Abstract
Adiponectin is secreted by adipose tissue and promotes insulin sensitivity. Low circulating adiponectin is associated with increased risk for preterm labor, but the influence of adiponectin on uterine myometrial physiology is unknown. We hypothesized that adiponectin receptors (AdipoRs) decrease myometrial contractility via AMPK to promote uterine quiescence in pregnancy. Using quantitative RT-PCR, we found that nonpregnant or pregnant human and mouse myometrium express AdipoR1 and AdipoR2 mRNAs. We confirmed AdipoR2 protein expression in human and mouse myometrium, with increased abundance in late mouse pregnancy. Both recombinant adiponectin and a pharmacologic AdipoR agonist, AdipoRon, potently inhibited uterine myometrial strip contractions in physiologic organ bath. The relaxation was independent of contractile stimulus (oxytocin, KCl, U46619). AdipoR agonists increased AMPK phosphorylation in pregnant mouse myometrium, and the direct AMPK activator A769662 also relaxed myometrial strips. However, the AMPK inhibitor dorsomorphin (compound C) blocked AMPK phosphorylation but did not abolish relaxation with either AdipoRon or A769662. In summary, adiponectin inhibits myometrial contractility consistent with the possibility that it is a previously unrecognized link between maternal metabolism and pregnancy maintenance. We also identify a separate role for AMPK regulating myometrial contractions that may influence labor onset.—Vyas, V., Guerra, D. D., Bok, R., Powell, T., Jansson, T., Hurt, K. J. Adiponectin links maternal metabolism to uterine contractility.
Keywords: adipoQ, myometrium, obstetric, oxytocin, parturition
Preterm birth (PTB) is a serious public health problem, accounting for 10–12% of all live births in the United States (1), with societal costs of more than $26 billion per year (2). Identifying quiescence mechanisms that suppress labor to prevent preterm parturition might lead to novel therapies to decrease PTB and the complications of prematurity. A retrospective study detected an association between PTB and lower maternal total and high-MW adiponectin compared with term delivery (3); however, that clinical study design could not identify physiologic mechanisms. Adiponectin is the most abundant hormone released by adipocytes (4), with wide-ranging effects on energy homeostasis (5), inflammation (6), and vascular tone (7). Circulating adiponectin is inversely correlated with body mass index (BMI) (8, 9), and decreased high-MW adiponectin is associated with insulin resistance (10). Plasma membrane adiponectin receptor (AdipoR) 1 and AdipoR2 mediate the cellular effects of adiponectin (11, 12). Although AdipoR signaling is complex (13), a major pathway in many cell types involves AMPK (14, 15). Because some hormones that regulate vascular tone also influence uterine smooth muscle, we explored whether adiponectin influences uterine contractility.
In pregnancy, circulating adiponectin decreases both with increasing BMI (9, 16, 17) and with advancing gestational age (8, 18). In contrast to its insulin-sensitizing effect in skeletal muscle and liver, adiponectin inhibits insulin signaling in the placenta to reduce amino acid transport (19, 20) and limit fetal growth (21). The decrease in maternal circulating adiponectin across gestation is consistent with the maternal weight gain and rapid fetal growth that occur in late gestation. Low adiponectin in pregnancy is associated with gestational diabetes (10), fetal macrosomia (22), and preeclampsia (23, 24). AdipoRs are expressed in the early pregnant uterus and decidua to facilitate implantation (25), but the expression and function of uterine AdipoRs later in pregnancy are not known. Mechanisms linking adiponectin with birth timing have not been reported.
We hypothesized that AdipoRs decrease myometrial contractility via AMPK signaling to promote uterine quiescence in pregnancy. We demonstrate that AdipoRs are expressed in human and mouse uterine smooth muscle during pregnancy. We show that AdipoR agonists promote uterine relaxation and activate myometrial AMPK in pregnant uterus, although myometrial AMPK and AdipoR may inhibit myometrial contractility by separate and distinct mechanisms. These data suggest previously unknown metabolic mechanisms by which AdipoRs and AMPK could maintain uterine quiescence during pregnancy.
MATERIALS AND METHODS
Reagents
AdipoRon (SML0998), KCl (P9333), oxytocin (O3251), protease inhibitor (SRE0055), phosphatase inhibitor (P5726) and U46619 (D8174) were from MilliporeSigma (Burlington, MA, USA). Purified recombinant mouse adiponectin was obtained from Enzo Life Sciences (ALX-522059-C050; Farmingdale, NY, USA). Dorsomorphin (3093) and A769662 were from R&D Systems (3336; Minneapolis, MN, USA). Antibodies for AMPK phosphorylated at Thr172 (2535S, 1:1000), total AMPK-α (2532S, 1:1000), and c-Myc (9B11, 1:500) were from Cell Signaling Technology (Danvers, MA, USA). AdipoR1 (ab70362 plus ab126611, both at 1:1000) and β-actin antibodies (ab8226, 1:10,000) were from Abcam (Cambridge, United Kingdom), and anti-AdipoR2 (A12777, 1:1000) was from ABclonal (Woburn, MA, USA). Anti–glyceraldehyde 3-phosphate dehydrogenase (GAPDH; GT239, 1:10,000) was from GeneTex (Irvine, CA, USA). The secondary antibody for AMPK blots (7074S, 1:5000) was from Cell Signaling Technology; the IRDye secondary antibodies for other blots (925-32210 or 925-68071, 1:10,000) were from Li-Cor Biosciences (Lincoln, NE, USA). RNA extraction (732-6870) and iScript cDNA synthesis (170-8890) were performed with Bio-Rad kits (Hercules, CA, USA). Taqman Master Mix (4369016) and SuperSignal West Pico Chemiluminescence substrate (34577) were from Thermo Fisher Scientific (Waltham, MA, USA).
Human tissue
All procedures were conducted with informed consent under institutional review board approval (03-1270). We collected uterine tissue from nonpregnant (NP) women undergoing benign hysterectomy or full-thickness biopsy of the upper edge of the hysterotomy incision from term pregnant women after cesarean delivery. Inclusion criteria were female, age 18–55 yr, NP undergoing hysterectomy for benign indications or pregnant >24 wk gestation and delivering by cesarean section. Exclusion criteria were unable to give informed consent (e.g., non-English speaking, developmental delay), HIV positive, hepatitis B or C infection, intrauterine infection, previous pelvic or gynecologic cancer, emergency cesarean section, increased risk of bleeding, or increased clinical risk from participation. Uterine tissue was cleaned, and endometrial and serosal tissue was removed. Uterine myometrium was dissected, snap frozen in liquid nitrogen, and stored at −80°C.
Animals
Animal protocols were approved by the Institutional Animal Care and Use Committee (protocol 00090). Ten-to-fifteen–week-old virgin or timed pregnant C57/BL6 female mice were obtained from Charles River Laboratories (Wilmington, MA, USA). NP females were primed with estradiol (0.1 mg/kg, E2758; MilliporeSigma) for 2 d to synchronize estrus (26). All animals were housed in the research vivarium under a 12-h light/dark cycle with ad libitum access to standard chow and water. Animals were euthanized humanely via CO2 asphyxiation and decapitation.
Measurement of myometrial contractility ex vivo
We rapidly dissected and cleaned the uterine horns from NP or pregnant mice at embryonic or embyonic/gestational day (E)15 or E18. The uterus was opened longitudinally in Krebs buffer on ice and scraped with a clean razor blade to remove endometrium. The myometrium was cut into equal strips and suspended in an organ bath chamber (PL3508B61/C-V; Radnoti, Covina, CA, USA). Strips were equilibrated in Krebs buffer bubbled with 95% O2 and 5% CO2 at 37°C with resting tension adjusted to 1 g. Tissue integrity was confirmed before and after all experiments using depolarizing 40 mM KCl; only strips that contracted both before and after treatments were analyzed. Contractile force was measured in grams of tension over time. Effects of pharmacologic agents such as adiponectin (0.1–10 µg/ml), AdipoRon (0.1–45 μM), and A769662 (0.1–70 µM) were observed in spontaneously contracting or oxytocin-stimulated tissue (2 nM oxytocin for pregnant, 4 nM for NP). Area under the curve (AUC; g · s), peak height, and cyclic frequency were obtained using Powerlab PL3508/P 8/35 (ADInstruments, Dunedin, New Zealand) and analyzed with LabChart software (ADInstruments). All organ bath experiments were repeated using strips from at least 3 different mice per condition. The number of replicates in figure legends denotes the number of individual strips. For Western blots, the treated myometrial strips were flash frozen in liquid nitrogen and stored at −80°C until used.
Characterization of AdipoR1 and AdipoR2 antibodies
AdipoR1 was cloned by RT-PCR from human telomerase reverse transcriptase immortalized uterine myometrial cells (hTERT) human myometrial smooth muscle cells (kind gift of Dr. J. Condon at Wayne State University) (27). AdipoR2 was cloned from a human expression plasmid (kind gift from Dr. L. Dong, University of Texas San Antonio Health Sciences Center San Antonio, TX, USA) (28). We introduced 5′ SalI and 3′ NotI sites by PCR and cloned AdipoR1 and AdipoR2 into pCMV-myc for N-terminal myc-tagged expression in HEK293T cells using TransFectin (Bio-Rad). We screened a large panel of commercial antibodies to identify AdipoR1 or AdipoR2 antibodies with no isoform cross-reactivity using myc-tagged AdipoR proteins.
