Abstract
Pulmonary immunosuppression often occurs after burn injury (BI). However, the reasons for BI-induced pulmonary immunosuppression are not clearly understood. Neutrophil recruitment and neutrophil extracellular trap (NET) formation (NETosis) are important components of a robust pulmonary immune response, and we hypothesized that pulmonary inflammation and NETosis are defective after BI. To test this hypothesis, we established a mouse model with intranasal LPS instillation in the presence or absence of BI (15% of body surface burn) and determined the degree of immune cell infiltration, NETosis, and the cytokine levels in the airways and blood on d 2. Presence of LPS recruited monocytes and large numbers of neutrophils to the airways and induced NETosis (citrullinated histone H3, DNA, myeloperoxidase). By contrast, BI significantly reduced LPS-mediated leukocyte recruitment and NETosis. This BI-induced immunosuppression is attributable to the reduction of chemokine (C-C motif) ligand (CCL) 2 (monocyte chemoattractant protein 1) and CCL3 (macrophage inflammatory protein 1α). BI also suppressed LPS-induced increase in IL-17A, IL-17C, and IL-17E/IL-25 levels in the airways. Therefore, BI-mediated reduction in leukocyte recruitment and NETosis in the lungs are attributable to these cytokines. Regulating the levels of some of these key cytokines represents a potential therapeutic option for mitigating BI-mediated pulmonary immunosuppression.—Sakuma, M., Khan, M. A. S., Yasuhara, S., Martyn, J. A., Palaniyar, N. Mechanism of pulmonary immunosuppression: extrapulmonary burn injury suppresses bacterial endotoxin–induced pulmonary neutrophil recruitment and neutrophil extracellular trap (NET) formation.
Keywords: lung, animal model, cytokines, LPS
Neutrophils are important innate immune cells that are required to maintain immunocompetence of various organs, including lung (1). Burn injury (BI) often causes pulmonary immunosuppression (2, 3), but the importance of neutrophils in BI-induced pulmonary immunosuppression is not clearly established. In our previous studies, we have shown that neutrophils are recruited to the airways upon LPS instillation and subsequently undergo cell death via neutrophil extracellular trap (NET) formation (NETosis) (4, 5). We have also conducted extensive studies on BI using mouse models to understand various immune components (6, 7). Recent studies suggest that T cells [particularly T helper (Th)17 cells] regulate BI-induced changes in immune response (8, 9). In the current study, we tested a hypothesis that neutrophils and NETosis are key components in BI-mediated pulmonary immunosuppression, and BI regulates specific chemokines and cytokines, which leads to the immunosuppression. To test this hypothesis, we combined these 2 mouse models (LPS-mediated pulmonary inflammation/NETosis and third-degree BI), characterized a unique animal model, and determined the specific cytokines responsible for BI-induced pulmonary immunosuppression.
The study of pulmonary immunosuppression is important because devastating BI causes significant morbidity and mortality worldwide. In the United States, 486,000 BI cases occurred in 2015 (10). A large proportion (41%) of BI-related mortality is attributable to pulmonary complications (11, 12). However, the pulmonary complications with thermal injuries are mostly reported as focusing on direct injuries to the lung, such as inhalation of smoke or direct thermal injuries to the lung. Pulmonary complications as a distant effect of body burn injuries are not known. In response to BI, immune cells are mobilized to the site of injury in an attempt to mitigate the tissue damage. Neutrophils are the major innate immune cells present in the circulation (50–70%), and they migrate to the site of injury within minutes. A substantial proportion of the neutrophil population (∼30%) is present as tissue marginalized pool. Because lungs have a large capillary bed, they harbor the major proportion of the marginalized pool (13, 14). Therefore, during BI, neutrophils would migrate into different sites and organs depending on specific cytokine response. Patients with BI often experience immunosuppression, and commensal microbes or their endotoxin could become pathogenic and cause infection and inflammation in various organs and cause sepsis. Gram-negative bacterial cell wall component, LPS, is a typical immunomodulatory molecule that causes inflammation and neutrophil recruitment (15, 16). BI-induced immunosuppression is not generic (17), and why the pulmonary immune system is dysfunctional during BI is not clearly established.
In response to LPS, airway cells secrete multiple cytokines with some functional overlap: these cytokines include inflammatory cytokines (e.g., IL-1β, IL-6, TNF-α, VEGF-A), monocyte chemokines [e.g., chemokine (C-C motif) ligand (CCL) 2 (monocyte chemoattractant protein [MCP]-1) and CCL4 (macrophage inflammatory protein [MIP]-1β)], neutrophil chemokine [e.g., CCL3 (MIP-1α), chemokine (C-X-C motif) ligand (CXCL) 1 (keratinocyte chemoattractant [KC]; IL-8 in humans), CXCL2 (MIP-2)], leukocyte cytokine [e.g., granulocyte-macrophage colony-stimulating factor (GM-CSF)], T-cell chemokine [e.g., IFN-γ–inducible protein-10 (IP-10) and CCL20 (MIP-3α)], and other T-cell cytokine (e.g., IL-2, IL-4, IL-9, Il-10, IL-12, IL-13, IL-15, IL-16, IL-17A, IL-17C, IL-17E/IL-25, IL-17F, IL-17-A/F, IL-21, IL-22, IL-23, IL-33, IFN-γ, and others) (18). Some of these cytokines are secreted by different cells and have different effector functions on target cells. In general, epithelial and endothelial cell layer permeability increases in response to IL-1β, IL-6, TNF-α, and VEGF-A, and neutrophils and monocytes migrate into the airways from the capillaries in response to immune cell–specific chemokines. In the airways, neutrophils help to clear infection or LPS and eventually resolve lung inflammation. Interaction between neutrophils and LPS also induces NETosis. During NETosis, neutrophils coat their granular proteins [e.g., myeloperoxidase (MPO)] onto their chromatin and release the DNA-protein strings as NETs (19–22). During NETosis, histones of neutrophil chromatin were also citrullinated [citrullinated histone H3 (CitH3)] and released as part of the NETs (23). These NETs could be taken up by macrophages and cleared from the sites of inflammation (24).
In this paper, for the first time, we show that neutrophils are recruited to the airways in response to LPS and undergo NETosis, but BI reduces neutrophil recruitment to the lung and NETosis. LPS-mediated production of cytokines and chemokines, such as CCL2, CCL3, IL-16, IL-12/IL-23p40, IL-17A, IL-17C, and IL-17E/IL-25, are reduced by BI. These alterations explain the reduction in leukocyte recruitment and NETosis in the lungs. Regulating some of these key cytokines is a potential therapeutic option for mitigating BI-mediated pulmonary immunosuppression. Even the small extrapulmonary body burn (15%) causes the risks of pulmonary complications in mice; hence, special attention to the lungs may be required during early stage of patient care because BI significantly suppresses endotoxin-induced pulmonary immune response.
MATERIALS AND METHODS
Mice and experimental groups
All of the animal experiments were performed per the protocol approved by the Subcommittee on Research Animal Care at Massachusetts General Hospital in accordance with the National Institutes of Health (NIH; Bethesda, MD, USA) guidelines. BALB/c mice (12–16 wk) were purchased from Charles River Laboratories (Wilmington, MA, USA) and housed to acclimatize for 1 wk. Mice were divided into 4 groups (n = 5–6/group): 1) sham burn + saline vehicle nasal instillation (sham), 2) burn + saline vehicle nasal instillation (BI), 3) sham burn + nasal LPS in saline instillation (LPS), and 4) burn + nasal LPS in saline instillation (BI + LPS). A third-degree BI and nasal LPS (from Escherichia coli 0111:B4) in saline instillation procedure were performed as previously described in refs. 4 and 25–27. Briefly, mice were anesthetized with a ketamine (100 mg/kg)/xylazine (10 mg/kg) mix. LPS (50 µg/kg) dissolved in 50 or 50 µl saline was administered intranasally. To make a BI to 15% of total body surface area, a portion of shaved back was exposed to water maintained at 80°C for 8 s. This exposure produced a third-degree burn to the skin but caused no direct injury to further deep tissues, such as muscle or internal organs like lung. Silver sulfadiazine 1% cream (Ascend Labs, Parsippany, NJ, USA) was applied to the burned area, and the animals were kept warm. They also received saline (1.0 ml per mouse, i.p.) as fluid resuscitation and buprenorphine (0.1 mg/kg, i.p.) as an analgesic. For sham burn on the back, the mice were treated after anesthesia by exposing the shaved area in lukewarm (37°C) water for 8 s. Thereafter, the animals were monitored for 2 d.
Bronchoalveolar lavage, Cytospin, and differential cell count
To obtain bronchoalveolar lavage (BAL), mice were exsanguinated under ketamine and xylazine anesthesia. BAL was then performed with 1 ml cold HBSS (Thermo Fisher Scientific, Waltham, MA, USA) with EDTA and Tris buffer, with 3 washes for each milliliter; the procedure was then repeated 3 times to obtain a total volume of 3 ml. BAL cells were deposited on slides (200 µl) using a Cytospin (Thermo Fisher Scientific). Differential cell counts were made from randomly taken images of Cytospin preparations stained with hematoxylin and eosin (H&E). All the cells in the image files were counted and adjusted for the individual mouse body weight. At least 300 cells were counted for each condition to determine the percentage of different types of cells.
The cell-free supernatant was collected after centrifugation of the rest of BAL fluid at 400 g for 10 min. The concentration of DNA present in these samples (50 µl) was determined by using the Quant-iT PicoGreen Double-Stranded DNA Quantitation Kit (Thermo Fisher Scientific). Remaining supernatant was stored frozen at −80°C as aliquots until further analyses.
Western blot analysis
For Western blotting of MPO, 40 μl each of BAL and homogenized lung tissue samples (right caudal lobe), NuPAGE 4–12% Bis-Tris Protein Gels (Thermo Fisher Scientific), and goat anti-human/mouse MPO antibody (R&D Systems, Minneapolis, MN, USA) were used for Western blots. Rabbit polyclonal anti-histone H3 (Abcam, Cambridge, United Kingdom) was used for CitH3 Western blotting. Mouse monoclonal anti–glyceraldehyde 3-phosphate dehydrogenase antibody (Abcam) was used for glyceraldehyde 3-phosphate dehydrogenase as loading control. Densitometry on the scanned blots was performed using ImageJ (NIH).
