Abstract
Adiponectin (APN), an adipocyte-derived adipokine, has been shown to limit lung injury originating from endothelial cell (EC) damage. Previously we reported that obese mice with low circulatory APN levels exhibited pulmonary vascular endothelial dysfunction. This study was designed to investigate the cellular and molecular mechanisms underlying the pulmonary endothelium-dependent protective effects of APN. Our results demonstrated that in APN−/− mice, there was an inherent state of endothelium mitochondrial dysfunction that could contribute to endothelial activation and increased susceptibility to LPS-induced acute lung injury (ALI). We noted that APN−/− mice showed decreased expression of mitochondrial biogenesis regulatory protein peroxisome proliferator-activated receptor γ coactivator 1-α (PGC-1α) and its downstream proteins nuclear respiratory factor 1, transcription factor A, mitochondrial, and Sirtuin (Sirt)3 and Sirt1 expression in whole lungs and in freshly isolated lung ECs from these mice at baseline and subjected to LPS-induced ALI. We further showed that treating APN−/− mice with PGC-1α activator pyrroloquinoline quinone enhances mitochondrial biogenesis and function in lung endothelium and attenuation of ALI. These results suggest that the pulmonary endothelium-protective properties of APN are mediated, at least in part, by an enhancement of mitochondrial biogenesis through a mechanism involving PGC-1α activation.—Shah, D., Torres, C., Bhandari, V. Adiponectin deficiency induces mitochondrial dysfunction and promotes endothelial activation and pulmonary vascular injury.
Keywords: PGC-1α mitochondria biogenesis, endothelial dysfunction, acute lung injury, adipokine
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) is a severe form of respiratory failure that develops abruptly in patients with other serious medical conditions (e.g., sepsis, major trauma, pneumonia). The hallmark of ALI is pulmonary vascular dysfunction characterized by endothelial cell (EC) inflammation and loss of endothelial barrier function resulting in inflammatory infiltrates, interstitial edema, alveolar flooding, and ultimately respiratory failure (1–3). Several adipocyte-secreted factors are believed to play an essential role in lung development (4), angiogenesis (5), and lung function (6). Among these, adiponectin (APN) has been shown to be involved in lung development (4), and its deficiency has been reported to accelerate pulmonary endothelial activation (7). We have previously reported that low circulatory APN levels in obese mice exhibited an inherent state of pulmonary vascular endothelial (VE) dysfunction, and these mice were more susceptible to LPS-induced ALI (8). Similarly, several other groups have shown that APN−/− mice are more susceptible to ALI in response to LPS (7), cecal ligation and puncture–induced sepsis (9), and hyperoxia exposure (10). Although the inhibitory effect of APN in proinflammatory cytokine milieu in the lungs in response to ALI is well established, the role of APN in mitochondrial-mediated endothelial dysfunction and pulmonary vascular injury is largely unknown.
In recent years, mitochondria have been identified as a key source of mitochondrial damage-associated molecular patterns (DAMPs), which play a crucial role in DAMP-mediated inflammation and organs injury (11). Mitochondrial DAMPs activate pathogen recognition receptors, such as toll-like membrane receptors and cytoplasmic nucleotide-binding oligomerization domain–like receptors to initiate the inflammatory cascades (12). Several clinical studies have reported that mitochondrial DNA (mt-DNA) released from dysfunctional mitochondria in response to stress or infection, or both, activates nucleotide-binding domain and leucine-rich repeat containing protein (NLRP3)-inflammasome in association with the severity of lung injury and mortality in sepsis-related ARDS (13–15). Similarly, preclinical studies have shown that the injection of mitochondrial lysates from dysfunctional mitochondria into rats causes lung inflammation (16) and that the preservation of mitochondrial function or activation of mitochondrial bioenergetics reduces the severity of lung injury (17). Recently, Hough et al. (18) have shown that pulmonary endothelial mitochondria regulate the barrier failure by activating uncoupling protein 2 in acid (HCl)-induced ALI. These studies indicate that mitochondria are involved in the regulation of lung inflammation and injury.
Although the protective effect of APN has been well established in preclinical models of ARDS, the APN-mediated protective mechanism effective in endothelial dysfunction is largely unknown. APN has been reported to modulate mitochondrial biogenesis and function in skeletal muscle (19) as well as in cardiovascular ECs (20). We hypothesize that APN deficiency may impair pulmonary endothelial mitochondrial function, which potentially promotes endothelial activation and increases the susceptibility to LPS-induced ALI. Our results clearly demonstrate that loss of APN impairs endothelial mitochondrial biogenesis and function, which promote endothelial activation and aggravate pulmonary vascular injury. We also found that administration of pyrroloquinoline quinone (PQQ) bypasses the effects of APN deficiency and enhances mitochondrial biogenesis through activation of regulatory protein peroxisome proliferator-activated receptor γ coactivator 1-α (PGC-1α), improves mitophagy, decreases NLRP3-inflammasome activation, and attenuates endothelium dysfunction and lung injury in response to LPS.
MATERIALS AND METHODS
Animals
This study was performed in accordance with U.S. National Institutes of Health (NIH; Bethesda, MD, USA) guidelines for the use of experimental animals. All animal studies were approved by the Institutional Animal Care and Use Committee of Drexel University College of Medicine (DUCOM), Philadelphia, PA, USA. Global APN−/− (21) and wild-type (WT) mice (C57BL6/J) of a similar genetic background as controls were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). Mice were bred, and the colony was maintained at the pathogen-free animal house facility at the DUCOM, Drexel University. In all reported experiments, male mice 10–12 wk of age were used.
Murine model of ALI
ALI was induced by administering 1-time intratracheal instillation of LPS (100 µg). Delivery of LPS (Escherichia coli O111: B4; List Biological Laboratories, Campbell, CA, USA) was performed using the tongue-pull maneuver in isoflurane-anesthetized mice as previously described in Shah et al. (22). For some experiments, APN−/− mice were treated via the intraperitoneal injection route with PQQ (20 mg/kg body weight) for 7 d (23, 24). Animals were euthanized at baseline (bsl) and 24 h after LPS administration, and bronchoalveolar lavage (BAL) fluid and lung tissues were harvested and stored at −80°C for subsequent analysis.
Analysis of BAL fluid
BAL was obtained by cannulating the trachea with a blunt 22-gauge needle and then lavaging the lungs 3 times with 1 ml of PBS (22). Total cell counts in BAL fluid were determined using the TC20 automated cell counter (Bio-Rad, Hercules, CA, USA). The differential cell counts were performed on cells cytocentrifuged onto glass slides (Thermo Fisher Scientific, Waltham, MA, USA) stained with the Hema 3 Staining System (Thermo Fisher Scientific), and cell differential was tabulated using light microscopy. Total protein concentration in the BAL fluid was measured using the Pierce BCA Assay Kit (Thermo Scientific), as previously described in Shah et al. (25).
ELISA
TNF-α, IL-6, IL-1β, and myeloperoxidase were quantified using commercially available DuoSet ELISA Kits (R&D Systems, Minneapolis, MN, USA), whereas the IgM level in BAL fluid was measured by a mouse IgM ELISA Kit (Abcam, Cambridge, MA, USA) according to the manufacturer’s instructions, as previously described in Shah et al. (22).
Assessment of lung endothelium permeability
Pulmonary EC permeability was determined by measuring the lung wet-to-dry weight ratio, as previously described in Shah et al. (26).
Real-time quantitative PCR analysis
Total RNA was isolated from the right lobe of the mice lungs using RNeasy Mini-Kit (Qiagen, Germantown, MD, USA). One microgram of total RNA was reverse transcribed using iScript Reverse Transcription (Bio-Rad), as recommended by the manufacturers. PCR condition for the transcript levels of pgc1α, nuclear respiratory factor 1 (nrf1), Sirtuin (Sirt)3, and the internal control hypoxanthine phosphoribosyltransferase 1 (hprt1) were analyzed using real-time RT-PCR in Master cycler gradient (7500 Sequence Detection System; Thermo Fisher Scientific) as previously described in Shah et al. (22). Primers used were as follows: mouse pgc1α, forward 5′-CGGAAATCATATCCAACCAG-3′ and reverse 5′-TGAGGACCGCTAGCAAGTTTG-3′; mouse nrf1, forward 5′-AGCACGGAGTGACCCAAAC-3′ and reverse 5′-TGTACGTGGCTACATGGACCT-3′; mouse mitochondrial transcription factor A (tfam), forward 5′-GGAATGTGGAGCGTGCTAAAA-3′ and reverse 5′-GCTGGAAAAACACTTCGGAATA-3′; mouse sirt3, forward 5′-CATATGGGCTGATGTGATGG-3′ and reverse 5′-GCCATATGGAGTAGGAACCTTG-3′: hprt1, forward 5′-TCCTCCTCAGACCGCTTTT-3′ and reverse 5′-CCTGGTTCATCATCGCTAATC-3′. The gene transcripts pgc1α, nrf1, tfam, and sirt3 expressions were normalized with the housekeeping gene hprt1.
