Abstract
Cerebral malaria (CM) from Plasmodium falciparum infection is associated with endothelial dysfunction and parasite sequestration. The glycocalyx (GCX), a carbohydrate-rich layer lining the endothelium, is crucial in vascular homeostasis. To evaluate the role of its loss in the pathogenesis of pediatric CM, we measured GCX degradation in Tanzanian children with World Health Organization–defined CM (n = 55), uncomplicated malaria (UM; n = 20), and healthy controls (HCs; n = 25). Urine GCX breakdown products [glycosaminoglycans (GAGs)] were quantified using dimethylmethylene blue (DMMB) and liquid chromatography–tandem mass spectrometry assays. DMMB-GAG and mass spectrometry (MS)-GAG (g/mol creatinine) were increased in CM and UM compared with HCs (P < 0.001), with no differences in DMMB-GAG and MS-GAG between CM and UM children or between those with and without a fatal outcome. In CM survivors, urinary GCX DMMB-GAG normalized by d 3. After adjusting for disease severity, DMMB-GAG was significantly associated with parasitemia [partial correlation coefficient (Pcorr) = 0.34; P = 0.01] and plasma TNF (Pcorr = 0.26; P = 0.04) and inversely with plasma and urine NO oxidation products [Pcorr = −0.31 (P = 0.01) and Pcorr = −0.26 (P = 0.03), respectively]. GCX breakdown is increased in children with falciparum malaria, with similar elevations in CM and UM. Endothelial GCX degradation may impair endothelial NO production, exacerbate adhesion-molecule expression, exposure, and parasite sequestration, and contribute to malaria pathogenesis.—Yeo, T. W., Bush, P. A., Chen, Y., Young, S. P., Zhang, H., Millington, D. S., Granger, D. L., Mwaikambo, E. D., Anstey, N. M., Weinberg, J. B. Glycocalyx breakdown is increased in African children with cerebral and uncomplicated falciparum malaria.
Keywords: Plasmodium falciparum, cerebral malaria, nitric oxide, endothelial glycocalyx
Malaria is an important global health problem with an estimated 219 million symptomatic cases and 435,000 deaths in 2017 (1), with over 90% in Sub-Saharan African children (2). Even with use of artesunate, the most effective antimalarial agent, the mortality rate from severe malaria in children remains at 8%, and up to 18% in cerebral malaria (CM). Delineating the pathogenic mechanisms of severe falciparum malaria is required to develop adjunctive agents to improve outcomes by targeting specific disease processes.
The vascular endothelium and microcirculation play major roles in the pathogenesis of malaria (3). Infected erythrocytes cytoadhere to endothelial receptors, resulting in parasite sequestration, impaired perfusion, tissue hypoxia, and organ damage (3). We have previously established that decreased vascular NO, endothelial activation, and dysregulated microcirculatory flow are key pathogenic mechanisms associated with disease severity and death in malaria (4–6); however, the underlying mechanisms are not fully understood.
The glycocalyx (GCX) is a gel-like layer that covers cellular surfaces throughout the body with largely homeostatic functions (7, 8). Structurally, the GCX is a matrix of macromolecules consisting mainly of glycoproteins and proteoglycans with various soluble molecules and proteins within (7, 8). Proteoglycans consist of a core protein with glycosaminoglycan (GAG) side chains attached (7, 8). Common GAGs include heparan sulfate (HS), chondroitin sulfate (CS), dermatan sulfate (DS), keratan sulfate, and hyaluronic acid (7). The endothelial GCX lines the luminal surface of endothelium throughout the vasculature, with an estimated thickness ranging from 0.5 to 5 μm, depending on vessel type (7, 8). The GAGs commonly found in the endothelial GCX include HS, CS, and hyaluronic acid, with HS and CS being the most common (7, 8), and the major core proteins include syndecans and glypicans (7, 8). The endothelial GCX has notable structural heterogeneity corresponding with multiple functions that support homeostasis. These include providing a barrier to regulate vascular permeability (9), stimulation of NO production by eNOS through HS-mediated transduction of flow-shear stress (10–12), and protection from adhesion of parasitized red cells and platelets to endothelial cells (13–15).
Breakdown of endothelial GCX impairs vascular homeostasis, resulting in endothelial dysfunction, decreased NO production, vascular leakage, and increased adhesion of leukocytes and platelets (16). GCX degradation is suspected to contribute to disease pathology in multiple conditions, including cardiovascular diseases (e.g., atherosclerosis, stroke, and hypertension), chronic kidney disease, pulmonary edema, and diabetes mellitus (8). GCX degradation is also implicated in the pathogenesis of complications in certain infectious diseases including sepsis (17) and dengue (18). In murine malaria models, endothelial GCX breakdown correlates with disease severity (19). GCX degradation is increased in adults with falciparum malaria (20), but there are no data in children, the age-group with the highest burden of disease and death from falciparum malaria (1). Here, we study aspects of the GCX in African children with uncomplicated, severe, and fatal falciparum malaria. The objective of this study was to determine if GCX breakdown occurs in pediatric Plasmodium falciparum malaria infections, and if so, the association with disease severity and measures of NO bioavailability.
MATERIALS AND METHODS
Human subjects
Healthy pediatric subjects and those diagnosed with malaria at the Muhimbili Medical Center (MMC) in Dar es Salaam, Tanzania, were prospectively enrolled as previously described in ref. 4. The study was approved by the Institutional Review Board at Duke University Medical Center and the MMC College Research and Publications Committee. The parents or guardians of all participant children provided informed consent.
Children aged 6 mo to 9 yr were recruited from pediatric or surgical units from 1994 to 1995. For the purposes of the GAG analyses, there were subsets of the following 3 groups from which remaining specimens were available for analyses: 1) healthy control (HC) subjects were afebrile, not acutely ill, with normal leukocyte counts; 2) patients with uncomplicated malaria (UM) were fully alert and febrile with blood P. falciparum ≥10,000 parasites/μl, normoglycemic, and with no other cause of fever, history of convulsions, or severe respiratory distress; and 3) CM were patients with coma (unarousable; Blantyre score of 2 or less persisting more than 30 min beyond last convulsion), and any level of P. falciparum parasitemia. The CM group included children who had complete recovery without neurologic complications as well as those with a fatal outcome or neurologic sequelae at discharge. Children with malaria received the standard of care treatment for malaria at the time of the study, which included quinine or chloroquine.
Clinical parameters and outcomes
We utilized a standardized data collection form to record these for all subjects during the course of the study. In some instances, limited sample volumes prevented performance of all desired assays. Admission laboratory testing included blood glucose and the automated blood parameters hemoglobin and white blood count.
GAG analyses
GAGs are stable over extended periods of time and various conditions including freezing. Indeed, Schmidt et al. (17) successfully used samples that had been stored many years for their GAG analyses, and Embery et al. (21) were able to extract intact GAGs (CS and hyaluronan) from Iguandodon dinosaur bones that were 125–135 million years old. For our studies in 1994–1995, urine was collected at enrollment (4) and then stored frozen at −80°C. We analyzed and measured GAG levels in urine using 2 methods as a measure of GCX degradation. For the first method, we measured total sulfated urinary GAG with a colorimetric dimethylmethylene blue (DMMB) assay as previously described in refs. 17 and 22). Briefly, urine was thawed at room temperature prior to the assay and centrifuged to remove all debris. Absorbance was determined at 525 nm, and we used a CS calibration curve to determine GAG concentration.
