Abstract
Objective:
The aim of this study was to investigate the microRNA-200b-3p expression in lung adenocarcinoma and the possible functional associations of microRNA-200b-3p with cell proliferation, migration, and invasion.
Methods:
Quantitative real-time polymerase chain reaction was used to detect the expression of microRNA-200b-3p in lung adenocarcinoma samples and in the human lung adenocarcinoma cell lines A549 and H1299. A549 and H1299 cells were transfected with either a microRNA-200b-3p mimic or a negative control microRNA or either an empty vector or an adenosine triphosphate-binding cassette transporter A-1 overexpression vector. A Cell Counting Kit-8 assay was employed to assess the ability of cell proliferation. Transwell assays and transwell-Matrigel invasion assay were, respectively, utilized to assess the capacity of migration and invasion in A549 and H1299 cells.
Results:
The results showed that microRNA-200b-3p expression was significantly upregulated in tumor tissues compared with that in adjacent normal tissues. Overexpression of microRNA-200b-3p promoted lung adenocarcinoma cell proliferation and metastasis. Furthermore, adenosine triphosphate-binding cassette transporter A-1 was a direct target of microRNA-200b-3p, and this binding was verified by luciferase reporter analysis. Overexpression of adenosine triphosphate-binding cassette transporter A-1 obviously suppressed lung adenocarcinoma cell proliferation, migration, and invasion. Lung adenocarcinoma cell phenotypes induced by microRNA-200b-3p overexpression could be partially remitted by the co-overexpression of microRNA-200b-3p and adenosine triphosphate-binding cassette transporter A-1.
Conclusion:
This study first identified that microRNA-200b-3p is upregulated in lung adenocarcinoma cells and associated with cell proliferation and metastasis. MicroRNA-200b-3p promoted lung adenocarcinoma cell proliferation and metastasis by suppressing adenosine triphosphate-binding cassette transporter A-1. MicroRNA-200b-3p may function as a novel molecular marker and therapeutic target for lung adenocarcinoma treatment.
Keywords: miR-200b-3p, lung adenocarcinoma (LUAD), ATP-binding cassette transporter A-1 (ABCA1), proliferation, metastasis
Introduction
Lung carcinoma is currently the most common cause of cancer incidence and mortality worldwide.1 Non-small-cell lung cancer (NSCLC) accounts for approximately 80% of all diagnosed lung carcinomas, and lung adenocarcinoma (LUAD) is the most frequent subtype.2 Although targeted therapy has been widely utilized, there are still patients having lung carcinoma with severe or metastatic disease after treatment. Therefore, new insight into the molecular mechanisms underlying LUAD and the identification of effective diagnostic and therapeutic targets for LUAD is still urgently needed.
MicroRNAs (miRNAs) are a class of highly conserved small noncoding RNAs that bind to the 3′-untranslated regions (3′-UTRs) of their target genes, leading to target gene suppression. Accumulating evidence indicates that the aberrant expression of miRNAs is related to diverse types of disease development and progression as well as LUAD. It was reported that microRNA-200b-3p (miR200b-3p) is a potential biomarker in LUAD, and that the level of miR-200b-3p was higher in LUAD than in control cells.3 MicroRNA-200b-3p mediates the regulation of TIMP4 expression in prostate cancer,4 but the exact mechanism needs to be investigated. It was found that miR-200b-3p suppressed epithelial–mesenchymal transition (EMT) and inhibited glioma tumor growth by downregulating ERK5.5 Additionally, miR-200b-3p in plasma could be a potential diagnostic biomarker in oral squamous cell carcinoma.6 Moreover, miR-200b-3p was able to inhibit the EMT of human pancreatic cancer in vivo and in vitro by targeting ZEB1.7 It was also reported that c-myc/miR-200b/PRDX2 loop regulated colorectal carcinoma progression and that its disruption enhanced tumor metastasis and chemotherapeutic resistance in colorectal cancer.8 MicroRNA-200b-3p was shown to be downregulated by the low expression of p73 in androgen-independent prostate cancer cells.9 Previous studies have shown the key role of miR-200b-3p in different cancers, but up until now, the detailed mechanism of how miR-200b-3p is regulated in LUAD and how miR-200b-3p affects the disease is largely unknown.
The goal of the current study was to explore the biological functions of miR-200b-3p in LUAD and to investigate the underlying mechanisms of action. We showed that miR-200b-3p directly targets and regulates the 3′-UTR of the human adenosine triphosphate (ATP)-binding cassette transporter A-1 (ABCA1) messenger RNA (mRNA) for the first time, which is downregulated in many cancers; ABCA1 inhibits cancer progression in many cancers; for example, overexpression of ABCA1 leads to curcumin resistance in M14 melanoma cells,10 and downregulated ABCA1 confers cisplatin resistance to NSCLC A549 cells.11 Here, we reported that miR-200b-3p is upregulated in LUAD compared to that in paracarcinoma tissue and found that the 3′-UTR of human ABCA1 mRNA is a target of miR-200b-3p. Collectively, we discovered that miR-200b-3p promotes cell proliferation and metastasis by directly targeting 3′-UTR of ABCA1 in LUAD.
Materials and Methods
Tumor Tissue Samples and Cell Lines
This study was approved by the human ethics and research ethics committees of 7th Medical Center of Peoples Liberation Army General Hospital. This study included 15 human LUAD samples and 15 corresponding adjacent normal tissue samples derived from patients who underwent surgery. The human LUAD cell lines A549 and H1299 and the human normal lung epithelial cells BEAS-2B were purchased from the Institute of Biochemistry and Cell Biology of the Chinese Academy of Sciences (Shanghai, China).