Western blotting
Whole lung tissue or lung ECs were homogenized in ice-cold RIPA buffer containing protease inhibitors (Complete mini; Roche, Basel, Switzerland) and phosphatase inhibitors (cOmplete mini; Roche) as previously described in Shah et al. (26). Cell lysate and lung homogenate were centrifuged (14,000 g) at 4°C for 15 min, and the supernatant was collected for further analysis. Thirty micrograms of protein was loaded onto each well, separated on 10% SDS-polyacrylamide gel, and then transferred onto a nitrocellulose membrane (Bio-Rad) using a Bio-Rad Mini-Blot transfer apparatus. Immunoblotting was performed at 4°C overnight using primary antibodies directed against intercellular adhesion molecule-1 (ICAM-1; R&D Systems), vascular cell adhesion molecule 1 (VCAM-1; Abcam), E-selectin (Abcam), VE-cadherin (Cell Signaling Technology, Danvers, MA, USA), β-Catenin (Cell Signaling Technology), phospho (p)-Src (Tyr416) (Cell Signaling Technology), PGC-1α (Abcam), TFAM (Cell Signaling Technology), NRF-1 (Cell Signaling Technology), Sirt3 (Cell Signaling Technology), Sirt1 (Abcam), microtubule-associated protein light chain 3 (LC3B; MilliporeSigma, Burlington, MA, USA), p62 (Cell Signaling Technology), PTEN-induced kinase 1 (Pink1; Abcam), Parkin (Cell Signaling Technology), VDAC (Cell Signaling Technology), NLRP3 (Cell Signaling Technology), Caspase-1 (Cell Signaling Technology), IL-1β (Cell Signaling Technology), IL-6 (Cell Signaling Technology), cleaved Caspase-3 (Cell Signaling Technology), and β-actin (Cell Signaling Technology), glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Cell Signaling Technology). Membranes were then incubated with a 1:5000 dilution of a secondary antibody (Li-Cor Biosciences, Lincoln, NE, USA) at room temperature for 1 h. Immunoblots were visualized using the Odyssey Infrared Imaging System (Li-Cor Biosciences). The band intensity on the western blots was analyzed using the ImageJ software (v.1.6; NIH).
Histologic analysis
Lung tissue samples were fixed overnight in 4% paraformaldehyde and subsequently embedded in paraffin. Lung tissue sections (5 μm) and hemotoxylin and eosin (H&E) staining were performed at the Department of Pathology Core Facility (DUCOM). A total of 4 to 5 random images per lung and 6 lungs per experimental group were characterized for measuring lung morphometric analysis.
Isolation of lung ECs
Lung ECs were isolated from whole lung tissues using CD45 and CD31 Microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany). In brief, a single-cell suspension was prepared from lung tissues using a Mouse Lung Dissociation Kit (Miltenyi Biotec). The single-cell suspension was passed through 40-µm nylon mesh and incubated with CD45 MicroBeads for 15 min. CD45 positive cells were removed by passing through a column (CD45+ cells bind to the column), and then the cell suspension was incubated with CD31 MicroBeads. CD31 positive cells bound to the column were collected. The purity of the ECs population was confirmed by flow cytometry, as previously described in Shah et al. (26).
Cell culture
Mouse lung microvascular ECs (MLECs) and medium were purchased from Cell Biologics (Chicago, IL, USA). Cells were maintained in 10-cm plastic dishes precoated with Gelatin-Based Coating Solution (Cell Biologics). MLECs were transfected with PGC-1α small interfering RNA and stimulated with LPS (1 µg/ml) (26). For some experiments, MLECs were pretreated with PQQ (30 µM) (27) and then stimulated with LPS (1 µg/ml). At specified time points, cell lysates were collected for analysis. For all the in-vitro experiments, MLECs were used from passages 4–6 to ensure their endothelial characteristics and experiments were performed at least in triplicate and repeated twice.
In vitro endothelial permeability assay
A transwell insert (0.4 µM, 12-mm diameter; Corning, Corning, NY, USA) was coated with collagen for 2 h at room temperature, MLECs were grown to confluence for a minimum 2 d, and cells were pretreated with PQQ for 6 h and then stimulated with LPS (1 µg/ml, 24 h) and FITC-dextran 70 KDa (1 mg/ml) in the top chamber. After 30 min, samples measured in aliquots were removed from the bottom compartment and the concentration of FITC-dextran was assessed using a Biotek Synergy [1H] plate reader (BioTek Instruments, Winooski, VT, USA), per the manufacturer’s protocol. All experiments were performed in quintuplicate and repeated 3 times.
Mitochondrial preparation and cytosolic extracts
Whole lung tissue and lung ECs were used for isolating mitochondrial and cytosolic fractions, as previously described in Ponnalagu et al. (28). Briefly, finely minced lungs tissue or ECs were homogenized in isolation buffer A (70 mM sucrose, 210 mM mannitol, 1 mM EDTA-Na2, and 50 mM Tris·HCl, pH 7.4) using a Potter-Elvejem homogenizer (10 rapid strokes). The homogenates were centrifuged at 1,300 g for 5 min. Pellets were dissolved in RIPA buffer and used for cytosolic fraction analysis. Supernatants were centrifuged at 10,000 g for 10 min at 4°C to collect the mitochondrial pellets and resuspended in 60 μl of mitochondrial isolation buffer.
Mitochondrial membrane potential measurements
The mitochondrial membrane potential was determined using JC-1 (5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide) fluorescent dye. In brief, ECs were plated in a 96-well plate (100 µl medium with 5000 cells/well) and transfected and stimulated. Fluorescence was measured using a fluorescence plate reader (BioTek Synergy [1H] Hybrid Reader). Potential-dependent accumulation of the dye in the mitochondria was indicated by a fluorescence emission shift from green (∼527 nm) to red (∼600 nm) following excitation at 488 nm.
ATP measurements
ATP was measured in lung tissues using a commercially available Colorimetric Kit (BioVision, Milpitas, CA, USA) according to the manufacturer’s instructions and as previously described in Lee et al. (29).
NAD+/NADH assay
NAD+/NADH was measured using the MilliporeSigma kit as per the manufacturer’s instructions.
Measurement of lung mechanics
Lung mechanics such as lung resistance, lung compliance, and tissue elastance were measured in anesthetized mice. Mice were mechanically ventilated with a tidal volume of 10 ml/kg and a positive end–expiratory pressure of 2 cm H2O using the flexiVent (Scireq, Montreal, QC, Canada) ventilation setup as previously described in McGovern et al. (30).
Statistical analysis
Statistics were performed using Prism v.8.1.2 (Graph Pad Software, La Jolla, CA, USA). Experimental data were analyzed for normal distributions. Two-group comparisons were analyzed by unpaired Student’s t test, and multiple-group comparisons were performed using 1-way ANOVA followed by Tukey post hoc analysis. Statistical significance was achieved when P < 0.05 at 95% confidence intervals. No animals were excluded from the analysis.
RESULTS
APN deficiency promotes the expression of lung endothelial adhesion markers
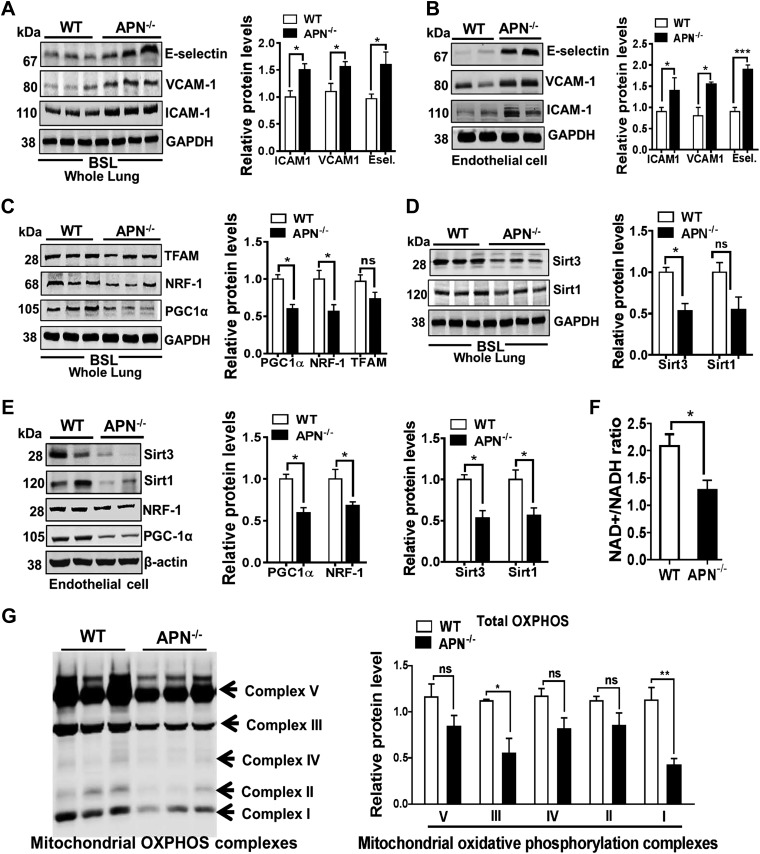
In our previous work, we showed that low APN obese mice exhibited increased expression of endothelial-specific adhesion markers ICAM-1, VCAM-1, and E-selectin in lung endothelium (8). To assess the functional role of APN in lung endothelial homeostasis, we have used APN−/− mice in this study and examined the expression of endothelial-specific adhesion markers (ICAM-1, VCAM-1, and E-selectin) in whole lung tissues and in freshly isolated lung ECs. As shown in Fig. 1, we found that protein and transcript levels of ICAM-1, VCAM-1, and E-selectin were significantly increased in lung tissues (Fig. 1A and Supplemental Fig. S1) and in freshly isolated lung ECs from APN−/− mice as compared with WT mice (Fig. 1B). Taken together, these findings indicate that APN deficiency promotes lung endothelial activation.
Figure 1.
