Abstract
Background and aims
The aim of this survey was to find out whether the perspectives of patients with inflammatory bowel disease concerning biosimilars have changed since the publication of our last survey carried out in 2014–2015.
Methods
An online survey consisting of 19 questions was made available by the European Federation of Crohn’s and Ulcerative Colitis Associations between July 2018 and December 2018. Only respondents who had heard of biosimilars were asked to respond to all of the questions.
Results
In total, 1619 patients with inflammatory bowel disease responded the questionnaire. Most respondents were from Europe (79%), followed by Asia (8%), South America (7%) and Africa (5%). Some 44% of them had heard of biosimilars, and only these respondents continued to the biosimilar-specific questions. Respondents worried significantly more about biosimilars being less effective than the originator (50% in current and 39% in previous survey, p = 0.0004). However, respondents were more likely to believe that biosimilars will have an impact on the management of inflammatory bowel disease (75% in current and 62% in previous survey).
Conclusions
Many patients with inflammatory bowel disease remain unfamiliar with biosimilars. Although patients still worry about different aspects regarding biosimilars, they also tend to be more confident that biosimilars will have an impact on the management of their disease. More patient education is still needed to raise awareness about biosimilars.
Keywords: Inflammatory bowel disease, biosimilars
Introduction
Biologics have improved the treatment of various inflammatory diseases, including inflammatory bowel disease (IBD). Biosimilars were first introduced in the European market in the early 2010s, and the initial approach to them of the European Crohn’s and Colitis Organisation (ECCO) and many national IBD medical societies was rather cautious.1 Further scientific evidence on the efficacy and safety of biosimilars, such as data from a large randomized control trial NOR-SWITCH, which included over 400 Norwegian patients with immune-mediated diseases and examined efficacy, safety and immunogenicity when switching from an infliximab originator to a less expensive biosimilar CT-P13 and showed that switching from the originator to the biosimilar was not inferior to continued originator treatment,2 have brought about a deeper understanding of the characteristics, development and regulatory approval of biosimilars. The more recent position statement by ECCO is more confident in terms of safety and efficacy of biosimilars, and considers switching from an originator acceptable.3
Patients with IBD have also been cautious regarding biosimilars. Our previous Biologics and Biosimilars survey, carried out in 2014–15 to assess patients’ knowledge about biosimilars and issues around them, showed that awareness on biosimilars was insufficient and there was much suspicion around them.4 It has long been recognized that patient perception is an important source of information when developing actions for quality improvement in health care, and that patient data can be used as a supplementary quality of care indicator.5,6 Therefore, the purpose of this survey was to find out whether patients’ perceptions on biosimilars have changed since our previous survey.
Materials and methods
The questionnaire
The original questionnaire, used in the previous survey,4 was developed by the European Federation of Crohn’s and Ulcerative Colitis Associations (EFCCA) in collaboration with experts in the field (Laurent Peyrin-Biroulet, Xavier Roblin, Silvio Danese and Luisa Avedano), and it consisted of 14 questions. It was carried out as an online survey in 2014–2015 and offered in nine languages (English, French, Italian, German, Spanish, Russian, Greek, Turkish and Hebrew). The national member associations of EFCCA were responsible for informing their membership about the survey. As the survey revealed that patients with IBD had doubt and concerns about the safety and efficacy of biosimilars, the decision was made to carry out a follow-up survey to find out whether patients’ perceptions have changed in recent years.
For the follow-up, five questions were added in the original questionnaire, and the final questionnaire therefore included 19 questions. Apart from English, the questionnaire was translated into seven languages and was available online from July to December 2018 on the EFCCA website. As during the previous survey, the national patient associations were responsible for informing their members about the survey. After basic demographic questions, only those respondents who had heard of biosimilars continued to the biosimilar-specific questions.
The participants
The participants of the survey were members of EFCCA member associations or persons following the communications of these associations. The recruitment was self-selective.
Statistical considerations
The response variables were categorical. Explanatory variables were integer age and binary disease. A binary logit model was used for the response variables that had only two possible values and a generalized logit model for the variables that had more than two possible values. In the case of the age or disease variable not being statistically significant, the variable with the greater p-value was removed from the model. In some questions, some observations were deleted as a result of missing values for the response or explanatory values. Some of the data from the previous survey were presented differently than previously to better allow for a comparison.
