Abstract
It has recently been identified that after motor cortex stroke, the ability of microglia processes to respond to local damage cues is lost from the thalamus, a major site of secondary neurodegeneration (SND). In this study, we combine a photothrombotic stroke model in mice, acute slice and fluorescent imaging to analyse the loss of microglia process responsiveness. The peri-infarct territories and thalamic areas of SND were investigated at time-points 3, 7, 14, 28 and 56 days after stroke. We confirmed the highly specific nature of non-responsive microglia processes to sites of SND. Non-responsiveness was at no time observed at the peri-infarct but started in the thalamus seven days post-stroke and persisted for 56 days. Loss of directed process extension is not a reflection of general functional paralysis as phagocytic function continued to increase over time. Additionally, we identified that somal P2Y12 was present on non-responsive microglia in the first two weeks after stroke but not at later time points. Finally, both classical microglia activation and loss of process extension are highly correlated with neuronal damage. Our findings highlight the importance of microglia, specifically microglia dynamic functions, to the progression of SND post-stroke, and their potential relevance as modulators or therapeutic targets during stroke recovery.
Keywords: Microglia motility, live cell multi-photon imaging, laser injury, thalamus, stroke recovery
Introduction
Over the last decade, our understanding of the dynamic nature of microglial process extension has been expanded by the use of multiphoton imaging. Microglia in the healthy brain continuously extend and retract their processes, surveying their microenvironment for signs of damage, infection and pathogen-associated molecules.1,2 Additionally, microglia respond to localised damage by rapidly extending their many fine processes towards the site of injury, effectively forming a three-dimensional seal around the site containing factors released from injured, dead and dying cells.3 This directed process extension has been shown consistently in rodent in-vivo and ex-vivo studies and is therefore considered a highly conserved microglia response.1–6
Our research team has been particularly interested in utilising in-vivo imaging to examine how microglia behave at sites post-stroke secondary neurodegeneration. The process of secondary neurodegeneration (SND) involves the progressive loss of tissue at sites that were connected to but not initially damaged by the primary infarction.8–9 SND has been observed in both rodents and humans,9–13 develops within days after and can last for weeks, months and even years after the primary infarction.14,15 We recently identified, using acute slice based imaging, that microglia at sites of secondary neurodegeneration exhibited an unusual response.16 Specifically, we identified that microglia within the degenerating thalamus had lost their typical process extension response towards laser-induced damage, when evaluated 14 days after injury. This was intriguing, as we had confirmed that the microglia were alive and responded normally within the thalamus of non-stroked animals. Highly interesting was also the observation that microglia within peri-infarct territory, immediately adjacent to the infarct site remained responsive to laser damage.
Morphological analysis (de-ramification) and/or the expression of marker proteins (CD11b, CD68) remain the most commonly used methods to assess microglial activation and their involvement in a specific pathological setting.17–19 Using these conventional metrics, we could not distinguish microglia process responsiveness from non-responsiveness. Both responsive microglia in the peri-infarct and non-responsive microglia at the site of SND exhibited a de-ramified morphology and increased levels of CD11b and CD68 expression. Therefore, it seems unlikely that the non-responsiveness is due to the transition of the cells to an amoeboid morphology. Although the classical metrics, such as morphological analysis, are still an appropriate approach to gauge the extent of microglia activation, the complete understanding of microglial behaviour requires a more in-depth analysis.
Since the discovery of fast, dynamic movement of microglial processes within the healthy and pathological brain, evidence supporting the interrelationship between movement and the execution of functional properties has continued to build.20–23 As such microglia process extension and retraction are considered essential microglia features and disturbances are being investigated in damage and disease in order to draw conclusion on functional variations.
The previous observation of specific impairment of microglial process extension within the thalamus at a single time point (14 days) after stroke left a number of important questions unanswered. Firstly, is the loss of process response truly specific to areas of SND or might the paralysis be apparent at an earlier or later time point within the cortex? This appeared to be a possibility, as our prior observation 14 days after stroke effectively evaluated the infarct territory and the thalamic areas of SND at different stages of damage progression, due to the delayed onset of microglia activation at sites of SND. A second question concerned the temporal development and the persistence of lost process movement within the thalamus. When does this form of non-responsiveness occur and how long does it last? Thirdly, is impaired process movement a reflection of a genuine/global functional paralysis or is process extension specifically inhibited? Finally, we had observed an unusual somal distribution of the P2Y12 receptor, which appeared predictive of impaired process movement. Would this be predictive at other time points other than 14 days after the infarction?
Given these questions, the aim of the current study was to analyse the spatiotemporal appearance and persistence of the non-responsive microglia phenotype and correlate its appearance to neuronal loss in areas of SND. We were additionally interested in relative localisation of P2Y12R as a putative biomarker of a non-responsive microglia phenotype. Specifically, we examined the thalamus, a major site of SND, following focal occlusion of the motor/somatosensory cortex. We examined differences in the cortex and thalamus at 3, 7, 14, 28 and 56 days post-injury using a combination of techniques including multiphoton imaging, functional phagocytosis assays and immunohistochemistry.
Materials and methods
Animals
All experiments were approved by the University of Newcastle Animal Care and Ethics Committee, and conducted in accordance with the New South Wales Animals Research Act and the Australian Code of Practice for the use of animals for scientific purposes. This study was planned and conducted according to the ARRIVE guidelines. Experiments were carried out in male heterozygous Cx3CR1GFP/WT mice (aged six to eight weeks), obtained from Jackson Laboratories, expressing EGFP under the control of the endogenous Cx3CR1 promotor. Cx3CR1 is ubiquitously expressed by microglia in the brain as well as in monocytes, dendritic cells, natural killer cells thorough the body.
Photothrombotic occlusion
Photothrombotic occlusions were induced within the somatosensory cortex, 2.2 mm lateral of bregma, using the photothrombotic protocol as previously described.24,25 Subsequent acute slice preparation or PFA fixation procedures were performed on days 3, 7, 14, 28 or 56 post-stroke. Animals without a visible cortical stroke in the somatosensory cortex were excluded from further analysis.
Acute slice preparation and two-photon imaging
Acute slice preparation and two-photon imaging were performed as described previously.16 Briefly, animals were perfused with ice cold cutting solution containing (in mM): 236 sucrose, 11 D-glucose, 25 NaHCO3, 2.5 KCl, 1 NaH2PO4, 5.3 MgCL2 and 1 CaCL2. Brains were extracted and sliced in ice cold cutting solution. Coronal sections (300 µm,) were cut using a vibratome (Leica VT 1200S) and subsequently incubated for 45 min at room temperature (RT) in artificial cerebral spinal fluid (aCSF) containing (in mM): 120 NaCl, 11 D- glucose, 26 NaHCO3, 2.5 KCl, 1 NaH2PO4, 1 MgCL2 and 2.5 CaCL2. All solutions were constantly oxygenated with 95% O2 and 5% CO2, adjusted to pH 7.3 and an osmolarity of 300 ± 5 mOsmol/kg (Osmomat 3000basic, Gonotec). Ex-vivo imaging of acute slices was performed at RT under constant perfusion of oxygenated aCSF on a multi-photon scanning microscope (MaiTai laser; Spectra Physics coupled to a Leica TCS SP8 MP microscope with a Leica HC 25 × /0.95 water objective with the Leica LAS X core software). Cortical brain sections between bregma 1.18 and −0.10 mm and thalamic brain sections between bregma 0.5 and −2.2 mm were selected for imaging. Brain regions and microglia activation were identified in fluorescent mode. Fluorescently labelled microglia were imaged at a depth of ∼100 µm within the tissue slice with a zoom of ×2. Z-stacks were 30 µm thick with a step size of 2 µm, resulting in a scan time of 1 min per stack. EGFP was excited at 950 nm and emission detected with the Leica Hyd RLD detector. Laser ablations were induced by zooming into the middle of the central z-plane (×48) and scanning the image at 100% laser power for 1 s. Consecutive z-stacks were taken every min for 15 min after induction of the laser damage creating 16 consecutive images (one prior to and 15 post laser damage) of microglia process responses after laser damage (Supplementary videos). After image acquisition, z-projections for each time point were created and aligned in their x-y plane using the StackReg plugin in Fiji in ImageJ (FIJI, 1997–2013; NIH; http://imagej.nih.gov/ij/). Image sets with a drift in x-y plane of more than 0.7 µm/min were excluded from analysis. A researcher blinded to both the time point and location of laser damage performed quantification of microglia process response to a laser ablation. Process response to laser damage was measured as an increase of average pixel intensity (I) within a 10 µm radius around the laser ablation at the time point t = 15 min after the induction of laser damage (process response = (It – It=1)/It=1) as previously described.16 Quantification was performed in MatLab using a cost made script. Briefly, 16 z-projection and x-y rectified image sets were uploaded into MatLab. The centre of laser damage was manually selected on time point t = 1 min (first image after laser damage) and the average pixel intensity for all consecutive images within the set was automatically assessed for the same 10 µm radius around the selected area Day 14, and the process response data were extracted from Kluge et al.16 Nthalamus = sham (14), 3 days (10), 7 days (7), 14 days (19), 28 days (10), 56 days (8); Ncortex = sham (17), 3 days (11), 7 days (5), 14 days (9), 28 days (8), 56 days (8).
