Abstract
The role of mitochondria in cancer continues to be debated and paradoxically implicated in opposing functions in tumor growth and tumor suppression. To understand this dichotomy, we explored the function of mitochondrial isocitrate dehydrogenase (IDH)2, a tricarboxylic acid cycle enzyme mutated in subsets of acute leukemias and gliomas, in cancer. Silencing of IDH2 in prostate cancer cells impaired oxidative bioenergetics, elevated reactive oxygen species (ROS) production, and promoted exaggerated mitochondrial dynamics. This was associated with increased subcellular mitochondrial trafficking, turnover of membrane focal adhesion complexes, and enhanced tumor cell migration and invasion, without changes in cell cycle progression. Mechanistically, loss of IDH2 caused ROS-dependent stabilization of hypoxia-inducible factor-1α in normoxia, which was required for increased mitochondrial trafficking and tumor cell movements. Therefore, IDH2 is a dual regulator of cancer bioenergetics and tumor cell motility. This pathway may reprogram mitochondrial dynamics to differentially adjust energy production or promote tumor cell invasion in response to microenvironment conditions.—Wang, Y., Agarwal, E., Bertolini, I., Ghosh, J. C., Seo, J. H., Altieri, D. C. IDH2 reprograms mitochondrial dynamics in cancer through a HIF-1α–regulated pseudohypoxic state.
Keywords: mitochondria, ROS, tumor cell motility, HIF-1α metastasis
Reprogramming of metabolic pathways is a ubiquitous hallmark of cancer (1), contributing to malignant traits and disease progression. Much effort has been devoted to the increased glucose utilization by transformed cells, even when oxygen is present [i.e., the Warburg effect (2)], and its implication for tumor growth (3). However, there is now evidence for greater complexity in cancer metabolism (4), as multiple bioenergetics pathways simultaneously coexist in a heterogeneous microenvironment and contribute to tumor- and context-specific responses (5).
This dynamic interplay is exemplified by a complex role of mitochondria in cancer (6). Previously considered dysfunctional or unimportant, we now know that mitochondria play an important role in malignancy, paradoxically associated with both tumor suppression (7) and tumor growth (8). How transformed cells balance this dichotomy is currently unknown, but recent data have pointed to oxidative bioenergetics (9), signaling by reactive oxygen species (ROS) (10), and exploitation of mitochondrial dynamics (11), an adaptive process that controls organelle size, shape, and subcellular distribution (12), as key tumor drivers and potential therapeutic targets.
In this context, isocitrate dehydrogenase (IDH)2 may embody the dichotomy of mitochondria in cancer. An essential component of the tricarboxylic acid (TCA) cycle, and thus potentially exploited for tumor bioenergetics (6), IDH2 is mutated in its substrate-binding pocket in subsets of acute leukemias and gliomas (13), suggesting a role in tumor suppression. Mechanistically, IDH2 mutations create a gain-of-function phenotype, subverting the oxidative decarboxylation of isocitrate to α-ketoglutarate with production of an oncometabolite, (R)-2-hydroxyglutarate (14), that competitively inhibits demethylases and dioxygenases (15).
Although this provides an actionable therapeutic target, and a small molecule inhibitor of mutant IDH2 is approved for clinical use (16), the function of wild-type (WT) IDH2 in cancer has remained controversial. Contrary to what is expected for a tumor suppressor, IDH2-deficient mice exhibit reduced tumor growth (17), and changes in IDH2 levels have been variously linked to tumor growth (18) or tumor suppression (19, 20). This pathway may involve ROS unbalance (21), as IDH2 serves a key antioxidant function in mitochondria by supplying NADPH for glutathione reductase (22) and the regeneration of the thioredoxin system (23).
In a recent short hairpin RNA (shRNA) screening, we identified IDH2 as a negative regulator of mitochondrial-directed tumor cell motility (11), anticipating potential roles of this pathway in tumor cell invasion and metastatic competence. In this study, we investigated the dichotomy of WT IDH2 in cancer bioenergetics and tumor cell movements.
MATERIALS AND METHODS
Cells and cell culture
Cell culture experiments were carried out in multiple model systems, including prostate adenocarcinoma PC3 and DU145 cells. Cells were obtained from the American Type Culture Collection (Manassas, VA, USA) and maintained in culture according to the supplier’s specifications. Conditioned medium used for cell invasion assays was prepared from exponentially growing cultures of NIH3T3 cells (ADCC) in Dulbecco’s modified Eagle medium supplemented with 4.5 g/L d-glucose, sodium pyruvate, 10 mM HEPES, and 10% fetal bovine serum for 48 h. Cell passaging was limited to <40 passages from receipt, and cell lines were authenticated by short tandem repeat profiling with AmpFlSTR Identifiler PCR Amplification Kit (Thermo Fisher Scientific, Waltham, MA, USA) at the Wistar Institute Genomics facility. Mycoplasma-free cultures were confirmed at the beginning of the studies and every 2 mo afterward by PCR amplification of cultures using Bioo Scientific Mycoplasma Primer Sets (375501) and Hot Start polymerase (Qiagen, Germantown, MD, USA).
Antibodies and reagents
Antibodies to focal adhesion (FA) kinase (FAK), phosphorylated FAK (Tyr925), dynamin-related protein 1 (Drp1), Ser616-phosphorylated Drp1, voltage-dependent anion channel, mitofusin 1, mitofusin 2, and hypoxia-inducible factor-1α (HIF-1α) were from Cell Signaling Technology (Danvers, MA, USA). Antibodies to peroxiredoxin 3 (Prx3), translocase of outer membrane (TOM)20, and β-actin were from Santa Cruz Biotechnology (Dallas, TX, USA). Antibodies to IDH2 and Prx3SO3 were from Thermo Fisher Scientific. An antibody to γ-H2A histone family member X (H2AX) was from MilliporeSigma (Burlington, MA, USA). Chemicals superoxide dismutase (SOD) mimetic (MnTBAP) and protein kinase B (Akt) inhibitor (MK-2206) were from MilliporeSigma. The TCA metabolites measurement kits for isocitrate, glutamic acid, α-ketoglutarate, and succinate (Succ) were from Enzo Life Sciences (Farmingdale, NY, USA).
Plasmid and small interfering RNA transfection
Gene knockdown experiments by small interfering RNA (siRNA) were carried out as previously described by Caino et al. (24). The following siRNA sequences were used: control, Ontarget Plus Nontargeting siRNA Pool (D-001810; Dharmacon, Lafayette, CO, USA), human Drp1 (L-012092; Dharmacon), HIF-1α (L-004959-00; Dharmacon), IDH1 (L-008294; Dharmacon), and IDH2 (L-004013; Dharmacon). Individual IDH2-directed siRNAs were synthesized by Dharmacon. IDH2 siRNA sequence #1 was 5′-GCAAGAACUAUGACGGAGAUU-3′, IDH2 siRNA sequence #2 was 5′-GAUGAGAUGACCCGUAUUAUU-3′, IDH2 siRNA sequence #3 was 5′-AGGCAGGAGCAGUGCGUUUUU-3′ targeting the 3′-UTR of human IDH2 mRNA, and IDH2 siRNA sequence #5 was 5′-UUAUAUUGCCCUUGGAACAUU-3′ targeting the 3′-UTR of human IDH2 mRNA. Tumor cell types were transfected with the various siRNA at 40 nM in Lipofectamine RNAiMax (Thermo Fisher Scientific) at a 1:1 ratio (volume siRNA 20 μM/volume Lipofectamine RNAiMax). After 72 h, transfected cells were validated for target protein knockdown by Western blotting and processed for functional experiments. The following human IDH2-directed shRNA TRCN0000027245 (D11), TRCN0000027296 (D12), and TRCN0000027225 (E1) were used to generate PC3 cells with stable IDH2 knockdown. An empty pLKO-based lentivirus was used as control, and selection of stable clones was carried out in the presence of puromycin (2 mg/ml). Plasmid cDNAs encoding WT IDH2 or IDH2 Arg172Lys (R172K) neomorphic mutant were from Addgene (Watertown, MA, USA). A loss-of-function Cys108Ser mutation was introduced in Prx3 using Stratagene QuickChange II XL Site-Directed Mutagenesis Kit (Agilent Technologies, Santa Clara, CA, USA) and confirmed by DNA sequencing. The sequence of primers for the Cys108Ser Prx3 mutant was as follows: forward, 5′-CTTTGGATTTCACCTTTGTGAGTCCTACAGAAATTGTTGCT-3′, and reverse, 5′-AGCAACAATTTCTGTAGGACTCACAAAGGTGAAATCCAAAG-3′.
Protein analysis
Protein lysates were prepared in RIPA buffer (150 mM NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris, pH 8.0) in the presence of EDTA-free Protease Inhibitor Cocktail (Roche, Basel, Switzerland) and Phosphatase Inhibitor Cocktail (Roche). Equal amounts of protein lysates were separated by SDS gel electrophoresis, transferred to PVDF membranes, and incubated with primary antibodies of various specificities. Protein bands were visualized by chemiluminescence.
Analysis of bioenergetics
Glucose concentrations were determined in a medium of PC3 cells using a glucose kit (MilliporeSigma). Briefly, 2 × 106 PC3 cells transfected with various siRNAs were seeded in 10-cm2 tissue culture dishes for 48 h, and 200-μl aliquots of culture medium were incubated with 1 ml assay mixture, containing 1.5 mM NAD, 1 mM ATP, 1.0 U/ml hexokinase, and 1.0 U/ml glucose-6-phosphate dehydrogenase (G6PDH). Glucose concentrations were determined by measuring the amount of reduced NAD to NADH by G6PDH and quantified by absorbance at 340 nm. Extracellular lactate concentrations were measured in siRNA-transfected PC3 cells using a colorimetric assay (Abcam, Cambridge, MA, USA), with quantification of lactate-dependent conversion of NADP to NADPH in the presence of excess lactate dehydrogenase (LDH) by absorbance at 450 nm.