Results
Respondent demographics
A total of 1643 respondents completed the survey. Some 61.8% (n = 1015) of them had Crohn’s disease (CD), 36.8% (n = 604) had ulcerative colitis (UC), 1.1% (n = 18) another inflammatory disease and 0.4% (n = 6) a rheumatic disease; this was very similar to the previous survey. Only respondents with IBD (n = 1619) were included in the analysis. Most respondents (45.5%) were 21–40 years old. Of the respondents, 2.0% were diagnosed in 1980 or before, 7.7% between 1981 and 1990, 16.2% between 1991 and 2000, 31.7% between 2001 and 2010, and 41.6% in 2011 or later. Most respondents were from Europe (78.9%), followed by Asia (7.8%), South America (6.8%) and Africa (5.3%). Because of the limited participation from other continents compared with Europe, a comparison between continents was left out.
Exposure to biologics and biosimilars (Questions 1–3)
Slightly more respondents than in the previous survey were currently being treated with anti-TNF, and slightly more had had the therapy discontinued due to either inefficacy or side effects. Significantly more respondents than in the previous survey had heard of biosimilars (44.0% in the current vs. 36.2% in the previous survey, p = 0.0001). Only those who had heard of biosimilars (n = 596) continued to the biosimilar-specific questions (Questions 4–19). For the overall results of Questions 1–3 and comparison with the previous survey, see Table 1.
Table 1.
Results of Questions 1–3 and comparison with the previous survey. In italics: p = 0.0001, otherwise p > 0.05.
| Question 1 | Current survey (n = 1322) | Previous survey (n = 1059) | |
|---|---|---|---|
| Exposure to anti-TNF therapy (infliximab (Remicade), adalimumab (Humira), certolizumab (Cimzia), golimumab (Simponi) | Currently treated with anti-TNF | 500 (58.9%) of CD patients (n = 849), 176 (37.2%) of UC patients (n = 473) | 347 (52.9%) of CD patients (n = 657), 130 (32.3%) of UC patients (n = 402) |
| Received anti-TNF in the past, therapy discontinued due to inefficacy | 104 (8.2%) | 73 6.9% | |
| Received anti-TNF in the past, therapy discontinued due to side effects | 108 (7.9%) | 74 7.0% | |
| Question 2 |
Current survey (n = 1221) | Previous survey | |
| Have you been previously or are you currently being treated with an infliximab biosimilar (INFLECTRA, REMSIMA or FLIXABI)? | Yes | 230 18.8% | n/a |
| Question 3 |
Current survey (n = 1355) | Previous survey (n = 1059) | |
| Have you ever heard of biosimilars? | Yes | 596 (44.0%) | 383 (36.2%) |
Concerns about biosimilars (Question 4)
As in the previous survey, the most common biosimilar-related concerns remained safety and efficacy. The worry about efficacy was significantly more common. For overall results and comparison with the previous survey, see Table 2.
Table 2.
Results of Question 4 and comparison with the previous survey. In italics underlined: p = 0.0004, otherwise p > 0.05.
| Question 4 | Current survey (n = 596) | Previous survey (n = 383) | |
|---|---|---|---|
| Concerning biosimilars, you worry (it is possible to choose more than one option): | (a) That the molecular basis of the biosimilar is different from that of the reference drug | 206 (34.6%) | 126 (32.9%) |
| (b) About safety profile (mainly infections and cancers) | 274 (46.0%) | 178 (46.5%) | |
| (c) About tolerability | 182 (30.5%) | 122 (31.9%) | |
| (d) That the biosimilar could be less effective than the reference drug | 299 (50.2%) | 148 (38.6%) | |
| (e) You have no specific concerns about biosimilars | 131 (22.0%) | 101 (26.4%) | |
Price, extrapolation and biosimilars in the market (Questions 5–7)
Respondents were surveyed on their views regarding the lower price of biosimilars, extrapolation, and biosimilars entering the market. Respondents were able to choose more than one option in the three questions. As they were able to only choose one option in these questions in the previous survey, the results are not statistically comparable, but demonstrate a more reliable division of opinions as respondents were able to choose all that applied. For results and comparison with the previous survey, see Table 3.
Table 3.
