Abstract
Background:
Stereotactic ablative radiotherapy (SABR) can deliver tumoricidal doses and achieve long-term control in early hepatocellular carcinoma (HCC). However, limited studies have investigated the safety and effectiveness of SABR in patients with advanced diseases that is unsuitable for transarterial chemoembolization (TACE).
Methods:
In this observational study, we reviewed the medical records of patients with Barcelona Clinic Liver Cancer (BCLC) stage C disease treated with linear accelerator-based SABR between 2008 and 2016. Their tumors were either refractory to TACE or TACE was contraindicated. Overall survival (OS), in-field progression-free survival (IFPFS), and out-field progression-free survival were calculated using Kaplan–Meier analysis. The Cox regression model was used to examine the effects of variables. Treatment-related toxicities were scored according to the Common Terminology Criteria for Adverse Events (version 4.03) and whether patients developed radiation-induced liver disease (RILD) after SABR.
Results:
This study included 32 patients. The mean maximal tumor diameter and tumor volumes were 4.7 cm and 135.9 ml, respectively. Patients received linear accelerator-based SABR with a median prescribed dose of 48 Gy (30–60 Gy) in three to six fractions. Based on the assessment of treatment response by using the Response Evaluation Criteria in Solid Tumors (version 1.1), 19% of patients achieved a complete response and 53% achieved a partial response. After a median follow-up of 18.1 months (4.0–65.9 months), 10, 19, and 9 patients experienced in-field failure, out-field hepatic recurrence, and extrahepatic metastases, respectively. The estimated 2-year OS and IFPFS rates were 54.4% and 62.7%, respectively. In a multivariate analysis, a pretreatment Cancer of the Liver Italian Program (CLIP) score of ⩾2 (p = 0.01) was a prognostic factor for shorter OS, and a biologically effective dose (BED) of < 85 Gy10 (p = 0.011) and a Child–Pugh score of ⩾6 (p = 0.014) were prognostic factors for inferior IFPFS. In this study five and eight patients developed classic and nonclassic RILD, respectively.
Conclusions:
SABR can serve as a salvage treatment for patients with HCC with BCLC stage C disease unsuitable for TACE, in particular, in those with a baseline CLIP score of ⩽1. A BED10 of ⩾85 Gy is an appropriate prescribed dose for tumor control. Because out-field relapse is the major cause of treatment failure, SABR in combination with novel systemic modalities should be investigated in future studies.
Keywords: Stereotactic ablative radiotherapy, hepatocellular carcinoma, Barcelona Clinic Liver Cancer stage C, transarterial chemoembolization, radiation-induced liver disease
Introduction
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related deaths worldwide. HCC is more common in the Asia-Pacific region than in other regions due to the higher prevalence of chronic hepatitis B virus infection in this region.1,2 Although surgical intervention and liver-directed nonsurgical therapies remain the mainstay of treatment for early-to-intermediate stage HCC, patients with HCC are typically diagnosed in the advanced stages of the disease, which are characterized by the presence of symptoms, portal vein thrombosis (PVT), or extrahepatic spread, for which radical treatments are often unfeasible and systemic therapy is the major treatment.1,3–6 Traditionally, since 2008, the tyrosine kinase inhibitor sorafenib has been the only standard treatment indicated for patients with HCC with Barcelona Clinic Liver Cancer (BCLC) stage C.7,8 Recently, systemic therapies, including multikinase and immune checkpoint inhibitors, have rapidly evolved and are now capable of prolonging the survival of patients with advanced HCC.1 However, intrahepatic tumor progression with consequent liver failure is the major cause of treatment failure.9,10
For patients with unresectable or multifocal HCCs, the reported objective response rate of transarterial chemoembolization (TACE) is 35–50%.11–13 TACE is considered a reasonable local treatment strategy because it delays intrahepatic tumor progression, therefore, providing potential survival benefits to selected patients with advanced HCC.10,14–16 However, patients with advanced HCC often present with a high tumor burden or macrovascular invasion, where TACE is less effective. Stereotactic ablative radiotherapy (SABR), a recent advancement in high-precision radiotherapy (RT), has been used as an alternative local therapy for early-stage HCC by delivering tumoricidal doses to hepatic tumors.17 Recently, early-phase trials have revealed that for patients with locally advanced HCC, SABR could provide substantial in-field tumor control while minimizing toxicity to the surrounding normal tissues.18,19
In this retrospective study, we analyzed the treatment outcomes of patients with advanced HCC unsuitable for TACE who received SABR to investigate the effectiveness and safety of SABR in these patients.
Methods
Patient eligibility
This retrospective study was conducted at Taipei Medical University Hospital and China Medical University Hospital in Taiwan. We reviewed the medical records of patients with HCC treated by SABR between January 2008 and December 2016. This research was reviewed and approved by the Taipei Medical University Joint Institutional Review Board (approval No.: N201706038). The need for informed consent was waived by the ethics committee according to ‘Article. 3 of the announcement named Range of Waiver of Informed Consent in Human Research from Ministry of Health and Welfare with official document No. 1010265083C on July 5, 2012’. This study is defined as minimal risk and the probability of harm or discomfort anticipated in this research are not greater than those ordinarily encountered in daily life. Thus, the exemption from obtaining consent does not affect the rights and interests of the research participant.
The inclusion criteria for this study were: histologically or radiologically confirmed HCC with BCLC stage C; SABR as the primary radical treatment modality for patients with advanced HCC refractory to TACE or with TACE contraindicated, and regular imaging follow-up 1–3 months after SABR and 3–6 months thereafter.