Cellular respiration
Oxygen consumption rates (OCRs) or extracellular acidification rates (ECARs) were quantified using an Agilent Seahorse XFe96 analyzer (Agilent Technologies). Briefly, PC3 cells (3 × 104) maintained in complete growth medium were transfected with various siRNAs and plated in each well of a Seahorse XFe96 cell culture plate (80-μl volume) for 24 h at 37°C in 5% CO2. The medium was then exchanged with XF base medium (Agilent Technologies) supplemented with 2 mM glutamine, 1 mM sodium pyruvate, and 10 mM glucose, pH 7.4, and equilibrated for 1 h at 37°C in a non-CO2 incubator before the experiment. Metabolic rates were monitored under basal conditions (before any addition) and after addition of oligomycin (1 μM), carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone (FCCP) (1 μM), and antimycin (0.5 μM), all dissolved in XF base medium. The 3 drugs were injected into the XFe96 sequentially, and OCR and ECAR were measured using 3 cycles of mixing (150 s), waiting (120 s), and measuring (210 s). This cycle was repeated following each injection.
Mitochondrial isolation
Mitochondrial fractions were prepared by a mitochondrial isolation kit for cultured cells (Thermo Fisher Scientific). Briefly, PC3 cells were homogenized by 70 strokes using a Dounce Tissue Grinder (MilliporeSigma) in isolation buffer A plus protease inhibitor cocktail. Cell extracts were collected into equal volumes of isolation buffer C with buffer A. Cell debris and nuclei were removed by centrifugation at 700 g for 10 min, and mitochondrial fractions were collected by centrifugation at 3000 g for 15 min.
ROS
PC3 cells (4 × 105) transfected with various siRNAs were stained with MitoSox Red mitochondrial superoxide indicator (5 μM; Thermo Fisher Scientific) or total CellRox Deep Red (5 μM; Thermo Fisher Scientific) for 10 min in complete medium, followed by washes with PBS, pH 7.4, and analyzed on a fluorescence-activated cell sorting (FACS)Calibur flow cytometer. Intact cells were gated in the forward scatter/side scatter (FSC/SSC) plot to exclude small debris.
Mitochondrial membrane potential
siRNA-transfected PC3 cells were washed 3 times in PBS, pH 7.4, and analyzed on a FACSCalibur flow cytometer, with the tetramethylrhodamine, ethyl ester (TMRE) signal as FL1. Intact cells were gated in the FSC/SSC plot to exclude small debris. The resulting FL1 data were plotted on a histogram.
Immunofluorescence
Cells were fixed in formalin/PBS (4% final concentration), pH 7.4, for 15 min at 22°C, permeabilized in 0.1% Triton X-100/PBS for 5 min, washed, and incubated in 5% normal goat serum (NGS; Vector Laboratories, Burlingame, CA, USA) diluted in 0.3 M glycine/PBS for 60 min. Cells were labeled with MitoTracker or an antibody to Ser616-phosphorylated Drp1 (Ser616) (1:100) in 5% NGS/0.3 M glycine/PBS and incubated for 18 h at 4°C. After 3 washes in PBS, secondary antibodies conjugated to tetramethylrhodamine (TRITC) or Alexa 488 (Thermo Fisher Scientific) were diluted 1:500 in 5% NGS/0.3 M glycine/PBS and added to cells for 1 h at 22°C. Slides were washed and mounted in DAPI-containing Prolong Gold Mounting Medium (Thermo Fisher Scientific).
Mitochondria time-lapse videomicroscopy
Cells (2 × 104) growing on high-optical-quality glass-bottom 35-mm plates (MatTek Corporation, Ashland, MA, USA) were incubated with 100 nM MitoTracker Deep Red FM Dye for 30 min and imaged on a Leica TCS SP8 × inverted laser scanning confocal microscope (Wetzlar, Germany) using a ×63 1.40 numerical aperture oil objective. Short-duration time-lapse sequences were carried out using a Tokai Hit Incubator (Fujinomiya, Japan) equilibrated to 37°C and 5% CO2 bidirectional scanning at 8000 Hz using a resonant scanner. Time lapse was performed for 2 min (3 s/frame). Individual 12-bit images were acquired using a white-light supercontinuum laser (0.2% at 645 nm) and HyD detectors at ×5 digital zoom with a pixel size of 70 × 70 nm. A pinhole setting of 1 airy unit provided a section thickness of 0.896 μm. Each time point was captured with a step size of 0.15 μm. At least 7 individual cells under each condition were collected for analysis. Initial postprocessing of the 3-dimensional (3D) sequences was carried out with Huygens Software (Scientific Volume Imaging, Hilversum, The Netherlands) to deconvolve the images, and then they were imported into Las X software to study fission and fusion events. A workflow capable of tracking the mitochondrial volume over time was designed on the Las X software platform (Leica). For each cell, the volume of mitochondria over time was analyzed in 4 different areas (with a mean of 10 mitochondria/area) in 3D images. Variations in mitochondrial volume were evaluated by fold change over time: a fold change >1.3 denoted a fusion event; a fold change <0.7 denoted a fission event. The mean fission and fusion events in the 4 different areas was used for each cell analyzed.
FA complex dynamics
PC3 cells transfected with various siRNAs were plated on high-optical-quality 35-mm glass-bottom plates and transduced with Talin-Red Fluorescent Protein (RFP) BacMam virus for 18 h. Time-lapse videomicroscopy was carried out using a Leica TCS SP8 Scanning Laser Confocal Microscope system with an HCX PL APO CS ×63 1.40 numerical aperture oil UV objective. Acquisition of live cells using an integrated Leica Las software was performed every 3 min per frame for a total interval of 2 h. Sequences were imported in ImageJ [National Institutes of Health (NIH), Bethesda, MD, USA] for further analysis. The initial and final frames were duplicated and assembled as composite images. FA was manually counted and classified (according to the presence in some or all the time frames) into 3 groups: decayed, new, and stable (merged areas). The analysis was carried out in 7 cells (about 150 FA complexes) per condition in 2 independent time-lapse experiments.
Tumor cell motility
For 2-dimensional (2D) cell motility studies, siRNA-transfected PC3 cells (2 × 104) were seeded in 4-well Ph+ Chambers (Ibidi, Gräfelfing, Germany) in complete medium and allowed to attach for 16 h at 37°C. Time-lapse videomicroscopy was performed over 10 h, with a time-lapse interval of 10 min, as previously described by Caino et al. (24). Stacks were imported into ImageJ Fiji (NIH) software for analysis, and at least 20 cells per condition were tracked using the Manual Tracking plugin for ImageJ Fiji. Tracking data were exported into the Chemotaxis and Migration Tool v.2.0 (Ibidi) for graphing and calculation of mean ± sd of speed and accumulated distance of movement. Directional cell migration was assessed in a wound-closure assay. Wounds in a monolayer of siRNA-transfected PC3 cells were made with a 10-μl pipette tip, cell debris were washed off, and cultures were maintained in complete medium containing 10% fetal bovine serum at 37°C and 5% CO2 for 24 h. Time-lapse imaging of migrating cells was performed using a TE300 Inverted Microscope (Nikon, Tokyo, Japan) equipped with an incubator set at 37°C, 5% CO2, and 95% relative humidity. Each image was acquired using a ×10 objective of the same fields at each 10-min interval for a total of 24 h.
Tumor cell invasion
Experiments were carried out essentially as previously described by Caino et al. (11) using Growth Factor Reduced Matrigel-coated 8-μm polyester (PET) Transwell chambers (Corning, Corning, NY, USA). Tumor cell types were seeded in duplicates onto the coated Transwell filters (1 × 105 cells/well) in medium containing 0.1% BSA, and conditioned medium from NIH3T3 fibroblasts was placed in the lower chamber as chemoattractant. Cells were allowed to invade for 16 h, noninvading cells were scraped off the topside of the membranes, and invaded cells on the Transwell insert were fixed in methanol. Membranes were mounted in medium containing DAPI (Vector Laboratories) and analyzed by fluorescence microscopy. Five random fields at ×10 magnification were collected for each membrane. Digital images were batch imported into NIS elements (Nikon), thresholded, and analyzed with the Analyze particles function.
Animal studies
Studies involving vertebrate animals (rodents) were carried out in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, MD, USA). Protocols were approved by the Institutional Animal Care and Use Committee of The Wistar Institute (112625 and 112610). For superficial xenograft tumor growth, PC3 cells stably transduced with control pLKO or IDH2-directed shRNA (5 × 106) were engrafted onto the flanks of 6–8-wk-old severe combined immunodeficient/beige mice (10–12 tumors per condition), and tumor growth was measured with a caliper at increasing time intervals. Animals were euthanized at d 37 postengraftment. For a liver metastasis model, 6–8-wk-old severe combined immunodeficient/beige mice (3 mice per experimental condition) were anesthetized with ketamine hydrochloride, the abdominal cavity was exposed by laparotomy, and PC3 cells (1 × 106) transduced with pLKO or IDH2-directed shRNA were injected into the spleen. Spleens were removed the first day after injection to minimize potentially confounding effects on metastasis due to variable growth of primary tumors. Following splenectomy, animals were euthanized after 11 d, and their livers were resected, fixed in formalin, and paraffin embedded. Serial liver sections 500 μm apart (n = 15/condition) were stained with hematoxylin and eosin and analyzed using NIS elements. Metastatic foci were counted manually, and the surface area of the foci was determined using NIS elements.
Statistical analysis
Data are expressed as means ± sd of multiple independent experiments or replicates of representative experiments out of a minimum of 2 or 3 independent determinations. A 2-tailed Student’s t test was used for 2-group comparative analyses. All statistical analyses were performed using Prism 8 (GraphPad Software, La Jolla, CA, USA) for Windows. A value of P < 0.05 was considered statistically significant.
