Abstract
Cytokines and chemokines play diverse roles in different organ systems. Family with sequence similarity 19, member A1-5 (FAM19A1-A5; also known as TAFA1-5) is a group of conserved chemokine-like proteins enriched in the CNS of mice and humans. Their functions are only beginning to emerge. Here, we show that the expression of Fam19a1-a5 in different mouse brain regions are induced or suppressed by unfed and refed states. The striking nutritional regulation of Fam19a family members in the brain suggests a potential central role in regulating metabolism. Using a knockout (KO) mouse model, we show that loss of FAM19A1 results in sexually dimorphic phenotypes. In male mice, FAM19A1 deficiency alters food intake patterns during the light and dark cycle. Fam19a1 KO mice are hyperactive, and locomotor hyperactivity is more pronounced in female KO mice. Behavior tests indicate that Fam19a1 KO female mice have reduced anxiety and sensitivity to pain. Spatial learning and exploration, however, is preserved in Fam19a1 KO mice. Altered behaviors are associated with elevated norepinephrine and dopamine turnover in the striatum. Our results establish an in vivo function of FAM19A1 and highlight central roles for this family of neurokines in modulating animal physiology and behavior.—Lei, X., Liu, L., Terrillion, C. E., Karuppagounder, S. S., Cisternas, P., Lay, M., Martinelli, D. C., Aja, S., Dong, X., Pletnikov, M. V., Wong, G. W. FAM19A1, a brain-enriched and metabolically responsive neurokine, regulates food intake patterns and mouse behaviors.
Keywords: chemokine-like, dopamine, locomotor hyperactivity, TAFA1
Cytokines and chemokines regulate diverse biologic processes that range from immunity (1, 2) to CNS control of food intake and metabolism (3–5). We have previously shown that the circulating plasma levels of a large number of cytokines, chemokines, and soluble cytokine receptors are regulated by physical activity and the metabolic states of mice (5, 6) and that some of these changes are functionally coupled to altered ingestive physiology.
Within the CNS, there is a group of 5 highly conserved, brain-enriched, chemokine-like proteins—family with sequence similarity 19, member A1-5 (FAM19A1-A5; also known as TAFA1-5)—originally identified by bioinformatics as being distantly related to macrophage inhibitory protein/C-C motif chemokine ligand 3. Each possesses 8–10 regularly spaced cysteine residues (7). The conservation between human and mouse FAM19A orthologs ranges from 86–100% amino acid identity. In contrast, the conservation between mouse FAM19A paralogs (comparing different family members) ranges from 44–69% amino acid identity. Recent genome-wide association studies in humans suggest a link between FAM19A2 and insulin sensitivity (8). Targeted disruption of the Fam19a2 gene in mice, however, revealed a role in modulating learning and memory, as well as anxious behavior (9). Biologic functions of other FAM19A family members have only begun to emerge. Genetic gain-of-function studies provide in vivo evidence for a protective role of FAM19A5 in the vasculature (10). Loss-of-function mouse models have uncovered somatosensory roles for FAM19A4/TAFA4 in pain sensation induced by mechanical and chemical stimuli (11, 12). Recent in vitro studies also highlight multiple roles for FAM19A family members in different cell types. For example, FAM19A1 was shown to be the ligand for GPCR 1 (13); binding of FAM19A1 to the N-terminal domain of GPCR1 activates Rho-associated protein kinase and its downstream signaling, leading to the suppression of neuronal stem cell proliferation and the promotion of neuronal differentiation in culture. Interestingly, several FAM19A family members have also been shown to modulate stromal and mesenchymal stem cell migration (14) as well as macrophage polarization (15), macrophage chemotaxis (16, 17), and phagocytosis (16). The physiologic significance of these in vitro findings, however, awaits further in vivo confirmation.
To identify novel secretory proteins within the CNS with potential metabolic function, we focused our analysis on secreted proteins in the brain that are understudied. Given that Fam19a family members are highly expressed in the nervous system, we explored their potential central role in modulating food intake and metabolism in peripheral tissues. We made the unexpected findings that Fam19a family member expression in different brain regions are highly responsive to unfed and refed states, which is suggestive of a metabolic role. Because Fam19a1 expression shows the most striking responsiveness to metabolic cues and its physiologic role in vivo is not known, we focused our functional studies on this protein using a genetic loss-of-function mouse model. Our studies provide important and novel insights on the emerging central functions of this class of enigmatic but highly conserved family of secretory proteins.
MATERIALS AND METHODS
Gene expression profiling
Gene expression profiling was performed using a TissueScan (OriGene Technologies, Rockville, MD, USA) human major tissue quantitative PCR (qPCR) array (HMRT303), mouse normal tissue qPCR array (MNRT501), human brain qPCR array (HBRT101), and mouse developmental tissue qPCR array (MDRT301). Primers used in these arrays are listed in Supplemental Table S1. For human FAM19A1-5, all expression data were normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH). For mouse Fam19a1-5, all expression data were normalized to Gapdh. Primary neonatal rat cortical neurons and astrocytes were isolated as previously described (18). Expression of rat Fam19a1-5 was normalized to β-actin.
RNA isolation and real-time PCR
Total RNA was isolated from tissue or cells using Trizol Reagent, and 2 µg of RNA were reverse transcribed using GoScript RT (Promega, Madison, WI, USA). Two nanograms per microliter cDNA from each sample was used in real-time PCR analysis using Sybr Green PCR master mix in a CFX Connect system (Bio-Rad, Hercules, CA, USA). Results were analyzed using the 2−∆∆Ct method (19).
cDNA constructs
C-terminal FLAG epitope-tagged mouse Fam19a1 (NP_877960), Fam19a2 (NP_001239316), Fam19a3 (NP_899047), Fam19a4 (NP_796207), Fam19a5 (NP_598857), as well as the N106A mutant of Fam19a5, were synthesized by GenScript (Piscataway, NJ, USA) and cloned into the BamHI and XhoI site of the mammalian expression vector, pcDNA3.1 (+). All constructs were verified by DNA sequencing. The plasmid for Fam19a2 encodes isoform 2 of the protein, and the plasmid for Fam19a5 encodes isoform 2 of the protein.
Cell culture and Western blot analysis
HEK 293 cells were cultured and transfected using Lipofectamine (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. Twenty-four hours post-transfection, the medium was replaced with Opti-MEM (Thermo Fisher Scientific). Both cell lysates and media from transfected cells were then collected 24 h later. Cell lysates were prepared by whole-cell extract buffer [20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM Na2EDTA, 0.5% NP-40, and 10% glycerol] containing SigmaFast protease inhibitor cocktail (MilliporeSigma, Burlington, MA, USA). For Western blot analyses, cell lysates and media were prepared in loading buffer (incubated at 95°C for 5 min), separated in 8–16% precast mini-Protean TGX gel (Bio-Rad), immunoblotted onto a PVDF membrane (Bio-Rad), blocked with 5% nonfat milk for 1 h, and then probed with mouse anti-FLAG M2 antibody (1:1000) overnight at 4°C. After washing, blots were exposed to anti-mouse secondary antibody conjugated to horseradish peroxidase for 1 h then developed in ECL (Amersham ECL select; GE Healthcare, Chicago, IL, USA). Bands were visualized with MultiImage III FluorChem Q (Alpha Innotech, San Leandro, CA, USA).
Mice
Eight-week-old C57BL/6J male mice were obtained from The Jackson Laboratory (Bar Harbor, ME, USA) and housed in polycarbonate cages on a 12-h light-dark photocycle with ad libitum access to water. Mouse tissues from different brain regions were collected from unfed and refed experiments. For the unfed group, standard chow pellets (Teklad 2018; Envigo, Huntingdon, United Kingdom) were removed for 16 h (beginning at 10 h into the light cycle), and mice were euthanized 2 h into the light cycle. For the refed group, mice were unfed for 16 h and refed with standard chow pellets for 3 h before being euthanized. Because of the nature of ad libitum food intake patterns (mice eat when they are hungry, so the exact timing, frequency, and size of meals is not consistent), the ad libitum fed group was not included.