Protein extraction and immunoblotting
Human or murine myometrium was homogenized in lysis buffer [25 mM Tris-HCl (pH 7.5 at 25°C) + 1 mM EGTA + 1 mM DTT + 0.4% (v/v) Triton X-100 + 1× protease and phosphatase inhibitors] using a Bullet Blender with 0.9–2.0 mm stainless steel beads (Next Advance, Troy, NY, USA). Homogenates were centrifuged for 10 min at 16,000 g, and the supernatant was retained. HEK293T cells were lysed directly. For membrane preparations, tissue was lysed in 250 mM sucrose + 50 mM 3-(N-morpholino)propane sulfonic acid (MOPS) + 2 mM EGTA + 2 mM EDTA + 1× protease inhibitor, pH 7.4, then centrifuged for 10 min at 14,000 g; the supernatant was centrifuged again for 60 min at 100,000 g, and the pellet was resuspended in 10 mM Tris (pH 8.0) + 1% (v/v) Triton X-100 + 150 mM NaCl. Protein concentration was determined with the Pierce BCA protein assay (Thermo Fisher Scientific). Lysates were resolved by 4–20% SDS-PAGE (Bio-Rad), transferred to Immobilon FL (MilliporeSigma), and then blocked with PBS + 5% milk (w/v) before incubating with primary antibody at 4°C overnight (AdipoR1, AdipoR2) or room temperature for 60 min (GAPDH, β-actin). Secondary antibodies were detected with an Odyssey CLx flatbed scanner (Li-Cor Biosciences), quantified with Image Studio 4.0 (Li-Cor Biosciences), and normalized to GAPDH orβ-actin. AMPK and phosphorylated AMPK blots were performed as above except that lysis buffer was 10 mM HEPES-Tris buffer pH 6.95 + 250 mM sucrose + 1× protease-phosphatase inhibitors and visualization was with SuperSignal West Pico reagent. AMPK signals were normalized to amido black total protein stain.
Quantitative RT-PCR
Total RNA was extracted (tissues, Bio-Rad; cells, Qiagen, Germantown, MD, USA), treated with DNase, and assessed for quality by RNA integrity assay; samples with RNA integrity number >7.5 were used. cDNA was synthesized using the iScript cDNA Kit (Bio-Rad). Taqman gene expression assays were performed for AdipoR1 (Taqman Mm01291334_mH-mouse; Hs00360422_m1-human), AdipoR2 (Taqman Mm01184032_m1-mouse; Hs00226105_m1-human), and adiponectin (Taqman Mm00456425_m1-mouse; Hs00605917_m1-human). The reference gene was ribosomal protein L13a (RPL13A; Taqman Mm01612987_g1-mouse; Hs04194366_g1-human). Mouse adipose tissue or pooled untreated hTERT cells were used as calibrator samples. The comparative ΔΔCt method was used for relative gene expression (29).
Statistical analysis
All data are presented as means ± sem or sd, as indicated, with n per condition and P value noted. Statistical analyses were performed in Prism 6 (GraphPad, La Jolla, CA, USA). Data central tendency was assessed by scatterplot and the D’Agostino-Pearson test. Parametric or nonparametric analyses were performed as appropriate with ANOVA or Kruskal-Wallis test. Posttesting was performed with Bonferroni’s multiple comparisons (organ bath experiments) or Dunn’s posttest. Statistical significance was defined at P < 0.05.
RESULTS
Identification of AdipoR1 and AdipoR2 in human and mouse myometrium
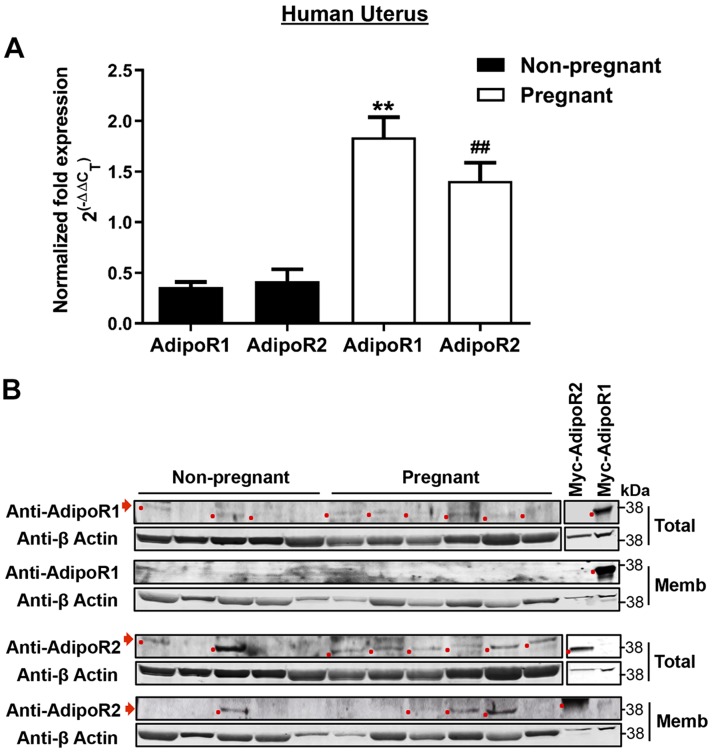
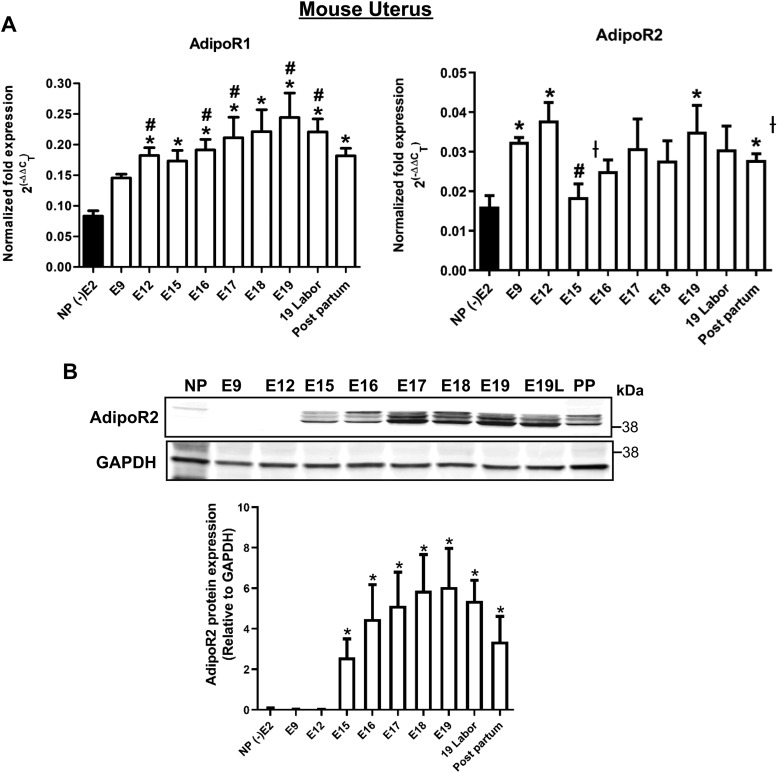
We obtained myometrial samples from NP women undergoing hysterectomy for benign indications and from pregnant women at term delivered by cesarean section. Patient demographics are summarized in Table 1. By quantitative RT-PCR, both AdipoR1 and AdipoR2 isoforms were expressed in human myometrium, with 3–4-fold higher expression in pregnant uterus (Fig. 1A). Adiponectin mRNA was not detected (unpublished results). To confirm protein expression, we validated antibodies for each receptor isoform (Supplemental Fig. S1) and performed Western blots on the same NP and pregnant specimens. A band consistent with AdipoR1 in total myometrial lysate did not enrich in membrane preparations, but we detected AdipoR2 in both total lysate and crude membrane fraction (Fig. 1B). The same experiments using mouse uterine myometrium gave similar results. Myometrial AdipoR1 and AdipoR2 mRNA expression increased in mouse pregnancy, especially in later gestation (Fig. 2A). AdipoR2 protein increased dramatically in late pregnancy. (Fig. 2B) We could not detect adiponectin mRNA (unpublished results) or AdipoR1 protein (Supplemental Fig. S1) in mouse uterus.
TABLE 1.
Patient demographic and clinical characteristics for uterine biopsy specimens
| Characteristic | NP (n = 6) | Pregnant (n = 7) | P |
|---|---|---|---|
| Age [yr (mean ± sd)] | 40.5 ± 4.9 | 28.7 ± 5.8 | 0.01 |
| Race and ethnicity [n (%)] | |||
| White | 2 (33) | 4 (57) | 0.39 |
| Asian | 1 (17) | 0 | |
| African American | 2 (33) | 0 | |
| Hispanic | 1 (17) | 3 (43) | |
| Gravidity, median | 3 | 3 | 0.71 |
| Parity (median) | 3 | 1 | 0.05 |
| Weight [kg (mean ± sd)] | 76.0 ± 16.5 | 81.8 ± 17.3 | 0.84 |
| BMI in kg/m2 at surgery or delivery (mean ± sd) | 29.4 ± 6.1 | 30.4 ± 7.8 | 1.00 |
| Gestational age [wk (mean ± sd)] | — | 39.1 ± 0.6 | — |
| Neonatal birth weight [g (mean ± sd)] | — | 3095.6 ± 352.2 | — |
| Premenopausal [n (%)] | 6 (100) | 7 (100) | — |
| Endometrial cycle stage by pathology report [n (%)] | |||
| Glandular | 1 (17) | — | — |
| Secretory | 3 (50) | — | — |
| Proliferative | 1 (17) | — | — |
| Unknown | 1 (17) | — | — |
| Not in labor [n (%)] | 6 (100) | 7 (100) | — |
| Indication for surgery [n (%)] | |||
| Abnormal uterine bleeding | 6 (100) | — | — |
| Uterine fibroids | 2 (33) | — | — |
| Pelvic pain | 1 (17) | — | — |
| Primary C-section for breech | — | 2 (25) | — |
| Repeat C-section | — | 4 (50) | — |
| Oligohydramnios | — | 1 (13) | — |
| Primary C-section for h/o fourth-degree laceration | — | 1 (13) | — |
Samples from NP women were obtained at benign hysterectomy surgeries. Samples from pregnant women were obtained from lower uterine segment biopsies collected after delivery by scheduled cesarean section. P value by Kruskal-Wallis or χ2 test, as appropriate. Total percentage per category may not be 100 because of rounding. For surgical indication, all diagnoses listed for the procedure are included (total count >N). C-section, cesarean section; h/o, history of.
Figure 1.
AdipoR1 and AdipoR2 are expressed in human uterine smooth muscle. A) mRNA for AdipoR1and AdipoR2 is detected in NP (n = 6) and pregnant (n = 7) human uterus. Data are means ± sem for fold difference in expression of the target gene relative to an adipose tissue calibrator using RPL13A as the reference gene. **P < 0.01 vs. NP AdipoR1, ##P < 0.01 vs. NP AdipoR2. B) AdipoR1 (top panels) and AdipoR2 (bottom panels) protein is detected in NP (n = 5) and pregnant (n = 6) human uterine smooth muscle. Tissue was prepared as total lysate (Total) or as crude membrane fraction (Memb) with β-actin as loading reference. Antibody specificity was confirmed using myc-tagged expression constructs (Supplemental Fig. S2).