The second method used isotope-dilution ultraperformance liquid chromatography (UPLC)–electrospray ionization tandem MS (MS/MS) to measure individual GAG including HS, CS, and DS as previously described in Zhang et al. (23). For the UPLC-MS/MS analyses, 25 μl aliquots were transferred to borosilicate injection vials. Following evaporation under nitrogen, residues were treated with 0.2 ml of 3 M HCl in methanol for 75 min at 65°C. Following evaporation, deuterium-labeled CS, DS, and HS methanolysate dimers in matrix were added as isotope-labeled internal standards, samples were separated on a UPLC Ethylene Bridged Hybrid (BEH) Amide 1.7-μm, 2.1 × 50–mm column (Waters, Milford, MA, USA) with gradient elution, and analytes were detected by selected reaction monitoring on a Xevo-TQ MS mass spectrometer equipped with an Acquity UPLC system (Waters). Peak area ratios of target analyte to the corresponding internal standard were converted to a concentration using calibration curves. Concentrations were normalized to urinary creatinine to correct for urine dilution as previously described in refs. 17 and 24. Urinary creatinine concentrations are known to be stable in frozen urine for over 10 yr (25). The sum of CS, DS, and HS concentrations was used as an estimate of the total GAG concentration. Urinary creatinine concentrations were determined by the alkaline picrate method (Sigma Diagnostics, Livonia, MI, USA). Concentrations of GAG were normalized to urinary creatinine (g/mol) in both the DMMB and the UPLC-MS/MS assays (22, 26).
NO production
The oxidation products of NO, nitrate and nitrite (NOx), were measured in plasma by capillary electrophoresis (4, 27) and in urine by the Griess reaction (4, 28), as previously reported. Because nitrate is retained in renal impairment, plasma and urine NOx concentrations were expressed as a ratio of plasma or urine creatinine. Plasma creatinine was measured by an Ektachem autoanalyzer (Kodak, Rochester, NY, USA) as previously reported in refs. 4 and 27. Plasma creatinine was also expressed as the percentage of mean for age (29). Diet was controlled in healthy subjects and in those with asymptomatic parasitemia to control for potential confounding results related to exogenous nitrate ingestion (4, 27).
Markers of disease severity
Parasite counts were determined using Giemsa-stained thick- and thin-blood films. Plasma TNF-α and IL-10 concentrations were determined by ELISA as previously described in Anstey et al. (4).
Statistical analysis
The intergroup differences for continuous variables were compared by ANOVA or the Kruskal-Wallis test depending on the distribution of the data. A priori post hoc pair-wise comparisons were used to compare CM, UM, and HC using the Sidak method. The Pearson’s or Spearman’s methods were used to determine correlation coefficients for continuous variables as appropriate for the distribution. Partial correlation coefficients (Pcorrs) were calculated adjusting for disease severity. Linear mixed-effect models were used to analyze longitudinal trends. Statistical analyses were conducted with Stata software (v.14; StataCorp, College Station, TX, USA). A 2-sided value of P < 0.05 was considered significant.
RESULTS
Patients
A total of 100 children who were part of a larger prospective study of 191 children had urine available for GAG analysis, including 25 HCs, 20 with UM, and 55 with CM. The clinical features of these children have been previously described in Anstey et al. (4). In brief, the mean age and weight were similar for each group, with the mean hemoglobin levels being inversely related to disease severity. In the CM group, 5 (9%) children had a fatal outcome and 8 (14.5%) had neurologic sequelae (1 with cortical blindness, quadriparesis, and deafness; 3 with cortical blindness and quadriparesis; 2 with cortical blindness; 1 with deafness; and 1 with inability to talk), with the remainder making a full recovery at the time of discharge. In the UM group, there were no fatalities, and in the HCs, there were 4 children with asymptomatic parasitemia. Baseline characteristics and laboratory results of the patients are summarized in Table 1.
TABLE 1.
Baseline demographic, hematologic, and biochemical features
| Group | HC (n = 25) | UM (n = 20) | CM (n = 55) | Pa |
|---|---|---|---|---|
| Male sex [n (%)] | 16 (64%) | 10 (50%) | 26 (47%) | 0.2 |
| Weight (kg) | 13.5 (4.8–22.7) | 12 (9–13) | 13 (9.5–15.0) | 0.3 |
| Blood pressure [mmHg (mean range)] | ||||
| Systolic | NA | NA | 104 (92–112) | NA |
| Diastolic | NA | NA | 60 (52–70) | NA |
| White blood cell count (×103 cells/µl) | 9.3 (5.9–14.8) | 10 (6.9–13.3) | 12.3 (8.0–17.6) | 0.02 |
| Hemoglobin (g/dl) | 10.7 (6.8–13.2) | 6 (4.9–8.5) | 6.7 (5.3–7.9) | <0.001 |
| Plasma creatinine level (µM) | 35.4 (26.5–53.0) | 35.4 (35.2–35.5) | 44.2 (35.36–53.0) | 0.001 |
| Creatinine (% of the mean for age) | 82.3 (4.8–96.3) | 97.4 (83.3–104.7) | 118.4 (100.8–136.9) | <0.001 |
| Creatinine [adjusted for bodyweight (µM/kg)] | 2.72 (1.99–3.32) | 3.11 (2.53–3.73) | 3.9 (3.3–5.44) | <0.001 |
| Parasite density (parasites/µl; geometric mean, 95% confidence interval) | 531 (26–9941)d | 38,525 (22,704–65,371) | 21,744 (11, 770–40,167) | <0.001 |
| Plasma NOx (µM) | 38.2 (21.1–120.5) | 38.15 (28.8–50.6) | 27.7 (21.1–37.8) | 0.004 |
| Plasma NOx/creatinine (µM/µM)b | 1.22 (0.48–3.48) | 0.74 (0.46–1.01) | 0.59 (0.36–0.71) | <0.001 |
| Urinary NOx/creatinine (µM/µM)b | 0.34 (0.12–0.58) | 0.15 (0.09–0.22) | 0.11 (0.08–0.16) | <0.001 |
| GAG-DMMB (g/mol creatinine)c | 5.4 (0.3–22.5) | 10.9 (3.9–33.6) | 12.8 (3.5–21.4) | <0.001 |
| GAG-MS (g/mol creatinine) | 13.6 (7.7–32.9) | 29.9 (18.8–62.9) | 24 (14.8–43.3) | <0.001 |
| CS-MS (g/mol creatinine) | 10.1 (5.5–25.0) | 19.6 (12.1–45.9) | 14.6 (7.6–28.5) | 0.004 |
| DS-MS (g/mol creatinine) | 2.1 (1.0–5.0) | 2.4 (1.2–5.1) | 2.4 (1.4–4.1) | 0.4 |
| HS-MS (g/mol creatinine) | 1.9 (1.1–6.3) | 4.9 (3.2–31.1) | 6.8 (3.9–12.8) | <0.001 |
| TNF (pg/ml) | 7.8 (7.8–7.8) | 7.8 (7.8–94.4) | 94.9 (7.8–186.6) | <0.001 |
| IL-10 (pg/ml) | 2.35 (1.9–154.7) | 124.1 (8.8–590.7) | 267.3 (2.35–2929.7) | <0.001 |
All values refer to median (interquartile range) unless otherwise specified. aANOVA or Kruskal-Wallis test, comparing the HC group, UM, and severe malaria groups; bcreatinine measured by Ektachem analyzer; curinary creatinine measured by alkaline picrate method; dn = 4 with a asymptomatic parasitemia. NA, not applicable.