Immunohistochemistry and confocal imaging
Free-floating, 30 µm PFA fixed sections were immuno-stained using standard protocols as previously described.16,26 After blocking in 3% bovine serum albumin, sections were incubated in primary antibody mouse anti-NeuN (#MAB377, Millipore, 1:500) or rabbit anti-P2Y12R (#55043A, AnaSpec EGT Group, 1:500) for 12 h at 4℃, followed by secondary antibody (Alexa-Fluor 594, goat anti-rabbit # R37117 and goat anti-mouse #A-11005, Thermo Fisher, 1:400) for 2 h at 25℃. Brain sections were washed with PBS in between each incubation step, mounted and cover slipped.
Confocal images were taken on a Leica TCS SP8 confocal microscope with a Leica HC 25 × /0.95 water, Leica HC PLC APO 40×/1.30 or 63×/1.40 OIL objective. Overview tile-scan images were taken and merged, using the LAX software and tile-scanning mode, generating z-stack images encompassing the entire thickness of the section at a step size of 1 µm. High-resolution images for morphological analysis and single cell representative images were taken at a step size of 0.5 µm spanning the entire section or 20 µm, respectively.
Morphological analysis
Morphological analysis was performed on maximum projection confocal images taken from 30 µm fixed Cx3CR1GFP/WT brain sections by a researcher blind to the group allocation. Quantitative analysis of soma and branch parameters was performed using MicroTrac in MatLab, a combined multilevel thresholding and minimum spanning tree-skeleton tracing approach, as described in Mahmoud and Sarah 27 ‘MicroTrac’ calculates the number of cells per image and several morphological parameters for every cell in an image individually before averaging them for each image. Metrics evaluated include the soma area, number of primary branches, total number of branch points, total branch length, cell area, cell solidity (=cell area/convex hull) and cell radius (spanning from the soma centre to the end of the longest branch). For a graphic illustration of the assessed parameters, please refer to Supplementary Figure 2. Joined cells which were not separated by the script, were manually excluded from the analysis. All cells within one image were reconstructed, analysed and results were averaged to create one data point per image. One image was taken per animal (n = 6).
Quantitative image analysis
Internalization of NeuN material and areas of cellular disturbances were analysed from whole slice z-stack projection images of Cx3CR1GFP/WT sections labelled for NeuN. For the internalization assay, a custom MatLab script was used to separate both colour channels and count the amount of NeuN pixels (red) and cell pixels (green), as well as NeuN pixels located inside the cell pixels (red in green) after Otsu’s thresholding. A minimum soma size of 30 µm2 for the green channel was set to exclude background particles and cell fragments. Internalization of NeuN material by microglia was analysed as % of co-localized NeuN pixels for each image (one image per animal, n = 6).
Areas of microglia activation and areas of NeuN loss were selected manually and using Fiji in ImageJ (FIJI, 1997–2013; NIH; http://imagej.nih.gov/ij/), one image per animal (nday 3 and 28 = 7; nday 7 and 14 = 5, nday 56 = 6).
Statistics and sample size calculation
Statistical comparisons and analysis between groups were made using GraphPad Prism 6 for Windows Version 6.01 statistical analysis software (GraphPad Software Inc., La Jolla, CA, USA). All values reported are mean ± STD. Data were analysed using one-way analysis of varience (ANOVA) followed by Tukey’s and Sidak’s post hoc comparison, respectively. P < 0.05 was considered statistically significant. Quantification of ex-vivo microglia response to laser damage, day 14 (Figure 1), is extracted from Kluge et al.16 and is highlighted in red.
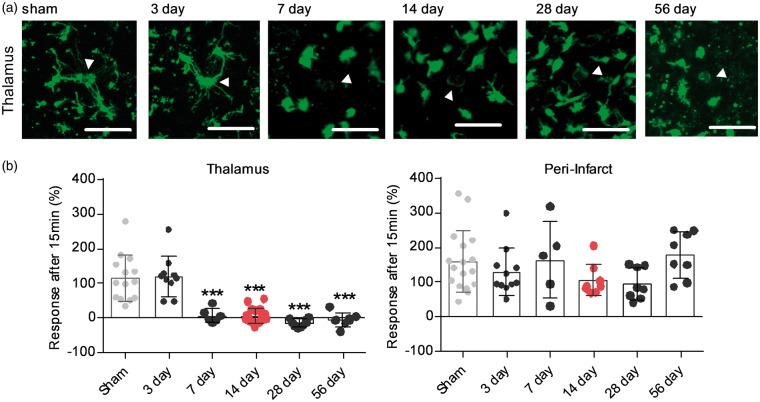
Figure 1.
Microglia process response to laser damage over time shows a specificity to the thalamus, starts at day 7 and peaks at day 28. (a) Acute slice based two-photon imaging showing microglia response following laser injury in the ipsilateral posterior complex (Po) of the thalamus for time-points 3, 7, 14, 28, 56 days post-stroke and sham-operated animals. Images were taken 15 min after laser damage, indicated by white arrow. Scale bar, 50 µm. (b) Quantification of microglia response 15 min after laser injury for the and peri-infarct territory ipsilateral Po over time. Response is measured as change in fluorescent material (average pixel intensity) within a 10 µm Ø around the centre of the laser-injury (white arrow). Data are shown as individual data points and as mean ± STD. N thalamus = sham (14), 3 days (10), 7 days (7), 14 days (19), 28 days (10), 56 days (8); Ncortex = sham (17), 3 days (11), 7 days (5), 14 days (9), 28 days (8), 56 days (8) ***p < 0.001 relative to sham and 3 days (one-way ANOVA followed by Tukey’s multiple comparisons). Dataset microglia process response day 14 is an excerpt from Kluge et al.16
Sample sizes were estimated using the formula to compare between groups with quantitate endpoints: . Using previous data on microglia response to laser damage at day 14 post-stroke, we obtained SD = 30 and an effect size of d = 50. Allowing a type 1 error of 5%, α = 0.05 with the power of 80%, β = 0.2, we calculated a sample size of six animals per group.
Results
Loss of microglia process extension to laser damage is specific to the thalamus, starts around day 7 and persists for up to 56 days post-stroke
We previously observed a loss of microglia process extension towards laser damage 14 days after stroke within the thalamus.16 This process non-responsiveness was only observed in areas of SND within the thalamus, as microglia in the peri-infarct territories retained their ability of directed process extension. To establish the time course and the regional specificity of impairment, we measured microglia process extension to laser injury at both the primary and secondary injury over time. As in our previous publication, we used multi-photon imaging of acute brain sections. This imaging technique allows the visualisation of cell movement deep within tissue samples with little photo-toxicity. Laser ablations were placed within the peri-infarct territory and the ipsilateral posterior complex (Po) of the thalamus 3, 7, 14, 28 and 56 days after a photothrombotic stroke occlusion and sham-operated animals, respectively. As the previous study indicated alterations in microglia responsiveness within the contralateral thalamus, this hemisphere was not used as an internal control in the current study. Process response was measured as an increase in fluorescent pixel intensity for a 10 µm circle around the laser damage.