Figure 2.
AdipoR1 and AdipoR2 are expressed in mouse uterine smooth muscle. A) mRNA for AdipoR1 and AdipoR2 is detected in NP (n = 4), pregnant (E9–19; n = 5), and laboring or postpartum mouse myometrium (n = 5). Data are means ± sem for fold difference in expression of the target gene relative to an adipose tissue calibrator, using RPL13A as the reference gene. AdipoR1: *P < 0.05 vs. NP, #P < 0.05 vs. E9. AdipoR2: *P < 0.05 vs. NP, #P < 0.05 vs. E9; †P < 0.05 vs. E12. B) Only AdipoR2 protein is detected by Western blot in pregnant (n = 5) but not NP (n = 5) mouse uterus. Quantification is the mean ratio of AdipoR2 to GAPDH. AdipoR1 protein was not detected in mouse uterus. PP, postpartum. *P < 0.05 vs. NP.
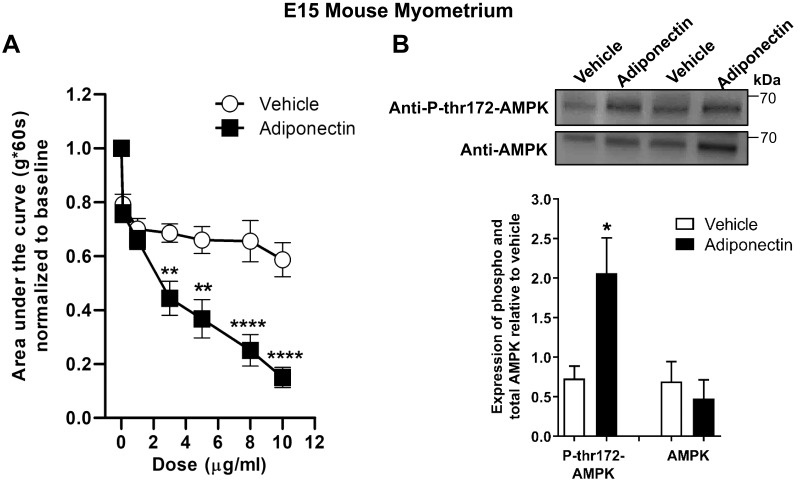
Recombinant adiponectin inhibits oxytocin-stimulated contractions in pregnant mouse uterine strips
We examined the effect of purified recombinant mouse adiponectin on oxytocin-treated E15 mouse uterine strips (E15 of timed pregnancy). Adiponectin significantly reduced contractile force at concentrations similar to reported plasma levels (2–20 µg/ml), mostly because of decreased peak tension (Fig. 3A and Supplemental Fig. S2). Because a major pathway for AdipoR signaling is AMPK activation, we examined AMPK Thr172 phosphorylation in adiponectin-treated uterine strips. AMPK Thr172 phosphorylation is associated with increased AMPK activity. We found 3–4-fold increased phosphorylated AMPK in strips treated with adiponectin, consistent with AdipoR activation (Fig. 3B). This confirms that adiponectin can elicit a rapid physiologic response in mouse uterus and that adiponectin activates AMPK in the myometrium.
Figure 3.
Purified adiponectin at physiologic concentrations relaxes oxytocin-stimulated contractions in E15 pregnant mouse myometrial strips and stimulates AMPK phosphorylation. A) Contractile tension as AUC is shown for oxytocin-stimulated myometrial strips treated with increasing cumulative concentrations of mouse adiponectin (0.1–10 µg/ml; n = 9) or with the equivalent volume of vehicle (n = 11). Representative tracings are shown in Supplemental Fig. S3. **P < 0.01, ****P < 0.0001. B) Representative blot shows duplicate preparations of adiponectin-treated myometrial strips from organ bath, expressing total AMPK (AMPK) and AMPK phosphorylated at Thr172 (P-thr172-AMPK). Strips were treated with 10 µg/ml adiponectin or equivalent volume of vehicle (n = 4–6 strips). *P < 0.05 vs. vehicle P-thr172-AMPK.
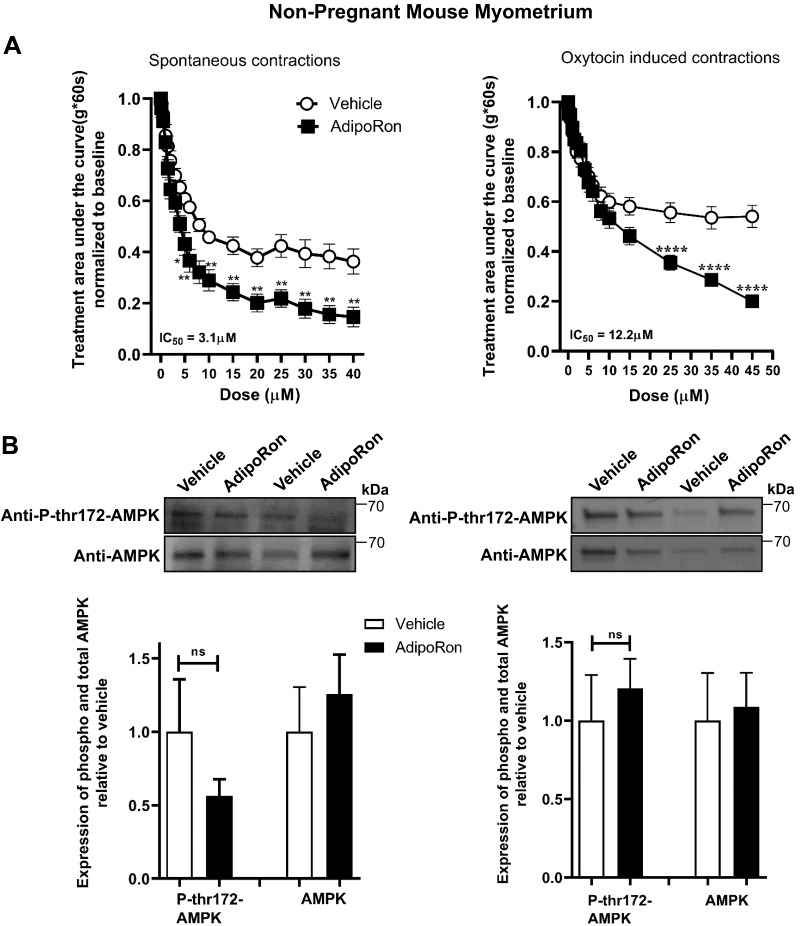
AdipoRon inhibits spontaneous and oxytocin-stimulated contractions in NP mouse uterine strips
To identify an economical pharmacologic approach to stimulate AdipoRs, we tested the AdipoR1 and AdipoR2 agonist AdipoRon (30) on myometrial contractility. For both spontaneous and oxytocin-induced contractions, AdipoRon relaxed uterine strips from NP mice (Fig. 4A and Supplemental Fig. S3); however, it did not alter AMPK phosphorylation (Fig. 4B). To confirm that estrogen priming did not influence AdipoR signaling, we examined NP estrus-phase nonprimed mice. By quantitative RT-PCR, estrogen increased AdipoR1 mRNA, but unprimed estrus-phase myometrium relaxed similarly to estrogen-primed strips (Supplemental Fig. S4). This supports our approach using estrogen-synchronized specimens for NP contractility experiments (26).
Figure 4.
The AdipoR agonist, AdipoRon, decreases myometrial contractility for both spontaneous and oxytocin-stimulated contractions in NP uterine strips. A) Contractile tension as AUC is shown for spontaneously contracting (left; n = 15) and oxytocin-stimulated (right; n = 12) myometrial strips treated with increasing cumulative concentrations of AdipoRon (0.1–45 µM) or the equivalent volume of vehicle. Representative tracings and analysis for peak height and cyclic frequency are shown in Supplemental Fig. S4. *P < 0.05, **P < 0.01, ****P < 0.0001. B) Representative blots show duplicate preparations of AdipoRon-treated myometrial strips from organ bath, expressing total AMPK (AMPK) and AMPK phosphorylated at Thr172 (P-thr172-AMPK). Strips were treated with 45 µM AdipoRon or equivalent volume of vehicle. Spontaneous contractions (n = 7) and oxytocin-induced contractions (n = 4). IC50, half maximal inhibition concentration; ns, not significant.