A strategic plan for cancer treatment for patients diagnosed with HCC was discussed by a multidisciplinary cancer team in both of the involved hospitals. Patients were deemed unsuitable for TACE if they had contraindications or were refractory to treatment.20 Contraindications included main or bilateral PVT without cavernous transformation, high bilirubin levels, and arterioportal or arteriovenous shunt. Tumors were considered refractory to TACE when targeted lesions demonstrated a progression or when viable parts over 50% appeared on computed tomography (CT) or magnetic resonance (MR) imaging, despite adequate TACE therapy being performed in two or more sessions within 6 months according to the consensus guideline of the Taiwan Liver Cancer Association.21
Stereotactic ablative radiotherapy
Patients who met the following criteria were eligible for SABR in Taipei Medical University Hospital and China Medical University Hospital: an Eastern Cooperative Oncology Group (ECOG) performance status of ⩽2; liver cirrhosis with a Child–Pugh score of A5–B7; normal liver reserve with a liver minus gross tumor volume (GTV) of >700 ml, and a distance of >1.0 cm between the tumor and the gastrointestinal tract.
The patients were immobilized with a vacuum cushion and a total body cover sheet, and they underwent forced shallow breathing through abdominal compression during CT simulation and RT. During CT simulation, four-dimensional CT images with a slice thickness of 3 mm were acquired after intravenous contrast injection. To define the targeted lesions for GTV delineation, the multiphase contrast-enhanced CT or MR images were imported into the planning system and fused with the images from CT simulation. For patients with tumor vascular invasion, the entire tumor was irradiated along with the involved vessels. The planning target volume (PTV) was determined by adding an internal margin and a setup margin to the GTV to compensate for the internal organ movement and positional uncertainties, respectively, according to the International Commission on Radiation Units Report 62.22 The dosimetry plans were calculated with Eclipse version 6.2 or 8.1 (Varian Medical Systems Inc., Palo Alto, CA, USA), Pinnacle (Philips Medical Systems, Inc. Milpitas, CA, USA), or TomoTherapy (TomoTherapy Inc., Madison, WI, USA). SABR was delivered using image-guided RT with a Varian Clinac iX linear accelerator (Varian Medical Systems Inc.), Elekta Synergy linear accelerator (Elekta AB, Stockholm, Sweden), or TomoTherapy accelerator (Accuray Inc., Sunnyvale, CA, USA) equipped with an online cone-beam CT device. The total prescribed dose ranged from 30 to 60 Gy in 3–6 fractions and was adapted according to liver and adjacent normal organ constraints. The aim was that 100% of the GTV and ⩾95% of the PTV were encompassed by the prescription isodose, which was normalized to the maximum dose. The protocol for the dose prescription and the normal tissue constraints are listed in appendix 1. The highest SABR doses that could maintain the normal tissue constraints were used.
To correlate the various fractionation schedules with treatment effectiveness, a biologically effective dose (BED) based on a linear-quadratic model was utilized.23 A BED at α/β of 10 (BED10) was calculated using the following formula: nd [1 + d/(α/β)], where n and d are the number of fractions and fraction size, respectively, and an α/β ratio of 10 Gy was assumed for liver tumors.
Follow-up and statistical analysis
The primary endpoint was overall survival (OS). The secondary endpoints were in-field progression-free survival (IFPFS) and out-field progression-free survival (OFPFS). Treatment responses were assessed using the Response Evaluation Criteria in Solid Tumors (version 1.1).24 Treatment-related toxicity was evaluated according to the Common Terminology Criteria for Adverse Events (CTCAE) (version 4.03).25 OS was defined as the duration from the SABR commencement date to the date of the last follow-up or death. IFPFS was defined as the duration from the SABR commencement date to the date of radiological progression, within the irradiated field or at the margin. OFPFS was defined as the duration between the SABR commencement date and the date of radiological progression outside the irradiated field. Contrast-enhanced CT or MR imaging was used to assess liver tumors. Extrahepatic diseases were evaluated using either CT or MR imaging, or bone scintigraphy for different sites. In the statistical analysis, the Wilcoxon signed-rank test and Mann–Whitney U test were used to compare continuous and ordinal variables for disease characteristics, respectively. The Chi-square test was used for comparing categorical variables. Univariate and multivariate analyses were performed using the Cox proportional hazards model. The Kaplan–Meier method was applied to plot survival curves, and the log-rank test was used to compare survival. A two-sided p value of <0.05 was considered statistically significant. Python 2.7 with SciPy module version 1.1.0 and Lifelines module version 0.14.6 were employed for the statistical analysis.
Results
Patients and treatment
A total of 32 patients with HCC with BCLC stage C disease met our inclusion criteria and were included in this study. All of the treated tumors were refractory to TACE or TACE was contraindicated. A total of 19 patients had portal vein tumor thrombus, and 3 others had extrahepatic metastases, 1 patient had both conditions. The remaining 11 patients were symptomatic with ECOG performance status classified as 1 or 2. The mean maximal tumor diameter was 4.7 ± 2.3 cm, and the mean GTV was 135.9 ± 250.3 ml. The median prescribed dose was 48 Gy (30–60 Gy). Patient characteristics are listed in Table 1.
Table 1.
Patient characteristics (n = 32).
