Abstract

The amphiphilic graphene derivative was prepared by covalent grafting of graphene oxide (GO) with isophorone diisocyanate and N,N-dimethylethanolamine and then noncovalent grafting of GO with sodium dodecylbenzenesulfonate. The results obtained from infrared spectroscopy, X-ray photoelectron spectroscopy, thermal gravimetric analysis, and X-ray diffraction analysis revealed that the short chains were successfully grafted onto the surface of GO. Subsequently, scanning electron microscopy and optical microscopy results showed that the modified GO (IP-GO) has the best dispersibility and compatibility than GO and reduced GO in the waterborne polyurethane matrix. The relationship between the corrosion resistance of composite coatings and the dispersibility of the graphene derivative and the compatibility of the graphene derivative with a polymer matrix were discussed. The anticorrosive properties were characterized by electrochemical impedance spectroscopy analysis and salt spray tests. Through a series of anticorrosion tests, it is concluded that the anticorrosion performance of a composite coating with 0.3 wt % IP-GO is significantly improved. The excellent anticorrosion performance is due to the perfect dispersion and good compatibility of IP-GO in waterborne polyurethane.
1. Introduction
Corrosion of metal surface, particularly oil pipeline, coastal transmission tower, ships, and other metal equipment, has an urgent need for anticorrosion. Every year, because of metal maintenance, it consumes a lot of workforce and resources.1−3 Electrochemical corrosion is one of the most common corrosion techniques for metals, which occurs at the interface of metal-electrolyte solutions. In order to prevent the corrosive substances (i.e., H2O, O2, and Cl–) from corroding the surface of the metal, the anticorrosion coatings (i.e., waterborne epoxy resin and waterborne polyurethane) have been often used for metal protection because of its physical barrier properties.4−7 With the increasing awareness of environmental protection, the strict control of volatile organic substances and harmful air pollutants by laws issued by various countries, the world coating industry is moving in the fast lane of water-based, solvent-free, and green development.8,9 Waterborne polyurethane (WPU) using water as a solvent has been widely accepted in the anticorrosion coating due to its excellent adhesion, chemical resistance, eco-friendliness, and good weathering resistance.10,11
However, the corrosive agents cannot be completely blocked by the organic coating, and more or less corrosive agents will enter the metal/coating interface. Therefore, attempts have been made to blend the modified organic coatings in many ways to provide better shielding against corrosive media. Addition of impermeable nanoparticles including Al2O3, ZnO, clay, graphene, and boron nitride into an epoxy coating as corrosive barrier substance media has been extensively discussed.12−15 These results have proven that the addition of nanoparticles could improve the barrier efficiency of organic coatings against corrosive media.
Graphene (Gr), a two-dimensional graphitic carbon material, is considered to be one of the most attractive materials, which has attracted enormous research interests in the reinforcement of composite materials for its remarkable performance, such as higher aspect ratio, low density, excellent mechanical strength,16 high thermal conductivity,17 and outstanding barrier property.18 In addition, graphene with the flexible surface performance19,20 can be used as a new barrier filler in corrosion-resistant polymer systems instead of the traditional scaly fillers. Because of the above advanced properties, Gr was more and more used in the field of achieving high efficiency corrosion resistance of composite coatings. Up to now, it has been reported that graphene grown by chemical vapor deposition (CVD-Gr) is a superior anticorrosion coating.21 Nevertheless, CVD-Gr coating cannot be used as a long-term anticorrosion barrier because it is not able to prevent the metal corrosion once defects and cracks occur in the atomically thin materials.22,23 It is unrealistic to prepare large-area defect-free graphene. Therefore, the use of graphene and its derivatives to enhance organic coatings is another effectual technique to achieve outstanding barrier properties. However, due to graphene and its derivatives, high aspect ratio, strong intermolecular forces, and easy agglomeration in the polymer limit their practical applications.19,24,25
In order to improve the dispersibility of graphene or graphene oxide (GO) in a polymer matrix, many methods have been tried to modify the surface of Gr, GO, or reduced GO (RGO) to improve their chemical affinity.18,26−30 Gr or GO has been treated by ultrasonic dispersion, in situ polymerization reduction, and chemical modification to enable better dispersion in the polymer matrix.31−34 Li et al.5 reported that graphene oxide modified with a titanate coupling agent exhibited self-alignment when a certain amount was added to the aqueous polyurethane, and the corrosion resistance was remarkably improved. Therefore, improving the dispersion and compatibility of graphene derivatives in polymers plays an important role in enhancing the properties of polymers.
The purpose of this investigation is to use GO nanosheets as a corrosion inhibitor filler in the WPU coating. For the sake of improving the dispersion and compatibility of GO in WPU, isophorone diisocyanate (IPDI) and N,N-dimethylethanolamine (DMEA) were covalently grafted onto GO to prepare modified graphene oxide (IP-GO), and a small amount of sodium dodecylbenzenesulfonate (SDBS) was used to graft IP-GO noncovalently. The waterborne polyurethane functionalized graphene composite anticorrosive coating was prepared and coated on a Q235 steel plate. Compared to the GO and RGO reinforced coating, the coating with a 0.3% high aspect ratio of functionalized graphene has the best anticorrosion effect.
2. Experimental Section
2.1. Materials
The ordinary carbon structural steels (Q235) were purchased from Baosteel Ltd. (China). Q235 steel panels were cut into a certain shape (80 mm × 40 mm × 1 mm). Before coating formation, the Q235 steel plate substrates were treated with a degreaser and ethanol in succession and then dried by nitrogen gas. Dibutyltin dilaurate (≥99.5%) and vitamin C (≥99.8%) were purchased from Guangdong Wengjiang Chemical Reagent Co., Ltd. (China) and Tianjin Guangfu Technology Development Co., Ltd. (China), respectively. N,N-Dimethylethanolamine (99%) was purchased from Tianjin Comemi Chemical Reagent Co., Ltd. (China). IPDI (99%) and SDBS were purchased from Aladdin Biochemical Technology Co., Ltd. (China). Natural graphite flake (99.95%) was purchased from Shanghai Yifan Graphite Co., Ltd. (China). KMnO4 (99.5%), H2SO4 (98%), NaNO3, H2O2 (30%), ammonia solution (25%), acetone (99.7%), and hydrochloric acid (37%) were bought from Tianjin Fengchuan Chemical Reagent Co., Ltd. (China). Waterborne polyurethane (NeoRez R-9679) with a solid content of 37% was purchased from DSM (Netherlands).