Cytokines analysis
Blood serum samples (25 μl; 1:1 dilution) and BAL supernatant (50 μl; undiluted) were analyzed for the cytokines. The Meso Scale Discovery U-Plex assay system was used for measuring GM-CSF, IFN-γ, IL-10, IL-12/IL-23p40, IL-12p70, IL-13, IL-15, IL-16, IL-17A, IL-17A/F, IL-17C, IL-17E/IL-25, IL-17F, IL-1β, IL-2, IL-21, IL-22, IL-23, IL-27p28/IL-30, IL-31, IL-33, IL-4, IL-5, IL-6, IL-9, IP-10, CXCL1 [KC/growth-regulated oncogene (GRO)], CCL2 (MCP-1), CCL3 (MIP-1α), CCL4 (MIP-1β), CXCL2 (MIP-2), CCL20 (MIP-3α), TNF-α, and VEGF-A. Mean signal intensity values were multiplied by the number of pixels from each band [U-Plex Biomarker Group 1 (ms) 35-Plex; Meso Scale Diagnostic, Rockville, MD, USA].
Immunofluorescent imaging
Cytospinned cells were fixed with 4% (v/v) paraformaldehyde for 10 min and rinsed with PBS. Nonspecific binding sites were blocked with 1% (w/v) bovine serum albumin (MilliporeSigma, Burlington, MA, USA) in PBS for 1 h at room temperature and then incubated with anti-MPO (0.2 μg/μl; R&D Systems) and CitH3 (1:100; Abcam) antibodies overnight at 4°C. After 3 washes in PBS, 5 min each, sections were incubated with a secondary antibody (donkey anti-goat 568 and donkey anti-rabbit 488, 1:500; Abcam) for 1 h at room temperature. They were then washed 3 times in PBS, 5 min each, and mounted using Vectashield (H-1200; Vector Laboratories, Burlingame, CA, USA).
Whole-lung tissue samples were fixed with 4% (v/v) paraformaldehyde at 4°C overnight, cryoprotected, and frozen in optimal cutting temperature compound (Sakura Finetek, Torrance, CA, USA). Cryosections 10 µm thick were blocked with 1% (w/v) bovine serum albumin in PBS and followed the same procedure with BAL. Images were taken using Zeiss LSM800 (Carl Zeiss, Oberkochen, Germany) equipped with ×40 lenses and operated by Zen blue software (Carl Zeiss).
Protein assay
Right caudal lobe was dissected, snap frozen, and kept at −80°C until homogenization. Mechanically grinded lung tissue was homogenized with 1 mM PMSF (Thermo Fisher Scientific), 1× proteinase inhibitor (MilliporeSigma), 0.5 M EDTA, and 1 M Tris in PBS. Bicinchoninic acid protein assays (Thermo Fisher Scientific) were used for determining protein concentration of BAL and lung homogenate supernatant.
Blood
Mice were anesthetized and injected with heparin (500 U/kg; Patterson Veterinary Supply, Fort Devens, MA, USA), and the blood was collected during exsanguination. Blood tubes were centrifuged (500 g for 10 min), and the cell-free plasma samples as supernatant were divided into aliquots, frozen, and stored at −80°C.
Statistical analyses
To test for statistical significance, 1-way ANOVA with Tukey’s multiple comparison tests were used. Values of P < 0.05 were considered to represent statistically significant differences. All statistical analyses were performed using Prism 8.0.1 for Mac (GraphPad Software, La Jolla, CA, USA). Final figures were assembled and labeled using Adobe Illustrator (Adobe, San Jose, CA, USA). Numerical values with 5, 4, and 3 decimals were rounded to the nearest 1000, 100, and 10, respectively, in the text.
RESULTS
BI reduces LPS-mediated neutrophil count in the airways
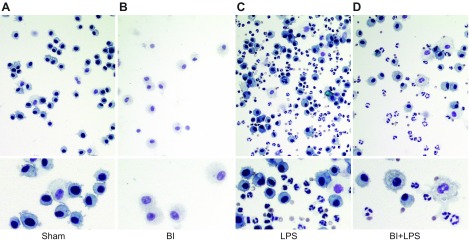
Effects of BI on neutrophil recruitment to the airways in the context of lung inflammation have not been clearly understood. To determine the mechanism of BI-induced pulmonary immunosuppression, we used a pulmonary inflammation and NETosis mouse model established in the Palaniyar laboratory (5, 28, 29) in combination with or without the BI mouse model extensively used in the J.A.M. laboratory (6, 7). In this combined model, we instilled LPS in saline or saline into the airways of the mice with sham burn (hereafter referred to as sham) or third-degree BI (15% total body surface area; hereafter referred to as BI). Two days after the procedure, peripheral blood was collected during exsanguination of the mice followed by the collection of BAL samples or lung samples. In the first set of experiments, cells present in the BAL samples were deposited on slides by centrifugation (Cytospin) and stained with H&E. Light microscopy analyses showed that the most of the immune cells present in the airways of sham or BI-alone mice were macrophages (Fig. 1A, B). LPS instillation resulted in a drastic increase in neutrophils in the airways of sham-treated mice (Fig. 1C). By contrast, BI reduced the LPS-mediated increase of immune cells; BI-mediated reduction of neutrophil count in the airways was obvious (Fig. 1D).
Figure 1.
BI reduces LPS-mediated neutrophil count in BAL. A, B) Cytospin H&E staining shows that most of the cells in BAL of sham (A) or BI alone (B) are macrophages. Intranasal instillation of LPS drastically increases the numbers of neutrophils into the airways. C) Macrophage numbers are also increased with LPS instillation. D) BI decreases LPS-mediated increase in both neutrophil and macrophage count. Lower panels represent magnified sections of the upper panels. Original images were captured at ×20 magnification.
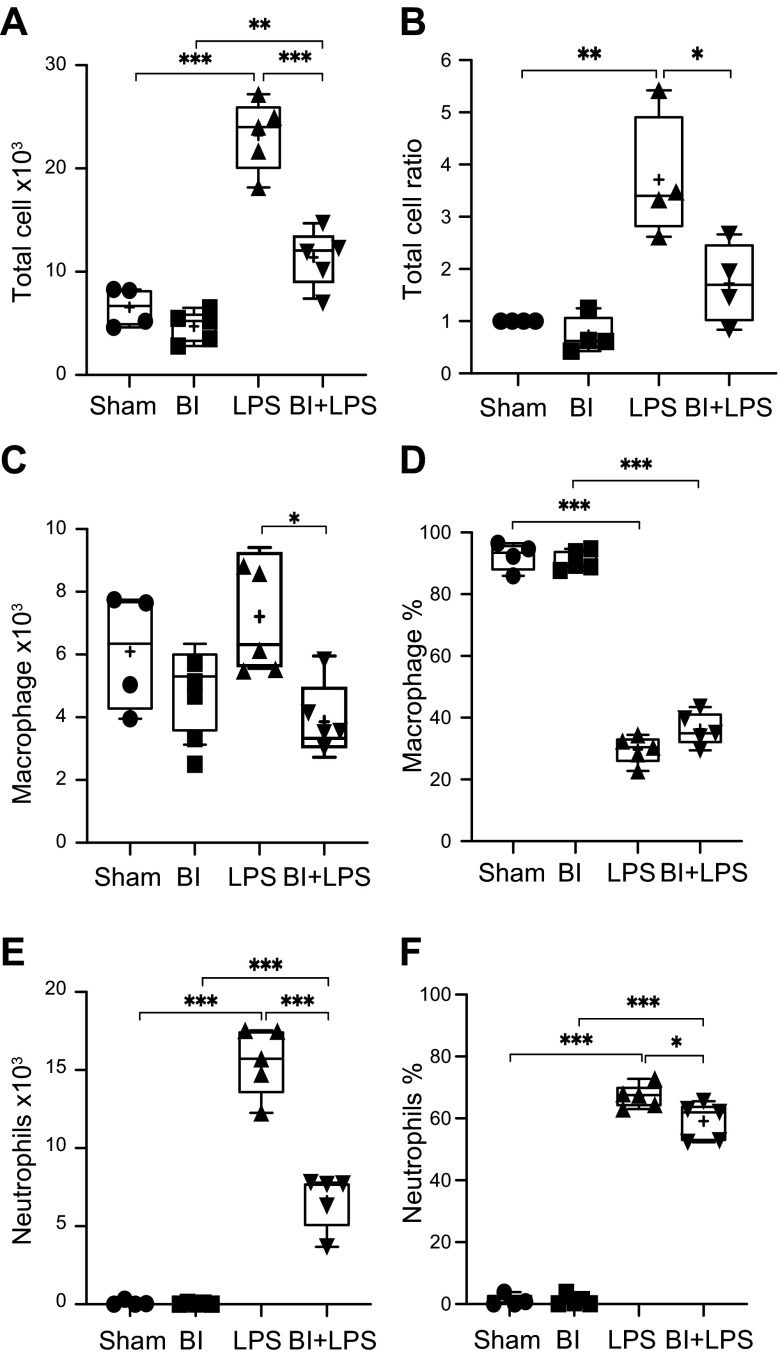
We quantified the cells present in the Cytospin preparations of the BAL samples to calculate total and differential cell counts. The total cell counts were divided by the body weight of each mouse to standardize the data set. These data show that BI alone compared with sham did not significantly alter total cell number (sham: 6600 ± 970, BI: 4700 ± 680; P = 0.7112; Fig. 2A). Compared with sham or BI alone, LPS instillation drastically increased total cell counts (23,000 ± 1540; P < 0.001). However, BI reduced LPS-mediated increase in the total cell count (11,200 ± 1300; P < 0.001; 2-fold; Fig. 2A, B). Differential cell analyses show that BI significantly reduced macrophage number (400 ± 490), particularly in the presence of LPS, compared with the LPS-alone group (6900 ± 740; P < 0.05; Fig. 2C). Nevertheless, macrophage percentage in the airways was similar between LPS and BI + LPS conditions (30–36%; Fig. 2D). BI alone did not increase neutrophil count (45 ± 25) in the airways compared with the baseline sham control (89 ±77; Fig. 2E). LPS instillation resulted in a massive increase in neutrophil count compared with both sham and BI-alone controls (15,600 ± 980; P < 0.001). BI significantly reduced LPS-mediated increase in neutrophil counts in the airways (6630 ± 790; P < 0.001; 2.4-fold; Fig. 2E). Such a reduction in neutrophil count resulted in a BI-mediated reduction in neutrophil percentage in the airways from 67 ± 1.69 to 59 ± 2.74% (Fig. 2F). Collectively, these data sets (Figs. 1 and 2) show that BI drastically reduced LPS-mediated increase in neutrophil counts and the percentage and, to some extent, macrophage counts in the airways (BAL).
Figure 2.
Quantification of total cells and differentiated cells in the airways. Sham and BI total cell count increases with LPS instillation. A) The increase of total cell number in LPS-mediated lung is decreased by BI significantly. B) LPS instillation significantly increases the total cell count, and BI decreases such effect. C) Differential cell analysis shows that BI further reduces macrophage numbers. D) Percentage of macrophages is similar between sham and BI, and LPS and BI + LPS. E) Neutrophil count drastically increases during LPS-mediated lung inflammation, and BI decreases the LPS-mediated increase in neutrophil count. F) LPS instillation increases neutrophil percentage, and BI decreases LPS-mediated increase in neutrophil percentage; n = 4 for sham, n = 5 for BI, LPS, and BI + LPS. +, mean. *P < 0.05, **P < 0.01, ***P < 0.001.