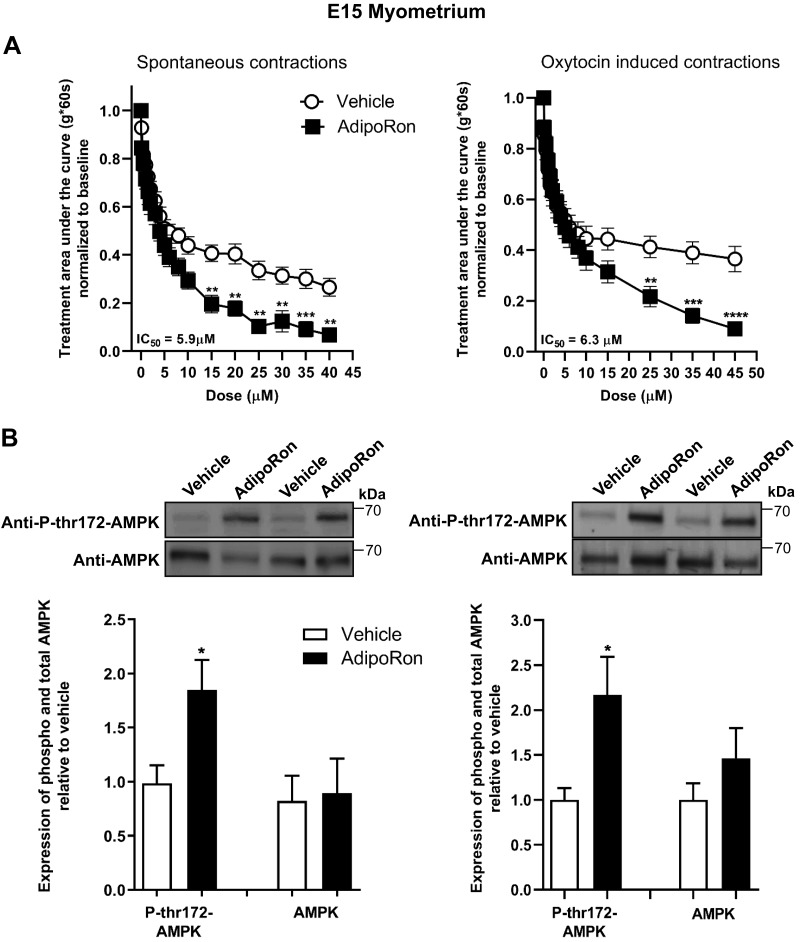
AdipoRon inhibits spontaneous and oxytocin-stimulated contractions in midpregnancy (E15) and late pregnancy (E18) mouse uterine strips
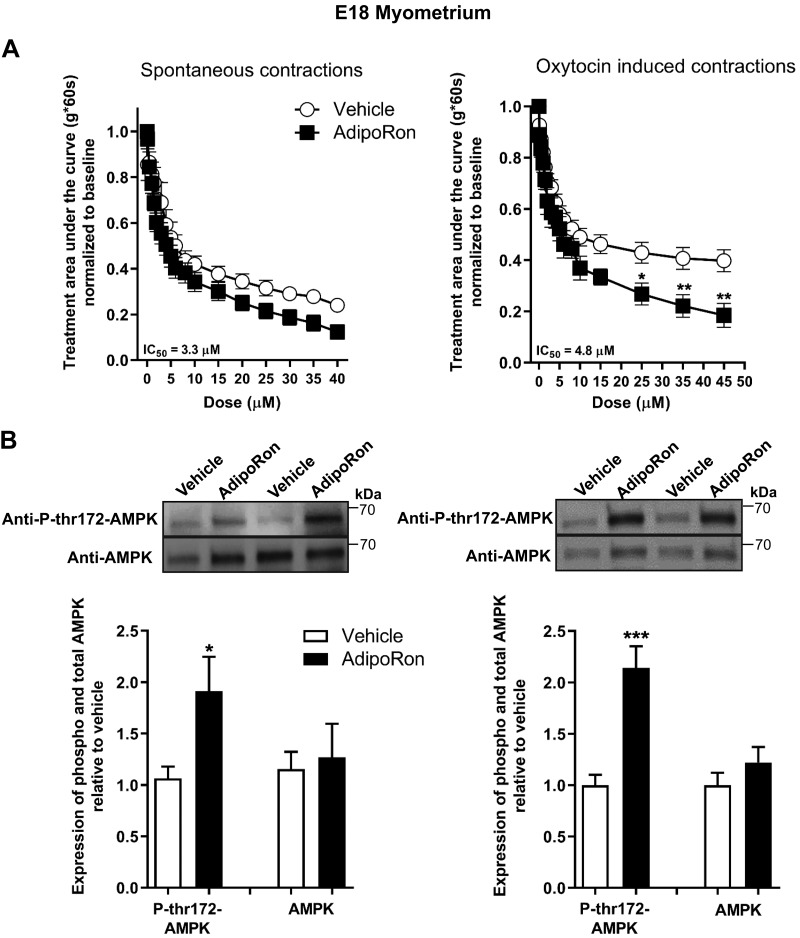
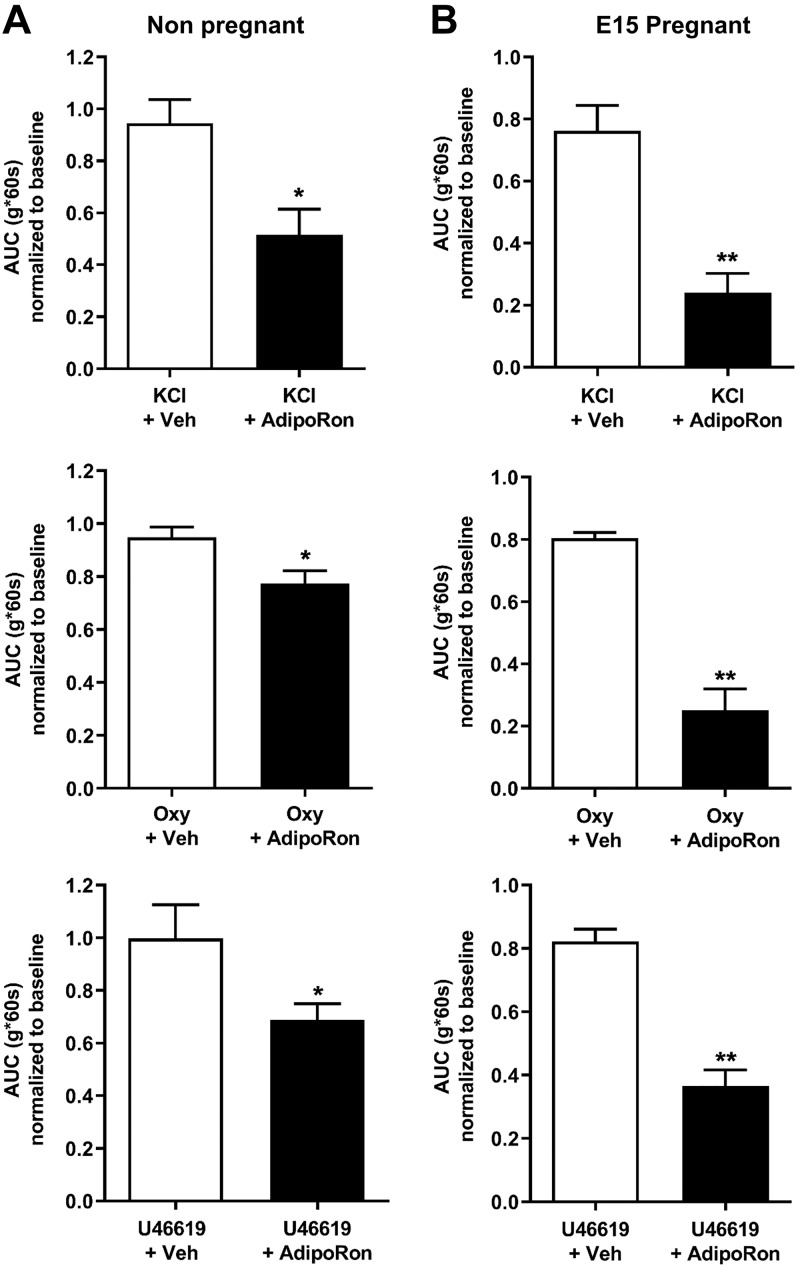
Similar to NP uterus, AdipoRon inhibited uterine strip contractions from midpregnancy (E15; Fig. 5A and Supplemental Fig. S5) with reduced peak tension; cyclic frequency was also reduced for oxytocin-stimulated strips. In late pregnancy (E18; Fig. 6A and Supplemental Fig. S6), AdipoRon only inhibited oxytocin-stimulated and not spontaneous contractions, partly because of the rapid loss of spontaneous contractility in this tissue. Unlike NP myometrial strips, AMPK phosphorylation increased markedly upon AdipoRon treatment of both E15 and E18 strips (Figs. 5B and 6B). We also determined the dependence of AdipoR tocolysis on the contractile stimulus by examining the effect of AdipoRon on uterine tone or contractions stimulated by KCl, oxytocin, or the thromboxane analog U46619. In both NP and pregnant (E15) uterine strips, AdipoRon inhibited contractions generated by all agents, with more pronounced relaxation for pregnant uterine strips (Fig. 7). Together these data show that stimulation of AdipoRs evokes potent tocolysis throughout mouse pregnancy and that uterine relaxation induced by AdipoRon is independent of the initiating contractile stimulus.
Figure 5.
The AdipoR agonist, AdipoRon, decreases myometrial contractility for both spontaneous and oxytocin-induced contractions in E15 uterine strips and activates AMPK. A) Contractile tension as AUC is shown for spontaneously contracting (left; n = 13) and oxytocin-stimulated (n = 12) myometrial strips treated with increasing cumulative concentrations of AdipoRon (0.1–45 µM) or the equivalent volume of vehicle. Representative tracings and analysis for peak height and cyclic frequency are shown in Supplemental Fig. S6. **P < 0.01, ***P < 0.001, ****P < 0.0001. B) Representative blots show duplicate preparations of AdipoRon-treated myometrial strips from organ bath, expressing total AMPK (AMPK) and AMPK phosphorylated at Thr172 (P-thr172-AMPK). Strips were treated with 45 µM AdipoRon or equivalent volume of vehicle. Spontaneous contractions (n = 9–12) and oxytocin-induced contractions (n = 8). IC50, half maximal inhibition concentration. *P < 0.05.
Figure 6.
The AdipoR agonist, AdipoRon, decreases myometrial contractility for oxytocin-induced contractions in E18 uterine strips and activates AMPK. A) Contractile tension as AUC is shown for spontaneously contracting (left; n = 14) and oxytocin-stimulated (n = 12) myometrial strips treated with increasing cumulative concentrations of AdipoRon (0.1–45 µM) or the equivalent volume of vehicle. Representative tracings and analysis for peak height and cyclic frequency are shown in Supplemental Fig. S7. *P < 0.05, **P < 0.01. B) Representative blots show duplicate preparations of AdipoRon-treated myometrial strips from organ bath, expressing total AMPK (AMPK) and AMPK phosphorylated at Thr172 (P-thr172-AMPK). Strips were treated with 45 µM AdipoRon or equivalent volume of vehicle. Spontaneous contractions (n = 12) and oxytocin-induced contractions (n = 6–8). IC50, half maximal inhibition concentration. *P < 0.05, ***P < 0.001.
Figure 7.
AdipoRon relaxes myometrial strips for several contractile stimuli. A) Contractile tension as AUC relative to baseline contractions for NP mouse uterus stimulated with KCl, oxytocin, or U46619, then treated with 1 dose of 25 µM AdipoRon or vehicle (n = 7–10). *P < 0.05. B) Similarly treated E15 pregnant mouse uterine strips demonstrate more marked tocolysis by 25 µM AdipoRon (n = 5–8). Oxy, oxytocin; Veh, vehicle (matched to treatment solvent). **P < 0.01.