2.2. Synthesis of GO and Reduced Graphene Oxide
GO was prepared by a strong oxidizing reaction through a modified Hummers’ method.35,36 Briefly, graphite flake (2.0 g) and NaNO3 were dispersed in 200 mL of H2SO4 in a beaker under constant stirring in an ice-water bath. After 30 min, 9.0 g of KMnO4 was tardily added to the mixture while keeping the temperature below 10 °C for an hour. The solution was stirred at 45 °C for 10 h. Then, 250 mL of deionized water was tardily added to the mixture; afterward, the mixture was heated to 90 °C and maintained at this temperature for 1.5 h. When the system was cooled down to 50–60 °C, 35 mL of H2O2 was added slowly. The mixture was cleaned twice with dilute hydrochloric acid and then continuously centrifuged with a large amount of deionized water until the pH reached 6–7. Then, a stable and homogeneous GO aqueous solution (3.3 mg/mL) was obtained by bath ultrasonication exfoliation. Finally, a GO powder was obtained by freeze-drying.
Reduced graphene oxide was successfully obtained by using a nontoxic and harmless chemical reduction method. Deionized water (60 mL) was added to the GO aqueous solution, and GO dispersion (2 mg/mL) was prepared by an ultrasonic bath for 1 h. Then, vitamin C (6 g) was progressively added and hereafter was placed in a constant temperature water bath at 85 °C by continuous stirring with a magnetic stirrer for 8 h, vacuum filtration, freeze-drying, and the resulting sample was labeled RGO.
2.3. Synthesis of IP-GO
IP-GO was obtained by a method of in situ polymerization. Typically, GO (0.7 g) was added into 200 mL of acetone and ultrasonically dispersed for 1 h. Then, the dispersion was transferred to a three-neck flask, and 80 g of IPDI and 0.01 g of dibutyltin dilaurate were added and reacted under vigorous electromagnetic stirring and nitrogen gas refluxing at 85 °C for 12 h. The temperature was lowered to 70 °C, and then 25 g of DMEA was added to the mixture for 1.5 h. The impurities were washed away with acetone and deionized water, and the product obtained by freeze-drying was named IP-GO. The procedure of the synthesis of IP-GO is schematically shown in Figure 1.
Figure 1.
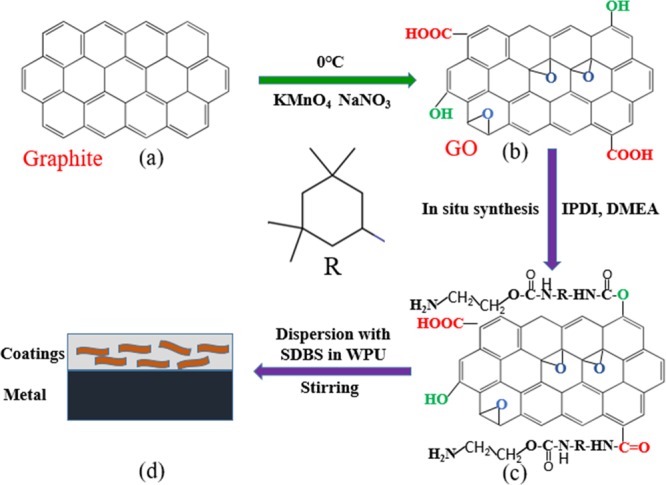
Schematic illustration of the synthesis of the functionalization graphene oxide (IP-GO) and coatings. (a) Graphite flake. (b) GO. (c) IP-GO. (d) Composite coatings on metal.
2.4. Preparation of the Composite Coatings
IP-GO (0.03 g) was dispersed with SDBS (0.03 g) in 8.5 mL of alcohol solution (5 wt %) and ultrasonically dispersed for 1 h, and then 10 g of WPU was added and stirred vigorously for 1 h with a magnetic stirrer. Then, WPU coatings with a solid content of 20% were obtained. The WPU composite coatings reinforced by GO, RGO, and IP-GO were named GO/WPU, RGO/WPU, and IP-GO/WPU coating, respectively. The Q235 steel were ultrasonically cleaned in a degreaser for 10 min and flushed with absolute ethanol for another 30 min. The reinforced WPU was coated on the surface of the Q235 steel (80 mm × 40 mm × 1 mm) by bar coating and air-dried for 2 h and then transferred to a vacuum oven and baked at 110 °C for 30 min.
2.5. Characterizations
The GO, RGO, and synthesized IP-GO was characterized by Fourier transform infrared (FT-IR) spectroscopy (TENSOR 37, Germany) over a wavelength range of 400–4000 cm–1 and X-ray diffraction (XRD; D8 DISCOVER, BRUKER, Germany). Thermogravimetric analysis (TGA; STA409PC, Germany) with a heating rate of 10 °C/min from room temperature to 700 °C under a N2 atmosphere and X-ray photoelectron spectroscopy (XPS; K-Aepna, USA) were introduced to further demonstrate the chemical composition of GO, RGO, and IP-GO. Transmission electron microscopy (TEM; TECNAI-20) were used to characterize the morphology of GO and P-GO. For TEM, the sample was dispersed in water to form a light yellow colloidal dispersion with a concentration of approximately 0.005 wt % and then dripped onto a microgrid and carbon support film. Laser confocal Raman spectroscopy (Raman; XploRA PLUS, Japan) was used to analyze the structures of GO and IP-GO.
Scanning electron microscopy (SEM; Hitachi S4800, Japan) was used to observe the cross section of the composite coating after brittle fracture in liquid nitrogen and to analyze the microdispersion of GO, RGO, and IP-GO nanosheets in the polymer. An optical microscope (OLYMPUS BX43, Japan) was used to analyze the dispersion of graphene derivatives in the polymer. The mechanical performance of the composite was tested by a universal testing machine (GMT4000, MTS Systems Co. Ltd., China).
The electrochemical corrosion measurement was performed in a 3.5 wt % NaCl solution by a three-electrode cell electrochemical workstation (CS350H, China). The electrochemical workstation comprises Pt wire, saturated calomel electrode (SCE), and Q235 steel (exposed area, 1 cm2) as the counter electrode, reference electrode, and working electrode, respectively. Electrochemical testing was started after testing for 1 h after OCP (open-circuit potential), and other systems were stabilized. For electrochemical impedance spectroscopy (EIS), the frequency range of 10 kHz to 0.1 Hz with a perturbation amplitude of 10 mV was used. The salt spray test was carried out on coated Q235 plates (40 mm × 80 mm) with a 5 wt % NaCl solution at 100% relative humidity at 35 °C according to ASTM B117-03.