BI reduces LPS-mediated NETosis by the pulmonary neutrophils
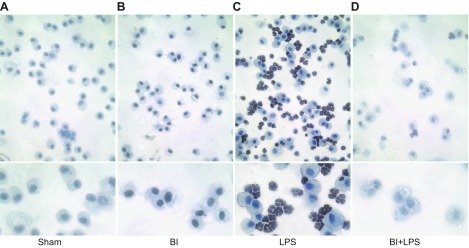
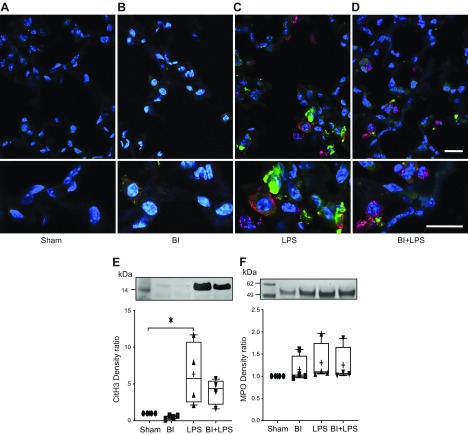
We have previously shown that the neutrophils recruited to the airways undergo NETosis (4). Therefore, we determined the markers of NETosis in pulmonary neutrophils using different methods. As CitH3 is a good marker of NETosis (4, 19, 30), we conducted an immunocytochemistry experiment using the BAL cells present on Cytospin preparations. 3,3′-Diaminobenzidine-based immunodetection using light microscopy shows that macrophages present in the airways of sham and BI mice did not have CitH3 (Fig. 3A, B). By contrast, the images clearly show that almost all of the neutrophils present in the BAL of the LPS-instilled mice contained CitH3, particularly in the nuclei (Fig. 3C; nuclear morphology and immunostaining). Fewer CitH3-positive neutrophils were detected in the airways during LPS-mediated inflammation with BI (BI + LPS; Fig. 3D). These images indicate that BI reduced the NETosis ability of pulmonary neutrophils.
Figure 3.
BI reduces LPS-mediated NETosis by the pulmonary neutrophils. A, B) Macrophages in BAL of sham (A) and BI (B) are not positive for NET marker CitH3. C) LPS instillation induces CitH3 formation in almost all of the neutrophils present in the BAL. D) CitH3 formation in neutrophils is less obvious in BI + LPS condition. Lower panels represent magnified sections of the upper panels. Original images were captured at ×20 magnification.
BI reduces the amount of NETs present in the airways during LPS-mediated lung inflammation
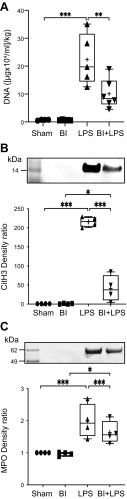
During NETosis, the MPO present in the cytoplasmic granules coats the chromatin (DNA:histone complexes); subsequently, these neutrophils release NETs coated with MPO and citrullinated histones into the airways (4, 31). Therefore, we first quantified the DNA content of the BAL supernatant (50 μl) by PicoGreen assays and standardized the values to the body weight (Fig. 4A). DNA content in sham, BI, LPS, and BI + LPS were 6500 ± 750, 6600 ± 620, 200,000 ± 40,800, and 101,000 ± 21,500 [μg/ml]/kg, respectively. DNA content in the LPS-alone group was significantly higher than sham (P < 0.001), BI-alone (P < 0.001), and BI + LPS (P < 0.01) groups.
Figure 4.
BI suppresses NETosis in the airways. A) PicoGreen analyses show that LPS instillation increases cell-free DNA, and BI suppresses such an effect. B, C) Western blot analyses show that BI has a similar effect on CitH3 (B) and MPO levels in the BAL (C); n = 4 for sham, n = 5 for BI, LPS, and BI + LPS. +, mean. *P < 0.05, **P < 0.01, ***P < 0.001.
Western blots of BAL supernatant samples (40 μl) show that a NET marker, CitH3, was readily detectable in the BAL samples of mice instilled with LPS in the presence or absence of BI (Fig. 4B). Analyses of CitH3 bands shows that LPS instillation (216 ± 13) generated the highest amount of CitH3 compared with sham (1 ± 0; P < 0.001), BI (0.84 ± 0.96, P < 0.001), or BI + LPS (41 ± 34, P < 0.01; Fig. 4B). BI suppressed LPS-mediated increase in CitH3 in the airways (LPS vs. BI + LPS; P < 0.001). We then analyzed the levels of another NET marker, MPO, in the BAL supernatant. The Western blots clearly show that LPS increased the amounts of MPO in the airways; BI significantly reduced LPS-mediated increase in MPO (P < 0.001; Fig. 4C). Therefore, these data indicate that large amounts of NETs are released into the airway surface lining fluid of mice instilled with LPS, but BI reduced the amount of NETosis during LPS-mediated lung inflammation.
BI reduces LPS-mediated increase in NETosis in the lung parenchyma
To determine whether NETs are trapped in different compartments of the lung parenchyma, such as airways or blood vessels, we examined the lung sections using immunohistochemistry and confocal microscopy. Animals were exsanguinated, and the blood vessels were washed with saline and fixed with paraformaldehyde via circulating these solutions through the heart with a syringe. The lungs were dissected from the exsanguinated animals, inflated to maintain alveoli at the open state, embedded in optimal cutting temperature compound, and cryosectioned for further analyses. Immunostaining of tissue sections for CitH3 (green) and MPO (orange) and staining of DNA with DAPI (blue) show that sham and BI mice did not have neutrophils or NETs in their lungs (Fig. 5A, B). In some sections, traces of CitH3 signal were detectable as small dots inside alveolar macrophages of the BI-alone condition. In the LPS conditions, NETotic neutrophils were detected exclusively in the alveoli (Fig. 5C, D). Macrophages that have taken up NET components (MPO, CitH3) were also detected in the alveoli. Although some capillaries contained neutrophils, they did not show any signs of NETosis (with MPO, but without CitH3).
Figure 5.
BI suppresses NETosis in the lung parenchyma, particularly in the airways. A–D) Immunohistochemical analysis shows that NETs are mainly present in the airways of the LPS-instilled lungs. E) Western blot analysis of unflushed lung homogenate samples shows that LPS instillation induces CitH3 formation, and BI suppresses such an effect. F) No significant differences are detectable in MPO levels. Scale bars, 20 μm.
To quantify the neutrophils/NET components present in the lungs, we harvested the lungs without flushing the blood from the lung capillary, extracted the total proteins from the lung tissues, and performed Western blots. CitH3 levels show that significant amounts of NETs were formed only in the LPS conditions (LPS alone and BI + LPS; Fig. 5E). Quantitative analyses show that sham and BI-alone conditions had no detectable levels of CitH3. By contrast, LPS-alone condition had the highest amounts of CitH3. BI + LPS condition had 5.3-fold less amount of CitH3 than LPS-alone condition (Fig. 5E). These Western blot analyses confirmed the immunohistochemistry experimental data.
MPO was detectable in all 4 conditions, indicating that neutrophils were mostly present in the pulmonary blood flow. Quantitative analyses of the MPO bands show that MPO was not significantly different among all the conditions (Fig. 5F). Therefore, neutrophils were circulating through the pulmonary vasculature in all 4 of the experimental conditions. LPS-induced NETosis and BI reduced LPS-mediated NETosis in the airway spaces.
BI does not alter LPS-mediated inflammatory and permeability-increasing cytokine response in the airways
The reasons for the differential neutrophilic infiltration during LPS-mediated inflammation by the BI are not clearly established. Hence, to determine the differences in cytokine response during these 4 experimental conditions, we analyzed the BAL supernatant (lavage in 3 ml; used without further dilution) and serum (used with 1:1 dilution). We have quantified 34 cytokines relevant to inflammation, using Meso scale U-Plex procedure, in BAL and serum to represent the airway compartment and blood, respectively. Precisely comparing BAL and serum is not recommended because of the difficulties in correctly estimating the dilution factor of the airway surface fluid in 3 ml BAL; nevertheless, airway surface fluid is considered to be diluted more than 10-fold during the BAL procedure. Therefore, the quantitative chemotactic gradient between the airways and blood is approximate. Hence, we did not compare the cytokine and chemokine levels between the BAL and serum. However, for most of these cytokines, the differences between the airway and blood were obvious.
We have conducted unbiased multiple comparisons to determine the differences among all 4 experimental conditions. However, only the most relevant comparisons are indicated in the graphs (e.g., sham vs. LPS; BI vs. BI + LPS; LPS vs. BI + LPS). In all the following sections, we present BAL and serum sample data in the left and right sides of the figures, respectively.
