Abstract
Aerobic physical exercise (EX) and controlling cardiovascular risk factors in midlife can improve and protect cognitive function in healthy individuals and are considered to be effective at reducing late-onset dementia incidence. By investigating commonalities between these preventative approaches, we sought to identify possible targets for effective interventions. We compared the efficacy of EX and simvastatin (SV) pharmacotherapy to counteract cognitive deficits induced by a high-cholesterol diet (2%, HCD) in mice overexpressing TGF-β1 (TGF mice), a model of vascular cognitive impairment and dementia. Cognitive deficits were found in hypercholesterolemic mice for object recognition memory, and both SV and EX prevented this decline. EX improved stimulus-evoked cerebral blood flow responses and was as effective as SV in normalizing endothelium-dependent vasodilatory responses in cerebral arteries. The up-regulation of galectin-3–positive microglial cells in white matter (WM) of HCD-fed TGF mice with cognitive deficits was significantly reduced by both SV and EX concurrently with cognitive recovery. Altered hippocampal neurogenesis, gray matter astrogliosis, or microgliosis did not correlate with cognitive deficits or benefits. Overall, results indicate that SV and EX prevented cognitive decline in hypercholesterolemic mice and that they share common sites of action in preventing endothelial cell dysfunction and reducing WM inflammation.—Trigiani, L. J., Royea, J., Tong, X.-K., Hamel, E. Comparative benefits of simvastatin and exercise in a mouse model of vascular cognitive impairment and dementia.
Keywords: white matter, inflammation, memory, cerebrovascular reactivity, cerebral blood flow
Alzheimer’s disease (AD) and vascular cognitive impairment and dementia (VCID) share similarities with regard to their cerebrovascular pathology (1, 2). In both AD and VCID, there is decreased capillary density, blood-brain barrier (BBB) tight junction proteins, and mitochondrial content in endothelial cells and increased amounts of vascular fibrosis and degenerating capillaries (3). Another commonality relates to altered levels of TGF-β1 in brain, plasma, cerebrospinal fluid, or brain vessels in AD and VCID (4) and TGF-β1 polymorphisms being associated with VCID (5) or with an increased risk for VCID and AD (6).
Interestingly, mice that overexpress a constitutively active form of TGF-β1 (TGF mice) in brain via a glial fibrillary acidic protein (GFAP) promotor recapitulate the cerebrovascular pathology seen in VCID (7). Chronic increases in TGF-β1 lead to the accumulation of basement membrane proteins in the brain vessel walls, resulting in smaller capillary endothelial cells and pericytes, degenerating capillaries (string vessel pathology), reduced endothelial-mediated dilatory function, baseline brain hypoperfusion, and impaired hyperemic response to increased neuronal activity (8–10). Cognitive deficits are absent (7, 9) or occasionally (11) apparent in aged TGF mice, whereas we recently found that they could be precipitated by combining a cardiovascular risk factor for dementia (namely, hypercholesterolemia) induced by feeding young TGF mice a high-cholesterol diet (HCD) (12).
Recent reports on dementia attribute declining incidence rates and slower cognitive decline to healthier lifestyle habits and control of cardiovascular risk factors with medication (13–16). A frequently recommended preventative strategy against dementia is moderate, regular physical exercise, a treatment whose benefits have been linked to increased hippocampal volume and neurogenesis (17), reduced inflammation and oxidative stress, increased clearance of toxic proteins, and restored cerebrovascular function and white matter (WM) integrity (18). Similarly, statins, whose primary role is to reduce cholesterol synthesis, when taken in midlife have been proposed, although not unanimously (19), to reduce the risk of developing dementia through various pleiotropic effects independent of their cholesterol-lowering properties (15). The Rotterdam study reported a hazard ratio of 0.57 for developing dementia with statin use and 1.05 for those using other lipid-lowering agents (19), and in a large United Kingdom cohort study, a relative risk of 0.29 was associated with risk of developing dementia when prescribed a statin compared with 0.72 for individuals with untreated hyperlipidemia (15). Interestingly, more recent studies suggest sex and race differences in statins’ ability to reduce AD incidence, with a particularly high efficacy for simvastatin (SV) (20). In adult AD mouse models, SV was effective in rescuing cerebrovascular function as well as learning and memory deficits through cholesterol-independent mechanisms (21, 22).