Microglia at the peri-infarct site extended their processes out towards the local damage at all measured time-points (Figure 1(b) and Supplementary Movie 4). In the ipsilateral thalamus, microglia processes remained responsive to laser-damage for up to three days after stroke, with processes extending towards the site of local damage (Figure 1 and Supplementary Movie 1). Seven days after stroke, microglia in the ipsilateral thalamus did not respond to laser damage by directed process extension and this non-responsiveness persisted for up to 56 days (Figure 1 and Supplementary Movies 2 and 3).
Spatiotemporal P2Y12 receptor expression after stroke
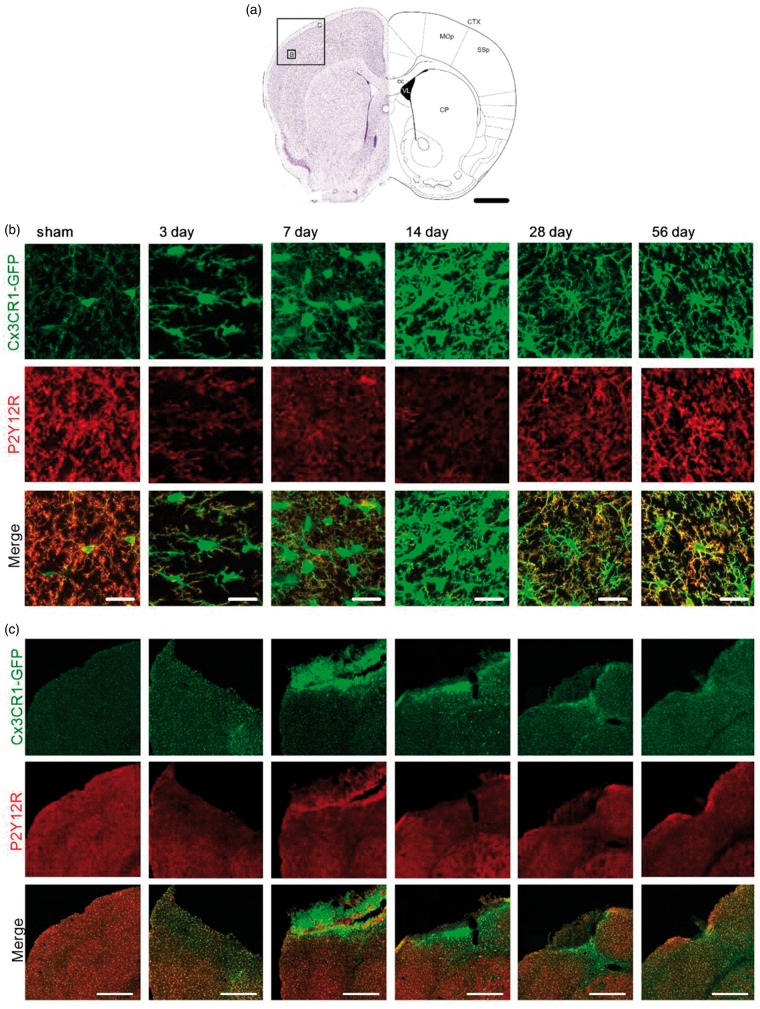
Our previous findings indicated that somal localisation of the purinergic P2Y12 receptor might be a predictor of non-responsive microglia processes. We therefore hypothesize that non-responsiveness is due to an alteration in P2Y12R and that somal P2Y12R can be used to classify the impaired microglia phenotype. To validate this hypothesis, we performed immuno-fluorescent labelling for P2Y12R on fixed Cx3CR1GFP/WT brain sections expressing GFP label microglia. The following fixed and immuno-labelled sections were imaged using a confocal microscope to obtain high-resolution images for further analysis. Overview of the tile scan images of the affected regions and high magnification images showing individual cells were taken from the infarct site at bregma 0.86 mm (Figure 2(a)), as well as the ipsilateral thalamus at bregma −2.00 mm (Figure 3(a)). Microglia cells in sham animals are equally distributed within the somatosensory motor cortex and thalamic regions, respectively (Figures 2(c) and 3(c)). Typically, P2Y12R labelling is predominantly located at the tips of ramified microglia processes (Figures 2(c) and 3(c)). At the peri-infarct site, P2Y12R expression was reduced 3–28 days after stroke (Figure 2(b)). Of note, the reduced P2Y12R expression was found to co-occur with an enhanced CX3CR1GFP signal (Figure 2(c)). Apart from reduced expression, we could not identify any obvious changes in the localisation of P2Y12R on microglia within the cortex at any time-point after stroke (Figure 2(b)).
Figure 2.
P2Y12 receptor expression profile at the primary infarct over time after stroke. (a) Schematic pictures adapted from Paxinos and Franklin50 illustrating the position and scale of images shown in (b) and (c) within the cerebral cortex. Confocal images of microglia were taken from the damaged, ipsilateral, hemispheres of the primary somatosensory and motor cortex areas at bregma 0.86 mm. Scale bar, 1 mm. CP: caudoputamen; cc: corpus callosum; CTX: cerebral cortex; MOp: primary motor area; SSp: primary somatosensory area. (b) Representative high-magnification maximum projection confocal images of the peri-infarct region within the SSp 3, 7 14, 28, 56 days after stroke and sham-operated animals as indicated in (a). Fixed brain slices of Cx3CR1GFP/WT mice expressing GFP-label microglia (top panel) were co-labelled with anti-P2Y12R antibody (middle panel). Merge in bottom panel (P2Y12R: red; GFP: green). Scale bars, 20 µm. (c) Representative maximum projection, tile scan, confocal images of cortical areas 3, 7 14, 28, 56 days after stroke and sham-operated animals. Fixed brain slices of Cx3CR1GFP/WT mice expressing GFP-label microglia (top panel) were co-labelled with anti-P2Y12R antibody (middle panel). Merge in bottom panel (P2Y12R: red; GFP: green). Scale bars, 500 µm.
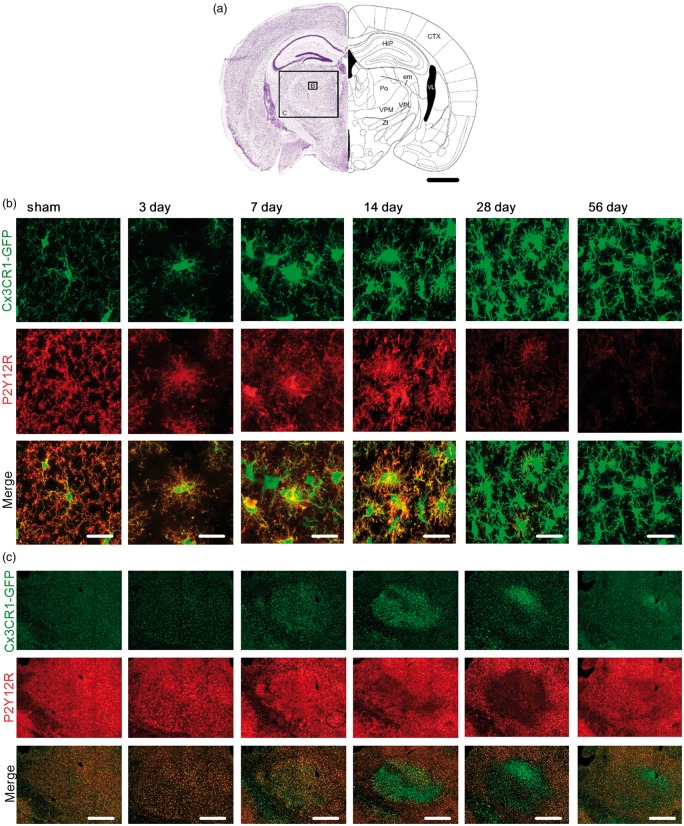
Figure 3.