Results of Questions 5–7 and comparison with the previous survey. In all comparisons p > 0.05.
| Question 5 | Current survey (n = 596) | Previous survey (n = 379) | |
|---|---|---|---|
| The biosimilar will be less expensive than the reference drug, you think that (it is possible to choose more than one option): | (a) These are good news because more patients will be treated with biologics | 293 (49.2%) | 120 (31.3%) |
| (b) The cost of a treatment should not come before its effectiveness or safety/tolerance | 395 (66.3%) | 209 (54.6%) | |
| (c) This will help cost savings | 131 (22.0%) | 30 (7.8%) | |
| (d) You don’t think that a lower cost will change something | 52 (8.7%) | 20 (5.2%) | |
| Question 6 |
Current survey (n = 596) | Previous survey (n = 379) | |
| The biosimilar of REMICADE (infliximab) has been successfully developed and used for the treatment of rheumatologic diseases. On June 27, 2013, the biosimilar of REMICADE (infliximab) received positive opinion from the European Medicines Agency (EMA) for the treatment of IBD by extrapolating data from rheumatoid arthritis (it is possible to choose more than one option): | (a) You think that it makes sense, because its efficacy and safety profile has been established for other chronic conditions than IBD | 126 (21.1%) | 50 (13.1%) |
| (b) You would prefer if it could be tested for inflammatory bowel diseases before extrapolating data from rheumatologic disorders | 335 (56.2%) | 116 (30.3%) | |
| (c) You trust the decisions made by regulatory agencies and you are not awaiting for data in IBD | 56 (9.4%) | 13 (3.4%) | |
| (d) You trust your treating physician who will make the decision to use biosimilars in your treatment | 568 (45.0%) | 104 (27.2%) | |
| (e) You trust your pharmacist to make the decision to use biosimilars in your treatment | 14 (2.4%) | 3 (0.8%) | |
| (f) You are waiting for more data in IBD before accepting to receive a biosimilar for either Crohn’s disease or ulcerative colitis | 202 (33.9%) | 93 (24.3%) | |
| Question 7 |
Current survey (n = 596) | Previous survey (n = 378) | |
| Now that biosimilars are coming to the market, you think (it is possible to choose more than one option): | (a) That patient associations should be informed and should be able to give their opinion | 371 (62.3%) | 96 (25.1%) |
| (b) That patients should systematically be given information | 467 (78.4%) | 164 (42.8%) | |
| (c) That we should wait for many patients to receive biosimilars in a real life setting before recommending its use in a large population of IBD patients | 254 (42.6%) | 87 (22.7%) | |
| (d) We should know in which country the drug has been tested/created before using it in your own country | 205 (34.4%) | 31 (8.1%) | |
Interchangeability with reference drug and same pharmacological name; biosimilars’ impact on IBD management (Questions 8–10)
In Question 8, respondents were surveyed on their views on interchangeability. In Question 9, it was explained to the respondents that the biosimilars will have the same pharmacological name as the reference drug, so there will be no way to distinguish it from the reference drug. In Question 10, respondents were asked about their views regarding biosimilars’ impact on IBD management. For overall results and comparison with the previous survey, see Table 4.
Table 4.
Results of Questions 8–10 and comparison with the previous survey. Underlined: p = 0.0002, in italics: p < 0.0001, otherwise p > 0.05. In Question 8, option (a) was divided into two different questions in the previous survey but combined into one in this one; the results of the previous survey were changed for the presentation in the current one. In Question 9, the wording was changed from “Do you think that the arrival of biosimilars will have an impact on the management of your disease?” in the previous survey to “Do you think that the arrival of biosimilars will have an impact on the management of IBD?” in the current one.
