Abstract
Evidence suggests that psychosocial stress negatively impacts immunological health in HIV-positive individuals. However, few studies have explored this association in substance-using older adults living with HIV (OALWH). We evaluated the effect of depression, loneliness, substance use problems, and HIV stigma on primary markers of immune function in a sample of 120 OALWH with substance-related issues. HIV stigma correlated with the greatest number of factors, including depression, loneliness, and substance use problems. Older age and antiretroviral adherence were associated with viral suppression, which was in turn associated with higher percentage of CD4 count. Multivariate path analyses demonstrated that lower HIV stigma and viral suppression were the only factors independently associated with higher percentage of CD4 count, with a significant indirect effect of adherence on CD4 through viral suppression. HIV stigma emerged as the most salient factor associated with both psychosocial well-being and immune health in the current study, suggesting that it is a critical factor to consider in future interventions for the rapidly growing population of OALWH.
Keywords: HIV, older adults, stigma, substance use, psychosocial, CD4 count
What Do We Already Know about This Topic?
The extant literature has suggested that older adults living with HIV (OALWH) are uniquely vulnerable to experiencing multiple, co-occurring psychosocial burdens (eg, depression, loneliness, HIV stigma, substance use) and that higher levels of psychosocial stress may underlie poorer physical health outcomes, such as higher viral load and lower CD4 count.
How Does Your Research Contribute to the Field?
The present study demonstrates that OALWH with chronic substance use issues experience multiple interrelated psychosocial challenges, and furthermore, it suggests that HIV stigma experiences play a particularly salient role in both the psychological and immunological health of OALWH.
What Are Your Research’s Implications toward Theory, Practice, or Policy?
Broadly, our research highlights the need to develop long-term, biopsychosocially minded models of care to address the complex health-care needs of the rapidly growing OALWH population, and more specifically, it suggests that interventions that emphasize HIV stigma reduction could optimize the overall health and well-being of OALWH.
Introduction
Wide variability in HIV disease progression continues to persist in the era of combination antiretroviral therapy (ART), suggesting that psychological and behavioral factors may underlie immunological differences among individuals with otherwise similar physical health profiles.1,2 The extant literature has implicated psychosocial stress as a key contributing factor to inflammatory processes and poorer immune function in people living with HIV.3-7 People living with HIV are disproportionately impacted by psychosocial stressors relative to the general population.8-10 For example, an estimated 40% to 42% of HIV-positive adults in the United States meet criteria for depression,11 40% engage in illicit drug use,12 and 15% engage in hazardous or heavy drinking.13 In contrast, 6.7% of the general US population are affected by major depression, 10% engage in illicit drug use, and 6.5% engage in heavy alcohol use.14 Additionally, people living with HIV face unique, HIV-related stressors, such as HIV stigma—a complex social process comprising discriminatory beliefs and stereotypes about HIV-positive persons that can subsequently become internalized.15 Recent national survey data suggest that the vast majority (80%) of US adults living with HIV have experienced HIV stigma.16 Given the substantial proportion of HIV-positive individuals affected by HIV stigma, it is critical to examine its impact as a psychosocial stressor on immunologic function, especially among the rapidly growing population of older adults living with HIV (OALWH).
The life expectancy of HIV-positive individuals in the United States is now comparable to that of the HIV-negative population,17 which has dramatically altered the demographic profile of the US HIV-positive population. In 2001, people aged 50 and older accounted for an estimated 17% of US adults living with HIV, which rose to 31% in 2008 and 47% in 2015.18,19 This epidemiological phenomenon is projected to endure in coming years, with recent data showing that OALWH currently comprise over 50% of the HIV-positive population in New York20 and San Francisco.21 Going forward, it will be essential to better understand the unique, often complex health-care needs of this growing population. In terms of physical health alone, evidence indicates that 91% of OALWH have at least 1 comorbid physical illness, and 77% have 2 or more comorbid conditions, which often carries the associated burden of polypharmacy.22,23 Empirical data concerning the prevalence of substance abuse among OALWH are currently limited, but preliminary work indicates that problems with drugs and alcohol remain a prevalent and persistent health burden for OALWH.24 Moreover, research has demonstrated that the difficulties typically associated with growing older (eg, multimorbidity, cognitive decline, dwindling social networks), compounded with the stress of living with a highly stigmatized illness, predispose OALWH to experiencing multiple, co-occurring psychosocial burdens.25-29 As an example, a study of 914 OALWH in New York City revealed that 40% of the sample reported symptoms of major depression, with 42% of the variance in depression explained by HIV stigma, age, loneliness, reduced energy, and decreased cognitive functioning.30 Similarly, a more recent study showed that HIV stigma and depression accounted for 41% of the variance in loneliness experienced by 146 Atlanta-based OALWH.31 A greater understanding of the degree and direction of influence imparted by these overlapping psychosocial factors on physical health is vital for achieving optimal health outcomes for the growing OALWH population.
Previous research has indicated a direct association between psychosocial factors and immune response.4,8 Evidence from studies on depression and immune health links chronic depression with greater decline in CD4 count, and higher odds of virologic failure, AIDS-related illness, and AIDS-related mortality.9,32 For example, a study of 765 HIV-positive women revealed that chronic depressive symptoms contributed a 2-fold increase in risk of death compared to those who reported limited or no depressive symptoms.33 Mounting evidence from interdisciplinary lines of research suggests that biological factors such as proinflammatory cytokines underlie the association between depression and immunological health.34,35 More specifically, empirical data have demonstrated that higher levels of cytokines increase risk for developing depression; that HIV-positive individuals with depression have elevated levels of proinflammatory cytokines; and that excess cytokine levels suppress immune functioning.11,36 Thus, the manifestation of depression can lead to an altered immune response, resulting in unfavorable HIV-related health outcomes—specifically higher viral load (VL) and lower CD4 counts.37 Similarly, substance use has been shown to predict more rapid disease progression, AIDS-related illness, and death38-40; stimulant use, in particular, can accelerate HIV disease progression, independent of ART adherence.41 While loneliness has been associated with depression in OALWH,30,42,43 additional research is needed to evaluate its influence on immune health.
Additionally, a limited but growing body of literature has suggested that HIV stigma may be directly linked to immunological health outcomes.44 As part of a large-scale, multisite effort to understand empirical associations between HIV stigma and the HIV care continuum, researchers examined data from a geographically diverse sample of 6401 men and women living with HIV and uncovered a significant association between self-reported HIV stigma experiences and the final, crucial step in the care cascade—viral suppression.45 Specifically, these observational results indicated that each unit increase in experienced HIV stigma was associated with a 13% increase in odds of detectable VL, even when adjusting for age, gender identity, sexual orientation, race/ethnicity, time in care, and geographic location. Recently published findings from a stigma-reduction intervention have portrayed a similar association between HIV stigma and VL.46,47 In cross-sectional analyses, 95% of participants endorsed experiencing HIV stigma, and moreover, higher experiences of HIV stigma were significantly associated with lower odds of VL suppression, while hypothesized indirect effects of depression and ART adherence failed to reach significance.47 In longitudinal analyses, the direct association between higher HIV stigma and lower odds of VL suppression persisted, while the hypothesized mediators of this association (depressive symptoms and social support) remained nonsignificant.46 Taken together, these intriguing findings suggest an inverse relationship between HIV stigma and HIV-related health—wherein higher degrees of subjective experiences of HIV-related stigma directly associate with objective measures of HIV VL. However, the potential psychosocial and behavioral mediators of this emergent association remain unclear, and to date, the possibility of a direct relationship between HIV stigma and HIV-related health outcomes has yet to be examined in a sample of OALWH.