P2Y12 receptor expression profile in the ipsilateral thalamus over time after stroke. (a) Schematic picture adapted from Paxinos and Franklin50 illustrating the position and scale of images shown in (b) and (c) within the thalamic area of the ipsilateral hemisphere. Specifically, confocal images of microglia were taken from the Po (Posterior complex), VPM (ventral posteriomedial nucleus) and VPL (Ventral posterolateral nucleus of the thalamus) at bregma – 2.00 mm. Scale bar, 1 mm. HIP: hippocampal region; CTX: cerebral cortex; VL: lateral ventricle; em: external medullary lamina of the thalamus; ZI: zona incerta. (b) Representative high-magnification maximum projection confocal images of the Po 3, 7 14, 28, 56 days after stroke and sham-operated animals as indicated in (a). Fixed brain slices of Cx3CR1GFP/WT mice expressing GFP-label microglia (top panel) were co-labelled with anti-P2Y12R antibody (middle panel). Merge in bottom panel (P2Y12R: red; GFP: green). Scale bar, 20 µm. (c) Representative maximum projection, tile scan, confocal images spanning the Po, VPM and VPL 3, 7 14, 28, 56 days after stroke and sham-operated animals. Fixed brain slices of Cx3CR1GFP/WT mice expressing GFP-label microglia (top panel) were co-labelled with anti-P2Y12R antibody (middle panel). Merge in bottom panel (P2Y12R: red; GFP: green). Scale bar, 500 µm.
In thalamic regions, microglia at days 7 and 14 after stroke, showed somal localisation of P2Y12R (Figure 3(b)). However, this expression profile was regionally specific in different nuclei of the thalamus (Figure 3(c)). Specifically, we observed clear somal clustering of P2Y12R in the posterior complex (Po) and ventral posterolateral nucleus (VPL) of the thalamus. In contrast, we could not find evidence of somal clustering in the ventral posteriomedial nucleus (VPM) despite the fact that Cx3CR1 signal was clearly altered. Instead, we found that the reduced P2Y12R expression in the VPM was very similar to that observed in the peri-infarct territories.
At days 28 and 56, somal clustering of P2Y12R was no longer observed in the Po and the VPL. In contrast, all microglia displayed a reduction in P2Y12R, similar to that observed at the infarct site.
Neuronal loss correlates with microglia activation
Neuronal degeneration and microglia activation are both hallmarks of post-stroke SND; however, to the best of our knowledge, their spatiotemporal appearance and progression has not been quantified simultaneously. To investigate their connection over time, we performed immuno-fluorescent labelling of NeuN, a marker for mature neurons, on brain sections of fixed Cx3CR1GFP/WT mice.
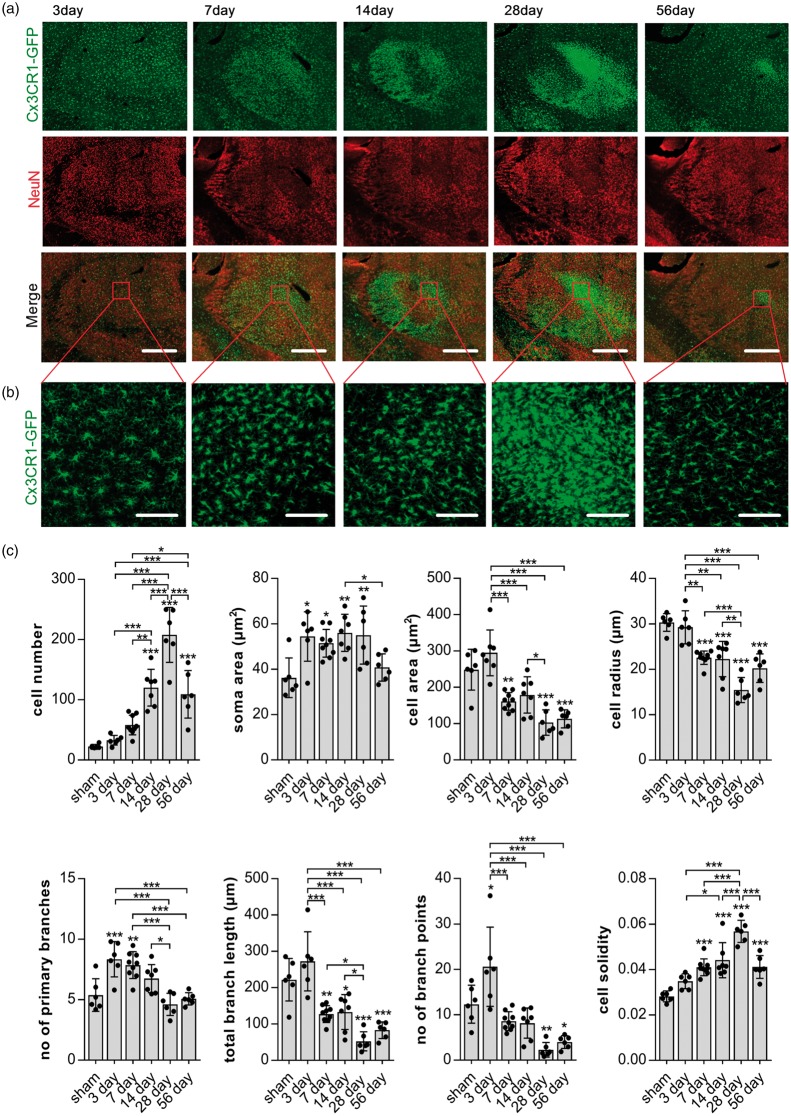
High-resolution confocal images spanning the entire thalamus at bregma −2.00 mm were taken and merged to create overview tile scan images for all time points (Figure 4(a)). This allowed the evaluation of the macroscopic structural changes within thalamic brain regions as well as an in-depth analysis of individual cellular changes with these areas (Figure 4(b)).
Figure 4.
NeuN loss co-localises with morphological microglia disturbances. (a) Representative maximum projection, tile scan, confocal images of thalamic areas 3, 7 14, 28, 56 days after stroke. Fixed brain slices of Cx3CR1GFP/WT mice expressing GFP-label microglia (top panel; green) were co-labelled with NeuN antibody (middle panel; red). Merge in bottom panel. Scale bars, 500 µm. Red square indicates area of morphological reconstruction as shown in (b). (b) Representative high-magnification maximum projection confocal images of the posterior nucleus of the thalamus as indicated by the red square in (a) used for morphological analysis. Scale bars, 100 µm. (c) Quantification of microglia morphology in the Po from images shown in (b) over time. Cells were reconstructed and analysed using MicroTrac software.28 All cells within one image were individually reconstructed and morphological parameters averaged for each image, one per animal. Data are shown as individual data points and as mean ± STD. (n = 6) ***p < 0.001, **p < 0.01, *p = 0.05 (one-way ANOVA followed by Tukey’s multiple comparisons).
Areas of microglia disturbances and neuronal loss, defined, as an increase in GFP labelling and a reduction in NeuN labelling, respectively, appeared to overlap across time and were predominantly located within the Po and VPL (Figure 4(a)).
For an in-depth analysis of microglia phenotypes, we firstly performed morphological analysis within the Po, the area of persistently reduced NeuN labelling using a single confocal image (Figure 4(b) and (c)). Parameters indicating morphological microglia activation (increase in cell number, increased soma area, decreased cell area and radius as well as reduction of branch length and branch points) were strongest 28 days after stroke (Figure 4(c)). Similar changes of these morphological parameters are visible 7, 14 and 56 days after stroke, even though not all parameters are significantly different to those of sham animals (cell number and number of branch point at day 7 and soma area at day 56). At days 14 and 56, parameters including cell number, cell area, cell radius, branch length and cell solidity are significantly different to sham animals and also when compared to 28 days. These data indicate a gradual increase in microglia activation from day 7 onward. A maximum of activation is reached on day 28, followed by a reduction at day 56 post-stroke.