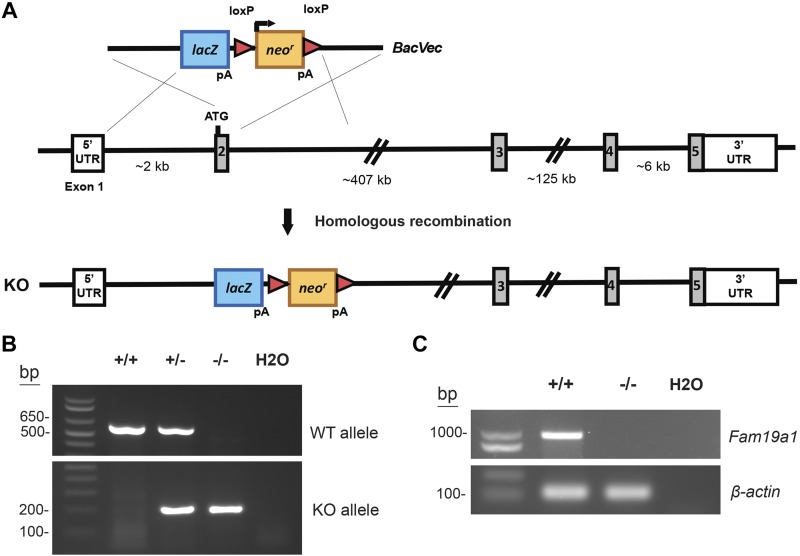
The Fam19a1 knockout (KO) mouse strain (Fam19a1tm1(KOMP)Vlcg) used in this study was created from ES cell clone 16914A-F3, generated by Regeneron Pharmaceuticals (Tarrytown, NY, USA), and obtained from the Knockout Mouse Project (KOMP) Repository (www.komp.org). Live mice were generated from Fam19a1 (+/−) sperm obtained from the KOMP Repository. Mouse Fam19a1 is located on chromosome 6 and consists of 5 exons and 4 introns. To generate Fam19a1-null mice, a total of 112 bp comprising exon 2 of the gene was replaced with a targeting cassette containing a β-galactosidase reporter gene, lacZ, and a neomycin-resistance gene. This strategy ensured that KO mice were devoid of the FAM19A1 protein. Genotyping primers for the Fam19a1 wild-type (WT) allele were: SU, 5′-GACC TCTGACAGAACTTAAG-3′ and SD, 5′-GATGGCGGGTTGCTCCTC-3′. The size of the amplified WT PCR product was 609 bp. Primers for the KO allele were: LacInf, 5′-GGTAAACTGGCTCGGATTAGGG3′ and LacInR, 5′-TTGACTGTAGCGGCTGATGTTG-3′. The size of the amplified KO PCR product was 210 bp. Fam19a1 KO mice were generated on a C57BL/6N genetic background. Semiquantitative PCR was used to confirm the absence of Fam19a1 transcript in KO mice. Forward primer (located in exon 1) and reverse primer (located in exon 5) used were: 5′-CTCGTCAGACAGAGGTGACG-3′ and 5′-CTCCAAATTTCACAGAGAAAACTCA-3′. The size of the amplified PCR product in KO mice was 970 bp. The PCR cycling conditions were denaturation at 95°C for 10 s, annealing at 61°C for 10 s, and extension at 72°C for 45 s. All mice used in this study were generated by crossing Fam19a1 (+/−) mice to obtain KO and WT littermates. Fam19a1 (−/−) mice and WT (+/+) littermates were housed in polycarbonate cages on a 12-h light/dark photocycle with ad libitum access to water and standard laboratory chow. At termination of the study, mice were unfed for 2 h before being euthanized. Tissues were collected, snap-frozen in liquid nitrogen, and kept at −80°C until analysis. All mouse protocols were submitted to and approved by the Institutional Animal Care and Use Committee of the Johns Hopkins University School of Medicine (protocol: MO16M431).
Body composition analysis
Body composition of WT and Fam19a1 KO mice (30 wk old) was determined using a quantitative magnetic resonance instrument (Echo-MRI-100; Echo Medical Systems, Houston, TX, USA) at The Johns Hopkins University School of Medicine mouse phenotyping core facility. Echo-MRI analyses measure total fat mass, lean mass, and water content.
Indirect calorimetry
WT and Fam19a1 KO mice (32 wk old) were used for simultaneous assessments of daily body weight change, food intake (corrected for spillage), physical activity, and whole-body metabolic profile in an open-flow indirect calorimeter [Comprehensive Laboratory Animal Monitoring System (CLAMS); Columbus Instruments, Columbus, OH, USA]. Data were collected for 3 d to confirm that mice were acclimated to the calorimetry chambers (indicated by stable body weights, food intake, and diurnal metabolic patterns), and data were analyzed from the fourth day. Meal pattern data were analyzed for average meal frequency and meal size; a meal was defined as being at least 0.04 g, and having a postmeal intermeal interval of at least 10 min, as previously described (20). A food intake event was considered a meal only when both criteria were met. Rates of Vo2 and Vco2 in each chamber were measured throughout the studies. Respiratory exchange ratio (RER = Vco2/Vo2) was calculated by CLAMS software (v.4.02) to estimate relative oxidation of carbohydrates (RER = 1.0) vs. fats (RER = 0.7), not accounting for protein oxidation. Energy expenditure (EE) was calculated as EE = Vo2 × [3.815 + (1.232 × RER)].
Open field
All mouse behavior tests were performed as previously described (21, 22) and analyzed with the experimenter blinded to genotype. Locomotor activity of male and female mice (12 wk old) was assessed over 30 min in a 40 × 40-cm activity chamber with infrared beams (San Diego Instruments, San Diego, CA, USA). Horizontal activity as well as time spent in the center or periphery of the chamber was automatically recorded.
Y-maze spontaneous alternation
Working memory was assessed in the Y-maze spontaneous alternation task. Male and female mice (13 wk old) were placed at the end of one arm of a Y-maze consisting of three 38-cm-long arms (San Diego Instruments) and allowed to explore the maze for 5 min. Distance traveled and entries into the arms were automatically recorded using ANY-maze Tracking Software (Stoelting, Wood Dale, IL, USA). The percent alternation was calculated using the equation % alternation = [correct alternations/(total arm entries – 2)] × 100.
Trace fear conditioning
Trace fear conditioning was conducted as previously described (21) on male and female mice at 16 wk old. Briefly, trace fear conditioning consisted of a habituation day, a training day, and a test day over 3 consecutive days. On the habituation day, mice were exposed to the shock box (Coulbourn Instruments, Holliston, MA, USA) for 10 min. On the training day, mice were placed in the shock box and given a 2-min habituation, after which a 20-s white noise tone (80 dB, 2000 Hz) was delivered. Twenty seconds following the termination of the tone, a scrambled 2-s 0.5 mA shock was delivered. The tone-shock pairing was repeated 3 additional times. Cued and context tests were performed 24 h after the training session. On the test day, mice were placed in the shock box for 3 min to measure freezing in response to the context. Mice were then placed in a separate context, and freezing in response to the 20-s white noise tone was measured. Freezing behavior was automatically scored using Cleversys Freezescan (Clever Sys, Reston, VA, USA).
Prepulse inhibition
Prepulse inhibition (PPI) was assessed as previously described (23) on male and female mice at 18 wk old. The experimental session consisted of a 5-min acclimatization period to a 70-dB background noise (continuous throughout the session), followed by the presentation of ten 40-ms, 120-dB white noise stimuli at a 20-s interstimulus interval (the habituation session). Upon the completion of the habituation session, each mouse was left in the enclosure for 5 min without any startle stimuli. Immediately after, the PPI session was begun. During each PPI session, mice were exposed to the following types of trials: pulse-alone (a 120-dB, 100-ms broadband burst); the omission of stimuli (no-stimulus trial); and 4 prepulse–pulse combinations (prepulse–pulse trials) consisting of a 20-ms broadband burst used as a prepulse and presented 80 ms before the pulse using one of the 4 prepulse intensities: 74, 78, 82, 86, and 90 dB. Each session consisted of 6 presentations of each trial type presented in a pseudorandom order. PPI was assessed as the percentage scores of PPI (%PPI): 100 × (mean startle amplitude on pulse-alone trials-mean startle amplitude on prepulse–pulse trials/mean startle amplitude on pulse-alone trials) for each mouse separately.
Hotplate assay
Male and female mice (21 wk old) were placed on an 11 × 11-cm black anodized aluminum plate heated to 52°C (IITC Life Science, Woodland Hills, CA, USA). The latency for each mouse to withdraw one of the hind limbs from the plate was recorded, and the mouse was returned to the home cage. If a mouse did not withdraw the hind limb after 30 s, it was removed from the hotplate and placed back in the home cage.
Behavioral itch assay
Female mice (16 wk old) were used for experimentation. All itch behavior was performed between 8 am and 12 pm. On the day before the experiment, mice were placed in the test chamber for 30 min before undergoing a series of 3 mock injections with 5-min breaks in between. On the day of the experiment, animals were allowed to acclimatize to the test chamber for 10 min before injection. Subcutaneous injection of histamine into the nape region of mice causes a robust scratching response due to itch (24). Pruritic compound (i.e., histamine; 1 mM dissolved in physiologic saline) was subcutaneously injected into the nape (50 μl volume), and scratching behavior was observed for 30 min. A bout of scratching was defined as a continuous scratching, not wiping, movement by either hind paw directed at the area of the injection site. Scratching behavior was quantified by counting the number of scratching bouts over the 30-min observation period.
Neurotransmitters in the striatum
Neurotransmitter concentrations were measured by HPLC with electrochemical detection as previously described (25). Briefly, mice were euthanized by decapitation, and the striatum was quickly removed. Striatal tissue was weighed and sonicated in 0.2 ml of ice-cold 0.01 mM perchloric acid containing 0.01% EDTA and 60 nM 3,4-dihydroxybenzylamine as an internal standard. After centrifugation at 15,000 g, 4°C for 30 min, the supernatant was passed through a 0.2-μm filter. Twenty microliters of the supernatant was analyzed in an HPLC column (Atlantis T3, 3 mm × 150 mm C-18 reverse-phase column; Waters, Milford, MA, USA) with detection by a dual-channel Coulchem III electrochemical detector (Model 5300; Thermo Fisher Scientific). Protein concentrations in the tissue homogenates were measured using the BCA Protein Assay Kit (Thermo Fisher Scientific). Data were normalized to protein concentrations (ng neurotransmitters/mg protein).
Statistical analysis
Comparisons between 2 groups of data were performed using 2-tailed Student’s t tests with 95% confidence intervals, and 2-way ANOVA tests were used to make comparisons involving changes over time (total activity, beam breaks, freezing, and PPI). Values were considered statistically significant when P < 0.05. All data are presented as means ± sem.