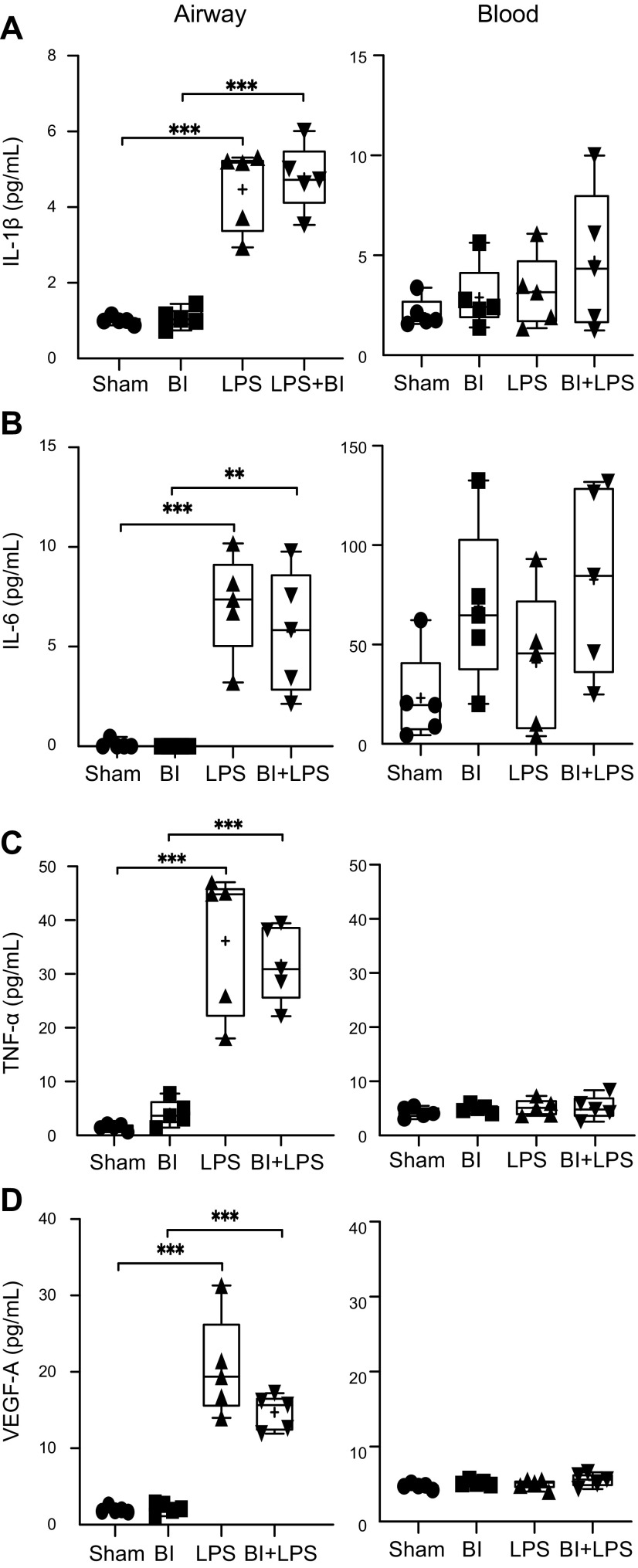
During LPS-mediated lung inflammation, LPS first encounters epithelial cells and alveolar macrophages. These cells get activated and secrete several inflammatory and permeability-increasing cytokines (e.g., IL-1β, IL-6, TNF-α, VEGF-A), which facilitate the formation of chemotactic gradient (32, 33). IL-6 has multiple effects: it increases VEGF-A, which directly increases endothelial cell permeability. It also helps to differentiate naive T cells into Th17 cells, which secrete many cytokines to regulate lung inflammation (34–36). Meso scale analyses show that all 4 of these cytokines increased in the airways after LPS instillation (Fig. 6). BI or BI + LPS did not affect the levels of these cytokines. None of these cytokines in serum differed significantly among these 4 conditions. Therefore, BI did not have a substantial effect on LPS-mediated increase in permeability of the airway lining.
Figure 6.
BI does not affect LPS-mediated increase in permeability-regulating cytokines in the BAL. LPS increases IL-1β (A), IL-6 (B), TNF-α (C), and VEGF-A (D) levels in the BAL. BI neither increases these cytokines nor alters the LPS-mediated increase in the levels of these cytokines. Although substantial amounts of IL-1β and IL-6 are measurable in the serum, levels of these cytokines are not significantly different among the 4 treatment conditions. TNF-α and VEGF-A levels are barely detectable in the serum; n = 5 for each condition. +, mean. **P < 0.01, ***P < 0.001 (P = 0.0781 for LPS vs. BI + LPS for VEGF-A in BAL).
BI differentially affects LPS-mediated increase of monocyte and neutrophil chemokines in the airways
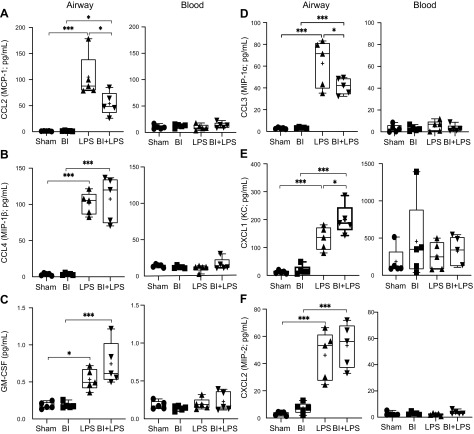
Activated airway epithelial cells and macrophages secrete specific chemokines such as CCL2 (MCP-1) and CCL4 (MIP-1β) to chemoattract monocytes (37, 38). CCL2 also acts as a neutrophil chemokine during LPS-mediated lung inflammation (39). Cytokine analysis showed that LPS increased CCL2 in the airways (Fig. 7A). However, BI substantially suppressed LPS-mediated increase in CCL2 levels. LPS increased CCL4 levels regardless of the presence or absence of BI (Fig. 7B). Therefore, the reduction in CCL2 was expected to decrease LPS-mediated monocyte/macrophage recruitment and neutrophil infiltration to the airways during BI.
Figure 7.
A–C) BI suppresses LPS-mediated increase in monocyte and neutrophil chemokine CCL2, but not CCL4 or monocyte differentiating cytokine GM-CSF, in the BAL. LPS increases the levels of CCL2 (MCP-1; A), CCL4 (MIP-1β; B), and GM-CSF (C). BI suppresses the LPS-mediated increase in the levels of CCL2, but not CCL4 and GM-CSF. These 3 cytokines are barely detectable in the serum. D–F) BI suppresses LPS-mediated increase in neutrophil chemokine CCL3 levels, but promotes CXCL1 levels, whereas it has no effect on CXCL2, in the BAL. LPS increases the levels of all 3 neutrophil chemokines CCL3 (MIP-1α; D), CXCL1 (KC; E), and CXCL2 (MIP-2; F). BI has opposing effects on the LPS-mediated increase in the levels of CCL3 and CXCL1. These 3 cytokines do not vary among the 4 treatment conditions in the serum; n = 5 for each condition. +, mean. *P < 0.05, ***P < 0.001 (P = 0.2265 for LPS vs. BI + LPS for GM-CSF, P = 0.7997 for LPS vs. BI + LPS for CXCL2 in BAL).
GM-CSF differentiates monocytes recruited to the lungs into M1 macrophages, in particular, and activates these macrophages to secrete IL-1, IL-6, TNF, IL-12, and IL-23 (40). LPS increased GM-CSF levels in the airways, and BI did not significantly alter LPS-mediated increase in GM-CSF (Fig. 7C). Therefore, monocyte differentiation into macrophages was likely not to be altered by BI. GM-CSF can also maintain the health of neutrophils, and the neutralization of GM-CSF reduces neutrophilic inflammation (41–44). Because the levels of GM-CSF in the BAL samples are the same in LPS and BI + LPS conditions, this cytokine may not be responsible for causing BI-mediated immunosuppression of the lung.
The major groups of cytokines secreted by the airway epithelium and the activated macrophages to recruit neutrophils are CCL3 (MIP-1α, same in human), CXCL1 (KC or Gro-α; CXCL3 or Gro-γ or MIP-2β in humans), and CXCL2 (MIP-2; MIP-2α or Gro-β in human). In humans, CXCL8 (IL-8 in human) is also involved in neutrophil chemotaxis (45, 46). Our cytokine analyses show that LPS-mediated increase in CCL3 levels were significantly suppressed by BI (Fig. 7D). By contrast, LPS-mediated increase in CXCL1 was promoted by BI (Fig. 7E). However, LPS-mediated increase in CXCL2 levels was not affected by BI (Fig. 7F). Therefore, the balance among these 3 chemokines would determine the level of neutrophil recruitment to the airways in our mouse model. LPS-induced macrophages are a major contributor of CCL3 (47). Decreased macrophages and neutrophil counts in the airways of mice with BI + LPS, compared with LPS, suggest that CCL3 was one of the key chemokines responsible for the overall BI-mediated reduction in neutrophil recruitment to these airways.
BI differentially affects LPS-mediated increase of T-cell cytokines and chemokines in the airways and serum
Other immune cells, such as Th17, can also help to recruit neutrophils to the lung (48–50). Th cells, particularly Th17 cells, play important roles during BI (51, 52). In the presence of bacteria/LPS, various cells in the lung parenchyma also secrete cytokines to recruit T cells to the inflamed tissue (e.g., CCL20, IL-16, IP-10). CCL20 (MIP-3α, the same in humans) is a strong chemoattractant, which recruits lymphocytes and dendritic cells toward the epithelial cells (53). Several cells in lung tissues and peripheral blood lymphocytes could secrete CCL20 in response to LPS and TNF-α (54). LPS increased CCL20, regardless of BI (Supplemental Fig. S1A). Hence, this chemokine should not affect the differential neutrophilic response to LPS in the lung during BI.
Many types of cells in the lung produce IL-16, which acts as a chemoattractant and an activator of various types of T cells (55). Cytokine analysis shows that LPS instillation into the airways increased IL-16 levels in both blood (serum) and the airways (Supplemental Fig. S1B). Notably, BI suppressed LPS-mediated increase in IL-16 in the airways. A similar trend is notable in the serum as well. However, airway IL-16 levels are substantially high. Hence, BI should be reducing the T-cell recruitment from the capillaries into the interstitium/alveoli during LPS-mediated inflammation.
T cells attracted from the blood need to attach to the endothelium before entering the lung parenchyma. IP-10 is a key cytokine, secreted by macrophages and endothelial cells, that promotes T-cell adhesion to endothelial cell (56). Notably, LPS-induced IP-10 levels in the BAL samples were suppressed by the BI (Supplemental Fig. S1C). Therefore, the reduction in IL-16 and IP-10 is expected to reduce T-cell recruitment to the LPS-mediated lung parenchyma during BI.
BI differentially affects LPS-mediated increase of T cell–differentiating cytokines in the airways and serum
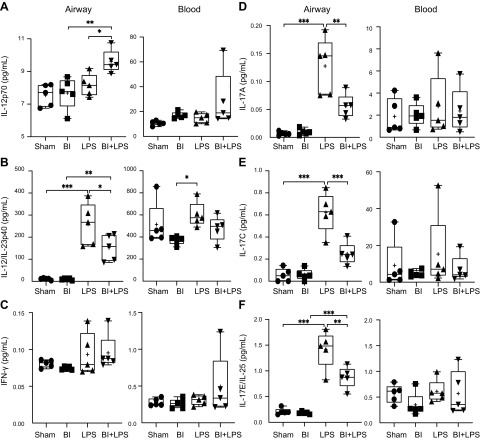
Lung cytokine environment affects T-cell differentiation and proliferation. In the lymphatic compartment, naive T cells differentiate into different subsets, whereas in interstitium, they proliferate. The key cytokines responsible for T-cell differentiation are IL-4, IL-6 (Fig. 6B), and IL-12 (Fig. 8A, B) (57). Circulating T cells that are attached to the endothelial surface then migrate into the interstitium. Dendritic cells and tissue macrophages present in this compartment direct the proliferation of various T-cell subsets.
Figure 8.
BI alters LPS-mediated increase of T cell–differentiating cytokines, as determined by IL-12p70 and IL-12/IL-23p40 in the BAL. A) BI promotes Th1 differentiation promoting cytokines IL-12p70 during LPS-mediated lung inflammation. B) LPS increases the levels of p40 subunit shared by IL-12 and IL-23; however, the amount of this subunit was low in BI + LPS condition. C) LPS or BI does not significantly affect the levels of IFN-γ. D–F) BI suppresses LPS-mediated Th17 response, as determined by IL-17 in the BAL. BI suppresses LPS-mediated increase in inflammatory cytokines IL-17A (D), IL-17C (E), and IL-17E/IL-25 (F) levels. LPS or BI does not significantly affect the levels of Th17-related cytokines in serum; n = 5 for each condition. +, mean. *P < 0.05, **P < 0.01, ***P < 0.001.