Past research has also demonstrated an indirect association, via ART adherence behaviors, between psychosocial factors and immune health outcomes.40,48,49 A large body of research designates ART adherence as a crucial factor for attaining optimal health outcomes, namely increasing CD4 count, achieving VL suppression, inhibiting progression to AIDS, and reducing morbidity and mortality.50-55 In fact, widespread use of modern ART regimens is commonly credited as the driving force behind improved longevity for HIV-positive individuals and the consequent growth of the OALWH population.17,56 However, OALWH who abuse substances typically exhibit poorer ART adherence and are at a higher risk for progressing to AIDS or death.40,57 Older adults living with HIV are also uniquely susceptible to experiencing a confluence of depression, HIV stigma, and social isolation,43 which are all independently associated with decreased ART adherence.11,58,59 Notably, Blashill et al60 recently reported that multiple psychosocial challenges synergistically influence odds of ART nonadherence, with each additional burden increasing the likelihood of nonadherence. Additional research is necessary to disentangle the downstream immunological effects of commonly co-occurring psychosocial issues in OALWH.
Negative psychosocial factors are rarely experienced in isolation among OALWH, and the co-occurrence of depression, loneliness, HIV stigma, and substance use problems may cumulatively hasten HIV disease progression. Further inquiry is needed to elucidate the contributions of discrete psychosocial influences on immune health. The present study aimed to expand the scope of the extant literature by examining the direct effects of several psychosocial factors—depression, loneliness, alcohol and drug use problems, and HIV stigma—on ART adherence, VL suppression, and percentage of CD4+ lymphocyte count in OALWH. Secondarily, we sought to examine the significance of the pathways from these psychosocial stressors through ART adherence to VL suppression and percentage of CD4 count.
Methods
Procedure
The present study analyzed baseline data from a larger research project, Wellness in Spirituality and Education, testing a behavioral intervention targeted to improve ART adherence and reduce substance use among OALWH. Participants were primarily recruited from AIDS service and community-based organizations across New York City and, to a lesser degree, internet-based advertisements and referrals. Inclusion criteria required participants to be (1) HIV positive, (2) 50 years of age or older, (3) prescribed ART, (4) suboptimally adherent (ie, missed ≥3 days in the past 30 days), (5) meeting dependence on drugs and/or alcohol, (6) currently using drugs and/or alcohol (ie, within the past year), and (7) English speaking. Further procedural details have been previously described.61
Ethical Approval and Informed Consent
The institutional review board of the City University of New York (protocol no. 29365-6) approved all study protocols and procedures. All participants provided written informed consent prior to study enrollment.
Measures
Participants completed a series of self-report measures via audio computer-assisted interviewing, a staff-guided timeline follow-back (TLFB) interview that captured medication adherence and substance use, and a blood draw to assess biological outcomes.
Demographics
Participants provided information on the following demographic characteristics: age, race, ethnicity, gender, sexual identity, employment status, annual income, education level, and relationship status (ie, single or partnered).
Depressive symptoms
The 15-item Geriatric Depression Scale (GDS-15) was used to assess depression.62 The GDS-15 employs a dichotomized response format (ie, yes or no) and scores may range between 0 and 15, with higher scores indicating higher depressive symptomology. This measure has been previously validated for assessing depression in OALWH.63
Loneliness
Loneliness was evaluated using the 8-item UCLA Loneliness Scale (ULS-8), which is a reliable and valid substitute for the longer original version.64 The ULS-8 asks participants to rate how often they felt affected by loneliness (eg, “I feel isolated from others”) in the past week on a 4-point scale, ranging from (1) = never to (4) = often. Higher scores indicate greater levels of loneliness.
HIV stigma
HIV stigma was measured with a condensed version of the HIV Stigma Scale.65 The 10-item HIV Stigma Scale66 has been used in prior research and demonstrates good validity and reliability.67,68 It includes items from each of the original measure’s 4 subscales (ie, Personalized Stigma, Disclosure, Negative-Self Image, and Public Attitudes), such as “Having HIV makes me feel unclean” and “I have lost friends by telling them I have HIV.” Participants rate how much they agree with each statement on a 5-point scale, with higher scores indicating greater levels of HIV stigma.
Alcohol use problems
Alcohol use problems were measured with the Alcohol Use Disorders Identification Test—Consumption, which is a brief, reliable tool for identifying individuals who engage in hazardous drinking or currently have an alcohol use disorder.69 Possible scores range from 0 to 12, with scores ≥3 and ≥4 indicating problem drinking in women and men, respectively.
Drug use problems
The 10-item Drug Abuse Screening Test (DAST) was used to assess problems with drug use.70 The DAST is widely used in both clinical screening and treatment research settings and has consistently demonstrated moderate to high levels of reliability and validity.71 The DAST scores range from 0 to 10, and scores of ≥3 indicate problematic use.
Antiretroviral therapy adherence
Trained research staff recorded daily medication adherence with a 30-day TLFB interview.72 Participants indicated the days they missed taking ART on a 30-day calendar, beginning with the day prior to their assessment, with the guidance of trained staff. The TLFB has demonstrated good test–retest reliability and convergent and discriminant validity for assessing event-level behaviors,73 and it has previously been used to assess ART adherence in substance-using populations.74
Viral load
Participants provided a blood sample on-site drawn by a certified phlebotomist for running HIV-1 RNA quantitative polymerase chain reaction (ie, VL) assays. Undetectable VL was defined as <200 copies/mL.
Percentage of CD4 count
Participant blood samples were also used to carry out lymphocyte panel analysis. The percentage of CD4 count per 100 cells (ie, percent CD4) was calculated via flow cytometry.