AMPK inhibits oxytocin-induced contractions from midpregnancy (E15) and late pregnancy (E18) mouse uterine strips
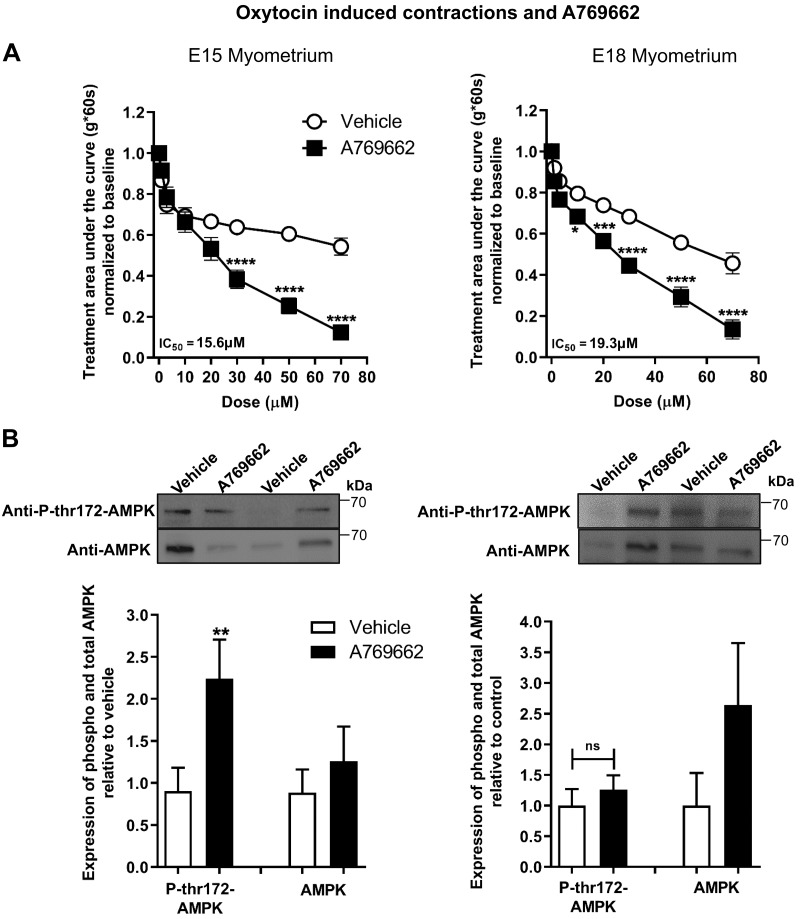
AdipoRon activated AMPK, assessed by AMPK Thr172 phosphorylation, in pregnant uterine strips. Therefore, we determined the effect of pharmacologic AMPK activation on oxytocin-induced myometrial contractions. In both E15 and E18 pregnant myometrial strips, the direct AMPK agonist A769662 significantly reduced the AUC (Fig. 8A). In E15 strips, A769662 also stimulated AMPK Thr172 phosphorylation; however, we observed no change in phosphorylation of AMPK in E18 strips (Fig. 8B).
Figure 8.
The direct AMPK activator, A769662, decreases myometrial contractility for oxytocin-induced contractions in E15 and E18 uterine strips. A) Contractile tension as AUC is shown for oxytocin-stimulated E15 (left; n = 6–7) and E18 (right, n = 4) myometrial strips treated with increasing cumulative concentrations of A769662 (1–70 µM) or the equivalent volume of vehicle. *P < 0.05, ***P < 0.001, ****P < 0.0001. B) Representative blots show duplicate preparations of A769662-treated myometrial strips from organ bath, expressing total AMPK (AMPK) and AMPK phosphorylated at Thr172 (P-thr172-AMPK). Strips were treated with 70 µM A769662 or equivalent volume of vehicle. E15 tissues (n = 6–7) and E18 tissues (n = 4). IC50, half maximal inhibition concentration; ns, not significant. **P < 0.01.
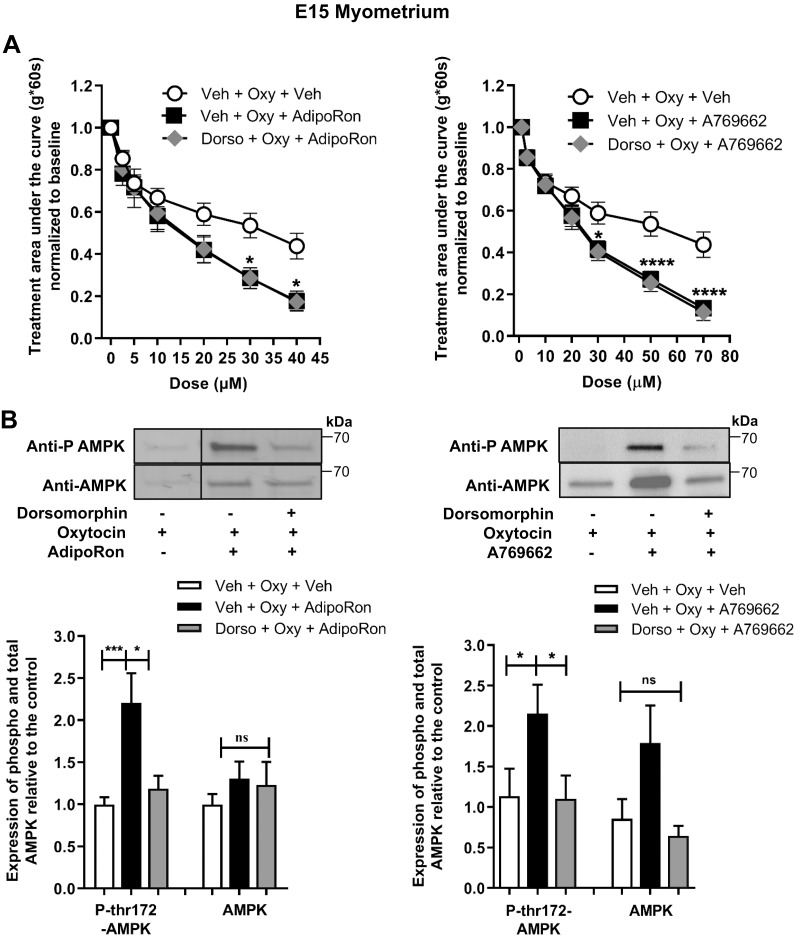
Pretreatment with dorsomorphin increases spontaneous contractile tone but does not affect AdipoRon or A769662 inhibition of oxytocin-stimulated contractions in E15 uterine strips
To test if AMPK mediates AdipoR tocolysis, we performed AdipoRon and A769662 concentration curves in the presence or absence of dorsomorphin (compound C), an inhibitor of AMPK. First, we assessed whether dorsomorphin influences spontaneous E15 myometrial contractile force. During pretreatment, we observed ∼1.8-fold increased AUC with dorsomorphin alone (Supplemental Fig. S7). However, dorsomorphin did not reduce the relaxation of E15 myometrium caused by either AdipoRon or A769662 (Fig. 9A), but dorsomorphin did block AMPK phosphorylation (Fig. 9B).
Figure 9.
AdipoRon and A769662 decrease myometrial contractility for oxytocin-induced contractions in E15 uterine strips independent of AMPK inhibition and phosphorylation. A) Contractile tension as AUC is shown for oxytocin-stimulated E15 myometrial strips treated with increasing cumulative concentrations of AdipoRon (left, 0.1–40 µM, n = 8–10) and A769662 (right, 1–70 µM, n = 7–9) or the equivalent volume of vehicle in the presence or absence of dorsomorphin (50 µM). *P < 0.05, ****P < 0.0001. B) Representative blots show preparations of AdipoRon- and A769662-treated myometrial strips from organ bath, expressing total AMPK (AMPK) and AMPK phosphorylated at thr172 (P AMPK or P-thr172-AMPK). Strips were treated with 40 µM AdipoRon (n = 12–16) and 70 µM A769662 (n = 8–10) or the equivalent volume of vehicle in presence or absence of 50 µM dorsomorphin. Dorso, dorsomorphin; ns, not significant; Oxy, oxytocin; Veh, vehicle. *P < 0.05, ***P < 0.001.
DISCUSSION
We have identified 2 previously unreported mechanisms inhibiting uterine myometrial contractions. First, adiponectin at physiologic concentrations or the AdipoR agonist AdipoRon relaxed uterine strips, suggesting that endogenous adiponectin and uterine AdipoRs could link maternal adiposity and metabolism to the onset of labor. Second, stimulating myometrial AMPK also relaxed myometrial strips, suggesting that local myometrial metabolic sensing (e.g., energy balance, AMP:ATP ratio) may influence labor onset or contraction efficiency. However, AdipoR stimulation of AMPK phosphorylation was not necessary for adiponectin tocolysis, indicating that adiponectin and AMPK may separately inhibit uterine contractions. Our findings imply that optimizing maternal adiponectin, stimulating uterine AdipoR, or increasing uterine AMPK activity might improve pregnancy outcomes by decreasing preterm labor. Furthermore, adiponectin might be a biomarker for PTB risk.
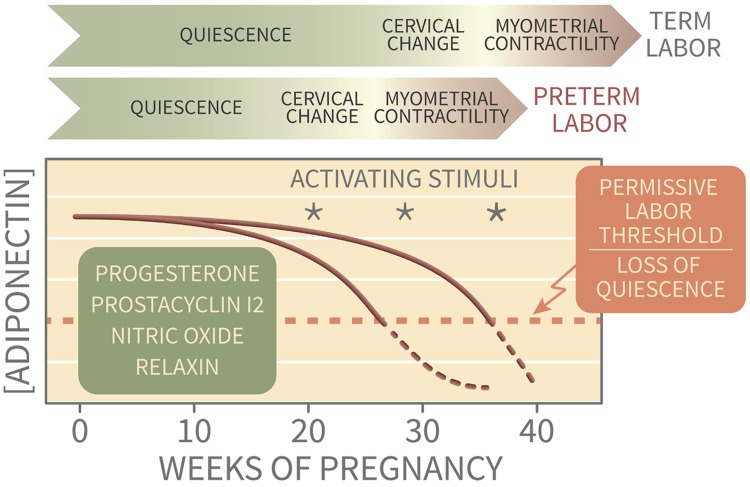
During pregnancy, adiponectin regulates placental nutrient exchange (19–21), and low maternal adiponectin may explain fetal overgrowth in maternal obesity. In the uterus, AdipoRs are expressed both in NP endometrium (31, 32) and in early pregnancy decidua (25). Our findings add adiponectin to a roster of endogenous uterine quiescence factors (e.g., progesterone, relaxin) that maintain pregnancy by counteracting procontractile factors such as distension, infection, hemorrhage, and rupture of membranes (33). The uterus may integrate the strength of initiating factors and the total opposing influence of numerous quiescence factors to determine progression to labor and delivery (Fig. 10). Thus, adiponectin may play a dual role in optimizing pregnancy through both placental and uterine signals. The effect of adiponectin on the trophoblast and the placenta is mediated by peroxisome proliferator–activated receptor-α (PPAR-α) (34). However, the rapid myometrial relaxation we observed in response to AdipoR activation is more consistent with a kinase signaling cascade rather than PPAR-α transcriptional regulation. Most adiponectin studies examine signaling or gene expression over hours to days (35). Importantly, our results do not preclude sustained changes in addition to acute tocolysis that might influence labor onset or efficiency. Those aspects require further investigation.