RESULTS
Brain-enriched expression of FAM19A family members
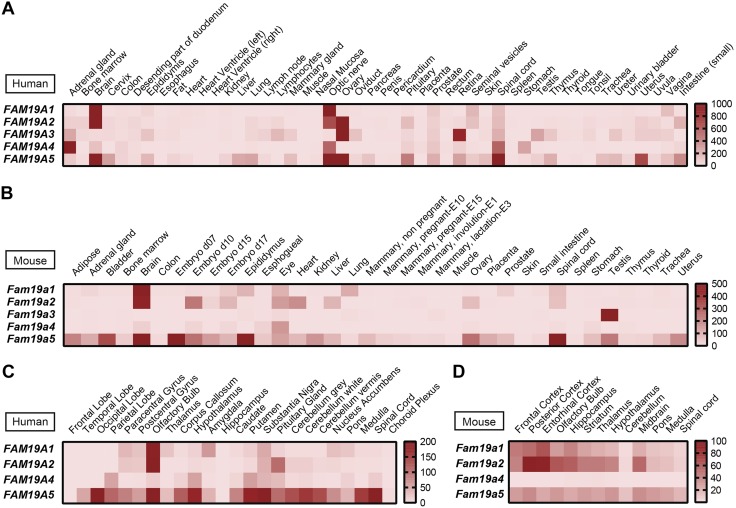
With the exception of FAM19A3, human FAM19A family members were highly and predominantly expressed in the brain, optic nerve, and spinal cord as measured by real-time qPCR analysis (Fig. 1A). The expression patterns in mouse tissues were similar in humans, with brain expressing the most Fam19a1, Fam19a2, and Fam19a5 (Fig. 1B). In different brain regions, human FAM19A1 and FAM19A2 showed similar expression patterns, whereas FAM19A5 showed a broader expression pattern throughout the CNS (Fig. 1C). With the exception of Fam19a4, Fam19a family members were widely expressed in the mouse CNS (Fig. 1D). Based on the Human Brain Transcriptome database (http://hbatlas.org/) (26), FAM19A1 and FAM19A2 showed similar dynamic expression patterns in different brain regions from early postnatal periods to adulthood (Supplemental Fig. S1). In contrast, the expression of human FAM19A3-A5 in different brain regions remained relatively constant from the postnatal period to adulthood (26). Within the brain (e.g., cortex), Fam19a1-a4 transcripts were mainly expressed by neurons and not astrocytes (Supplemental Fig. S2). In contrast, Fam19a5 transcript was expressed by both neurons and astrocytes. Our data confirm and extend the brain-enriched expression patterns of the FAM19A family in human and mouse (7, 11, 13, 15, 17) and provide greater spatial and temporal resolution of transcript expression.
Figure 1.
Gene expression of FAM19A1-A5 in human and mouse tissues and in different regions of human and mouse brain. High expression of FAM19A1, FAM19A2, FAM19A4, and FAM19A5 transcript in brain, optic nerve, and spinal cord as measured by TissueScan human major tissue qPCR array (A) and mouse normal tissue qPCR array (B); similar expression patterns of FAM19A1 and FAM19A2 in different brain regions as measured by TissueScan human brain qPCR array (C) and mouse developmental tissue qPCR array (D). The heat maps were generated based on the relative mRNA expression levels in different samples obtained from real-time qPCR analyses.
Expression of Fam19a in the brain is regulated by metabolic state
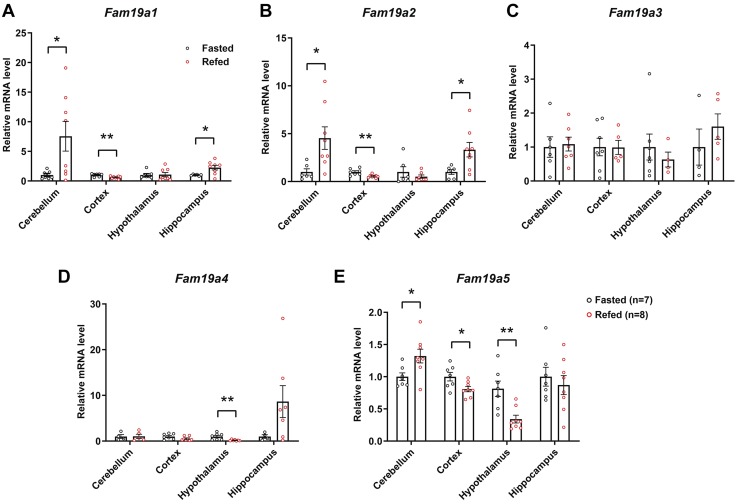
We next addressed whether the expression of Fam19a family members in different brain regions are responsive to unfed and refed states, 2 opposing physiologic and metabolic states, in mice. We observed similar changes in Fam19a1 and Fam19a2 expression in response to unfed and refed states; both were strikingly up-regulated in the cerebellum and hippocampus and down-regulated in the cortex in the refed relative to unfed state (Fig. 2A, B). Expression of Fam19a3 in the brain, however, remained unchanged by the unfed and refed states (Fig. 2C). In contrast, hypothalamic expression of Fam19a4 was significantly suppressed by refeeding (Fig. 2D). Expression of Fam19a5 was modestly increased in the cerebellum and decreased in the cortex and hypothalamus in the refed relative to the unfed state (Fig. 2E). These data indicate that Fam19a expression in different brain regions can be dynamically regulated by changes in nutritional state.
Figure 2.
Altered expression of Fam19a1-a5 in different mouse brain regions in response to unfed and refed states. Real-time PCR analysis of Fam19a1 (A), Fam19a2 (B), Fam19a3 (C), Fam19a4 (D), and Fam19a5 (E) expression in the cerebellum, cortex, hypothalamus, and hippocampus of mice subjected to being unfed overnight (unfed group; n = 7) or unfed overnight followed by 3 h of refeeding (refed group; n = 8). Expression levels were normalized to β-actin. Data are expressed as means ± sem. Two-tailed Student’s t tests were used for comparing gene expression of unfed vs. refed states in different brain regions. *P < 0.05, **P < 0.01.
Secretion and glycosylation of FAM19A family members
All human FAM19A family members contain a signal peptide at the N terminal and have been shown to be secreted when expressed in heterologous COS7L cells (7). Human and mouse FAM19A1-A5 are conserved and share a high degree of amino acid identity of 99, 97, 87, 93, and 99% between the 2 species. All mouse FAM19A proteins were robustly secreted when expressed in heterologous HEK 293 cells (Supplemental Fig. S3A), consistent with previous reports of human FAM19A proteins (7, 13, 15, 16). The mature secreted FAM19A1-A4 were ∼15 kDa, whereas the mature secreted FAM19A5 was ∼20 kDa. The doublet bands of FAM19A5 suggest that the protein is glycosylated. Mouse FAM19A5 contains 1 potential N-linked glycosylation site (Asn-106) that conforms to the N-X-S/T motif. This site was indeed glycosylated; mutant protein in which Asn-106 was replaced with Ala migrated with an apparent molecular mass smaller than the WT protein (Supplemental Fig. S3B).
FAM19A1 deficiency alters metabolic parameters and physical activity
Of the FAM19A family members, Fam19a1 shows one of the most dynamic expression patterns in response to unfed and refed states, and its role in vivo is presently unclear. For this reason, we chose to use a genetic loss-of-function mouse model to determine the biologic function of FAM19A1 in vivo (Fig. 3A). Two sets of primers were designed to amplify a sequence, including exon 2 of the WT allele and a sequence spanning the downstream deletion site in the lacZ gene to confirm the genotype of WT and KO mice, respectively (Fig. 3B). PCR analysis (using primers located in exons 1 and 5) further confirmed the absence of Fam19a1 mRNA in the cortex of KO animals (Fig. 3C). To ensure that loss of FAM19A1 did not affect the expression of other related family members, we quantified the mRNA expression of Fam19a2-a5. The expression of Fam19a2-a5 in the cortex or hippocampus was not significantly different between WT and Fam19a1 KO mice (Supplemental Fig. S4). Fam19a1 KO mice were born at the expected Mendelian ratio and appeared normal with no gross developmental abnormalities.
Figure 3.
Generation of Fam19a1 KO mice. A) Schematic showing the gene targeting strategy used to generate Fam19a1 KO mice. B) PCR genotyping results showing the successful generation of WT (+/+), heterozygous (+/−), and homozygous KO (−/−) mice. C) The absence of Fam19a1 mRNA in cortex of KO mice was confirmed by PCR using primer pairs (located in exons 1 and 5) specific for Fam19a1.