In the current study, we sought to identify common mechanisms through which aerobic physical exercise (EX) and SV prevent cognitive decline. Overall, our findings demonstrate that both interventions improved endothelial-mediated vasodilation and selectively reduced WM inflammation concurrently with cognitive benefits.
MATERIALS AND METHODS
Animals and treatments
Heterozygous transgenic mice overexpressing a constitutively active form of TGF-β1 under the control of the GFAP promoter on a C57BL/6J background (TGF mice, line T64) (23) were used with their age-matched wild-type (WT) littermate controls, both in approximately equal numbers of males and females (45.5 and 43.8% males in cohort 1 and cohort 2, respectively). Transgene expression was confirmed with touchdown PCR using tail-extracted DNA. A first cohort (hereby referred to as cohort 1) consisted of 1 group of adult (∼3 mo, end point ∼6–7 mo of age) WT mice fed a standard diet (n = 14) and 4 randomized groups of TGF mice fed either a standard diet (n = 8), an HCD (n = 8, supplemented with 2% cholesterol and 0.5% cholic acid; Envigo, Huntingdon, United Kingdom), an HCD concurrently with EX (12 h, during dark phase, n = 11) using a 17.5-cm-diameter running wheel (61,706; Living World, QC, Canada), or an HCD concurrently with the cholesterol-lowering drug SV (n = 10, ∼40 mg/kg body weight/d; Cedarlane, Burlington, ON, Canada). SV was activated per the manufacturer’s protocol and added to the drinking water at a concentration of 0.04% (12).
In a separate cohort of mice (hereby referred to as cohort 2), we investigated the effects of a more translationally relevant EX regimen (5 d/wk, 2 h/night access to running wheels) on cognitive function in mice fed an HCD. Young male and female WT and TGF mice (3–4 mo old) were used. Two groups were fed a standard lab chow diet (WT n = 13, TGF n = 11); the remaining 4 were fed the HCD, 2 of which were not treated (WT HCD n = 12, TGF HCD n = 10); and the other 2 were given access to running wheels (7:00–9:00 pm) equipped with a magnetic odometer (WT HCD EX n = 12, TGF HCD EX n = 10). Mice ran ∼2–3 km each per night over the entire course of the study until euthanized. Mice were housed together (n = 3–5/cage) in a 12-h light/dark cycle with food and water ad libitum, and those that exercised were housed in large cages to accommodate 1 wheel/mouse. Experiments were approved by the Animal Ethics Committee of the Montreal Neurologic Institute at McGill University) and complied with the Canadian Council on Animal Care.
Blood and brain cholesterol measurement
Prior to being intracardially perfused, mice were anesthetized with isoflurane and 1 ml of blood extracted from the heart. Blood samples were centrifuged (15 min, 15,000 rpm), and serum was removed (∼300 μl) and sent for biochemical analysis of serum cholesterol levels at the Comparative Medicine and Animal Resources Centre at McGill University (CMARC). Brain cholesterol levels were measured in the cortex (10 mg samples) with a Cholesterol/Cholesteryl Ester Assay Kit (ab65359; Abcam, Cambridge, MA, USA). According to the manufacturer’s protocol, brain tissue was homogenized and centrifuged, the resultant supernatant was dried using a speed vacuum and then diluted and incubated with manufacturer’s reagents, and sample absorbance (570 nM) was read using a microplate reader (Molecular Devices, SpectraMax Plus 384, CA, USA).