RESULTS
IDH2 regulation of tumor metabolism
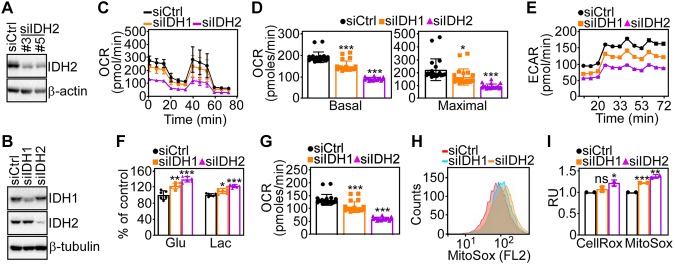
We began this study by looking at the effect of IDH2 targeting on tumor bioenergetics. For these experiments, we first characterized individual (Fig. 1A) or pooled (Fig. 1B) IDH2-directed siRNA sequences that efficiently silence IDH2 protein levels in prostate adenocarcinoma PC3 cells compared with nontargeting siRNA transfectants (Fig. 1A, B). IDH2 silencing in these conditions caused the accumulation of TCA intermediates, glutamate and isocitrate, whereas downstream metabolites, α-ketoglutarate and Succ, were reduced (Supplemental Fig. S1A). Consistent with these data, IDH2 knockdown resulted in lower OCRs in PC3 cells (Fig. 1C), a marker of oxidative phosphorylation, with decrease in both basal and maximal respiratory capacity (Fig. 1D) as well as ECAR (Fig. 1E). Conversely, silencing of cytosolic IDH1 (Fig. 1B) minimally affected OCR or ECAR in PC3 cells (Fig. 1C–E).
Figure 1.
IDH2 regulation of mitochondrial bioenergetics. A) Prostate adenocarcinoma PC3 cells were transfected with control nontargeting siRNA (siCtrl) or 2 independent IDH2-directed siRNAs (#3 and #5) and analyzed by Western blotting. B) PC3 cells were transfected with siCtrl or IDH1- or IDH2-directed siRNA and analyzed by Western blotting. C, D) PC3 cells transfected as in B were analyzed for OCRs on a Seahorse XFe96 Bioenergetics Flux Analyzer [representative tracings; n = 2 (C)], and basal (left) and maximal (right) respiratory capacities were quantified (D). Means ± sd (n = 22). *P < 0.01, ***P < 0.0001. E) The conditions are as in B, and transfected PC3 cells were analyzed for ECARs on a Seahorse XFe96 Bioenergetics Flux Analyzer. Representative tracings (n = 2). F) PC3 cells transfected as in B were analyzed for glucose consumption (Glu) or lactate production (Lac). Means ± sd (n = 6). *P = 0.01, **P = 0.001, ***P < 0.0001. G) The conditions are as in B, and siRNA-transfected PC3 cells were analyzed for the rate of ATP production on a Seahorse XFe96 Bioenergetics Flux Analyzer. Means ± sd (n = 22). ***P < 0.001. H, I) PC3 cells transfected as in B were analyzed for total ROS (CellRox) or mitochondrial ROS (MitoSox) production by flow cytometry [representative tracings of MitoSox reactivity (H)] and quantified (I). FL2, fluorescence laser 2; Ns, not significant; RU, relative units. Means ± sd (n = 3). *P = 0.04, **P = 0.006, ***P < 0.0001.
As an independent approach, we next established clones of PC3 cells transduced with control shRNA or 3 independent IDH2-directed shRNAs. Stable IDH2 silencing in these settings (Supplemental Fig. S1B) also reduced OCR (Supplemental Fig. S1C), affecting basal and maximal respiration (Supplemental Fig. S1D), and attenuated ECAR (Supplemental Fig. S1E). In parallel, IDH2-targeted cells exhibited increased glucose consumption and lactate generation compared with control transfectants (Fig. 1F). Despite these compensatory changes, loss of IDH2 resulted in decreased ATP production (Fig. 1G and Supplemental Fig. S1F) and greater production of total ROS as well as mitochondrial-derived ROS (Fig. 1H, I). siRNA knockdown of cytosolic IDH1 also induced glycolytic reprogramming in PC3 cells (Fig. 1F) and was similarly associated with reduced ATP generation (Fig. 1G) and elevation of total and mitochondrial ROS (Fig. 1H, I), albeit less prominently than IDH2 loss. Despite higher ROS levels, IDH2 knockdown cells exhibited no significant changes in γ-H2AX reactivity, a marker of DNA damage and repair (Supplemental Fig. S1G), or mitochondrial inner membrane potential compared with control transfectants (Supplemental Fig. S1H).
IDH2 regulation of mitochondrial dynamics
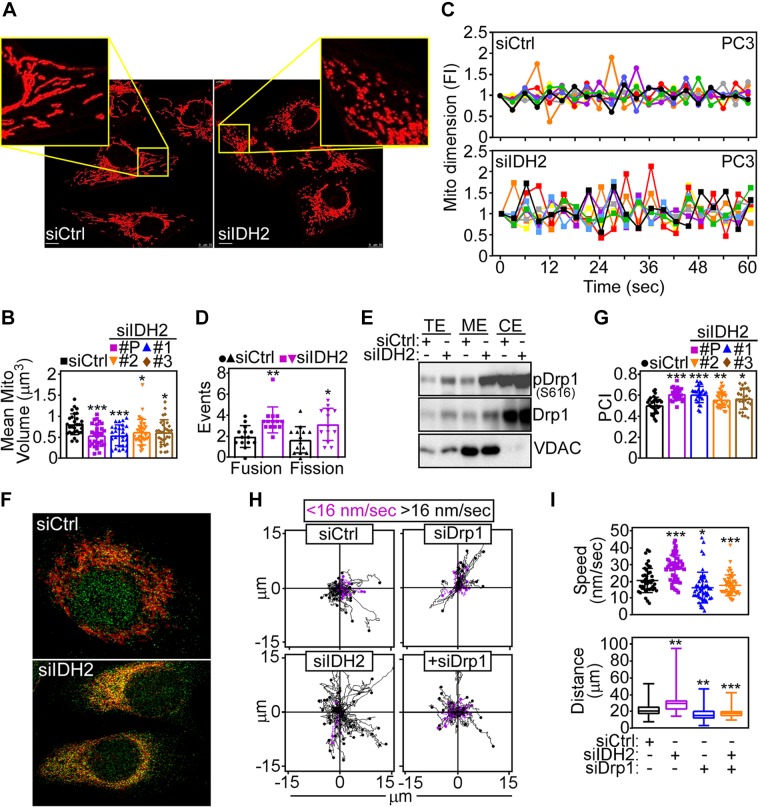
Recent studies have shown that nontoxic levels of ROS may affect mitochondrial dynamics (25), and this possibility was next investigated for IDH2 signaling. Compared with control transfectants, IDH2 knockdown reduced mitochondrial volume in PC3 cells, suggestive of organelle fission (Fig. 2A,B). Accordingly, loss of IDH2 significantly increased mitochondrial dynamics in PC3 (Fig. 2C and Supplemental Fig. S2A, B) as well as DU145 (Supplemental Fig. S2C) cells, characterized by heightened frequency of fission and fusion events (Fig. 2D). Biochemically, IDH2-targeted cells exhibited increased expression of Drp1, a key effector of mitochondrial fission (12) (Supplemental Fig. S2D), and differential modulation of mitochondrial fusion regulators mitofusin 1 and 2 (Supplemental Fig. S2E).
Figure 2.
IDH2 controls mitochondrial dynamics. A) PC3 cells transfected with siCtrl or siIDH2 were analyzed for mitochondrial morphology by confocal laser microscopy. Representative images. Scale bars, 10 μm. Insets, magnification of indicated areas. B) PC3 cells transfected with siCtrl, 3 independent IDH2-directed siRNAs (1, 2, 3), or IDH2-directed pooled siRNA (P) were quantified for mitochondrial volume. Means ± sd (n = 29–33). *P = 0.01, ***P = 0.0002–0.0003. C) The conditions are as in A, and siRNA-transfected PC3 cells were analyzed for changes in mitochondrial dimension indicative of organelle fusion (>1.3-fold change in mitochondrial volume, positive y scale) or fission (<0.7-fold change in mitochondrial volume, negative y scale), and events were quantified continuously by time-lapse videomicroscopy at the indicated time intervals. Each tracing corresponds to an individual cell. Representative experiment (n = 2). D) The conditions are as in C, and mitochondrial fusion and fission events (60-s interval) were quantified in siRNA-transfected DU145 cells. Means ± sd (n = 13–14). *P = 0.01, **P = 0.001. E) PC3 cells transfected with siCtrl or siIDH2 were fractionated in total (TE), cytosolic (CE), or mitochondrial (ME) extracts and analyzed by Western blotting. F) PC3 cells transfected as in A were labeled with MitoTracker plus an antibody to Ser616-phosphorylated Drp1 and analyzed for signal colocalization by confocal fluorescence microscopy. Representative images (n = 3). G) The experimental conditions are as in F, and colocalization of Ser616-phosphorylated Drp1 and MitoTracker was quantified with determination of a Pearson’s correlation index (PCI). Means ± sd (n = 24–31). *P = 0.01, **P = 0.009, ***P < 0.0001. H) PC3 cells transfected with siCtrl or siIDH2 were analyzed for mitochondrial motility in 2D contour plots in the presence or absence of Drp1-directed siRNA (siDrp1). Each tracing corresponds to the movement of an individual mitochondrion. The cutoff velocities for slow-moving or fast-moving (<16 nm/s or >16 nm/s, respectively) mitochondria are indicated. Representative experiment (n = 2). I) The conditions are as in H, and the speed of mitochondrial movements (top; n = 45–50) and total distance traveled by individual mitochondria (bottom; n = 47–50) were quantified. FI, fold increase; siCtrl, control nontargeting siRNA; VDAC, voltage-dependent anion channel. *P = 0.01, **P = 0.001-0.003, ***P < 0.0001.