3. Results and Discussion
3.1. Characterization of Functionalized GO Nanosheets
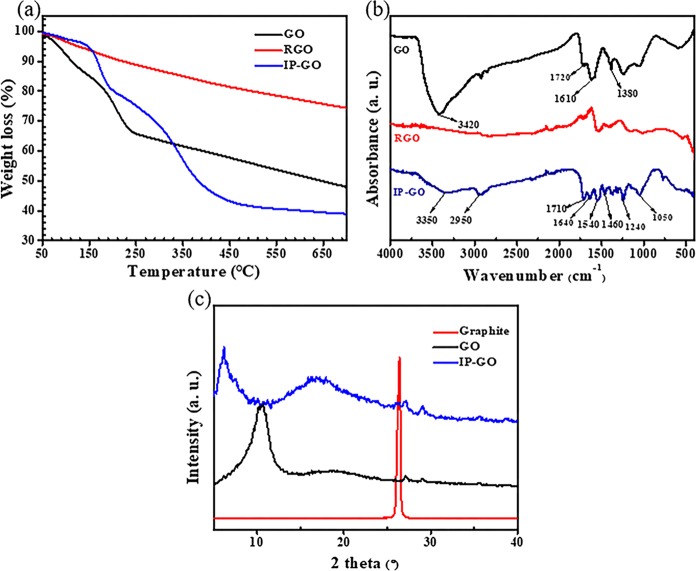
TGA, XPS, XRD, and FT-IR were used to demonstrate the successful grafting of IPDI and DMEA to the edge and surface of GO. The GO surface includes a large amount of oxygen-containing functional groups, which provides reactive sites for IPDI. A schematic representation of the characterization of IPDI and DMEA grafted onto the GO surface is displayed in Figure 1. TGA was used to analyze the thermal stability of the GO, RGO, and IP-GO. The results obtained from TGA measurements can also be used to analyze the amount of IPDI and DMEA chains grafted onto GO sheets, as shown in Figure 2a. The weight loss of more than 35% was observed in the range of 50–300 °C for GO. Furthermore, there was a significant weight reduction when the temperature increased to 200 °C. This reduction was primarily the result of unstable oxygen-containing groups decomposing into CO2 or other vapors. The hydroxyl carboxyl and epoxy groups on the edge and surface of the RGO were removed, so RGO exhibited high thermal stability with a mass loss of about 26.2% at a temperature below 700 °C. IP-GO showing lower loss than GO at 50–330 °C due to the polymer chains grafted on the GO surface replaced the thermal instability of hydroxyl and carboxyl groups and alleviated the rate of thermal decomposition. However, at temperatures above 330 °C, the thermal loss of IP-GO is much higher than GO, which is mainly due to the thermal decomposition of polymer chains grafted on the GO surface. The residual carbon rate of GO was 48.21%, while that of IP-GO was significantly reduced (39.02%). It is also proved that the grafting reaction did take place in GO lamellae during the modification process. In addition, compared with the initial decomposition temperature of TGA, the thermal stability of surface groups of GO modified by IPDI and DMEA was greatly improved. Molecular chains were successfully grafted onto oxygen-containing groups of GO, which significantly increased the initial thermal decomposition temperature of GO.
Figure 2.
Characterization of GO, RGO, and IP-GO by (a) TGA and (b) FT-IR spectra analysis and (c) XRD analysis of graphite, GO, and IP-GO.
The GO treated with IPDI and DMEA can be derivatized by forming an amide bond and a urethane bond with the hydroxyl group at the edge and the surface oxygen-containing groups, respectively.37,38 The formation of new chemical bonds of these chemical changes about GO and modified GO derivatives that exhibit characteristic IR spectra can be analyzed by FT-IR spectroscopy. Figure 2b displays the spectra of GO, RGO, and IP-GO. In the infrared absorption spectra of GO, a high absorption band at 1720 cm–1 corresponding to the C=O stretching of a carboxyl group appears and the O–H deformation vibration appears at 1380 cm–1. The resonance at 1610 cm–1 may result from two aspects: for one thing, it can be attributed to the stretching of the adsorbed water molecules, and for another, it may contain the deformation vibration of C=C.39−41 A broad and intense adsorption at about 3420 cm–1 is attributed to the stretching vibration of −OH groups on the GO surface. The characteristic peaks of RGO are weakened after being reduced.
Compared to GO, some new characteristic IR spectra appeared in IP-GO after treatment with IPDI and DMEA. Compared with the C=O characteristic peaks (1720 cm–1) in GO, the C=O characteristic peaks in IP-GO move to a low wavenumber, which can be attributed to the interaction stretching between the carbamate bond of C=O on the surface of IP-GO and the carbonyl group in the carboxyl group. The new stretch at 1640 cm–1 corresponds to an amide carbonyl-stretching mode (the so-called amide I vibration stretch). The band peak at 1540 cm–1 can be assigned to carbamate esters and the coupling of the C–N stretching vibration with the CHN deformation vibration (the so-called amide II vibration stretch).42 The new peak at 2950 cm–1 is attributed to the methyl group (C–H, from N,N-dimethylethanolamine) stretching vibrations. The new adsorption peaks at 1240 and 1050 cm–1 can be assigned to the C–N stretching. Obviously, FT-IR spectroscopy indicates the successful preparation of IP-GO.
TEM and SEM were utilized to observe the morphology of GO and P-GO. The typical structures of GO after chemical exfoliation are seen in Figures S1 and S2, showing that the surface of GO was relatively smooth with characteristic crumples. The covalent graft modification of GO by IPDI and DMEA did not destroy the structure of GO nanosheets, and the surface still showed a slightly folded structure. The GO nanosheet structure was not destroyed in high-power SEM images, and the observed results were consistent with TEM images.
XRD spectra are often used to characterize the interlayer distance of GO and graphene oxide derivatives, as shown in Figure 2c. It is found that the strong (0 0 2) diffraction peaks of graphite and GO are at 2θ = 26.4° and 2θ = 10.6°, suggesting an interlay distance of 0.34 and 0.84 nm, respectively. However, the diffraction peak of IP-GO was replaced by a low-angle peak (2θ = 6.4°) with decreased intensity, in which the interlayer distance was about 1.40 nm. These results show that the short chain grafted to the surface of the GO prevented the polymerization of graphene lamellae and increased the interlayer distance. Further, a broad peak appears at about 2θ = 17°, meaning that the modified graphene sheets show a slight re-growth after drying. The typical Raman spectra of GO and IP-GO are compared in Figure S3.