Behavioral tests
Morris water maze
Spatial cognitive function was tested in the Morris water maze (MWM) (24), as previously described (12). A pool (1.4 m diameter) was filled with water (18 ± 1°C) made opaque with inert white tempera paint. For the first maze (MWM1), performed after 3 mo of treatment, there were 3 visible platform (15 cm diameter) training days during which the mice were given 3 trials of 60 s separated by ∼45 min to find the platform. Before conducting the 5-d learning phase, the cues in the room were changed, as was the location of the platform, which was completely submerged (1 cm below the surface of the water). Mice were given 3 trials of 90 s to find the hidden platform. On the final day, a probe trial was conducted to assess spatial memory. The platform was removed from the pool, and mice were given 60 s of free swim during which the time they spent, and distance swam in the quadrant that previously contained the platform was recorded, as well as the number of times they would have landed on the platform if it were present. In cohort 2, a second MWM was conducted (MWM2) after 4 mo of treatment, whereby the platform and visual cue locations were altered, and only 1 visible platform day was performed. All data were recorded using the 2020 Plus Tracking System and Water 2020 Software (Ganz FC62D video camera; HVS Image, Buckingham, United Kingdom).
Novel object recognition
Following the MWM (MWM2 for cohort 2), mice were habituated (10 min) to the novel object recognition (NOR) testing arena, a white plastic box (45 × 45 × 50 cm) equipped with a webcam. The following day, mice underwent both the familiarization and test phases. During familiarization, each mouse was given 5 min to explore 2 identical objects placed in opposite corners of the arena, equidistant from the center. Two hours after familiarization, 1 object was replaced with a novel object, and another 5 min exploration period was performed. Behavior was recorded using iSpy software (https://www.ispyconnect.com) and ODLog (http://www.macropodsoftware.com/odlog/) to record the time spent exploring each object. Both the location of the novel object and the objects were counterbalanced. An investigation ratio was calculated by dividing the time spent with the novel object by the total time investigating both objects during the test phase.
Laser Doppler flowmetry
Changes in cerebral blood flow (CBF) evoked by whisker stimulation were measured with laser Doppler flowmetry (LDF; Transonic Systems, Ithaca, NY, USA) 3 d following the end of behavioral testing. Mice (n = 4–6/group) were anesthetized (ketamine 85 mg/kg, i.p., and xylazine 3 mg/kg, i.p.) and placed in a stereotaxic frame, and the bone was thinned over the left somatosensory cortex. CBF was continuously recorded using an LDF probe before, during, and after stimulation (20 s) of the right whiskers with an electric toothbrush (8–10 Hz), as previously described (21). In total, 4–6 recordings were acquired, and maximal responses were averaged for each mouse. Mice were sutured and allowed to recover for 3 d before being used for vascular reactivity experiments. Whisker-evoked CBF responses were expressed as the peak percentage change relative to mean baseline value taken 1 min prior to whisker stimulation.
Cerebrovascular reactivity
At 3 d following LDF experiments, reactivity of isolated middle or posterior cerebral artery segments (n = 3–5/group) was measured with online video microscopy (Living Systems Instrumentation, Burlington, VT, USA), as previously described (25). Vessel segments were gradually pressurized over 10 min to reach 60 mmHg and allowed to acquire their resting tone diameter. Dilatory responses to acetylcholine (ACh, 10−10−10−4 M; MilliporeSigma, Burlington, MA, USA), calcitonin gene-related peptide (CGRP, 10−10−10−6 M; American Peptide, Sunnyvale, CA, USA), the transient receptor potential cation channel subfamily V member 4 (TRPV4) channel opener GSK1016790A (10−10−10−5 M; MilliporeSigma), and the ATP-sensitive potassium (KATP) channel opener levcromakalim (Lev, 10−10−10−4 M; Tocris Bioscience, Bristol, United Kingdom) were tested on preconstricted vessels (phenylephrine 2 × 10−7 M; MilliporeSigma). Responses were expressed as percentage change in diameter from preconstricted vessels and plotted as a function of vasodilator concentration. Maximal responses (EAmax) and the concentration eliciting half EAmax (pD2 value or −log EC50) were used to compare vasodilator efficacy and potency, respectively.