Based on these data, we next asked whether Drp1 contributed to IDH2 regulation of mitochondrial fission. First, siRNA silencing of IDH1, as opposed to IDH2, had no effect on Drp1 levels in tumor cells (Supplemental Fig. S2E). Instead, IDH2 depletion increased the recruitment of total Drp1 as well as Ser616-phosphorylated (i.e., active) Drp1 (12) to mitochondria by Western blotting of isolated subcellular fractions (Fig. 2E) and colocalization studies with a mitochondrial marker, MitoTracker, by confocal microscopy (Fig. 2F, G). Functionally, this was associated with greater mitochondrial motility in IDH2 knockdown cells (Fig. 2H), with faster speed of organelle movements and longer distance traveled by individual mitochondria compared with control transfectants (Fig. 2I). Mechanistically, siRNA silencing of Drp1 was sufficient to reverse the increase in subcellular mitochondrial motility in IDH2-targeted cells (Fig. 2H), restoring the speed of mitochondrial movements and the total distance traveled by individual mitochondria to levels of control transfectants (Fig. 2I).
IDH2 regulation of mitochondrial trafficking regulates membrane dynamics and tumor cell movements
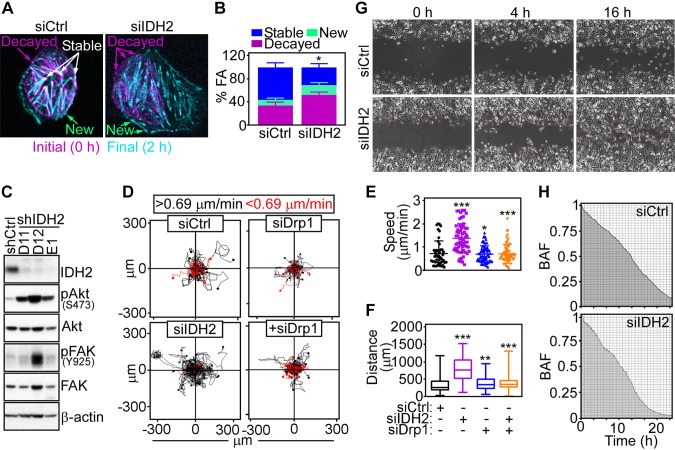
Increased mitochondrial trafficking has been linked to tumor cell motility (11). Consistent with this, siRNA knockdown of IDH2 enhanced FA complex dynamics in tumor cells (Fig. 3A), a prerequisite of cell movements (26), increasing the fraction of new and decayed FA complexes while reducing stable FA complexes (Fig. 3B). In addition, IDH2 loss caused increased phosphorylation of cell motility kinases, Akt (Ser473) and FAK (Tyr925), in PC3 cells (Fig. 3C). Together, this resulted in increased 2D tumor cell motility (Fig. 3D), quantitatively characterized by faster speed of cell movements (Fig. 3E) and longer distance traveled by individual cells (Fig. 3F). Consistent with the data above, silencing of Drp1 was sufficient to reverse the increase in 2D cell motility after IDH2 knockdown (Fig. 3D) and normalized quantitative parameters of cell movements to levels of control transfectants (Fig. 3E, F). Finally, PC3 cells silenced for IDH2 exhibited increased directional migration in a wound-closure assay (Fig. 3G, H).
Figure 3.
IDH2 regulation of tumor cell movements. A) PC3 cells transfected with siCtrl or siIDH2 were labeled with Talin-Red Fluorescent Protein (RFP) and analyzed for FA complex dynamics by time-lapse videomicroscopy. Representative merged frames at 0 h (magenta) and 2 h (cyan) are shown (n = 2). Arrows, position of new, stable, and decayed FA complexes. B) The conditions are as in A, and the percentage of new, stable, or decayed FA complexes was quantified per each condition (siCtrl, n = 12; siIDH2, n = 7). *P = 0.04. C) PC3 cells stably transduced with shCtrl or 3 independent IDH2-directed shRNAs (D11, D12, E1) were analyzed by Western blotting. P, phosphorylated. D) PC3 cells were transfected with siCtrl or siIDH2 and analyzed for cellular motility in 2D contour plots in the presence or absence of Drp1-directed siRNA (siDrp1). Each tracing corresponds to the movements of an individual cell. The cutoff velocities for slow-moving or fast-moving cells (<0.69 or >0.69 μm/min, respectively) are indicated. E, F) The conditions are as in D, and the speed of cell motility n = 45–66 (E)] and total distance traveled by individual cells [n = 58–66 (F)] was quantified. *P = 0.01, **P = 0.003, ***P < 0.0001. G, H) PC3 cells transfected with siCtrl or siIDH2 were analyzed for directional cell migration in a wound-closure assay (G), and the area covered by cell migration was quantified at the indicated time intervals (H). Representative images (n = 3). BAF, binary area fraction; shCtrl, control nontargeting shRNA; siCtrl, control nontargeting siRNA.
Control of tumor cell migration and invasion by IDH2
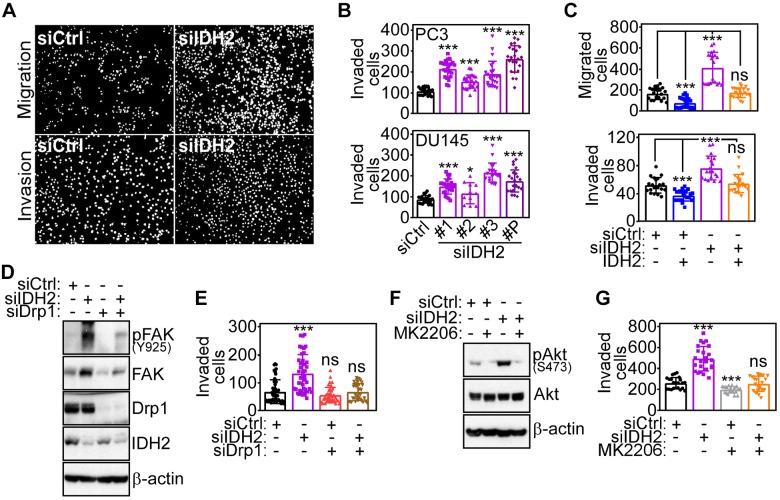
Based on these data, we next asked whether IDH2 levels also affected tumor cell invasion across artificial basement membranes. Accordingly, siRNA knockdown of IDH2 but not IDH1 strongly increased prostate cancer cell migration and invasion across Matrigel-coated inserts (Fig. 4A and Supplemental Fig. S3A). Similar results were obtained using multiple, independent IDH2-directed siRNA sequences, which also resulted in increased prostate cancer cell invasion (Fig. 4B). This response was specific because reconstitution of IDH2 knockdown cells with non-siRNA inhibitable IDH2 cDNA reversed the increase in tumor cell migration and invasion in PC3 (Fig. 4C) and DU145 (Supplemental Fig. S3B) cells indistinguishably from control transfectants.
Figure 4.
Requirements IDH2 regulation of tumor cell motility. A) PC3 cells transfected with siCtrl or siIDH2 were analyzed for cell migration (top) or invasion across Matrigel-coated Transwell inserts (bottom). Representative images of DAPI-stained nuclei of migrated or invaded cells are shown. B) PC3 (top, n = 21–25) or DU145 (bottom, n = 22–24) cells were transfected with siCtrl, 3 independent IDH2-directed siRNAs (#1, #2, #3), or IDH2-directed pooled siRNA (P) and analyzed for Matrigel invasion. Means ± sd. *P = 0.02, ***P < 0.0001. C) PC3 cells transfected as in A were reconstituted with IDH2 cDNA and analyzed for cell migration (top, n = 21–22) or Matrigel invasion (bottom, n = 21–22). Means ± sd. ***P < 0.0001. D, E) PC3 cells transfected with siCtrl or siIDH2 were further transfected with Drp1-directed siRNA (siDrp1) and analyzed by Western blotting (D) or Matrigel invasion [n = 37–45 (E)]. Means ± sd. ***P < 0.0001. F, G) PC3 cells transfected with siCtrl or siIDH2 were incubated with small molecule Akt inhibitor, MK2206, and analyzed by Western blotting (F) or Matrigel invasion (G). siCtrl, control nontargeting siRNA; ns, not significant; p, phosphorylated. Means ± sd (n = 22–24). ***P < 0.0001.
The requirements of IDH2 modulation of tumor cell movements were further investigated. First, siRNA knockdown of Drp1 prevented the increase in FAK phosphorylation (Fig. 4D) and tumor cell invasion (Fig. 4E) induced by IDH2 knockdown. Similarly, preincubation of PC3 cells with a small molecule Akt inhibitor, MK2206, suppressed Akt phosphorylation (Fig. 4F) and blocked the increase in tumor cell invasion associated with IDH2 loss (Fig. 4G). Finally, reconstitution of IDH2 knockdown cells with WT IDH2 or oncogenic R172K IDH2 mutant (Supplemental Fig. S3C) comparably normalized tumor cell invasion to levels of control transfectants (Supplemental Fig. S3D).