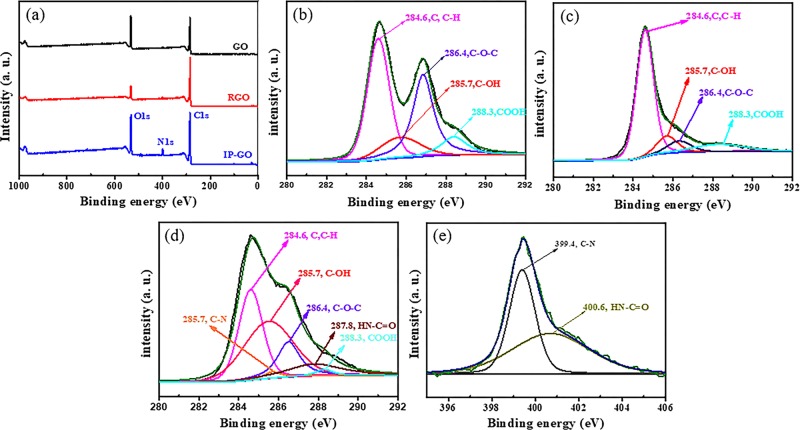
The modification and reduction of GO nanosheets were characterized by XPS. The chemical element and bond of the modification and reduction of GO nanosheets were assessed by XPS characterization. Figure 3a exhibits the C 1s, O 1s, and N 1s binding energies at about 286.0, 532.4, and 398.0 eV, respectively. Compared to GO, it can be clearly seen from Figure 3a that an obvious peak of N at 398.0 eV is displayed on the image of IP-GO. Figure 3b,c shows that C 1s spectra of GO and RGO exhibit the binding energies at 284.6, 285.7, 286.4, and 288.3 eV assigned to C–C, C–OH, −C–O–C–, and COOH, respectively. In virtue of the loss of the oxygen-containing functional groups, compared with GO, the atomic ratio of O/C of RGO decreases significantly. Compared to GO, the XPS survey of IP-GO in Figure 3d shows two peaks detected in the C 1s at 285.7 and 287.8 eV associated with C–N and HN—C=O, respectively. There are two peaks in the N 1s of IP-GO: C–N (399.4 eV) and HN—C=O (400.6 eV) (as shown in Figure 3e). These above information can testify that IPDI has grafted onto the surface of GO by forming an amide bond group.
Figure 3.
XPS survey images of (a) GO, RGO, and IP-GO, C 1s spectra of (b) GO, (c) RGO, and (d) IP-GO, and N 1s spectra of (e) IP-GO.
3.2. Characterization of WPU, GO/WPU, RGO/WPU, and IP-GO/WPU Composite Coatings
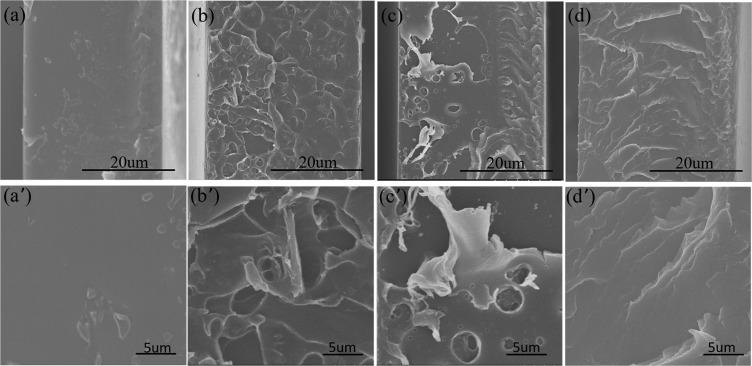
Digital photos of water (upper part) and waterborne polyurethane (lower part) dispersion of GO and IP-GO after 15 days are exhibited in Figure S4. The dispersibility of the graphene derivative in the WPU coating and the compatibility with the polymer matrix affect the morphology of the composite coatings. This was investigated by studying the fracture morphology of these samples by SEM analysis. SEM images of the composite coatings are exhibited in Figure 4a–d at low magnification and Figure 4a′–d′ at high magnification. The thickness of these coatings is about 35 ± 5 μm. The neat WPU coating shows a relatively smooth surface, while the GO, RGO, and IP-GO strengthened WPU coatings show a coarser surface due to the distribution of the graphene derivatives and are easily seen at low magnification. The fractured surfaces of GO/WPU and RGO/WPU coatings show the high roughness and some component pull-out has been observed, which indicates a weak interaction between GO and RGO with the polymer interface and weak compatibility. The IP-GO/WPU presents a smoother surface than GO/WPU and RGO/WPU coatings, suggesting no agglomerates of IP-GO in the polymer matrix and the strong interface force of IP-GO nanosheets with the matrix. The molecular chain grafted on the GO surface interacting with the WPU matrix increased the dispersibility and compatibility of IP-GO in the polymer. IP-GO shows a more regular arrangement in the polymer, while RGO show aggregation and a random arrangement, which can be seen in high-power images.
Figure 4.
SEM micrographs of fractured surfaces of the coatings: (a, a′) WPU, (b, b′) GO, (c, c′) RGO, and (d, d′) IP-GO coatings (top panel, low magnification; bottom panel, high magnification).
Optical images can also be used to derive the dispersion of graphene and its derivatives in the WPU matrix, as shown in Figure 5. Optical microscopes have different colors in their images due to their different substrates. Figure 5a is an optical picture of pure WPU with a uniform color distribution. The dark color in Figure 5b–d was caused by the presence of graphene. Figure 5b,c shows the phenomenon of agglomeration of graphene in the WPU matrix. In Figure 5d, the IP-GO was particularly well dispersed in the WPU matrix. The test results are consistent with the SEM results.
Figure 5.
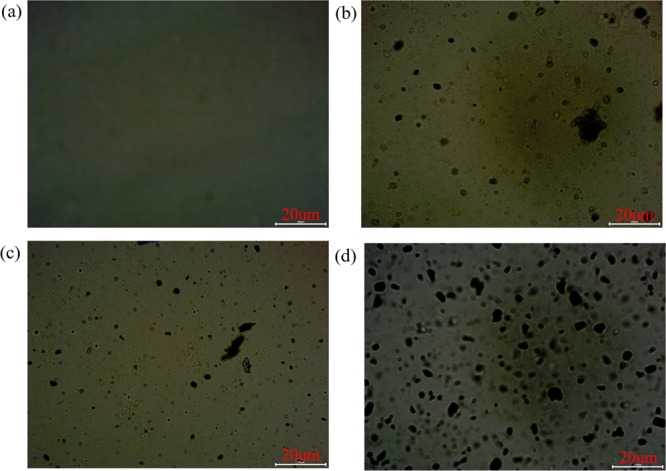
Optical microscope images of (a) pure WPU, (b) GO, (c) RGO, and (d) IP-GO reinforced WPU composite coatings.