| Question 8 |
Current survey (n = 588) | Previous survey (n = 379) | |
| In the future, biosimilars could be interchangeable with the reference drug: | (a) You are opposed to this idea if the patient is not aware of this decision but accept if the patient is systematically informed | 182 (31.0%) | 167 (44.0%) |
| (b) You might accept this exchange if the drug is delivered by your usual pharmacist | 23 (3.9%) | 4 (1.0%) | |
| (c) You accept this exchange if your treating physician gives his approval | 196 (33.3%) | 115 (30.3%) | |
| (d) You accept this exchange if EBM (evidence-based medicine) data are available | 187 (31.8%) | 93 (24.5%) | |
| Question 9 |
Current survey (n = 590) | Previous survey (n = 379) | |
| Question 9: The biosimilar will have the same pharmacological name as the reference drug, so, when prescribed, there will be no way to distinguish it from the reference drug: | (a) You wish to know if you receive the biosimilar or the reference drug | 277 (47.0%) | 169 (44.6%) |
| (b) You don’t mind as long as the biosimilar has the same efficacy and safety profile as the reference drug | 65 (11.0%) | 85 (22.4%) | |
| (c) You would like to be informed about it, but you trust the pharmacist if he delivers it or your treating physician if he prescribes it | 90 (15.3%) | 45 (11.9%) | |
| (d) You wish to have all the necessary information before the drug is administered and obtain written information (e.g. card) to be used for future care | 158 (26.8%) | 80 (21.1%) | |
| Question 10 |
Current survey (n = 555) | Previous survey (n = 379) | |
| Do you think that the arrival of biosimilars will have an impact on the management of IBD: | (a) Yes, completely | 96 (17.3%) | 46 (12.1%) |
| (b) Probably | 258 (46.5%) | 139 (36.7%) | |
| (c) Maybe a little | 64 (11.5%) | 49 (12.9%) | |
| (d) Not at all | 28 (5.1%) | 27 (7.1%) | |
| (e) Don’t know | 109 (19.6%) | 118 (31.1%) | |
Biosimilar prescribed by the treating physician or handed out by pharmacist and after starting the treatment (Questions 11–13)
Respondents were surveyed about their views on being prescribed biosimilars by their treating physician, on the pharmacist handing out the biosimilar, changing the initial prescription without the consent of the prescribing physician, and regarding their behaviour after starting a biosimilar treatment. For results and comparison with the previous survey, see Table 5.
Table 5.
Results of Questions 11–13 and comparison with the previous survey. In all comparisons p > 0.05.
| Question 11 |
Current survey (n = 589) | Previous survey (n = 379) | |
| If a biosimilar is prescribed and explained to you by your treating physician: | (a) You will be fully confident | 205 (34.8%) | 123 (32.4%) |
| (b) You will be worried but you will accept the treatment | 219 (37.2%) | 146 (38.5%) | |
| (c) You will probably not accept it and express yourself on this matter | 72 (12.2%) | 48 (12.7%) | |
| (d) You will ask another physician | 43 (7.3%) | 23 (6.1%) | |
| (e) You don’t know | 50 (8.5%) | 39 (10.3%) | |
| Question 12 |
Current survey (n = 587) | Previous survey (n = 376) | |
| If the pharmacist hands out the biosimilar, changing the initial prescription without the consent of the prescribing physician: | (a) You will accept it because of the lower cost of the biosimilar | 21 (3.6%) | 13 (3.5%) |
| (b) You will accept it because of available scientific evidence | 64 (10.9%) | 72 (19.2%) | |
| (c) You disagree, but you acknowledge that you will have to accept it | 111 (18.9%) | 52 (13.8%) | |
| (d) You will try to obtain the reference drug | 391 (66.6%) | 239 (63.6%) | |
| Question 13 |
Current survey (n = 586) | Previous survey (n = 379) | |
| After starting a treatment with biosimilar: | (a) You will carefully follow the treatment | 330 (56.3%) | 200 (52.7%) |
| (b) You will be worried and will probably stop the treatment at the first doubt or adverse event | 111 (18.9%) | 75 (19.8%) | |
| (c) You will be worried, but the fact that the treatment has been approved by the European Medicines Agency is reassuring | 145 (24.7%) | 104 (27.4%) | |
Biosimilars and generic drugs (Questions 14 and 15)
Respondents were surveyed about their perceptions regarding biosimilars and generic drugs. Results and comparisons with the previous survey are shown in Table 6.
Table 6.
Results of Questions 14 and 15 and comparison with the previous survey. In all comparisons p > 0.05.
| Question 14 |
Current survey (n = 588) | Previous survey (n = 380) | |
| You believe that biosimilars (Generic: a drug product that is comparable to brand/reference listed drug product in dosage form, strength, route of administration, quality and performance characteristics, and intended use, containing the same active ingredients): | (a) Are like generic drugs | 152 25.9% | 116 (30.6%) |
| (b) Are close to generic drugs | 175 29.8% | 127 (33.3%) | |
| (c) Are not at all like generics | 149 25.3% | 69 (18.2%) | |
| (d) You don’t know | 112 19.1% | 68 (17.9%) | |
| Question 15 |
Current survey (n = 589) | Previous survey (n = 379) | |
| Regarding generic treatments: (Generic: a drug product that is comparable to brand/reference listed drug product in dosage form, strength, route of administration, quality and performance characteristics, and intended use, containing the same active ingredients): | (a) You take them without worries | 227 38.5% | 126 (33.3%) |
| (b) You accept to take them but you have some doubts | 208 35.3% | 128 (33.8%) | |
| (c) You refuse them when you can | 97 16.5% | 78 (20.6%) | |
| (d) You have never thought about this | 28 4.8% | 30 (7.9%) | |
| (e) You don’t know | 29 4.9% | 17 (4.5%) | |
Quality of information and communication on biosimilars (Questions 16 and 17; n = 555 and n = 575, respectively)
A question was added in the current survey about how the respondents would grade, on a scale from 0 (very poor) to 10 (excellent), the quality of information/communication that they received so far on biosimilars. Results are shown in Figure 1.