ROS regulation of tumor cell motility and invasion
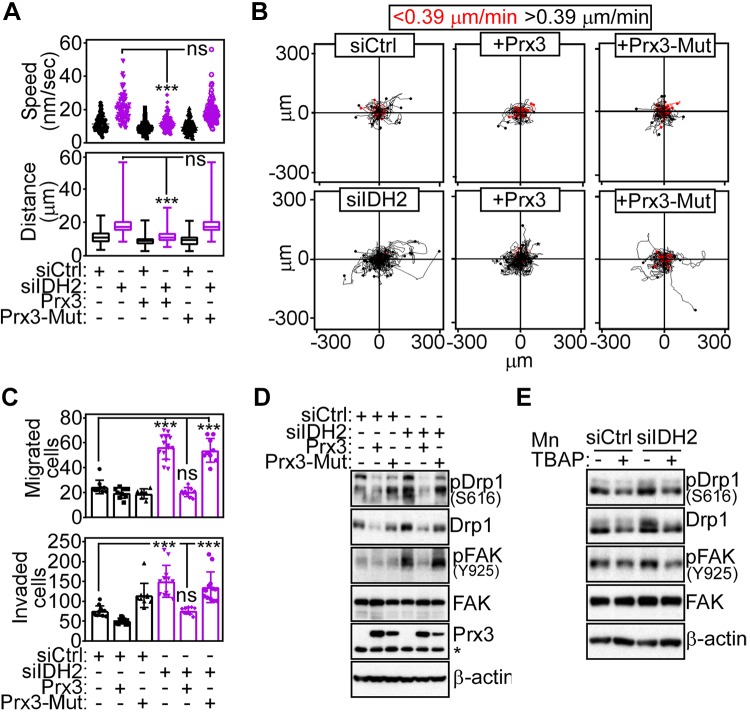
Next, we explored upstream mechanisms of IDH2 control of tumor cell motility, and we focused on potential ROS unbalance in this process. Accordingly, transfection of the antioxidant Prx3 (Supplemental Fig. S4A, B) or treatment with the superoxide scavenger MnTBAP (Supplemental Fig. S4C, D) reversed the increase in mitochondrial ROS induced by IDH2 loss. Reconstitution of IDH2-targeted cells with Prx3, but not a loss-of-function C108S Prx3 mutant, normalized mitochondrial motility (Supplemental Fig. S4E) and restored the speed of organelle trafficking and the total distance traveled by individual mitochondria to levels of control transfectants (Fig. 5A). Downstream of this response, ROS buffering with Prx3 also corrected the increase in 2D cell motility induced by IDH2 loss (Fig. 5B), normalized quantitative parameters of cell movements (Supplemental Fig. S4F), and abrogated the heightened tumor cell migration and invasion observed in these settings (Fig. 5C). Conversely, reconstitution with loss-of-function C108S Prx3 mutant had no effect on mitochondrial movements (Fig. 5A and Supplemental Fig. S4E), 2D tumor cell motility (Fig. 5B and Supplemental Fig. S4F), or cell migration and invasion (Fig. 5C). Biochemically, re-expression of Prx3, but not C108S Prx3 mutant, prevented the accumulation of Ser616-phosphorylated Drp1 as well as the increase in Tyr925-phosphorylated FAK in IDH2 knockdown cells (Fig. 5D). ROS scavenging with MnTBAP gave similar results, reducing the accumulation of Ser616-phosphorylated Drp1 and FAK phosphorylation on Tyr925 (Fig. 5E) as well as tumor cell migration and invasion to levels of control transfectants (Supplemental Fig. S4G).
Figure 5.
ROS regulation of IDH2-directed tumor cell motility. A) PC3 cells transfected with siCtrl or siIDH2 were reconstituted with Prx3 or loss-of-function Cys108Ser (C108S) Prx3 mutant (Prx3-Mut) and quantified for speed of mitochondrial movements (top, n = 85–92) or distance traveled by individual mitochondria (bottom, n = 85–92). ***P < 0.0001. B) The conditions are as in A, and reconstituted PC3 cells were analyzed for cell motility in 2D contour plots. Each tracing corresponds to the movement of an individual cell. The cutoff velocities for slow-moving or fast-moving (<0.39 μm/min or >0.39 μm/min, respectively) cells are shown. Representative experiment (n = 3). C, D) PC3 cells transfected with siCtrl or siIDH2 and reconstituted as in A were analyzed for cell migration [top n = 10–14 (C)] or Matrigel invasion [bottom n = 10–12 (C)] or Western blotting (D). Means ± sd. ***P < 0.0001. E) PC3 cells transfected with siCtrl or siIDH2 were incubated with the ROS scavenger, MnTBAP, and analyzed by Western blotting. Ns, not significant; p, phosphorylated.
IDH2 targeting induces an HIF-1α–dependent pseudohypoxic state
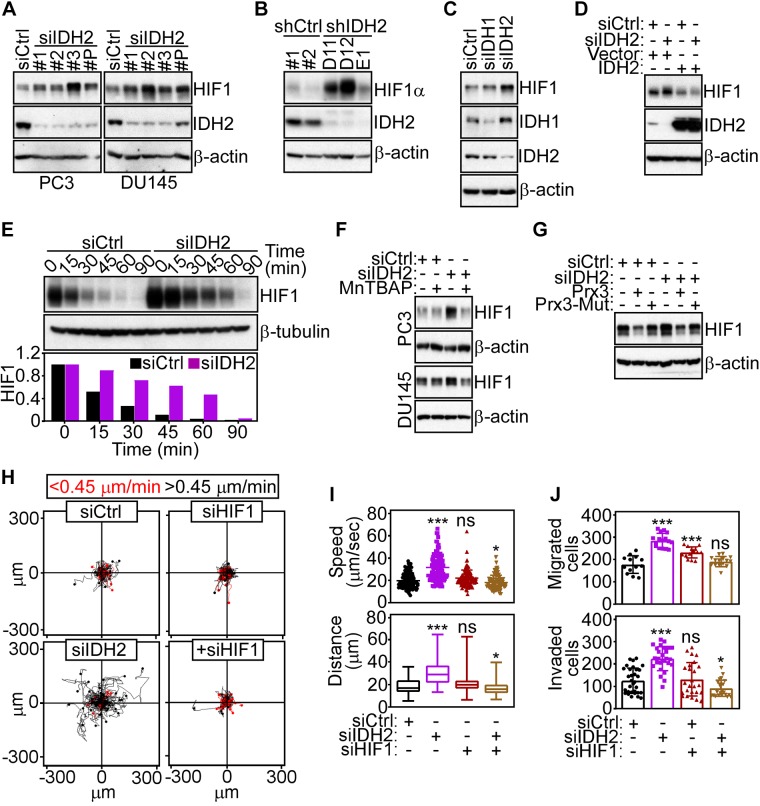
Next, we searched for downstream effectors of ROS signaling that regulated mitochondrial dynamics and tumor cell motility. We found that IDH2 silencing by siRNA (Fig. 6A) or shRNA (Fig. 6B) resulted in increased expression of HIF-1α in normoxic conditions. siRNA silencing of IDH1 had no effect on HIF-1α levels (Fig. 6C). Reconstitution of IDH2-silenced cells with IDH2 cDNA restored HIF-1α expression to levels of control transfectants (Fig. 6D), supporting the specificity of this response. In terms of mechanisms, loss of IDH2 resulted in a 4-fold increase in HIF-1α half-life compared with control transfectants (Fig. 6E), without significant changes in HIF-1α mRNA expression (Supplemental Fig. S5A). Despite the higher HIF-1α levels in these settings, there was no significant increase in mRNA (Supplemental Fig. S5B) or protein (Supplemental Fig. S5C) expression of established HIF-1α target genes, including LDHA, aldolase A (ALDOA), pyruvate dehydrogenase kinase, isozyme 1 (PDHK1), or hexokinase 2 (HK-II). As control, HIF-1α stabilized in response to hypoxia resulted in increased mRNA expression of LDHA and ALDOA, whereas IDH2 mRNA was unchanged (Supplemental Fig. S5D). Consistent with a role of ROS in this pathway, treatment with MnTBAP (Fig. 6F) or transfection with Prx3, but not C108S Prx3 mutant (Fig. 6G), prevented the increase in HIF-1α levels in IDH2 knockdown cells.
Figure 6.
HIF-1α regulation by IDH2. A) PC3 (left) or DU145 (right) cells transfected with siCtrl, 3 independent IDH2-directed siRNAs (1, 2, 3), or pooled IDH2-directed siRNA (P) were analyzed by Western blotting. B) PC3 cells stably transduced with 2 independent control shRNAs (shCtrl #1 and #2) or 3 IDH2-directed shRNAs (D11, D12, and E1) were analyzed by Western blotting. C) PC3 cells transfected with siCtrl, siIDH1, or siIDH2 were analyzed by Western blotting. D) PC3 cells transfected with siCtrl or siIDH2 were reconstituted with vector or IDH2 cDNA and analyzed by Western blotting. E) PC3 cells transfected as in C were analyzed at the indicated time intervals by Western blotting. Bar graph (bottom), densitometric quantification of HIF-1α protein bands. F) PC3 (top) or DU145 (bottom) cells transfected with siCtrl or siIDH2 were incubated with the ROS scavenger, MnTBAP, and analyzed by Western blotting. G) PC3 cells transfected with siCtrl or siIDH2 were reconstituted with Prx3 or loss-of-function C108S Prx3 mutant (Prx3-Mut) and analyzed by Western blotting. H) PC3 cells transfected with siCtrl, siIDH2, or siHIF-1α were analyzed for cell motility in 2D contour plots. Each line corresponds to the movements of an individual cell. The cutoff velocities for slow-moving or fast-moving (<0.45 μm/min or >0.45 μm/min) cells are indicated. Representative experiment (n = 2). I) The conditions are as in H, and the speed of cell movements (top, n = 58–64) and total distance traveled by individual cells (bottom, n = 58–64) was quantified per each condition. *P = 0.01, ***P < 0.0001. J) The conditions are as in H, and siRNA-transfected PC3 cells were analyzed for cell migration (top, n = 13–16) or Matrigel invasion (bottom, n = 23–31). Ns, not significant; shCtrl, control nontargeting shRNA; siCtrl, control nontargeting siRNA. Means ± sd. ***P = 0.0002 to P < 0.0001.