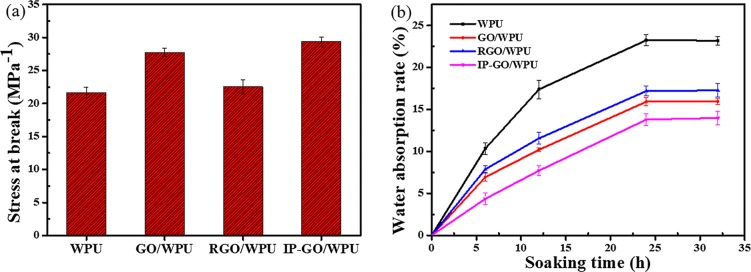
Figure 6a presents the tensile strength for neat WPU and graphene derivative reinforced WPU composite coatings. The tensile strength of WPU has been significantly improved after the enhancement of GO and IP-GO incorporation. Compared to the IP-GO/WPU coating, the RGO/WPU composite coating has a low tensile strength due to the weak dispersion and compatibility of RGO in WPU. Figure 6b shows the water absorption rate of neat WPU and graphene derivative reinforced WPU composite coatings. Pure waterborne polyurethanes have a high water absorption rate because they contain a large amount of hydrophilic functional groups. Due to the impermeability of the graphene derivative to water molecules, the water absorption of the composite coating is significantly reduced after the addition of the graphene derivative. The water absorption of the composite coatings is directly related to the dispersion of the graphene derivative in the coatings. Because the IP-GO/WPU composite has the best combination and compatibility with the matrix, it has the lowest water absorption and the highest efficiency for blocking the diffusion of water molecules in the coating. Therefore, we have obtained that IP-GO has better dispersibility in the coating and has the best barrier effect on corrosive media, which is also consistent with the SEM observations.
Figure 6.
(a) Tensile strength and (b) water absorption rate test of pure WPU, GO, RGO, and IP-GO reinforced WPU composite coatings.
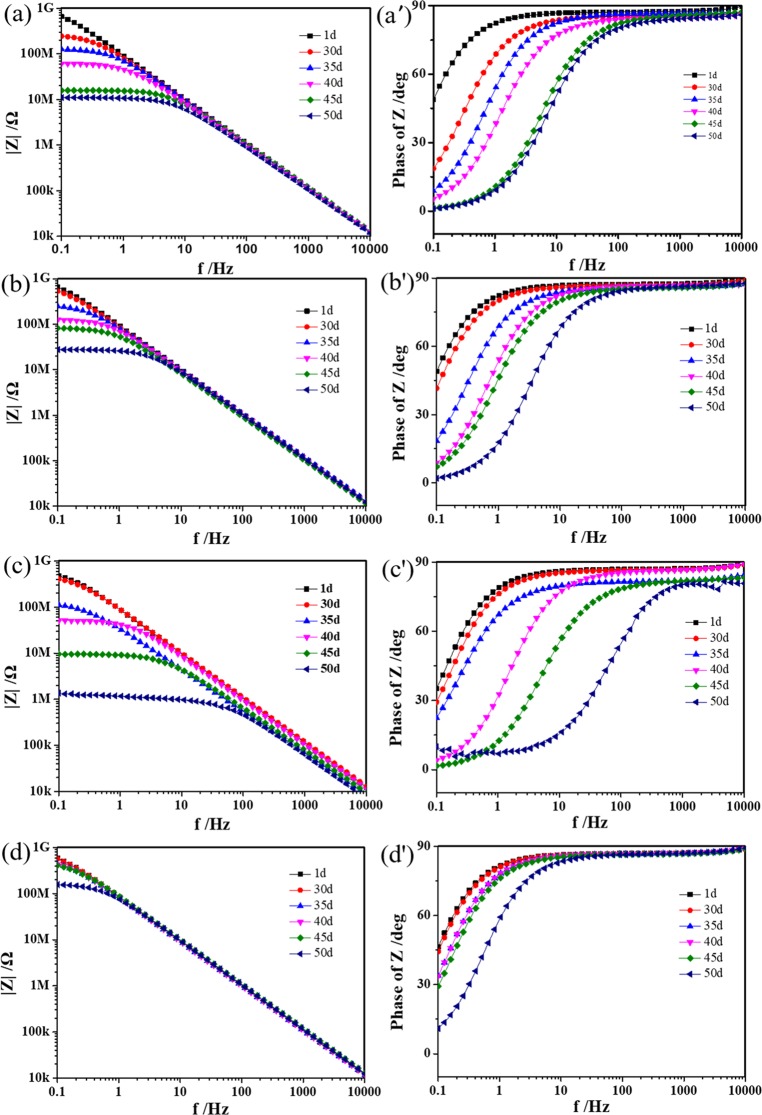
EIS is one of the techniques to effectively evaluate the corrosion process of coatings and to gain insight into the corrosion performance of coating on metals. The higher impedance at lower frequencies (e.g., |Z|0.1Hz) in the Bode modulus plot is associated with increased corrosion resistance, and the slight decrease in value over time generally indicates that the coating degrades as the environment changes. For a complete coating without defects, the phase angle in the Bode plot should be nearly 90° due to the higher electrical resistance of the coating. The EIS spectra of the Bode modulus plot (left) and Bode phase plot (right) of the WPU, GO/WPU, and RGO/WPU coatings and IP-GO/WPU composite coatings with different exposure durations are shown in Figure 7. Due to the purely resistive nature of the coating, the coating had a high impedance modulus in the low-frequency region and a phase angle of approximately 90° in the high-frequency region at the beginning. As the electrolyte solution gradually penetrated into the coating, the impedance modulus of the low-frequency region continued to decrease due to the parallel capacitance of the coating (as shown in Figure 8b). The addition of 0.3 wt % GO obviously improved the corrosion resistance of the composites. Compared with the pure WPU coating (Figure 7a,a′), the GO/WPU coating (Figure 7b,b′) had higher impedance after 50 days of immersion in 3.5 wt % salt water and the phase angle in the high-frequency region was close to 90°. However, due to the poor dispersion of RGO in the polymer, the integrity of the coating was lowered and the efficiency of the coating decreased. The impedance of RGO/WPU coating decreased below 107 Ω after 50 days of immersion, and two time constants appeared in the Bode phase plot, indicating that the corrosion medium had invaded the metal/coating interface (Figure 7c,c′). Quite differently, after 50 days of immersion in 3.5 wt % salt water, the impedance modulus of the IP-GO/WPU coating (Figure 7d,d′) remained above 108 Ω, and the phase angle in the high-frequency region was near 90°, showing the highest efficiency for anticorrosion.
Figure 7.
EIS spectra of the four coatings with different immersion durations: Bode phase plot (left panel) and Bode modulus plot (right panel) of (a, a′) the pure WPU coating, (b, b′) the GO coating, (c, c′) the RGO coating, and (d, d′) the IP-GO coating, respectively.
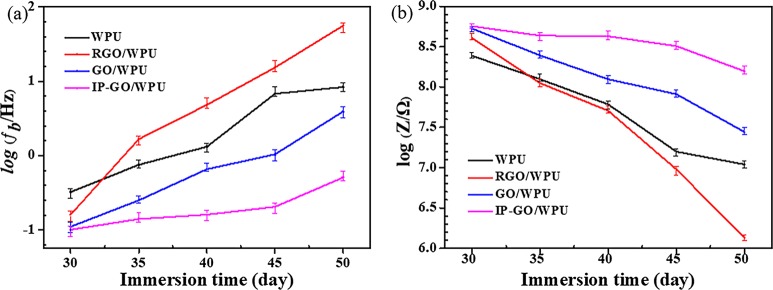
Figure 8.
