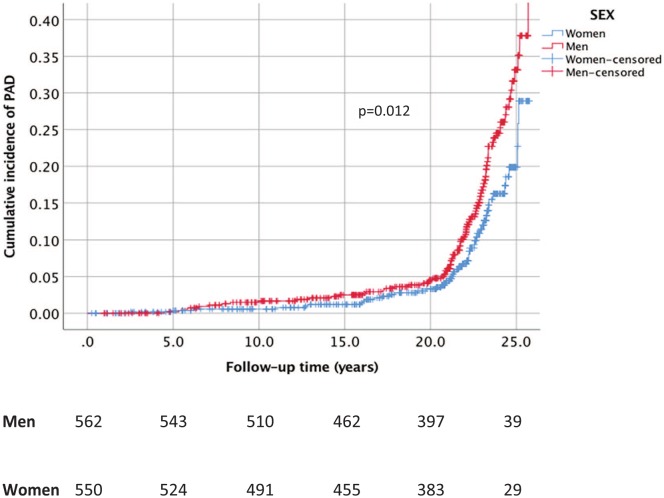
In the study population of 1112 patients with DM, 136 (12.2%) were diagnosed with PAD, during a median follow up of 19.7 years (interquartile range of 12.9–22.4). Baseline characteristics of diet variables and lifestyle factors for individuals with and without incident PAD are shown in Table 1. The cumulative incidences of PAD in men and women were 15.5% and 8.9%, respectively (p = 0.012 in Kaplan–Meier analysis, Figure 2). Patients with DM developing PAD during follow up were more often smokers (p < 0.001) and there was a trend for hypertension (p = 0.086) at baseline. Individuals with DM developing PAD had a lower intake of fish and shellfish (p = 0.036).
Table 1.
Baseline characteristics of study participants with DM with and without incident symptomatic PAD in the Malmö Diet and Cancer cohort. Data are n (%), mean (SD) or median (IQR).
| Incident PAD, DM (n = 136) | No PAD, DM
(n = 976) |
p value | |
|---|---|---|---|
| Male sex (%) | 87 (64) | 475 (49) | 0.001 |
| Age (years) | 61.37 (6.26) | 60.72 (6.90) | 0.294 |
| Total energy intake (kcal/day) | 2090 (589.06) | 2191 (677.10) | 0.136 |
| BMI (kg/m2) | 27.74 (4.36) | 28.15 (4.76) | 0.419 |
| Hypertension | 129 (94.9) | 878 (90.3) | 0.086 |
| Alcohol consumption | 0.950 | ||
| <265 g/week | 52 (38.2) | 343 (35.1) | |
| 265–722 | 43 (31.6) | 368 (37.7) | |
| >722 | 41 (30.1) | 265 (27.2) | |
| Smoking status | <0.001 | ||
| Never | 28 (20.7) | 373 (38.3) | |
| Former or current | 107 (79.3) | 602 (61.7) | |
| Leisure-time physical activity | 0.149 | ||
| <447.5 MET-h/week | 77 (57.9) | 497 (51.4) | |
| 447.5–742.5 | 37 (27.8) | 299 (30.9) | |
| >742.5 | 19 (14.3) | 171 (17.7) | |
| Educational level | 0.140 | ||
| <9 years | 77 (56.6) | 482 (49.6) | |
| Elementary (9–10 years) ± upper secondary school (9–13 years) | 39 (28.7) | 320 (33.0) | |
| University | 20 (14.7) | 169 (17.4) | |
| Saturated fat (E%) | 14.31 (3.71) | 14.74 (3.74) | 0.328 |
| Polyunsaturated fat (E%) | 6.23 (1.47) | 6.09 (1.58) | 0.414 |
| Fish and shellfish (g/week) | 248.93 (244.83) | 300.64 (267.60) | 0.036 |
| Fiber (g/MJ) | 2.36 (0.78) | 2.35 (0.74) | 0.923 |
| Fruits and berries (g/1000 kcal) | 78.68 (63.88) | 81.55 (59.73) | 0.479 |
| Vegetables (g/1000 kcal) | 85.29 (64.44) | 83.97 (59.63) | 0.659 |
| Sucrose (E%) | 5.26 (2.67) | 5.92 (3.35) | 0.107 |
| Whole grains (servings/1000 kcal) | 0.23 (0.36) | 0.28 (0.32) | 0.814 |
| Refined grains (servings/1000 kcal) | 1.12 (0.61) | 1.10 (0.55) | 0.848 |
BMI, body mass index; DM, diabetes mellitus; IQR, interquartile range; MET, metabolic equivalent of task; PAD, peripheral arterial disease; SD, standard deviation.