HIF-1α–directed reprogramming of mitochondrial dynamics
In a first set of experiments, we found that HIF-1α stabilization induced by IDH2 knockdown resulted in increased recruitment of Drp1 as well as Ser616-phosphorylated Drp1 to mitochondria (Supplemental Fig. S6A). Reciprocally, knockdown of HIF-1α prevented the recruitment of Ser616-phosphorylated Drp1 to mitochondria in IDH2 knockdown cells (Supplemental Fig. S6A). We next looked at a potential relationship between HIF-1α and Drp1 signaling in mitochondrial dynamics. First, increased HIF-1α levels induced by oxidative stress (H2O2) did not significantly affect Drp1 expression (Supplemental Fig. S6B), suggesting that Drp1 is not a direct target of HIF-1α. Instead, siRNA silencing of Drp1 was sufficient to blunt HIF-1α induction in response to IDH2 knockdown (Supplemental Fig. S6C). Consistent with mitochondrial damage in these settings, silencing of Drp1 elevated mitochondrial ROS generation in a response further augmented by simultaneous knockdown of IDH2 (Supplemental Fig. S6D). Functionally, knockdown of HIF-1α potently inhibited the increase in mitochondrial motility induced by IDH2 silencing (Supplemental Fig. S6E) and normalized the speed of mitochondrial movements and total distance traveled by individual mitochondria to levels of control transfectants (Supplemental Fig. S6F). To examine a role of Drp1 in this response, we next reconstituted siRNA-silenced prostate cancer cells with Drp1 cDNA. Re-expression of Drp1 in these settings restored the increase in mitochondrial motility (Supplemental Fig. S6E) and quantitative parameters of mitochondrial movements (Supplemental Fig. S6F) in PC3 cells simultaneously silenced for IDH2 and HIF-1α. Similarly, HIF-1α silencing reversed the increase in 2D cell motility induced by IDH2 knockdown (Fig. 6H, I) and suppressed the increase in tumor cell migration and Matrigel invasion similarly to control transfectants (Fig. 6J).
An IDH2–HIF-1α mitochondrial axis controls tumor cell invasion in vivo
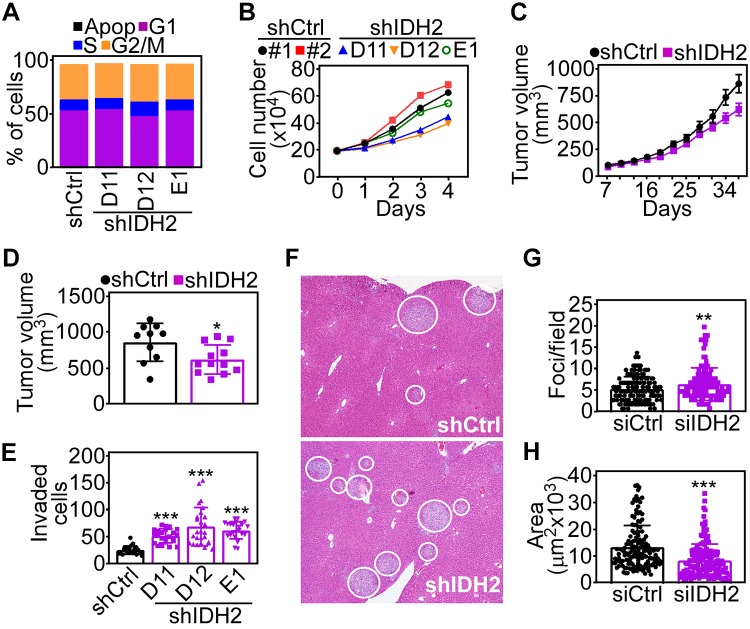
Finally, we looked at the impact of the IDH2 pathway on tumor progression. First, we found that shRNA silencing of IDH2 did not affect cell cycle transitions in PC3 cells (Fig. 7A) and only slightly reduced tumor cell proliferation throughout a 72-h time interval (Fig. 7B). When engrafted subcutaneously on the flanks of immunocompromised mice, PC3 cells with stable IDH2 knockdown gave rise to exponentially growing xenograft tumors, comparable in size and growth kinetics to control transfectants (Fig. 7C). By the end of the experiment, IDH2 depletion only modestly reduced tumor size compared with control shRNA-expressing tumors (Fig. 7D). Conversely, stable IDH2 knockdown increased tumor cell invasion across Matrigel-coated Transwell inserts (Fig. 7E), in agreement with the data above. When analyzed for systemic dissemination, intrasplenic injection of IDH2 knockdown PC3 cells gave rise to extensive metastatic foci in the liver 11 d after reconstitution (Fig. 7F). Morphometric quantification of this response demonstrated that IDH2 knockdown increased the number of metastatic foci (Fig. 7G), whereas the overall surface area of liver metastases was reduced compared with control transfectants (Fig. 7H).
Figure 7.
IDH2 regulation of tumor cell invasion in vivo. A) PC3 cells stably transduced with shCtrl or 3 independent IDH2-directed shRNAs (shIDH2 D11, D12, E1) were analyzed by propidium iodide staining and flow cytometry. The percentage of cells in each cell cycle phase is indicated. Apop, apoptotic. B) The conditions are as in A, and stably transduced PC3 cells were analyzed for cell proliferation by direct cell counting at the indicated time intervals. C) PC3 cells stably transduced with shCtrl or shIDH2 were engrafted subcutaneously on the flank of immunocompromised mice, and tumor growth was measured with a caliper at the indicated time intervals. Means ± sd (shCtrl, n = 10; shIDH2, n = 12). D) The conditions are as in C, and tumor volume in each animal group was determined at d 37 postengraftment. Means ± sd. *P = 0.02. E) PC3 cells stably transduced as in A were analyzed for Matrigel invasion. Means ± sd (n = 22–26). ***P < 0.0001. F) PC3 cells stably transduced as in C were injected into the spleen of immunocompromised mice, and metastatic foci to the liver were identified after 11 d by hematoxylin and eosin staining and light microscopy. Representative images; white circles, metastatic foci. G, H) The conditions are as in F, and the number [shCtrl, n = 142, shIDH2, n = 162 (G)] and surface area [shCtrl, n = 142; shIDH2, n = 162 (H)] of liver metastatic foci was quantified in each animal group. shCtrl, control nontargeting shRNA. Means ± sd. **P = 0.004, ***P < 0.0001.
DISCUSSION
In this study, we have shown that loss of endogenous IDH2 results in defective oxidative bioenergetics, decreased ATP production, and aberrant generation of ROS in model prostate cancer cells. In turn, this causes exaggerated mitochondrial dynamics and greater mitochondrial motility, sustaining FA complex turnover, phosphorylation of oncogenic kinases, and increased tumor cell migration and invasion. Mechanistically, this pathway involved ROS-mediated stabilization of HIF-1α in normoxia, which was required for heightened mitochondrial dynamics and greater tumor cell movements.
Considerable effort has been devoted to characterize mutant IDH2 as a validated therapeutic target in gliomas and acute leukemias (13, 14), but the function of WT IDH2 in cancer has remained controversial. The data presented here uncovered a new role for IDH2 as an endogenous inhibitor of tumor cell migration and invasion, buffering ROS signals (22) and suppressing mitochondrial dynamics (12). This conclusion is at variance with a proposed oncogenic role of IDH2 in lung cancer (18), but it is in line with other data pointing to an antitumorigenic function of this pathway (20), including inhibition of cell migration and invasion (27) and modulation of matrix metalloproteinase activity (28).
Mitochondrial dynamics is broadly exploited in cancer (12) and linked to key traits of disease progression, including MAPK- (29) and Ras-dependent (30) tumor growth, cell cycle transitions (31), cancer stemness (32, 33), and tumor cell invasion and metastasis (25, 34). Mechanistically, endogenous IDH2 inhibited upstream steps in this process, dampening the activating phosphorylation of Drp1 on Ser616 and its recruitment to the mitochondrial outer membrane. As a result, decreased IDH2 expression caused exaggerated cycles of mitochondrial fusion and fission, which in turn promoted faster and more extensive subcellular organelle movements. Mechanistically, this may reflect the ability of smaller, “fissed” mitochondria to travel more efficiently to specialized subcellular compartments (11) and fuel membrane lamellipodia dynamics and FA complex turnover as key antecedents of cell motility (35–37). In the case of IDH2, this may involve modulation of both antero- and retrograde mitochondrial movements, as no significant accumulation of stationary mitochondria was observed at the peripheral or cortical cytoskeleton of IDH2-targeted cells. This phenotype is at variance with the function of another endogenous inhibitor of mitochondrial trafficking exploited in cancer [i.e., syntaphilin (38)], whose loss during tumor progression results in prominent accumulation of stationary mitochondria at the cortical cytoskeleton coupled to increased tumor cell migration and invasion in vivo (11).
A key requirement of tumor cell motility triggered by IDH2 loss was increased generation of mitochondrial ROS. This is consistent with a key antioxidant function of IDH2 (39), regulating NADPH for glutathione reductase (22) and the thioredoxin system (23). There is evidence that IDH2-ROS signaling is exploited in cancer, as deregulated ROS production in IDH2 knockout mice generates oxidative stress in stromal and malignant cells and increased tumor growth (17). Although it is established that tumors produce more mitochondrial ROS than normal tissues, how this response affects malignant behavior continues to be debated (40), and paradoxically linked to pro- (41) or antitumorigenic signaling (42). Here, ROS produced after IDH2 depletion did not induce markers of DNA damage (i.e., γ-H2AX or defective cell cycle progression). Instead, nontoxic ROS generated in these settings signaled increased subcellular mitochondrial trafficking and greater tumor cell motility and invasion (35). This is consistent with other data on the role of nontoxic ROS in cell motility (43), epithelial-mesenchymal transition (44), and metastatic dissemination in vivo (10). Similar to the IDH2 pathway, loss of syntaphilin has also been associated with ROS-dependent increased subcellular mitochondrial trafficking and heightened tumor cell movements (11).