APN−/− mice show up-regulated expression of endothelial adhesion markers and are related to mitochondrial dysfunctional in lung endothelium. A, B) Western blot analysis for ICAM-1, VCAM-1, and E-selectin (Esel.) in the whole lung tissues and in freshly isolated lung ECs in APN−/− and WT control mice at bsl. GAPDH is used as loading control. C–E) Western blot analysis for PGC-1α, NRF-1, TFAM, Sirt3, and Sirt1 expression in whole lung tissues and in freshly isolated lung ECs in APN−/− and WT control mice at bsl (GAPDH and β-actin are used as loading controls). F) NAD+/NADH ratio in the lungs of APN−/− and WT control mice at bsl. G) Expression of mitochondrial oxidative phosphorylation complexes (I, II, III, IV, and V) in the lungs of APN−/− and WT control mice at bsl. Ns, not significant. Densitometry analysis is shown on the right. Data are expressed as means ± se; n = 6 in each group. *P < 0.05, **P < 0.01, ***P < 0.001.
APN deficiency induces mitochondrial dysfunction in lung endothelium
APN deficiency has been reported to decrease mitochondrial biogenesis and impair mitochondrial function, which has been linked to dysfunction in various organs/tissues such as skeletal muscle and cardiovascular ECs (19, 20). However, the role of APN on mitochondrial function and its effects on pulmonary endothelial homeostasis is unknown. We hypothesized that APN deficiency may promote endothelial activation by tempering the function of mitochondria in lung ECs. Consistent with our hypothesis, we found that APN−/− mice exhibited the decreased mitochondrial biogenesis regulator PGC-1α at both the protein (Fig. 1C) and transcript (Supplemental Fig. S2) levels in whole lungs as well as in freshly lung ECs isolated from APN−/− mice (Fig. 1E). APN deficiency-mediated diminished mitochondrial biogenesis is further supported by decreased molecules involved in transcription such as Nrf1 and molecules involved in mt-DNA replication/translation such as TFAM in whole lungs (Fig. 1C) and in freshly isolated lung ECs (Fig. 1E). In addition, we also noted the decreased expression of Sirt1 and Sirt3 proteins in whole lung tissue as well as in freshly isolated lung ECs from APN−/− mice as compared with WT control mice (Fig. 1D, E).
Moreover, we found that the decreased mitochondrial biogenesis was also associated with diminished mitochondrial function as demonstrated by lower NAD+/NADH (Fig. 1F) and reduced expression of mitochondrial complexes I, II, III, and V (Fig. 1G) in the lungs of APN−/− mice as compared with WT mice at bsl. These results suggest that APN deficiency induces mitochondrial dysfunction in lung endothelium.
Mitochondrial dysfunction relates to impaired mitophagy and activates NLRP3-inflammasome in lung endothelium
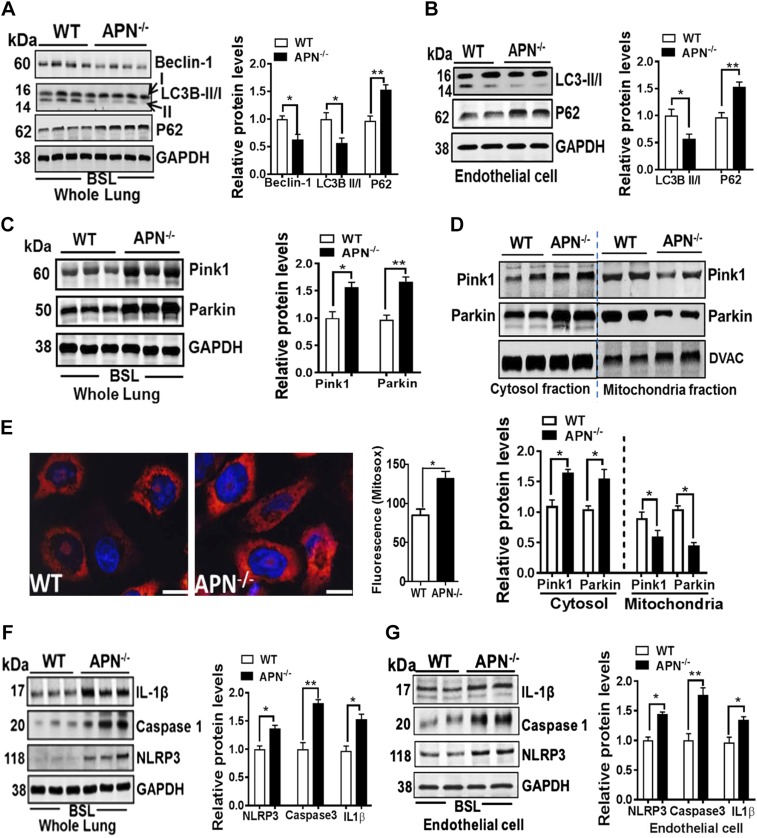
The maintenance of high-quality mitochondria is also dependent on the turnover and degradation of low-functioning or damaged mitochondria in the lysosome by a process termed as mitophagy (i.e., autophagy of mitochondria), which is a sensor for NLRP3-inflammasome activation (12, 31). As shown in Fig. 2, APN−/− mice showed decreased mitophagy in lung endothelium as manifested by reduced expression of beclin-1 and LC3B-II/I and increased expression of p62, or sequestosome, proteins in whole lungs (Fig. 2A) and in isolated lung ECs (Fig. 2B) as compared with WT mice at bsl. The impaired clearance of mitophagy is mechanistically linked to compromised translocation of Parkin from cytosol to outer mitochondrial membrane by Pink1 (31). In support of this, our data showed an increased expression of Parkin in cytosol fraction but a decreased expression in mitochondrial fraction in the lungs of APN−/− mice (Fig. 2C, D). Moreover, we found that impaired endothelial mitophagy was associated with increased accumulation of mitochondrial reactive oxygen species (mt-ROS; MitoSox, Fig. 2E) and activated NLRP3-inflammasome as demonstrated by increased cleaved caspase-1, IL-1β, and NLRP3 in whole lung tissues (Fig. 2F) as well as in isolated lung ECs (Fig. 2G) from the APN−/− mice.
Figure 2.
Mitochondrial dysfunction in APN−/− mice also relates to impaired mitophagy and NLRP3-inflammasome activation in lung endothelium. A, B) Western blot analysis for autophagy markers beclin-1, LC3B-II/I ratio, p62 proteins in whole lung tissues and in freshly isolated lung ECs in APN−/− and WT control mice at bsl. GAPDH is used as loading control. C, D) Western blot analysis for mitophagy markers Pink1 and Parkin in whole lung tissues and in cytosol and mitochondrial fractions isolated from lungs of APN−/− and WT control mice at bsl. GAPDH and voltage-dependent anion channels (DVAC) are used as loading control. E) Increased mt-ROS production (MitoSox) in isolated ECs from the lungs of APN−/− and WT control mice are shown in red and merged with nuclear stain DAPI in blue. F, G) Western blot analysis for NLRP3-inflammasome activation markers NLRP3, activated caspase-1, and cleaved IL-1β in the whole lung tissues and in freshly isolated lung ECs in APN−/− and WT control mice at bsl. GAPDH is used as loading control. Densitometry analyses are shown on the right; n = 6–8 in each group. Data are expressed as means ± se. Scale bars, 200 µm. *P < 0.05, **P < 0.01.
Mitochondrial dysfunction at bsl increases the susceptibility to LPS-induced lung injury in APN-deficient mice
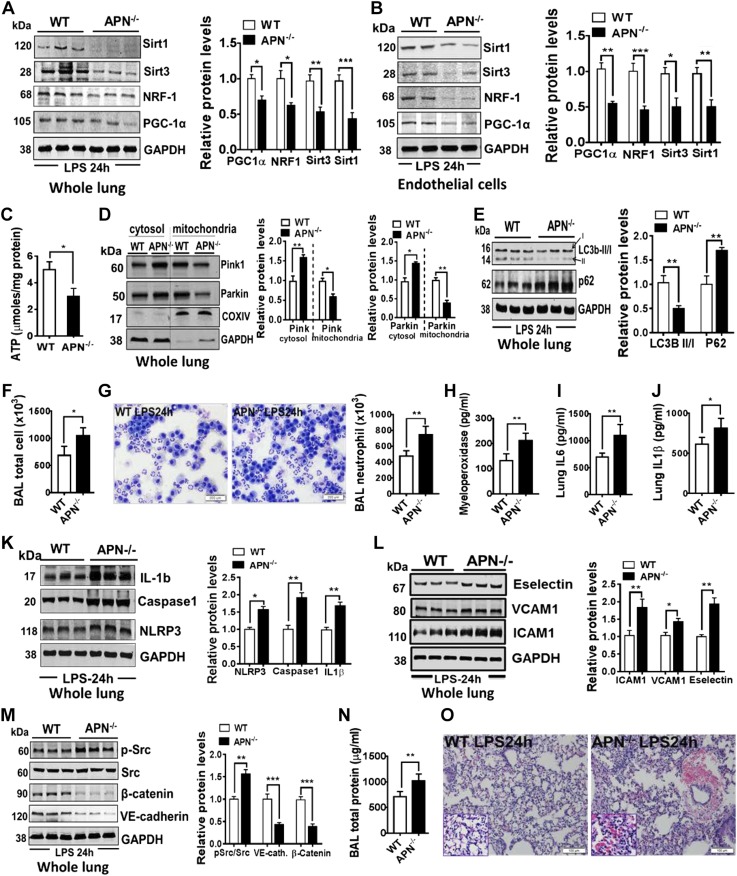
We first determined whether LPS further exacerbated mitochondrial dysfunction in APN−/− mice as compared with WT mice. As shown in Fig. 3, LPS instillation in APN−/− mice caused further diminished mitochondrial biogenesis as demonstrated by reduced expression of PGC-1α, NRF-1, TFAM, Sirt3, and Sirt1 proteins in whole lungs (Fig. 3A) as well as in freshly isolated lung ECs (Fig. 3B). These decreased mitochondrial biogenesis parameters were also associated with compromised mitochondrial function as evident by decreased intracellular ATP content in the lungs (Fig. 3C) of ALI APN−/− mice. We next determined whether LPS-augmented mitochondrial dysfunction in APN−/− mice is also related to impaired mitophagy to exacerbate lung inflammation and pulmonary vascular leakage (32). We found that in response to LPS, APN−/− mice showed further decreased mitophagy as exhibited by reduced recruitment of Parkin in the mitochondrial compartment and increased accumulation in the cytosol as compared with WT mice (Fig. 3D). The decreased mitophagy is further supported by diminished ratio of LC3B-II/I (Fig. 3E) and increased cytosolic accumulation of p62/sequestosome in the lungs of APN−/− ALI mice (Fig. 3E).