| Characteristics | Number |
|---|---|
| Age, years, median (range) | 67 (42–91) |
| Sex | |
| Male | 24 (75) |
| Female | 8 (25) |
| ECOG performance status | |
| 0 | 13 (41) |
| 1–2 | 19 (59) |
| Hepatitis status | |
| HBV-related | 19 |
| HCV-related | 13 |
| Alcohol-related | 2 |
| Child–Pugh score | |
| 5 | 27 (84) |
| 6 | 3 (9) |
| 7 | 2 (6) |
| CLIP score | |
| 0 | 3 (9) |
| 1 | 17 (53) |
| 2 | 8 (25) |
| 3 | 2 (6) |
| 4 | 2 (6) |
| AJCC (seventh edition) stage | |
| I | 2 (6) |
| II | 9 (28) |
| III | 18 (56) |
| IV | 3 (9) |
| Portal vein thrombosis | |
| Yes | 19 (59) |
| No | 13 (41) |
| Extrahepatic metastasis | |
| Yes | 3 (9) |
| No | 29 (91) |
| AFP level, ng/dl (range) | 27.4 (1.9–48653.0) |
| Sequential use of sorafenib | |
| No | 15 (47) |
| Yes | 17 (53) |
| Maximal tumor diameter, cm (mean ± SD) | 4.7 ± 2.3 |
| Gross tumor volume, ml (mean ± SD) | 135.9 ± 250.3 |
| Treated tumor number | |
| 1 | 25 (78) |
| 2 | 6 (19) |
| 3 | 1 (3) |
| Total prescribed dose, Gy, median (range) | 48 (30–60) |
| Number of fractions, median (range) | 6 (3–6) |
| BED10, Gy, median (range) | 86 (45–120) |
| Normal liver reserve, ml, median (range) | 997.0 (716.0–1647.0) |
| Mean normal liver dose, Gy, median (range) | 11.68 (6.27–19.54) |
| Indication | |
| TACE-refractory | 13 (41) |
| TACE contraindicated | 19 (59) |
AFP, alpha-fetoprotein; AJCC, American Joint Committee on Cancer; BED10, biologically effective dose at an alpha/beta ratio of 10; CLIP, Cancer of the Liver Italian Program; ECOG, Eastern Cooperative Oncology Group; HBV, hepatitis B virus; HCV, hepatitis C virus; SABR, stereotactic ablative radiotherapy; SD, standard deviation; TACE, transarterial chemoembolization.
Tumor response and failure pattern
The Response Evaluation Criteria in Solid Tumors (version 1.1)24 was used to assess the initial treatment response and the overall best treatment response after SABR. The results revealed that 18 patients (56%) achieved a complete response (CR) or partial response (3 had a CR), and 14 (44%) had stable or progressive disease (1 exhibited progressive disease) at their first imaging evaluation 1–3 months after SABR. A total of 6 (19%) achieved a CR and 17 (53%) achieved a partial response during the post-treatment imaging follow-up. The overall best treatment response rate was 72%, and the median time to the maximal response was 5.5 months (1.2–11.6 months). Figure 1 shows representative images before and after SABR for a patient who achieved CR.
Figure 1.
Representative images for a patient with hepatocellular carcinoma and portal vein tumor thrombosis before (red arrows) and 1 month after (green arrows) stereotactic ablative radiotherapy (SABR).
After a median follow-up of 18.1 months (4.0–65.9 months), 10 patients (31%) were diagnosed with in-field intrahepatic failure, and 19 (59%) experienced intrahepatic failure outside the PTV. A total of 9 (28%) patients had extrahepatic distant metastasis. The median time to in-field tumor progression was 11.9 months (1.6–44.5 months).
Progression-free survival and overall survival
At the time of analysis, 4 patients survived without evidence of tumor progression, and 14 survived with recurrent disease. A total of 14 patients (44%) died due to tumor progression. The median OS was 33.7 months. As depicted in Figure 2, the estimated 2-year OS, IFPFS, and OFPFS rates were 54.4%, 62.7%, and 27.8%, respectively. Table 2 summarizes the results of the univariate analysis of OS and IFPFS. A baseline Cancer of the Liver Italian Program (CLIP) score of ⩾2, pretreatment alpha-fetoprotein levels, tumor diameter, and tumor volume were associated with poor OS.
Figure 2.

Survival outcomes of patients with advanced hepatocellular carcinoma unsuitable for transarterial chemoembolization who were treated with stereotactic ablative radiotherapy. OS, overall survival; PFS, progression-free survival.
Table 2.
Univariate analysis of overall survival and in-field progression-free survival.
| Variables | IFPFS |
OS |
||
|---|---|---|---|---|
| HR | p value | HR | p value | |
| Age (continuous) | 0.99 | 0.79 | 1.04 | 0.15 |
| Sex (female versus male) | 0.63 | 0.48 | 1.71 | 0.42 |
| ECOG (0 versus 1/2) | 2.32 | 0.29 | 2.98 | 0.16 |
| HBV (no versus yes) | 0.70 | 0.57 | 0.67 | 0.46 |
| HCV (no versus yes) | 1.01 | 0.99 | 1.05 | 0.93 |
| Child–Pugh score (5 versus 6/7) | 4.11 | 0.048* | 2.72 | 0.14 |
| CLIP score (⩽1 versus ⩾2) | 1.40 | 0.65 | 30.07 | 0.002* |
| Portal vein thrombosis (no versus yes) | 0.94 | 0.93 | 0.69 | 0.54 |
| Intrahepatic metastasis (no versus yes) | 1.36 | 0.66 | 1.63 | 0.40 |
| Extrahepatic metastasis (no versus yes) | 2.02 | 0.52 | 2.31 | 0.29 |
| AFP level (continuous) | 1.00 | 0.95 | 1.00 | 0.035* |
| Sequential use of sorafenib (no versus yes) | 1.40 | 0.61 | 1.41 | 0.55 |
| Maximal tumor diameter (continuous) | 1.07 | 0.69 | 1.39 | 0.016* |
| Gross Tumor volume (continuous) | 1.00 | 0.68 | 1.003 | 0.001* |
| Treated tumor number (1 versus 2/3) | 1.28 | 0.71 | 2.14 | 0.16 |
| BED10 (<85 Gy versus ⩾85 Gy) | 0.23 | 0.025* | 0.43 | 0.12 |
| TACE evaluation (contraindicated versus refractory) | 1.06 | 0.93 | 1.45 | 0.54 |
AFP, alpha-fetoprotein; BED10, biologically effective dose at an alpha/beta ratio of 10; CLIP, Cancer of the Liver Italian Program; ECOG, Eastern Cooperative Oncology Group; HBV, hepatitis B virus; HCV, hepatitis C virus; HR, hazard ratio; IFPFS, in-field progression-free survival; OS, overall survival; TACE, transarterial chemoembolization.
Statistically significant.