MicroRNA-200b-3p and ABCA1 Expression Analysis of LUAD Tissue in the Database
The starBase Pan-Cancer Analysis Platform (http://starbase.sy su.edu.cn/panCancer.php) was utilized, and the mRNA or miRNA expression profiles in LUAD were extracted by cancer genome mapping (The Cancer Genome Atlas [TCGA]). MicroRNA-200b-3p–ABCA1 interactions were identified in LUAD from cancer genome mapping (TCGA), and coexpression analysis was also performed with the starBase Pan-Cancer Analysis Platform (http://starbase.sysu.edu.cn/panMirCoExp.php).
Quantitative Real-Time Polymerase Chain Reaction
Total RNA was extracted from the indicated cells with TRIzol Reagent (Invitrogen, Shanghai, China) in accordance with the manufacturer’s instructions and was then performed to reverse transcribe into complementary DNA. The quantification of genes and miRNAs was detected by a quantitative real-time polymerase chain reaction kit (Life Technologies, Shanghai, China) with the QuantStudio 6 Flex Real-Time PCR System (Life Technologies) using a SYBR Green Kit (TaKaRa, Tokyo, Japan). U6 was the internal reference for miRNA. Glyceraldehyde 3-phosphate dehydrogenase was the internal reference for ABCA1, and the relative expression of genes and miRNAs was quantified. The primers for miR-200b-3p were 5′-GCTGCTGAATTCCATCTAATTTCCAAAAG-3′ and 5′-TATTATGGATCCGCCCCCAGGGCAATGGG-3′. The primers for ABCA1 were 5′-CCTGACCGGGTTGTTCCC-3′ (forward primer) and 5′-TTCTGCCGGATGGTGCTC-3′ (reverse primer).
Cell Culture and Transfection
Cells were cultured in Dulbecco modified Eagle medium (DMEM; Gibco, Grand Island, New York) containing 10% fetal bovine serum (10% FBS), 100 U/mL penicillin, and 100 mg/mL streptomycin (Gibco) and incubated in humidified air at 37°C with 5% CO2. The miR-200b-3p mimic and the miR-200b-3p negative control (miR-200b-3p NC) mimic were purchased from GenePharma Co, Ltd (Shanghai, China); ABCA1 overexpression vector and blank vector were obtained from Vigene Biosiciences Co, Ltd. (Shandong, China). Cells (2 × 105 cells/well) were seeded into 6-well plates, mixed and placed overnight in CO2 incubator at 37°C. The plasmid to be transfected was diluted with 2 μg using 100 μL serum-free medium. Cells were transfected with either the miR-200b-3p mimic or the miR-200b-3p NC or with either the overexpression vector or the blank vector by using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions.
Cell Counting Kit-8 Assay
The ability of proliferation in cell lines A549/H1299 was determined by a Cell Counting Kit-8 (CCK-8; Dojindo, Kumamoto, Japan). The A549 and H1299 cells (5 × 105 cells/well) were seeded in 24-well plates. After 1 hour incubating with 10% CCK-8, the cell viability was determined by measuring the absorbance of 450 nm. The proliferation rates of A549 and H1299 cells were measured at 0, 1, 2, 3, and 4 days after transfection.
Transwell Migration/Invasion Assay
A549 and H1299 cells were grown in DMEM containing 10% FBS to ∼60% confluence and were transfected with the corresponding miRNA mimic or negative control or gene overexpression construct. Cells were collected by trypsinization after 48 hours. A density of 2 × 104 cells in 200 µL of serum-free medium was placed into the upper transwell chamber (Costar, High Wycombe, United Kingdom). Cell migration and invasion analysis was performed with 8-mm pore size culture inserts placed into the wells of 24-well culture plates. Complete medium (500 μL) containing 10% FBS was placed to the lower chambers as a chemoattractant. After culturing for 24 hours, the cells on the upper surface of the membrane were completely removed using a cotton swab. Then, the cells were fixed in 4% paraformaldehyde and stained with 4′,6-diamidino-2-phenylindole for 15 to 20 minutes. The migrating or invading cells were counted under a microscope and photographed. Each experiment was performed at least 3 times.
Western Blot Analysis
Total protein was extracted from LUAD tissues or cells, and concentration was determined by bicinchoninic acid assay; 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis was used to separate the total protein, and then the protein was transferred from membrane onto polyvinylidene difluoride membranes (Bio-Rad, Hercules, California). The bolts were incubated with primary anti-ABCA1 (ab18180) and anti-GAPDH (ab8245) antibodies overnight at 4°C. Then the blots incubated with the secondary antibody (Santa Cruz Biotechnology, California) for 1 hour at room temperature. Protein bands were visualized using the enhanced chemiluminescence kit according to the manufacturer’s instructions; GAPDH was used as a loading control. Western blot protein grayscale values were detected and analyzed by ImageJ.
Luciferase Assay
The A549 and H1299 cells (5 × 105 cells/well) were seeded into 24-well plates. Then they were incubated for 24 hours. For the luciferase reporter gene assay, the A549 and H1299 cells were cotransfected with ABCA1 wild-type reporter plasmid and a mutant reporter plasmid as well as a miR-200b-3p mimic and miR-200b-3p NC mimic with Lipofectamine 2000. The activities of firefly and Renilla luciferase were detected by dual-luciferase reporter assay 48 hours posttransfection (Promega, Shanghai, China).
Statistical Analysis
Student t test was used between 2 groups, and the one-way analysis of variance was used among multiple groups. Data are displayed as the means ± the standard deviations from at least 3 independent experiments. P < .05 was considered statistically significant.