Figure 10.
Conceptual model of adiponectin as a metabolic quiescence factor. Risk for labor, with uterine contractions and cervical change, may be determined by the balance of multiple quiescence factors during early pregnancy and intermittent activating stimuli throughout gestation. As pregnancy progresses and maternal weight gain and obesity increase, adiponectin levels fall, thereby removing a progestational factor and increasing susceptibility to myometrial activation. In normal parturition, the quiescence and activating factors are optimized for delivery at term.
AMPK is a major AdipoR signal transducer and has been proposed to regulate smooth muscle contraction via myosin light chain kinase desensitization (36), altered NO signaling (37), potassium channel activation (38, 39), and decreased calcium entry or release (38). We were unable to detect protein expression of AdipoR1 that is typically associated with AMPK activation, but AMPK was significantly activated by adiponectin in pregnant myometrium. Although initial studies suggested that AdipoR1 signals via AMPK and AdipoR2 stimulates PPAR-α, recent work shows overlap between AdipoR1 and AdipoR2 pathways (12, 40). There are also descriptions of tissue-specific AdipoR signaling (13). Activation of both AdipoR and AMPK-α1 has been reported to relax vascular smooth muscle (41, 42). However, in our study, the lack of inhibition by dorsomorphin in E15 myometrium and the relaxation of both spontaneous and oxytocin-induced contractions without concomitant AMPK phosphorylation in NP myometrium indicate that AdipoR myometrial effects are unlikely to occur through AMPK. AdipoR1 protein was not detected in the NP myometrium, but AdipoRon caused uterine relaxation. It is possible, therefore, that AdipoR1 expression is below the threshold for antibody detection or that off-target effects of AdipoRon are involved. Experiments using selective genetic knockout mice could address this. Simultaneous AMPK-dependent and AMPK-independent AdipoR signaling has been reported (43), and AdipoR can influence other receptor signaling pathways (44) that cause myometrial relaxation. Unfortunately, dorsomorphin is the only available AMPK inhibitor, and although widely employed its selectivity is not fully confirmed (45). In our experiments dorsomorphin markedly inhibited AMPK phosphorylation yet failed to reverse the relaxation caused by AdipoRon or A769662. We cannot exclude that myometrial AdipoRs regulate uterine contractions through AMPK mechanisms that are independent of AMPK phosphorylation. Phosphorylation-independent allosteric AMPK activation has been described (46–48), but not specifically for AdipoR signaling. Our data show that AMPK activation can be sufficient for myometrial relaxation. We speculate that AMPK could be activated by episodic elevated AMP:ATP during hypoxic-ischemic labor contractions to enhance relaxation and ATP recovery. Uterine AMPK may similarly participate in uterine preconditioning such as during hypoxia-induced force increase (49).
Our findings imply that adiponectin could mediate adipose-uterus crosstalk, linking maternal metabolism to parturition onset. Obese pregnant women typically have low circulating adiponectin levels, which may contribute to altered birth timing or dysfunctional labor. Obesity is associated with medically indicated and spontaneous PTB (50–52) and also influences pregnancy length, causing increased postterm induction and slow labor progress (53). Obese parturients demonstrate impaired myometrial contractility (54, 55) and increased risk for unplanned cesarean delivery because of labor dystocia (53). Racial and ethnic differences in maternal adipokines have been identified (56), but their effect on PTB disparities is unknown. Although underweight women (BMI <18.5 kg/m2) with elevated adiponectin also have increased risk for spontaneous PTB (57), the mechanisms may be different. Either low or high maternal adiponectin might dysregulate uterine contractility. Also, the combined effects of other adipokines with uterine AdipoR expression and adiponectin stimulation are unknown.
Adiponectin is just one adipokine that alters uterine contractions (53). Maternal circulating leptin, secreted by both adipose tissue and the placenta, increases with BMI and gestational age (58) and is associated with PTB risk (59) and labor duration (60). In addition, leptin inhibits myometrial contractions ex vivo (61, 62). Whether leptin resistance occurs in the uterus is unknown, as are the interactions between myometrial adiponectin and leptin pathways (63). Changes in visfatin and apelin during pregnancy (secreted by adipose tissue and the placenta) are variable (64–66), whereas ghrelin is expressed in the uterine myometrium and decreases during labor (67). Visfatin, apelin, and ghrelin all inhibit uterine contractions ex vivo (62, 68–70), and visfatin has been associated with preterm labor (71). The effect of other adipokines on myometrium, such as omentin (72) and resistin (73), have not been reported. The unique contribution of adipose procontractile inflammation mediators (e.g., TNF-α and IL-6) on uterine contractions is also not established (74, 75).
In summary, we demonstrate that adiponectin and AMPK can independently inhibit myometrial contractility ex vivo, findings that call for in vivo study of these pathways in regulating gestational length and suppressing preterm labor. Adiponectin and AMPK may be gentle brakes on myometrial contractility that, together with other quiescence signals, oppose activating factors and reduce the risk of preterm parturition. Adiponectin likely plays a role throughout normal pregnancy, with decreasing quiescence effects near term in women with adequate nutrition and acquired energy stores to support offspring success (76). These evolutionarily conserved energy sensing mechanisms, originally tuned to optimize birth timing in a favorable environment, may be dysregulated by the high-calorie modern Western diet and elevated baseline adiposity, thus contributing to obstetric complications, dysfunctional labor, and prematurity.
ACKNOWLEDGMENTS
The authors acknowledge graphic design support from KimenDesign4Research (https://www.kimendesign4research.com/). This work was supported by U.S. National Institutes of Health (NIH)/Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Grant HD065007 (to T.J. and T.P.), an NIH/NICHD T32 Training Grant HD007186-37 (to D.D.G.), a Society for Maternal-Fetal Medicine/American Association of Obstetricians and Gynecologists (SMFM/AAOGF) Scholar Award (to K.J.H.), and a Ferring Innovation Grant (to K.J.H.). The authors declare no conflicts of interest.
Glossary
- AdipoR
adiponectin receptor
- AUC
area under the curve
- BMI
body mass index
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- NP
nonpregnant
- PPAR-α
peroxisome proliferator–activated receptor-α
- PTB
preterm birth
- RPL13A
ribosomal protein L13a
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
D. D. Guerra, T. Powell, T. Jansson, and K. J. Hurt designed research; V. Vyas, D. D. Guerra, and K. J. Hurt analyzed data; V. Vyas, D. D. Guerra, and R. Bok performed research; and V. Vyas, T. Powell, T. Jansson, and K. J. Hurt wrote the manuscript.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Martin J. A., Hamilton B. E., Osterman M. J. K. (2018) Births in the United States, 2017. NCHS Data Brief (318) 1–8 [PubMed] [Google Scholar]
- 2.Frey H. A., Klebanoff M. A. (2016) The epidemiology, etiology, and costs of preterm birth. Semin. Fetal Neonatal. Med. 21, 68–73 [DOI] [PubMed] [Google Scholar]
- 3.Mazaki-Tovi S., Romero R., Vaisbuch E., Erez O., Mittal P., Chaiworapongsa T., Kim S. K., Pacora P., Yeo L., Gotsch F., Dong Z., Nhan-Chang C. L., Jodicke C., Yoon B. H., Hassan S. S., Kusanovic J. P. (2009) Dysregulation of maternal serum adiponectin in preterm labor. J. Matern. Fetal Neonatal. Med. 22, 887–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scherer P. E., Williams S., Fogliano M., Baldini G., Lodish H. F. (1995) A novel serum protein similar to C1q, produced exclusively in adipocytes. J. Biol. Chem. 270, 26746–26749 [DOI] [PubMed] [Google Scholar]
- 5.Yamauchi T., Kamon J., Minokoshi Y., Ito Y., Waki H., Uchida S., Yamashita S., Noda M., Kita S., Ueki K., Eto K., Akanuma Y., Froguel P., Foufelle F., Ferre P., Carling D., Kimura S., Nagai R., Kahn B. B., Kadowaki T. (2002) Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 8, 1288–1295 [DOI] [PubMed] [Google Scholar]
- 6.Engeli S., Feldpausch M., Gorzelniak K., Hartwig F., Heintze U., Janke J., Möhlig M., Pfeiffer A. F., Luft F. C., Sharma A. M. (2003) Association between adiponectin and mediators of inflammation in obese women. Diabetes 52, 942–947 [DOI] [PubMed] [Google Scholar]
- 7.Fésüs G., Dubrovska G., Gorzelniak K., Kluge R., Huang Y., Luft F. C., Gollasch M. (2007) Adiponectin is a novel humoral vasodilator. Cardiovasc. Res. 75, 719–727 [DOI] [PubMed] [Google Scholar]
- 8.Mazaki-Tovi S., Kanety H., Pariente C., Hemi R., Wiser A., Schiff E., Sivan E. (2007) Maternal serum adiponectin levels during human pregnancy. J. Perinatol. 27, 77–81 [DOI] [PubMed] [Google Scholar]