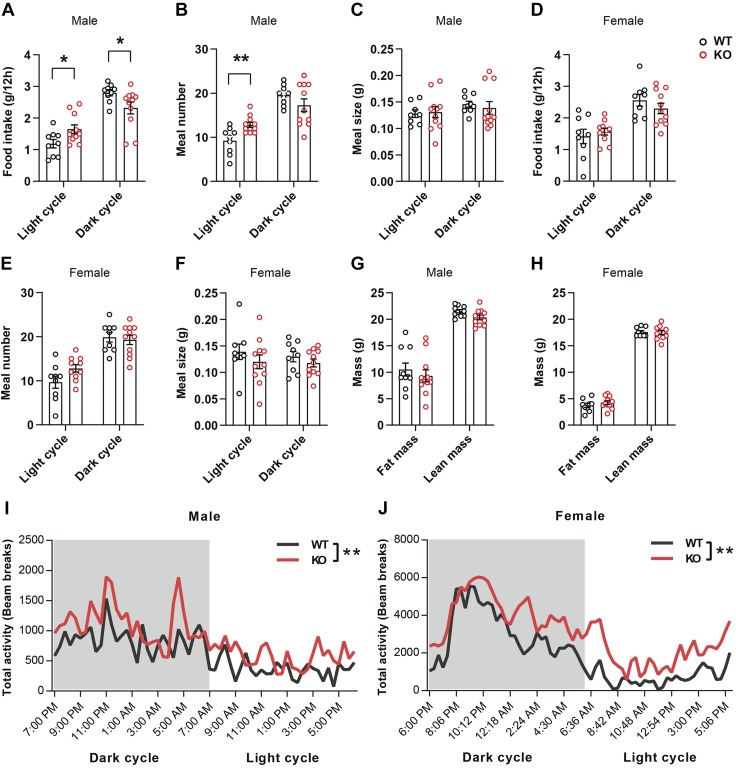
Because Fam19a1 expression in the brain is responsive to unfed and refed states (Fig. 2), we first tested whether the KO mice had any overt metabolic alterations. The metabolic parameters of both male and female mice fed a standard chow were examined using the indirect calorimetry method (Table 1). In male mice, although total food intake over a 24-h period was not different between genotypes, the pattern of food intake was altered: Fam19a1 KO male mice had significantly increased food intake in the light cycle and reduced food intake in the dark cycle (Fig. 4A and Table 1). Increased food intake was due to increased meal number and not meal size (Fig. 4B, C). In female mice, however, neither food intake nor meal number and size were different between genotypes (Fig. 4D–F). Body composition analysis revealed no difference in whole-body fat and lean mass in WT and KO male mice (Fig. 4G) nor any differences in metabolic rates (Vo2), substrate oxidation (RERs), or EE (Table 1). Total physical and ambulatory activity of KO male mice was markedly elevated in the light cycle relative to WT littermates, and ambulatory activity was also significantly higher in the dark cycle (Fig. 4I and Table 1). Surprisingly, although there were no differences in food intake and body composition in KO female mice (Fig. 4H), total physical and ambulatory activity was strikingly elevated over a 24-h period (Fig. 4J and Table 1). The effect was much more pronounced in the light cycle and trended higher in the dark cycle. Increased physical and locomotor activity likely accounted for the significant increase in metabolic rate (Vo2) and EE in KO female mice (Table 1). Thus, loss of FAM19A1 differentially affects food intake, metabolic rate, physical activity, and EE in male and female mice.
TABLE 1.
Indirect calorimetry analysis of WT and Fam19a1 KO mice
| Light cycle |
Dark cycle |
|||
|---|---|---|---|---|
| Variable | WT | KO | WT | KO |
| Male | n = 10 | n = 11 | n = 10 | n = 11 |
| Food intake (g/12 h) | 1.19 ± 0.13 | 1.65 ± 0.14* | 2.80 ± 0.10 | 2.32 ± 0.20* |
| Vo2 (ml/kg/h) | 2256.10 ± 58.78 | 2379.69 ± 60.35 | 2597.21 ± 94.70 | 2633.07 ± 124.16 |
| Vco2 (ml/kg/h) | 2024.79 ± 63.44 | 2169.77 ± 65.76 | 2436.67 ± 96.59 | 2462.61 ± 136.57 |
| RER | 0.90 ± 0.01 | 0.90 ± 0.01 | 0.94 ± 0.01 | 0.93 ± 0.01 |
| EE (kcal/kg/h) | 11.10 ± 0.30 | 11.75 ± 0.31 | 12.91 ± 0.48 | 13.08 ± 0.64 |
| Ambulatory activity (beam breaks) | 5345 ± 876 | 10,279 ± 2103* | 12,954 ± 1539 | 19,074 ± 2259* |
| Total activity (beam breaks) | 11,961 ± 1117 | 20,988 ± 3358* | 25,605 ± 2153 | 33,805 ± 3612 |
| Female | n = 9 | n = 11 | n = 9 | n = 11 |
| Food intake (g/12 h) | 1.58 ± 0.18 | 1.57 ± 0.11 | 2.56 ± 0.18 | 2.30 ± 0.17 |
| Vo2 (ml/kg/h) | 2959.16 ± 74.04 | 3265.49 ± 94.63* | 3701.04 ± 146.19 | 3941.75 ± 98.30 |
| Vco2 (ml/kg/h) | 2661.96 ± 91.49 | 2948.67 ± 102.09 | 3426.74 ± 109.70 | 3623.73 ± 99.88 |
| RER | 0.90 ± 0.02 | 0.90 ± 0.02 | 0.93 ± 0.02 | 0.92 ± 0.02 |
| EE (kcal/kg/h) | 14.57 ± 0.38 | 16.09 ± 0.47* | 18.34 ± 0.68 | 19.50 ± 0.47 |
| Ambulatory activity (beam breaks) | 16,337 ± 3298 | 51,912 ± 6902*** | 82,149 ± 20,174 | 108,007 ± 9472 |
| Total activity (beam breaks) | 25,237 ± 3019 | 70,765 ± 7915*** | 106,736 ± 23,230 | 142,613 ± 10,515 |
Data are expressed as means ± sem. Two-tailed Student’s t tests were used for comparing data of WT mice vs. KO mice. *P < 0.05, ***P < 0.001.
Figure 4.
Altered food intake patterns and increased physical activity in Fam19a1 KO mice. A–F) Food intake, meal number, and meal size during light and dark cycles in WT and KO male mice (A–C) and female mice (D–F). G, H) Echo-MRI measurements of fat and lean mass in WT and KO mice; 2-tailed Student’s t tests were used for comparing data of WT vs. KO mice. I, J) Physical activity (in the metabolic cage) during light and dark cycle in WT and Fam19a1 KO mice. Two-way ANOVA tests were used for comparing overall activity of WT vs. KO mice. Male mice: WT, n = 9; KO, n = 11. Female mice: WT, n = 9; KO, n = 11. Data are expressed as means ± sem. *P < 0.05, **P < 0.01.
Increased locomotor activity and reduced anxiety in Fam19a1-deficient mice
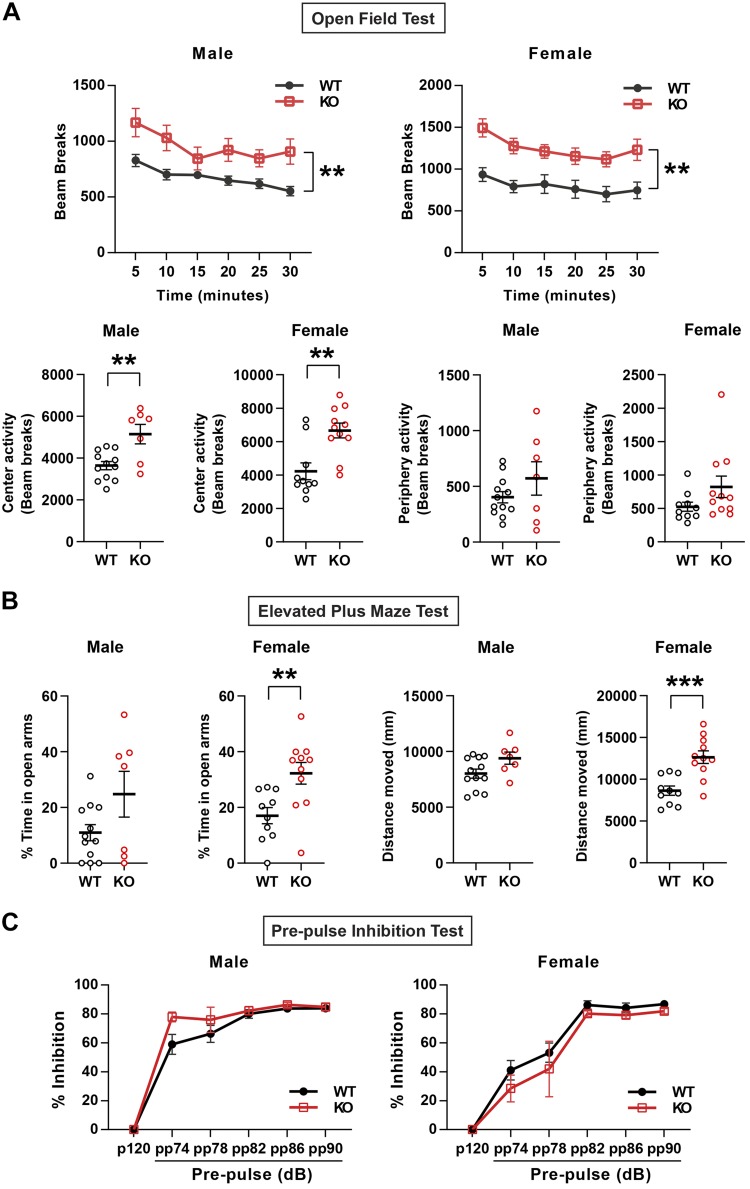
Additional behavioral tests were performed to further examine increased activity of male and female KO mice. In an open field test, both male and female Fam19a1 KO mice showed significantly increased total and central locomotor activity over the 30-min period (Fig. 5A). In elevated plus maze (EPM) tests, the KO female mice spent significantly more time in the open arms and increased distance traveled in the EPM (Fig. 5B). These results suggest that FAM19A1 deficiency leads to enhanced locomotor activity and reduced anxiety in a sex-dependent manner.
Figure 5.
Reduced anxiety in Fam19a1 KO mice. A) Total activity (2-way ANOVA tests) as well as activity spent in the center and periphery (2-tailed Student’s t tests) in WT and KO mice as measured in open field tests. B) Percentage of time spent in open arms and total distance moved in WT and KO mice as measured by EPM tests (2-tailed Student’s t tests). C) Percentage of inhibition during PPI tests with different prestimulus in male and female WT and Fam19a1 KO mice (2-way ANOVA tests). Male mice: WT, n = 12; KO, n = 7. Female mice: WT, n = 10; KO, n = 11. Data are expressed as means ± sem. **P < 0.01, ***P < 0.001.