Figure 2.
Cumulative incidence of symptomatic peripheral arterial disease (PAD) in relation to sex among participants with diabetes mellitus in the Malmö Diet and Cancer cohort.
Correlation between fish and shellfish and potential risk factors for PAD
There was a significant correlation between fish and shellfish consumption and age (r = 0.11, p < 0.001), alcohol consumption (r = 0.18, p < 0.001), leisure-time physical activity (r = 0.094, p = 0.002) and educational level (r = 0.086, p = 0.004; Table 2).
Table 2.
Correlation between fish or shellfish consumption and potential risk factors for peripheral arterial disease among individuals with diabetes mellitus at baseline.
| Fish or shellfish consumption |
||
|---|---|---|
| r | p | |
| Potential risk factors | ||
| Age | 0.11 | <0.001 |
| Male sex | 0.059 | 0.050 |
| Body mass index | 0.050 | 0.097 |
| Hypertension | 0.046 | 0.12 |
| Smoking | −0.001 | 0.96 |
| Alcohol consumption | 0.18 | <0.001 |
| Leisure-time physical activity | 0.094 | 0.002 |
| Educational level | 0.086 | 0.004 |
Factors associated with incident symptomatic PAD among patients with DM
In the Cox regression multivariable analysis, smoking (HR of 1.96, 95% CI of 1.28–3.00) was associated with an increased risk of PAD (Table 3), and there was a trend that a higher intake of fish and shellfish (HR of 0.99, 95% CI of 0.99–1.00; p = 0.051) was associated with a decreased risk of PAD.
Table 3.
Factors associated with incident symptomatic peripheral arterial disease among patients with diabetes mellitus in the Malmö Diet and Cancer cohort.
| HR (95% CI) | p value | |
|---|---|---|
| Age | 1.01 (0.98–1.03) per year | 0.64 |
| Male sex | 1.36 (0.95–1.95) | 0.090 |
| Hypertension | 1.86 (0.87–4.02) | 0.11 |
| Smoking | 1.96 (1.28–3.0) | 0.002 |
| Fish and shellfish intake | 0.99 (0.99–1.00) per additional gram per week | 0.051 |
All five variables were analyzed using the Cox regression analysis.
CI, confidence interval; HR, hazard ratio.
Discussion
In the present study of individuals with DM from the MDCS, a trend towards a protective effect of high intake of fish and shellfish upon risk of PAD could be demonstrated.
Previous studies on how different dietary components affect the risk of PAD among individuals with DM have been scarce, and most reports have focused on the association between traditional cardiovascular risk factors and incident PAD.16 Moreover, incident PAD has seldom been a prespecified endpoint in the study protocols and associations of dietary components with incident PAD have almost always been based on post hoc analyses and not on individuals with DM exclusively, thereby downgrading the evidence.17
In particular, no prospective longitudinal studies evaluating the role of isolated dietary compounds and incident PAD among individuals with DM have previously been published. A Mediterranean diet pattern, characterized by high consumption of plant-based foods, olive oil as the main source of fat, moderate consumption of fish, dairy products and poultry, low consumption of red and processed meat, and low-to-moderate consumption of wine with meals,18 has been recommended by the American Diabetes Association and the American Heart Association for improving glycemic control and reducing cardiovascular risk in type 2 DM.19 The relationship between a Mediterranean diet and the risk of PAD has been studied in a cross-sectional Italian study, showing that the highest Mediterranean diet score was associated with a significant 56% risk reduction of symptomatic PAD among individuals with type 2 DM.20 With fish being a well-known dietary component in the Mediterranean diet, such results are in line with the present study findings. The Mediterranean diet has also been shown to lower markers of inflammation and blood lipids, which in turn reduces the burden and