Variations of impedance data including (a) log(fb) and (b) log|Z| at 0.1 Hz of WPU, GO/WPU, RGO/WPU, and IP-GO/PU coatings as a function of immersion time in a 3.5 wt % NaCl solution at pH = 7.
The breakpoint frequency (fb) corresponds to the frequency of the 45° phase angle that was obtained from the Bode phase plot, which is often used to analyze information about coating delamination.43 It has been reported that the reaction at the metal/coating interface of coatings could be analyzed by the variation of fb with immersion durations.44 As the corrosive medium passes through the composite coating to the metal/coating interface, the chemical corrosion reaction begins in the reactive site of the metal surface. The values of fb for the four paint coatings increased to higher values with the immersion duration, as can be found in Figure 8a. The fb shifted to higher values, suggesting the increase in the delaminated area. The fb of the coating after the addition of the RGO nanosheets moved toward the high-frequency region compared to that of the pure aqueous polyurethane coating. The GO addition delayed the increase in fb. However, the fb of the coating decreased the most after the addition of IP-GO, and the value remained at a lower position after 50 days of soaking. The diffusion of the corrosive medium to the surface of the metal/substrate is the main cause of corrosion of the metal. After electrochemical corrosion of the interface between the metal and the coating, the hydroxyl anion will accumulate at the cathode sites. This can accelerate the delamination and shedding of the coating.
The anticorrosive performance of the composite coatings was certificated by the salt spray test. The images of the coatings after the salt spray test for 25 days are exhibited in Figure 9. The large area of the brown corrosion product was accumulated on the surface of the pure WPU, GO/WPU, and RGO/WPU coatings after the 25 days salt spray test. The surface of the IP-GO/WPU coating only showed slight corrosion after the same conditions of the salt spray test. Both of the consequences from the salt spray test and EIS test have shown that the IP-GO composite coatings have the best anticorrosion performance, although the two techniques have different accuracies and different ways of characterizing corrosion.
Figure 9.
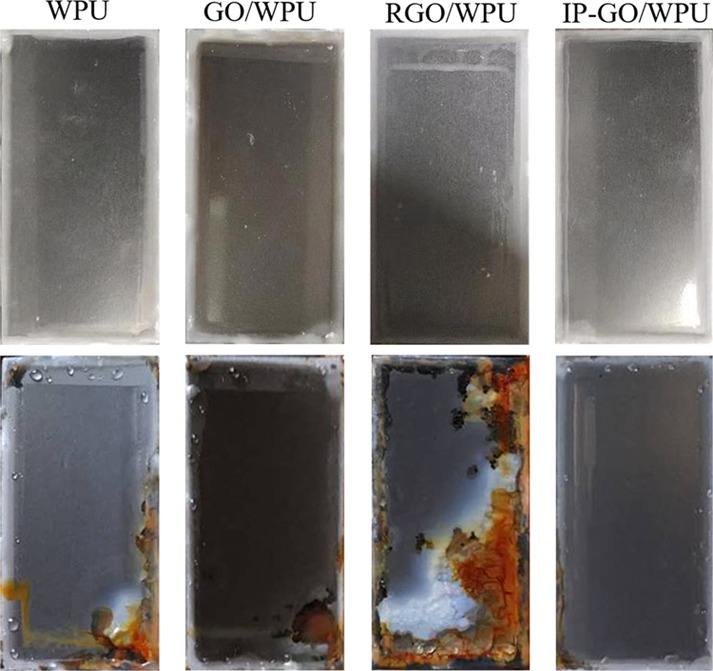
Optical images of Q235 samples coated with WPU, GO/WPU, RGO/WPU, and IP-GO/PU composite coatings before (top panel) and after (bottom panel) subjected to the salt spray test for 480 h.
Based on the above analysis, the excellent corrosion resistance of the graphene derivative anticorrosive coating is due to three factors: (i) maintenance of a high specific surface area of graphene, (ii) excellent dispersion of the graphene derivative in the composite material matrix, and (iii) compatibility of the polymer with graphene derivative sexuality and excellent interface combination. Graphene derivative can be inhibited by forming an electrolyte outside the barrier network in the polymer as schematically illustrated in Figure 10, and the functionalized graphene derivative forms a protective network in the WPU matrix, delaying the time that the corrosive medium enters the interface of the coating matrix. The functionalized graphene derivative is capable of achieving a greater degree of dispersion in the WPU matrix, giving full play to the action of each piece of graphene derivative. Large-scale agglomeration of the graphene derivative fails to provide a protective network and sometimes destroys the continuity of the coating to accelerate corrosion. Therefore, the corrosion resistance of the coating is directly related to the good dispersion of the graphene derivative in the polymer matrix.
Figure 10.
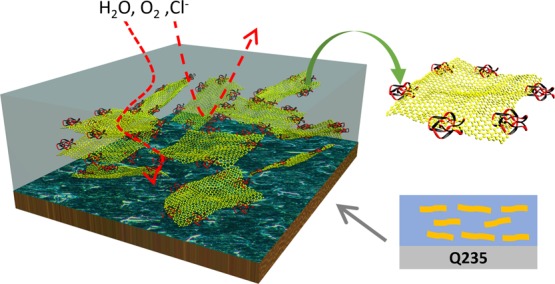
Schematic illustration of the corrosion protection mechanism of IP-GO composite coatings on the metal substrate.
4. Conclusions
The amphiphilic graphene derivative was prepared by covalent grafting of GO with IPDI and DMEA and then noncovalent grafting of GO with SDBS. Characterization results indicate that IPDI and DMEA were grafted on the surface of GO by forming carbamate ester bonds. Compared to other graphene derivatives, the IP-GO enhanced its dispersion and compatibility in WPU. The composite materials of 0.3 wt % GO, RGO, and IP-GO nanosheets combination with the WPU, of which the IP-GO polyurethane composite had the highest mechanical properties and the lowest water absorption. The anticorrosion efficiency of the coating was significantly improved after the addition of GO and IP-GO, but the increase in IP-GO was more pronounced. The results show that IP-GO can form a perfect network structure in the polymer to block the intrusion of corrosive media.
Acknowledgments
The authors gratefully acknowledge financial support from the Natural Science Foundation of Tianjin China (grant no. 19JCZDJC37900), the Science and Technology Plans of Tianjin China (grant no. 18PTSYJC00180), and the National Natural Science Foundation of China (grant no. 21878230).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.9b02687.