Western blots and ELISA of inflammatory cytokines
Western blots were performed on cerebral cortex samples (n = 4 mice/group) dissected and frozen from mice used in reactivity experiments. Tissue samples were dissolved in 400 μl lysis buffer (18.5 ml distilled H2O, 400 μl 1 M Tris-HCl, 600 μl 5 M NaCl, 200 μl 10% Nonidet P-40, 200 μl glycerol, 100 μl 200 mM NaV, and 2 protease inhibitor cocktail tablets). Samples were homogenized and centrifuged and proteins were assayed (Bio-Rad, Hercules, CA, USA), separated by SDS gel (25 μg/sample, 10% acrylamide), and transferred to a nitrocellulose membrane. The nitrocellulose membrane was incubated (1 h, room temperature) in Tris-buffered saline–Tween blocking buffer (50 mM NaCl, 0.1% Tween 20, containing 7% skim milk). Membranes were incubated overnight with either mouse anti–β-actin (1:16,000; MilliporeSigma) or rabbit anti-occludin (1:500; Abcam). Membranes were incubated (1 h) with horseradish peroxidase–conjugated secondary anti-mouse (1:1000) or -rabbit (1:2000) antibody. Membranes were developed using WesternSure Premium Chemiluminescent substrate (Li-Cor Kit; Li-Cor Biosciences, Lincoln, NE, USA) and scanned using a C-Digit Blot Phosphor Imager (Scanner Storm 860; GE Healthcare, Waukesha, WI, USA). Image densitometry was analyzed with Image Studio Digit software v.5 (Li-Cor). ELISAs were performed on cortical samples weighing ∼10 mg and processed according to the protocol provided by Mesoscale V-Plex Kit (210,377, K152A0H-1) to measure levels of IL-6, TNF-α, and IL-1β.
Immunohistochemistry
The remaining mice from each group (n = 4–5/group) were anesthetized (65 mg/kg of sodium pentobarbital, i.p.) and perfused intracardially using 4% paraformaldehyde in phosphate buffer (0.1 M, pH 7.4). Brains were postfixed (4% paraformaldehyde overnight), cryoprotected (30% sucrose, 48 h), and then frozen in isopentane and stored at −80°C. Coronal sections (25-μm thickness, 2–3 sections/mouse) were obtained on a freezing microtome and immunostained (overnight) as floating sections with primary antibodies as follows: rabbit anti-GFAP (a marker of activated astrocytes, 1:800; Agilent Technologies, Santa Clara, CA, USA), rabbit anti-ionized calcium-binding adaptor molecule 1 (Iba-1, a marker of microglial cells, 1:300; Wako Pure Chemicals, Osaka, Japan), rat anti–galectin-3 (Gal-3, a marker of activated microglia in response to injury, 1:1500; Cedarlane), rabbit anti-Ki67 (a marker for newborn cells, 1:1000; Leica Microsystems, Buffalo Grove, IL, USA), and goat anti-doublecortin (DCX, a marker for immature neurons, 1:1000; Santa Cruz Biotechnology, Dallas, TX, USA). After rinsing, sections were incubated (30 min) with species-specific cyanine (Cy)2-tagged secondary antibodies for immunofluorescence (1:400) or biotinylated antibodies (1:200; Vector Laboratories, Burlingame, CA, USA), followed by incubation in the avidin-biotin complex (1 h 15 min; Vector Laboratories) and 3′3-diaminobenzidine (2 min; Vector Laboratories) for DCX and Ki67 immunohistochemistry. Sections were mounted on gelatin-coated slides, coverslipped with Mowiol (MilliporeSigma), or dehydrated and coverslipped with Permount (Fisher Scientific, catalogue number SP15-500, New Jersey, USA). Low-power digital images were taken with a light microscope (Leitz Aristoplan; Leica Microsystems) equipped for epifluorescence and analyzed using MetaMorph (6.1r3; Universal Imaging, Bedford Hills, NY, USA) where the areas of interest were manually delineated. The barrel cortex was used for cortical analyses, as this is the area in which CBF responses were recorded during whisker stimulation. The internal capsule was used for WM measurements, as it is an easily identifiable and large WM tract in the mouse brain. Furthermore, the internal capsule is an early myelinated tract, meaning it is likely that other smaller WM tracts myelinated later during development would show similar results. The percentage area occupied by GFAP or Gal-3–immunopositive material in the barrel cortex or internal capsule was measured using MetaMorph. The number of positive DCX cells in the dentate gyrus of the hippocampus was counted under the microscope. For microgliosis, a further analysis of the size of individual cortical Iba-1–positive microglial cells (10 individual cells/mouse randomly selected) was completed with ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
All in vivo and vascular reactivity experiments were performed blind by the experimenter. For cohort 1, 1-way ANOVAs were used followed by Newman-Keuls post hoc multiple comparison tests. Two-way ANOVAs (factors being genotype: WT or TGF and treatment: normal chow, HCD, or HCD EX) were used for cohort 2, followed by Newman-Keuls post hoc multiple comparison tests. All statistical analyses were performed using Prism 7 (GraphPad Software, La Jolla, CA, USA), and a value of P ≤ 0.05 was considered statistically significant. Effect sizes (η2) were calculated using the sum of squares.