Analytic Plan
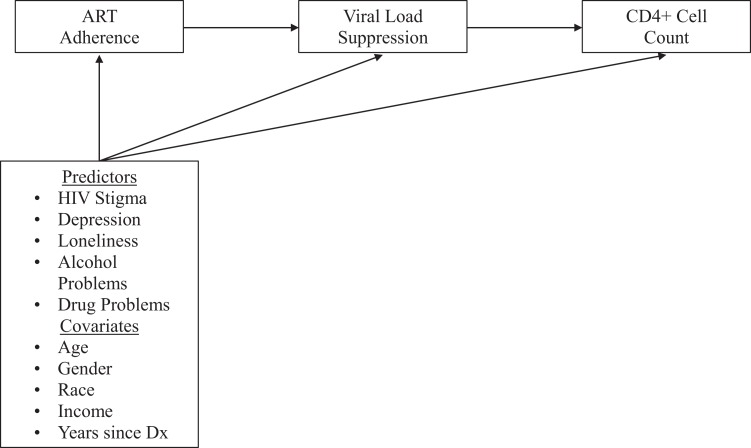
To analyze the association between psychosocial factors and CD4 count, we examined bivariate Pearson correlations. Subsequently, multivariate path analysis in Mplus version 8 was used to examine the outcomes of ART adherence, VL suppression, and percent CD4 count with psychosocial variables as predictors of each, adjusting for gender, race/ethnicity, income, age, and years since HIV diagnosis (Figure 1). We adjusted for these covariates based on the understanding that each might exert its own influence on the 3 outcomes. Antiretroviral therapy adherence was modeled as a continuous percentage, VL suppression as a dichotomous outcome with a probit link (with ART adherence as a predictor), and percent CD4 count as a continuous outcome (with VL suppression as a predictor). Models were run with Bayesian estimation using 5000 iterations; besides this, the Mplus defaults were used (ie,
Figure 1.
Hypothesized multivariate path model of psychosocial stressors and HIV-related health outcomes.
Results
The sociodemographic characteristics of the sample are presented in Table 1. The sample of 120 OALWH was predominantly Black (76%) and reported earning less than US$20 000 per year (80%). On average, participants were 54.6 years of age (standard deviation [SD] = 4.1; range = 50-66). The sample was nearly evenly split in terms of having postsecondary educational experiences or not, and there was diversity in terms of gender and sexual orientation, with women representing approximately one-third of the sample and gay and bisexual men comprising 43% of the sample.
Table 1.
Demographic Characteristics of the Sample.a
| n (%) | |
|---|---|
| Race and ethnicity | |
| Black | 91 (75.8) |
| Latino | 11 (9.2) |
| White | 9 (7.5) |
| Multiracial/other | 9 (7.5) |
| Education | |
| High school or less | 57 (47.5) |
| Some college | 63 (52.5) |
| Gender and sexual identity | |
| Straight men | 29 (24.2) |
| Gay and bisexual men | 52 (43.3) |
| Women | 39 (32.5) |
| Income | |
| Less than US$20 000 | 96 (80.0) |
| US$20 000 or more | 24 (20.0) |
| M (SD) | |
| Age | 54.6 (4.1) |
Abbreviations: M, mean; SD, standard deviation.
a N = 120.
Descriptive statistics for and bivariate correlations among the variables of interest are displayed in Table 2. In regard to HIV-related health characteristics, mean length of time living with HIV was 17.1 years (SD = 6.8), past-month mean adherence was 60% (ie, ART was taken on 18/30 days), and just under half (49.2%) of participants were virally suppressed. Bivariate analyses revealed several associations. Age was significantly associated with greater likelihood of having an undetectable VL at baseline. Greater drug-related problems were also significantly associated with greater alcohol-related problems, as well as with more loneliness. As expected, higher ART adherence was associated with greater likelihood of viral suppression and being virally suppressed was associated with higher percentages of CD4+ lymphocyte count, while ART adherence and percentage of CD4 count were not themselves significantly associated. Notably, HIV stigma was associated with the greatest number of other variables. HIV stigma was positively associated with experiences of depressive symptoms, loneliness, alcohol problems, and drug use problems and was negatively associated with years since HIV diagnosis and percentage of CD4 count.
Table 2.
Bivariate Correlations between Demographic Covariates, Psychosocial Predictors, and HIV-Related Health Outcomes.a
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Age | - | ||||||||||||
| 2. Gender (1 = female) | 0.08 | - | |||||||||||
| 3. Race (1 = White) | −0.05 | −0.55b | - | ||||||||||
| 4. Income (1 = US$30 000+) | −0.08 | −0.12 | −0.02 | - | |||||||||
| 5. Years since HIV diagnosis | 0.13 | −0.11 | 0.03 | −0.07 | - | ||||||||
| 6. HIV stigma | −0.10 | −0.06 | −0.22 | 0.14 | −0.19c | - | |||||||
| 7. Depressive symptoms | −0.03 | 0.11 | −0.12 | −0.02 | 0.09 | 0.36d | - | ||||||
| 8. Loneliness | −0.06 | −0.22 | −0.01 | −0.2 | −0.04 | 0.39d | 0.53d | - | |||||
| 9. Alcohol use problems | 0.06 | −0.13 | −0.09 | 0.17 | 0.10 | 0.19b | −0.01 | 0.04 | - | ||||
| 10. Drug use problems | 0.13 | −0.09 | −0.24 | 0.02 | −0.03 | 0.33d | 0.15 | 0.26b | 0.25d | - | |||
| 11. ART adherence | 0.12 | 0.16 | −0.03 | 0.07 | −0.06 | −0.06 | 0.04 | 0.06 | −0.07 | 0.01 | - | ||
| 12. Viral load (1 = undetectable) | 0.26c | 0.13 | −0.19 | −0.33 | 0.02 | 0.12 | 0.04 | 0.01 | −0.02 | 0.07 | 0.39d | - | |
| 13. CD4 count percent | 0.10 | 0.20 | −0.01 | −0.08 | −0.12 | −0.18c | 0.00 | −0.03 | 0.01 | −0.07 | 0.12 | 0.23c | - |
| M | 54.5 | - | - | - | 17.1 | 22.4 | 11.4 | 18.2 | 5.8 | 6.8 | 0.6 | - | 25.6 |
| % | - | 32.8% | 13.4% | 6.7% | - | - | - | - | - | - | - | 49.2% | - |
| Cronbach α | - | - | - | - | - | 0.87 | 0.83 | 0.86 | 0.77 | 0.78 | - | - | - |
Abbreviation: ART, antiretroviral therapy.
a N = 120. Correlations calculated with Mplus Estimator = Bayes.
b P < .01.
c P < .05.
d P < .001.
The results of the multivariate path analyses are displayed in Table 3. Regressing the ART adherence percentage onto the sociodemographic and psychosocial stressors, we found none that emerged as independently significant predictors, which was consistent with the bivariate findings. Nonetheless, the model as a whole explained 12% of the variability in ART adherence, which was statistically significant. Regressing whether or not individuals had an undetectable VL onto the sociodemographic factors, psychosocial stressors, and ART adherence, the only variable that emerged as an independently significant predictor was ART adherence—individuals with higher levels of adherence also had a higher likelihood of being virally suppressed. These findings were primarily consistent with the bivariate findings, whereby adherence was strongly associated with VL suppression, though age no longer emerged as independently significant in the multivariate model, which predicted 37% of the variability in VL suppression despite few independently significant predictors. Finally, regressing percent CD4 onto the sociodemographic factors, psychosocial stressors, and being virally suppressed, we found that lower levels of stigma and being virally suppressed were the only 2 variables that were positively associated with greater percent CD4 which mirrored the bivariate associations. The combined model explained 20% of the variability in percent CD4. We conducted sensitivity analyses by dichotomizing adherence at 80% and found no meaningful changes in the pattern of findings in predicting the adherence outcome (adherence remained a significant predictor of VL).