Microglia three days after stroke show classical changes of activation only in terms of an increased soma size but no increase in cell number, cell area or radius when compared to sham. Interestingly, however, the changes in branch structure three days after stroke are significantly different to both sham animals and alterations observed at subsequent time-points. The total branch length and number of branch points three days after stroke have increased instead of decreasing when compared to other time-points after stroke. Additionally, microglia three days after stroke show a significant increase in the number of primary branches compared to those of microglia in the sham thalamus.
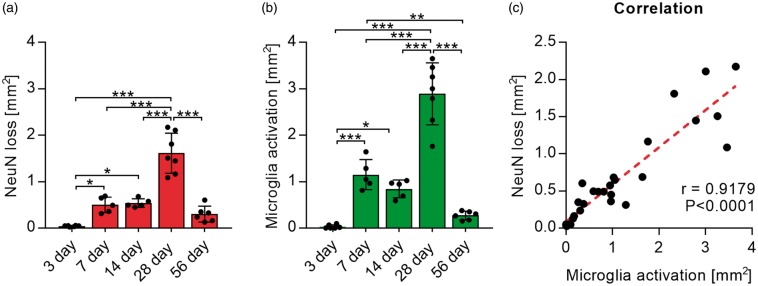
To investigate the progression of neuronal disturbances and loss in connection to microglia activation, we next assessed the areas affected by microglia disturbances and NeuN loss in the thalamus over time. Areas of increased GFP labelling and reduced NeuN labelling were selected manually, using Fiji is just ImageJ (1997–2013; NIH; http://imagej.nih.gov/ij/), for each co-labelled whole slice tile scan image. Three days after stroke, no measurable disturbance in NeuN or GFP signal was visible, while all other time points displayed a degree of microglia activation and reduction in NeuN labelling (Figure 5(a)). The largest areas of both were visible 28 days after stroke and reduced 56 days after stroke. Areas of neuronal loss and microglia activation co-localize and sizes were correlated using correlation analysis. Pearson correlation showed a significant correlation (r = 0.9179, p < 0.0001) between the area of microglia activation and the area of reduced NeuN labelling (Figure 5(b)).
Figure 5.
Areas of microglia activation and NeuN loss correlate. (a) Quantification of manually selected areas of NeuN loss (left graph) and manually selected areas of microglia activation/GFP signal increase (right graph) over time. Data are shown as individual data points and as mean ± STD. (nday 3 and 28 = 7; nday 7 and 14 = 5, nday 56 = 6) ***p < 0.001, **p < 0.01, *p = 0.05 (one-way ANOVA followed by Tukey’s multiple comparisons). (b) Scatter graph and correlation (r) between areas of microglia activation and NeuN loss (Pearson’s correlation coefficient).
Increased internalization of NeuN material 56 days after stroke
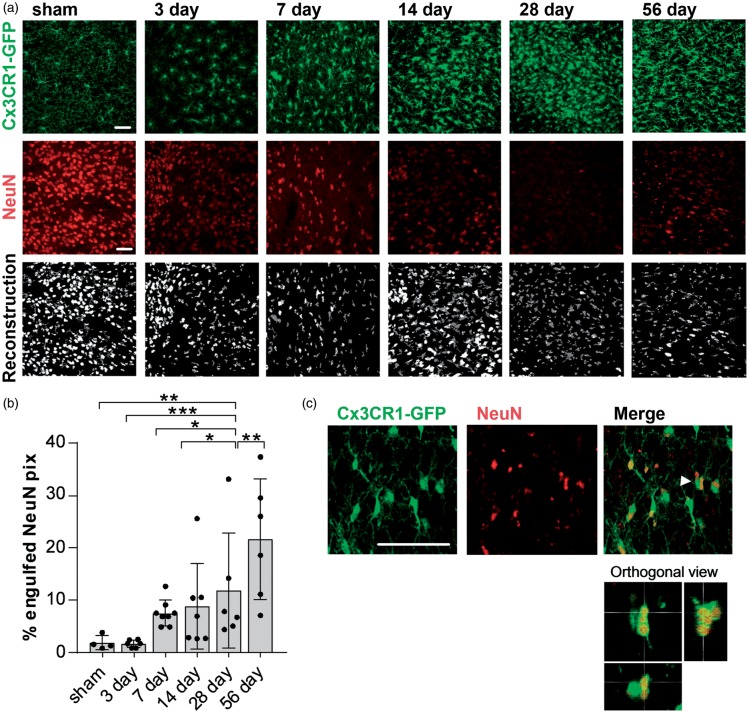
An important microglia function is the phagocytosis of cells or scavenging of cellular debris. The observed reduction of NeuN positive cells in the thalamus, particularly in the Po, starting seven days and persisting for up to two months after the initial stroke leads us to investigate the uptake of NeuN material by microglia in areas of SND. Confocal images of the Po, Cx3CR1GFP/WT tissue labelled for NeuN, were taken for all time-points and microglia, and NeuN signal was reconstructed, respectively (Figure 6(a)). Co-localisation was analyzed as % engulfed NeuN pixels. Full engulfment was verified using orthogonal view (Figure 6(c)); 56 days after stroke, NeuN material is being increasingly engulfed by microglia in the ipsilateral (IL) thalamus compared to both sham and to the contralateral (CL) hemispheres (Figure 6(b)). Other time-points after stroke, namely 3, 7, 14 and 28, showed trends towards an increased uptake of NeuN material, however not significantly different to sham animals.
Figure 6.
Increased NeuN phagocytosis in the posterior complex of the thalamus 56 days after stroke. (a) Representative confocal images of the posterior complex of the thalamus (Po) 3, 7 14, 28, 56 days after stroke used for the quantification of engulfed NeuN material. Top and middle panels indicate the original images for Cx3CR1-GFP and NeuN labelling, bottom panel shows the reconstruction (dark grey: GFP; white: NeuN). Scale bar, 50 µm. (b) Quantification of engulfed NeuN pixels (in %) by microglia in the Po for all time points after stroke and in sham-operated animals. Data are shown as individual data points and as mean ± STD. (n = 6) ***p < 0.001, **p < 0.01, *p < 0.05 (one-way ANOVA followed by Tukey’s multiple comparisons). (c) Representative higher magnification confocal images and orthogonal view (bottom panel) of the Po 56 days after stroke to determine full engulfment of NeuN material (red). Arrow shows cell visualized in orthogonal view. Scale bars, 50 µm.
Discussion
The current study has made a number of significant and original observations. Firstly, we have robustly confirmed the highly site specific nature of non-responsive microglia processes to areas of SND. We identify that this phenomenon starts around seven days and persist for up to two months after the primary infarct but does not occur at the primary infarct site itself. Secondly, we show that the impairment of microglial processes extension is not due to a complete functional paralysis, as we observed continuous engagement in phagocytosis across the time when the process non-responsiveness was present. We also show a strong correlation between classical morphological microglia activation and neuronal disturbances within the thalamus over time. Both microglia activation and neuronal degeneration peak 28 days after stroke correlate with impaired process extension. Interestingly, we also show a hyper-ramified microglia morphology pre-ceding neuronal disturbances in the thalamus at day 3. As a secondary objective, we wished to determine if somal P2Y12R localisation was a consistent predictor of non-responsiveness. However, P2Y12R was not consistently localised to the soma of microglia with impaired process movement over time. Collectively, these results are transformative for our current knowledge about microglia behaviour at sites of SND. We have identified that the impairment of process extension is specific to sites of SND, highly persistent and does not represent a form of general functional paralysis.