Total sulfated GAG concentration in urine and disease severity
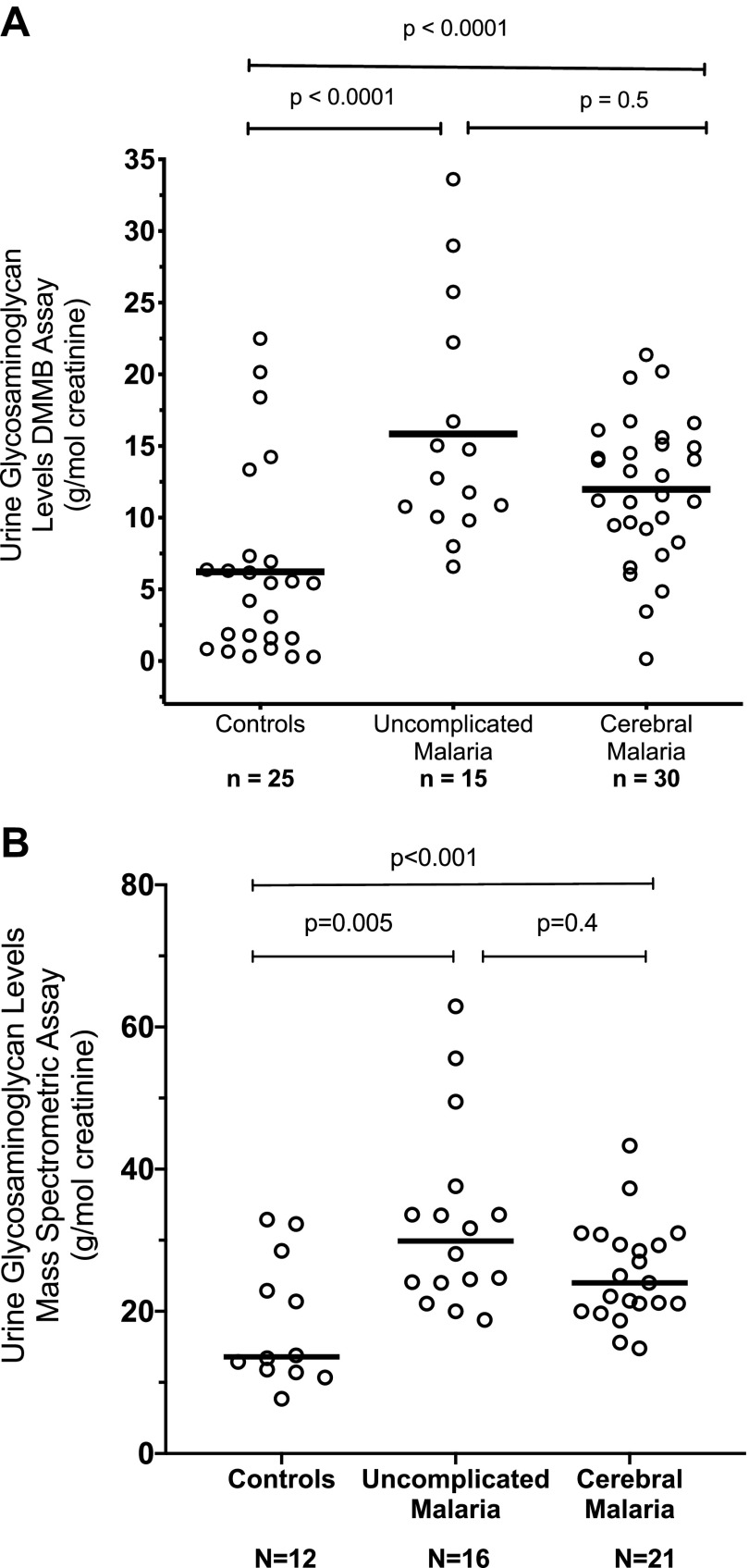
There were 36 children with CM with urine collected upon enrollment, and an additional 19 CM children had urine collected ∼24 h later. In the children with UM or CM, urinary excretion of total GAG as measured by the DMMB colorimetric assay (Fig. 1A) and UPLC-MS/MS (Fig. 1B) were significantly elevated compared with HCs (P < 0.001 by ANOVA). However, there was no significant difference between the children with UM and CM (P = 0.4) on post hoc pair-wise comparison. Within the group of children with CM, there was no significant difference between survivors who made a full recovery and those who had a fatal outcome or neurologic sequelae (P = 0.6).
Figure 1.
A) Urinary GAG levels measured by DMMB colorimetric assay in African children enrolled as HCs, UM, or CM. The horizontal bars indicate the median value. P < 0.001 by ANOVA among the 3 groups with post hoc pair-wise comparison between UM with CM and UM with HC indicated. B) Urinary GAG levels (sum of CS, DS, and HS) measured by a mass-spectrometric method assay in African children enrolled as HCs, UM, or CM. The horizontal bars indicate the median value. P < 0.001 by ANOVA with post hoc pair-wise comparison between UM with CM and UM with HC indicated.
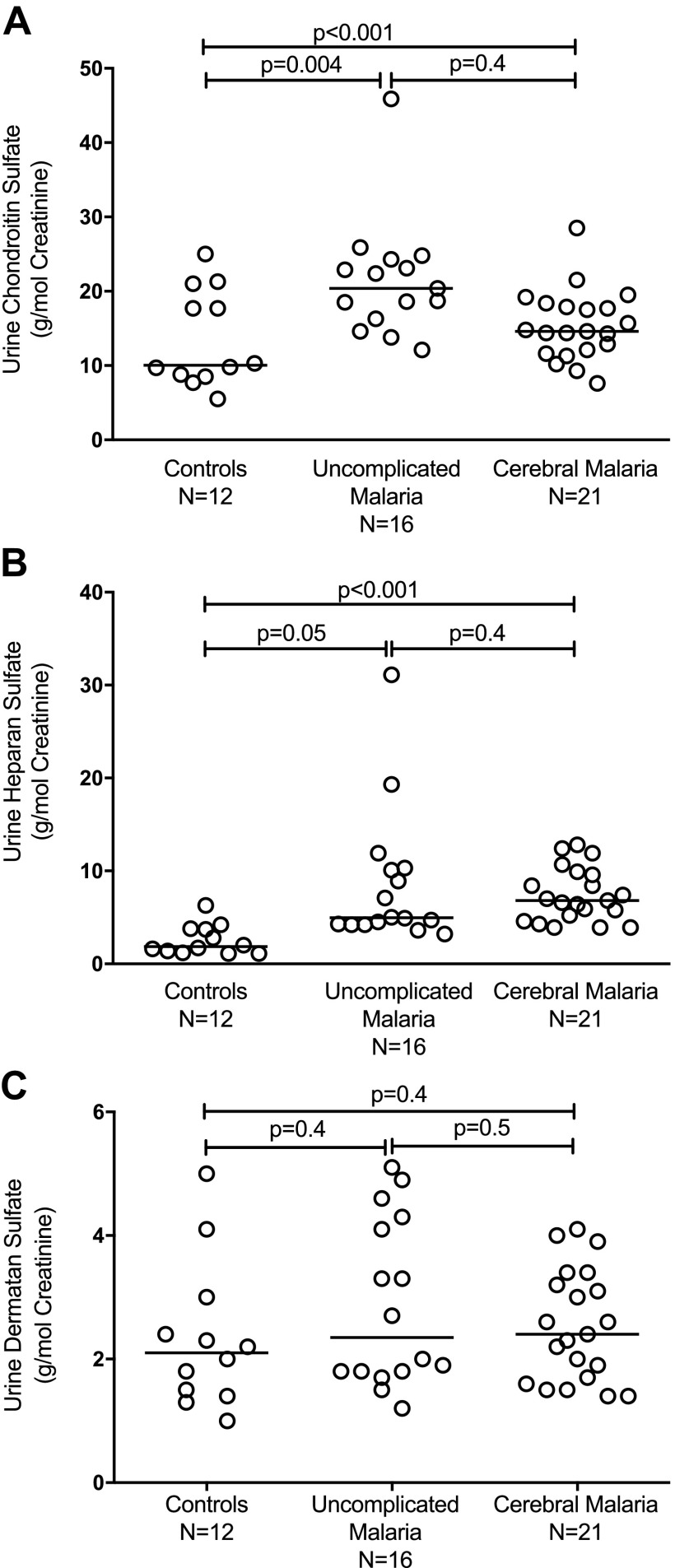
Levels of CS were significantly elevated in the UM and CM groups compared with HC subjects (P < 0.001) (Fig. 2A). Levels of HS were also significantly elevated in UM and CM compared with HCs (P < 0.001) (Fig. 2B). In contrast, levels of DS were similar in patients with UM and CM compared with HCs (P = 0.4) (Fig. 2C). There were no significant differences between CS and HS urinary levels between the CM children and those with UM (P = 0.4 respectively). To rule out the possibility that GCX breakdown products originated from the parasite or infected red blood cells (RBCs) in patients with malaria, we used the DMMB assay to test supernatant medium from in vitro cultures of P. falciparum growing in human RBCs. The culture supernatants from uninfected RBCs and P. falciparum–infected RBC cultures did not contain GAG (unpublished results).