Figure 3.
Mitochondrial dysfunction in lung endothelium of APN−/− mice is associated with increased susceptibility to LPS-induced lung injury. A, B) Western blot analysis for mitochondrial biogenesis markers PGC-1α, NRF-1, Sirt3, and Sirt1 in whole lungs and in freshly isolated lung ECs from APN−/− and WT mice in response to LPS. GAPDH is used as loading control. C) ATP levels in whole lungs of APN−/− and WT in response to LPS; n = 8 in each group. D) Western blot analysis for Pink1 and Parkin in cytosol and mitochondrial fractions isolated from lungs of APN−/− and WT mice after instillation of LPS into the lungs; n = 6–8 in each group. E) Western blot analysis for p62 and LC3B-II/I in the whole lungs of APN−/− and WT mice after instillation of LPS into the lungs; n = 6–8 in each group. F, G) Total and neutrophil cell counts in the BAL fluid; n = 8 in each group. H) Myeloperoxidase levels in lungs; n = 6 in each group. I, J) ELISA for IL-6 and IL-1β in lungs of APN−/− and WT mice after instillation of LPS into the lungs; n = 8 in each group. K) Western blot analysis for NLRP3, activated caspase-1, and cleaved IL-1β in the whole lung tissues; n = 6 in each group. L) Western blot analysis for ICAM-1, VCAM-1, and E-selectin in the whole lung tissues; n = 6 in each group. M) Western blot analysis for phosphor (p)-Src/Src, β-catenin, and VE-cadherin in whole lung tissues from APN−/− and WT mice after instillation of LPS into the lungs; n = 6–8 in each group. N) Total protein concentration in BAL fluid; n = 8 in each group. O) Representative image of H&E-stained lungs; n = 5 in each group. Densitometry analyses are shown on the right. Data are expressed as means ± se. Scale bars, 100 µm. *P < 0.05, **P < 0.01, ***P < 0.001.
Finally, we assessed whether mitochondrial dysfunction and impaired mitophagy was also related to increased susceptibility to LPS-induced lung injury in APN−/− mice. We found that dysfunctional mitochondrial in APN−/− ALI mice showed aggravated lung inflammation as manifested by increased total immune cells (Fig. 3F), neutrophils infiltration into lung (Fig. 3G, H), enhanced proinflammatory cytokines (IL-6 and IL-1β; Fig. 3I, J), NLRP3-inflammasome activation markers, activated IL-1β, cleaved caspase-1 and NLRP3 in the lungs (Fig. 3K), and increased endothelial-specific adhesion markers ICAM-1, VCAM-1, and E-selectin (Fig. 3L) in the lungs of APN−/− ALI mice. This augmented lung ECs activation was also associated with decreased endothelial barrier function as manifested by decreased endothelial adherens junctions proteins VE-cadherin and β-Catenin (Fig. 3M), increased total protein (Fig. 3N), and IgM proteins (Supplemental Fig. S3A) in BAL fluid, as well as increased lung wet-dry weight ratio (Supplemental Fig. S3B) in APN−/− ALI. Consistent with this lung endothelium injury pattern, lung H&E staining consistently showed an enhanced accumulation of perivascular fluid surrounding pulmonary blood vessels and intrapulmonary hemorrhage (Fig. 3O) in APN−/− ALI mice as compared with WT ALI mice.
Our results suggest that APN deficiency induces mitochondrial dysfunction and leads to activation of ECs and decreases endothelial barrier function in response to LPS. For proof of concept, we determined whether the suppression of PGC-1α expression in lung ECs using small interfering RNA approach also decreases LPS-induced endothelial barrier function. As shown in Supplemental Fig. S4, the suppression of PGC-1α expression in ECs led to activation of endothelium (Supplemental Fig. S4A) and the reduction of endothelial barrier function as was evident by an increased FITC-dextran leaking through LPS-exposed endothelial monolayers (Supplemental Fig. S4B). These data suggest that mitochondria are involved in lung ECs dysfunction and pulmonary vascular injury.
PQQ enhances mitochondrial biogenesis and reverses lung endothelial activation in APN-deficient mice
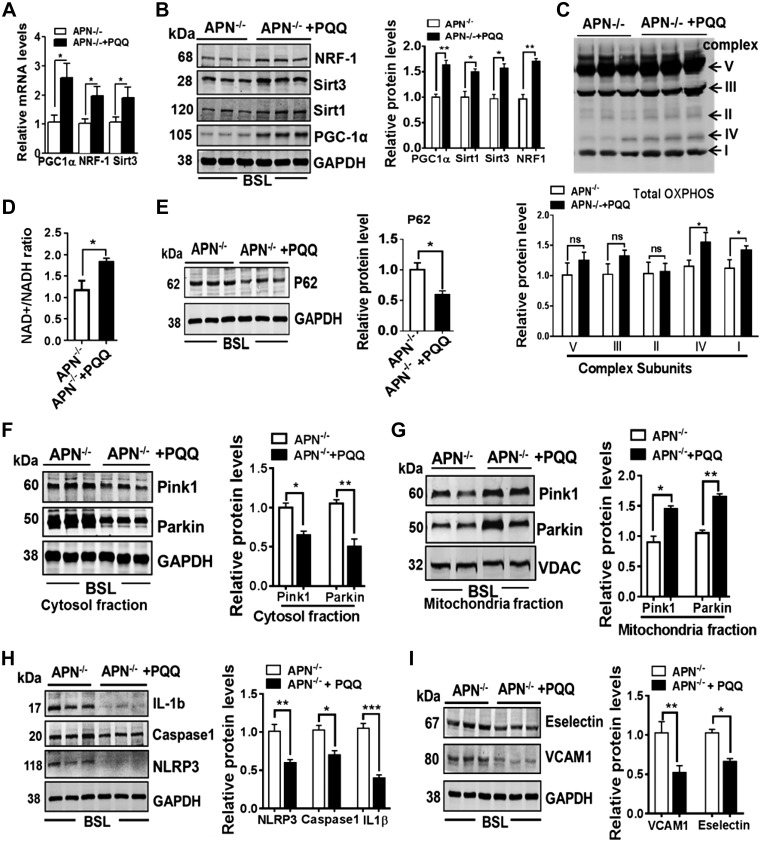
PQQ is a small polyphenolic compound found in plant foods. Among the various protective effects shown by PQQ, induction of mitochondrial biogenesis and function by increasing activity of PGC-1α has been shown in several preclinical and clinical studies (33, 34). Consistent with these reports, we also found that APN−/− mice treated with PQQ significantly enhanced mitochondrial biogenesis through phosphorylation of cAMP response element-binding protein at Ser133 (Supplemental Fig. S5), activating PGC-1α and increased NRF-1, TFAM, and Sirt3 expression in the whole lung tissues (Fig. 4A, B). This improved mitochondrial biogenesis was linked to increased expression of mitochondrial complexes IV, I (Fig. 4C) and NAD+/NADH ratio (Fig. 4D) in the lungs of APN−/− mice treated with PQQ as compared with APN−/− mice treated with PBS. We further noted that PQQ treatment also augmented mitophagy in APN−/− mice as evidenced by decreased cytosolic p62 (Fig. 4E), Pink1, and Parkin levels (Fig. 4F), and increased Pink1 and Parkin levels (Fig. 4G) in the lungs of APN−/− mice. Moreover, we also found that the increased mitochondrial biogenesis and mitophagy was also linked to decreased NLRP3-inflammasome activation (Fig. 4H) and reduced endothelial activation markers ICAM-1, VCAM-1, and E-selectin (Fig. 4I) in APN−/− mice treated with PQQ as compared with APN−/− mice treated with PBS. All together these results suggest that restoration of mitochondrial biogenesis and function reverses endothelial phenotypes caused by APN deficiencies in mice.
Figure 4.
Restoration of mitochondrial biogenesis reverses lung endothelial activation in APN-deficient mice. A, B) Transcript (PGC-1α, NRF-1, Sirt3) and protein expression (PGC-1α, NRF-1, Sirt1, and Sirt3) in the lungs of APN−/− mice treated with and without PQQ; n = 6 in each group. C) Expression of mitochondrial oxidative phosphorylation complexes (I, II, III, IV, and V) in the lungs of APN−/− mice treated with and without PQQ; n = 6 in each group. D) NAD+/NADH ratio in the lungs of APN−/− mice treated with and without PQQ; n = 8 in each group. E) Western blot analysis for p62 protein in the lungs of APN−/− mice treated with and without PQQ; n = 6 in each group. F, G) Western blot analysis for Pink1 and Parkin in cytosol and mitochondrial fractions isolated from lungs of APN−/− mice treated with and without PQQ; n = 6 in each group. H) Western blot analysis for NLRP3, activated caspase-1, and cleaved IL-1β in whole lung tissues; n = 6 in each group. *P < 0.05, **P < 0.01. I) Western blot analysis for VCAM-1 and E-selectin in whole lung tissues; n = 6 in each group. Ns, not significant. Densitometry analyses are shown on the right. Data are expressed as means ± se. *P < 0.05, **P < 0.01, ***P < 0.001.