Results
MicroRNA-200b-3p is Upregulated in LUAD Tissues and Cells
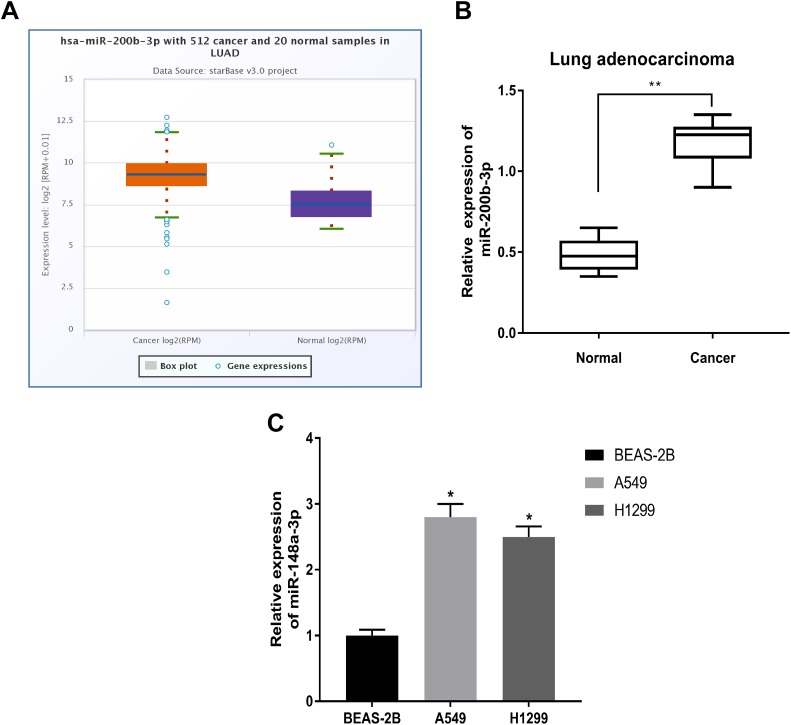
To investigate the role of miR-200b-3p in LUAD tissues, we first analyzed miR-200b-3p expression in human LUAD samples from TCGA. We observed that miR-200b-3p was significantly upregulated in the 512 LUAD samples compared with the expression in the 20 normal samples (Figure 1A). Moreover, we also detected the expression levels of miR-200b-3p in LUAD clinical samples we collected from surgery. As expected, the level of miR-200b-3p expression was elevated in the LUAD samples compared with the expression in normal samples (Figure 1B). Moreover, we also detected the expression levels of miR-200b-3p in LUAD cells (A549 and H1299), and the results were consistent with those in LUAD tissues. The expression levels of miR-200b-3p in both type of LUAD cells (A549 and H1299) were higher than that in human normal lung epithelial cell BEAS-2B (Figure 1C), these results indicated that miR-200b-3p is upregulated in LUAD tissues and cells.
Figure 1.
The expression of miR-200b-3p in LUAD tissues and cells. A, The analysis of miR-200-3p in LUAD samples from TCGA. B, Quantitative polymerase chain reaction quantification of miR-200b-3p in LUAD tissues and adjacent normal tissues. C, Quantitative polymerase chain reaction quantification of miR-200b-3p in LUAD cells (A549 and H1299) and the human normal lung epithelial cells BEAS-2B. *P < .05, **P < .01. LUAD indicates lung adenocarcinoma; TCGA, The Cancer Genome Atlas
MicroRNA-200b-3p Promotes LUAD cell Proliferation and Metastasis
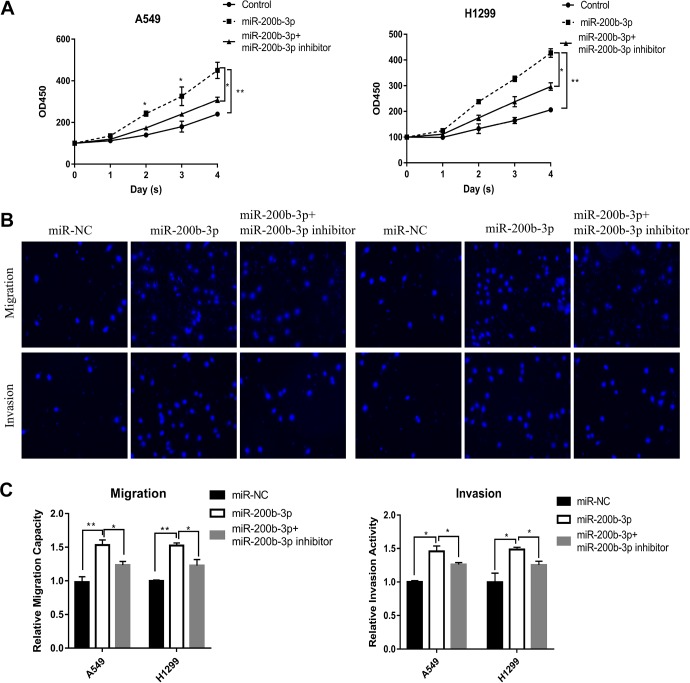
To further explore the function of miR-200b-3p in LUAD, we measured the effects of miR-200b-3p on the LUAD cell proliferation by using CCK8 assay. Our results showed that overexpression of miR-200b-3p remarkably increased the proliferation of both A549 and H1299 cells compared to the proliferation of the control cells, but the proliferation of both A549 and H1299 decreased significantly after miR-200b-3p inhibitor treatment compared to the proliferation of the miR-200b-3p-transfected cells (Figure 2A). In addition, we also investigated the role of miR-200b-3p in LUAD cell metastasis by using transwell analysis, and the results revealed that miR-200b-3p mimic treatment resulted in an increase in the migration capacity and invasive ability of A549 and H1299 cells compared with those of the cells in NC mimic group; what’s more, the miR-200b-3p inhibitor significantly weakened the effects of the miR-200b-3p mimic on the migration capacity and invasive ability of A549 and H1299 cells (Figure 2B), in accordance with the quantified results shown in Figure 2C.
Figure 2.