According to the results of the multivariate analysis (Table 3), a baseline CLIP score of ⩾2 [p = 0.01, hazard ratio (HR): 17.89, CI 95% 1.99–161.25] was a prognostic factor for shorter OS (Figure 3), and a BED10 of <85 Gy (p = 0.011, HR: 6.38, CI 95% 1.54–26.43) and a Child–Pugh score of ⩾6 (p = 0.014, HR: 7.09, CI 95%: 1.49–33.73) were prognostic factors for inferior IFPFS (Figure 4). Patients with a Child–Pugh score of ⩾6 received a SABR dose with a lower mean BED10; however, the difference was not significant (75.9 ± 19.85 versus 83.84 ± 14.85, p = 0.756). The estimated 2-year OS rates of patients with CLIP scores of ⩽1 and ⩾2 were 83.3% and 0% (p < 0.001), respectively, and the 2-year IFPFS rates of those receiving a BED10 of ⩾85 Gy and BED10 of <85 Gy were 74.4% and 33.2% (p = 0.027), respectively. As demonstrated in Figure 5, a CLIP score of ⩾2 was the only independent factor for out-field failure (p = 0.01, HR: 5.03, CI 95% 1.47–17.18).
Table 3.
Multivariate analysis of overall survival, in-field progression-free survival, and out-field progression-free survival using the Cox regression model.
| Variables | IFPFS |
OS |
OFPFS |
|---|---|---|---|
| HR (CI 95%) p value | HR (CI 95%) p value | HR (CI 95%) p value | |
| Child–Pugh score (5 versus 6/7) | 7.09 (1.49–33.73) 0.014* | ||
| CLIP score (⩽1 versus ⩾2) | 17.89 (1.99–161.25) 0.01* | 5.03 (1.47–17.18) 0.01* | |
| BED10 (<85 Gy versus ⩾85 Gy) | 0.16 (0.04–0.65) 0.011* |
IFPFS, in-field progression-free survival; OS, overall survival; OFPFS, out-field progression-free survival; CLIP, Cancer of the Liver Italian Program; BED10, biologically effective dose at an alpha/beta ratio of 10; HR, hazard ratio; CI, confidence interval.
Statistically significant.
Figure 3.
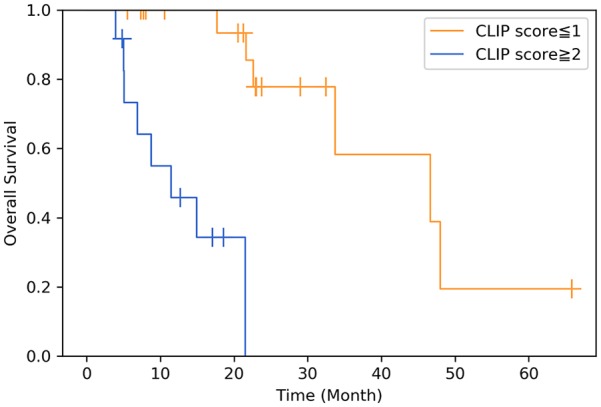
Overall survival in patients with a Cancer of the Liver Italian Program (CLIP) score of ⩾2 versus ⩽1 (p = 0.01).
Figure 4.
In-field progression-free survival (IFPFS) in patients who received a biologically effective dose at an alpha/beta ratio of 10 (BED10) ⩾85 Gy and BED10 <85 Gy (a), and those with Child–Pugh scores of 5 and ⩾6 (b) (p = 0.011 and p = 0.001, respectively).
Figure 5.

Out-field progression-free survival (OFPFS) in patients with a Cancer of the Liver Italian Program (CLIP) score of ⩾2 versus ⩽1 (p = 0.01).
Toxicities
All patients received the allocated SABR schedule without any interruption caused by treatment-related toxicities. Acute toxicities observed within 3 months following SABR are listed in appendix 2. In general, most adverse effects were grade 1 or 2 and were transient. They tended to ameliorate eventually after SABR. Only one patient developed a duodenal ulcer in the 5 months after SABR, however, the symptoms were alleviated after medication. None of the patients in this study experienced SABR-related toxicities of the biliary tract. According to the definitions of radiation-induced liver disease (RILD),26 classic RILD is characterized by anicteric hepatomegaly and ascites, or elevated alkaline phosphatase greater than twice the upper normal limit or baseline value approximately 2 weeks to 3 months after hepatic irradiation, and nonclassic RILD, which is observed in patients with underlying chronic hepatic diseases including cirrhosis or viral hepatitis, may present with elevated liver transaminases greater than five times the upper normal limit, or CTCAE grade 4 levels in those with baseline values exceeding five times the upper normal limit, or a worsening Child–Pugh score of >2 without the presence of classic RILD within 3 months of completing hepatic RT. In this study, five (16%) and eight (25%) patients had classic and nonclassic RILD events, respectively. However, none of the patients died of hepatic failure due to SABR. The detailed characteristics of the patients who developed RILD are presented in appendix 3. Table 4 summarizes the characteristics of patient and tumor related factors associated with the development of classic or nonclassic RILD. In summary, the higher mean normal liver dose (p = 0.011) and the baseline CLIP score of ⩾2 (p = 0.033) were two significant factors in the development of classic RILD. The baseline Child–Pugh score of ⩾6 (p = 0.049) was significantly associated with the occurrence of nonclassic RILD.
Table 4.