TEM images of GO and IP-GO, SEM images of GO and IP-GO, Raman spectra of GO and IP-GO, and digital photos of water and waterborne polyurethane dispersion of GO and IP-GO (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Gianni L.; Gigante G. E.; Cavallini M.; Adriaens A. Corrosion of bronzes by extended wetting with single versus mixed acidic pollutants. Materials 2014, 7, 3353–3370. 10.3390/ma7053353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller J.; Laïk B.; Guillot I. α-CuSn bronzes in sulphate medium: Influence of the tin content on corrosion processes. Corros. Sci. 2013, 77, 46–51. 10.1016/j.corsci.2013.07.025. [DOI] [Google Scholar]
- Poggi G.; Toccafondi N.; Melita L. N.; Knowles J. C.; Bozec L.; Giorgi R.; Baglioni P. Calcium hydroxide nanoparticles for the conservation of cultural heritage: new formulations for the deacidification of cellulose-based artifacts. Appl. Phys. A: Mater. Sci. Process. 2013, 114, 685–693. 10.1007/s00339-013-8172-7. [DOI] [Google Scholar]
- González-García Y.; González S.; Souto R. M. Electrochemical and structural properties of a polyurethane coating on steel substrates for corrosion protection. Corros. Sci. 2007, 49, 3514–3526. 10.1016/j.corsci.2007.03.018. [DOI] [Google Scholar]
- Li Y.; Yang Z.; Qiu H.; Dai Y.; Zheng Q.; Li J.; Yang J. Self-aligned graphene as anticorrosive barrier in waterborne polyurethane composite coatings. J. Mater. Chem. A 2014, 2, 14139–14145. 10.1039/C4TA02262A. [DOI] [Google Scholar]
- Liu X.; Xiong J.; Lv Y.; Zuo Y. Study on corrosion electrochemical behavior of several different coating systems by EIS. Prog. Org. Coat. 2009, 64, 497–503. 10.1016/j.porgcoat.2008.08.012. [DOI] [Google Scholar]
- Monetta T.; Belluci F.; Nicodemo L.; Nicolais L. Protective properties of epoxy-based organic coatings on mild steel. Prog. Org. Coat. 1993, 21, 353–369. 10.1016/0033-0655(93)80050-K. [DOI] [Google Scholar]
- Lindeboom J. Air-drying high solids alkyd pants for decorative coating. Prog. Org. Coat. 1998, 34, 147–151. 10.1016/S0300-9440(98)00034-4. [DOI] [Google Scholar]
- Weiss K. D. Paint and coatings: a mature industry transition. Prog. Polym. Sci. 1997, 22, 203–245. 10.1016/S0079-6700(96)00019-6. [DOI] [Google Scholar]
- Chattopadhyay D. K.; Raju K. V. S. N. Structural engineering of polyurethane coatings for high performance applications. Prog. Polym. Sci. 2007, 32, 352–418. 10.1016/j.progpolymsci.2006.05.003. [DOI] [Google Scholar]
- Li J. H.; Hong R. Y.; Li M. Y.; Li H. Z.; Zheng Y.; Ding J. Effects of ZnO nanoparticles on the mechanical and antibacterial properties of polyurethane coatings. Prog. Org. Coat. 2009, 64, 504–509. 10.1016/j.porgcoat.2008.08.013. [DOI] [Google Scholar]
- Cui Y.; Kundalwal S. I.; Kumar S. Gas barrier performance of graphene/polymer nanocomposites. Carbon 2016, 98, 313–333. 10.1016/j.carbon.2015.11.018. [DOI] [Google Scholar]
- Pourhashem S.; Vaezi M. R.; Rashidi A.; Bagherzadeh M. R. Exploring corrosion protection properties of solvent based epoxy-graphene oxide nanocomposite coatings on mild steel. Corros. Sci. 2017, 115, 78–92. 10.1016/j.corsci.2016.11.008. [DOI] [Google Scholar]
- Rostami M.; Rasouli S.; Ramezanzadeh B.; Askari A. Electrochemical investigation of the properties of Co doped ZnO nanoparticle as a corrosion inhibitive pigment for modifying corrosion resistance of the epoxy coating. Corros. Sci. 2014, 88, 387–399. 10.1016/j.corsci.2014.07.056. [DOI] [Google Scholar]
- Golru S. S.; Attar M. M.; Ramezanzadeh B. Studying the influence of nano-Al2O3 particles on the corrosion performance and hydrolytic degradation resistance of an epoxy/polyamide coating on AA-1050. Prog. Org. Coat. 2014, 77, 1391–1399. 10.1016/j.porgcoat.2014.04.017. [DOI] [Google Scholar]
- Lee C.; Wei X.; Kysar J. W.; Hone J.; Hone J. Measurement of the elastic properties and intrinsic strength of monolayer graphen. Science 2008, 321, 385–388. 10.1126/science.1157996. [DOI] [PubMed] [Google Scholar]
- Balandin A. A.; Ghosh S. G.; Bao W.; Calizo I.; Teweldebrhan D.; Miao F.; Lau C. N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. 10.1021/nl0731872. [DOI] [PubMed] [Google Scholar]
- Compton O. C.; Kim S.; Pierre C.; Torkelson J. M.; Nguyen S. T. Crumpled graphene nanosheets as highly effective barrier property enhancers. Adv. Mater. 2010, 22, 4759–4763. 10.1002/adma.201000960. [DOI] [PubMed] [Google Scholar]
- Georgakilas V.; Otyepka M.; Bourlinos A. B.; Chandra V.; Kim N.; Kemp K. C.; Hobza P.; Zboril R.; Kim K. S. Functionalization of graphene: covalent and non-covalent approaches, derivatives and applications. Chem. Rev. 2012, 112, 6156–6214. 10.1021/cr3000412. [DOI] [PubMed] [Google Scholar]
- Pu J.; Wan S.; Lu Z.; Zhang G.-A.; Wang L.; Zhang X.; Xue Q. Controlled water adhesion and electrowetting of conducting hydrophobic graphene/carbon nanotubes composite films on engineering materials. J. Mater. Chem. A 2013, 1, 1254–1260. 10.1039/C2TA00344A. [DOI] [Google Scholar]
- Mišković-Stanković V.; Jevremović I.; Jung I.; Rhee K. Electrochemical study of corrosion behavior of graphene coatings on copper and aluminum in a chloride solution. Carbon 2014, 75, 335–344. 10.1016/j.carbon.2014.04.012. [DOI] [Google Scholar]
- Schriver M.; Regan W.; Gannett W. J.; Zaniewski A. M.; Crommie M. F.; Zettl A. Graphene as a long-term metal oxidation barrier: worse than nothing. ACS Nano 2013, 7, 5763. 10.1021/nn4014356. [DOI] [PubMed] [Google Scholar]