Figure 2.
A) Urinary CS levels measured by a mass-spectrometric method assay in African children enrolled as HCs, UM, or CM. The horizontal bars indicate the median value. P < 0.001 by ANOVA with post hoc pair-wise comparison between UM with CM and UM with HC indicated. B) Urinary HS levels measured by a mass-spectrometric method assay in African children enrolled as HCs, UM, or CM. The horizontal bars indicate the median value. P < 0.001 by ANOVA with post hoc pair-wise comparison between UM with CM and UM with HC indicated. C) Urinary DS levels measured by a mass-spectrometric method assay in African children enrolled as HCs, UM, or CM. The horizontal bars indicate the median value. P = 0.4 by ANOVA with post hoc pair-wise comparison between UM with CM and UM with HC.
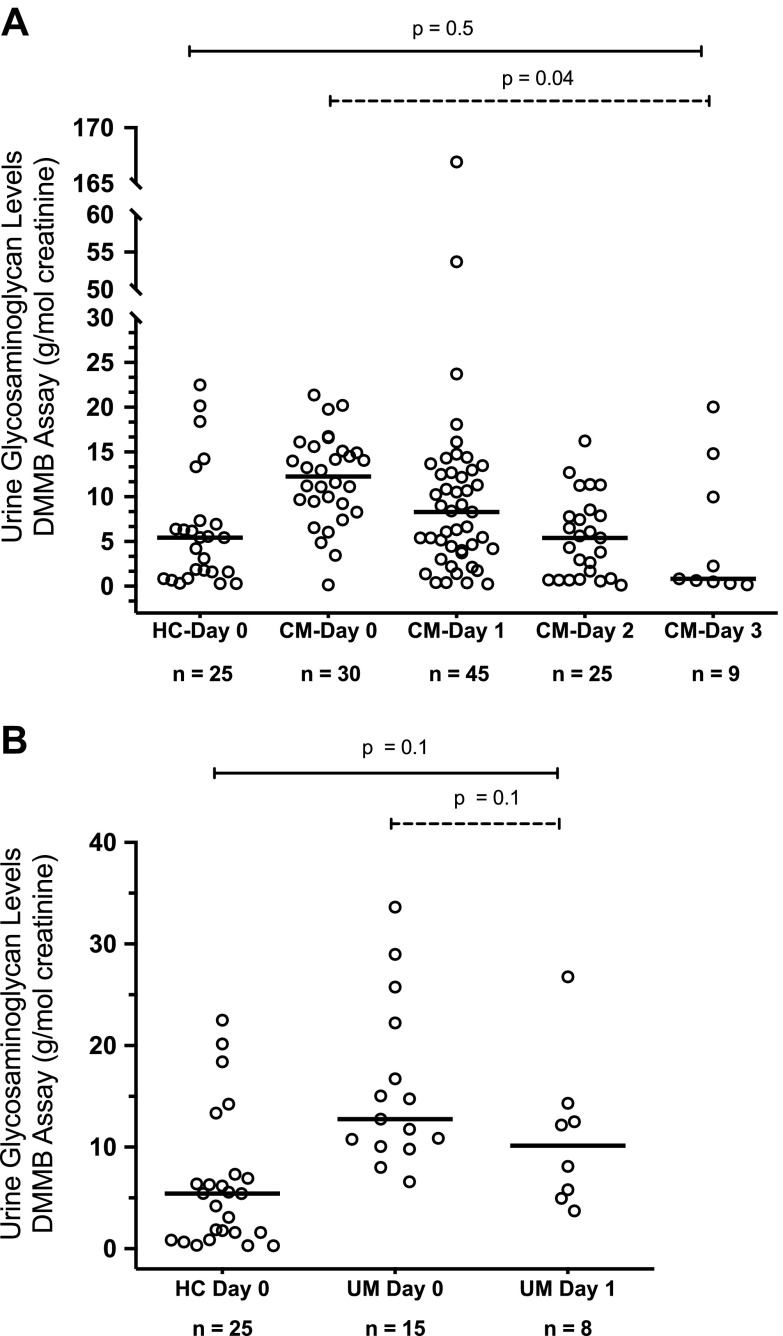
In several children with CM, we were able to measure urinary DMMB-GAG levels daily until discharge for up to 3 d (d 0–3). In this group, urinary DMMB-GAG:creatinine ratios decreased from the day of enrollment to control levels by d 3 (P = 0.04) (Fig. 3A). We were able to measure levels for only 2 d in several children with UM because their length of stay was relatively short, with no significant decrease in urine DMMB-GAG levels from enrollment d 0 to 1 (Fig. 3B).
Figure 3.
A) The urinary GAG levels measured by the DMMB colorimetric assay in HC children and children with CM from day of enrollment (d 0) until d 3. Dashed line indicates longitudinal change in DMMB-GAG levels by linear mixed-effects analysis (P = 0.04). Solid line indicates difference between DMMB- GAG levels at d 3 compared with controls (Mann-Whitney U test, P = 0.5). B) The urinary GAG levels measured by DMMB colorimetric assay in HC children and children with UM from day of enrollment (d 0) to d 1. Dashed line indicates longitudinal change in DMMB-GAG levels by linear mixed-effects analysis (P = 0.1). Solid line indicates difference between DMMB-GAG levels at d 1 compared with controls (Mann-Whitney U test, P = 0.1).
Total sulfated GAG concentration in urine and NO production
Plasma and urine oxidation products of NO (NOx), expressed as a ratio of plasma or urine creatinine, respectively, were significantly reduced in CM and UM compared with the HC group (Table 1), as we reported earlier (4). There was a significant inverse association between plasma and urinary NOx levels and DMMB-GAG [Pcorr = −0.31 (P = 0.01) and Pcorr = −0.26 (P = 0.03)], but not MS-GAG (P = 0.2) after adjusting for disease severity (Table 2). There were no significant associations between plasma and urinary NOx levels with urinary CS or HS levels.
TABLE 2.
Pcorr for urinary GAGs and biomarkers of severity
| Urinary GAG | Biomarker | Pcorr | P |
|---|---|---|---|
| GAG-DMMB | Plasma NOx | −0.31 | 0.01 |
| Urinary NOx | −0.26 | 0.03 | |
| TNF | 0.26 | 0.04 | |
| IL-10 | 0.36 | 0.005 | |
| Peripheral parasitemia | 0.34 | 0.01 | |
| Plasma creatinine | 0.12 | 0.2 | |
| GAG-MS | Plasma NOx | 0.19 | 0.2 |
| Urinary NOx | 0.21 | 0.2 | |
| TNF | 0.35 | 0.02 | |
| IL-10 | 0.44 | 0.005 | |
| Peripheral parasitemia | 0.10 | 0.4 | |
| Creatinine | 0.13 | 0.2 |
Each Pcorr adjusted for disease severity.
Total sulfated GAG concentration in urine and biomarkers of severity
Biomarkers of severity measured during the study included peripheral parasitemia, plasma creatinine, the cytokines TNF-α and IL-10 (Table 1). We were unable to measure plasma P. falciparum histidine-rich protein 2 (PfHRP2) levels. PfHRP2 is a better indicator of total-body burden of parasite biomass than blood parasitemia (30). PfHRP2 measurement was not available at the time of the study (1994–1995), and we did not have any plasma or serum available to measure. In brief, creatinine, TNF-α, and IL-10 concentrations were significantly increased in children with CM compared with levels in HC children. In addition, creatinine, TNF-α, and IL-10 concentrations were significantly higher in children with CM compared with levels in children with UM. There was no significant difference in peripheral parasite density between UM and CM subjects (Table 1).