RESULTS
HCD increased cholesterol blood levels but not brain cholesterol
Total cholesterol levels were measured in mice from cohort 1 and were significantly increased (∼2-fold) in all groups fed the HCD compared with mice fed a standard diet (Table 1), in line with our recent study (12). This was driven by raised LDL, whereby WT and TGF controls had hardly detectable levels never surpassing 0.12 mM, whereas mice fed an HCD had values up to 2.06 ± 0.33 mM. HDL levels did not differ between groups, nor did brain cholesterol levels.
TABLE 1.
Plasma and brain cholesterol levels in mice from cohort 1
| Variable | WT | TGF | TGF HCD | TGF HCD SV | TGF HCD EX |
|---|---|---|---|---|---|
| LDL (mM) | 0.12 ± 0.01 | 0.11 ± 0.02 | 1.69 ± 0.47* | 2.06 ± 0.33** | 1.36 ± 0.19* |
| HDL (mM) | 2.57 ± 0.16 | 2.40 ± 0.12 | 2.60 ± 0.27 | 2.75 ± 0.58 | 2.79 ± 0.14 |
| Total cholesterol (mM) | 2.57 ± 0.16 | 2.77 ± 0.12 | 4.55 ± 0.23*** | 5.05 ± 0.25*** | 4.52 ± 0.26*** |
| Brain cholesterol (µg/100 µg cortex) | 1.25 ± 0.05 | 1.23 ± 0.09 | 1.29 ± 0.24 | 1.48 ± 0.29 | 1.32 ± 0.09 |
Data are means ± sem. Serum samples spun down from peripheral blood samples acquired prior to perfusion were analyzed for LDL, HDL, and total cholesterol. A 10-mg piece of cortex was dissected from brains used for reactivity experiments to measure total cholesterol (n = 4–5/group). *P < 0.05, **P < 0.01, ***P < 0.001, indicate differences between group fed an HCD and groups fed a standard diet.