Our spatiotemporal analysis of microglia responsiveness to laser injury is the first data set to clearly identify a long lasting (up to two months) impairment of process movement specific to areas within the ipsilateral thalamus (Figure 1 and Supplementary Movies 1 to 3). Strikingly, lack of process response was not, at any time-point, observed within the peri-infarct regions of the infarction (Figure 1(b) and Supplementary Movie 4). This result strongly suggests that the loss of directed microglia process extension is not a transient or global feature of microglia undergoing activation but rather a specific feature to sites of SND.
An increasing number of studies describing the alterations in microglia motility connected to a specific pathology, inflammatory setting or neuronal activity, highlight the close connection of microglia dynamic properties to their respective environment.20,28,29 Given this strong relationship of microglia functions, particularly motility, to their environment, our results suggest that the non-responsive microglia phenotype is specific to the environment present at sites of SND.30
To our knowledge, a complete loss of directed process extension has been described only in the pathologies of a neurodegenerative nature, Alzheimer’s and Parkinson’s disease, respectively.31–33 Although certainly different in many aspects, post-stroke SND is more closely related to neurodegenerative disorders such as Alzheimer’s and Parkinson’s disease than to the primary infarct, particularly in terms of the specificity of neuronal damage, the progressive nature and the macroscopic microglia response. An important difference is that ischemic stroke effects all cell types on a global scale, whereas post-stroke SND, much like neurodegenerative pathologies, selectively effect neurons causing progressive neuronal cell death, synaptic damage and neuro-inflammation.14,34–36 Factors released at sites of post-stroke SND, including the loss of neuronal viability signals, might be similar to those released in neurodegenerative pathologies and therefore contribute to motile impairment. However, further research will be required to determine exactly how similar or distinct the non-responsive microglia phenotypes across different neurodegenerative pathologies are.
Another important difference between the primary injury and SND after stroke is the formation of a dense cellular barrier exclusively observed at the primary infarct site. Consistent with the previous reports, we show the formation of a dense microglia structure at the edge of the infarction developing around the third day after the stroke with a subsequent reduction coinciding with reduced microglia activation in the surrounding peri-infarct area after one month (Figure 2(c)). This cell barrier most likely functions as a border between the damaged area, generally described as the unsalvageable core of the infarct, and the surrounding tissue. There is no evidence of such a dense cell structure forming in post-stroke SND, even though both neuronal loss and activated microglia appear very strongly localized to distinct nuclei within the thalamus (Po and VPN) (Figure 3(c)). While inflammation and glia activation are features of both the infarct and SND, lack of this prominent structure despite a very distinct area of neuronal loss supports the idea of different mechanisms and environments present at both damage areas.
Directed process extension is mediated via binding of ATP, released from the injury site, to the purinergic P2Y12 receptor on microglia.1–3 A direct contribution of P2Y12R in the loss of process movement therefore seemed likely. We previously showed a strong transition of P2Y12R from the process tips to the cell soma of non-responsive microglia and therefore hypothesised that somal P2Y12R might be a predictor of this microglia phenotype. However, here we show that the localisation of P2Y12R seemed sensitive to the onset of process non-responsiveness rather than being persistently located to the soma. Although somal P2Y12R was not detected at the infarct, it was also only increased to the cell soma in the first and second week after stroke (Figures 2 and 3). After day 14, microglia displayed a reduced expression of P2Y12R consistent with classical microglia activation and the observations at the peri-infarct site. Additionally, we observed that only activated microglia within the Po and VPL had an increased somal labelling for P2Y12R. The involvement of P2Y12R remains unclear, and assomal localisation of P2Y12R might be a part of the initial transitioning process into a non-responsive phenotype. Additionally, the initial re localisation of P2Y12 to the soma might lead to a disruption of downstream signalling pathways at later stages. The activation of the PI3 kinase signalling pathway and generation of an outward potassium current are crucial for process extensions.22,37 The investigation of these pathways and its components provides a promising future direction for a potential mechanistic explanation of impaired process dynamics.
Given this new evidence, a disruption of P2Y12R localisation alone cannot explain impaired process dynamics. Accordingly, non-responsiveness might be due to an interference of the ATP gradient required for sensing and detection. In an acute slice study, the microglia process response towards an injury site was disrupted via bath application of ATP/ADP.38 Microglia extended their processes to the edge of the slice rather than towards injured neurons within the slice. This is an important finding, highlighting that excess of extracellular ATP and ADP can mask additional ATP release from an injury site and disrupt the formation of an ATP gradient required for directed process extension. A similar saturation of ATP at sites of SND might also cause the impaired process extension observed here. Increased ATP outflow and concentration in the striatum of rats were shown during ischemia in-vivo.39 Both the ATP hydrolysing enzyme and the metabolite adenosine are upregulated in ischemic models of stroke.40,41 Reduced clearance, increased excitotoxicity and ATP release via neurons and astrocytes at sites of post stroke SND can result in further accumulation of ATP and saturation of the microenvironment. A disruption of the ATP gradient potentially led to a compromised detection mechanism causing non-responsiveness. This idea is also supported by previous evidence showing that processes non-responsive to laser damage maintain normal baseline motility and these microglia maintain their phagocytic function.16 However, the disturbances of ATP outflow and a potential saturation of ATP specific to the thalamus after stroke require further investigation.
Here we have identified that rather than representing a global functional paralysis phenomena, microglia in the thalamus appear to be specifically impaired in their directed process extension. Microglia show both phagocytosis of apoptotic cells and internalization of NeuN material (Figure 6 and Supplementary Figure 1) at all time-points after stroke. Crucially, however, we observed that phagocytosis continued to increase significantly two months after stroke at the very same time that impaired process response persisted. This, in our view, provides compelling evidence to suggest that the paralysis is quite specific. Further, we have identified that phagocytosis, which has not to our knowledge been investigated this long after the infarction process, appears to be ramping up. Together with the observed reduction in neuronal loss, this indicates that repair processes are ongoing within the thalamus many months after the primary injury and has implications for potential treatment options in the recovery process after stroke.
We also performed in-depth morphological analysis of microglia in connection with neuronal damage in the thalamus over time. Based on the labelling for NeuN, a marker of mature neurons, neuronal loss was consistently reduced in the Po, the same thalamic nuclei most severely affected by microglia disturbances and therefore selected for morphological microglia analysis. Parameters indicating classical activation (de-ramification and increased soma size) were detectable 7–56 days and peaked 28 days after stroke (Figure 4). The area of microglia activation and area of neuronal loss, selected manually from whole brain sections, are strongly correlated and aligned with the time course of both morphological alterations as well as non-responsive microglia to laser damage (Figure 5). The close spatiotemporal correlation between microglia activation, impaired dynamics and neuronal damage highlights the potential importance of microglia and process response as a modulator of post-stroke SND.
Another very interesting observation is a potential priming of microglia prior to neuronal loss in the thalamus. Morphological analysis reveals the appearance of a hyper-ramified microglia phenotype, with increased branch structures, three days after stroke before transitioning into a classically activated morphology (Figure 4). Microglia hyper-ramification is associated with a state of alertness or priming and often observed in neurodegenerative pathologies, aging as well as CNS trauma.42,43 Priming is a state of mostly pro-inflammatory microglia, driven by changes in the microenvironment and considered to trigger an exaggerated inflammatory response after a secondary damage stimulus.43,44 The occurrence of hyper-ramified microglia at sites of SND provides yet another link to neurodegeneration but most importantly, it provides evidence suggesting a negative contribution of microglia to SND. A potential exaggerated immune response due to the early priming might contribute to neuronal dysfunction and degeneration. It is to be noted that subpopulations of hyper-ramified microglia can also be found at the infarction but their presence is considered a reflection of the complex pro-and anti-inflammatory functions performed at the infarct site, rather than a priming event.45 It remains unclear what triggers microglia hyper-ramification in areas of SND as early as three days after stroke. Neuronal damage can cause this microglia alteration; however, we did not detect neuronal damage as per NeuN labelling or TUNEL assay at this time point after stroke (Figure 1 and Supplementary Figure 1). TUNEL positive cells have been reported in the thalamus as early as one day after a middle cerebral artery occlusion; however, the peak level was reached seven days after the insult.9 These findings show a delayed cell death within the thalamus following microglia disturbances and thus implicating microglia in the processes that contribute to neuronal damage.