There was a weak but statistically significant association between DMMB-GAG (Pcorr = 0.26; P = 0.04) and total MS-GAG with TNF-α levels after adjusting for disease severity (Pcorr = 0.35, P = 0.02) (Table 2). There was also a significant association with urinary CS levels and TNF-α (Pcorr = 0.39; P = 0.01) but not with HS or DS. In addition, there was a significant association between IL-10 with DMMB-GAG (Pcorr = 0.36; P = 0.005) and with total MS-GAG (Pcorrs = 0.44; P = 0.005) (Table 2). Urinary levels of CS, DS, or HS levels were not significantly associated with IL-10 levels. We showed earlier that children with UM and severe malaria have extremely high plasma levels of IL-10 and that plasma IL-10 levels correlate significantly with measures of malaria severity (31). It is notable that our prior studies showed that in a different infectious disease (human dengue), there is evidence of GCX degradation and that levels of plasma HS significantly correlate with plasma IL-10 (18).
Furthermore, there was a significant association between peripheral parasitemia with DMMB-GAG (Pcorr = 0.34; P = 0.01) but not MS-GAG (P = 0.4) after adjusting for disease severity (Table 2). Because of the difference in muscle masses between younger and older children, we used plasma creatinine expressed as a percentage of mean for age and plasma creatinine adjusted for weight. There was no significant association between the 2 measures of creatinine with DMMB-GAG or MS-GAG levels (Table 2) or with CS, HS, and DS concentrations.
DISCUSSION
In African children with CM and UM, the urinary levels of GAGs, a measure of GCX breakdown, were increased compared with levels in HC children. However, there was no difference between the groups of children with CM and those with UM. There was also no difference in GCX breakdown within the CM group between the children with a fatal outcome or neurologic sequelae and survivors without sequelae. Elevated urinary DMMB-GAG levels in patients with CM normalized over 3 d following antimalarial treatment. The inverse association between plasma NOx and GCX breakdown products is consistent with findings showing that GCX damage alters endothelial shear-stress signaling and reduces vascular NO release (10–12). The association between TNF-α and GAG levels suggests that this cytokine may contribute to the pathogenesis of endothelial GCX breakdown. The increased inflammation and GCX damage may alter parasite sequestration as seen by the association between peripheral parasite density and GAG levels.
We have previously assessed microvascular flow and vascular NO bioavailability in both African and Indonesian children with severe malaria, CM, or UM (32, 33). Consistent with the GCX findings in the present study, we earlier found that microvascular flow and vascular NO were decreased in children with malaria compared with HCs but there was no significant greater impairment in CM compared with UM (6, 33). In contrast, in studies of Indonesian adults with malaria, we observed significant differences in microvascular flow, vascular NO bioavailability, and urinary GAG concentrations between those with severe malaria compared with those with moderately severe disease (which also differed from controls) (5, 20, 32), A possible explanation for this difference may be that vascular activation and dysfunction are more generalized in adults with malaria compared with children with CM in whom the activation and dysfunction may be more focused in the cerebral circulation.
When individual GCX breakdown products were analyzed, we found that levels of urinary HS and CS GAG are increased in those with CM and UM, whereas DS is not increased. This may be because HS and CS are the major components of the endothelial GCX, whereas only very low levels of DS are present in endothelial GCX (34). This is consistent with comparable urinary HS, CS, and DS findings in Indonesian adults with falciparum malaria (20). Although the evidence of elevated HS and CS in urine is consistent with breakdown of endothelial GCX as the major contributor, detection of GCX breakdown products in urine does not rule out the possibility that GCX in sites other than endothelium may be involved in malaria.
Elevated urinary GAGs have been demonstrated in other infectious diseases including bacterial sepsis and dengue, and the elevated GAG levels are considered as an indicator of GCX damage (17, 18). Schmidt and colleagues reported that elevated urinary GAG concentrations early after diagnosis in patients with sepsis were predictive of renal dysfunction and that levels of urinary HS are a marker of early mortality in those with sepsis (17). Breakdown of GCX has also been proposed to have significance in the pathophysiology of experimental malaria (35, 36). In a murine malaria model comparing experimental CM with uncomplicated disease, increased breakdown of GCX (as detected by transmission electron microscopy imaging), and increased circulating plasma GAG concentrations were seen in CM (19). In addition, in vitro studies have shown that cytoadhesion of P. falciparum–infected RBCs to CD36 receptors is impaired by the presence of endothelial GCX (14). Furthermore, in vitro studies using flow-based assays have demonstrated that degradation of endothelial cell GCX increases cytoadhesion (37).
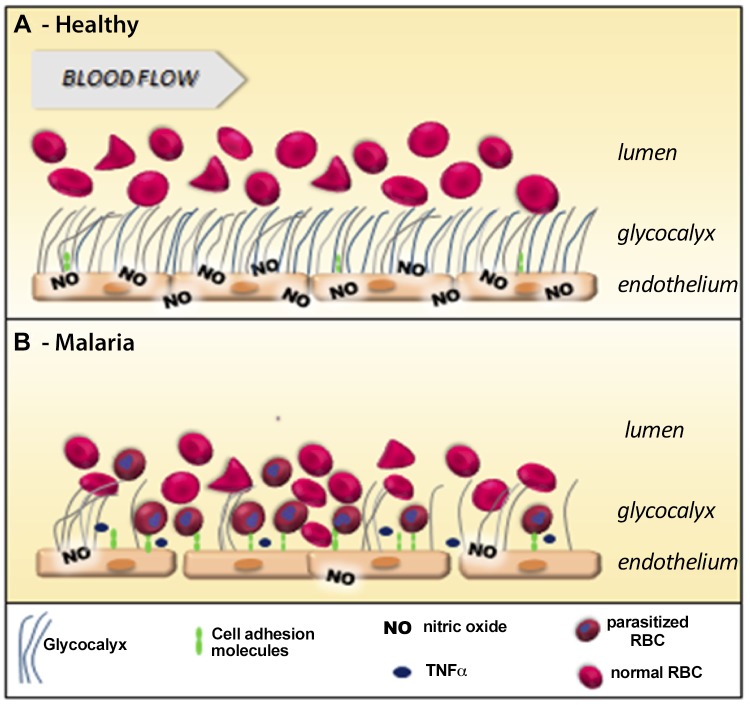
Breakdown of the GCX during blood-stage malaria may promote multiple aspects of malaria pathogenesis as shown in Fig. 4. Major consequences include a reduction in shear-stress signaling, causing impaired vascular NO formation and bioavailability, and an increase in endothelial cell activation with increases in both expression and exposure of endothelial adhesion receptors, all of which exacerbate cytoadhesion of infected RBCs and therefore parasite sequestration. All of these processes result in increased microvascular obstruction and decreased microcirculatory flow leading to inadequate oxygen delivery and organ damage. We have previously demonstrated that total and vascular NO production are decreased in proportion to disease severity in African and Indonesian patients with malaria (4, 38). Multiple factors reduce NO bioavailability in clinical malaria. These include: 1) low plasma concentrations of l-arginine and its precursors (5, 39, 40), 2) decreased NOS2 protein and mRNA expression (4, 31), 3) NO quenching by cell-free hemoglobin (41), 4) low levels of the NOS cofactor tetrahydrobiopterin (42, 43), and 5) competitive NOS inhibition by asymmetric dimethylarginine (44, 45). The association between NO metabolite levels and GAG levels suggests that reduced flow-mediated endothelial NO formation caused by impaired GCX signal transduction could also contribute. In support of this, the time course of resolution in GCX degradation in the children surviving CM is consistent with the recovery of NO-dependent endothelial dysfunction seen previously in severe malaria (46).
Figure 4.
Deleterious effects of GCX degradation on malaria pathogenesis. A) In healthy vessels, endothelial cell (EC) GCX provides a barrier and promotes vessel homeostasis. In addition, EC GCX transduces shear-stress signals resulting in formation of the vasodilator NO by endothelial NO synthase. The healthy GCX provides a physical barrier, separating red cells from ECs and reducing quenching of NO by red cell hemoglobin. Constitutive EC adhesion molecules are embedded within the GCX and are not exposed to passing red cells, leukocytes, and platelets. B) Degradation of EC GCX may impact aspects critical to malaria pathogenesis. Loss of GCX-mediated transduction of sheer stress reduces flow-mediated EC NO formation that can result in vasoconstriction, Weibel-Palade Body exocytosis, and microvascular dysfunction. Closer proximity of red cells to ECs increases NO quenching by red cells as well as cell-free hemoglobin. Loss of GCX exposes EC adhesion molecules previously embedded within the GCX and, by reducing NO-mediated inhibition, increases endothelial expression of inducible EC adhesion molecules, with both increased expression and increased exposure of adhesion molecules promoting EC adhesion of parasitized RBCs and microvascular sequestration.