Against the backdrop of a ubiquitous Warburg effect (2), it has been difficult to conclusively assign a role of mitochondria in cancer. Although compelling evidence points to oxidative bioenergetics as a tumor driver (6, 8), including metastatic competence (9, 10, 35), other data have suggested that this pathway may actually function in tumor suppression (7) as restoring oxidative phosphorylation antagonized tumor growth (45), at least in certain malignancies (46). As shown here, IDH2 may embody this apparent dichotomy by enabling oxidative bioenergetics and thus tumor metabolism (8) while inhibiting tumor cell movements as a prerequisite of metastatic potential (12).
As uncovered here, the switch between these 2 seemingly opposing functions was the creation of a pseudohypoxic state, in which ROS produced after IDH2 loss stabilized HIF-1α levels in tumor cells even under conditions of normoxia (47), which enabled increased mitochondrial trafficking and heightened tumor cell movements. The mechanistic underpinning of these responses remains to be elucidated. A link between HIF-1α signaling and Drp1-directed mitochondrial dynamics, including subcellular organelle trafficking, has not been previously proposed, and HIF-1α stabilized after IDH2 loss was not associated with transcriptional up-regulation of established HIF-1α target genes. Compelling evidence has demonstrated an important role of HIF-1α in transcriptional control of mitochondrial oxidative metabolism (48, 49). In addition, there is precedent for a HIF-1α–directed pseudohypoxic state as a tumor driver, in particular in response to mutations in TCA genes Succ dehydrogenase and fumarate hydratase (50). Consistent with this scenario, HIF-1α signaling has been linked to multiple steps in the metastatic cascade (51), promoting epithelial-mesenchymal transition (52), tumor cell movements (53, 54), and assembly of a premetastatic niche (55).
In sum, the dual role of WT IDH2 in cancer, sustaining oxidative metabolism while antagonizing tumor cell movements, may exemplify how transformed cells reprogram mitochondrial functions in response to microenvironment conditions to fulfill energy needs for cell proliferation (8) vs. mitochondrial dynamics (12) to sustain tumor cell movements (11, 34). In this context, the central role of mitochondrial dynamics (12) in tumor cell migration and invasion may provide an actionable therapeutic target to limit metastatic dissemination in patients with advanced cancer (56).
ACKNOWLEDGMENTS
The authors thank James Hayden and Frederick Keeney (The Wistar Institute Imaging Core Facility) for assistance with time-lapse videomicroscopy. This work was supported by U.S. National Institutes of Health, National Cancer Institute Grants P01 CA140043 and R35 CA220446 (to D.C.A.). The support for Shared Resources utilized in this study was provided by Cancer Center Support Grant (CCSG) P30 CA010815. The authors declare no conflicts of interest.
Glossary
- 2D
2-dimensional
- 3D
3-dimensional
- Akt
protein kinase B
- Drp1
dynamin-related protein 1
- ECAR
extracellular acidification rate
- FA
focal adhesion
- FAK
focal adhesion kinase
- H2AX
H2A histone family member X
- HIF-1α
hypoxia-inducible factor-1α
- IDH
isocitrate dehydrogenase
- LDH
lactate dehydrogenase
- MK-2206
Akt inhibitor
- MnTBAP
superoxide dismutase (SOD) mimetic
- NGS
normal goat serum
- OCR
oxygen consumption rate
- Prx3
peroxiredoxin 3
- ROS
reactive oxygen species
- shRNA
short hairpin RNA
- siRNA
small interfering RNA
- Succ
succinate
- TCA
tricarboxylic acid cycle
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
Y. Wang and D. C. Altieri conceived the project; Y. Wang performed experiments of ROS production, mitochondrial metabolic reprogramming, tumor cell motility, and invasion and modulation of HIF-1α function; E. Agarwal performed experiments of focal adhesion complex dynamics and metastatic dissemination in vivo; I. Bertolini performed experiments of mitochondrial dynamics; J. C. Ghosh performed experiments of mitochondrial bioenergetics, and J. H. Seo performed experiments of Drp1 regulation of mitochondrial trafficking; and Y. Wang and D. C. Altieri analyzed data and wrote the manuscript.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Hanahan D., Weinberg R. A. (2011) Hallmarks of cancer: the next generation. Cell 144, 646–674 [DOI] [PubMed] [Google Scholar]
- 2.Ward P. S., Thompson C. B. (2012) Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell 21, 297–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gatenby R. A., Gillies R. J. (2004) Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer 4, 891–899 [DOI] [PubMed] [Google Scholar]
- 4.Lehuédé C., Dupuy F., Rabinovitch R., Jones R. G., Siegel P. M. (2016) Metabolic plasticity as a determinant of tumor growth and metastasis. Cancer Res. 76, 5201–5208 [DOI] [PubMed] [Google Scholar]
- 5.Tabassum D. P., Polyak K. (2015) Tumorigenesis: it takes a village. Nat. Rev. Cancer 15, 473–483 [DOI] [PubMed] [Google Scholar]
- 6.Vyas S., Zaganjor E., Haigis M. C. (2016) Mitochondria and cancer. Cell 166, 555–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gottlieb E., Tomlinson I. P. (2005) Mitochondrial tumour suppressors: a genetic and biochemical update. Nat. Rev. Cancer 5, 857–866 [DOI] [PubMed] [Google Scholar]
- 8.Anderson R. G., Ghiraldeli L. P., Pardee T. S. (2018) Mitochondria in cancer metabolism, an organelle whose time has come? Biochim. Biophys. Acta Rev. Cancer 1870, 96–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.LeBleu V. S., O’Connell J. T., Gonzalez Herrera K. N., Wikman H., Pantel K., Haigis M. C., de Carvalho F. M., Damascena A., Domingos Chinen L. T., Rocha R. M., Asara J. M., Kalluri R. (2014) PGC-1α mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat. Cell Biol. 16, 992–1003, 1–15; erratum: 1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Porporato P. E., Payen V. L., Pérez-Escuredo J., De Saedeleer C. J., Danhier P., Copetti T., Dhup S., Tardy M., Vazeille T., Bouzin C., Feron O., Michiels C., Gallez B., Sonveaux P. (2014) A mitochondrial switch promotes tumor metastasis. Cell Rep. 8, 754–766 [DOI] [PubMed] [Google Scholar]
- 11.Caino M. C., Seo J. H., Aguinaldo A., Wait E., Bryant K. G., Kossenkov A. V., Hayden J. E., Vaira V., Morotti A., Ferrero S., Bosari S., Gabrilovich D. I., Languino L. R., Cohen A. R., Altieri D. C. (2016) A neuronal network of mitochondrial dynamics regulates metastasis. Nat. Commun. 7, 13730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Senft D., Ronai Z. A. (2016) Regulators of mitochondrial dynamics in cancer. Curr. Opin. Cell Biol. 39, 43–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiang S., Weigelt B., Wen H. C., Pareja F., Raghavendra A., Martelotto L. G., Burke K. A., Basili T., Li A., Geyer F. C., Piscuoglio S., Ng C. K., Jungbluth A. A., Balss J., Pusch S., Baker G. M., Cole K. S., von Deimling A., Batten J. M., Marotti J. D., Soh H. C., McCalip B. L., Serrano J., Lim R. S., Siziopikou K. P., Lu S., Liu X., Hammour T., Brogi E., Snuderl M., Iafrate A. J., Reis-Filho J. S., Schnitt S. J. (2016) IDH2 mutations define a unique subtype of breast cancer with altered nuclear polarity. Cancer Res. 76, 7118–7129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waitkus M. S., Diplas B. H., Yan H. (2018) Biological role and therapeutic potential of IDH mutations in cancer. Cancer Cell 34, 186–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.M Gagné L., Boulay K., Topisirovic I., Huot M. É., Mallette F. A. (2017) Oncogenic activities of IDH1/2 mutations: from epigenetics to cellular signaling. Trends Cell Biol. 27, 738–752 [DOI] [PubMed] [Google Scholar]
- 16.Amaya M. L., Pollyea D. A. (2018) Targeting the IDH2 pathway in acute myeloid leukemia. Clin. Cancer Res. 24, 4931–4936 [DOI] [PubMed] [Google Scholar]
- 17.Kim S., Kim S. Y., Ku H. J., Jeon Y. H., Lee H. W., Lee J., Kwon T. K., Park K. M., Park J. W. (2014) Suppression of tumorigenesis in mitochondrial NADP(+)-dependent isocitrate dehydrogenase knock-out mice. Biochim. Biophys. Acta 1842, 135–143 [DOI] [PubMed] [Google Scholar]
- 18.Li J., He Y., Tan Z., Lu J., Li L., Song X., Shi F., Xie L., You S., Luo X., Li N., Li Y., Liu X., Tang M., Weng X., Yi W., Fan J., Zhou J., Qiang G., Qiu S., Wu W., Bode A. M., Cao Y. (2018) Wild-type IDH2 promotes the Warburg effect and tumor growth through HIF1α in lung cancer. Theranostics 8, 4050–4061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zou X., Zhu Y., Park S. H., Liu G., O’Brien J., Jiang H., Gius D. (2017) SIRT3-mediated dimerization of IDH2 directs cancer cell metabolism and tumor growth. Cancer Res. 77, 3990–3999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lian C. G., Xu Y., Ceol C., Wu F., Larson A., Dresser K., Xu W., Tan L., Hu Y., Zhan Q., Lee C. W., Hu D., Lian B. Q., Kleffel S., Yang Y., Neiswender J., Khorasani A. J., Fang R., Lezcano C., Duncan L. M., Scolyer R. A., Thompson J. F., Kakavand H., Houvras Y., Zon L. I., Mihm M. C., Jr., Kaiser U. B., Schatton T., Woda B. A., Murphy G. F., Shi Y. G. (2012) Loss of 5-hydroxymethylcytosine is an epigenetic hallmark of melanoma. Cell 150, 1135–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weinberg F., Hamanaka R., Wheaton W. W., Weinberg S., Joseph J., Lopez M., Kalyanaraman B., Mutlu G. M., Budinger G. R., Chandel N. S. (2010) Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc. Natl. Acad. Sci. USA 107, 8788–8793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jo S. H., Son M. K., Koh H. J., Lee S. M., Song I. H., Kim Y. O., Lee Y. S., Jeong K. S., Kim W. B., Park J. W., Song B. J., Huh T. L. (2001) Control of mitochondrial redox balance and cellular defense against oxidative damage by mitochondrial NADP+-dependent isocitrate dehydrogenase. J. Biol. Chem. 276, 16168–16176; erratum: 26732 [DOI] [PubMed] [Google Scholar]