MicroRNA-200b-3p overexpression promoted LUAD cell proliferation and metastasis. A, Cell Counting Kit-8 assay measured the proliferation of the miR-200b-3p-transfected LUAD cells A549 and H1299. *P < .05, **P < .01. B, Representative transwell migration assays and transwell invasion assays after transfection with either the miR-200b-3p mimic or miR-NC or either the miR-200b-3p mimic or inhibitor in A549 or H1299. C< Quantitative representation of the transwell migration assay and transwell invasion assay after transfection with either the miR-200b-3p mimic or miR-NC or either the miR-200b-3p mimic or inhibitor in A549 or H1299 cells. Assays were performed in triplicate. *P < .05, **P < .01. Means ± SEMs are shown. Statistical analysis was conducted using ANOVA. ANOVA indicates analysis of variance; LUAD, lung adenocarcinoma; miR-NC, microRNA-negative control; SEM, standard error of the mean.
ABCA1 is a Direct Target of miR-200b-3p
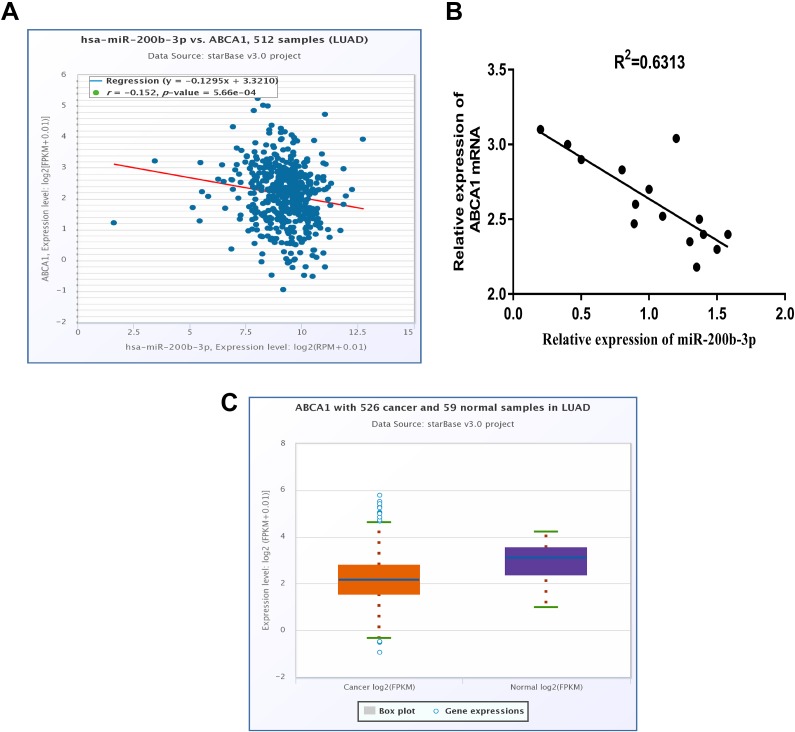
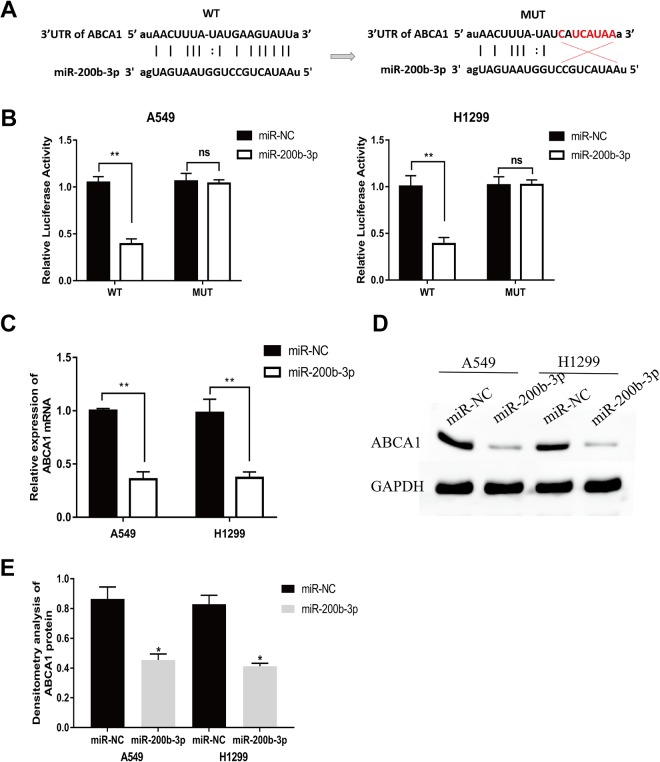
MicroRNA target prediction software (starbase v3.0) was used to search for the target genes of miR-200b-3p and found that HMGB3, ZEB1, and ABCA1 were all highly regulated by miR-200b-3p, then, through the detection of the influence of overexpressed miR-200b-3p on the mRNA expression of these 3 target genes, it was found that miR-200b-3p had the most significant effect on ABCA1 (Figure S1). Thus, we detected the expression of miR-200b-3p and ABCA1 in 15 clinical samples. We found that ABCA1 had a negative correlation with miR-200b-3p (Figure 3A and B), and that ABCA1 expression was downregulated in LUAD tumors from TCGA (Figure 3C). Furthermore, a complementary region of miR-200b-3p was identified in the 3′-UTR of ABCA1 (Figure 4A). Thus, the luciferase plasmid including the wild-type ABCA1 3′-UTR fragment or the mutated ABCA1 3′-UTR fragment was constructed. Our results showed that miR-200b-3p significantly decreased the luciferase activity compared to the luciferase activity of the control miRNA in both A549 and H1299 cells (Figure 4B). Importantly, miR-200b-3p had no effect on the mutated 3′-UTR fragment (Figure 4B), suggesting that miR-200b-3p directly targeted ABCA1. In addition, both the mRNA and protein levels of ABCA1 were significantly declined in A549 or H1299 cells transfected with miR-200b-3p mimics (Figure 4C, D, and F).
Figure 3.