Various mediators of GCX breakdown have been proposed. Several with possible relevance to malaria include TNF-α (47–49), angiopoietin 2 (50), and exocytosed lysosome-like organelles (51, 52). In experimental sepsis, degradation of endothelial GCX is linked to TNF-α–related activation of heparinase, resulting in loss of endothelial HS (49). Plasma TNF-α and IL-10 levels are elevated in malaria and are associated with disease severity (4). The association between TNF-α and IL-10 with GAG levels suggests that dysregulated cytokine expression in malaria may contribute to GCX damage and breakdown. We and other groups have previously shown that the angiogenic cytokine angiopoietin 2 is increased in severe malaria in Indonesian adults and African children and is a reliable prognostic marker for a fatal outcome (53, 54). Although this could not be measured in the current study because of insufficient sample volumes, angiopoietin 2 enhances TNF-α–mediated pathology and mediates endothelial GCX breakdown and thus may also play a pathogenic role in GCX loss in malaria (50).
Our study has several limitations: 1) lack of available plasma prevented us from measuring PfHRP-2, a parameter that is a better indicator of total-body parasite mass than blood parasitemia levels; 2) the observational nature of the study does not allow us to assign a mechanistic effect between GCX breakdown and disease severity and the rate of recovery. However, a mechanistic link is supported by the previous association of protection from severe malaria in African children with a gene polymorphism for syndecan 1, a key component of the endothelial GCX (55); 3) the use of urinary GAG concentrations as a marker of GCX damage does not allow us to assess the contribution from specific vascular types or vascular beds such as the cerebral circulation that may be especially important in pediatric CM. Remaining plasma was not available to measure GCX degradation by measuring plasma components, and we cannot exclude a predominantly renal source of urinary metabolites. However, the same urinary measures of GCX degradation correlated with plasma GCX when measured in adult severe falciparum malaria (20); 4) urinary GAG levels do not inform us about the actual thickness of the endothelial GCX and about increased GCX turnover and production rates that might maintain normal GCX structure; and 5) using urinary creatinine levels for normalization of urinary GAG levels is somewhat controversial. Waikar and others (56) detailed the potential errors of using normalization using urinary creatinine when studying urine as a biomarker for acute kidney injury (AKI) in a setting in which the patient has renal insufficiency. A commentary accompanying that article by Goldstein (57) as well as other recent papers [e.g., (58)] note that normalization using urinary creatinine in studies of urine biomarkers is commonly done, but they agree that this may give erroneous results in those with renal insufficiency. However, in a study of patients undergoing cardiovascular surgery, Wang et al. (59) noted that using urinary creatinine for normalization was useful in predicting AKI and overall patient outcome. Also, children with uncomplicated falciparum malaria enrolled in our study did not have evidence of renal dysfunction, making AKI a less likely explanation for the elevated urinary GAG:creatinine in this group. Measurement of plasma GCX degradation products would not be confounded by altered urinary creatinine excretion. However, remaining plasma was not available to measure plasma GCX degradation products in the children in the current study.
In summary, we show that GCX breakdown as measured by urinary GAG levels in African children is increased in both CM and UM and that this breakdown is associated with NO insufficiency. Based on in vitro models and animal studies, administration of agents that attenuate or repair GCX breakdown and damage may be of potential benefit as adjunctive therapies in malaria.
ACKNOWLEDGMENTS
The authors thank all patients and their families for participation in this research and the staff at the Duke–Muhimbili Clinical Research Laboratory and the Muhimbili Medical Center. This study was supported by the U.S. National Institutes of Health, National Heart, Lung, and Blood Institute (Grant R01 HL130763-01), the National Health and Medical Research Council of Australia (Grants 1132975, 1098334, and Fellowship 1135820 to N.M.A.), the Durham and Salt Lake City Veterans Affairs (VA) Medical Centers (to J.B.W. and D.L.G.), the Singapore National Medical Research Council (CSA INV 15nov007 to T.W.Y.), and a fellowship from the American Society of Tropical Medicine and Hygiene (to N.M.A.). The authors declare no conflicts of interest.
Glossary
- AKI
acute kidney injury
- CM
cerebral malaria
- CS
chondroitin sulfate
- DS
dermatan sulfate
- DMMB
dimethylmethylene blue
- GAG
glycosaminoglycan
- GCX
glycocalyx
- HC
healthy control
- HS
heparan sulfate
- MS
mass spectrometry
- MS/MS
tandem MS
- NOx
nitrate and nitrite
- Pcorr
partial correlation coefficient
- PfHRP2
Plasmodium falciparum histidine-rich protein 2
- RBC
red blood cell
- UM
uncomplicated malaria
- UPLC
ultraperformance liquid chromatography
AUTHOR CONTRIBUTIONS
T. W. Yeo, D. L. Granger, E. D. Mwaikambo, N. M. Anstey, and J. B. Weinberg designed the study; N. M. Anstey, D. L. Granger, E. D. Mwaikambo, and J. B. Weinberg conducted the clinical study; T. W. Yeo, D. L. Granger, E. D. Mwaikambo, N. M. Anstey, and J. B. Weinberg analysed the data; P. A. Bush, Y. Chen, S. P. Young, H. Zhang, and D. S. Millington developed and performed the glycosaminoglycan assays; and all authors contributed toward writing of the manuscript.