Clinical parameters associated with classic (n = 5) and nonclassic (n = 8) radiation-induced liver disease.
| Variables | Classic RILD |
Nonclassic RILD |
|---|---|---|
| p value | p value | |
| Child–Pugh score (5 versus 6/7) | 4/27 versus 1/5 0.77 | 5/27 versus 3/5 0.049* |
| CLIP score (⩽1 versus ⩾2) | 1/20 versus 4/12 0.033* | 5/20 versus 3/12 1.00 |
| Sequential use of sorafenib (no versus yes) | 1/14 versus 4/18 0.24 | 3/14 versus 5/18 0.68 |
| GTV volume, ml (mean ± SD) | 135.6 ± 260.9 versus 137.6 ± 182.9 0.48 | 89.5 ± 126.6 versus 274.9 ± 420.5 0.18 |
| Treated tumor number (1 versus 2/3) | 3/25 versus 2/7 0.29 | 7/25 versus 1/7 0.46 |
| Normal liver reserve, ml, median (range) | 1001.0 (716.0–1486.2) versus 948.1 (759.0–1647.0) 0.45 |
1025.5 (759.0–1647.0) versus 810.6 (716.0–1486.2) 0.053 |
| Mean normal liver dose, Gy, median (range) | 11.52 (6.27–16.77) versus 14.54 (11.43–19.54) 0.011* | 11.61 (6.27–19.54) versus 12.43 (8.71–14.95) 0.47 |
| Total prescribed dose, Gy, median (range) | 48 (30–60) versus 48 (36–50) 0.41 | 48 (30–60) versus 38.5 (30–54) 0.12 |
| Baseline platelet count, k/μl median (range) | 109 (37–418) versus 86 (48–145) 0.25 | 113.5 (37–247) versus 55.5 (37–418) 0.061 |
CLIP, Cancer of the Liver Italian Program; GTV, gross tumor volume; RILD, radiation-induced liver disease; SD, standard deviation.
Note: Categorical variables were examined using the Chi-square test and continuous variables were examined using the Wilcoxon signed-rank test and the Mann–Whitney U test.
Statistically significant.
Discussion
In patients with advanced HCC unsuitable for hepatectomy or liver transplantation, various treatment options or combinations are available, including systemic therapy, TACE, radioembolization with yttrium-90, and external beam RT. In addition, the heterogeneous spectrum of BCLC stage C disease and the heterogeneity of the inclusion criteria across studies render the comparison of different trials challenging. Although targeted therapies with a tumoricidal drug such as sorafenib can eradicate cancer cells, the survival benefit of sorafenib alone is limited in patients with advanced HCC,7,8 primarily because of the consequent disease progression within the liver.9,10 In contrast, liver-directed therapy remains challenging, in particular, in patients with vascular tumor thrombosis or a large tumor burden because TACE is unsatisfactory for eliminating tumors.11–13 Therefore, there is a need for more effective local treatment modalities that can improve therapeutic responses and survival. With advances in radiation techniques, SABR has emerged as a method that can deliver higher biological doses to hepatic tumors with superior preservation of the adjacent organs, therefore, resulting in more favorable treatment outcomes than those obtained with conventional RT.27–29
This paper presents a cohort-based analysis of SABR for BCLC stage C disease unsuitable for TACE. The results of this study highlighted that optimal patient selection and prescribed dose can be achieved. Based on our findings, with regard to optimal patient selection, patients with a baseline CLIP score of ⩽1 could obtain superior survival benefits from liver SABR. In addition, the optimal prescribed dose BED10 ⩾85 Gy is recommended for long-term tumor control.
The response rate, survival, and local control in our study are comparable with those reported in previous studies.18,30–34 Several studies have indicated various prognostic factors for HCC, including baseline CLIP score, Child–Pugh score, and presence of PVT in patients with cirrhotic liver receiving SABR.18,30,34,35 PVT did not affect the outcomes of our cohort because of two potential reasons. First, patients with PVT were treated for a macrovascular invasion of the partial portal trunk, and imaging studies revealed that 53% (10/19) of patients with PVT were complete or partial responders, which contributed to a longer in-field progression-free interval (median, 11.9 months) following SABR and the deferral of tumor thrombi-related liver failure. Second, over half of the patients developed hepatic recurrence outside the PTV, which led to an increased risk of hepatic failure. Thus, the effect of PVT was diluted. Because out-field failures remain a major cause of mortality, SABR in combination with novel systemic modalities is a potential treatment strategy that should be investigated in prospective studies. In a recent phase I/II study, treatment with a PD-1 inhibitor (nivolumab) resulted in substantial objective response rates of 15%–20%, irrespective of the type of therapy, in patients with advanced HCC.36 Of note, the disease control rate reached 64% in the dose-expansion phase. Therefore, the effectiveness of SABR in combination with immunotherapies, including checkpoint inhibitors, should be urgently examined and including combined therapy may reduce recurrence outside the PTVs and maximize OS.
As mentioned previously in this text, different research groups employ various prescribed doses and treatment planning strategies,29 therefore, information on optimal treatment doses remains limited. Some studies have employed SABR alone, and other studies have included TACE as part of the treatment modalities. Variations have also been noted in tumor size. These differences are attributed to the geographic variability in etiology and treatment availability. Therefore, prescribed doses are expected to vary between studies. Although BED ⩾100 Gy was associated with improved outcomes, multiple factors contributed to the dose selection, with treatment-favorable patients receiving higher doses.37 In general, fixed doses are employed for small tumors (median diameter ≈ 3 cm), and adapted doses are employed for larger tumors according to the normal liver tolerance, as determined by the tumor size and normal liver volume.29 Owing to the differences in treatment intention, both prescription approaches have their own rationale. In our study cohort, TACE was unsuitable for all patients and they had a mean tumor diameter of 4.7 cm. We adopted an isotoxic SABR, which was developed at the Princess Margaret Hospital of the University of Toronto, the prescribed SABR dose was adjusted according to the tolerance of the adjacent normal organs.38 In a pioneering phase I/II trial with a similar SABR approach conducted by Bujold and colleagues18 the median time to in-field tumor progression was 6 months, and in our cohort the median time was 11.9 months. The results might be ascribed to the lower prescribed doses (median, 30 versus 48 Gy) in that study due to the involvement of larger targets compared with our study (median values of maximal tumor diameter, 7.2 versus 4.7 cm).