Although most certainly involved, the exact role and contribution of microglia to the progression of post-stroke SND remain unclear. Studies which modulate the outcome of SND display either a beneficial or detrimental effects of both neuronal loss and the extent of microglial activation.24,46–48 The close correlation between microglia activation and neuronal loss indicates possible detrimental role of microglia potentially via the loss of process extension. This idea is supported by previous findings highlighting the importance of directed process extension as a neuroprotective microglia feature.49 It appears possible that the loss of directed process extension obstructs the microglial repair response and therefore contributes to the severity of neuronal loss in the areas of SND. Further investigations selectively targeting microglial activation in situ are required to determine the functional status of microglia, the extent of microglial process response to the contribution to SND and their release of inflammatory and anti-inflammatory modulators.
In conclusion, our study shows that microglia at sites of SND lose their ability of directed process extension towards local injury sites but maintain phagocytic functions particularly in the late stages after stroke. This behaviour supports the concept of a disruption of ATP gradient specific to the environment of SND rather than general functional paralysis of microglia.
Finally, both microglia activation and process non-responsiveness are highly correlated to neuronal damage. The findings of this study highlight the importance of microglia, specifically microglia dynamic functions in the progression of SND post-stroke, and their relevance as modulators or therapeutic targets for stroke recovery.
Supplemental Material
Supplemental material, sj-vid-1-jcb-10.1177_0271678X18797346 for Spatiotemporal analysis of impaired microglia process movement at sites of secondary neurodegeneration post-stroke by Murielle G Kluge, Mahmoud Abdolhoseini, Katarzyna Zalewska, Lin Kooi Ong, Sarah J Johnson, Michael Nilsson and Frederick R Walker in Journal of Cerebral Blood Flow & Metabolism
Supplemental Material
Supplemental material, sj-vid-2-jcb-10.1177_0271678X18797346 for Spatiotemporal analysis of impaired microglia process movement at sites of secondary neurodegeneration post-stroke by Murielle G Kluge, Mahmoud Abdolhoseini, Katarzyna Zalewska, Lin Kooi Ong, Sarah J Johnson, Michael Nilsson and Frederick R Walker in Journal of Cerebral Blood Flow & Metabolism
Supplemental Material
Supplemental material for Spatiotemporal analysis of impaired microglia process movement at sites of secondary neurodegeneration post-stroke by Murielle G Kluge, Mahmoud Abdolhoseini, Katarzyna Zalewska, Lin Kooi Ong, Sarah J Johnson, Michael Nilsson and Frederick R Walker in Journal of Cerebral Blood Flow & Metabolism
Availability of data and materials
Original imaging datasets analysed during this current study are available from the corresponding author on reasonable request. This manuscript contains an original dataset from Kluge et al.16 (Figure 1(b) – dataset microglia process response day 14).
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Health and Medical Research Council (NHMRC) of Australia, Hunter Medical Research Institute, Faculty of Health and Medicine Pilot Grant and The University of Newcastle, Australia. F.R.W. and M.N. also acknowledge the ongoing support from NHMRC Centre for Research Excellence in Stroke Recovery and Rehabilitation.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
MK and FRW designed the research; MK performed all imaging experiments and data analysis with assistance and contributions from LKO; MK and KZ performed image processing; SJJ and MA wrote custom made MatLab scripts for image analysis; MK and FRW prepared the first draft of the manuscript; All authors contributed to the final version of the manuscript; The study was supervised by FRW and MN.
Supplementary material
Supplementary material for this paper can be found at the journal website: http://journals.sagepub.com/home/jcb
References
- 1.Davalos D, Grutzendler J, Yang G, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci 2005; 8: 752–758. [DOI] [PubMed] [Google Scholar]
- 2.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 2005; 308: 1314–1318. [DOI] [PubMed] [Google Scholar]
- 3.Hines DJ, Hines RM, Mulligan SJ, et al. Microglia processes block the spread of damage in the brain and require functional chloride channels. Glia 2009; 57: 1610–1618. [DOI] [PubMed] [Google Scholar]
- 4.Sieger D, Moritz C, Ziegenhals T, et al. Long-range Ca2+ waves transmit brain-damage signals to microglia. Dev Cell 2012; 22: 1138–1148. [DOI] [PubMed] [Google Scholar]
- 5.Haynes SE, Hollopeter G, Yang G, et al. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci 2006; 9: 1512–1519. [DOI] [PubMed] [Google Scholar]
- 6.Dissing-Olesen L, LeDue JM, Rungta RL, et al. Activation of neuronal NMDA receptors triggers transient ATP-mediated microglial process outgrowth. J Neurosci 2014; 34: 10511–10527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Block F, Dihne M, Loos M. Inflammation in areas of remote changes following focal brain lesion. Prog Neurobiol 2005; 75: 342–365. [DOI] [PubMed] [Google Scholar]
- 8.Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci 1999; 22: 391–397. [DOI] [PubMed] [Google Scholar]
- 9.Wei L, Ying DJ, Cui L, et al. Necrosis, apoptosis and hybrid death in the cortex and thalamus after barrel cortex ischemia in rats. Brain Res 2004; 1022: 54–61. [DOI] [PubMed] [Google Scholar]
- 10.Dihne M, Grommes C, Lutzenburg M, et al. Different mechanisms of secondary neuronal damage in thalamic nuclei after focal cerebral ischemia in rats. Stroke 2002; 33: 3006–3011. [DOI] [PubMed] [Google Scholar]
- 11.Ogawa T, Yoshida Y, Okudera T, et al. Secondary thalamic degeneration after cerebral infarction in the middle cerebral artery distribution: evaluation with MR imaging. Radiology 1997; 204: 255–262. [DOI] [PubMed] [Google Scholar]
- 12.Nakane M, Tamura A, Sasaki Y, et al. MRI of secondary changes in the thalamus following a cerebral infarct. Neuroradiology 2002; 44: 915–920. [DOI] [PubMed] [Google Scholar]
- 13.Nakane M, Teraoka A, Asato R, et al. Degeneration of the ipsilateral substantia nigra following cerebral infarction in the striatum. Stroke 1992; 23: 328–332. [DOI] [PubMed] [Google Scholar]
- 14.Zhang J, Zhang Y, Xing S, et al. Secondary neurodegeneration in remote regions after focal cerebral infarction: a new target for stroke management? Stroke 2012; 43: 1700–1705. [DOI] [PubMed] [Google Scholar]
- 15.Gerhard A, Schwarz J, Myers R, et al. Evolution of microglial activation in patients after ischemic stroke: a [11C](R)-PK11195 PET study. Neuroimage 2005; 24: 591–595. [DOI] [PubMed] [Google Scholar]
- 16.Kluge MG, Kracht L, Abdolhoseini M, et al. Impaired microglia process dynamics post-stroke are specific to sites of secondary neurodegeneration. Glia 2017 2017/08/25. [DOI] [PubMed]
- 17.Eggen BJ, Raj D, Hanisch UK, et al. Microglial phenotype and adaptation. J Neuroimmune Pharmacol 2013; 8: 807–823. [DOI] [PubMed] [Google Scholar]
- 18.Fumagalli S, Perego C, Pischiutta F, et al. The ischemic environment drives microglia and macrophage function. Front Neurol 2015; 6: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walker FR, Beynon SB, Jones KA, et al. Dynamic structural remodelling of microglia in health and disease: a review of the models, the signals and the mechanisms. Brain Behav Immun 2014; 37: 1–14. [DOI] [PubMed] [Google Scholar]