- 9.Nien J. K., Mazaki-Tovi S., Romero R., Erez O., Kusanovic J. P., Gotsch F., Pineles B. L., Gomez R., Edwin S., Mazor M., Espinoza J., Yoon B. H., Hassan S. S. (2007) Plasma adiponectin concentrations in non-pregnant, normal and overweight pregnant women. J. Perinat. Med. 35, 522–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams M. A., Qiu C., Muy-Rivera M., Vadachkoria S., Song T., Luthy D. A. (2004) Plasma adiponectin concentrations in early pregnancy and subsequent risk of gestational diabetes mellitus. J. Clin. Endocrinol. Metab. 89, 2306–2311 [DOI] [PubMed] [Google Scholar]
- 11.Yamauchi T., Kamon J., Ito Y., Tsuchida A., Yokomizo T., Kita S., Sugiyama T., Miyagishi M., Hara K., Tsunoda M., Murakami K., Ohteki T., Uchida S., Takekawa S., Waki H., Tsuno N. H., Shibata Y., Terauchi Y., Froguel P., Tobe K., Koyasu S., Taira K., Kitamura T., Shimizu T., Nagai R., Kadowaki T. (2003) Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 423, 762–769; erratum: 431, 1123 [DOI] [PubMed] [Google Scholar]
- 12.Yamauchi T., Nio Y., Maki T., Kobayashi M., Takazawa T., Iwabu M., Okada-Iwabu M., Kawamoto S., Kubota N., Kubota T., Ito Y., Kamon J., Tsuchida A., Kumagai K., Kozono H., Hada Y., Ogata H., Tokuyama K., Tsunoda M., Ide T., Murakami K., Awazawa M., Takamoto I., Froguel P., Hara K., Tobe K., Nagai R., Ueki K., Kadowaki T. (2007) Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat. Med. 13, 332–339 [DOI] [PubMed] [Google Scholar]
- 13.Ruan H., Dong L. Q. (2016) Adiponectin signaling and function in insulin target tissues. J. Mol. Cell Biol. 8, 101–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou L., Deepa S. S., Etzler J. C., Ryu J., Mao X., Fang Q., Liu D. D., Torres J. M., Jia W., Lechleiter J. D., Liu F., Dong L. Q. (2009) Adiponectin activates AMP-activated protein kinase in muscle cells via APPL1/LKB1-dependent and phospholipase C/Ca2+/Ca2+/calmodulin-dependent protein kinase kinase-dependent pathways. J. Biol. Chem. 284, 22426–22435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoon M. J., Lee G. Y., Chung J. J., Ahn Y. H., Hong S. H., Kim J. B. (2006) Adiponectin increases fatty acid oxidation in skeletal muscle cells by sequential activation of AMP-activated protein kinase, p38 mitogen-activated protein kinase, and peroxisome proliferator-activated receptor alpha. Diabetes 55, 2562–2570 [DOI] [PubMed] [Google Scholar]
- 16.Haghiac M., Basu S., Presley L., Serre D., Catalano P. M., Hauguel-de Mouzon S. (2014) Patterns of adiponectin expression in term pregnancy: impact of obesity. J. Clin. Endocrinol. Metab. 99, 3427–3434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lekva T., Roland M. C. P., Michelsen A. E., Friis C. M., Aukrust P., Bollerslev J., Henriksen T., Ueland T. (2017) Large reduction in adiponectin during pregnancy is associated with large-for-gestational-age newborns. J. Clin. Endocrinol. Metab. 102, 2552–2559 [DOI] [PubMed] [Google Scholar]
- 18.Combs T. P., Berg A. H., Rajala M. W., Klebanov S., Iyengar P., Jimenez-Chillaron J. C., Patti M. E., Klein S. L., Weinstein R. S., Scherer P. E. (2003) Sexual differentiation, pregnancy, calorie restriction, and aging affect the adipocyte-specific secretory protein adiponectin. Diabetes 52, 268–276 [DOI] [PubMed] [Google Scholar]
- 19.Jones H. N., Jansson T., Powell T. L. (2010) Full-length adiponectin attenuates insulin signaling and inhibits insulin-stimulated amino acid transport in human primary trophoblast cells. Diabetes 59, 1161–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosario F. J., Schumacher M. A., Jiang J., Kanai Y., Powell T. L., Jansson T. (2012) Chronic maternal infusion of full-length adiponectin in pregnant mice down-regulates placental amino acid transporter activity and expression and decreases fetal growth. J. Physiol. 590, 1495–1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aye I. L., Rosario F. J., Powell T. L., Jansson T. (2015) Adiponectin supplementation in pregnant mice prevents the adverse effects of maternal obesity on placental function and fetal growth. Proc. Natl. Acad. Sci. USA 112, 12858–12863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Retnakaran R., Ye C., Hanley A. J., Connelly P. W., Sermer M., Zinman B., Hamilton J. K. (2012) Effect of maternal weight, adipokines, glucose intolerance and lipids on infant birth weight among women without gestational diabetes mellitus. CMAJ 184, 1353–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Masuyama H., Nakatsukasa H., Takamoto N., Hiramatsu Y. (2007) Correlation between soluble endoglin, vascular endothelial growth factor receptor-1, and adipocytokines in preeclampsia. J. Clin. Endocrinol. Metab. 92, 2672–2679 [DOI] [PubMed] [Google Scholar]
- 24.Mazaki-Tovi S., Romero R., Vaisbuch E., Kusanovic J. P., Erez O., Gotsch F., Chaiworapongsa T., Than N. G., Kim S. K., Nhan-Chang C. L., Jodicke C., Pacora P., Yeo L., Dong Z., Yoon B. H., Hassan S. S., Mittal P. (2009) Maternal serum adiponectin multimers in preeclampsia. J. Perinat. Med. 37, 349–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim S. T., Marquard K., Stephens S., Louden E., Allsworth J., Moley K. H. (2011) Adiponectin and adiponectin receptors in the mouse preimplantation embryo and uterus. Hum. Reprod. 26, 82–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buxton I. L., Milton D., Barnett S. D., Tichenor S. D. (2010) Agonist-specific compartmentation of cGMP action in myometrium. J. Pharmacol. Exp. Ther. 335, 256–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Condon J., Yin S., Mayhew B., Word R. A., Wright W. E., Shay J. W., Rainey W. E. (2002) Telomerase immortalization of human myometrial cells. Biol. Reprod. 67, 506–514 [DOI] [PubMed] [Google Scholar]
- 28.Wang C., Xin X., Xiang R., Ramos F. J., Liu M., Lee H. J., Chen H., Mao X., Kikani C. K., Liu F., Dong L. Q. (2009) Yin-Yang regulation of adiponectin signaling by APPL isoforms in muscle cells. J. Biol. Chem. 284, 31608–31615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rao X., Huang X., Zhou Z., Lin X. (2013) An improvement of the 2ˆ(-delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat. Bioinforma. Biomath. 3, 71–85 [PMC free article] [PubMed] [Google Scholar]
- 30.Okada-Iwabu M., Yamauchi T., Iwabu M., Honma T., Hamagami K., Matsuda K., Yamaguchi M., Tanabe H., Kimura-Someya T., Shirouzu M., Ogata H., Tokuyama K., Ueki K., Nagano T., Tanaka A., Yokoyama S., Kadowaki T. (2013) A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. Nature 503, 493–499 [DOI] [PubMed] [Google Scholar]
- 31.Takemura Y., Osuga Y., Yamauchi T., Kobayashi M., Harada M., Hirata T., Morimoto C., Hirota Y., Yoshino O., Koga K., Yano T., Kadowaki T., Taketani Y. (2006) Expression of adiponectin receptors and its possible implication in the human endometrium. Endocrinology 147, 3203–3210 [DOI] [PubMed] [Google Scholar]
- 32.Dos Santos E., Serazin V., Morvan C., Torre A., Wainer R., de Mazancourt P., Dieudonné M. N. (2012) Adiponectin and leptin systems in human endometrium during window of implantation. Fertil. Steril. 97, 771–778.e1 [DOI] [PubMed] [Google Scholar]
- 33.Longo M., Jain V., Vedernikov Y. P., Garfield R. E., Saade G. R. (2003) Effects of recombinant human relaxin on pregnant rat uterine artery and myometrium in vitro. Am. J. Obstet. Gynecol. 188, 1468–1474, discussion 1474–1476 [DOI] [PubMed] [Google Scholar]
- 34.Aye I. L., Gao X., Weintraub S. T., Jansson T., Powell T. L. (2014) Adiponectin inhibits insulin function in primary trophoblasts by PPARα-mediated ceramide synthesis. Mol. Endocrinol. 28, 512–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perrini S., Laviola L., Cignarelli A., Melchiorre M., De Stefano F., Caccioppoli C., Natalicchio A., Orlando M. R., Garruti G., De Fazio M., Catalano G., Memeo V., Giorgino R., Giorgino F. (2008) Fat depot-related differences in gene expression, adiponectin secretion, and insulin action and signalling in human adipocytes differentiated in vitro from precursor stromal cells. Diabetologia 51, 155–164 [DOI] [PubMed] [Google Scholar]
- 36.Horman S., Morel N., Vertommen D., Hussain N., Neumann D., Beauloye C., El Najjar N., Forcet C., Viollet B., Walsh M. P., Hue L., Rider M. H. (2008) AMP-activated protein kinase phosphorylates and desensitizes smooth muscle myosin light chain kinase. J. Biol. Chem. 283, 18505–18512 [DOI] [PubMed] [Google Scholar]
- 37.Chen Z. P., Mitchelhill K. I., Michell B. J., Stapleton D., Rodriguez-Crespo I., Witters L. A., Power D. A., Ortiz de Montellano P. R., Kemp B. E. (1999) AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett. 443, 285–289 [DOI] [PubMed] [Google Scholar]
- 38.Schneider H., Schubert K. M., Blodow S., Kreutz C. P., Erdogmus S., Wiedenmann M., Qiu J., Fey T., Ruth P., Lubomirov L. T., Pfitzer G., Mederos Y Schnitzler M., Hardie D. G., Gudermann T., Pohl U. (2015) AMPK dilates resistance arteries via activation of SERCA and BKCa channels in smooth muscle. Hypertension 66, 108–116 [DOI] [PubMed] [Google Scholar]