PQQ treatment attenuates LPS-induced ALI in APN-deficient mice
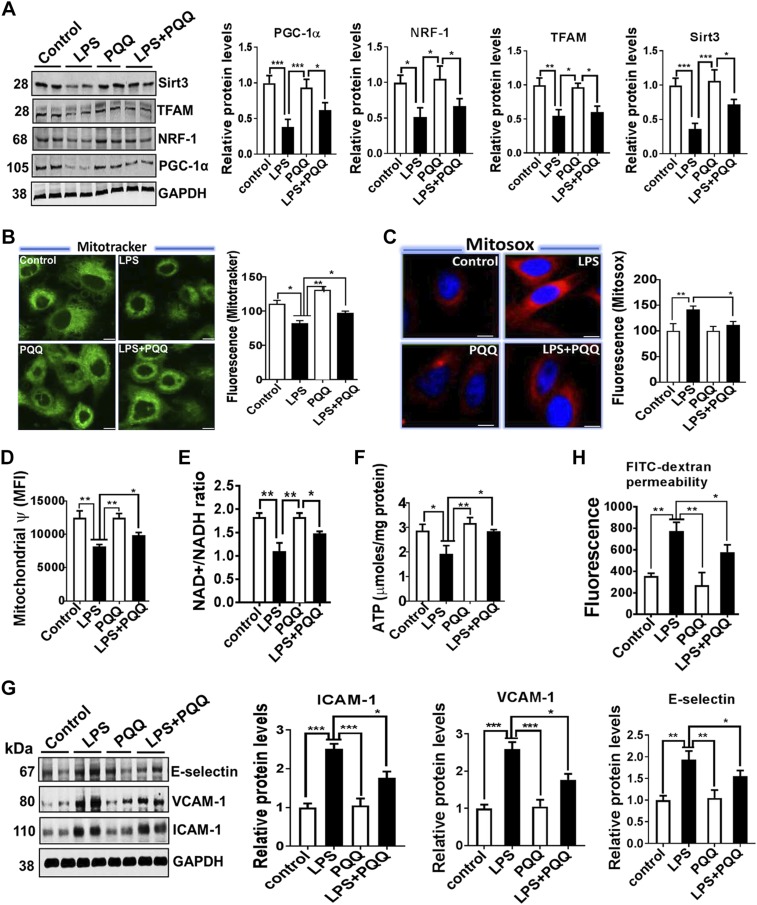
To study the effect of PQQ on lung inflammation and endothelial barrier function, we carried out in-vitro and in-vivo studies in response to LPS and assessed these parameters. As shown in Fig. 5, PQQ pretreatment improved mitochondrial biogenesis as demonstrated by increased PGC-1α, NRF-1, TFAM, Sirt3 (Fig. 5A), and mitotracker staining (Fig. 5B) in MLECs stimulated with LPS. The improved mitochondrial biogenesis is also associated with reduced mt-ROS accumulation (MitoSox, Fig. 5C), enhanced mitochondrial membrane potential (Fig. 5D), increased NAD+/NADH ratio (Fig. 5E), and ATP (Fig. 5F) in MLECs pretreated with PQQ and stimulated with LPS. Consistent with these changes in mitochondrial biogenesis and function, we also found that PQQ treatment markedly attenuated the expression of endothelial activation markers ICAM-1, VCAM-1, and E-selectin (Fig. 5G) in MLECs pretreated with PQQ and stimulated with LPS. This reduced endothelial activation by PQQ treatment also enhanced endothelial barrier function as evident by a decreased FITC-dextran leaking through LPS-exposed endothelial monolayers (Fig. 5H).
Figure 5.
Up-regulation of mitochondrial biogenesis and function in lung ECs mitigates endothelial activation and barrier function in response to LPS. A) Western blot analysis for PGC-1α, NRF-1, TFAM, and Sirt3 in lung ECs pretreated with PQQ and stimulated with LPS (1 μg/ml for 24 h). B) Confocal microscopy analysis of mitochondria morphology in lung ECs stained with MitoTracker Green. Quantification of MitoTracker Green is shown on the right. C) Decreased mt-ROS production (MitoSox) as shown in red and merged with nuclear stain DAPI in blue in lung ECs treated with PQQ and stimulated with LPS. D–F) Mitochondrial membrane potential, NAD+/NADH, and ATP content in lung ECs pretreated with PQQ and stimulated with LPS. G) Western blot analysis for ICAM-1, VCAM-1, and E-selectin in lung ECs treated with PQQ and stimulated with LPS. H) Endothelial permeability measured by FITC-dextran permeability to lung ECs treated with PQQ and stimulated with LPS. MFI, mean fluorescence intensity. Densitometry analyses are shown on the right; n = 4 in each group. *P < 0.05, **P < 0.01, ***P < 0.001.
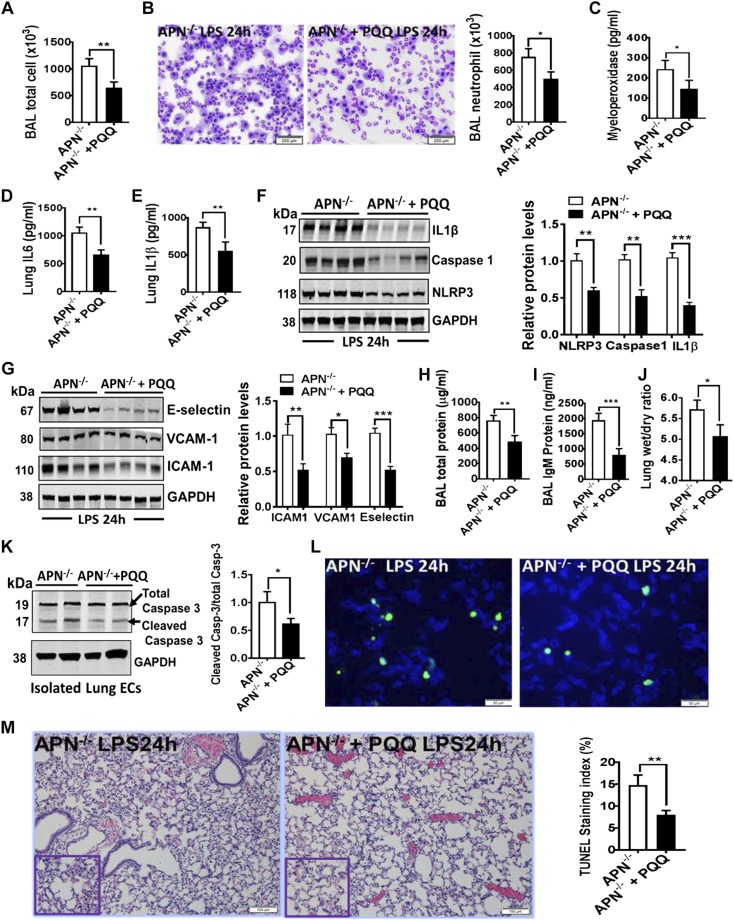
Like the in vitro results, APN−/− mice pretreated with PQQ attenuated LPS-induced lung inflammation, as demonstrated by decreased total neutrophil cell counts in BAL fluid, decreased inflammatory cytokines (IL-6 and IL-1β) as well as NLRP3-inflammasome markers (NLRP3, cleaved caspase-1, and IL-1β) in lungs (Fig. 6A–F). Importantly, PQQ pretreatment to APN−/− mice also showed the decreased expression of endothelial activations markers (Fig. 6G), improved endothelial barrier function, as demonstrated by reduced BAL total protein level (Fig. 6H) and BAL IgM protein level (Fig. 6I) and by decreased lung wet-dry ratio (Fig. 6J) as compared with APN−/− mice pretreated with PBS (vehicle), and followed intratracheal instillation of LPS. We also detected the decreased cell death as displayed by reduced cleaved caspase-3 (Fig. 6K) and terminal deoxynucleotidyl transferase dUTP nick end labeling staining (Fig. 6L) in lungs of APN−/− mice pretreated with PQQ and challenged with LPS. We further found that APN−/− mice pretreated with PQQ also significantly decreased ALI as evident by improved lung histology (Fig. 6M) as compared with APN−/− mice pretreated with PBS. Taken together, these findings indicate that PQQ bypasses the effects of APN deficiency and enhances mitochondrial biogenesis through activation of PGC-1α, improves mitophagy, decreases NLRP3-inflammasome activation, and attenuates lung endothelium dysfunction and injury.
Figure 6.