Adenosine triphosphate-binding cassette transporter A-1 is a predicted target of miR-200b-3p in LUAD. A, Adenosine triphosphate-binding cassette transporter A-1 has a negative correlation with miR-200b-3p in 512 LUAD samples from starBase v3.0; r = -0.152. B, Adenosine triphosphate-binding cassette transporter A-1 has a negative correlation with miR-200b-3p in 15 LUAD tissue samples used in this study. R 2 = 0.6313; C, The expression of ABCA1 is decreased in 526 LUAD tumor samples compared with that in 59 normal samples from starBase v3.0. ABCA1 indicates adenosine triphosphate-binding cassette transporter A-1; LUAD, lung adenocarcinoma; miR-200b-3p; microRNA-200b-3p.
Figure 4.
MicroRNA-200b-3p targets ABCA1 in LUAD cells A, The predicted sequence complementation between miR-200b-3p and ABCA1. B, Analysis of the relative luciferase activities of ABCA1-WT and ABCA1-MUT in A5491 and H1299 cells after transfection with miR-200b-3p mimics. **P < .01. C, The mRNA level of ABCA1 significantly decreased with time after transfection with miR-200b-3p mimics in A5491 and H1299 cells by qRT-PCR. **P < .01. D, The protein level of ABCA1 significantly decreased with time after transfection with miR-200b-3p mimics in A549 and H1299 cells as detected by a Western blot. E, Densitometry analysis of ABCA1 protein after transfection with miR-200b-3p mimics in A549 and H1299 cells. ABCA1 indicates adenosine triphosphate-binding cassette transporter A-1; LUAD, lung adenocarcinoma; miR-200b-3p; miR-200b-3p; microRNA-200b-3p; MUT, mutant; qRT-PCR, quantitative real-time polymerase chain reaction; WT, wild type.
ABCA1 Inhibits the Proliferation and Metastasis of LUAD Cells
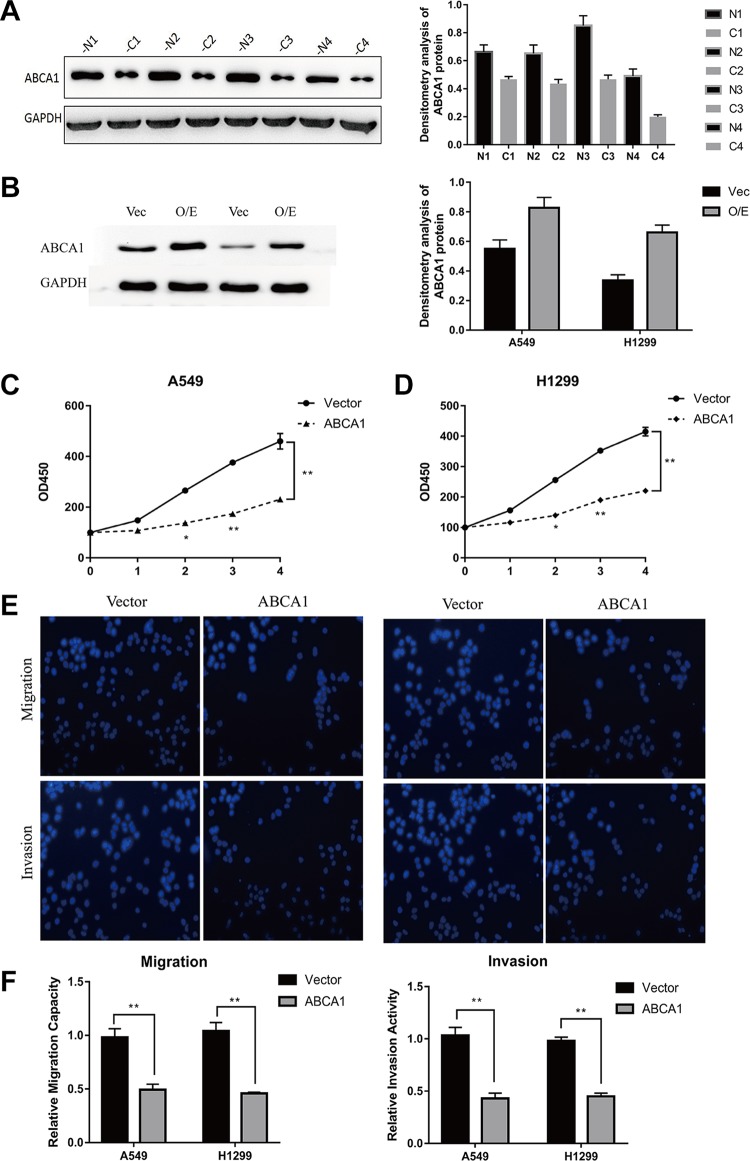
We next examined the ABCA1 expression in human LUAD samples using Western blot. The results demonstrated that ABCA1 was significantly decreased in LUAD tissues compared to that in adjacent normal tissues (Figure 5A). Then, we constructed the ABCA1 plasmid to overexpress ABCA1 in LUAD cell lines and examined the expression levels in both LUAD cell lines A549 and H1299. The results showed that ABCA1 expression at the protein level was significantly elevated in ABCA1-transfected A549 and H1299 cells as evaluated by a Western blot (Figure 5B). Functional analysis revealed that ABCA1 overexpression caused proliferation inhibition both in A549 and H1299 cells (Figure 5C and D). What’s more, both the migration and invasion abilities of A549 and H1299 cells transfected with the ABCA1 overexpression plasmid were significantly impaired (Figure 5E and F), which was contrary to the phenotype resulting from the miR-200b-3p overexpression (Figure 2B and C). These results suggested that ABCA1 can inhibit the proliferation and metastasis of LUAD cells.
Figure 5.