Normal sensorimotor gating in Fam19a1 KO mice
Because Fam19a1 is predominantly expressed in the CNS, we determined whether loss of FAM19A1 would affect sensorimotor gating as measured by PPI of the acoustic startle, deficits in which has been reported in many animal models for and patients with various neuropsychiatric disorders [e.g., obsessive-compulsive disorder, bipolar disorder, schizophrenia, and Huntington’s Disease (27–29)]. We observed no differences between genotypes of either sex (Fig. 5C).
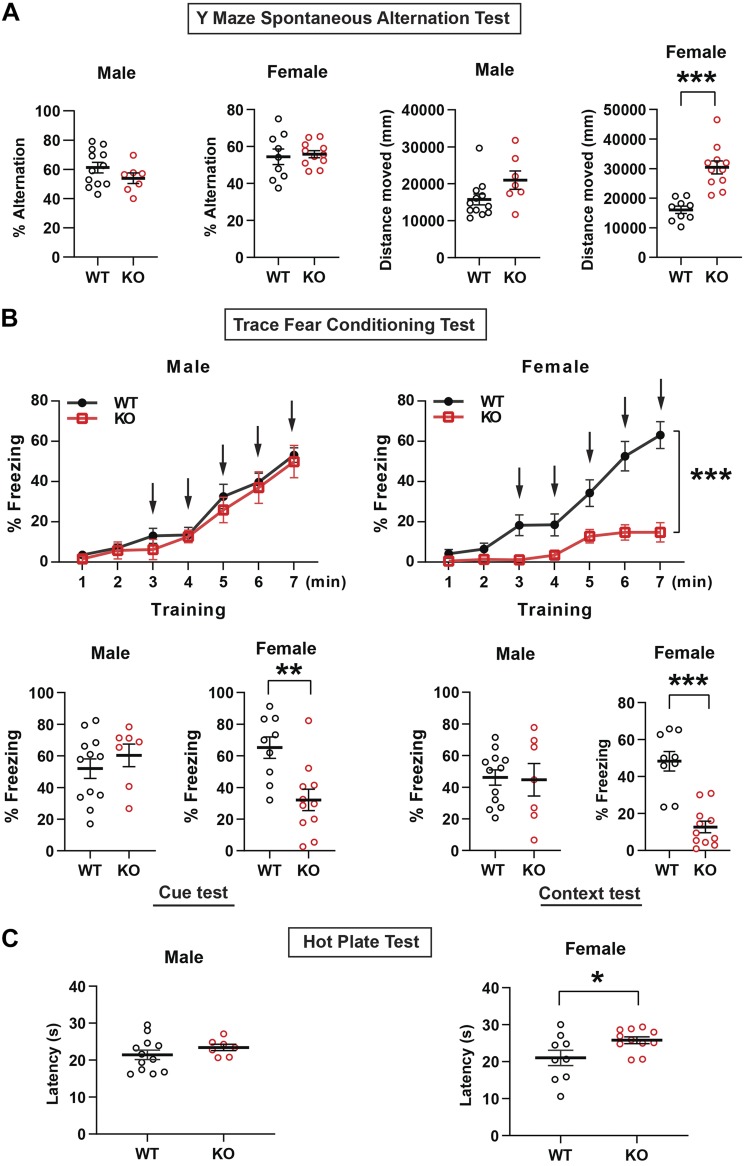
Spatial learning and exploration are preserved in Fam19a1-null mice
Because mouse Fam19a1 transcript is highly expressed in the brain (Fig. 1D), we sought to evaluate learning and memory in KO mice. No difference was observed between WT or KO mice of either sex in the Y-maze spontaneous alternation test for short-term spatial working memory (Fig. 6A), with female KO mice traveling a significantly longer distance (Fig. 6A). These results suggest that FAM19A1 deficiency did not affect the acquisition of short-term spatial memory and exploratory behavior in new environments.
Figure 6.
Spatial learning, trace fear conditioning, and hot plate tests in Fam19a1 KO mice. A) Percentage of alternation and total distance moved in WT and KO mice during the Y-maze spontaneous alternation tests (2-tailed Student’s t tests). B) Percentage of freezing in training (2-way ANOVA tests), cue test, and context test (2-tailed Student’s t tests) in WT and KO mice during trace fear conditioning tests. C) Time of latency in male and female WT and Fam19a1 KO mice during hot plate test (2-tailed Student’s t tests). Male mice: WT, n = 12; KO, n = 7. Female mice: WT, n = 9; KO, n = 11. Data are expressed as means ± sem. *P < 0.05, **P < 0.01, ***P < 0.001.
To detect possible, more subtle alterations in learning and memory, we performed trace fear conditioning tests. In male mice, there was no difference between genotypes in the percentage of freezing during training, nor were there differences in the cue- or context-dependent freezing (Fig. 6B), indicating that hippocampal-dependent learning is preserved in the absence of FAM19A1. Female mice, however, had dramatically reduced frequency of freezing during training, precluding an unambiguous interpretation of reduced fear conditioning in female KO mice (Fig. 6B).
Reduced pain threshold sensitivity
Decreased freezing response to shock in female mice was already apparent during the first application of foot shock, before any fear conditioning took place, suggesting that Fam19a1 KO female mice might have reduced threshold pain sensitivity. Single-cell RNA sequencing of the mouse lumbar dorsal root ganglions has shown that Fam19a1 is expressed in different types of sensory neurons involved in itch and pain (30). To further examine whether FAM19A1-deficient mice have altered sensitivity to pain, we subjected WT and Fam19a1 KO mice to the hot plate test. Although the time of latency between male WT and KO mice was not different, female KO mice had significantly longer times of latency (Fig. 6C), suggesting reduced pain in response to heat. The scratching response in the behavioral itch assay was not different between WT and Fam19a1 KO female mice injected with histamine (Supplemental Fig. S5).
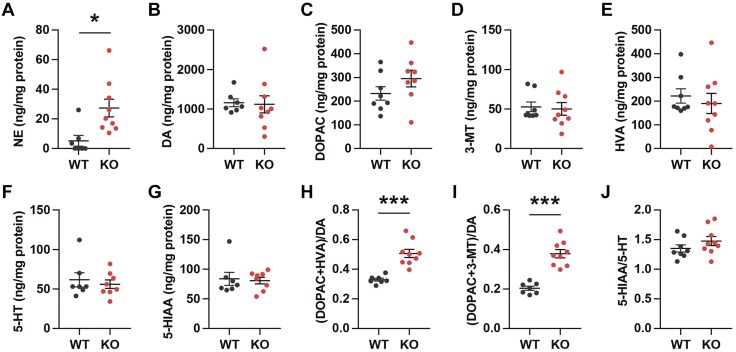
Increased dopamine turnover in the striatum of Fam19a1 KO female mice
Next, we addressed whether neurochemical changes underlie the behavioral changes observed in Fam19a1 KO mice. Alterations in dopamine and other monoamines in the striatum are associated with locomotor hyperactivity (31, 32). We measured monoamines and their metabolites in the striatum of WT and KO mice (Fig. 7). Norepinephrine (NE), but not dopamine, levels in the striatum were significantly elevated in Fam19a1 KO female mice (Fig. 7A, B). The levels of dopamine metabolites 3,4-dihydroxyphenylacetic acid, 3-methoxytyramine, and homovanillic acid were not significantly different between WT and KO mice (Fig. 7C–E). Both serotonin and its metabolite 5-hydroxyindoleacetic acid (5-HIAA) were also not significantly altered in the KO animals compared with WT controls (Fig. 7F, G). Dopamine turnover (based on the ratio of dopamine metabolites to dopamine), however, was significantly increased in Fam19a1 KO mice relative to WT controls (Fig. 7H, I). The turnover of serotonin (based on the ratio of serotonin metabolite to serotonin) was not different between genotypes (Fig. 7J). These results suggest that increased NE level and dopamine turnover may, at least in part, account for the observed locomotor hyperactivity seen in the KO animals.
Figure 7.
Increased dopamine turnover in the striatum in female Fam19a1 KO mice. A–G) Monoamines and their metabolites [NE (A), dopamine (B, DA), 3,4-dihydroxyphenylacetic acid (C, DOPAC), 3-methoxytyramine (D, 3-MT), homovanillic acid (E, HVA), 5-hydroxytryptamine (F, 5-HT; serotonin), and 5-HIAA (G)] concentrations in the striatum were measured by HPLC with electrochemical detection. H, I) Dopamine turnover rate (the ratio of the dopamine metabolites to dopamine. J) Serotonin turnover rate (the ratio of serotonin metabolite 5-HIAA to 5-HT). WT, n = 8; KO, n = 9. Data are expressed as means ± sem. Two-tailed Student’s t tests were used for comparing data of WT vs. KO mice. *P < 0.05, ***P < 0.001.
DISCUSSION
In our efforts to discover novel secreted proteins in the CNS with metabolic function, we identified Fam19a family transcripts whose expression in the brain is responsive to metabolic cues induced by unfed and refed states. Of the 5 family members, Fam19a1 and Fam19a2 show the most dynamic and striking changes in transcript expression. Interestingly, we observed a 5–8-fold induction of Fam19a1 and Fam19a2 in the mouse cerebellum in the refed/postprandial relative to the unfed state. Motor coordination is one of the best described functions of the cerebellum (33). Unlike the hypothalamus (34), the role of cerebellum in food intake regulation is much less understood (35–37). Leptin receptor and the insulin-responsive glucose transporter type 4 are expressed in the cerebellum, and their expression can be modulated by diet (38–40). The cerebellum can signal to the hypothalamus to control food intake (41–44), and human studies suggest a role for the cerebellum in food intake regulation (45, 46).