development of cardiovascular disease.21 A healthy diet, rich in fish, might help to achieve and maintain body weight goals, reach individual glycemic, blood pressure, and lipid targets, and to some extent prevent diabetic complications.22 It is possible that high consumers of fish and shellfish have a different lifestyle to low consumers, which might help explain the putative protective effects towards PAD development. Consuming less fish at the expense of more saturated fats and meat products appears to be associated with the progression of PAD.23 Apart from studies on the Mediterranean diet, there is high-level evidence showing that diet patterns such as dietary approaches to stop hypertension and a low-fat diet is beneficial for primary and secondary prevention of cardiovascular disease.24
The present study has several limitations and strengths that deserve clarification. A limitation is the low number of participants with DM which may not have rendered sufficient power to attain statistical significance in some of the analyses. Another limitation is that the study was limited to symptomatic PAD cases only. It would have been of value to determine the ankle-brachial index, both at baseline and at follow up to identify participants with asymptomatic PAD. This could both have helped exclude patients with prevalent asymptomatic PAD at baseline, and rendered a larger sample size of incident asymptomatic PAD cases, possibly strengthening the associated trend between a high intake of fish and shellfish and a reduced risk of PAD development. The study cohort focused on dietary habits in a middle-aged Swedish population. However, self-reported dietary habits are prone to be misreported to some extent and might have changed during follow up, and individuals with DM are known to be dietary changers.25 On the other hand, the study population was homogenous since it only included individuals with DM at baseline. Other confounders not accounted for are changes in smoking habits, antihypertensive medications and anti-atherosclerotic agents during follow-up time. The shown correlation between fish and shellfish consumption and educational level is interesting, but associations of properly defined socioeconomic status26 and dietary components and development of PAD in this cohort were not evaluated and were not within the scope of this study. The main strengths of this study are its longitudinal study design and the extensive (19.7 years) duration of follow up.
In conclusion, the present study found a trend towards a protective effect of higher intake of fish and shellfish against incident symptomatic PAD among individuals with DM.
Footnotes
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Erika Lilja  https://orcid.org/0000-0001-9965-8133
https://orcid.org/0000-0001-9965-8133
Contributor Information
Erika Lilja, Department of Clinical Sciences, Malmö, Lund University, Ruth Lundskogs gata 10, 205 02 Malmö, Sweden.
Sara Bergwall, Department of Clinical Sciences, Malmö, Lund University, Malmö, Sweden.
Emily Sonestedt, Department of Clinical Sciences, Malmö, Lund University, Malmö, Sweden.
Anders Gottsäter, Department of Clinical Sciences, Malmö, Lund University, Malmö, Sweden; Vascular Centre, Department of Cardiothoracic and Vascular Surgery, Skåne University Hospital, Malmö, Sweden.
Stefan Acosta, Department of Clinical Sciences, Malmö, Lund University, Malmö, Sweden; Vascular Centre, Department of Cardiothoracic and Vascular Surgery, Skåne University Hospital, Malmö, Sweden.
References
- 1. Nationella Diabetesregistret. Årsrapport 2018, www.ndr.nu/pdfs/Arsrapport_NDR_2018.pdf (2018, accessed 18 May 2019).
- 2. Centers for Disease Control and Prevention. National diabetes statistics report 2017, www.cdc.gov/diabetes/data/statistics-report/index.html (2017, accessed 3 June 2019).