Adenosine triphosphate-binding cassette transporter A-1 overexpression inhibits LUAD cell proliferation and metastasis. A, Western blot analysis and the densitometry analysis of ABCA1 expression in LUAD tissues (C) and adjacent normal tissues (N). B, Western blot analysis and densitometry analysis of ABCA1 overexpression in the human LUAD cell lines A549 and H1299. C and D, Cell Counting Kit-8 assays of A549 and H1299 cells after transfection with an ABCA1 overexpression construct or an empty vector. **P < .01. E, Representatives of transwell migration assays and transwell invasion assays after transfection with an ABCA1 overexpression construct or an empty vector in A549 and H1299. F, Quantitative representation of the transwell migration assay and transwell invasion assay after transfection with an ABCA1 overexpression construct or an empty vector in A549 and H1299 cells. Assays were performed in triplicate. *P < .05, **P < .01. Means ± SEMs are shown. Statistical analysis was conducted using ANOVA. ABCA1 indicates adenosine triphosphate-binding cassette transporter A-1; ANOVA, analysis of variance; LUAD, lung adenocarcinoma; SEM, standard error of the mean.
MiR-200b-3p Facilitates LUAD Cell Growth, Migration, and Invasion by Targeting ABCA1
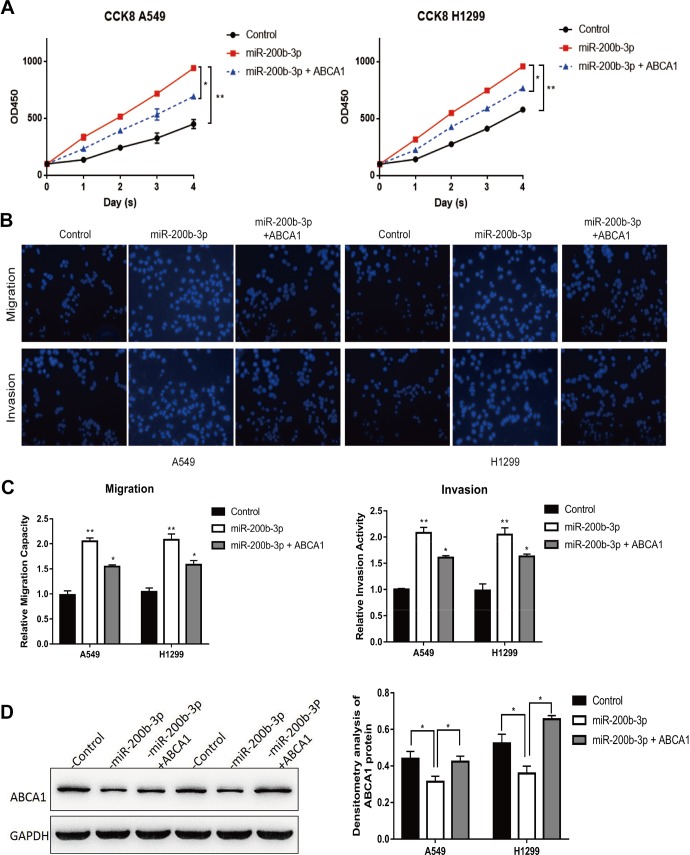
To verify our hypothesis that miR-200b-3p directly targeted ABCA1, we co-overexpressed ABCA1 and miR-200b-3p in A549 and H1299 cells. The CCK8 assay demonstrated that overexpression of miR-200b-3p significantly inhibited LUAD cell proliferation while co-overexpression of miR-200b-3p and ABCA1 partially restored the LUAD cell growth (Figure 6A), suggesting that miR-200b-3p functioned possibly through targeting ABCA1. In addition, we also assessed the migration capacity and invasion abilities of LUAD cells with the coexpression of miR-200b-3p and ABCA1. Our data showed that the impaired migration and invasion abilities of LUAD cells due to miR-200b-3p overexpression could be partially reversed by co-overexpression of miR-200b-3p and ABCA1 (Figure 6B and C). A Western blot revealed that overexpression of miR-200b-3p significantly inhibited the ABCA1 expression while co-overexpression of miR-200b-3p and ABCA1 almost restored the ABCA1 protein levels in A549 and H1299 cells (Figure 6D). Taken together, these results indicated that miR-200b-3p promotes the growth, migration, and invasion of LUAD cells by directly targeting the expression of ABCA1.
Figure 6.
Co-overexpression of ABCA1 and miR-200b-3p partially restored miR-200b-3p overexpression-induced effects. A, Cell Counting Kit-8 cell viability analysis of A549 and H1299 cells transfected with an empty vector, A549 and H1299 cells with an miR-200b-3p mimic, and A549 and H1299 cells co-overexpressing miR-200b-3p and ABCA1. *P < .05, **P < .01. B, Cell migration and cell invasion analysis of A549 and H1299 cells transfected with an empty vector, A549 and H1299 cells transfected with miR-200b-3p mimic, and A549 and H1299 cells co-overexpressing miR-200b-3p and ABCA1. C, Quantitative representation of the transwell migration assay and transwell invasion assay after transfection with an empty vector, miR-200b-3p mimic, miR-200b-3p mimic, and ABCA1-overexpressing vector in A549 and H1299. Assays were performed in triplicate. *P < .05, **P < .01. Means ± SEMs are shown. Statistical analysis was conducted using ANOVA. D, Western blot analysis and densitometry analysis of ABCA1 expression in empty vector control, miR-200b-3p-overexpressing, ABCA1, and miR-200b-3p co-overexpressing A549 and H1299 cells. ABCA1 indicates adenosine triphosphate-binding cassette transporter A-1; ANOVA, analysis of variance; miR-200b-3p, microRNA-200b-3p; SEM, standard error of the mean.
Discussion
Lung cancer is one of the most common malignant tumors in the world and is the main cause of cancer-related deaths.2 Lung adenocarcinoma is the most common type of lung cancer, accounting for 30% to 35% of primary lung cancer cases.12 Therefore, it is of great significance to explore the molecular mechanisms of LUAD in the diagnosis and treatment of lung cancer.