In our study, toxicities were tolerable and similar to profiles described in previous studies.18,30–34 None of the study patients died of treatment-related hepatic failure despite 16% and 25% of patients developing classic and nonclassic RILD, respectively. We discovered that the mean normal liver doses and baseline CLIP scores significantly affected classic RILD, and the baseline Child–Pugh scores were associated with the occurrence of nonclassic RILD after treatment. SABR should be cautiously administered when used concurrently with sorafenib, and this therapy is not recommended outside clinical trials because considerable toxicities were observed in the high-risk group.39 In a retrospective toxicity analysis,40 baseline Child–Pugh scores and higher liver doses were strongly associated with an increase in the Child–Pugh score of ⩾2 3 months after SABR. In combination, these findings indicate that SABR-related hepatic toxicities can be minimized through meticulous selection criteria. Taking into consideration the higher incidence of RILD in our study, the current dose prescription scheme and patient selection should be further optimized for patients with BCLC stage C disease, with the prescribed dose delivered in three to six fractions.
This study has several limitations. First, the results should be interpreted with caution due to the limited sample size. A larger, prospective study is required to confirm the dose-response curve for SABR for advanced HCC. In particular, the optimal SABR dose for tumors with vascular invasion should be investigated further. However, the conclusions detailed in this study may be strengthened by the fact that data from two institutes were pooled and the same treatment strategy and clinical stages were used. In addition, inconsistency in sorafenib use might lead to a bias in the results. Finally, the causes of the higher incidence of RILDs should be investigated to determine whether the current regimen should be adapted for Asian patients, given that most of the patients with HCC had underlying chronic hepatic diseases. However, the effectiveness of SABR in BCLC stage C patients should be clarified before the results of the RTOG 1112 trial are reported. Our study results may have implications in decision-making when initiating SABR or stratifying patients in future clinical trials. When SABR is used to treat hepatic tumors refractory to TACE or where TACE is contraindicated in patients with advanced HCC, physicians can use our results as a reference to treat patients more meticulously and effectively. The early prediction of treatment effectiveness and potential liver toxicities would permit individualized therapy for patients requiring hepatic RT. Of note, most BCLC stage C disease developed out-field failures despite substantial intrahepatic tumor control with SABR in this study, this highlights the importance for future trials to use combined systemic therapies, including novel multikinase and immune checkpoint inhibitors, to improve OS rates.
Conclusion
For patients with HCC with BCLC stage C disease that is unsuitable for TACE, SABR can achieve substantial tumor control and can serve as a salvage treatment. Patients with a baseline CLIP score of ⩽1 can obtain superior survival benefits. In addition, a BED10 of ⩾85 Gy is an appropriate prescribed dose for tumor control. Because out-field relapse is the major cause of treatment failure, SABR in combination with novel systemic therapies is a potential treatment strategy that should be investigated in future studies.
Appendix
Appendix 1.
Protocol for the dose prescription and adjacent normal organ constraints for stereotactic ablative radiotherapy.
(a) Total dose prescription and normal liver constraint.
| Prescription dose | Mean normal liver dose |
|
|---|---|---|
| 3–4 fractions | 5–6 fractions | |
| ⩾50 – ⩽60 Gy | ⩽13 Gy | ⩽15 Gy |
| ⩾40 – <50 Gy | ⩽16 Gy | ⩽18 Gy |
| ⩾30 – <40 Gy | ⩽19 Gy | ⩽21 Gy |
(b) Adjacent normal organ constraints.
| Nonliver OARs | 3–4 fractions | 5–6 fractions |
|---|---|---|
| esophagus max (0.5 ml) | 24 Gy | 32 Gy |
| stomach max (0.5 ml) | 22.5 Gy | 30 Gy |
| duodenum max (0.5 ml) | 22.5 Gy | 30 Gy |
| small bowel max (0.5 ml) | 22.5 Gy | 30 Gy |
| large bowel max (0.5 ml) | 24 Gy | 32 Gy |
| spinal cord + 5 mm max (0.5 ml) | 18 Gy | 25 Gy |
| kidneys mean dose (bilateral) | 10 Gy | 12 Gy |
max, maximum dose; OAR, organ at risk.
Appendix 2.
Acute toxicity, except liver toxicity, within 3 months of SABR according to CTCAE version 4.03 (n = 32).
| Toxicity | Grade 1 | Grade 2 | Grade 3 | Grade 4/5 |
|---|---|---|---|---|
| Fatigue | 6 (19) | 1 (3) | 0 | 0 |
| Anorexia | 4 (13) | 0 | 0 | 0 |
| Nausea/vomiting | 5 (16) | 0 | 0 | 0 |
| Abdominal pain | 7 (22) | 0 | 0 | 0 |
| Diarrhea | 2 (6) | 0 | 0 | 0 |
CTCAE, Common Terminology Criteria for Adverse Events; SABR, stereotactic ablative radiotherapy.
Appendix 3.
Details for the patients with radiation-induced liver disease (n = 13).
| No. | RILD | Total dose, Gy/fractions | Normal liver reserve, ml | Mean normal liver dose, Gy | CP score | CLIP score |
|---|---|---|---|---|---|---|
| 1 | classic | 50/5 | 1098.3 | 14.09 | 7 | 2 |
| 2 | classic | 48/6 | 807.0 | 14.54 | 5 | 3 |
| 3 | classic | 48/6 | 1647.0 | 11.43 | 5 | 2 |
| 4 | classic | 45/5 | 948.1 | 15.77 | 5 | 2 |
| 5 | classic | 36/6 | 759.0 | 19.54 | 5 | 1 |
| 6 | nonclassic | 54/6 | 716.0 | 8.71 | 5 | 1 |
| 7 | nonclassic | 50/5 | 810.6 | 12.43 | 5 | 1 |
| 8 | nonclassic | 50/5 | 1076.4 | 10.44 | 6 | 0 |
| 9 | nonclassic | 42/6 | 915.0 | 13.72 | 5 | 3 |
| 10 | nonclassic | 35/5 | 1486.2 | 13.66 | 5 | 4 |
| 11 | nonclassic | 35/5 | 727.5 | 14.95 | 6 | 4 |
| 12 | nonclassic | 32/4 | 906.7 | 10.66 | 5 | 1 |
| 13 | nonclassic | 30/5 | 740.4 | 10.02 | 7 | 1 |
CP, Child–Pugh; CLIP, Cancer of the Liver Italian Program; RILD, radiation-induced liver disease.