Th2 cells
IL-4 is a key cytokine responsible for differentiating naive T cells into Th2 cells, which secrete IL-4, IL-5, IL-13, and IL-31(58–61). IL-4, IL-13, and IL-31 induce goblet cells and submucosal glands to secrete mucus into the airways during inflammation (62–64). IL-5 levels increase in the airways during Th2-mediated allergic inflammation and eosinophilic activation (65). Neither BI nor LPS increased IL-5 levels in the airways (Supplemental Fig. S2A). However, LPS increased IL-4, IL-13, and IL-31 levels in the airways, and BI did not affect LPS-mediated increase of these cytokines (Supplemental Fig. S2B–D). Therefore, BI does not affect LPS-induced Th2 response relevant to mucus secretion and stimulation of epithelial cells.
IL-33 is an IL-1 family cytokine, which can be secreted by many cell types, particularly by damaged lung epithelial cells. IL-33 induces IL-5 production by Th2 cells (66). Levels of IL-5 and IL-33 were not altered by LPS or BI (Supplemental Fig. S2A, E). Th9 and several other types of cells secrete IL-9, which promotes Th cell proliferation and suppresses apoptosis (67). IL-9 promotes goblet cell metaplasia and chemokine production; it also increases IL-13 production (68, 69). LPS increased IL-9 levels in the BAL, and BI did not affect IL-9 production (Supplemental Fig. S2F). None of the experimental conditions significantly altered IL-9 levels in the serum. IL-15 is another key cytokine produced by several types of mononuclear cells, and it promotes the survival of T cells by increasing antiapoptotic proteins such as B-cell lymphoma (Bcl)2 and Bcl-extra large (xL) (70). LPS increased IL-15 levels in the BAL, and BI did not inhibit LPS-mediated increase in IL-15 (Supplemental Fig. S2G). IL-2 is a key cytokine that differentially regulates the survival of different Th subsets; it promotes the survival of Th1 and Th2 cells but antagonizes Th17 cell survival (71, 72). Notably, IL-2 levels in the BAL were only increased in the BI + LPS condition (Supplemental Fig. S2H). Therefore, BI is expected to inhibit Th17-related cytokines during LPS-mediated lung inflammation.
Th1 cells
Activated macrophages, dendritic cells, and neutrophils secrete IL-12 (IL-12α, p35 + IL-12β, p40, p70; active heterodimer), which differentiates naive Th cells into Th1 cells (73, 74). IL-12/IL-23p40 is a p40 subunit shared by both IL-12 and IL-23 (73, 75). IL-12p70, the fully assembled biologically active form, was not elevated in the LPS-alone condition in the airways (Fig. 8A). However, IL-12p70 was significantly increased in BI + LPS compared with BI-alone or LPS-alone condition, implying that IL-12p70 was fully assembling only in the BI + LPS double-hit condition. Levels of the free p40 subunit (IL-12/IL-23p40) were high in LPS condition, but BI lowered the LPS-induced increase of this subunit, both in serum and in the airways, although the differences are clearer in the airways (Fig. 8B). These data suggest that BI is conducive for promoting Th1 differentiation during LPS-mediated lung inflammation.
IL-23 (p40 and p19 subunits) is a key cytokine secreted by dendritic cells and activated macrophages (73). LPS increased IL-23 levels, and BI did not affect this response (Supplemental Fig. S3A). Therefore, the presence of IL-12/IL-23p40 subunits at higher levels in LPS condition and low levels in BI + LPS condition suggest that BI promotes the assembly of IL-12 but does not affect IL-23 assembly during LPS-mediated inflammation.
IL-27 has 2 subunits, p40 (related to p40 subunit of IL-12 and IL-23) and p28 (related to the p35 subunit of IL-12). Endothelial cells, monocytes, and dendritic cells produce IL-27, which induces IL-12 receptor expression on naive CD4+ T cells, sensitizing them for subsequent IL-12-induced Th1 differentiation. It also up-regulates IL-10 in regulatory T cells and suppresses Th17 cell activation (76–79). No significant differences in IL-27 were detected in the experimental conditions, although BI may promote IL-27 production (Supplemental Fig. S3B). By contrast, IFN-γ, a cytokine mainly produced by Th1 cells and IL-12–stimulated neutrophils (80), was not different among the 4 experimental conditions (Fig. 8C). IL-10 is a cytokine produced by regulatory T cells or macrophages during the resolution phase of the LPS-mediated lung inflammation (81). Levels of IL-10 were not different among the 4 conditions (Supplemental Fig. S3C). Therefore, BI may be inducing a specific Th1 response during LPS-mediated lung inflammation.
Th17 cells
Several Th17 cytokines were readily detectable in LPS condition, but such an effect was significantly blunted during BI, as evident by the suppression of IL-17A, IL-17C, and IL-17E/IL-25 (Fig. 8D–F). LPS-mediated increase in IL-17F (homodimer) and IL-17A/F (Supplemental Fig. S3D, E, heterodimer) levels was not significantly altered by BI (Supplemental Fig. S3D, E). These 2 cytokine complexes are moderate inducers of downstream signaling in target cells (82, 83). Other Th17 cytokines such as IL-21 and IL-22 also increased in LPS condition, but BI did not suppress the effect of LPS on these cytokines (Supplemental Fig. S3F, G). Therefore, BI suppressed key effector functions of Th17 cells, particularly by lowering the levels of IL-17A, IL-17C, and IL-17E/IL-25 during LPS-mediated lung inflammation.
DISCUSSION
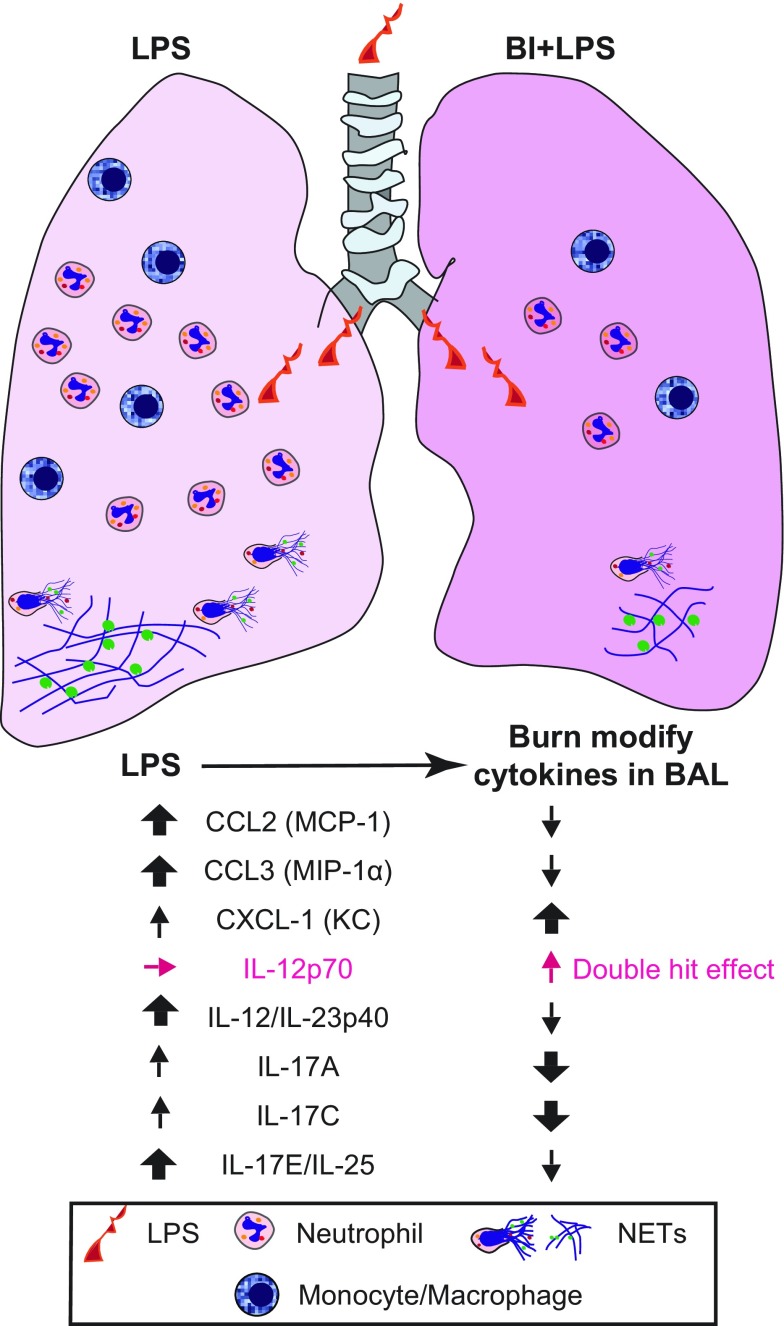
It has been well documented that BI induces pulmonary immunosuppression (84–86). However, the cytokines and differential immune cell response that regulate such defects have not been clearly understood. Here, we describe a mouse model to study pulmonary immunosuppression in the context of third-degree BI. In this model, presence of LPS increased monocyte/macrophage and neutrophil counts in the airways. Such an increase resulted in NETosis. BI, on its own, did not significantly affect immune cell homeostasis of the airways. However, BI drastically suppressed monocyte/macrophage and, in particular, neutrophil counts and NETosis in the presence of LPS in the airways. BI specifically suppresses LPS-mediated increase in CCL2, CCL3, IL-12/IL-23p40, IL-17A, IL-17C, and IL-17E/IL-25 in the airways (Fig. 9). Collectively, these changes are reminiscent of significant neutrophilic immunosuppression, perhaps combined with macrophage and Th17-mediated immune response. Therefore, the data presented in this study unravel the key reasons for BI-induced immunosuppression.
Figure 9.
A model summarizing the BI-mediated pulmonary immunosuppression.
Cytokines are the key regulators of inflammation that recruit immune cells to the site of infection or injury (87–89). Cytokine production of injured host helps to recruit neutrophils and other immune cells to the site of BI (28, 90). Bacterial endotoxin such as LPS is known to generate a robust cytokine production and subsequent recruitment of immune cells to the airways. The data sets show that the LPS model is working well (Figs. 1–8). BI alone imparted no significant differences in airway cytokine levels or immune cell counts. Nevertheless, it is clear that specific cytokine balance is altered when endotoxin is present in the airways during BI. Reduction in CCL2 reflects the reduction in monocyte/macrophage levels and also potentially in neutrophil levels. Reduction in CCL3 in the airways reflects the reduction in neutrophil count. In addition, all the key IL-17 cytokine levels are also reduced by BI. IL-17 is known as a key cytokine secreted by mainly Th17 cells that promote neutrophil production and subsequent recruitment to the lungs (48–50). Th17 plays important roles in immune response during BI (8, 91–93). Therefore, the neutrophil-Th17 axis could be one of the key regulators of pulmonary immune deficiency. It is also worth noting that recent studies show that neutrophils could also produce IL-17 under certain conditions (94). Therefore, it is possible that both Th17 and neutrophils could contribute to the pool of IL-17. Nevertheless, it is clear that reduction in the levels of CCL3, CCL2, and IL-17–related cytokines are important features of BI-mediated reduction in neutrophil counts in the airways.