- 20.Tremblay ME, Majewska AK. A role for microglia in synaptic plasticity? Commun Integr Biol 2011; 4: 220–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parkhurst CN, Gan W-B. Microglia dynamics and function in the CNS. Curr Opin Neurobiol 2010; 20: 595–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swiatkowski P, Murugan M, Eyo UB, et al. Activation of microglial P2Y12 receptor is required for outward potassium currents in response to neuronal injury. Neuroscience 2016; 318: 22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gomez-Nicola D, Perry VH. Microglial dynamics and role in the healthy and diseased brain: a paradigm of functional plasticity. Neuroscientist 2015; 21: 169–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ong LK, Zhao Z, Kluge M, et al. Chronic stress exposure following photothrombotic stroke is associated with increased levels of amyloid beta accumulation and altered oligomerisation at sites of thalamic secondary neurodegeneration in mice. J Cereb Blood Flow Metab 2016; 37: 1338–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patience MJ, Zouikr I, Jones K, et al. Photothrombotic stroke induces persistent ipsilateral and contralateral astrogliosis in key cognitive control nuclei. Neurochem Res 2015; 40: 362–371. [DOI] [PubMed] [Google Scholar]
- 26.Tynan RJ, Beynon SB, Hinwood M, et al. Chronic stress-induced disruption of the astrocyte network is driven by structural atrophy and not loss of astrocytes. Acta Neuropathol 2013; 126: 75–91. [DOI] [PubMed] [Google Scholar]
- 27.Mahmoud Abdolhoseini FRW and Sarah J Johnson. Automated tracing of microglia using multilevel thresholding and minimum spanning trees. In: 38th international conference of IEEE engineering in medicine and biology society (EMBC), Orlando, FL, 16–20 August, August 2016. [DOI] [PubMed]
- 28.Hristovska I, Pascual O. Deciphering resting microglial morphology and process motility from a synaptic prospect. Front Integr Neurosci 2015; 9: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Avignone E, Lepleux M, Angibaud J, et al. Altered morphological dynamics of activated microglia after induction of status epilepticus. J Neuroinflammation 2015; 12: 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luo XG, Chen SD. The changing phenotype of microglia from homeostasis to disease. Transl Neurodegener 2012; 1: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krabbe G, Halle A, Matyash V, et al. Functional impairment of microglia coincides with Beta-amyloid deposition in mice with Alzheimer-like pathology. PLoS One 2013; 8: e60921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gyoneva S, Swanger SA, Zhang J, et al. Altered motility of plaque-associated microglia in a model of Alzheimer's disease. Neuroscience 2016; 330: 410–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gyoneva S, Shapiro L, Lazo C, et al. Adenosine A2A receptor antagonism reverses inflammation-induced impairment of microglial process extension in a model of Parkinson's disease. Neurobiol Dis 2014; 67: 191–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Northington FJ, Ferriero DM, Graham EM, et al. Early neurodegeneration after hypoxia-ischemia in neonatal rat is necrosis while delayed neuronal death is apoptosis. Neurobiol Dis 2001; 8: 207–219. [DOI] [PubMed] [Google Scholar]
- 35.Friedlander RM. Apoptosis and caspases in neurodegenerative diseases. N Engl J Med 2003; 348: 1365–1375. [DOI] [PubMed] [Google Scholar]
- 36.Ong LK, Walker FR, Nilsson M. Is stroke a neurodegenerative condition? A critical review of secondary neurodegeneration and amyloid-beta accumulation after stroke. Aims Med Sci 2017; 4: 1–16. [Google Scholar]
- 37.Wu LJ, Vadakkan KI, Zhuo M. ATP-induced chemotaxis of microglial processes requires P2Y receptor-activated initiation of outward potassium currents. Glia 2007; 55: 810–821. [DOI] [PubMed] [Google Scholar]
- 38.Kurpius D, Nolley EP, Dailey ME. Purines induce directed migration and rapid homing of microglia to injured pyramidal neurons in developing hippocampus. Glia 2007; 55: 873–884. [DOI] [PubMed] [Google Scholar]
- 39.Melani A, Turchi D, Vannucchi MG, et al. ATP extracellular concentrations are increased in the rat striatum during in vivo ischemia. Neurochem Int 2005; 47: 442–448. [DOI] [PubMed] [Google Scholar]
- 40.Matsumoto K, Graf R, Rosner G, et al. Flow thresholds for extracellular purine catabolite elevation in cat focal ischemia. Brain Res 1992; 579: 309–314. [DOI] [PubMed] [Google Scholar]
- 41.Braun N, Zhu Y, Krieglstein J, et al. Upregulation of the enzyme chain hydrolyzing extracellular ATP after transient forebrain ischemia in the rat. J Neurosci 1998; 18: 4891–4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perry VH, Cunningham C, Holmes C. Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev Immunol 2007; 7: 161–167. [DOI] [PubMed] [Google Scholar]
- 43.Norden DM, Muccigrosso MM, Godbout JP. Microglial priming and enhanced reactivity to secondary insult in aging, and traumatic CNS injury, and neurodegenerative disease. Neuropharmacology 2015; 96: 29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perry VH, Holmes C. Microglial priming in neurodegenerative disease. Nat Rev Neurol 2014; 10: 217–224. [DOI] [PubMed] [Google Scholar]
- 45.Morrison HW, Filosa JA. A quantitative spatiotemporal analysis of microglia morphology during ischemic stroke and reperfusion. J Neuroinflammation 2013; 10: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schroeter M, Zickler P, Denhardt DT, et al. Increased thalamic neurodegeneration following ischaemic cortical stroke in osteopontin-deficient mice. Brain 2006; 129: 1426–1437. [DOI] [PubMed] [Google Scholar]
- 47.Zuo X, Hou Q, Jin J, et al. Inhibition of cathepsin b alleviates secondary degeneration in ipsilateral thalamus after focal cerebral infarction in adult rats. J Neuropathol Exp Neurol 2016; 75: 816–826. [DOI] [PubMed] [Google Scholar]
- 48.Jones KA, Zouikr I, Patience M, et al. Chronic stress exacerbates neuronal loss associated with secondary neurodegeneration and suppresses microglial-like cells following focal motor cortex ischemia in the mouse. Brain Behav Immun 2015; 48: 57–67. [DOI] [PubMed] [Google Scholar]
- 49.Eyo UB, Peng J, Swiatkowski P, et al. Neuronal hyperactivity recruits microglial processes via neuronal NMDA receptors and microglial P2Y12 receptors after status epilepticus. J Neurosci 2014; 34: 10528–10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.G. Paxinos KF. The mouse brain in stereotaxic coordinates, San Diego, CA: Elsevier, 2001. [Google Scholar]
- 51.Abdolhoseini M, Walker F, Johnson S. Automated tracing of microglia using multilevel thresholding and minimum spanning trees. IEEE Eng Med Bio 2016, pp. 1208–1211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-vid-1-jcb-10.1177_0271678X18797346 for Spatiotemporal analysis of impaired microglia process movement at sites of secondary neurodegeneration post-stroke by Murielle G Kluge, Mahmoud Abdolhoseini, Katarzyna Zalewska, Lin Kooi Ong, Sarah J Johnson, Michael Nilsson and Frederick R Walker in Journal of Cerebral Blood Flow & Metabolism
Supplemental material, sj-vid-2-jcb-10.1177_0271678X18797346 for Spatiotemporal analysis of impaired microglia process movement at sites of secondary neurodegeneration post-stroke by Murielle G Kluge, Mahmoud Abdolhoseini, Katarzyna Zalewska, Lin Kooi Ong, Sarah J Johnson, Michael Nilsson and Frederick R Walker in Journal of Cerebral Blood Flow & Metabolism
Supplemental material for Spatiotemporal analysis of impaired microglia process movement at sites of secondary neurodegeneration post-stroke by Murielle G Kluge, Mahmoud Abdolhoseini, Katarzyna Zalewska, Lin Kooi Ong, Sarah J Johnson, Michael Nilsson and Frederick R Walker in Journal of Cerebral Blood Flow & Metabolism
Data Availability Statement
Original imaging datasets analysed during this current study are available from the corresponding author on reasonable request. This manuscript contains an original dataset from Kluge et al.16 (Figure 1(b) – dataset microglia process response day 14).