Table 3.
Results of the Multivariate Model Predicting Behavioral and Biological Health Outcomes for HIV-Positive Older Adults.a
| ART Adherence | Undetectable Viral Load | Percentage of CD4 Count | |||||||
|---|---|---|---|---|---|---|---|---|---|
| B | 95% CI | β | B | 95% CI | β | B | 95% CI | β | |
| Age | 0.82 | −0.46 to 2.12 | 0.11 | 0.04 | −0.03 to 0.11 | 0.13 | 0.10 | −0.49 to 0.67 | 0.03 |
| Gender (1 = female) | 8.05 | −4.05 to 19.53 | 0.13 | 0.00 | −0.57 to 0.57 | 0.00 | 2.89 | −2.08 to 8.11 | 0.11 |
| Race (1 = White) | −0.17 | −16.41 to 15.65 | 0.00 | −0.41 | −1.15 to 0.33 | −0.11 | 1.17 | −5.39 to 8.07 | 0.03 |
| Income (1 = US$30 000+) | 7.44 | −13.49 to 28.99 | 0.07 | −1.04 | −2.20 to 0.01 | −0.22 | 1.68 | −7.32 to 11.08 | 0.03 |
| Years since HIV diagnosis | −0.19 | −0.99 to 0.58 | −0.04 | 0.01 | −0.03 to 0.05 | 0.05 | −0.33 | −0.67 to 0.01 | −0.17 |
| HIV stigma | −0.36 | −1.29 to 0.63 | −0.08 | 0.04 | −0.01 to 0.08 | 0.19 | −0.51 | −0.92 to −0.09 | −0.26b |
| Depressive symptoms | −0.06 | −1.10 to 0.99 | −0.01 | 0.00 | −0.06 to 0.05 | −0.02 | 0.15 | −0.31 to 0.61 | 0.07 |
| Loneliness | 0.68 | −0.49 to 1.86 | 0.13 | −0.02 | −0.08 to 0.04 | −0.10 | 0.13 | −0.38 to 0.64 | 0.06 |
| Alcohol use problems | −0.49 | −2.25 to 1.30 | −0.05 | −0.02 | −0.11 to 0.07 | −0.04 | 0.37 | −0.40 to 1.14 | 0.09 |
| Drug use problems | 0.08 | −2.14 to 2.22 | 0.01 | 0.00 | −0.11 to 0.11 | 0.00 | −0.17 | −1.15 to 0.79 | −0.03 |
| ART adherence | - | - | - | 0.02 | 0.01 to 0.03 | 0.40c | - | - | - |
| Viral load (1 = undetectable) | - | - | - | - | - | - | 2.54 | 0.13 to 4.95 | 0.25b |
| R 2 | 0.12c | 0.37c | 0.20c | ||||||
Abbreviations: ART, antiretroviral therapy; 95% CI, 95% credibility interval.
a N = 120. Analyses were conducted as simultaneous regressions using Bayes estimation. Undetectable viral load was treated as a binary variable with a logit link.
b P < .05.
c P < .001.
Because no psychosocial factors significantly predicted the adherence or VL outcomes, we did not test indirect effects related to these variables. However, we did identify a significant indirect effect of adherence on CD4 count through VL suppression (B = 3.83, 95% credibility interval: 0.21-8.98, β = 0.09).
Discussion
The present study examined the influence of several psychosocial stressors—depression, substance abuse, loneliness, and HIV stigma—on 3 HIV-related health outcomes—ART adherence, VL suppression, and percentage of CD4+ lymphocyte count—in a sample of OALWH with a history of substance-related problems. Bivariate analyses revealed a high degree of association among all psychosocial stressors, which supports the idea that OALWH are vulnerable to experiencing a unique set of co-occurring psychosocial challenges and is consistent with HIV syndemics.75 As expected, bivariate and multivariate models showed significant associations between higher ART adherence and VL suppression, and in turn, VL suppression was associated with higher percent CD4, which follows existing research on the immune-enhancing action of ART.50,51 In contrast to our expectation that multiple stressors would additively impact immunologic function, HIV stigma emerged as the sole psychosocial influence on a marker of disease progression in both bivariate and multivariate analyses. HIV stigma was the only psychosocial factor associated with percent CD4 lymphocyte in bivariate analyses, and at the multivariate level, lower HIV stigma remained independently associated with greater percent CD4, that is, participants who subjectively reported experiencing less HIV stigma yielded objectively better immune health results, adjusting for all other psychosocial factors, sociodemographics, and VL suppression.
The primary aim of this study was to evaluate the direct effects of co-occurring psychosocial stressors on 3 HIV-related health outcomes in OALWH. Bivariate results supported the presence of multiple, interrelated psychosocial burdens, but the co-occurrence of these challenges did not appear to additively influence immune health. Instead, our results identified HIV stigma as the singular psychosocial predictor of percent CD4 while none of the psychosocial variables was significantly associated with ART adherence or VL suppression.
The lack of a direct influence of HIV stigma on ART adherence or VL implies a potentially direct link between HIV stigma and percent CD4, or, alternatively, the existence of a mediating factor not explored within the current analyses. This finding diverges from previous work suggesting that HIV stigma negatively impacts HIV-related health behaviors (eg, ART adherence), and secondarily, nonadherence facilitates HIV disease progression.76,77 However, the notion of a direct link between HIV stigma and physical health has met preliminary support in a limited number of similar, but methodologically diverse studies.78 For example, Lipira et al47 found that higher HIV stigma predicted lower odds of VL suppression, independent of ART nonadherence. Relatedly, Kay et al44 observed that both ART nonadherence and experienced HIV stigma predicted VL nonsuppression; however, the authors concluded that adherence did not mediate the association between HIV stigma and VL. Likewise, in the first study to examine HIV stigma’s impact on physical health, Earnshaw and colleagues79 found that enacted HIV stigma (ie, experiencing discrimination as a result of being HIV positive) was associated with having a CD4 count <200, while anticipated HIV stigma (ie, expectations of discrimination from others due to one’s HIV status) was associated with greater chronic illness comorbidity. In a subsequent study, Earnshaw et al80 demonstrated that enacted HIV stigma was directly associated with higher self-reported HIV symptom severity ratings. Despite variation in stigma terminology and outcome variables, these studies converge on the idea that HIV stigma has a direct and detrimental effect on physical health. Whether the association between HIV stigma and CD4 count is mediated by biological processes, or another, presently unknown third variable, remains an important question for future research. One possible mechanism underlying this association is a stigma-induced, proinflammatory response similar to that observed in HIV-positive individuals with depression.11 In other words, the chronic stress associated with HIV stigma may trigger increases in circulating proinflammatory cytokines, and chronically high levels of cytokines would produce immunosuppressant effects. Nonetheless, the current results suggest that HIV stigma may critically compromise immune health in OALWH.