Neutrophils recruited to the airways are known to undergo NETosis (4). Significant amounts of NETs are detectable in the BAL samples after LPS instillation (Figs. 3 and 4). Examination of BAL cells shows that chromatin in the nuclei of these neutrophils contains CitH3 (Fig. 3), suggesting that the pulmonary neutrophils are primed to undergo NETosis, which is consistent with the point that neutrophils recruited to the airways release NETs with CitH3 in vivo (95). Compared with the LPS condition, the BI + LPS airway condition has a lower number of neutrophils and less degree of CitH3 in the nuclei. Therefore, BI also suppresses the ability of the airway neutrophils to undergo NETosis. When the neutrophils undergo NETosis, they release NETs (96). Presence of NETs could be deduced by determining NET markers such as DNA, CitH3, and MPO (97). Analyses of BAL supernatant shows that the airways of LPS-instilled mice contain significantly higher amounts of NETs than other conditions, such as sham, BI, and BI + LPS, and the amount of NETs is lower in the airways with BI + LPS compared with the LPS-instilled condition without burn (Fig. 4). Therefore, we consider that the amounts of NETs largely reflect the number of neutrophils present in the airways and the BI-mediated suppression of NETosis. Once the NETs are released, they are cleared from the airways. Macrophages have been considered as the phagocytes that clear the NETs (24). Alveolar macrophages present with neutrophils in the airways contain NET markers (CitH3) on their surface or in the cytoplasmic comportments, suggesting that these phagocytes participate in NET clearance (Fig. 5). Most of the NETs and NET-forming neutrophils are detected in the airways. Hence, the airway compartment is susceptible for BI-induced pulmonary immunosuppression.
In summary, this study shows that neutrophils and NETs are important components of BI-induced pulmonary immunosuppression. Reduced cytokines and chemokines that recruit neutrophils during BI is a major contributor of the immunosuppression (Figs. 6–8). This reduction results in reduced neutrophil recruitment and eventual NETosis (Fig. 9). This new information could help us to understand the pulmonary immunosuppression in burn patients and pave the way for devising novel therapeutic approaches to promote neutrophil recruitment to the lungs during pulmonary immunosuppression. The risks of extrapulmonary burn, even small body surface, to pulmonary complications exist from the early days of injuries, and hence, special attention is required during patient care because of the BI-induced neutrophilic pulmonary immunosuppression.
ACKNOWLEDGMENTS
Most of the work for this study was performed at Shriners Hospitals for Children–Boston. The authors thank Dr. Yong-Ming Yu and Dr. Sarah S. Kelangi (Massachusetts General Hospital and Shriners Hospitals for Children–Boston) for administrative support. The authors also thank the Scadden laboratory (Massachusetts General Hospital) for the use their equipment. Animals were housed at Center for Comparative Medicine (CCM) in Shriners Hospitals for Children–Boston. Immunofluorescent imaging was performed at the Microscopy Core of the Program in Membrane Biology, which is partially supported by a Centre for the Study of Inflammatory Bowel Disease Grant DK043351 and Boston Area Diabetes and Endocrinology Research Center (BADERC) Award DK057521. The Zeiss LSM 800 Airyscan confocal microscope was purchased using U.S. National Institutes of Health (NIH) Shared Instrumentation Grant 1S10OD021577-01. Pulmonary neutrophil extracellular trap formation (NETosis) studies of N.P. were supported by Canadian Institutes of Health Research (MOP-111012). This work was also partially supported by NIH National Institute of General Medical Sciences Grant RO1 GM 11847, and from Shriners Hospital Philanthropy (86300 and 85124 to J.A.M.). The authors thank Dr. Anil V. Nair and Dr. Richard Bouley (both from Massachusetts General Hospital) for technical support at the Core. Meso scale analysis was performed at the Metabolism and Mitochondrial Research Core (Beth Israel Deaconess Medical Center, Boston, MA, USA). The authors also thank Dr. Xiaowen Liu (Beth Israel Deaconess Medical Center) for technical support at the Core. The authors declare no conflicts of interest.
Glossary
- BAL
bronchoalveolar lavage
- BI
burn injury
- BI + LPS
burn injury with nasal LPS in saline instillation
- CCL
chemokine (C-C motif) ligand
- CitH3
citrullinated histone H3
- CXCL
chemokine (C-X-C motif) ligand
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- GRO
growth-regulated oncogene
- H&E
hematoxylin and eosin
- IP
IFN-γ–inducible protein-10
- KC
keratinocyte chemoattractant
- MCP
monocyte chemoattractant protein
- MIP
macrophage inflammatory protein
- MPO
myeloperoxidase
- NETosis
NET formation
- NET
neutrophil extracellular trap
- Th
T helper
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
M. Sakuma conducted experiments, analyzed the data, generated the figures, and drafted the manuscript; M. A. S. Khan helped to set up the animal procedures and assays; S. Yasuhara interpreted the data and edited the manuscript; J. A. Martyn obtained funding support, supervised the study, interpreted the data, and edited the manuscript; and N. Palaniyar conceived the idea, conducted experiments, interpreted the data, drafted and edited the manuscript, and supervised the study.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Balamayooran G., Batra S., Fessler M. B., Happel K. I., Jeyaseelan S. (2010) Mechanisms of neutrophil accumulation in the lungs against bacteria. Am. J. Respir. Cell Mol. Biol. 43, 5–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robins E. V. (1989) Immunosuppression of the burned patient. Crit. Care Nurs. Clin. North Am. 1, 767–774 [PubMed] [Google Scholar]
- 3.Davis C. S., Albright J. M., Carter S. R., Ramirez L., Kim H., Gamelli R. L., Kovacs E. J. (2012) Early pulmonary immune hyporesponsiveness is associated with mortality after burn and smoke inhalation injury. J. Burn Care Res. 33, 26–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Douda D. N., Jackson R., Grasemann H., Palaniyar N. (2011) Innate immune collectin surfactant protein D simultaneously binds both neutrophil extracellular traps and carbohydrate ligands and promotes bacterial trapping. J. Immunol. 187, 1856–1865 [DOI] [PubMed] [Google Scholar]
- 5.Yildiz C., Palaniyar N., Otulakowski G., Khan M. A., Post M., Kuebler W. M., Tanswell K., Belcastro R., Masood A., Engelberts D., Kavanagh B. P. (2015) Mechanical ventilation induces neutrophil extracellular trap formation. Anesthesiology 122, 864–875 [DOI] [PubMed] [Google Scholar]
- 6.Khan M. A. S., Khan M. F., Kashiwagi S., Kem W. R., Yasuhara S., Kaneki M., Tompkins R. G., Martyn J. A. J. (2017) An ALPHA7 nicotinic acetylcholine receptor agonist (GTS-21) promotes C2C12 myonuclear accretion in association with release of interleukin-6 (IL-6) and improves survival in burned mice. Shock 48, 227–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma L., Zhou Y., Khan M. A. S., Yasuhara S., Martyn J. A. J. (2019) Burn-induced microglia activation is associated with motor neuron degeneration and muscle wasting in mice. Shock 51, 569–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rendon J. L., Choudhry M. A. (2012) Th17 cells: critical mediators of host responses to burn injury and sepsis. J. Leukoc. Biol. 92, 529–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim A., Lang T., Xue M., Wijewardana A., Jackson C., Vandervord J. (2017) The role of Th-17 cells and γδ T-cells in modulating the systemic inflammatory response to severe burn injury. Int. J. Mol. Sci. 18, E758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Burn Association Burn incidence and treatment in the United States. Available at: http://www.ameriburn.org/resources_factsheet.php
- 11.Pruitt B. A., Jr., Erickson D. R., Morris A. (1975) Progressive pulmonary insufficiency and other pulmonary complications of thermal injury. J. Trauma 15, 369–379 [PubMed] [Google Scholar]
- 12.Silva L., Garcia L., Oliveira B., Tanita M., Festti J., Cardoso L., Lavado L., Grion C. (2016) Acute respiratory distress syndrome in burn patients: incidence and risk factor analysis. Ann. Burns Fire Disasters 29, 178–182 [PMC free article] [PubMed] [Google Scholar]
- 13.Doerschuk C. M., Allard M. F., Martin B. A., MacKenzie A., Autor A. P., Hogg J. C. (1987) Marginated pool of neutrophils in rabbit lungs. J. Appl. Physiol. (1985) 63, 1806–1815 [DOI] [PubMed] [Google Scholar]
- 14.Hogg J. C. (1987) Neutrophil kinetics and lung injury. Physiol. Rev. 67, 1249–1295 [DOI] [PubMed] [Google Scholar]
- 15.Reber L. L., Gillis C. M., Starkl P., Jönsson F., Sibilano R., Marichal T., Gaudenzio N., Bérard M., Rogalla S., Contag C. H., Bruhns P., Galli S. J. (2017) Neutrophil myeloperoxidase diminishes the toxic effects and mortality induced by lipopolysaccharide. J. Exp. Med. 214, 1249–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayashi F., Means T. K., Luster A. D. (2003) Toll-like receptors stimulate human neutrophil function. Blood 102, 2660–2669 [DOI] [PubMed] [Google Scholar]
- 17.Schwacha M. G., Chaudry I. H. (2002) The cellular basis of post-burn immunosuppression: macrophages and mediators. Int. J. Mol. Med. 10, 239–243 [PubMed] [Google Scholar]