Figure 1.
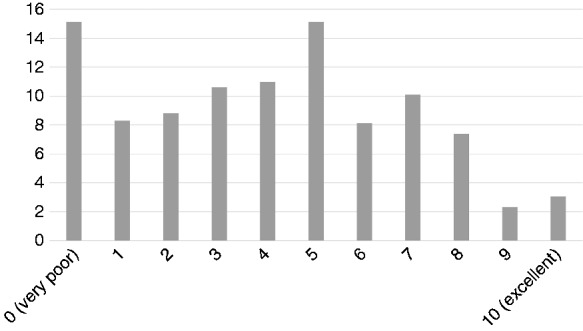
Quality of information and communication on biosimilars (%).
In another new question, the respondents were asked whether they have been systematically informed by their doctor if they were receiving biosimilars. Some 23.0% of the respondents felt they had, and 35.1% felt they had not; for 41.9%, the question was not applicable.
Biosimilar efficacy and side effects in patients who have been switched (Questions 18 and 19, n = 570 and n = 569, respectively)
In two more new questions, respondents were asked about their experiences on efficacy and side effects if they had been switched from Remicade to a biosimilar. Some 10.2% of the respondents reported to be experiencing the same efficacy, and 12.8% reported not. For 77.0% of the respondents, the question was not applicable. Some 7.9% of the respondents reported experiencing more side effects and 13.2% not experiencing more side effects than before. For 78.9% of the respondents, the question was not applicable.
Discussion
In our previous survey,4 one of the most striking results was the patients’ unfamiliarity with biosimilars; 45.0% of the respondents were currently treated with biologic medications, but only 36.2% had heard of biosimilars. This time, more respondents (58.9%) were currently being treated with biologics, and significantly more (44.0%) had also heard of biosimilars. Still, a large proportion of those being treated with biologics were unfamiliar with biosimilars.
Furthermore, the respondents of the previous survey seemed generally sceptical about biosimilars; only 26.4% of the respondents had no specific concerns. In the current survey, 22% had no specific concerns; however, the difference was not statistically significant. Clearly, patient information and education in recent years has not greatly succeeded in clearing suspicions and worries around biosimilars. Future initiatives should focus on delivering more awareness and information. Interestingly, although the worries remain, respondents were significantly more likely to believe that biosimilars would have an impact on the management of IBD in the current survey than in the previous one.
These findings may explain, at least in part, the nocebo effect, defined as a negative effect of a pharmacological or non-pharmacological medical treatment that is induced by patients’ expectations, and that is unrelated to the physiological action of the treatment.7,8
Furthermore, it seems that patients with immune-mediated inflammatory diseases may in general have poorer knowledge than oncology patients on biosimilar-related topics. In a study9 of over 600 French patients treated for rheumatic inflammatory diseases that assessed patients’ information about biosimilars, it was found that biosimilars were largely unknown to patients: 57% of the respondents did not know what biosimilars were. Respondents also worried about efficacy (60%), safety (57%) and non-similar molecular structure (46%) compared with the originator. On the other hand, an American study10 on 79 oncology patients showed that over 70% of the respondents were aware of the correct definition of biosimilars. The study showed a good level of knowledge and awareness among the participants. While the questionnaire used in these surveys was not identical to ours, the results give an idea of differences in awareness between oncology patients and patients with immune-mediated diseases. This is important, as information and a good understanding on biosimilars seem to be associated with better adherence to biosimilars.9
The new questions in the current survey showed that patients experience dissatisfaction in the quality and sufficiency of information and communication regarding biosimilars. Future research should clarify exactly what patients feel is missing, and how the missing information could be brought to the patients in the most efficient way. Initiatives should be directed at both patients and health care personnel to improve biosimilar awareness among patients with IBD.