The lack of association between the remaining psychosocial predictors and immune outcomes was surprising. Previous literature has demonstrated that depression, substance use, and social isolation are typically associated with ART nonadherence and consequently, unfavorable HIV-related health outcomes.37,60 One possible explanation is that this particular sample, who had, on average, been living with HIV and substance use problems for a substantial amount of time, developed personal coping strategies for concurrently managing chronic physical and psychosocial burdens. Combined with years of experience with ART regimens, it is conceivable that these participants developed a unique resilience to traditional adherence barriers. Age was also positively associated with having an undetectable VL, corroborating previous empirical findings that older, more “ART-experienced” individuals are more likely to be virally suppressed.81,82 On the other hand, the lack of significant findings for these other variables may have been due to sample features and methodology. For example, suboptimal ART adherence was required to participate in this study, and just under half (49.2%) of the participant sample had reached VL suppression. Relatedly, the sample overall had high levels of psychosocial stressors. Consequently, it remains equally plausible that the potential negative influences of depression, substance use, and loneliness were masked by their relatively high levels combined with the relatively low ART adherence rates consistently reported across the participant sample.
A related aim of this study was to assess the indirect effects of concurrent psychosocial stressors on immune health outcomes in OALWH, via the downstream effect of ART adherence on VL and, subsequently, VL on CD4 count. We confirmed our hypotheses that greater adherence would predict VL suppression and in turn, VL suppression would predict higher CD4 levels, which bolsters prior work demonstrating the immune-enhancing effects of ART adherence.51,55 Moreover, we demonstrated a significant indirect effect of adherence on CD4 through VL suppression, despite no direct association between ART adherence and percent CD4, suggesting the link between these operates through VL suppression. Exploring the possibility of these indirect pathways remains an important avenue for future research.
Results from the bivariate analyses suggest that OALWH manage numerous interrelated psychosocial challenges. Specifically, higher levels of depressive symptoms were associated with higher levels of loneliness; increased loneliness was associated with increased drug problems; more drug problems were associated with more alcohol problems; and higher HIV stigma experiences were strongly associated with higher levels of depression, loneliness, and both drug and alcohol problems. These results align with previous literature describing the intersection of physical, psychological, and social conditions that place OALWH at a higher risk for experiencing numerous, co-occurring psychosocial issues.22,26,43 The interconnected nature of these burdens is also highly consistent with decades of empirical insight gleaned from HIV syndemics literature. This line of research centers on the idea that HIV-positive individuals endure marginalizing social experiences at disproportionate rates, and these adverse experiences “snowball” over time, ultimately exacerbating interrelated, co-occurring health burdens.75,83 Essentially, the negative aspects of individual health conditions and challenges synergistically interact to worsen overall health outcomes and well-being. As an example, Glynn et al84 explored the association between the number of syndemics endorsed by a diverse sample of HIV-positive adults (mean age = 50) and HIV-related health outcomes, which revealed that each syndemic factor (including HIV stigma) significantly increased the odds of suboptimal ART adherence, transmission risk behavior, and detectable VL. In the context of the present results, it may be concluded that depression, drug and alcohol problems, loneliness, and HIV stigma represent syndemic factors that decrease overall health in OALWH. Notably, HIV stigma was the only psychosocial stressor that was significantly associated with each of the other psychosocial variables in the current study, further highlighting the significant role of HIV stigma in the well-being of OALWH. There is a relative paucity of research describing how HIV stigma affects OALWH specifically, but existing data have demonstrated that HIV stigma is negatively associated with psychological well-being in aging individuals.28,42
These results underscore the importance of considering HIV stigma in the testing and development of effective interventions for OALWH with substance use-related problems and, more broadly, add to a growing literature on the negative impact of HIV stigma.28,80,85 Adults aged 50 and older will soon represent the majority of the HIV-positive population in the United States, yet our health-care system is currently unequipped to effectively address the complex health-care needs of OALWH, especially those who struggle with substance use issues. For example, a 2009 study found that just 7% of US substance abuse treatment facilities offer targeted intervention programs for older adults.86 Ostensibly, OALWH may be motivated to avoid these limited treatment options due to HIV stigma concerns. Indeed, a more recent study revealed that, among OALWH currently using illicit drugs, 37% had never enrolled in a substance use treatment program, 35% had been in treatment at least once, and only 28% were currently in treatment.87 Thus, there is a clear and present need to develop effective substance use intervention programs for OALWH that also have a strong emphasis on psychosocial stressors common among OALWH, particularly HIV stigma. Given the breadth of social, mental, and physical health burdens commonly faced by the aging HIV-positive population, the need to develop comprehensive models of long-term care for OALWH is becoming increasingly apparent.88 Ideally, an integrative HIV primary care setting would offer OALWH a one-stop shop to receive routine medical, substance use, and mental health care from expert providers, trained to understand the unique challenges of growing older with HIV. The present results support the idea that effectively addressing HIV stigma in such an environment could produce tangible improvements in the biopsychosocial health of OALWH.
Strengths and Limitations
These findings should be regarded within the context of several potential limitations. The present study was derived from a larger investigation aimed at evaluating the efficacy of a substance use intervention. Consequently, the recruited sample consisted of OALWH interested in reducing substance use behaviors. Moreover, this relatively small sample was largely comprised of participants who were Black, sexual minority men of low socioeconomic status, living in New York City who, on average, had been living with HIV for a relatively long time. As such, the current results may not be generalizable to the broader population of OALWH. However, in terms of intervention development, the unique sociodemographic characteristics of this high-risk sample may also serve as a significant strength of the study. This sample represents a historically underserved and currently understudied subset of the HIV-positive population, although the majority of participants (ie, sexual minority men of color) represent the group most impacted by HIV.89 Arguably, these individuals stand to gain the most from targeted, stigma-focused intervention research and practice—the efficacy and effectiveness of which is dependent upon the depth and nuance of our understanding of these individuals’ health needs.
Although self-report data may present concerns, our multimethod approach remains a notable strength of the study, allowing us to integrate self-report, psychosocial data and objective, biological data to form conclusions about the biopsychosocial impact of HIV stigma. Future research should consider implementing objective measures of adherence (eg, pill counts) to complement objective measures of immune health. Finally, the present cross-sectional study exclusively analyzed baseline data, limiting our ability to establish temporal order and durability between variables. Going forward, researchers should consider implementing observational, longitudinal designs.