REFERENCES
- 1.WHO (2018) World Malaria Report 2018, WHO, Geneva, Switzerland [Google Scholar]
- 2.White N. J., Pukrittayakamee S., Hien T. T., Faiz M. A., Mokuolu O. A., Dondorp A. M. (2014) Malaria. Lancet 383, 723–735 [DOI] [PubMed] [Google Scholar]
- 3.Miller L. H., Ackerman H. C., Su X. Z., Wellems T. E. (2013) Malaria biology and disease pathogenesis: insights for new treatments. Nat. Med. 19, 156–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anstey N. M., Weinberg J. B., Hassanali M. Y., Mwaikambo E. D., Manyenga D., Misukonis M. A., Arnelle D. R., Hollis D., McDonald M. I., Granger D. L. (1996) Nitric oxide in Tanzanian children with malaria: inverse relationship between malaria severity and nitric oxide production/nitric oxide synthase type 2 expression. J. Exp. Med. 184, 557–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yeo T. W., Lampah D. A., Gitawati R., Tjitra E., Kenangalem E., McNeil Y. R., Darcy C. J., Granger D. L., Weinberg J. B., Lopansri B. K., Price R. N., Duffull S. B., Celermajer D. S., Anstey N. M. (2007) Impaired nitric oxide bioavailability and L-arginine reversible endothelial dysfunction in adults with falciparum malaria. J. Exp. Med. 204, 2693–2704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yeo T. W., Lampah D. A., Kenangalem E., Tjitra E., Weinberg J. B., Granger D. L., Price R. N., Anstey N. M. (2014) Decreased endothelial nitric oxide bioavailability, impaired microvascular function, and increased tissue oxygen consumption in children with falciparum malaria. J. Infect. Dis. 210, 1627–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reitsma S., Slaaf D. W., Vink H., van Zandvoort M. A., oude Egbrink M. G. (2007) The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch. 454, 345–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tarbell J. M., Cancel L. M. (2016) The glycocalyx and its significance in human medicine. J. Intern. Med. 280, 97–113 [DOI] [PubMed] [Google Scholar]
- 9.Henry C. B., Duling B. R. (1999) Permeation of the luminal capillary glycocalyx is determined by hyaluronan. Am. J. Physiol. 277, H508–H514 [DOI] [PubMed] [Google Scholar]
- 10.Florian J. A., Kosky J. R., Ainslie K., Pang Z., Dull R. O., Tarbell J. M. (2003) Heparan sulfate proteoglycan is a mechanosensor on endothelial cells. Circ. Res. 93, e136–e142 [DOI] [PubMed] [Google Scholar]
- 11.Yen W., Cai B., Yang J., Zhang L., Zeng M., Tarbell J. M., Fu B. M. (2015) Endothelial surface glycocalyx can regulate flow-induced nitric oxide production in microvessels in vivo. PLoS One 10, e0117133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tarbell J. M., Pahakis M. Y. (2006) Mechanotransduction and the glycocalyx. J. Intern. Med. 259, 339–350 [DOI] [PubMed] [Google Scholar]
- 13.Mulivor A. W., Lipowsky H. H. (2002) Role of glycocalyx in leukocyte-endothelial cell adhesion. Am. J. Physiol. Heart Circ. Physiol. 283, H1282–H1291 [DOI] [PubMed] [Google Scholar]
- 14.Hempel C., Wang C. W., Kurtzhals J. A. L., Staalsø T. (2017) Binding of Plasmodium falciparum to CD36 can be shielded by the glycocalyx. Malar. J. 16, 193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chappell D., Brettner F., Doerfler N., Jacob M., Rehm M., Bruegger D., Conzen P., Jacob B., Becker B. F. (2014) Protection of glycocalyx decreases platelet adhesion after ischaemia/reperfusion: an animal study. Eur. J. Anaesthesiol. 31, 474–481 [DOI] [PubMed] [Google Scholar]
- 16.Nieuwdorp M., Meuwese M. C., Vink H., Hoekstra J. B., Kastelein J. J., Stroes E. S. (2005) The endothelial glycocalyx: a potential barrier between health and vascular disease. Curr. Opin. Lipidol. 16, 507–511 [DOI] [PubMed] [Google Scholar]
- 17.Schmidt E. P., Overdier K. H., Sun X., Lin L., Liu X., Yang Y., Ammons L. A., Hiller T. D., Suflita M. A., Yu Y., Chen Y., Zhang F., Cothren Burlew C., Edelstein C. L., Douglas I. S., Linhardt R. J. (2016) Urinary glycosaminoglycans predict outcomes in septic shock and acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 194, 439–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang T. H., Alonso S., Ng L. F., Thein T. L., Pang V. J., Leo Y. S., Lye D. C., Yeo T. W. (2017) Increased serum hyaluronic acid and heparan sulfate in dengue fever: association with plasma leakage and disease severity. Sci. Rep. 7, 46191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hempel C., Hyttel P., Kurtzhals J. A. (2014) Endothelial glycocalyx on brain endothelial cells is lost in experimental cerebral malaria. J. Cereb. Blood Flow Metab. 34, 1107–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yeo T. W., Weinberg J. B., Lampah D. A., Kenangalem E., Bush P., Chen Y., Price R. N., Young S., Zhang H. Y., Millington D., Granger D. L., Anstey N. M. (2019) Glycocalyx breakdown is associated with severe disease and fatal outcome in Plasmodium falciparum malaria. [E-pub ahead of print] Clin. Infect. Dis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Embery G., Milner A. C., Waddington R. J., Hall R. C., Langley M. S., Milan A. M. (2003) Identification of proteinaceous material in the bone of the dinosaur Iguanodon. Connect. Tissue Res. 44(Suppl1), 41–46 [PubMed] [Google Scholar]
- 22.Sun X., Li L., Overdier K. H., Ammons L. A., Douglas I. S., Burlew C. C., Zhang F., Schmidt E. P., Chi L., Linhardt R. J. (2015) Analysis of total human urinary glycosaminoglycan disaccharides by liquid chromatography-tandem mass spectrometry. Anal. Chem. 87, 6220–6227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang H., Wood T., Young S. P., Millington D. S. (2015) A straightforward, quantitative ultra-performance liquid chromatography-tandem mass spectrometric method for heparan sulfate, dermatan sulfate and chondroitin sulfate in urine: an improved clinical screening test for the mucopolysaccharidoses. Mol. Genet. Metab. 114, 123–128 [DOI] [PubMed] [Google Scholar]
- 24.Auray-Blais C., Bhérer P., Gagnon R., Young S. P., Zhang H. H., An Y., Clarke J. T., Millington D. S. (2011) Efficient analysis of urinary glycosaminoglycans by LC-MS/MS in mucopolysaccharidoses type I, II and VI. Mol. Genet. Metab. 102, 49–56 [DOI] [PubMed] [Google Scholar]
- 25.Remer T., Montenegro-Bethancourt G., Shi L. (2014) Long-term urine biobanking: storage stability of clinical chemical parameters under moderate freezing conditions without use of preservatives. Clin. Biochem. 47, 307–311 [DOI] [PubMed] [Google Scholar]
- 26.Husdan H., Rapoport A. (1968) Estimation of creatinine by the Jaffe reaction. A comparison of three methods. Clin. Chem. 14, 222–238 [PubMed] [Google Scholar]
- 27.Leone A., Kelm M. (1996) Capillary electrophoretic and liquid chromatographic analysis of nitrite and nitrate. In Methods in Nitric Oxide Research (Feelisch M. M., Stamler J. S., eds.), pp. 499–508, Wiley-Blackwell, Chichester, UK [Google Scholar]
- 28.Granger D. L., Taintor R. R., Boockvar K. S., Hibbs J. B., Jr (1996) Measurement of nitrate and nitrite in biological samples using nitrate reductase and Griess reaction. Methods Enzymol. 268, 142–151 [DOI] [PubMed] [Google Scholar]
- 29.Schwartz G. J., Haycock G. B., Spitzer A. (1976) Plasma creatinine and urea concentration in children: normal values for age and sex. J. Pediatr. 88, 828–830 [DOI] [PubMed] [Google Scholar]
- 30.Dondorp A. M., Desakorn V., Pongtavornpinyo W., Sahassananda D., Silamut K., Chotivanich K., Newton P. N., Pitisuttithum P., Smithyman A. M., White N. J., Day N. P. (2005) Estimation of the total parasite biomass in acute falciparum malaria from plasma PfHRP2. PLoS Med. 2, e204; erratum: 390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weinberg J. B., Volkheimer A. D., Rubach M. P., Florence S. M., Mukemba J. P., Kalingonji A. R., Langelier C., Chen Y., Bush M., Yeo T. W., Granger D. L., Anstey N. M., Mwaikambo E. D. (2016) Monocyte polarization in children with falciparum malaria: relationship to nitric oxide insufficiency and disease severity. Sci. Rep. 6, 29151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yeo T. W., Lampah D. A., Kenangalem E., Tjitra E., Price R. N., Anstey N. M. (2013) Impaired skeletal muscle microvascular function and increased skeletal muscle oxygen consumption in severe falciparum malaria. J. Infect. Dis. 207, 528–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yeo T. W., Florence S. M., Kalingonji A. R., Chen Y., Granger D. L., Anstey N. M., Mwaikambo E. D., Weinberg J. B. (2017) Decreased microvascular function in Tanzanian children with severe and uncomplicated falciparum malaria. Open Forum Infect. Dis. 4, ofx079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weinbaum S., Tarbell J. M., Damiano E. R. (2007) The structure and function of the endothelial glycocalyx layer. Annu. Rev. Biomed. Eng. 9, 121–167 [DOI] [PubMed] [Google Scholar]