PQQ-mediated improved mitochondrial biogenesis and function alleviates LPS-induced ALI in APN−/− mice. A, B) Total and neutrophil cell counts in the BAL fluid from APN−/− ALI mice with and without treatment of PQQ; n = 8 in each group. C) Myeloperoxidase levels in lungs; n = 8 in each group. D, E) ELISA for IL-6 and IL-1β in lungs of APN−/− ALI mice with and without treatment of PQQ; n = 8 in each group. F) Western blot analysis for NLRP3, activated caspase-1, and cleaved IL-1β in the whole lung tissues of APN−/− ALI mice with and without treatment of PQQ; n = 6 in each group. G) Western blot analysis for ICAM-1, VCAM-1, and E-selectin in the whole lung tissues of APN−/− ALI mice with and without treatment of PQQ. Densitometry analysis is shown on the right; n = 8 in each group. H, I) Total and IgM protein concentration in BAL fluid; n = 8 in each group. J) Lung wet-dry ratio in APN−/− ALI mice with and without treatment of PQQ; n = 8 in each group. K) Western blot analysis for cleaved caspase-3 (Casp-3) in lungs of APN−/− ALI mice with and without treatment of PQQ; n = 6 in each group. L) Representative terminal deoxynucleotidyl transferase dUTP nick end labeling staining (green color) of apoptotic cells in lungs of APN−/− ALI mice with and without treatment of PQQ; n = 4 in each group. M) Representative image of H&E-stained lungs of APN−/− ALI mice with and without treatment of PQQ; n = 5 in each group. Densitometry analyses are shown on the right. Data are expressed as means ± se. Scale bars, 200 µm (B); 100 µm (L). *P < 0.05, **P < 0.01, ***P < 0.001.
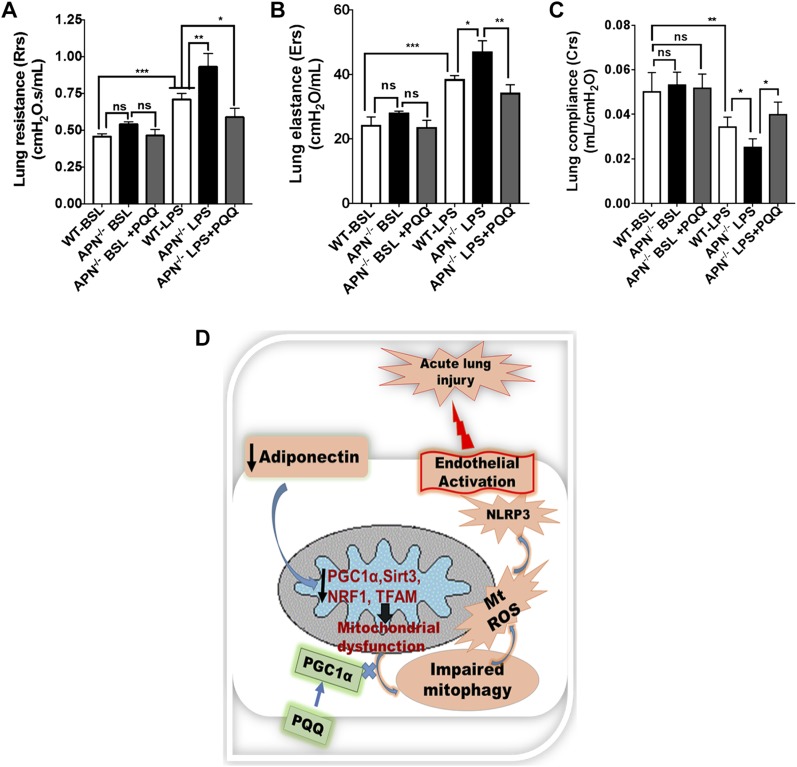
PQQ treatment improves lung mechanics in APN-deficient ALI mice
Lastly, we determined whether PQQ-mediated improved mitochondrial biogenesis and bioenergetics also improves lung mechanics in APN−/− mice. Although, we found enhanced mitochondrial dysfunction and endothelium activated in APN−/− mice as compared with WT mice at bsl (Fig. 1), lung mechanics parameters such as lung resistance, elastance, and compliance were not significantly different in these mice at bsl (Fig. 7A–C). However, in response to LPS, lung resistance and elastance were significantly increased, whereas lung compliance was remarkably decreased in ALI APN−/− mice as compared with ALI WT mice (Fig. 7A–C). Interestingly the enhanced mitochondrial biogenesis by PQQ treatment to APN−/− mice showed improved lung mechanics as demonstrated by diminished lung resistance and elastance with enhanced lung compliance as compared with APN-deficient ALI mice treated with PBS (Fig. 7A–C). These data suggest that mitochondrial homeostasis is required for normal physiologic function of lung. Figure 7D shows a diagram of our proposed hypothesis and results.
Figure 7.
PQQ treatment improves lung mechanics of APN−/− ALI mice. A) Lung resistance (Rrs) measured by flexiVent at bsl and after LPS-induced ALI in APN−/− mice treated with and without PQQ; n = 6 in each group. B) Lung elastance (Ers); n = 6 in each group. C) Lung compliance (Crs); n = 6 in each group. D) Schematic illustration of the proposed sequence of events in APN-deficient mice that leads to mitochondrial dysfunction and impaired mitophagy, which together activate lung endothelium and predispose to injury in response to LPS. The restoration of mitochondrial biogenesis by PQQ treatment establishes endothelial homeostasis and attenuates LPS-induced ALI in APN-deficient mice. Ns, not significant. *P < 0.05, **P < 0.01, ***P < 0.001.
DISCUSSION
APN, an adipocyte-derived adipokine, has been shown to tonically inhibit EC activation and to limit lung injury originated from EC damage (7, 35). Over the past decade, efforts in the field have focused on the connection between APN and lung endothelial dysfunction; however, the mechanism is largely unknown. Here, we provide evidence that APN deficiency decreases pulmonary endothelial mitochondrial biogenesis, impairs mitochondrial function, which potentially promotes EC activation, and increases the susceptibility to LPS-induced ALI. We showed that pretreatment of APN-deficient mice with PQQ enhances mitochondrial biogenesis through activation of PGC-1α, improves mitophagy, decreases NLRP3-inflammasome activation, and attenuates endothelial dysfunction and lung injury.
Previous preclinical studies have shown that APN protects lung ECs activation and pulmonary vascular injury through inhibition of inflammatory cytokines milieu in the lungs in response to injury (7, 10, 21, 36). Among these studies, Ouedraogo et al. (21) and Konter et al. (7) specified that APN-deficient mice showed changes in lung endothelial phenotype such as activated and inflamed endothelium at bsl. The Konter group isolated lung ECs from APN-deficient mice at bsl and reported that APN deficiency promoted endothelial activation through increased inflammation in lung endothelium. The Ouedraogo group showed that APN deficiency induces endothelial dysfunction mediated by loss of endothelial NO and increased leukocyte-endothelium interactions. Consistent with these reports, we also found the lung endothelial phenotypic changes in APN-deficient mice in the freshly isolated lung ECs at bsl. Our data showed an increase in transcript and protein expressions of endothelial-specific adhesion markers ICAM-1, VCAM-1, and E-selectin in whole lungs and freshly isolated ECs from the lungs of APN−/− mice. These results confirmed that APN deficiency promotes lung endothelial activation.
Previous studies indicated that APN may be directly protective of endothelial activation and lung injury (7, 21). Our results showed that APN acts as a signaling molecule and controls lung endothelial inflammation and activation. We report for the first time that APN deficiency produces a series of changes in mitochondrial biogenesis regulatory protein PGC-1α and its upstream and downstream protein (NRF-1, TFAM, Sirt3, and Sirt1) expressions in whole lungs and in lung ECs in APN−/− mice at bsl. The decreased mitochondrial biogenesis in lung ECs also showed the compromised mitochondrial function such as low intracellular ATP content, reduced expression of mitochondrial complexes I/II, III/V, as well as impaired mitophagy in the lung ECs of APN−/− mice at bsl. We also noted that APN deficiency-mediated mitochondrial dysfunction in lung ECs is related to ECs activation at bsl in APN−/− mice. Analogous to these findings, several lines of evidence from human subjects suggest that circulating extracellular mt-DNA released from dysfunctional mitochondria play important roles in trauma and severe illness, leading to ARDS (15, 37–40). Similarly, a preclinical study has shown that the injection of mitochondrial lysates in the rat causes lung inflammation (16). We found that endothelial mitochondrial dysfunction in APN−/− mice also showed an increased NLRP3-inflammasome activation. Although, we did not demonstrate how NLRP3-inflammasome is activated in APN−/− mice; however, release of mt-DNA and mt-ROS from damaged or dysfunctional mitochondrial have been well established as a danger signal for inflammasome activation (41–43). We speculate that NLRP3-inflammasome activation mediated by damaged mitochondria in ECs may be, in part, involved in the lung ECs activation in these mice.
APN has been reported to modulate the mitochondrial biogenesis and function through increased expression of PGC-1α as well as by enhanced PGC-1α activity in various tissues (19, 20). Notably, PGC-1α heterozygote (PGC-1α+/−) mice are more susceptible to Staphylococcus aureus–induced sepsis (44). Our results indicate that APN deficiency promotes mitochondrial dysfunction and induces endothelial activation in lungs. We hypothesized that restoration of mitochondrial biogenesis and function may bypass the effects of APN deficiency and attenuate lung endothelium dysfunction and injury. We utilized PQQ, a specific inducer or activator of PGC-1α, to restore mitochondrial biogenesis in lung endothelium of APN−/− mice and studied the endothelial phenotype at bsl and vascular injury in response to LPS-mediated ALI. PQQ is a small polyphenolic compound found in plant foods and has been shown to increase expression and activity of PGC-1α as well as nuclear respiratory factor activation (NRF-1, TFAM) and Sirt3 expression to elicit mitochondrial biogenesis in several preclinical and clinical studies (27, 33, 34). Consistent with these reports, our data demonstrate that pretreatment of PQQ to APN−/− mice restored mitochondrial biogenesis and function and diminished lung endothelial activation at bsl. The restored mitochondrial bioenergetics in lung endothelial also diminished lung inflammation and protected against LPS-induced pulmonary vascular injury in APN−/− mice pretreated with PQQ. We also noted that PQQ treatment to ECs reduced mt-ROS accumulation and improved clearance of damaged or dysfunctional mitochondria by augmented mitophagy; however, currently we do not know how PQQ causes these effects on lung endothelium. Previous studies have reported that sepsis induced in PGC-1α+/− mice showed decreased antioxidant enzyme activity with increased induction and accumulation of mt-ROS (44), and the overexpression of PGC-1α led to the induction of the mitochondrial antioxidant defenses and a reduction of oxidative stress in vascular ECs (45). We postulate that PQQ-mediated increased PGC-1α expression may be involved in enhanced mitophagy and decreased mt-ROS accumulation to control NLRP3-inflammasome activation in lung endothelium; however, additional studies are needed to confirm it.