This survey had some limitations. The survey was self-selective, only available online and in eight languages, which may have affected the participant population. Some questions were rephrased or response options altered from the previous survey, which made the previous and current survey partly statistically incomparable. Furthermore, differences in availability of treatment modalities between countries may have affected the responses of the participants.
In conclusion, the current survey shows that although awareness on biosimilars has somewhat improved, there is room for larger improvements. Furthermore, although patients with IBD are more familiar with biosimilars, worrying about their efficacy as compared with the reference drugs is significantly more common than in our previous survey. While awareness-raising around biosimilars has succeeded, it has failed in clearing the air of suspicions around biosimilars. Stronger cooperation between patient organisations and health care practitioners could help improve the situation.
Acknowledgements
The authors would like to acknowledge Ms. Johanna Ikonen for her support in the statistical analysis.
Conflicts of interest
LBP reports honoraria from AbbVie, Janssen, Genentech, Ferring, from Tillots, Pharmacosmos, Celltrion, Takeda, Boerhinger Ingelheim, Pfizer, Index Pharmaceuticals, Sandoz, Celgene, Biogen, Samsung Bioepis, Alma, Sterna, Nestle, Enterome, Allergan, MSD, Roche, Arena, Gilead, Hikma, Amgen; grants from Abbvie, MSD, Takeda; stock options: CT-SCOUT. SD has served as a speaker, a consultant and an advisory board member for Abbvie, Ferring, Hospira, Johnson & Johnson, Merck, Millennium Takeda, Mundipharma, Pfizer Inc, Tigenix, UCB Pharma, and Vifor. SL has received consulting fees from Pfizer. No further conflicts of interest to declare.
Ethics approval
Not required.
Funding
This project was sponsored by Pfizer Inc.
Informed consent
Not applicable.
References
- 1.Danese S, Gomollon F and Governing Board and Operational Board of ECCO. ECCO position statement: The use of biosimilar medicines in the treatment of inflammatory bowel disease (IBD). J Crohns Colitis 2013; 7: 586–589. [DOI] [PubMed] [Google Scholar]
- 2.Jørgensen KK, Olsen IC, Goll GL, et al. Switching from originator infliximab to biosimilar CT-P13 compared with maintained treatment with originator infliximab (NOR-SWITCH): A 52-week, randomised, double-blind, non-inferiority trial. Lancet 2017; 389: 2304–2316. [DOI] [PubMed] [Google Scholar]
- 3.Danese S, Fiorino G, Raine T, et al. ECCO position statement on the use of biosimilars for inflammatory bowel disease – an update. J Crohns Colitis 2017; 11: 26–34. [DOI] [PubMed] [Google Scholar]
- 4.Peyrin-Biroulet L, Lönnfors S, Roblin X, et al. Patient perspectives on biosimilars: A survey by the European Federation of Crohn’s and Ulcerative Colitis Associations. J Crohns Colitis 2017; 11: 128–133. [DOI] [PubMed] [Google Scholar]
- 5.Levine AS, Plume SK, Nelson EC. Transforming patient feedback into strategic actions plans. Qual Manag Health Care 1997; 5: 28–40. [PubMed] [Google Scholar]
- 6.Cleary PD, McNeil BJ. Patient satisfaction as an indicator of quality care. Inquiry 1988; 25: 25–36. [PubMed] [Google Scholar]
- 7.Pouillon L, Danese S, Hart A, et al. Consensus report: clinical recommendations for the prevention and management of the nocebo effect in biosimilar-treated IBD patients. Aliment Pharmacol Ther 2019; 49: 1181–1187. [DOI] [PubMed] [Google Scholar]
- 8.Pouillon L, Socha M, Demore B, et al. The nocebo effect: A clinical challenge in the era of biosimilars. Expert Rev Clin Immunol 2018; 14: 739–749. [DOI] [PubMed] [Google Scholar]
- 9.Frantzen L, Cohen JD, Tropé S, et al. Patients’ information and perspectives on biosimilars in rheumatology: A French nation-wide survey. Joint Bone Spine 2019; 86: 491–496. [DOI] [PubMed] [Google Scholar]
- 10.Ismailov RM, Khasanova ZD, Gascon P. Knowledge and awareness of biosimilars among oncology patients in Colorado, USA. Future Oncol 2019; 15: 2577–2584. [DOI] [PubMed] [Google Scholar]