To elucidate the function of Fam19a1, which was primarily expressed in the brain of both humans and mice and was highly responsive to unfed and refed states in mice, we employed a genetic loss-of-function mouse model. Intriguingly, we observed altered food intake patterns in male, but not female, KO mice. In the light cycle when mice are generally resting, we observed elevated food intake in male Fam19a1 KO mice due to increased meal frequency. In contrast, in the dark cycle when mice are typically awake and active, we observed reduced food intake in the Fam19a1 KO male mice relative to WT controls. Intriguingly, the food intake patterns we observed in KO male mice are reminiscent of the evening hyperphagia syndrome found in humans in which consumption of ≥25% of total daily calories occurs after evening meals (47), analogous to mice (a nocturnal species) eating more during the light cycle. Functional MRI in humans with evening hyperphagia syndrome demonstrated changes in multiple brain regions, including the cerebellum (48). Thus, our data suggest that cerebellum-derived FAM19A1 may play a role in food intake regulation in a sex-dependent manner. Although the expression of Fam19a1 did not change in the hypothalamus in response to unfed/refed states, we could not rule out specific nuclei and cell types within the hypothalamus that can potentially modulate the food intake patterns we observed in KO male mice (49). Interestingly, single-cell sequencing of Agouti-related protein– and Proopiomelanocortin-expressing neurons revealed higher expression of Fam19a1 in Agouti-related protein neurons within the mouse hypothalamus in the ad libitum fed state (50). Whether FAM19A1 derived from these orexigenic or anorexigenic neurons affects food intake remains to be determined. An alternative explanation for increased food intake seen in Fam19a1 KO male mice could be due to increased physical activity in the light cycle. The fact that female Fam19a1 KO mice have an even higher physical activity level in the light cycle without any significant changes in food intake patterns suggests that the altered food intake patterns seen in male mice are not simply secondary to enhanced physical activity. Instead, our data are consistent with the notion that FAM19A1 may participate in altering the drive to initiate and reinitiate energy intake rather than affecting the progression to satiation during meals. Given the complexity of appetite control (51), future studies will help pinpoint which food intake circuit in the brain is regulated by FAM19A1.
In addition to altering food intake patterns, FAM19A1 deficiency also results in multiple changes in mouse behavior. Fam19a1 KO mice of either sex are hyperactive irrespective of the light/dark circadian cycle. The degree of locomotor hyperactivity is more pronounced in female compared with male KO animals. Elevated NE in the brain can cause locomotor hyperactivity (52). It is also known that dopamine signaling in the striatum profoundly affects locomotor activity (31, 32). Accordingly, dopamine-receptor KO mice with persistently high levels of extracellular dopamine are hyperactive (53), and, conversely, dopamine-deficient mice are severely hypoactive (54). Indeed, when we examined monoamine neurotransmitters and their metabolite levels in the striatum, we observed a significant increase in NE level as well as enhanced dopamine turnover in the Fam19a1 KO mice relative to WT littermates; these changes could account for the enhanced locomotor activity seen in the Fam19a1 KO mice. Whether FAM19A1 regulates NE and dopamine synthesis and turnover in the striatum directly or indirectly remains to be determined. Genetic mouse models with locomotor hyperactivity due to increased dopamine turnover in the brain often also have learning and memory deficits (31, 32). Despite locomotor hyperactivity, Fam19a1 KO mice have normal short-term spatial recognition and learning as judged by Y-maze spontaneous alternation tests.
Fam19a1-deficient female, but not male, mice exhibited less anxiety-like behavior assessed in the open field and EPM tests. The observed differences between WT and Fam19a1 KO mice in these behavioral tests cannot simply be due to increased locomotor activity. In the open field tests, both male and female KO mice have significantly higher locomotor activity. However, in the EPM tests, only the female KO mice spent significantly more time in the open arms compared with WT littermates, a behavior suggestive of less anxiety. Short-term spatial recognition and exploration, as measured in the Y-maze spontaneous alternation test, was preserved in Fam19a1 KO animals. No group differences were found in the trace fear conditioning test (55, 56), suggesting that FAM19A1 deficiency did not impair hippocampal-dependent associative learning and memory. In striking contrast to male mice, Fam19a1 KO female mice showed decreased freezing in response to the first (before any learning) and subsequent electric shocks during the training period. Locomotor hyperactivity in the female mice could be a confounding factor in interpreting the fear memory test; however, because the male mice have normal fear memory and freezing behavior, this suggests the alternative possibility that the KO female mice have reduced sensitivity to pain induced by electric shock. Consistent with reduced sensitivity to electrical foot shock, Fam19a1 KO female mice also show decreased pain sensitivity assessed in the hot plate test. Single-cell RNA sequencing of the mouse dorsal root ganglions reveals 11 types of sensory neurons, and Fam19a1 is considered a unique marker for 2 major types of sensory neurons potentially involved in pain and itch (30). Although histamine-induced itch scratching was not different between WT and Fam19a1 KO mice, our hot plate and electrical shock data suggest that FAM19A1 is involved in pain sensitivity.
In humans, FAM19A1 is dynamically expressed in different areas within the neocortex during development from embryo to adulthood (26). Deep single-cell RNA sequencing of 2 areas (primary visual cortex and anterior lateral motor cortex) of the mouse neocortex also revealed specific expression of Fam19a1 in the lysosome-associated membrane protein 5 (LAMP5)-positive neurons (57). LAMP5 (and its ortholog unc-46 in Caenorhabditis elegans) defines a subclass of GABAergic neurons (58–60). Interestingly, Lamp5 KO mice showed decreased anxiety in the EPM tests (59), a phenotype we also observed in Fam19a1 KO female mice. In FAM19A1-deficient mice, we did not observe any alteration in sensorimotor gating as assessed in the PPI of acoustic startle response test. Humans suffering from neuropsychiatric disorders commonly have deficits in sensorimotor gating (61–63). Likewise, animal models of neuropsychiatric disorders (related to obsessive-compulsive disorder, bipolar disorder, and schizophrenia) also have significantly altered sensorimotor gating as revealed by the acoustic startle response (27–29). In PPI tests, we found no difference between WT and KO mice of either sex, suggesting that FAM19A1 deficiency did not affect sensorimotor gating.
In summary, we identified a group of brain-enriched, chemokine-like secretory proteins whose expression is responsive to metabolic cues. Employing a KO mouse model, we demonstrated novel and sexually dimorphic roles for FAM19A1 in modulating food intake patterns, locomotor activity, anxiety-like behaviors, and sensitivity to pain induced by heat or electric shock. These results underscore the importance of sex as a significant biologic variable influencing phenotypic outcomes (64–66). Our studies demonstrate an in vivo physiologic function of FAM19A1 (TAFA1) and provide the foundation for future mechanistic studies aimed at further understanding FAM19A1-regulated brain circuits and mouse behavior. In a broader context, our current studies and previous findings highlight the importance of this family of neurokines in regulating ingestive and somatosensory physiology as well as animal behavior.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Valina Dawson and Ted Dawson (Johns Hopkins University School of Medicine) for providing resources to measure brain monoamine levels. This work was supported by a grant from the U.S. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (DK084171 to G.W.W.). The authors declare no conflicts of interest.
Glossary
- 5-HIAA
5-hydroxyindoleacetic acid
- DA
dopamine
- EE
energy expenditure
- EPM
elevated plus maze
- FAM19A1-5
family with sequence similarity 19, member A1-5
- KO
knockout
- LAMP5
lysosome-associated membrane protein 5
- NE
norepinephrine
- PPI
prepulse inhibition
- qPCR
quantitative PCR
- RER
respiratory exchange ratio
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
X. Lei and G. W. Wong contributed to the experimental design; X. Lei, L. Liu, C. E. Terrillion, S. S. Karuppagounder, P. Cisternas, M. Lay, and S. Aja performed the experiments; D. C. Martinelli, X. Dong, and M. V. Pletnikov provided technical and intellectual input; and X. Lei and G. W. Wong analyzed and interpreted the data and wrote the manuscript.