Lung mechanics are an extremely useful readout in ARDS models to evaluate the therapeutic potential of drugs to rescue the compromised lung function parameters in ALI mice (46, 47). Although our data showed that APN−/− mice at bsl showed endothelial activation phenotype, these mice did not show significant compromised lung function parameters as compared with WT mice. These results suggest and are consistent with previous reports that mild inflammation does not change lung function measurement in mice (48). We detected significant changes in lung mechanics parameters (lung compliance, resistance, and elastase) in both WT and APN−/− ALI mice after LPS instillation with further compromised lung function in APN−/− mice. Interestingly, the restoration of mitochondrial homeostasis by PQQ treatment to APN−/− ALI mice improved lung mechanics parameters such as lung compliance, resistance, and elastase in this study.
Our study is primarily focused on the effects of mitochondrial dysfunction in endothelial activation and pulmonary vascular injury in ALI mice. Another group of investigators has recently reported that mitochondrial biogenesis related gene Sirt3−/− mice developed severe ALI and WT mice pretreated with Sirt3 activator viniferin restored mitochondrial bioenergetics in macrophages and attenuated ALI (17). Likewise, Hough et al. (18) have shown that pulmonary endothelial mitochondria regulate the barrier failure by activating uncoupling protein 2 in acid (HCl)-induced ALI. These findings suggest that mitochondria homeostasis is crucial for proper functioning of lung cell types, such as endothelial, and immune cell types, such as macrophages. Thus, it would be interesting to know whether APN deficiency also impairs mitochondrial homeostasis in lung epithelial cells and contributes to lung inflammation and injury.
In summary, our data show that the pulmonary endothelium-protective properties of APN are mediated, at least in part, by an enhancement of mitochondrial biogenesis and function through a mechanism involving PGC-1α in lung endothelium.
ACKNOWLEDGMENTS
This work was supported by the Departmental of Pediatrics and Drexel University Fund (282656 to D.S. and V.B.), U.S. National Institutes of Health, National Institute on Aging 1R56AG056696-01 (to C.T.), and the Commonwealth Universal Research Enhancement Program (CURE; 450095-5626 to D.S., C.T., and V.B.). The authors declare no conflicts of interest.
Glossary
- ALI
acute lung injury
- APN
adiponectin
- ARDS
acute respiratory distress syndrome
- BAL
bronchoalveolar lavage
- bsl
baseline
- DAMP
damage-associated molecular pattern
- DUCOM
Drexel University College of Medicine
- EC
endothelial cell
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- H&E
hemotoxylin and eosin
- Hprt1
hypoxanthine phosphoribosyltransferase 1
- ICAM-1
intercellular adhesion molecule 1
- LC3B
microtubule-associated protein light chain 3
- MLEC
mouse lung microvascular endothelial cell
- mt-DNA
mitochondrial DNA
- NLRP3
nucleotide-binding domain and leucine-rich repeat containing protein
- NRF-1
nuclear respiratory factor 1
- PGC-1α
regulatory protein peroxisome proliferator-activated receptor γ coactivator 1-α
- Pink1
PTEN-induced kinase 1
- PQQ
pyrroloquinoline quinone
- mt-ROS
mitochondrial reactive oxygen species
- Sirt
Sirtuin
- TFAM
mitochondrial transcription factor A
- VCAM-1
vascular cell adhesion molecule 1
- VE
vascular endothelial
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
D. Shah conceived and designed the study; D. Shah performed experiments; D. Shah, C. Torres, and V. Bhandari analyzed the data; D. Shah wrote the manuscript; D. Shah, C. Torres, and V. Bhandari edited the manuscript; and all authors have approved the final version of the submitted manuscript.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Matthay M. A., Ware L. B., Zimmerman G. A. (2012) The acute respiratory distress syndrome. J. Clin. Invest. 122, 2731–2740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rubenfeld G. D., Caldwell E., Peabody E., Weaver J., Martin D. P., Neff M., Stern E. J., Hudson L. D. (2005) Incidence and outcomes of acute lung injury. N. Engl. J. Med. 353, 1685–1693 [DOI] [PubMed] [Google Scholar]
- 3.Mora-Rillo M., Arsuaga M., Ramírez-Olivencia G., de la Calle F., Borobia A. M., Sánchez-Seco P., Lago M., Figueira J. C., Fernández-Puntero B., Viejo A., Negredo A., Nuñez C., Flores E., Carcas A. J., Jiménez-Yuste V., Lasala F., García-de-Lorenzo A., Arnalich F., Arribas J. R.; La Paz-Carlos III University Hospital Isolation Unit (2015) Acute respiratory distress syndrome after convalescent plasma use: treatment of a patient with Ebola virus disease contracted in Madrid, Spain. Lancet Respir. Med. 3, 554–562 [DOI] [PubMed] [Google Scholar]
- 4.Kang N. Y., Ivanovska J., Tamir-Hostovsky L., Belik J., Gauda E. B. (2018) Chronic intermittent hypoxia in premature infants: the link between low fat stores, adiponectin receptor signaling and lung injury. Adv. Exp. Med. Biol. 1071, 151–157 [DOI] [PubMed] [Google Scholar]
- 5.Piao L., Yu C., Xu W., Inoue A., Shibata R., Li X., Nan Y., Zhao G., Wang H., Meng X., Lei Y., Goto H., Ouchi N., Murohara T., Kuzuya M., Cheng X. W. (2018) Adiponectin/AdiopR1 signal inactivation contributes to impaired angiogenesis in mice of advanced age. Int. J. Cardiol. 267, 150–155 [DOI] [PubMed] [Google Scholar]
- 6.Eising J. B., Uiterwaal C. S., Evelein A. M., Visseren F. L., van der Ent C. K. (2014) Relationship between leptin and lung function in young healthy children. Eur. Respir. J. 43, 1189–1192 [DOI] [PubMed] [Google Scholar]
- 7.Konter J. M., Parker J. L., Baez E., Li S. Z., Ranscht B., Denzel M., Little F. F., Nakamura K., Ouchi N., Fine A., Walsh K., Summer R. S. (2012) Adiponectin attenuates lipopolysaccharide-induced acute lung injury through suppression of endothelial cell activation. J. Immunol. 188, 854–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah D., Romero F., Duong M., Wang N., Paudyal B., Suratt B. T., Kallen C. B., Sun J., Zhu Y., Walsh K., Summer R. (2015) Obesity-induced adipokine imbalance impairs mouse pulmonary vascular endothelial function and primes the lung for injury. Sci. Rep. 5, 11362 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Xu L., Bao H. G., Si Y. N., Han L., Zhang R., Cai M. M., Shen Y. (2013) Effects of adiponectin on acute lung injury in cecal ligation and puncture-induced sepsis rats. J. Surg. Res. 183, 752–759 [DOI] [PubMed] [Google Scholar]
- 10.Sliman S. M., Patel R. B., Cruff J. P., Kotha S. R., Newland C. A., Schrader C. A., Sherwani S. I., Gurney T. O., Magalang U. J., Parinandi N. L. (2013) Adiponectin protects against hyperoxic lung injury and vascular leak. Cell Biochem. Biophys. 67, 399–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naik E., Dixit V. M. (2011) Mitochondrial reactive oxygen species drive proinflammatory cytokine production. J. Exp. Med. 208, 417–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cloonan S. M., Choi A. M. (2016) Mitochondria in lung disease. J. Clin. Invest. 126, 809–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brealey D., Brand M., Hargreaves I., Heales S., Land J., Smolenski R., Davies N. A., Cooper C. E., Singer M. (2002) Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet 360, 219–223 [DOI] [PubMed] [Google Scholar]
- 14.Japiassú A. M., Santiago A. P., d’Avila J. C., Garcia-Souza L. F., Galina A., Castro Faria-Neto H. C., Bozza F. A., Oliveira M. F. (2011) Bioenergetic failure of human peripheral blood monocytes in patients with septic shock is mediated by reduced F1Fo adenosine-5′-triphosphate synthase activity. Crit. Care Med. 39, 1056–1063 [DOI] [PubMed] [Google Scholar]
- 15.Nakahira K., Kyung S. Y., Rogers A. J., Gazourian L., Youn S., Massaro A. F., Quintana C., Osorio J. C., Wang Z., Zhao Y., Lawler L. A., Christie J. D., Meyer N. J., Mc Causland F. R., Waikar S. S., Waxman A. B., Chung R. T., Bueno R., Rosas I. O., Fredenburgh L. E., Baron R. M., Christiani D. C., Hunninghake G. M., Choi A. M. (2013) Circulating mitochondrial DNA in patients in the ICU as a marker of mortality: derivation and validation. PLoS Med. 10, e1001577; discussion e1001577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Q., Itagaki K., Hauser C. J. (2010) Mitochondrial DNA is released by shock and activates neutrophils via p38 map kinase. Shock 34, 55–59 [DOI] [PubMed] [Google Scholar]