- 35.Hempel C., Pasini E. M., Kurtzhals J. A. L. (2016) Endothelial glycocalyx: shedding light on malaria pathogenesis. Trends Mol. Med. 22, 453–457 [DOI] [PubMed] [Google Scholar]
- 36.Hempel C., Sporring J., Kurtzhals J. A. L. (2019) Experimental cerebral malaria is associated with profound loss of both glycan and protein components of the endothelial glycocalyx. FASEB J. 32, 2058–2071 [DOI] [PubMed] [Google Scholar]
- 37.Introini V., Carciati A., Tomaiuolo G., Cicuta P., Guido S. (2018) Endothelial glycocalyx regulates cytoadherence in Plasmodium falciparum malaria. J. R. Soc. Interface 15, 20180773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weinberg J. B., Lopansri B. K., Mwaikambo E., Granger D. L. (2008) Arginine, nitric oxide, carbon monoxide, and endothelial function in severe malaria. Curr. Opin. Infect. Dis. 21, 468–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lopansri B. K., Anstey N. M., Weinberg J. B., Stoddard G. J., Hobbs M. R., Levesque M. C., Mwaikambo E. D., Granger D. L. (2003) Low plasma arginine concentrations in children with cerebral malaria and decreased nitric oxide production. Lancet 361, 676–678 [DOI] [PubMed] [Google Scholar]
- 40.Rubach M. P., Zhang H., Florence S. M., Mukemba J. P., Kalingonji A. R., Anstey N. M., Yeo T. W., Lopansri B. K., Thompson J. W., Mwaikambo E. D., Young S., Millington D. S., Weinberg J. B., Granger D. L. (2019) Kinetic and cross-sectional studies on the genesis of hypoargininemia in severe pediatric Plasmodium falciparum malaria. Infect. Immun. 87, e00655-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yeo T. W., Lampah D. A., Tjitra E., Gitawati R., Kenangalem E., Piera K., Granger D. L., Lopansri B. K., Weinberg J. B., Price R. N., Duffull S. B., Celermajer D. S., Anstey N. M. (2009) Relationship of cell-free hemoglobin to impaired endothelial nitric oxide bioavailability and perfusion in severe falciparum malaria. J. Infect. Dis. 200, 1522–1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yeo T. W., Lampah D. A., Kenangalem E., Tjitra E., Price R. N., Weinberg J. B., Hyland K., Granger D. L., Anstey N. M. (2015) Impaired systemic tetrahydrobiopterin bioavailability and increased dihydrobiopterin in adult falciparum malaria: association with disease severity, impaired microvascular function and increased endothelial activation. PLoS Pathog. 11, e1004667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rubach M. P., Mukemba J., Florence S., Lopansri B. K., Hyland K., Volkheimer A. D., Yeo T. W., Anstey N. M., Weinberg J. B., Mwaikambo E. D., Granger D. L. (2015) Impaired systemic tetrahydrobiopterin bioavailability and increased oxidized biopterins in pediatric falciparum malaria: association with disease severity. PLoS Pathog. 11, e1004655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yeo T. W., Lampah D. A., Tjitra E., Gitawati R., Darcy C. J., Jones C., Kenangalem E., McNeil Y. R., Granger D. L., Lopansri B. K., Weinberg J. B., Price R. N., Duffull S. B., Celermajer D. S., Anstey N. M. (2010) Increased asymmetric dimethylarginine in severe falciparum malaria: association with impaired nitric oxide bioavailability and fatal outcome. PLoS Pathog. 6, e1000868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weinberg J. B., Yeo T. W., Mukemba J. P., Florence S. M., Volkheimer A. D., Wang H., Chen Y., Rubach M., Granger D. L., Mwaikambo E. D., Anstey N. M. (2014) Dimethylarginines: endogenous inhibitors of nitric oxide synthesis in children with falciparum malaria. J. Infect. Dis. 210, 913–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yeo T. W., Lampah D. A., Gitawati R., Tjitra E., Kenangalem E., McNeil Y. R., Darcy C. J., Granger D. L., Weinberg J. B., Lopansri B. K., Price R. N., Duffull S. B., Celermajer D. S., Anstey N. M. (2008) Recovery of endothelial function in severe falciparum malaria: relationship with improvement in plasma L-arginine and blood lactate concentrations. J. Infect. Dis. 198, 602–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Henry C. B., Duling B. R. (2000) TNF-alpha increases entry of macromolecules into luminal endothelial cell glycocalyx. Am. J. Physiol. Heart Circ. Physiol. 279, H2815–H2823 [DOI] [PubMed] [Google Scholar]
- 48.Nieuwdorp M., Meuwese M. C., Mooij H. L., van Lieshout M. H., Hayden A., Levi M., Meijers J. C., Ince C., Kastelein J. J., Vink H., Stroes E. S. (2009) Tumor necrosis factor-alpha inhibition protects against endotoxin-induced endothelial glycocalyx perturbation. Atherosclerosis 202, 296–303 [DOI] [PubMed] [Google Scholar]
- 49.Schmidt E. P., Yang Y., Janssen W. J., Gandjeva A., Perez M. J., Barthel L., Zemans R. L., Bowman J. C., Koyanagi D. E., Yunt Z. X., Smith L. P., Cheng S. S., Overdier K. H., Thompson K. R., Geraci M. W., Douglas I. S., Pearse D. B., Tuder R. M. (2012) The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat. Med. 18, 1217–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lukasz A., Hillgruber C., Oberleithner H., Kusche-Vihrog K., Pavenstädt H., Rovas A., Hesse B., Goerge T., Kümpers P. (2017) Endothelial glycocalyx breakdown is mediated by angiopoietin-2. Cardiovasc. Res. 113, 671–680 [DOI] [PubMed] [Google Scholar]
- 51.Song J. W., Zullo J., Lipphardt M., Dragovich M., Zhang F. X., Fu B., Goligorsky M. S. (2018) Endothelial glycocalyx-the battleground for complications of sepsis and kidney injury. Nephrol. Dial. Transplant. 33, 203–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zullo J. A., Fan J., Azar T. T., Yen W., Zeng M., Chen J., Ratliff B. B., Song J., Tarbell J. M., Goligorsky M. S., Fu B. M. (2016) Exocytosis of endothelial lysosome-related organelles hair-triggers a patchy loss of glycocalyx at the onset of sepsis. Am. J. Pathol. 186, 248–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yeo T. W., Lampah D. A., Gitawati R., Tjitra E., Kenangalem E., Piera K., Price R. N., Duffull S. B., Celermajer D. S., Anstey N. M. (2008) Angiopoietin-2 is associated with decreased endothelial nitric oxide and poor clinical outcome in severe falciparum malaria. Proc. Natl. Acad. Sci. USA 105, 17097–17102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lovegrove F. E., Tangpukdee N., Opoka R. O., Lafferty E. I., Rajwans N., Hawkes M., Krudsood S., Looareesuwan S., John C. C., Liles W. C., Kain K. C. (2009) Serum angiopoietin-1 and -2 levels discriminate cerebral malaria from uncomplicated malaria and predict clinical outcome in African children. PLoS One 4, e4912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Manjurano A., Sepúlveda N., Nadjm B., Mtove G., Wangai H., Maxwell C., Olomi R., Reyburn H., Drakeley C. J., Riley E. M., Clark T. G.; Collaboration With MalariaGEN (2015) USP38, FREM3, SDC1, DDC, and LOC727982 gene polymorphisms and differential susceptibility to severe malaria in Tanzania. J. Infect. Dis. 212, 1129–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Waikar S. S., Betensky R. A., Emerson S. C., Bonventre J. V. (2012) Imperfect gold standards for kidney injury biomarker evaluation. J. Am. Soc. Nephrol. 23, 13–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goldstein S. L. (2010) Urinary kidney injury biomarkers and urine creatinine normalization: a false premise or not? Kidney Int. 78, 433–435 [DOI] [PubMed] [Google Scholar]
- 58.Tang K. W., Toh Q. C., Teo B. W. (2015) Normalisation of urinary biomarkers to creatinine for clinical practice and research--when and why. Singapore Med. J. 56, 7–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang J. J., Chi N. H., Huang T. M., Connolly R., Chen L. W., Chueh S. J., Kan W. C., Lai C. C., Wu V. C., Fang J. T., Chu T. S., Wu K. D. (2018) Urinary biomarkers predict advanced acute kidney injury after cardiovascular surgery. Crit. Care 22, 108 [DOI] [PMC free article] [PubMed] [Google Scholar]