REFERENCES
- 1.Turner M. D., Nedjai B., Hurst T., Pennington D. J. (2014) Cytokines and chemokines: at the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta 1843, 2563–2582 [DOI] [PubMed] [Google Scholar]
- 2.Schwartz D. M., Bonelli M., Gadina M., O’Shea J. J. (2016) Type I/II cytokines, JAKs, and new strategies for treating autoimmune diseases. Nat. Rev. Rheumatol. 12, 25–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zorrilla E. P., Sanchez-Alavez M., Sugama S., Brennan M., Fernandez R., Bartfai T., Conti B. (2007) Interleukin-18 controls energy homeostasis by suppressing appetite and feed efficiency. Proc. Natl. Acad. Sci. USA 104, 11097–11102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reed J. A., Clegg D. J., Smith K. B., Tolod-Richer E. G., Matter E. K., Picard L. S., Seeley R. J. (2005) GM-CSF action in the CNS decreases food intake and body weight. J. Clin. Invest. 115, 3035–3044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petersen P. S., Lei X., Seldin M. M., Rodriguez S., Byerly M. S., Wolfe A., Whitlock S., Wong G. W. (2014) Dynamic and extensive metabolic state-dependent regulation of cytokine expression and circulating levels. Am. J. Physiol. Regul. Integr. Comp. Physiol. 307, R1458–R1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Little H. C., Tan S. Y., Cali F. M., Rodriguez S., Lei X., Wolfe A., Hug C., Wong G. W. (2018) Multiplex quantification identifies novel exercise-regulated myokines/cytokines in plasma and in glycolytic and oxidative skeletal muscle. Mol. Cell. Proteomics 17, 1546–1563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tom Tang Y., Emtage P., Funk W. D., Hu T., Arterburn M., Park E. E., Rupp F. (2004) TAFA: a novel secreted family with conserved cysteine residues and restricted expression in the brain. Genomics 83, 727–734 [DOI] [PubMed] [Google Scholar]
- 8.Walford G. A., Gustafsson S., Rybin D., Stančáková A., Chen H., Liu C. T., Hong J., Jensen R. A., Rice K., Morris A. P., Mägi R., Tönjes A., Prokopenko I., Kleber M. E., Delgado G., Silbernagel G., Jackson A. U., Appel E. V., Grarup N., Lewis J. P., Montasser M. E., Landenvall C., Staiger H., Luan J., Frayling T. M., Weedon M. N., Xie W., Morcillo S., Martínez-Larrad M. T., Biggs M. L., Chen Y. D., Corbaton-Anchuelo A., Færch K., Gómez-Zumaquero J. M., Goodarzi M. O., Kizer J. R., Koistinen H. A., Leong A., Lind L., Lindgren C., Machicao F., Manning A. K., Martín-Núñez G. M., Rojo-Martínez G., Rotter J. I., Siscovick D. S., Zmuda J. M., Zhang Z., Serrano-Rios M., Smith U., Soriguer F., Hansen T., Jørgensen T. J., Linnenberg A., Pedersen O., Walker M., Langenberg C., Scott R. A., Wareham N. J., Fritsche A., Häring H. U., Stefan N., Groop L., O’Connell J. R., Boehnke M., Bergman R. N., Collins F. S., Mohlke K. L., Tuomilehto J., März W., Kovacs P., Stumvoll M., Psaty B. M., Kuusisto J., Laakso M., Meigs J. B., Dupuis J., Ingelsson E., Florez J. C. (2016) Genome-wide association study of the modified stumvoll insulin sensitivity index identifies BCL2 and FAM19A2 as novel insulin sensitivity loci. Diabetes 65, 3200–3211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X., Shen C., Chen X., Wang J., Cui X., Wang Y., Zhang H., Tang L., Lu S., Fei J., Wang Z. (2018) Tafa-2 plays an essential role in neuronal survival and neurobiological function in mice. Acta Biochim. Biophys. Sin. (Shanghai) 50, 984–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y., Chen D., Zhang Y., Wang P., Zheng C., Zhang S., Yu B., Zhang L., Zhao G., Ma B., Cai Z., Xie N., Huang S., Liu Z., Mo X., Guan Y., Wang X., Fu Y., Ma D., Wang Y., Kong W. (2018) Novel adipokine, FAM19A5, inhibits neointima formation after injury through sphingosine-1-phosphate receptor 2. Circulation 138, 48–63 [DOI] [PubMed] [Google Scholar]
- 11.Delfini M. C., Mantilleri A., Gaillard S., Hao J., Reynders A., Malapert P., Alonso S., François A., Barrere C., Seal R., Landry M., Eschallier A., Alloui A., Bourinet E., Delmas P., Le Feuvre Y., Moqrich A. (2013) TAFA4, a chemokine-like protein, modulates injury-induced mechanical and chemical pain hypersensitivity in mice. Cell Rep. 5, 378–388 [DOI] [PubMed] [Google Scholar]
- 12.Kambrun C., Roca-Lapirot O., Salio C., Landry M., Moqrich A., Le Feuvre Y. (2018) TAFA4 reverses mechanical allodynia through activation of GABAergic transmission and microglial process retraction. Cell Rep. 22, 2886–2897 [DOI] [PubMed] [Google Scholar]
- 13.Zheng C., Chen D., Zhang Y., Bai Y., Huang S., Zheng D., Liang W., She S., Peng X., Wang P., Mo X., Song Q., Lv P., Huang J., Ye R. D., Wang Y. (2018) FAM19A1 is a new ligand for GPR1 that modulates neural stem-cell proliferation and differentiation. [E-pub ahead of print] FASEB J. [DOI] [PubMed] [Google Scholar]
- 14.Jafari A., Isa A., Chen L., Ditzel N., Zaher W., Harkness L., Johnsen H. E., Abdallah B. M., Clausen C., Kassem M. (2019) TAFA2 induces skeletal (stromal) stem cell migration through activation of rac1-p38 signaling. Stem Cells 37, 407–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shao Y., Deng T., Zhang T., Li P., Wang Y. (2015) FAM19A3, a novel secreted protein, modulates the microglia/macrophage polarization dynamics and ameliorates cerebral ischemia. FEBS Lett. 589, 467–475 [DOI] [PubMed] [Google Scholar]
- 16.Wang W., Li T., Wang X., Yuan W., Cheng Y., Zhang H., Xu E., Zhang Y., Shi S., Ma D., Han W. (2015) FAM19A4 is a novel cytokine ligand of formyl peptide receptor 1 (FPR1) and is able to promote the migration and phagocytosis of macrophages. Cell. Mol. Immunol. 12, 615–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park M. Y., Kim H. S., Lee M., Park B., Lee H. Y., Cho E. B., Seong J. Y., Bae Y. S. (2017) FAM19A5, a brain-specific chemokine, inhibits RANKL-induced osteoclast formation through formyl peptide receptor 2. Sci. Rep. 7, 15575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Byerly M. S., Petersen P. S., Ramamurthy S., Seldin M. M., Lei X., Provost E., Wei Z., Ronnett G. V., Wong G. W. (2014) C1q/TNF-related protein 4 (CTRP4) is a unique secreted protein with two tandem C1q domains that functions in the hypothalamus to modulate food intake and body weight. J. Biol. Chem. 289, 4055–4069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 20.Wei Z., Lei X., Petersen P. S., Aja S., Wong G. W. (2014) Targeted deletion of C1q/TNF-related protein 9 increases food intake, decreases insulin sensitivity, and promotes hepatic steatosis in mice. Am. J. Physiol. Endocrinol. Metab. 306, E779–E790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terrillion C. E., Abazyan B., Yang Z., Crawford J., Shevelkin A. V., Jouroukhin Y., Yoo K. H., Cho C. H., Roychaudhuri R., Snyder S. H., Jang M. H., Pletnikov M. V. (2017) DISC1 in astrocytes influences adult neurogenesis and hippocampus-dependent behaviors in mice. Neuropsychopharmacology 42, 2242–2251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abazyan B., Nomura J., Kannan G., Ishizuka K., Tamashiro K. L., Nucifora F., Pogorelov V., Ladenheim B., Yang C., Krasnova I. N., Cadet J. L., Pardo C., Mori S., Kamiya A., Vogel M. W., Sawa A., Ross C. A., Pletnikov M. V. (2010) Prenatal interaction of mutant DISC1 and immune activation produces adult psychopathology. Biol. Psychiatry 68, 1172–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pletnikov M. V., Ayhan Y., Nikolskaia O., Xu Y., Ovanesov M. V., Huang H., Mori S., Moran T. H., Ross C. A. (2008) Inducible expression of mutant human DISC1 in mice is associated with brain and behavioral abnormalities reminiscent of schizophrenia. Mol. Psychiatry 13, 173–186, 115 [DOI] [PubMed] [Google Scholar]
- 24.Dong X., Dong X. (2018) Peripheral and central mechanisms of itch. Neuron 98, 482–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karuppagounder S. S., Xiong Y., Lee Y., Lawless M. C., Kim D., Nordquist E., Martin I., Ge P., Brahmachari S., Jhaldiyal A., Kumar M., Andrabi S. A., Dawson T. M., Dawson V. L. (2016) LRRK2 G2019S transgenic mice display increased susceptibility to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-mediated neurotoxicity. J. Chem. Neuroanat. 76(Pt B), 90–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang H. J., Kawasawa Y. I., Cheng F., Zhu Y., Xu X., Li M., Sousa A. M., Pletikos M., Meyer K. A., Sedmak G., Guennel T., Shin Y., Johnson M. B., Krsnik Z., Mayer S., Fertuzinhos S., Umlauf S., Lisgo S. N., Vortmeyer A., Weinberger D. R., Mane S., Hyde T. M., Huttner A., Reimers M., Kleinman J. E., Sestan N. (2011) Spatio-temporal transcriptome of the human brain. Nature 478, 483–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geyer M. A. (1999) Assessing prepulse inhibition of startle in wild-type and knockout mice. Psychopharmacology (Berl.) 147, 11–13 [DOI] [PubMed] [Google Scholar]
- 28.Ouagazzal A. M., Jenck F., Moreau J. L. (2001) Drug-induced potentiation of prepulse inhibition of acoustic startle reflex in mice: a model for detecting antipsychotic activity? Psychopharmacology (Berl.) 156, 273–283 [DOI] [PubMed] [Google Scholar]
- 29.Swerdlow N. R., Light G. A. (2016) Animal models of deficient sensorimotor gating in schizophrenia: are they still relevant? Curr. Top. Behav. Neurosci. 28, 305–325 [DOI] [PubMed] [Google Scholar]