Conclusions
Findings from the current study extend the literature by highlighting the critical influence of HIV stigma on a primary marker of immune functioning— percent CD4 count—in OALWH, along with its significant impact on a range of other co-occurring psychosocial stressors. This result may help to explain the variability in disease progression that persists even in the era of highly effective ART. Perhaps more importantly, this study provides preliminary support for the notion that reducing HIV stigma could improve the biopsychosocial health of OALWH, thus improving overall well-being and HIV-related morbidity and mortality rates among this vulnerable population. HIV stigma represents an essential factor to address in future research endeavors and in the development of structural and individual interventions with OALWH, particularly within substance use interventions. As this population continues to grow and expand, it will be increasingly vital to develop a comprehensive understanding of the degree and direction of influence of psychosocial factors on immune health, in order to optimize the physical, mental, and immunological health and well-being of OALWH.
Acknowledgments
The authors would like to acknowledge the contributions of the WISE Project Team: Tyrel Starks, Ethan Fusaris, Sitaji Gurung, Margaret Wolff, Zak Hill-Whilton, Theresa Navalta, Michael Castro, Ruben Jimenez, Chloe Mirzayi, Anita Viswanath, David Marcotte, Chris Hietikko, Kailip Boonrai, and our team of research assistants, recruiters, and interns. The authors also thank the participants who volunteered their time for this study.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclose receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institute on Drug Abuse [R01-DA029567, K01-DA039030].
ORCID iD: H. Jonathon Rendina, PhD, MPH  https://orcid.org/0000-0002-0148-2852
https://orcid.org/0000-0002-0148-2852
Jeffrey T. Parsons, PhD  https://orcid.org/0000-0002-6875-7566
https://orcid.org/0000-0002-6875-7566
References
- 1. Ironson GH, Hayward HS. Do positive psychosocial factors predict disease progression in HIV-1? A review of the evidence. Psychosom Med. 2008;70(5):546–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Langford SE, Ananworanich J, Cooper DA. Predictors of disease progression in HIV infection: a review. AIDS Res Ther. 2007;4(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Evans DL, Leserman J, Perkins DO, et al. Severe life stress as a predictor of early disease progression in HIV infection. Am J Psychiatry. 1997;154(5):630–634. [DOI] [PubMed] [Google Scholar]
- 4. Leserman J. Role of depression, stress, and trauma in HIV disease progression. Psychosom Med. 2008;70(5):539–545. [DOI] [PubMed] [Google Scholar]
- 5. Fuster D, Cheng DM, Quinn EK, et al. Inflammatory cytokines and mortality in a cohort of HIV-infected adults with alcohol problems. AIDS. 2014;28(7):1059–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Antoni MH. Stress management effects on psychological, endocrinological, and immune functioning in men with HIV infection: empirical support for a psychoneuroimmunological model. Stress. 2003;6(3):173–188. [DOI] [PubMed] [Google Scholar]
- 7. Perez GK, Cruess DG, Kalichman SC. Effects of stress on health in HIV/AIDS In: Contrada RJ, Baum A, eds. The Handbook of Stress Science. New York, NY: Springer; 2011:447–460. [Google Scholar]
- 8. Chida Y, Vedhara K. Adverse psychosocial factors predict poorer prognosis in HIV disease: a meta-analytic review of prospective investigations. Brain Behav Immun. 2009;23(4):434–445. [DOI] [PubMed] [Google Scholar]
- 9. Farinpour R, Miller EN, Satz P, et al. Psychosocial risk factors of HIV morbidity and mortality: findings from the Multicenter AIDS Cohort Study (MACS). J Clin Exp Neuropsychol. 2003;25(5):654–670. [DOI] [PubMed] [Google Scholar]
- 10. Whetten K, Reif S, Whetten R, Murphy-McMillan LK. Trauma, mental health, distrust, and stigma among HIV-positive persons: implications for effective care. Psychosom Med. 2008;70(5):531–538. [DOI] [PubMed] [Google Scholar]
- 11. Nanni MG, Caruso R, Mitchell AJ, Meggiolaro E, Grassi L. Depression in HIV infected patients: a review. Curr Psychiatry Rep. 2015;17(1):530. [DOI] [PubMed] [Google Scholar]
- 12. Chander G, Himelhoch S, Moore RD. Substance abuse and psychiatric disorders in HIV-positive patients: epidemiology and impact on antiretroviral therapy. Drugs. 2006;66(6):769–789. [DOI] [PubMed] [Google Scholar]
- 13. Turner BJ, Fleishman JA, Wenger N, et al. Effects of drug abuse and mental disorders on use and type of antiretroviral therapy in HIV-infected persons. J Gen Intern Med. 2001;16(9):625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Substance Abuse and Mental Health Services Administration. Summary of the Effects of the 2015. National Survey on Drug Use and Health Questionnaire Redesign: Implications for Data Users. CBHSQ Methodology Report https://www.samhsa.gov/data/sites/default/files/NSDUH-TrendBreak-2015.pdf. Accessed January 15, 2019. [PubMed]
- 15. Kalichman SC, Simbayi LC, Cloete A, Mthembu PP, Mkhonta RN, Ginindza T. Measuring AIDS stigmas in people living with HIV/AIDS: the Internalized AIDS-Related Stigma Scale. AIDS Care. 2009;21(1):87–93. [DOI] [PubMed] [Google Scholar]
- 16. Baugher AR, Beer L, Fagan JL, et al. Prevalence of internalized HIV-related stigma among HIV-infected adults in care, United States, 2011–2013. AIDS Behav. 2017;21(9):2600–2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Antiretroviral Therapy Cohort Collaboration. Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. Lancet HIV. 2017;4(8):e349–e356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Centers for Disease Control and Prevention, Division of HIV/AIDS Prevention. HIV Surveillance Report. 2011. https://www.cdc.gov/hiv/pdf/statistics_2011_HIV_Surveillance_Report_vol_23.pdf. Accessed January 15, 2019.
- 19. Centers for Disease Control and Prevention, Division of HIV/AIDS Prevention. HIV Surveillance Report. 2016. https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-report-2016-vol-28.pdf. Accessed January 15, 2019.