Footnotes
Author Note: This study was presented as an oral presentation at the 103rd Scientific Assembly and Annual Meeting of Radiological Society of North America, 26 November 26 to 1 December 2017, Chicago, Illinois, USA.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declare that there are no conflicts of interest.
ORCID iD: Hsin-Lun Lee  https://orcid.org/0000-0002-2905-7858
https://orcid.org/0000-0002-2905-7858
Contributor Information
Hsin-Lun Lee, The PhD Program for Translational Medicine, College of Medical Science and Technology, Taipei; Medical University and Academia Sinica, Taipei Medical University, Taipei; Department of Radiation Oncology, Taipei Medical University Hospital, Taipei Medical University, Taipei; Taipei Cancer Center, Taipei Medical University, Taipei.
Jo-Ting Tsai, Department of Radiation Oncology, Shuang Ho Hospital, Taipei Medical University, New Taipei City; Department of Radiology, School of Medicine, College of Medicine, Taipei Medical University, Taipei.
Chun-You Chen, Taipei Cancer Center, Taipei Medical University, Taipei; Department of Radiation Oncology, Wan Fang Hospital, Taipei Medical University, Taipei.
Ying-Chun Lin, Department of Radiation Oncology, China Medical University Hospital, Taichung.
Chin-Beng Ho, Cancer Center, Camillians Saint Mary’s Hospital Luodong, Yilan.
Lai-Lei Ting, Department of Radiation Oncology, Taipei Medical University Hospital, Taipei Medical University, Taipei.
Chia-Chun Kuo, Department of Radiation Oncology, Taipei Medical University Hospital, Taipei Medical University, Taipei; Department of Radiation Oncology, Wan Fang Hospital, Taipei Medical University, Taipei.
I-Chun Lai, The PhD Program for Translational Medicine, College of Medical Science and Technology, Taipei; Medical University and Academia Sinica, Taipei Medical University, Taipei; Division of Radiation Oncology, Department of Oncology, Taipei Veterans General Hospital, Taipei.
Chun-Yu Lin, Department of Medical Imaging, Taipei Medical University Hospital, Taipei Medical University, Taipei.
Jui-Hsiang Tang, Division of Gastroenterology and Hepatology, Department of Internal Medicine, Taipei Medical University Hospital, Taipei Medical University, Taipei.
Yu-Min Huang, Department of Surgery, College of Medicine, Taipei Medical University, Taipei; Division of Gastrointestinal Surgery, Department of Surgery, Taipei Medical University Hospital, Taipei Medical University, Taipei.
Wei-Yu Kao, Division of Gastroenterology and Hepatology, Department of Internal Medicine, Taipei Medical University Hospital, Taipei Medical University, Taipei; Department of Internal Medicine, School of Medicine, College of Medicine, Taipei Medical University; Graduate Institute of Clinical Medicine, College of Medicine, Taipei Medical University, Taipei.
Sheng-Wei Cheng, Graduate Institute of Clinical Medicine, College of Medicine, Taipei Medical University, Taipei; Division of Gastroenterology and Hepatology, Department of Internal Medicine, Wan Fang Hospital, Taipei Medical University, Taipei.
Chia-Ning Shen, Genomics Research Center, Academia Sinica, No. 128, Academia Road, Section 2, Nankang District, 11529, Taipei City; The PhD Program for Translational Medicine, College of Medical Science and Technology, Taipei; Medical University and Academia Sinica, Taipei Medical University, Taipei; Graduate Institute of Clinical Medicine, College of Medicine, Taipei Medical University, Taipei.
Shang-Wen Chen, Department of Radiation Oncology, China Medical University Hospital, No. 2, Yude Road, North District, 40447, Taichung City; Department of Radiology, School of Medicine, College of Medicine, Taipei Medical University, Taipei; Graduate Institute of Biomedical Sciences, School of Medicine, College of Medicine, China Medical University, Taichung.
Jeng-Fong Chiou, Taipei Cancer Center, Taipei Medical University, No.250, Wu Hsing Street, Xinyi District, 110, Taipei City; The PhD Program for Translational Medicine, College of Medical Science and Technology, Taipei Medical University and Academia Sinica, Taipei; Medical University, Taipei Department of Radiation Oncology, Taipei; Department of Radiation Oncology, Taipei Medical University Hospital, Taipei Medical University, Taipei; Department of Radiology, School of Medicine, College of Medicine, Taipei Medical University, Taipei.