It is reported that miRNAs had diagnostic, prognostic, and therapeutic potential for LUAD.13-17 Our data presented that the increased expression of miR-200b-3p facilitated the development and metastasis of LUAD, suggesting an oncogenic role of miR-200b-3p that was mediated by the direct target of ABCA1. MicroRNA dysregulation is one of the major pathogenic factors of LUAD, and it may be possible to intervene with the dysregulated genes using miRNA mimics or antagonists in clinical applications.18-20 As a result, it is important to identify cancer-specific miRNAs. Our results showed that miR-200b-3p was significantly upregulated in human LUAD tissues, indicating that miR-200b-3p acted as an oncogene in LUAD. However, no special studies have examined the mechanistic role of miR-200b-3p in LUAD. Thus, it is very important to explore the expression levels and regulatory mechanisms of miR-200b-3p in LUAD to obtain a comprehensive understanding of the role of miR-200b-3p in LUAD. In addition to the facilitator role of miR-200b-3p in LUAD proliferation, our results also indicated that miR-200b-3p overexpression promoted the migration and invasion of A549 and H1299 cells, suggesting a stimulative effect of miR-200b-3p on cancer cell migration and invasion. In the future, we will perform study in vivo on the function of miR-200b-3p in LUAD metastasis to validate the results in this study.
Adenosine triphosphate-binding cassette transporter A-1 is actually is a cholesterol exporter that can mediate the transfer of cellular cholesterol.21 The dysregulation of cholesterol homeostasis in many human cancers is associated with the loss of ABCA1.22,23 Adenosine triphosphate-binding cassette transporter A-1 acts as a tumor suppressor gene in many cancers, such as colon cancer24 and breast cancer.21 However, the function of ABCA1 in LUAD has not been studied.
The analysis from the starBase v3.0 project uncovered a negative correlation between miR-200b-3p and ABCA1 among 512 LUAD samples (Figure 3A); and there was also a negative correlation between miR-200b-3p and ABCA1 among 15 clinical samples (Figure 3B), ABCA1 was decreased in 526 LUAD samples compared to that in 59 normal samples (Figure 3C). We also performed a luciferase reporter assay to show that miR-200b-3p functioned by suppressing ABCA1 (Figure 4B). Our data revealed that ABCA1 expression was downregulated in human LUAD tissues (Figure 5A), which was consistent with previous findings from a TCGA database analysis. Furthermore, restoration of ABCA1 expression rescued the cell growth promotion caused by miR-200b-3p overexpression. Indeed, a mountain of evidence seems to indicate that the downregulation of ABCA1 is involved in tumor initiation, progression, metastasis, and drug susceptibility.22,25-29 Our results provided further evidence that ABCA1 overexpression was involved in the suppression of tumor growth, migration, and invasion (Figure 5C-E). Consistently, our data showed that ABCA1 inhibition induced by miR-200b-3p overexpression led to the improvement of migration and invasion ability in LUAD cells (Figure 4C-E).
To sum up, our study revealed that miR-200b-3p was upregulated in LUAD and promoted LUAD cell proliferation, migration, and invasion by targeting ABCA1. This novel miR-200b-3p/ABCA1 axis improves our understanding of the mechanisms underlying LUAD. Since miR-200b-3p overexpression promoted the proliferation, migration, and invasion of LUAD cells, and miRNA mimics or antagonists are becoming investigated as therapeutic targets in the context of molecular networks in the future, miR-200b-3p may serve as a potential therapeutic target for LUAD treatment.
Supplemental Material
Supplementary_material for MiR-200b-3p Functions as an Oncogene by Targeting ABCA1 in Lung Adenocarcinoma by Keqiang Liu, Weiqiang Zhang, Jian Tan, Jingbo Ma and Jing Zhao in Technology in Cancer Research & Treatment
Acknowledgments
The authors thank for Genex Health Co, Ltd (Beijing, China) assistance during the preparation of this manuscript.
Abbreviations
- ABCA1
adenosine triphosphate-binding cassette transporter A-1
- ATP
adenosine triphosphate
- CCK-8
Cell Counting Kit-8
- DMEM
Dulbecco modified Eagle medium
- EMT
epithelial–mesenchymal transition
- FBS
fetal bovine serum
- LUAD
lung adenocarcinoma
- miRNA
microRNA
- miR-200b-3p
microRNA-200b-3p
- mRNA
messenger RNA
- NC
negative control
- NSCLC
non-small-cell lung cancer
- 3′-UTR
3′-untranslated region
Authors’ Note: This study was approved by the 7th Medical Center of Peoples Liberation Army General Hospital Ethical Committee (approval no. 2018633). All patients provided the verbal consent prior to enrollment in the study.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from Wu Jieping Medical Fund (320.6750.15047).