- Zhou F.; Li Z.; Shenoy G. J.; Li L.; Liu H. Enhanced room-temperature corrosion of copper in the presence of graphene. ACS Nano 2013, 7, 6939–6947. 10.1021/nn402150t. [DOI] [PubMed] [Google Scholar]
- Cheng H.; Ye M.; Zhao F.; Hu C.; Zhao Y.; Liang Y.; Chen N.; Chen S.; Jiang L.; Qu L. A General and extremely simple remote approach toward graphene bulks with in situ multifunctionalization. Adv. Mater. 2016, 28, 3305–3312. 10.1002/adma.201505431. [DOI] [PubMed] [Google Scholar]
- Kim K.-S.; Jeon I.-Y.; Ahn S.-N.; Kwon Y.-D.; Baek J.-B. Edge-functionalized graphene-like platelets as a co-curing agent and a nanoscale additive to epoxy resin. J. Mater. Chem. 2011, 21, 7337. 10.1039/c0jm03504a. [DOI] [Google Scholar]
- Chai G.-L.; Qiu K.; Qiao M.; Titirici M.-M.; Shang C.; Guo Z. Active sites engineering leads to exceptional ORR and OER bifunctionality in P,N Co-doped graphene frameworks. Energy Environ. Sci. 2017, 10, 1186–1195. 10.1039/C6EE03446B. [DOI] [Google Scholar]
- Hua D.; Rai R. K.; Zhang Y.; Chung T.-S. Aldehyde functionalized graphene oxide frameworks as robust membrane materials for pervaporative alcohol dehydration. Chem. Eng. Sci. 2017, 161, 341–349. 10.1016/j.ces.2016.12.061. [DOI] [Google Scholar]
- Lin Y. H.; Lee T.-C.; Hsiao Y.-S.; Lin W.-K.; Whang W.-T.; Chen C.-H. Facile synthesis of diamino-modified graphene/polyaniline semi-interpenetrating networks with practical high thermoelectric performance. ACS Appl. Mater. Interfaces 2018, 10, 4946–4952. 10.1021/acsami.7b14890. [DOI] [PubMed] [Google Scholar]
- Sainsbury T.; Gnaniah S.; Spencer S. J.; Mignuzzi S.; Belsey N. A.; Paton K. R.; Satti A. Extreme mechanical reinforcement in graphene oxide based thin-film nanocomposites via covalently tailored nanofiller matrix compatibilization. Carbon 2017, 114, 367–376. 10.1016/j.carbon.2016.11.061. [DOI] [Google Scholar]
- Williams G.; Seger B.; Kamat P. V. TiO2-Graphene nanocomposites. UV-assisted photocatalytic reduction of graphene oxide. ACS Nano 2008, 2, 1487–1491. 10.1021/nn800251f. [DOI] [PubMed] [Google Scholar]
- Yu D.; Yang Y.; Durstock M.; Baek J.-B.; Dai L. Soluble P3HT-grafted graphene for efficient bilayer–heterojunction photovoltaic devices. ACS Nano 2010, 4, 5633–5640. 10.1021/nn101671t. [DOI] [PubMed] [Google Scholar]
- Pang H.; Chen T.; Zhang G.; Zeng B.; Li Z.-M. An electrically conducting polymer/graphene composite with a very low percolation threshold. Mater. Lett. 2010, 64, 2226–2229. 10.1016/j.matlet.2010.07.001. [DOI] [Google Scholar]
- Wang X.; Zhi L.; Müllen K. Transparent, conductive graphene electrodes for dye-sensitized solar cells. Nano Lett. 2008, 8, 323–327. 10.1021/nl072838r. [DOI] [PubMed] [Google Scholar]
- Wei T.; Luo G.; Fan Z.; Zheng C.; Yan J.; Yao C.; Li W.; Zhang C. Preparation of graphene nanosheet/polymer composites using in situ reduction-extractive dispersion. Carbon 2009, 47, 2296–2299. 10.1016/j.carbon.2009.04.030. [DOI] [Google Scholar]
- Luo Z.-J.; Geng H.-Z.; Zhang X.; Du B.; Ding E. X.; Wang J.; Lu Z.; Sun B.; Wang J.; Liu J. A timesaving, low-cost, high-yield method for the synthesis of ultrasmall uniform graphene oxide nanosheets and their application in surfactants. Nanotechnology 2016, 27, 055601 10.1088/0957-4484/27/5/055601. [DOI] [PubMed] [Google Scholar]
- Wang J.; Geng H.-Z.; Luo Z.-J.; Zhang S.; Zhang J.; Liu J.; Yang H.-J.; Ma S.; Sun B.; Wang Y.; Da S.-X.; Fu Y.-Q. Preparation, characterization, and chemical-induced hydrophobicity of thermostable amine-modified graphene oxide. RSC Adv. 2015, 5, 105393–105399. 10.1039/C5RA19166A. [DOI] [Google Scholar]
- Blagbrough I. S.; Mackenzie N. E.; Ortiz C.; Scott A. I. The condensation reaction between isocyanates and carboxylic acids. A practical synthesis of substituted amides and anilides. Tetrahedron Lett. 1986, 27, 1251–1254. 10.1016/S0040-4039(00)84230-6. [DOI] [Google Scholar]
- Stankovich S.; Piner R. D.; Nguyen S. T.; Ruoff R. S. Synthesis and exfoliation of isocyanate-treated graphene oxide nanoplatelets. Carbon 2006, 44, 3342–3347. 10.1016/j.carbon.2006.06.004. [DOI] [Google Scholar]
- Cataldo F. Structural analogies and differences between graphite oxide and C60 and C70 polymeric oxides (fullerene ozopolymers). Fullerenes, Nanotubes, Carbon Nanostruct. 2003, 11, 1–13. 10.1081/FST-120018670. [DOI] [Google Scholar]
- Mermoux M.; Chabre Y.; Rousseau A. FTIR and 13C NMR study of graphite oxide. Carbon 1991, 29, 469–474. 10.1016/0008-6223(91)90216-6. [DOI] [Google Scholar]
- Szabó T.; Berkesi O.; Dékány I. DRIFT study of deuterium-exchanged graphite oxide. Carbon 2005, 43, 3186–3189. 10.1016/j.carbon.2005.07.013. [DOI] [Google Scholar]
- Luo X.; Zhong J.; Zhou Q.; Du S.; Yuan S.; Liu Y. Cationic reduced graphene oxide as self-aligned nanofiller in the epoxy nanocomposite coating with excellent anticorrosive performance and its high antibacterial activity. ACS Appl. Mater. Interfaces 2018, 10, 18400–18415. 10.1021/acsami.8b01982. [DOI] [PubMed] [Google Scholar]
- Amirudin A.; Thierry D. Application of electrochemical impedance spectroscopy to study the degradation of polymer-coated metals. Prog. Org. Coat. 1995, 26, 1–28. 10.1016/0300-9440(95)00581-1. [DOI] [Google Scholar]
- Scully J. R. Electrochemical impedanceof organic-coated steel: correlation of impedance parameterswith long-term coating deterioration. J. Electrochem. Soc. 1989, 136, 979–989. 10.1149/1.2096897. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.