References
- 1. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet 2018; 391: 1301–1314. [DOI] [PubMed] [Google Scholar]
- 2. El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012; 142: 1264–1273 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology 1998; 28: 751–755. [DOI] [PubMed] [Google Scholar]
- 4. Yeung YP, Lo CM, Liu CL, et al. Natural history of untreated nonsurgical hepatocellular carcinoma. Am J Gastroenterol 2005; 100: 1995–2004. [DOI] [PubMed] [Google Scholar]
- 5. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2018; 69: 182–236. [DOI] [PubMed] [Google Scholar]
- 6. Vogel A, Cervantes A, Chau I, et al. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018; 29: iv238–iv255. [DOI] [PubMed] [Google Scholar]
- 7. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359: 378–390. [DOI] [PubMed] [Google Scholar]
- 8. Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009; 10: 25–34. [DOI] [PubMed] [Google Scholar]
- 9. Uka K, Aikata H, Takaki S, et al. Clinical features and prognosis of patients with extrahepatic metastases from hepatocellular carcinoma. World J Gastroenterol 2007; 13: 414–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yoo DJ, Kim KM, Jin YJ, et al. Clinical outcome of 251 patients with extrahepatic metastasis at initial diagnosis of hepatocellular carcinoma: does transarterial chemoembolization improve survival in these patients? J Gastroenterol Hepatol 2011; 26: 145–154. [DOI] [PubMed] [Google Scholar]
- 11. Llovet JM, Real MI, Montana X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet 2002; 359: 1734–1739. [DOI] [PubMed] [Google Scholar]
- 12. Forner A, Reig ME, de Lope CR, et al. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis 2010; 30: 61–74. [DOI] [PubMed] [Google Scholar]
- 13. Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology 2003; 37: 429–442. [DOI] [PubMed] [Google Scholar]
- 14. Chung GE, Lee JH, Kim HY, et al. Transarterial chemoembolization can be safely performed in patients with hepatocellular carcinoma invading the main portal vein and may improve the overall survival. Radiology 2011; 258: 627–634. [DOI] [PubMed] [Google Scholar]
- 15. Choi GH, Shim JH, Kim MJ, et al. Sorafenib alone versus sorafenib combined with transarterial chemoembolization for advanced-stage hepatocellular carcinoma: results of propensity score analyses. Radiology 2013; 269: 603–611. [DOI] [PubMed] [Google Scholar]
- 16. Pinter M, Hucke F, Graziadei I, et al. Advanced-stage hepatocellular carcinoma: transarterial chemoembolization versus sorafenib. Radiology 2012; 263: 590–599. [DOI] [PubMed] [Google Scholar]
- 17. Su TS, Liang P, Liang J, et al. Long-term survival analysis of stereotactic ablative radiotherapy versus liver resection for small hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2017; 98: 639–646. [DOI] [PubMed] [Google Scholar]
- 18. Bujold A, Massey CA, Kim JJ, et al. Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J Clin Oncol 2013; 31: 1631–1639. [DOI] [PubMed] [Google Scholar]
- 19. Feng M, Suresh K, Schipper MJ, et al. Individualized adaptive stereotactic body radiotherapy for liver tumors in patients at high risk for liver damage: a phase 2 clinical trial. JAMA Oncol 2018; 4: 40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kudo M, Matsui O, Izumi N, et al. Transarterial chemoembolization failure/refractoriness: JSH-LCSGJ criteria 2014 update. Oncology 2014; 87(Suppl. 1): 22–31. [DOI] [PubMed] [Google Scholar]
- 21. Surveillance Group, Diagnosis Group, Staging Group, et al. Management consensus guideline for hepatocellular carcinoma: 2016 updated by the Taiwan Liver Cancer Association and the Gastroenterological Society of Taiwan. J Formos Med Assoc 2018; 117: 381–403. [DOI] [PubMed] [Google Scholar]
- 22. Bethesda. ICRU Report 62. Prescribing, recording, and reporting photon beam therapy (Supplement to ICRU Report 50). J ICRU. 1999. [Google Scholar]
- 23. Larson DA, Flickinger JC, Loeffler JS. The radiobiology of radiosurgery. Int J Radiat Oncol Biol Phys 1993; 25: 557–561. [DOI] [PubMed] [Google Scholar]
- 24. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–247. [DOI] [PubMed] [Google Scholar]
- 25. National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.03. 2010, https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf
- 26. Pan CC, Kavanagh BD, Dawson LA, et al. Radiation-associated liver injury. Int J Radiat Oncol Biol Phys 2010; 76: S94–S100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Andolino DL, Johnson CS, Maluccio M, et al. Stereotactic body radiotherapy for primary hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2011; 81: e447–e453. [DOI] [PubMed] [Google Scholar]
- 28. Klein J, Dawson LA. Hepatocellular carcinoma radiation therapy: review of evidence and future opportunities. Int J Radiat Oncol Biol Phys 2013; 87: 22–32. [DOI] [PubMed] [Google Scholar]
- 29. Sanuki N, Takeda A, Kunieda E. Role of stereotactic body radiation therapy for hepatocellular carcinoma. World J Gastroenterol 2014; 20: 3100–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lo CH, Yang JF, Liu MY, et al. Survival and prognostic factors for patients with advanced hepatocellular carcinoma after stereotactic ablative radiotherapy. PLoS One 2017; 12: e0177793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sanuki N, Takeda A, Oku Y, et al. Stereotactic body radiotherapy for small hepatocellular carcinoma: a retrospective outcome analysis in 185 patients. Acta Oncol 2014; 53: 399–404. [DOI] [PubMed] [Google Scholar]
- 32. Kang JK, Kim MS, Cho CK, et al. Stereotactic body radiation therapy for inoperable hepatocellular carcinoma as a local salvage treatment after incomplete transarterial chemoembolization. Cancer 2012; 118: 5424–5431. [DOI] [PubMed] [Google Scholar]
- 33. Huertas A, Baumann AS, Saunier-Kubs F, et al. Stereotactic body radiation therapy as an ablative treatment for inoperable hepatocellular carcinoma. Radiother Oncol 2015; 115: 211–216. [DOI] [PubMed] [Google Scholar]
- 34. Que J, Kuo HT, Lin LC, et al. Clinical outcomes and prognostic factors of cyberknife stereotactic body radiation therapy for unresectable hepatocellular carcinoma. BMC Cancer 2016; 16: 451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Prospective validation of the CLIP score: a new prognostic system for patients with cirrhosis and hepatocellular carcinoma. The Cancer of the Liver Italian Program (CLIP) Investigators. Hepatology 2000; 31: 840–845. [DOI] [PubMed] [Google Scholar]
- 36. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017; 389: 2492–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Robbins JR, Schmid RK, Hammad AY, et al. Stereotactic body radiation therapy for hepatocellular carcinoma: practice patterns, dose selection and factors impacting survival. Cancer Med 2019; 8: 928–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dawson LA, Eccles C, Craig T. Individualized image guided iso-NTCP based liver cancer SBRT. Acta Oncol 2006; 45: 856–864. [DOI] [PubMed] [Google Scholar]
- 39. Brade AM, Ng S, Brierley J, et al. Phase 1 Trial of sorafenib and stereotactic body radiation therapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2016; 94: 580–587. [DOI] [PubMed] [Google Scholar]
- 40. Velec M, Haddad CR, Craig T, et al. Predictors of liver toxicity following stereotactic body radiation therapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2017; 97: 939–946. [DOI] [PubMed] [Google Scholar]