- 39.Yoshida H., Bao L., Kefaloyianni E., Taskin E., Okorie U., Hong M., Dhar-Chowdhury P., Kaneko M., Coetzee W. A. (2012) AMP-activated protein kinase connects cellular energy metabolism to KATP channel function. J. Mol. Cell. Cardiol. 52, 410–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ito R., Higa M., Goto A., Aoshima M., Ikuta A., Ohashi K., Yokoyama S., Ohno Y., Egawa T., Miyata H., Goto K. (2018) Activation of adiponectin receptors has negative impact on muscle mass in C2C12 myotubes and fast-type mouse skeletal muscle. PLoS One 13, e0205645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goirand F., Solar M., Athea Y., Viollet B., Mateo P., Fortin D., Leclerc J., Hoerter J., Ventura-Clapier R., Garnier A. (2007) Activation of AMP kinase alpha1 subunit induces aortic vasorelaxation in mice. J. Physiol. 581, 1163–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hong K., Lee S., Li R., Yang Y., Tanner M. A., Wu J., Hill M. A. (2016) Adiponectin receptor agonist, AdipoRon, causes vasorelaxation predominantly via a direct smooth muscle action. Microcirculation 23, 207–220 [DOI] [PubMed] [Google Scholar]
- 43.Wang Y., Liang B., Lau W. B., Du Y., Guo R., Yan Z., Gan L., Yan W., Zhao J., Gao E., Koch W., Ma X. L. (2017) Restoring diabetes-induced autophagic flux arrest in ischemic/reperfused heart by ADIPOR (adiponectin receptor) activation involves both AMPK-dependent and AMPK-independent signaling. Autophagy 13, 1855–1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fang X., Fetros J., Dadson K. E., Xu A., Sweeney G. (2009) Leptin prevents the metabolic effects of adiponectin in L6 myotubes. Diabetologia 52, 2190–2200 [DOI] [PubMed] [Google Scholar]
- 45.Dasgupta B., Seibel W. (2018) Compound C/dorsomorphin: its use and misuse as an AMPK inhibitor. Methods Mol. Biol. 1732, 195–202 [DOI] [PubMed] [Google Scholar]
- 46.Gowans G. J., Hawley S. A., Ross F. A., Hardie D. G. (2013) AMP is a true physiological regulator of AMP-activated protein kinase by both allosteric activation and enhancing net phosphorylation. Cell Metab. 18, 556–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suter M., Riek U., Tuerk R., Schlattner U., Wallimann T., Neumann D. (2006) Dissecting the role of 5′-AMP for allosteric stimulation, activation, and deactivation of AMP-activated protein kinase. J. Biol. Chem. 281, 32207–32216 [DOI] [PubMed] [Google Scholar]
- 48.Scott J. W., Ling N., Issa S. M., Dite T. A., O’Brien M. T., Chen Z. P., Galic S., Langendorf C. G., Steinberg G. R., Kemp B. E., Oakhill J. S. (2014) Small molecule drug A-769662 and AMP synergistically activate naive AMPK independent of upstream kinase signaling. Chem. Biol. 21, 619–627 [DOI] [PubMed] [Google Scholar]
- 49.Alotaibi M., Arrowsmith S., Wray S. (2015) Hypoxia-induced force increase (HIFI) is a novel mechanism underlying the strengthening of labor contractions, produced by hypoxic stresses. Proc. Natl. Acad. Sci. USA 112, 9763–9768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cnattingius S., Villamor E., Johansson S., Edstedt Bonamy A. K., Persson M., Wikström A. K., Granath F. (2013) Maternal obesity and risk of preterm delivery. JAMA 309, 2362–2370 [DOI] [PubMed] [Google Scholar]
- 51.Khatibi A., Brantsaeter A. L., Sengpiel V., Kacerovsky M., Magnus P., Morken N. H., Myhre R., Gunnes N., Jacobsson B. (2012) Prepregnancy maternal body mass index and preterm delivery. Am. J. Obstet. Gynecol. 207, 212.e1–217 [DOI] [PubMed] [Google Scholar]
- 52.Kim S. S., Mendola P., Zhu Y., Hwang B. S., Grantz K. L. (2017) Spontaneous and indicated preterm delivery risk is increased among overweight and obese women without prepregnancy chronic disease. BJOG 124, 1708–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carlson N. S., Hernandez T. L., Hurt K. J. (2015) Parturition dysfunction in obesity: time to target the pathobiology. Reprod. Biol. Endocrinol. 13, 135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arrowsmith S., Wray S., Quenby S. (2011) Maternal obesity and labour complications following induction of labour in prolonged pregnancy. BJOG 118, 578–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Crankshaw D. J., O’Brien Y. M., Crosby D. A., Morrison J. J. (2017) Maternal body mass index and spontaneous contractility of human myometrium in pregnancy. J. Perinatol. 37, 492–497 [DOI] [PubMed] [Google Scholar]
- 56.Chen X., Scholl T. O. (2015) Ethnic differences in maternal adipokines during normal pregnancy. Int. J. Environ. Res. Public Health 13, ijerph13010008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ehrenberg H. M., Iams J. D., Goldenberg R. L., Newman R. B., Weiner S. J., Sibai B. M., Caritis S. N., Miodovnik M., Dombrowski M. P.; Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network (MFMU) (2009) Maternal obesity, uterine activity, and the risk of spontaneous preterm birth. Obstet. Gynecol. 113, 48–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang M. J. (2005) Interrelationships of maternal serum leptin, body mass index and gestational age. J. Chin. Med. Assoc. 68, 452–457 [DOI] [PubMed] [Google Scholar]
- 59.Fakor F., Sharami S. H., Milani F., Mirblouk F., Kazemi S., Pourmarzi D., Ebrahimi H., Heirati S. F. (2016) The association between level of maternal serum leptin in the third trimester and the occurrence of moderate preterm labor. J. Turk. Ger. Gynecol. Assoc. 17, 182–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carlhäll S., Källén K., Thorsell A., Blomberg M. (2018) Maternal plasma leptin levels in relation to the duration of the active phase of labor. Acta Obstet. Gynecol. Scand. 97, 1248–1256 [DOI] [PubMed] [Google Scholar]
- 61.Moynihan A. T., Hehir M. P., Glavey S. V., Smith T. J., Morrison J. J. (2006) Inhibitory effect of leptin on human uterine contractility in vitro. Am. J. Obstet. Gynecol. 195, 504–509 [DOI] [PubMed] [Google Scholar]
- 62.Mumtaz S., AlSaif S., Wray S., Noble K. (2015) Inhibitory effect of visfatin and leptin on human and rat myometrial contractility. Life Sci. 125, 57–62 [DOI] [PubMed] [Google Scholar]
- 63.Cui H., López M., Rahmouni K. (2017) The cellular and molecular bases of leptin and ghrelin resistance in obesity. Nat. Rev. Endocrinol. 13, 338–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Morgan S. A., Bringolf J. B., Seidel E. R. (2008) Visfatin expression is elevated in normal human pregnancy. Peptides 29, 1382–1389 [DOI] [PubMed] [Google Scholar]
- 65.Mazaki-Tovi S., Romero R., Kusanovic J. P., Vaisbuch E., Erez O., Than N. G., Chaiworapongsa T., Nhan-Chang C. L., Pacora P., Gotsch F., Yeo L., Kim S. K., Edwin S. S., Hassan S. S., Mittal P. (2009) Maternal visfatin concentration in normal pregnancy. J. Perinat. Med. 37, 206–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kourtis A., Gkiomisi A., Mouzaki M., Makedou K., Anastasilakis A. D., Toulis K. A., Gerou S., Gavana E., Agorastos T. (2011) Apelin levels in normal pregnancy. Clin. Endocrinol. (Oxf.) 75, 367–371 [DOI] [PubMed] [Google Scholar]
- 67.Fuglsang J., Skjaerbaek C., Espelund U., Frystyk J., Fisker S., Flyvbjerg A., Ovesen P. (2005) Ghrelin and its relationship to growth hormones during normal pregnancy. Clin. Endocrinol. (Oxf.) 62, 554–559 [DOI] [PubMed] [Google Scholar]
- 68.Hehir M. P., Morrison J. J. (2012) The adipokine apelin and human uterine contractility. Am. J. Obstet. Gynecol. 206, 359.e1–359.e5 [DOI] [PubMed] [Google Scholar]
- 69.Mostafa A. F., Samir S. M. (2013) What is the effect of ghrelin on rat uterine contractility in vitro? J. Basic Clin. Physiol. Pharmacol. 24, 137–142 [DOI] [PubMed] [Google Scholar]
- 70.Hehir M. P., Glavey S. V., Morrison J. J. (2008) Uterorelaxant effect of ghrelin on human myometrial contractility. Am. J. Obstet. Gynecol. 198, 323.e1–323.e5 [DOI] [PubMed] [Google Scholar]
- 71.Mazaki-Tovi S., Romero R., Vaisbuch E., Erez O., Chaiworapongsa T., Mittal P., Kim S. K., Pacora P., Gotsch F., Dong Z., Hassan S. S., Kusanovic J. P. (2009) Maternal plasma visfatin in preterm labor. J. Matern. Fetal Neonatal. Med. 22, 693–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Barker G., Lim R., Georgiou H. M., Lappas M. (2012) Omentin-1 is decreased in maternal plasma, placenta and adipose tissue of women with pre-existing obesity. PLoS One 7, e42943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Suto M., Maeda K., Sato M., Kaji T., Irahara M. (2019) Plasma adipokine concentrations in overweight/obese pregnant women: a longitudinal study. Gynecol. Endocrinol. 35, 242–246 [DOI] [PubMed] [Google Scholar]
- 74.Curry A. E., Vogel I., Drews C., Schendel D., Skogstrand K., Flanders W. D., Hougaard D., Olsen J., Thorsen P. (2007) Mid-pregnancy maternal plasma levels of interleukin 2, 6, and 12, tumor necrosis factor-alpha, interferon-gamma, and granulocyte-macrophage colony-stimulating factor and spontaneous preterm delivery. Acta Obstet. Gynecol. Scand. 86, 1103–1110 [DOI] [PubMed] [Google Scholar]
- 75.Robertson S. A., Christiaens I., Dorian C. L., Zaragoza D. B., Care A. S., Banks A. M., Olson D. M. (2010) Interleukin-6 is an essential determinant of on-time parturition in the mouse. Endocrinology 151, 3996–4006 [DOI] [PubMed] [Google Scholar]
- 76.Williams T. C., Drake A. J. (2019) Preterm birth in evolutionary context: a predictive adaptive response? Philos. Trans. R. Soc. Lond. B Biol. Sci. 374, 20180121 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.