- 20. Xia Q, Westheimer E, Robbins RS, Torian LV. Persons living with diagnosed HIV in New York City: over 50% over 50 years old. AIDS Care. 2018;30(4):531–534. [DOI] [PubMed] [Google Scholar]
- 21. O’Keefe KJ, Scheer S, Chen M-J, Hughes AJ, Pipkin S. People fifty years or older now account for the majority of AIDS cases in San Francisco, California. AIDS Care. 2013;25(9):1145–1148. [DOI] [PubMed] [Google Scholar]
- 22. Brennan M, Karpiak S, Cantor M, Shippy R. Older Adults with HIV: an In-depth Examination of an Emerging Population. New York: Nova Science Publishers; 2009. [Google Scholar]
- 23. High KP, Brennan-Ing M, Clifford DB, et al. HIV and aging: state of knowledge and areas of critical need for research. A report to the NIH Office of AIDS Research by the HIV and Aging Working Group. J Acquir Immune Defic Syndr. 2012;60(suppl 1): 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Green TC, Kershaw T, Lin H, et al. Patterns of drug use and abuse among aging adults with and without HIV: a latent class analysis of a US veteran cohort. Drug Alcohol Depend. 2010;110(3):208–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Morgan EE, Iudicello JE, Weber E, et al. Synergistic effects of HIV infection and older age on daily functioning. J Acquir Immune Defic Syndr. 2012;61(3):341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wing EJ. HIV and aging. Int J Infect Dis. 2016;53:61–68. [DOI] [PubMed] [Google Scholar]
- 27. Karpiak SE, Havlik R. Are HIV-infected older adults aging differently? Interdiscip Top Gerontol Geriatr. 2017;42:11–27. [DOI] [PubMed] [Google Scholar]
- 28. Porter KE, Brennan-Ing M, Burr JA, Dugan E, Karpiak SE. Stigma and psychological well-being among older adults with HIV: the impact of spirituality and integrative health approaches. Gerontologist. 2017;57(2):219–228. [DOI] [PubMed] [Google Scholar]
- 29. Sankar A, Nevedal A, Neufeld S, Berry R, Luborsky M. What do we know about older adults and HIV? A review of social and behavioral literature. AIDS Care. 2011;23(10):1187–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Grov C, Golub SA, Parsons JT, Brennan M, Karpiak SE. Loneliness and HIV-related stigma explain depression among older HIV-positive adults. AIDS Care. 2010;22(5):630–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yoo-Jeong M, Hepburn K, Holstad M, Haardörfer R, Waldrop-Valverde D. Correlates of loneliness in older persons living with HIV. AIDS Care. 2019:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schuster R, Bornovalova M, Hunt E. The influence of depression on the progression of HIV: direct and indirect effects. Behav Modif. 2012;36(2):123–145. [DOI] [PubMed] [Google Scholar]
- 33. Ickovics JR, Hamburger ME, Vlahov D, et al. Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women: longitudinal analysis from the HIV Epidemiology Research Study. JAMA. 2001;285(11):1466–1474. [DOI] [PubMed] [Google Scholar]
- 34. Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71(2):171–186. [DOI] [PubMed] [Google Scholar]
- 35. Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65(9):732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rivera-Rivera Y, Vázquez-Santiago FJ, Albino E, Sánchez MD, Rivera-Amill V. Impact of depression and inflammation on the progression of HIV disease. J Clin Cell Immunol. 2016;7(3):423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ironson G, O’Cleirigh C, Fletcher MA, et al. Psychosocial factors predict CD4 and viral load change in men and women with human immunodeficiency virus in the era of highly active antiretroviral treatment. Psychosom Med. 2005;67(6):1013–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cook JA, Burke-Miller JK, Cohen MH, et al. Crack cocaine, disease progression, and mortality in a multi-center cohort of HIV-1 positive women. AIDS. 2008;22(11):1355–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kapadia F, Vlahov D, Wu Y, et al. Impact of drug abuse treatment modalities on adherence to ART/HAART among a cohort of HIV seropositive women. Am J Drug Alcohol Abuse. 2008;34(2):161–170. [DOI] [PubMed] [Google Scholar]
- 40. Cohn SE, Jiang H, McCutchan JA, et al. Association of ongoing drug and alcohol use with non-adherence to antiretroviral therapy and higher risk of AIDS and death: results from ACTG 362. AIDS Care. 2011;23(6):775–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Carrico AW. Substance use and HIV disease progression in the HAART era: implications for the primary prevention of HIV. Life Sci. 2011;88(21-22):940–947. [DOI] [PubMed] [Google Scholar]
- 42. Shippy RA, Karpiak SE. The aging HIV/AIDS population: fragile social networks. Aging Ment Health. 2005;9(3):246–254. [DOI] [PubMed] [Google Scholar]
- 43. Chambers LA, Wilson MG, Rueda S, Gogolishvili D, Shi MQ, Rourke SB. Evidence informing the intersection of HIV, aging and health: a scoping review. AIDS Behav. 2014;18(4):661–675. [DOI] [PubMed] [Google Scholar]
- 44. Kay ES, Rice WS, Crockett KB, Atkins GC, Batey DS, Turan B. Experienced HIV-related stigma in health care and community settings: mediated associations with psychosocial and health outcomes. J Acquir Immune Defic Syndr. 2018;77(3):257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Christopoulos KA, Neilands TB, Hartogensis W, et al. Internalized HIV stigma is associated with concurrent viremia and poor retention in a cohort of US patients in HIV care. J Acquir Immune Defic Syndr. 2019;82(2):116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kemp CG, Lipira L, Huh D, et al. HIV stigma and viral load among African-American women receiving treatment for HIV. AIDS. 2019;33(9):1511–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lipira L, Williams EC, Huh D, et al. HIV-related stigma and viral suppression among African-American women: exploring the mediating roles of depression and ART nonadherence. AIDS Behav. 2019;23(8):2025–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Attonito J, Dévieux JG, Lerner BDG, Hospital MM, Rosenberg R. Antiretroviral treatment adherence as a mediating factor between psychosocial variables and HIV viral load. J Assoc Nurses AIDS Care. 2014;25(6):626–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Carrico AW, Riley ED, Johnson MO, et al. Psychiatric risk factors for HIV disease progression: the role of inconsistent patterns of anti-retroviral therapy utilization. J Acquir Immune Defic Syndr. 2011;56(2):146–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133(1):21–30. [DOI] [PubMed] [Google Scholar]
- 51. García dOP, Knobel H, Carmona A, Guelar A, López-Colomés JL, Caylà JA. Impact of adherence and highly active antiretroviral therapy on survival in HIV-infected patients. J Acquir Immune Defic Syndr. 2002;30(1):105–110. [DOI] [PubMed] [Google Scholar]
- 52. Mills EJ, Nachega JB, Buchan I, et al. Adherence to antiretroviral therapy in sub-Saharan Africa and North America: a meta-analysis. JAMA. 2006;296(6):679–690. [DOI] [PubMed] [Google Scholar]
- 53. Dybul M, Fauci AS, Bartlett JG, Kaplan JE, Pau AK. Guidelines for using antiretroviral agents among HIV-infected adults and adolescents. Ann Intern Med. 2002;137(5 pt 2):381–433. [DOI] [PubMed] [Google Scholar]
- 54. Chesney M. Adherence to HAART regimens. AIDS Patient Care STDs. 2003;17(4):169–177. [DOI] [PubMed] [Google Scholar]