- 18.Rimington T. L., Hodge E., Billington C. K., Bhaker S., K C B., Kilty I., Jelinsky S., Hall I. P., Sayers I. (2017) Defining the inflammatory signature of human lung explant tissue in the presence and absence of glucocorticoid. F1000 Res. 6, 460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D. S., Weinrauch Y., Zychlinsky A. (2004) Neutrophil extracellular traps kill bacteria. Science 303, 1532–1535 [DOI] [PubMed] [Google Scholar]
- 20.Fuchs T. A., Abed U., Goosmann C., Hurwitz R., Schulze I., Wahn V., Weinrauch Y., Brinkmann V., Zychlinsky A. (2007) Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 176, 231–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takei H., Araki A., Watanabe H., Ichinose A., Sendo F. (1996) Rapid killing of human neutrophils by the potent activator phorbol 12-myristate 13-acetate (PMA) accompanied by changes different from typical apoptosis or necrosis. J. Leukoc. Biol. 59, 229–240 [DOI] [PubMed] [Google Scholar]
- 22.Yipp B. G., Petri B., Salina D., Jenne C. N., Scott B. N., Zbytnuik L. D., Pittman K., Asaduzzaman M., Wu K., Meijndert H. C., Malawista S. E., de Boisfleury Chevance A., Zhang K., Conly J., Kubes P. (2012) Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat. Med. 18, 1386–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu J., Zhang X., Pelayo R., Monestier M., Ammollo C. T., Semeraro F., Taylor F. B., Esmon N. L., Lupu F., Esmon C. T. (2009) Extracellular histones are major mediators of death in sepsis. Nat. Med. 15, 1318–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farrera C., Fadeel B. (2013) Macrophage clearance of neutrophil extracellular traps is a silent process. J. Immunol. 191, 2647–2656 [DOI] [PubMed] [Google Scholar]
- 25.Lee H. Y., Kaneki M., Andreas J., Tompkins R. G., Martyn J. A. (2011) Novel mitochondria-targeted antioxidant peptide ameliorates burn-induced apoptosis and endoplasmic reticulum stress in the skeletal muscle of mice. Shock 36, 580–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rittirsch D., Flierl M. A., Day D. E., Nadeau B. A., McGuire S. R., Hoesel L. M., Ipaktchi K., Zetoune F. S., Sarma J. V., Leng L., Huber-Lang M. S., Neff T. A., Bucala R., Ward P. A. (2008) Acute lung injury induced by lipopolysaccharide is independent of complement activation. J. Immunol. 180, 7664–7672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khan M. A., Farkhondeh M., Crombie J., Jacobson L., Kaneki M., Martyn J. A. (2012) Lipopolysaccharide upregulates α7 acetylcholine receptors: stimulation with GTS-21 mitigates growth arrest of macrophages and improves survival in burned mice. Shock 38, 213–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta S., Feng L., Yoshimura T., Redick J., Fu S. M., Rose C. E., Jr (1996) Intra-alveolar macrophage-inflammatory peptide 2 induces rapid neutrophil localization in the lung. Am. J. Respir. Cell Mol. Biol. 15, 656–663 [DOI] [PubMed] [Google Scholar]
- 29.Huang S., Paulauskis J. D., Godleski J. J., Kobzik L. (1992) Expression of macrophage inflammatory protein-2 and KC mRNA in pulmonary inflammation. Am. J. Pathol. 141, 981–988 [PMC free article] [PubMed] [Google Scholar]
- 30.Delgado-Rizo V., Martínez-Guzmán M. A., Iñiguez-Gutierrez L., García-Orozco A., Alvarado-Navarro A., Fafutis-Morris M. (2017) Neutrophil extracellular traps and its implications in inflammation: an overview. Front. Immunol. 8, 81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng O. Z., Palaniyar N. (2013) NET balancing: a problem in inflammatory lung diseases. Front. Immunol. 4, 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thurley K., Gerecht D., Friedmann E., Höfer T. (2015) Three-dimensional gradients of cytokine signaling between T cells. PLOS Comput. Biol. 11, e1004206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sedgwick J. B., Menon I., Gern J. E., Busse W. W. (2002) Effects of inflammatory cytokines on the permeability of human lung microvascular endothelial cell monolayers and differential eosinophil transmigration. J. Allergy Clin. Immunol. 110, 752–756 [DOI] [PubMed] [Google Scholar]
- 34.Wilson N. J., Boniface K., Chan J. R., McKenzie B. S., Blumenschein W. M., Mattson J. D., Basham B., Smith K., Chen T., Morel F., Lecron J. C., Kastelein R. A., Cua D. J., McClanahan T. K., Bowman E. P., de Waal Malefyt R. (2007) Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat. Immunol. 8, 950–957 [DOI] [PubMed] [Google Scholar]
- 35.Acosta-Rodriguez E. V., Napolitani G., Lanzavecchia A., Sallusto F. (2007) Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat. Immunol. 8, 942–949 [DOI] [PubMed] [Google Scholar]
- 36.Tsai H. C., Velichko S., Hung L. Y., Wu R. (2013) IL-17A and Th17 cells in lung inflammation: an update on the role of Th17 cell differentiation and IL-17R signaling in host defense against infection. Clin. Dev. Immunol. 2013, 267971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fillion I., Ouellet N., Simard M., Bergeron Y., Sato S., Bergeron M. G. (2001) Role of chemokines and formyl peptides in pneumococcal pneumonia-induced monocyte/macrophage recruitment. J. Immunol. 166, 7353–7361 [DOI] [PubMed] [Google Scholar]
- 38.Smith R. E., Strieter R. M., Zhang K., Phan S. H., Standiford T. J., Lukacs N. W., Kunkel S. L. (1995) A role for C-C chemokines in fibrotic lung disease. J. Leukoc. Biol. 57, 782–787 [DOI] [PubMed] [Google Scholar]
- 39.Williams A. E., José R. J., Mercer P. F., Brealey D., Parekh D., Thickett D. R., O’Kane C., McAuley D. F., Chambers R. C. (2017) Evidence for chemokine synergy during neutrophil migration in ARDS. Thorax 72, 66–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Douda D. N., Farmakovski N., Dell S., Grasemann H., Palaniyar N. (2009) SP-D counteracts GM-CSF-mediated increase of granuloma formation by alveolar macrophages in lysinuric protein intolerance. Orphanet J. Rare Dis. 4, 29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Puljic R., Benediktus E., Plater-Zyberk C., Baeuerle P. A., Szelenyi S., Brune K., Pahl A. (2007) Lipopolysaccharide-induced lung inflammation is inhibited by neutralization of GM-CSF. Eur. J. Pharmacol. 557, 230–235 [DOI] [PubMed] [Google Scholar]
- 42.Lenzo J. C., Turner A. L., Cook A. D., Vlahos R., Anderson G. P., Reynolds E. C., Hamilton J. A. (2012) Control of macrophage lineage populations by CSF-1 receptor and GM-CSF in homeostasis and inflammation. Immunol. Cell Biol. 90, 429–440 [DOI] [PubMed] [Google Scholar]
- 43.Vlahos R., Bozinovski S., Chan S. P., Ivanov S., Lindén A., Hamilton J. A., Anderson G. P. (2010) Neutralizing granulocyte/macrophage colony-stimulating factor inhibits cigarette smoke-induced lung inflammation. Am. J. Respir. Crit. Care Med. 182, 34–40 [DOI] [PubMed] [Google Scholar]
- 44.Kariya S., Okano M., Higaki T., Makihara S., Haruna T., Eguchi M., Nishizaki K. (2013) Neutralizing antibody against granulocyte/macrophage colony-stimulating factor inhibits inflammatory response in experimental otitis media. Laryngoscope 123, 1514–1518 [DOI] [PubMed] [Google Scholar]
- 45.Lin F., Nguyen C. M., Wang S. J., Saadi W., Gross S. P., Jeon N. L. (2004) Effective neutrophil chemotaxis is strongly influenced by mean IL-8 concentration. Biochem. Biophys. Res. Commun. 319, 576–581 [DOI] [PubMed] [Google Scholar]
- 46.Harada A., Sekido N., Akahoshi T., Wada T., Mukaida N., Matsushima K. (1994) Essential involvement of interleukin-8 (IL-8) in acute inflammation. J. Leukoc. Biol. 56, 559–564 [PubMed] [Google Scholar]
- 47.VanOtteren G. M., Standiford T. J., Kunkel S. L., Danforth J. M., Burdick M. D., Abruzzo L. V., Strieter R. M. (1994) Expression and regulation of macrophage inflammatory protein-1 alpha by murine alveolar and peritoneal macrophages. Am. J. Respir. Cell Mol. Biol. 10, 8–15 [DOI] [PubMed] [Google Scholar]
- 48.Rathore J. S., Wang Y. (2016) Protective role of Th17 cells in pulmonary infection. Vaccine 34, 1504–1514 [DOI] [PubMed] [Google Scholar]
- 49.Laan M., Cui Z. H., Hoshino H., Lötvall J., Sjöstrand M., Gruenert D. C., Skoogh B. E., Lindén A. (1999) Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J. Immunol. 162, 2347–2352 [PubMed] [Google Scholar]
- 50.Gurczynski S. J., Moore B. B. (2018) IL-17 in the lung: the good, the bad, and the ugly. Am. J. Physiol. Lung Cell. Mol. Physiol. 314, L6–L16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Neely C. J., Maile R., Wang M. J., Vadlamudi S., Meyer A. A., Cairns B. A. (2011) Th17 (IFNγ- IL17+) CD4+ T cells generated after burn injury may be a novel cellular mechanism for postburn immunosuppression. J. Trauma 70, 681–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Inatsu A., Kogiso M., Jeschke M. G., Asai A., Kobayashi M., Herndon D. N., Suzuki F. (2011) Lack of Th17 cell generation in patients with severe burn injuries. J. Immunol. 187, 2155–2161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thorley A. J., Goldstraw P., Young A., Tetley T. D. (2005) Primary human alveolar type II epithelial cell CCL20 (macrophage inflammatory protein-3alpha)-induced dendritic cell migration. Am. J. Respir. Cell Mol. Biol. 32, 262–267 [DOI] [PubMed] [Google Scholar]
- 54.Ovrevik J., Låg M., Holme J. A., Schwarze P. E., Refsnes M. (2009) Cytokine and chemokine expression patterns in lung epithelial cells exposed to components characteristic of particulate air pollution. Toxicology 259, 46–53 [DOI] [PubMed] [Google Scholar]
- 55.Cruikshank W., Little F. (2008) lnterleukin-16: the ins and outs of regulating T-cell activation. Crit. Rev. Immunol. 28, 467–483 [DOI] [PubMed] [Google Scholar]
- 56.Campanella G. S., Grimm J., Manice L. A., Colvin R. A., Medoff B. D., Wojtkiewicz G. R., Weissleder R., Luster A. D. (2006) Oligomerization of CXCL10 is necessary for endothelial cell presentation and in vivo activity. J. Immunol. 177, 6991–6998 [DOI] [PubMed] [Google Scholar]