- 30.Usoskin D., Furlan A., Islam S., Abdo H., Lönnerberg P., Lou D., Hjerling-Leffler J., Haeggström J., Kharchenko O., Kharchenko P. V., Linnarsson S., Ernfors P. (2015) Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat. Neurosci. 18, 145–153 [DOI] [PubMed] [Google Scholar]
- 31.Beninger R. J. (1983) The role of dopamine in locomotor activity and learning. Brain Res. 287, 173–196 [DOI] [PubMed] [Google Scholar]
- 32.Palmiter R. D. (2008) Dopamine signaling in the dorsal striatum is essential for motivated behaviors: lessons from dopamine-deficient mice. Ann. N. Y. Acad. Sci. 1129, 35–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manto M., Bower J. M., Conforto A. B., Delgado-García J. M., da Guarda S. N., Gerwig M., Habas C., Hagura N., Ivry R. B., Mariën P., Molinari M., Naito E., Nowak D. A., Oulad Ben Taib N., Pelisson D., Tesche C. D., Tilikete C., Timmann D. (2012) Consensus paper: roles of the cerebellum in motor control--the diversity of ideas on cerebellar involvement in movement. Cerebellum 11, 457–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morton G. J., Cummings D. E., Baskin D. G., Barsh G. S., Schwartz M. W. (2006) Central nervous system control of food intake and body weight. Nature 443, 289–295 [DOI] [PubMed] [Google Scholar]
- 35.Zhu J. N., Wang J. J. (2008) The cerebellum in feeding control: possible function and mechanism. Cell. Mol. Neurobiol. 28, 469–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahler P., Guastavino J. M., Jacquart G., Strazielle C. (1993) An unexpected role of the cerebellum: involvement in nutritional organization. Physiol. Behav. 54, 1063–1067 [DOI] [PubMed] [Google Scholar]
- 37.Mendoza J., Pévet P., Felder-Schmittbuhl M. P., Bailly Y., Challet E. (2010) The cerebellum harbors a circadian oscillator involved in food anticipation. J. Neurosci. 30, 1894–1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koros C., Boukouvalas G., Gerozissis K., Kitraki E. (2009) Fat diet affects leptin receptor levels in the rat cerebellum. Nutrition 25, 85–87 [DOI] [PubMed] [Google Scholar]
- 39.Guan X. M., Hess J. F., Yu H., Hey P. J., van der Ploeg L. H. (1997) Differential expression of mRNA for leptin receptor isoforms in the rat brain. Mol. Cell. Endocrinol. 133, 1–7 [DOI] [PubMed] [Google Scholar]
- 40.El Messari S., Leloup C., Quignon M., Brisorgueil M. J., Penicaud L., Arluison M. (1998) Immunocytochemical localization of the insulin-responsive glucose transporter 4 (Glut4) in the rat central nervous system. J. Comp. Neurol. 399, 492–512 [DOI] [PubMed] [Google Scholar]
- 41.Li B., Guo C. L., Tang J., Zhu J. N., Wang J. J. (2009) Cerebellar fastigial nuclear inputs and peripheral feeding signals converge on neurons in the dorsomedial hypothalamic nucleus. Neurosignals 17, 132–143 [DOI] [PubMed] [Google Scholar]
- 42.Wen Y. Q., Zhu J. N., Zhang Y. P., Wang J. J. (2004) Cerebellar interpositus nuclear inputs impinge on paraventricular neurons of the hypothalamus in rats. Neurosci. Lett. 370, 25–29 [DOI] [PubMed] [Google Scholar]
- 43.Zhu J. N., Li H. Z., Ding Y., Wang J. J. (2006) Cerebellar modulation of feeding-related neurons in rat dorsomedial hypothalamic nucleus. J. Neurosci. Res. 84, 1597–1609 [DOI] [PubMed] [Google Scholar]
- 44.Zhu J. N., Zhang Y. P., Song Y. N., Wang J. J. (2004) Cerebellar interpositus nuclear and gastric vagal afferent inputs reach and converge onto glycemia-sensitive neurons of the ventromedial hypothalamic nucleus in rats. Neurosci. Res. 48, 405–417 [DOI] [PubMed] [Google Scholar]
- 45.Berman S. M., Paz-Filho G., Wong M. L., Kohno M., Licinio J., London E. D. (2013) Effects of leptin deficiency and replacement on cerebellar response to food-related cues. Cerebellum 12, 59–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tataranni P. A., Gautier J. F., Chen K., Uecker A., Bandy D., Salbe A. D., Pratley R. E., Lawson M., Reiman E. M., Ravussin E. (1999) Neuroanatomical correlates of hunger and satiation in humans using positron emission tomography. Proc. Natl. Acad. Sci. USA 96, 4569–4574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Allison K. C., Lundgren J. D., O’Reardon J. P., Geliebter A., Gluck M. E., Vinai P., Mitchell J. E., Schenck C. H., Howell M. J., Crow S. J., Engel S., Latzer Y., Tzischinsky O., Mahowald M. W., Stunkard A. J. (2010) Proposed diagnostic criteria for night eating syndrome. Int. J. Eat. Disord. 43, 241–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lundgren J. D., Patrician T. M., Breslin F. J., Martin L. E., Donnelly J. E., Savage C. R. (2013) Evening hyperphagia and food motivation: a preliminary study of neural mechanisms. Eat. Behav. 14, 447–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Campbell J. N., Macosko E. Z., Fenselau H., Pers T. H., Lyubetskaya A., Tenen D., Goldman M., Verstegen A. M., Resch J. M., McCarroll S. A., Rosen E. D., Lowell B. B., Tsai L. T. (2017) A molecular census of arcuate hypothalamus and median eminence cell types. Nat. Neurosci. 20, 484–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Henry F. E., Sugino K., Tozer A., Branco T., Sternson S. M. (2015) Cell type-specific transcriptomics of hypothalamic energy-sensing neuron responses to weight-loss. eLife 4, e09800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Andermann M. L., Lowell B. B. (2017) Toward a wiring diagram understanding of appetite control. Neuron 95, 757–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jones M. D., Hess E. J. (2003) Norepinephrine regulates locomotor hyperactivity in the mouse mutant coloboma. Pharmacol. Biochem. Behav. 75, 209–216 [DOI] [PubMed] [Google Scholar]
- 53.Giros B., Jaber M., Jones S. R., Wightman R. M., Caron M. G. (1996) Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature 379, 606–612 [DOI] [PubMed] [Google Scholar]
- 54.Zhou Q. Y., Palmiter R. D. (1995) Dopamine-deficient mice are severely hypoactive, adipsic, and aphagic. Cell 83, 1197–1209 [DOI] [PubMed] [Google Scholar]
- 55.Bangasser D. A., Waxler D. E., Santollo J., Shors T. J. (2006) Trace conditioning and the hippocampus: the importance of contiguity. J. Neurosci. 26, 8702–8706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Song C., Detert J. A., Sehgal M., Moyer J. R., Jr (2012) Trace fear conditioning enhances synaptic and intrinsic plasticity in rat hippocampus. J. Neurophysiol. 107, 3397–3408 [DOI] [PubMed] [Google Scholar]
- 57.Tasic B., Yao Z., Graybuck L. T., Smith K. A., Nguyen T. N., Bertagnolli D., Goldy J., Garren E., Economo M. N., Viswanathan S., Penn O., Bakken T., Menon V., Miller J., Fong O., Hirokawa K. E., Lathia K., Rimorin C., Tieu M., Larsen R., Casper T., Barkan E., Kroll M., Parry S., Shapovalova N. V., Hirschstein D., Pendergraft J., Sullivan H. A., Kim T. K., Szafer A., Dee N., Groblewski P., Wickersham I., Cetin A., Harris J. A., Levi B. P., Sunkin S. M., Madisen L., Daigle T. L., Looger L., Bernard A., Phillips J., Lein E., Hawrylycz M., Svoboda K., Jones A. R., Koch C., Zeng H. (2018) Shared and distinct transcriptomic cell types across neocortical areas. Nature 563, 72–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koebis M., Urata S., Shinoda Y., Okabe S., Yamasoba T., Nakao K., Aiba A., Furuichi T. (2019) LAMP5 in presynaptic inhibitory terminals in the hindbrain and spinal cord: a role in startle response and auditory processing. Mol. Brain 12, 20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tiveron M. C., Beurrier C., Céni C., Andriambao N., Combes A., Koehl M., Maurice N., Gatti E., Abrous D. N., Kerkerian-Le Goff L., Pierre P., Cremer H. (2016) LAMP5 fine-tunes GABAergic synaptic transmission in defined circuits of the mouse brain. PLoS One 11, e0157052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schuske K., Palfreyman M. T., Watanabe S., Jorgensen E. M. (2007) UNC-46 is required for trafficking of the vesicular GABA transporter. Nat. Neurosci. 10, 846–853 [DOI] [PubMed] [Google Scholar]
- 61.Braff D. L., Geyer M. A., Light G. A., Sprock J., Perry W., Cadenhead K. S., Swerdlow N. R. (2001) Impact of prepulse characteristics on the detection of sensorimotor gating deficits in schizophrenia. Schizophr. Res. 49, 171–178 [DOI] [PubMed] [Google Scholar]
- 62.Perry W., Minassian A., Feifel D., Braff D. L. (2001) Sensorimotor gating deficits in bipolar disorder patients with acute psychotic mania. Biol. Psychiatry 50, 418–424 [DOI] [PubMed] [Google Scholar]
- 63.Ahmari S. E., Risbrough V. B., Geyer M. A., Simpson H. B. (2012) Impaired sensorimotor gating in unmedicated adults with obsessive-compulsive disorder. Neuropsychopharmacology 37, 1216–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miller L. R., Marks C., Becker J. B., Hurn P. D., Chen W. J., Woodruff T., McCarthy M. M., Sohrabji F., Schiebinger L., Wetherington C. L., Makris S., Arnold A. P., Einstein G., Miller V. M., Sandberg K., Maier S., Cornelison T. L., Clayton J. A. (2017) Considering sex as a biological variable in preclinical research. FASEB J. 31, 29–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miller V. M. (2014) Why are sex and gender important to basic physiology and translational and individualized medicine? Am. J. Physiol. Heart Circ. Physiol. 306, H781–H788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Varlamov O., Bethea C. L., Roberts C. T., Jr (2015) Sex-specific differences in lipid and glucose metabolism. Front. Endocrinol. (Lausanne) 5, 241 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.