- 55. Broder S. The development of antiretroviral therapy and its impact on the HIV-1/AIDS pandemic. Antiviral Res. 2010;85(1):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bhaskaran K, Hamouda O, Sannes M, et al. Changes in the risk of death after HIV seroconversion compared with mortality in the general population. JAMA. 2008;300(1):51–59. [DOI] [PubMed] [Google Scholar]
- 57. Parsons JT, Starks TJ, Millar BM, Boonrai K, Marcotte D. Patterns of substance use among HIV-positive adults over 50: implications for treatment and medication adherence. Drug Alcohol Depend. 2014;139:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sweeney SM, Mitzel LD, Vanable PA. Impact of HIV-related stigma on medication adherence among persons living with HIV. Current Opin Psychol. 2015;5:96–100. [Google Scholar]
- 59. Turan B, Fazeli P, Raper JL, Mugavero MJ, Johnson MO. Social support and moment-to-moment changes in treatment self-efficacy in men living with HIV: psychosocial moderators and clinical outcomes. Health Psychol. 2016;35(10):1126–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Blashill AJ, Bedoya CA, Mayer KH, et al. Psychosocial syndemics are additively associated with worse ART adherence in HIV-infected individuals. AIDS Behav. 2015;19(6):981–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Millar BM, Starks TJ, Gurung S, Parsons JT. The impact of comorbidities, depression, and substance use problems on quality of life among older adults living with HIV. AIDS Behav. 2017;21(6):1684–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a Geriatric Depression Screening Scale: a preliminary report. J Psychiatr Res. 1982;17(1):37–49. [DOI] [PubMed] [Google Scholar]
- 63. Heckman TG, Barcikowski R, Ogles B, et al. A telephone-delivered coping improvement group intervention for middle-aged and older adults living with HIV/AIDS. Ann Behav Med. 2006;32(1):27–38. [DOI] [PubMed] [Google Scholar]
- 64. Hays RD, DiMatteo MR. A short-form measure of loneliness. J Pers Assess. 1987;51(1):69–81. [DOI] [PubMed] [Google Scholar]
- 65. Berger BE, Ferrans CE, Lashley FR. Measuring stigma in people with HIV: psychometric assessment of the HIV stigma scale. Res Nurs Health. 2001;24(6):518–529. [DOI] [PubMed] [Google Scholar]
- 66. Wright K, Naar-King S, Lam P, Templin T, Frey M. Stigma scale revised: reliability and validity of a brief measure of stigma for HIV+ youth. J Adolesc Health. 2007;40(1):96–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Li MJ, Murray JK, Suwanteerangkul J, Wiwatanadate P. Stigma, social support, and treatment adherence among HIV-positive patients in Chiang Mai, Thailand. AIDS Educ Prev. 2014;26(5):471–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kuhns LM, Hotton AL, Garofalo R, et al. An index of multiple psychosocial, syndemic conditions is associated with antiretroviral medication adherence among HIV-positive youth. AIDS Patient Care STDs. 2016;30(4):185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Dawson DA, Grant BF, Stinson FS, Zhou Y. Effectiveness of the derived alcohol use disorders identification test (AUDIT-C) in screening for alcohol use disorders and risk drinking in the US general population. Alcohol Clin Exp Res. 2005;29(5):844–854. [DOI] [PubMed] [Google Scholar]
- 70. Skinner HA. The Drug Abuse Screening Test. Addict Behav. 1982;7(4):363–371. [DOI] [PubMed] [Google Scholar]
- 71. Yudko E, Lozhkina O, Fouts A. A comprehensive review of the psychometric properties of the drug abuse screening test. J Subst Abuse Treat. 2007;32(2):189–198. [DOI] [PubMed] [Google Scholar]
- 72. Sobell LC, Sobell MB. Timeline follow-back. In: Litten RZ, Allen JP, eds. Measuring Alcohol Consumption. Totowa, NJ: Springer; 1992;41–72. [Google Scholar]
- 73. Weinhardt LS, Carey MP, Maisto SA, Carey KB, Cohen MM, Wickramasinghe SM. Reliability of the Timeline Followback sexual behavior interview. Ann Behav Med. 1998;20(1):25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Parsons JT, Rosof E, Mustanski B. Patient-related factors predicting HIV medication adherence among men and women with alcohol problems. J Health Psychol. 2007;12(2):357–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Singer M. AIDS and the health crisis of the U.S. urban poor; the perspective of critical medical anthropology. Soc Sci Med. 1994;39(7):931–948. [DOI] [PubMed] [Google Scholar]
- 76. Sumari-de Boer IM, Sprangers MA, Prins JM, Nieuwkerk PT. HIV stigma and depressive symptoms are related to adherence and virological response to antiretroviral treatment among immigrant and indigenous HIV infected patients. AIDS Behav. 2012;16(6):1681–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Vanable PA, Carey MP, Blair DC, Littlewood RA. Impact of HIV-related stigma on health behaviors and psychological adjustment among HIV-positive men and women. AIDS Behav. 2006;10(5):473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Rueda S, Mitra S, Chen S, et al. Examining the associations between HIV-related stigma and health outcomes in people living with HIV/AIDS: a series of meta-analyses. BMJ Open. 2016;6(7): e011453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Earnshaw VA, Smith LR, Chaudoir SR, Amico KR, Copenhaver MM. HIV stigma mechanisms and well-being among PLWH: a test of the HIV stigma framework. AIDS Behav. 2013;17(5):1785–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Earnshaw VA, Lang SM, Lippitt M, Jin H, Chaudoir SR. HIV stigma and physical health symptoms: do social support, adaptive coping, and/or identity centrality act as resilience resources? AIDS Behav. 2015;19(1):41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Barclay TR, Hinkin CH, Castellon SA, et al. Age-associated predictors of medication adherence in HIV-positive adults: health beliefs, self-efficacy, and neurocognitive status. Health Psychol. 2007;26(1):40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Hinkin CH, Hardy DJ, Mason KI, et al. Medication adherence in HIV-infected adults: effect of patient age, cognitive status, and substance abuse. AIDS. 2004;18(suppl 1):S19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Mustanski B, Garofalo R, Herrick A, Donenberg G. Psychosocial health problems increase risk for HIV among urban young men who have sex with men: preliminary evidence of a syndemic in need of attention. Ann Behav Med. 2007;34(1):37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Glynn TR, Safren SA, Carrico AW, et al. High levels of syndemics and their association with adherence, viral non-suppression, and biobehavioral transmission risk in Miami, a US city with an HIV/AIDS epidemic. AIDS Behav. 2019;23(11):2956–2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Emlet CA, Brennan DJ, Brennenstuhl S, Rueda S, Hart TA, Rourke SB. The impact of HIV-related stigma on older and younger adults living with HIV disease: does age matter? AIDS Care. 2015;27(4):520–528. [DOI] [PubMed] [Google Scholar]
- 86. Han B, Gfroerer JC, Colliver JD, Penne MA. Substance use disorder among older adults in the United States in 2020. Addiction. 2009;104(1):88–96. [DOI] [PubMed] [Google Scholar]
- 87. Ompad DC, Giobazolia TT, Barton SC, et al. Drug use among HIV+ adults aged 50 and older: findings from the GOLD II Study. AIDS Care. 2016;28(11):1373–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Siegler EL, Brennan-Ing M. Adapting systems of care for people aging with HIV. J Assoc Nurs AIDS Care. 2017;28(5):698–707. [DOI] [PubMed] [Google Scholar]
- 89. Centers for Disease Control and Prevention. HIV Among Gay and Bisexual Men. https://www.cdc.gov/hiv/group/msm/index.html. Accessed December 21, 2018.