- 57.Zhang Y., Zhang Y., Gu W., Sun B. (2014) TH1/TH2 cell differentiation and molecular signals. Adv. Exp. Med. Biol. 841, 15–44; erratum: E1–E2 [DOI] [PubMed] [Google Scholar]
- 58.Berger A. (2000) Th1 and Th2 responses: what are they? BMJ 321, 424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seder R. A., Paul W. E. (1994) Acquisition of lymphokine-producing phenotype by CD4+ T cells. Annu. Rev. Immunol. 12, 635–673 [DOI] [PubMed] [Google Scholar]
- 60.Barner M., Mohrs M., Brombacher F., Kopf M. (1998) Differences between IL-4R alpha-deficient and IL-4-deficient mice reveal a role for IL-13 in the regulation of Th2 responses. Curr. Biol. 8, 669–672 [DOI] [PubMed] [Google Scholar]
- 61.Huang J., Yue H., Jiang T., Gao J., Shi Y., Shi B., Wu X., Gou X. (2019) IL-31 plays dual roles in lung inflammation in an OVA-induced murine asthma model. Biol. Open 8, bio036244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lai H., Rogers D. F. (2010) New pharmacotherapy for airway mucus hypersecretion in asthma and COPD: targeting intracellular signaling pathways. J. Aerosol Med. Pulm. Drug Deliv. 23, 219–231 [DOI] [PubMed] [Google Scholar]
- 63.Kang J. W., Lee Y. H., Kang M. J., Lee H. J., Oh R., Min H. J., Namkung W., Choi J. Y., Lee S. N., Kim C. H., Yoon J. H., Cho H. J. (2017) Synergistic mucus secretion by histamine and IL-4 through TMEM16A in airway epithelium. Am. J. Physiol. Lung Cell. Mol. Physiol. 313, L466–L476 [DOI] [PubMed] [Google Scholar]
- 64.Ding F., Liu B., Zou W., Tian D., Li Q., Dai J., Luo Z., Fu Z. (2018) LPS exposure in early life protects against mucus hypersecretion in ovalbumin-induced asthma by down-regulation of the IL-13 and JAK-STAT6 pathways. Cell. Physiol. Biochem. 46, 1263–1274 [DOI] [PubMed] [Google Scholar]
- 65.Jakiela B., Szczeklik W., Plutecka H., Sokolowska B., Mastalerz L., Sanak M., Bazan-Socha S., Szczeklik A., Musial J. (2012) Increased production of IL-5 and dominant Th2-type response in airways of Churg-Strauss syndrome patients. Rheumatology (Oxford) 51, 1887–1893 [DOI] [PubMed] [Google Scholar]
- 66.Cayrol C., Girard J. P. (2018) Interleukin-33 (IL-33): a nuclear cytokine from the IL-1 family. Immunol. Rev. 281, 154–168 [DOI] [PubMed] [Google Scholar]
- 67.Kundu-Raychaudhuri S., Abria C., Raychaudhuri S. P. (2016) IL-9, a local growth factor for synovial T cells in inflammatory arthritis. Cytokine 79, 45–51 [DOI] [PubMed] [Google Scholar]
- 68.Vermeer P. D., Harson R., Einwalter L. A., Moninger T., Zabner J. (2003) Interleukin-9 induces goblet cell hyperplasia during repair of human airway epithelia. Am. J. Respir. Cell Mol. Biol. 28, 286–295 [DOI] [PubMed] [Google Scholar]
- 69.Whittaker L., Niu N., Temann U. A., Stoddard A., Flavell R. A., Ray A., Homer R. J., Cohn L. (2002) Interleukin-13 mediates a fundamental pathway for airway epithelial mucus induced by CD4 T cells and interleukin-9. Am. J. Respir. Cell Mol. Biol. 27, 593–602 [DOI] [PubMed] [Google Scholar]
- 70.Berard M., Brandt K., Bulfone-Paus S., Tough D. F. (2003) IL-15 promotes the survival of naive and memory phenotype CD8+ T cells. J. Immunol. 170, 5018–5026 [DOI] [PubMed] [Google Scholar]
- 71.Martinez G. J., Nurieva R. I., Yang X. O., Dong C. (2008) Regulation and function of proinflammatory TH17 cells. Ann. N. Y. Acad. Sci. 1143, 188–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kelly E., Won A., Refaeli Y., Van Parijs L. (2002) IL-2 and related cytokines can promote T cell survival by activating AKT. J. Immunol. 168, 597–603 [DOI] [PubMed] [Google Scholar]
- 73.Sun L., He C., Nair L., Yeung J., Egwuagu C. E. (2015) Interleukin 12 (IL-12) family cytokines: role in immune pathogenesis and treatment of CNS autoimmune disease. Cytokine 75, 249–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Trinchieri G., Pflanz S., Kastelein R. A. (2003) The IL-12 family of heterodimeric cytokines: new players in the regulation of T cell responses. Immunity 19, 641–644 [DOI] [PubMed] [Google Scholar]
- 75.Cooper A. M., Khader S. A. (2007) IL-12p40: an inherently agonistic cytokine. Trends Immunol. 28, 33–38 [DOI] [PubMed] [Google Scholar]
- 76.Colgan J., Rothman P. (2006) All in the family: IL-27 suppression of T(H)-17 cells. Nat. Immunol. 7, 899–901 [DOI] [PubMed] [Google Scholar]
- 77.Iwasaki Y., Fujio K., Okamura T., Yamamoto K. (2015) Interleukin-27 in T cell immunity. Int. J. Mol. Sci. 16, 2851–2863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stumhofer J. S., Silver J. S., Laurence A., Porrett P. M., Harris T. H., Turka L. A., Ernst M., Saris C. J., O’Shea J. J., Hunter C. A. (2007) Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat. Immunol. 8, 1363–1371 [DOI] [PubMed] [Google Scholar]
- 79.Pot C., Jin H., Awasthi A., Liu S. M., Lai C. Y., Madan R., Sharpe A. H., Karp C. L., Miaw S. C., Ho I. C., Kuchroo V. K. (2009) Cutting edge: IL-27 induces the transcription factor c-Maf, cytokine IL-21, and the costimulatory receptor ICOS that coordinately act together to promote differentiation of IL-10-producing Tr1 cells. J. Immunol. 183, 797–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ethuin F., Gérard B., Benna J. E., Boutten A., Gougereot-Pocidalo M. A., Jacob L., Chollet-Martin S. (2004) Human neutrophils produce interferon gamma upon stimulation by interleukin-12. Lab. Invest. 84, 1363–1371 [DOI] [PubMed] [Google Scholar]
- 81.Inoue G. (2000) Effect of interleukin-10 (IL-10) on experimental LPS-induced acute lung injury. J. Infect. Chemother. 6, 51–60 [DOI] [PubMed] [Google Scholar]
- 82.Gu C., Wu L., Li X. (2013) IL-17 family: cytokines, receptors and signaling. Cytokine 64, 477–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wright J. F., Bennett F., Li B., Brooks J., Luxenberg D. P., Whitters M. J., Tomkinson K. N., Fitz L. J., Wolfman N. M., Collins M., Dunussi-Joannopoulos K., Chatterjee-Kishore M., Carreno B. M. (2008) The human IL-17F/IL-17A heterodimeric cytokine signals through the IL-17RA/IL-17RC receptor complex. J. Immunol. 181, 2799–2805 [DOI] [PubMed] [Google Scholar]
- 84.Hampson P., Dinsdale R. J., Wearn C. M., Bamford A. L., Bishop J. R. B., Hazeldine J., Moiemen N. S., Harrison P., Lord J. M. (2017) Neutrophil dysfunction, immature granulocytes, and cell-free DNA are early biomarkers of sepsis in burn-injured patients: a prospective observational cohort study. Ann. Surg. 265, 1241–1249 [DOI] [PubMed] [Google Scholar]
- 85.Kaufman T., Magosevich D., Moreno M. C., Guzman M. A., D’Atri L. P., Carestia A., Fandiño M. E., Fondevila C., Schattner M. (2017) Nucleosomes and neutrophil extracellular traps in septic and burn patients. Clin. Immunol. 183, 254–262 [DOI] [PubMed] [Google Scholar]
- 86.Korkmaz H. I., Ulrich M. M. W., Vogels S., de Wit T., van Zuijlen P. P. M., Krijnen P. A. J., Niessen H. W. M. (2017) Neutrophil extracellular traps coincide with a pro-coagulant status of microcirculatory endothelium in burn wounds. Wound Repair Regen. 25, 609–617 [DOI] [PubMed] [Google Scholar]
- 87.Hur J., Yang H. T., Chun W., Kim J. H., Shin S. H., Kang H. J., Kim H. S. (2015) Inflammatory cytokines and their prognostic ability in cases of major burn injury. Ann. Lab. Med. 35, 105–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tanimoto N., Terasawa M., Nakamura M., Kegai D., Aoshima N., Kobayashi Y., Nagata K. (2007) Involvement of KC, MIP-2, and MCP-1 in leukocyte infiltration following injection of necrotic cells into the peritoneal cavity. Biochem. Biophys. Res. Commun. 361, 533–536 [DOI] [PubMed] [Google Scholar]
- 89.Guillot L., Carroll S. F., Homer R., Qureshi S. T. (2008) Enhanced innate immune responsiveness to pulmonary Cryptococcus neoformans infection is associated with resistance to progressive infection. Infect. Immun. 76, 4745–4756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Singer M., Sansonetti P. J. (2004) IL-8 is a key chemokine regulating neutrophil recruitment in a new mouse model of Shigella-induced colitis. J. Immunol. 173, 4197–4206 [DOI] [PubMed] [Google Scholar]
- 91.Sasaki J. R., Zhang Q., Schwacha M. G. (2011) Burn induces a Th-17 inflammatory response at the injury site. Burns 37, 646–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Griffin G. K., Newton G., Tarrio M. L., Bu D. X., Maganto-Garcia E., Azcutia V., Alcaide P., Grabie N., Luscinskas F. W., Croce K. J., Lichtman A. H. (2012) IL-17 and TNF-α sustain neutrophil recruitment during inflammation through synergistic effects on endothelial activation. J. Immunol. 188, 6287–6299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xu S., Cao X. (2010) Interleukin-17 and its expanding biological functions. Cell. Mol. Immunol. 7, 164–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ferretti S., Bonneau O., Dubois G. R., Jones C. E., Trifilieff A. (2003) IL-17, produced by lymphocytes and neutrophils, is necessary for lipopolysaccharide-induced airway neutrophilia: IL-15 as a possible trigger. J. Immunol. 170, 2106–2112 [DOI] [PubMed] [Google Scholar]
- 95.Cortjens B., de Boer O. J., de Jong R., Antonis A. F., Sabogal Piñeros Y. S., Lutter R., van Woensel J. B., Bem R. A. (2016) Neutrophil extracellular traps cause airway obstruction during respiratory syncytial virus disease. J. Pathol. 238, 401–411 [DOI] [PubMed] [Google Scholar]
- 96.Yipp B. G., Kubes P. (2013) NETosis: how vital is it? Blood 122, 2784–2794 [DOI] [PubMed] [Google Scholar]
- 97.Masuda S., Nakazawa D., Shida H., Miyoshi A., Kusunoki Y., Tomaru U., Ishizu A. (2016) NETosis markers: quest for specific, objective, and quantitative markers. Clin. Chim. Acta 459, 89–93 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.