- 3. Hiatt WR, Goldstone J, Smith SC, et al. Atherosclerotic peripheral vascular disease symposium II: nomenclature for vascular diseases. Circulation 2008; 118: 2826–2829. [DOI] [PubMed] [Google Scholar]
- 4. Writing Group Members, Mozaffarian D, Benjamin EJ, et al. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation 2016; 133: e38–e360. [DOI] [PubMed] [Google Scholar]
- 5. Weir-McCall JR, Duce SL, Gandy SJ, et al. Whole body cardiovascular magnetic resonance imaging to stratify symptomatic and asymptomatic atherosclerotic burden in patients with isolated cardiovascular disease. BMC Med Imaging 2016; 16: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Steg PG, Bhatt DL, Wilson PW, et al. One-year cardiovascular event rates in outpatients with atherothrombosis. JAMA 2007; 297: 1197–1206. [DOI] [PubMed] [Google Scholar]
- 7. Hirsch AT, Criqui MH, Treat-Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA 2001; 286: 1317–1324. [DOI] [PubMed] [Google Scholar]
- 8. Norgren L, Hiatt WR, Dormandy JA, et al. Inter-society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg 2007; 45: S5–S67. [DOI] [PubMed] [Google Scholar]
- 9. Britton KA, Mukamal KJ, Ix JH, et al. Insulin resistance and incident peripheral artery disease in the Cardiovascular Health Study. Vasc Med 2012; 17: 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yu E, Rimm E, Qi L, et al. Diet, lifestyle, biomarkers, genetic factors, and risk of cardiovascular disease in the nurses’ health studies. Am J Public Health 2016; 106: 1616–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mitrou PN, Kipnis V, Thiébaut ACM, et al. Mediterranean dietary pattern and prediction of all-cause mortality in a US population: results from the NIH-AARP Diet and Health Study. JAMA Intern Med 2007; 167: 2461–2468. [DOI] [PubMed] [Google Scholar]
- 12. Manjer J, Elmståhl S, Janzon L, et al. Invitation to a population-based cohort study: differences between subjects recruited using various strategies. Scand J Public Health 2002; 30: 103–112. [DOI] [PubMed] [Google Scholar]
- 13. Drake I, Gullberg B, Ericson U, et al. Development of a diet quality index assessing adherence to the Swedish nutrition recommendations and dietary guidelines in the Malmö Diet and Cancer cohort. Public Health Nutr 2011; 14: 835–845. [DOI] [PubMed] [Google Scholar]
- 14. Brugård Konde Å, Bjerselius R, Haglund L, et al. Swedish dietary guidelines—risk and benefit management report. Report No. 5/2015, 6 June 2015. Uppsala, Sweden: Livsmedelsverket (National Food Agency). [Google Scholar]
- 15. Fatemi S, Gottsäter A, Zarrouk M, et al. Lp-PLA2 activity and mass and CRP are associated with incident symptomatic peripheral arterial disease. Sci Rep 2019; 9: 5609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Althouse AD, Abbott JD, Forker AD, et al. Risk factors for incident peripheral arterial disease in type 2 diabetes: results from the Bypass Angioplasty Revascularization Investigation in type 2 Diabetes (BARI 2D) Trial. Diabetes Care 2014; 37: 1346–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ruiz-Canela M, Estruch R, Corella D, et al. Association of Mediterranean diet with peripheral artery disease: the PREDIMED randomized trial. JAMA 2014; 311: 415–417. [DOI] [PubMed] [Google Scholar]
- 18. Esposito K, Maiorino MI, Bellastella G, et al. Mediterranean diet for type 2 diabetes: cardiometabolic benefits. Endocrine 2017; 56: 27–32. [DOI] [PubMed] [Google Scholar]
- 19. Fox CS, Golden SH, Anderson C, et al. Update on prevention of cardiovascular disease in adults with type 2 diabetes mellitus in light of recent evidence: a scientific statement from the American heart association and the American diabetes association. Diabetes Care 2015; 38: 1777–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ciccarone E, Di Castelnuovo A, Salcuni M, et al. A high-score Mediterranean dietary pattern is associated with a reduced risk of peripheral arterial disease in Italian patients with type 2 diabetes. J Thromb Haemost 2003; 1: 1744–1752. [DOI] [PubMed] [Google Scholar]
- 21. Widmer RJ, Flammer AJ, Lerman LO, et al. The Mediterranean diet, its components, and cardiovascular disease. Am J Med 2015; 128: 229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Look AHEAD Research Group, Wing RR, Bolin P, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 2013; 369: 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Redmond ML, Dong F, Goetz J, et al. Food insecurity and peripheral arterial disease in older adult populations. J Nutr Health Aging 2016; 20: 989–995. [DOI] [PubMed] [Google Scholar]
- 24. Nosova EV, Conte MS, Grenon SM. Advancing beyond the “heart-healthy diet” for peripheral arterial disease. J Vasc Surg 2015; 61: 265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Paisley J, Beanlands H, Goldman J, et al. Dietary change: what are the responses and roles of significant others? J Nutr Educ Behav 2008; 40: 80–88. [DOI] [PubMed] [Google Scholar]
- 26. Sonestedt E, Wirfält E, Gullberg B, et al. Past food habit change is related to obesity, lifestyle and socioeconomic factors in the Malmö Diet and Cancer cohort. Public Health Nutr 2005; 8: 876–885. [DOI] [PubMed] [Google Scholar]