- 17.Kurundkar D., Kurundkar A. R., Bone N. B., Becker E. J., Jr., Liu W., Chacko B., Darley-Usmar V., Zmijewski J. W., Thannickal V. J. (2019) SIRT3 diminishes inflammation and mitigates endotoxin-induced acute lung injury. JCI Insight 4, 120722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hough R. F., Islam M. N., Gusarova G. A., Jin G., Das S., Bhattacharya J. (2019) Endothelial mitochondria determine rapid barrier failure in chemical lung injury. JCI Insight 4, 124329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qiao L., Kinney B., Yoo H. S., Lee B., Schaack J., Shao J. (2012) Adiponectin increases skeletal muscle mitochondrial biogenesis by suppressing mitogen-activated protein kinase phosphatase-1. Diabetes 61, 1463–1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan W., Zhang H., Liu P., Wang H., Liu J., Gao C., Liu Y., Lian K., Yang L., Sun L., Guo Y., Zhang L., Dong L., Lau W. B., Gao E., Gao F., Xiong L., Wang H., Qu Y., Tao L. (2013) Impaired mitochondrial biogenesis due to dysfunctional adiponectin-AMPK-PGC-1α signaling contributing to increased vulnerability in diabetic heart. Basic Res. Cardiol. 108, 329 [DOI] [PubMed] [Google Scholar]
- 21.Ouedraogo R., Gong Y., Berzins B., Wu X., Mahadev K., Hough K., Chan L., Goldstein B. J., Scalia R. (2007) Adiponectin deficiency increases leukocyte-endothelium interactions via upregulation of endothelial cell adhesion molecules in vivo. J. Clin. Invest. 117, 1718–1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah D., Das P., Alam M. A., Mahajan N., Romero F., Shahid M., Singh H., Bhandari V. (2019) MicroRNA-34a promotes endothelial dysfunction and mitochondrial-mediated apoptosis in murine models of acute lung injury. Am. J. Respir. Cell Mol. Biol. 60, 465–477 [DOI] [PubMed] [Google Scholar]
- 23.Kumar N., Kar A. (2015) Pyrroloquinoline quinone (PQQ) has potential to ameliorate streptozotocin-induced diabetes mellitus and oxidative stress in mice: a histopathological and biochemical study. Chem. Biol. Interact. 240, 278–290 [DOI] [PubMed] [Google Scholar]
- 24.Kumar N., Kar A. (2015) Pyrroloquinoline quinone ameliorates oxidative stress and lipid peroxidation in the brain of streptozotocin-induced diabetic mice. Can. J. Physiol. Pharmacol. 93, 71–79 [DOI] [PubMed] [Google Scholar]
- 25.Shah D., Romero F., Summer R. (2016) Diet-induced obesity induces endoplasmic reticulum stress in the lung endothelium and predispose to LPS induced acute lung injury. Am. J. Respir. Crit. Care Med. 193, A7868 (abstr.) [Google Scholar]
- 26.Shah D., Romero F., Zhu Y., Duong M., Sun J., Walsh K., Summer R. (2015) C1q deficiency promotes pulmonary vascular inflammation and enhances the susceptibility of the lung endothelium to injury. J. Biol. Chem. 290, 29642–29651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chowanadisai W., Bauerly K. A., Tchaparian E., Wong A., Cortopassi G. A., Rucker R. B. (2010) Pyrroloquinoline quinone stimulates mitochondrial biogenesis through cAMP response element-binding protein phosphorylation and increased PGC-1alpha expression. J. Biol. Chem. 285, 142–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ponnalagu D., Gururaja Rao S., Farber J., Xin W., Hussain A. T., Shah K., Tanda S., Berryman M., Edwards J. C., Singh H. (2016) Molecular identity of cardiac mitochondrial chloride intracellular channel proteins. Mitochondrion 27, 6–14 [DOI] [PubMed] [Google Scholar]
- 29.Lee S. J., Song E. S., Cho H. J., Choi Y. Y., Ma J. S., Cho Y. K. (2017) Rapid regression of obstructive cardiac rhabdomyoma in a preterm neonate after sirolimus therapy. Biomed. Hub 2, 460813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGovern T. K., Robichaud A., Fereydoonzad L., Schuessler T. F., Martin J. G. (2013) Evaluation of respiratory system mechanics in mice using the forced oscillation technique. J. Vis. Exp. 75, e50172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rüb C., Wilkening A., Voos W. (2017) Mitochondrial quality control by the Pink1/Parkin system. Cell Tissue Res. 367, 111–123 [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y., Sauler M., Shinn A. S., Gong H., Haslip M., Shan P., Mannam P., Lee P. J. (2014) Endothelial PINK1 mediates the protective effects of NLRP3 deficiency during lethal oxidant injury. J. Immunol. 192, 5296–5304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harris C. B., Chowanadisai W., Mishchuk D. O., Satre M. A., Slupsky C. M., Rucker R. B. (2013) Dietary pyrroloquinoline quinone (PQQ) alters indicators of inflammation and mitochondrial-related metabolism in human subjects. J. Nutr. Biochem. 24, 2076–2084 [DOI] [PubMed] [Google Scholar]
- 34.Stites T., Storms D., Bauerly K., Mah J., Harris C., Fascetti A., Rogers Q., Tchaparian E., Satre M., Rucker R. B. (2006) Pyrroloquinoline quinone modulates mitochondrial quantity and function in mice. J. Nutr. 136, 390–396 [DOI] [PubMed] [Google Scholar]
- 35.Van Meurs M., Castro P., Shapiro N. I., Lu S., Yano M., Maeda N., Funahashi T., Shimomura I., Zijlstra J. G., Molema G., Parikh S. M., Aird W. C., Yano K. (2012) Adiponectin diminishes organ-specific microvascular endothelial cell activation associated with sepsis. Shock 37, 392–398 [DOI] [PubMed] [Google Scholar]
- 36.Xu B., O’Donnell M., O’Donnell J., Yu J., Zhang Y., Sartor M. A., Koenig R. J. (2016) Adipogenic differentiation of thyroid cancer cells through the Pax8-PPARγ fusion protein is regulated by thyroid transcription factor 1 (TTF-1). J. Biol. Chem. 291, 19274–19286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kung C. T., Hsiao S. Y., Tsai T. C., Su C. M., Chang W. N., Huang C. R., Wang H. C., Lin W. C., Chang H. W., Lin Y. J., Cheng B. C., Su B. Y., Tsai N. W., Lu C. H. (2012) Plasma nuclear and mitochondrial DNA levels as predictors of outcome in severe sepsis patients in the emergency room. J. Transl. Med. 10, 130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johansson P. I., Nakahira K., Rogers A. J., McGeachie M. J., Baron R. M., Fredenburgh L. E., Harrington J., Choi A. M. K., Christopher K. B. (2018) Plasma mitochondrial DNA and metabolomic alterations in severe critical illness. Crit. Care 22, 360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee Y. L., Obiako B., Gorodnya O. M., Ruchko M. V., Kuck J. L., Pastukh V. M., Wilson G. L., Simmons J. D., Gillespie M. N. (2017) Mitochondrial DNA damage initiates acute lung injury and multi-organ system failure evoked in rats by intra-tracheal Pseudomonas Aeruginosa. Shock 48, 54–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Q., Raoof M., Chen Y., Sumi Y., Sursal T., Junger W., Brohi K., Itagaki K., Hauser C. J. (2010) Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464, 104–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Q., Zhang D., Hu D., Zhou X., Zhou Y. (2018) The role of mitochondria in NLRP3 inflammasome activation. Mol. Immunol. 103, 115–124 [DOI] [PubMed] [Google Scholar]
- 42.Zhou R., Yazdi A. S., Menu P., Tschopp J. (2011) A role for mitochondria in NLRP3 inflammasome activation. Nature 469, 221–225; erratum: 475, 122 [DOI] [PubMed] [Google Scholar]
- 43.Gurung P., Lukens J. R., Kanneganti T. D. (2015) Mitochondria: diversity in the regulation of the NLRP3 inflammasome. Trends Mol. Med. 21, 193–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cherry A. D., Suliman H. B., Bartz R. R., Piantadosi C. A. (2014) Peroxisome proliferator-activated receptor γ co-activator 1-α as a critical co-activator of the murine hepatic oxidative stress response and mitochondrial biogenesis in Staphylococcus aureus sepsis. J. Biol. Chem. 289, 41–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Valle I., Alvarez-Barrientos A., Arza E., Lamas S., Monsalve M. (2005) PGC-1alpha regulates the mitochondrial antioxidant defense system in vascular endothelial cells. Cardiovasc. Res. 66, 562–573 [DOI] [PubMed] [Google Scholar]
- 46.Henderson W. R., Chen L., Amato M. B. P., Brochard L. J. (2017) Fifty years of research in ARDS. Respiratory mechanics in acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 196, 822–833 [DOI] [PubMed] [Google Scholar]
- 47.Rocco P. R., Momesso D. P., Figueira R. C., Ferreira H. C., Cadete R. A., Légora-Machado A., Koatz V. L., Lima L. M., Barreiro E. J., Zin W. A. (2003) Therapeutic potential of a new phosphodiesterase inhibitor in acute lung injury. Eur. Respir. J. 22, 20–27 [DOI] [PubMed] [Google Scholar]
- 48.Reiss L. K., Kowallik A., Uhlig S. (2011) Recurrent recruitment manoeuvres improve lung mechanics and minimize lung injury during mechanical ventilation of healthy mice. PLoS One 6, e24527 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.