ORCID iD: Keqiang Liu  https://orcid.org/0000-0003-0589-6101
https://orcid.org/0000-0003-0589-6101
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. [DOI] [PubMed] [Google Scholar]
- 3. Maemura K, Watanabe K, Ando T, et al. Altered editing level of microRNAs is a potential biomarker in lung adenocarcinoma. Cancer Sci. 2018;109(10):3326–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Janiak M, Paskal W, Rak B, et al. TIMP4 expression is regulated by miR-200b-3p in prostate cancer cells. APMIS. 2017;125(2):101–105. [DOI] [PubMed] [Google Scholar]
- 5. Wu J, Cui H, Zhu Z, Wang L. MicroRNA-200b-3p suppresses epithelial-mesenchymal transition and inhibits tumor growth of glioma through down-regulation of ERK5. Biochem Biophys Res Commun. 2016;478(3):1158–1164. [DOI] [PubMed] [Google Scholar]
- 6. Sun G, Cao Y, Wang P. et al. MiR-200b-3p in plasma is a potential diagnostic biomarker in oral squamous cell carcinoma. Biomarkers. 2018;23(2):137–141. [DOI] [PubMed] [Google Scholar]
- 7. Gui Z, Luo F, Yang Y, Shen C, Li S, Xu J. Oridonin inhibition and miR-200b-3p/ZEB1 axis in human pancreatic cancer. Int J Oncol. 2017;50(1):111–120. [DOI] [PubMed] [Google Scholar]
- 8. Lv Z, Wei J, You W, et al. Disruption of the c-Myc/miR-200b-3p/PRDX2 regulatory loop enhances tumor metastasis and chemotherapeutic resistance in colorectal cancer. J Transl Med. 2017;15(1):257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. He M, Liu Y, Deng X, et al. Down-regulation of miR-200b-3p by low p73 contributes to the androgen-independence of prostate cancer cells. Prostate. 2013;73(10):1048–1056. [DOI] [PubMed] [Google Scholar]
- 10. Bachmeier BE, Iancu CM, Killian PH, et al. Overexpression of the ATP binding cassette gene ABCA1 determines resistance to curcumin in M14 melanoma cells. Mol Cancer. 2009;8:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ma Y, Li X, Cheng S, Wei W, Li Y. MicroRNA-106a confers cisplatin resistance in non-small cell lung cancer A549 cells by targeting adenosine triphosphatase-binding cassette A1. Mol Med Rep. 2015;11(1):625–632. [DOI] [PubMed] [Google Scholar]
- 12. Wan L, Zhu L, Xu J, et al. MicroRNA-409-3p functions as a tumor suppressor in human lung adenocarcinoma by targeting c-Met. Cell Physiol Biochem. 2014;34(4):1273–1290. [DOI] [PubMed] [Google Scholar]
- 13. Vansteenkiste J, Dooms C, Mascaux C, Nackaerts K. Screening and early detection of lung cancer. Ann Oncol. 2012;23(suppl 10): x320–x327. [DOI] [PubMed] [Google Scholar]
- 14. Liu Y, Wang F, Xu P. MiR-590 accelerates lung adenocarcinoma migration and invasion through directly suppressing functional target OLFM4. Biomed Pharmacother. 2017;86:466–474. [DOI] [PubMed] [Google Scholar]
- 15. Lv Q, Hu JX, Li YJ, et al. MiR-320a effectively suppresses lung adenocarcinoma cell proliferation and metastasis by regulating STAT3 signals. Cancer Biol Ther. 2017;18(3):142–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rani S, Gately K, Crown J, O’Byrne K, O’Driscoll L. Global analysis of serum microRNAs as potential biomarkers for lung adenocarcinoma. Cancer Biol Ther. 2013;14(12):1104–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang N, Guo H, Dong Z, et al. Establishment and validation of a 7-microRNA prognostic signature for non-small cell lung cancer. Cancer Manag Res. 2018;10:3463–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Detassis S, Grasso M, Del Vescovo V, Denti MA. MicroRNAs make the call in Can Personal Med . Front Cell Dev Biol. 2017;5:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rodriguez-Aguayo C, Monroig P, Redis RS, et al. Regulation of hnRNPA1 by microRNAs controls the miR-18a-K-RAS axis in chemotherapy-resistant ovarian cancer. Cell Discov. 2017;3:17029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pulito C, Mori F, Sacconi A, et al. Metformin-induced ablation of microRNA 21-5p releases Sestrin-1 and CAB39 L antitumoral activities. Cell Discov. 2017;3:17022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schimanski S, Wild PJ, Treeck O, et al. Expression of the lipid transporters ABCA3 and ABCA1 is diminished in human breast cancer tissue. Horm Metab Res. 2010;42(2):102–109. [DOI] [PubMed] [Google Scholar]
- 22. Lee BH, Taylor MG, Robinet P, et al. Dysregulation of cholesterol homeostasis in human prostate cancer through loss of ABCA1. Cancer Res. 2013;73(3):1211–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Solomon KR, Allott EH, Freeman MR, Freedland SJ. Words of wisdom. Re: Dysregulation of cholesterol homeostasis in human prostate cancer through loss of ABCA1. Eur Urol. 2013;63(6):1128–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bi DP, Yin CH, Zhang XY, Yang NN, Xu JY. MiR-183 functions as an oncogene by targeting ABCA1 in colon cancer. Oncol Rep. 2016;35(5):2873–2879. [DOI] [PubMed] [Google Scholar]
- 25. Prochazka L, Koudelka S, Dong LF, et al. Mitochondrial targeting overcomes ABCA1-dependent resistance of lung carcinoma to α-tocopheryl succinate. Apoptosis. 2013;18(3):286–299. [DOI] [PubMed] [Google Scholar]
- 26. Fukuchi J, Hiipakka RA, Kokontis JM, et al. Androgenic suppression of ATP-binding cassette transporter A1 expression in LNCaP human prostate cancer cells. Cancer Res. 2004;64(21):7682–7685. [DOI] [PubMed] [Google Scholar]
- 27. Park S, Shimizu C, Shimoyama T, et al. Gene expression profiling of ATP-binding cassette (ABC) transporters as a predictor of the pathologic response to neoadjuvant chemotherapy in breast cancer patients. Breast Cancer Res Treat. 2006;99(1):9–17. [DOI] [PubMed] [Google Scholar]
- 28. Chen JH, Zheng YL, Xu CQ, et al. Valproic acid (VPA) enhances cisplatin sensitivity of non-small cell lung cancer cells via HDAC2 mediated down regulation of ABCA1. Biol Chem. 2017;398(7):785–792. [DOI] [PubMed] [Google Scholar]
- 29. Chou JL, Huang RL, Shay J, et al. Hypermethylation of the TGF-β target, ABCA1 is associated with poor prognosis in ovarian cancer patients. Clin Epigen. 2015;7:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary_material for MiR-200b-3p Functions as an Oncogene by Targeting ABCA1 in Lung Adenocarcinoma by Keqiang Liu, Weiqiang Zhang, Jian Tan, Jingbo Ma and Jing Zhao in Technology in Cancer Research & Treatment