- 23.Lillig C. H., Lönn M. E., Enoksson M., Fernandes A. P., Holmgren A. (2004) Short interfering RNA-mediated silencing of glutaredoxin 2 increases the sensitivity of HeLa cells toward doxorubicin and phenylarsine oxide. Proc. Natl. Acad. Sci. USA 101, 13227–13232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caino M. C., Chae Y. C., Vaira V., Ferrero S., Nosotti M., Martin N. M., Weeraratna A., O’Connell M., Jernigan D., Fatatis A., Languino L. R., Bosari S., Altieri D. C. (2013) Metabolic stress regulates cytoskeletal dynamics and metastasis of cancer cells. J. Clin. Invest. 123, 2907–2920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seo J. H., Agarwal E., Bryant K. G., Caino M. C., Kim E. T., Kossenkov A. V., Tang H. Y., Languino L. R., Gabrilovich D. I., Cohen A. R., Speicher D. W., Altieri D. C. (2018) Syntaphilin ubiquitination regulates mitochondrial dynamics and tumor cell movements. Cancer Res. 78, 4215–4228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roussos E. T., Condeelis J. S., Patsialou A. (2011) Chemotaxis in cancer. Nat. Rev. Cancer 11, 573–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu X., Liu Y., Qin C., Pan Z., Luo J., Yu A., Cheng Z. (2014) Up-regulated isocitrate dehydrogenase 1 suppresses proliferation, migration and invasion in osteosarcoma: in vitro and in vivo. Cancer Lett. 346, 114–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu D. (2016) Isocitrate dehydrogenase 2 inhibits gastric cancer cell invasion via matrix metalloproteinase 7. Tumour Biol. 37, 5225–5230 [DOI] [PubMed] [Google Scholar]
- 29.Kashatus J. A., Nascimento A., Myers L. J., Sher A., Byrne F. L., Hoehn K. L., Counter C. M., Kashatus D. F. (2015) Erk2 phosphorylation of Drp1 promotes mitochondrial fission and MAPK-driven tumor growth. Mol. Cell 57, 537–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Serasinghe M. N., Wieder S. Y., Renault T. T., Elkholi R., Asciolla J. J., Yao J. L., Jabado O., Hoehn K., Kageyama Y., Sesaki H., Chipuk J. E. (2015) Mitochondrial division is requisite to RAS-induced transformation and targeted by oncogenic MAPK pathway inhibitors. Mol. Cell 57, 521–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qian W., Choi S., Gibson G. A., Watkins S. C., Bakkenist C. J., Van Houten B. (2012) Mitochondrial hyperfusion induced by loss of the fission protein Drp1 causes ATM-dependent G2/M arrest and aneuploidy through DNA replication stress. J. Cell Sci. 125, 5745–5757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie Q., Wu Q., Horbinski C. M., Flavahan W. A., Yang K., Zhou W., Dombrowski S. M., Huang Z., Fang X., Shi Y., Ferguson A. N., Kashatus D. F., Bao S., Rich J. N. (2015) Mitochondrial control by DRP1 in brain tumor initiating cells. Nat. Neurosci. 18, 501–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen H., Chan D. C. (2017) Mitochondrial dynamics in regulating the unique phenotypes of cancer and stem cells. Cell Metab. 26, 39–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao J., Zhang J., Yu M., Xie Y., Huang Y., Wolff D. W., Abel P. W., Tu Y. (2013) Mitochondrial dynamics regulates migration and invasion of breast cancer cells. Oncogene 32, 4814–4824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caino M. C., Ghosh J. C., Chae Y. C., Vaira V., Rivadeneira D. B., Faversani A., Rampini P., Kossenkov A. V., Aird K. M., Zhang R., Webster M. R., Weeraratna A. T., Bosari S., Languino L. R., Altieri D. C. (2015) PI3K therapy reprograms mitochondrial trafficking to fuel tumor cell invasion. Proc. Natl. Acad. Sci. USA 112, 8638–8643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cunniff B., McKenzie A. J., Heintz N. H., Howe A. K. (2016) AMPK activity regulates trafficking of mitochondria to the leading edge during cell migration and matrix invasion. Mol. Biol. Cell 27, 2662–2674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Desai S. P., Bhatia S. N., Toner M., Irimia D. (2013) Mitochondrial localization and the persistent migration of epithelial cancer cells. Biophys. J. 104, 2077–2088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ohno N., Chiang H., Mahad D. J., Kidd G. J., Liu L., Ransohoff R. M., Sheng Z. H., Komuro H., Trapp B. D. (2014) Mitochondrial immobilization mediated by syntaphilin facilitates survival of demyelinated axons. Proc. Natl. Acad. Sci. USA 111, 9953–9958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou L., Wang F., Sun R., Chen X., Zhang M., Xu Q., Wang Y., Wang S., Xiong Y., Guan K. L., Yang P., Yu H., Ye D. (2016) SIRT5 promotes IDH2 desuccinylation and G6PD deglutarylation to enhance cellular antioxidant defense. EMBO Rep. 17, 811–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sabharwal S. S., Schumacker P. T. (2014) Mitochondrial ROS in cancer: initiators, amplifiers or an Achilles’ heel? Nat. Rev. Cancer 14, 709–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gorrini C., Harris I. S., Mak T. W. (2013) Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 12, 931–947 [DOI] [PubMed] [Google Scholar]
- 42.Piskounova E., Agathocleous M., Murphy M. M., Hu Z., Huddlestun S. E., Zhao Z., Leitch A. M., Johnson T. M., DeBerardinis R. J., Morrison S. J. (2015) Oxidative stress inhibits distant metastasis by human melanoma cells. Nature 527, 186–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hurd T. R., DeGennaro M., Lehmann R. (2012) Redox regulation of cell migration and adhesion. Trends Cell Biol. 22, 107–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Radisky D. C., Levy D. D., Littlepage L. E., Liu H., Nelson C. M., Fata J. E., Leake D., Godden E. L., Albertson D. G., Nieto M. A., Werb Z., Bissell M. J. (2005) Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature 436, 123–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bhat T. A., Kumar S., Chaudhary A. K., Yadav N., Chandra D. (2015) Restoration of mitochondria function as a target for cancer therapy. Drug Discov. Today 20, 635–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cancer Genome Atlas Research Network (2013) Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature 499, 43–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keith B., Johnson R. S., Simon M. C. (2011) HIF1α and HIF2α: sibling rivalry in hypoxic tumour growth and progression. Nat. Rev. Cancer 12, 9–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fukuda R., Zhang H., Kim J. W., Shimoda L., Dang C. V., Semenza G. L. (2007) HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell 129, 111–122 [DOI] [PubMed] [Google Scholar]
- 49.Papandreou I., Cairns R. A., Fontana L., Lim A. L., Denko N. C. (2006) HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 3, 187–197 [DOI] [PubMed] [Google Scholar]
- 50.Hoekstra A. S., Bayley J. P. (2013) The role of complex II in disease. Biochim. Biophys. Acta 1827, 543–551 [DOI] [PubMed] [Google Scholar]
- 51.Semenza G. L. (2016) The hypoxic tumor microenvironment: a driving force for breast cancer progression. Biochim. Biophys. Acta 1863, 382–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang M. H., Wu M. Z., Chiou S. H., Chen P. M., Chang S. Y., Liu C. J., Teng S. C., Wu K. J. (2008) Direct regulation of TWIST by HIF-1alpha promotes metastasis. Nat. Cell Biol. 10, 295–305 [DOI] [PubMed] [Google Scholar]
- 53.Rankin E. B., Fuh K. C., Castellini L., Viswanathan K., Finger E. C., Diep A. N., LaGory E. L., Kariolis M. S., Chan A., Lindgren D., Axelson H., Miao Y. R., Krieg A. J., Giaccia A. J. (2014) Direct regulation of GAS6/AXL signaling by HIF promotes renal metastasis through SRC and MET. Proc. Natl. Acad. Sci. USA 111, 13373–13378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sahlgren C., Gustafsson M. V., Jin S., Poellinger L., Lendahl U. (2008) Notch signaling mediates hypoxia-induced tumor cell migration and invasion. Proc. Natl. Acad. Sci. USA 105, 6392–6397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cox T. R., Rumney R. M. H., Schoof E. M., Perryman L., Høye A. M., Agrawal A., Bird D., Latif N. A., Forrest H., Evans H. R., Huggins I. D., Lang G., Linding R., Gartland A., Erler J. T. (2015) The hypoxic cancer secretome induces pre-metastatic bone lesions through lysyl oxidase. Nature 522, 106–110 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 56.Pienta K. J., Robertson B. A., Coffey D. S., Taichman R. S. (2013) The cancer diaspora: metastasis beyond the seed and soil hypothesis. Clin. Cancer Res. 19, 5849–5855 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.