HCD worsened cognitive performance: effects of SV and EX
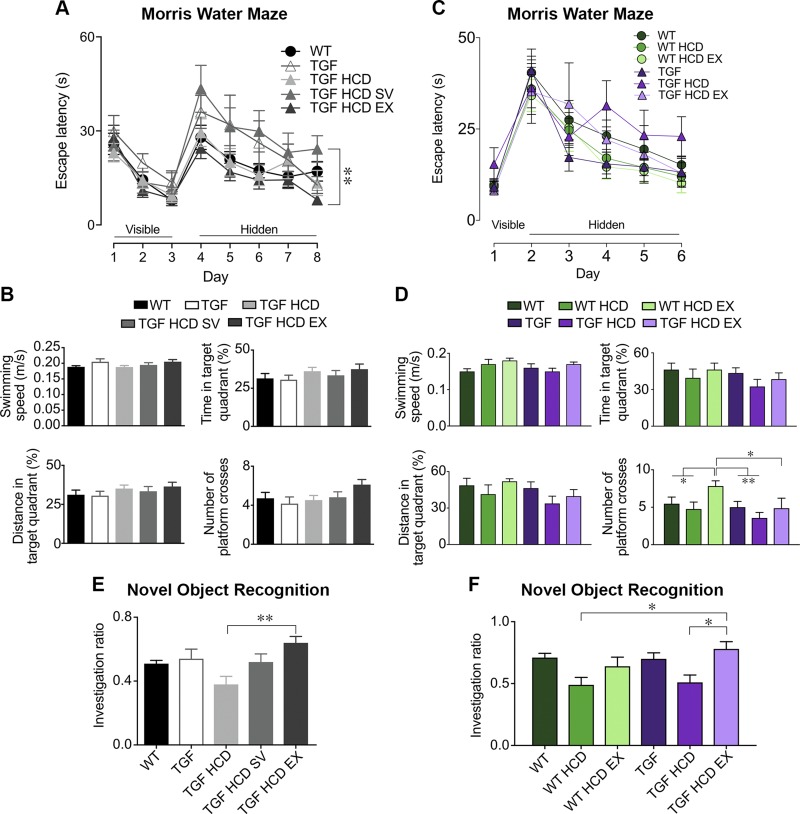
Because high levels of LDL have been identified as a risk factor for late-onset dementia (26, 27), and individuals with nonfamilial hypercholesterolemia have a 2.9% increased risk of developing dementia (28), we investigated whether cognitive deficits could arise in HCD-fed mice and whether SV and EX could be protective. After 3 mo of HCD in cohort 1, the last spatial learning day significantly differed between the 2 treatment groups, whereby mice allowed to freely exercise were faster than those treated with SV in locating the hidden platform [Fig. 1A, B, F(4,48) = 3.35, P < 0.05, η2 = 0.15]. In cohort 2, both MWM1 performed after 3 mo (unpublished results) and MWM2 tested after 4 mo of treatment (Fig. 1C, D) revealed that HCD-fed TGF mice tended to take longer at locating the hidden platform on the last 3 learning days, but it did not reach significance (P ≥ 0.21). All groups of mice from both cohorts had similar capacity in remembering the location of the platform in the probe trial, but TGF HCD mice from cohort 2 behaved more poorly in all parameters measured. SV and EX had no effect in the probe trial (Fig. 1B, D), except for WT HCD mice submitted to limited EX, which had significantly more crossings over the previous location of the hidden platform than all other groups (Fig. 1D).
Figure 1.
HCD worsened cognitive performance, whereas treatment with SV and EX maintained cognitive abilities. A–D) There was no strong adverse effect of HCD on the spatial MWM in either cohort 1 (A, B) or cohort 2 (C, D) in either WT or TGF mice. E, F) However, there was a clear trend in both cohorts for the HCD to worsen object recognition memory and for SV to prevent the decline (E), whereas mice that exercised reach cognitive performance comparable to that of WT mice (F). *P < 0.05, **P < 0.01.
In the NOR test, TGF mice fed an HCD in both cohorts performed more poorly than their counterparts fed a standard diet, although not significantly (P = 0.13 for cohort 1, P = 0.20 for cohort 2) (Fig. 1E, F), yet recognition memory was significantly better in HCD-fed TGF mice allowed to exercise in both cohorts. In cohort 1, TGF HCD mice performed worse than TGF controls [ANOVA F(4,41) = 4.40, P < 0.01, η2 = 0.30], and concurrent SV administration prevented a decline in performance. Multiple comparisons revealed a significant difference between TGF HCD and TGF HCD EX groups (Fig. 1E, P < 0.01). In cohort 2, 2-way ANOVA for the NOR test was significant, showing a main effect of treatment F(2,52) = 8.69, P < 0.001, η2 = 0.55, and multiple comparisons showed a significant benefit of EX in TGF HCD mice (Fig. 1F, P < 0.05).
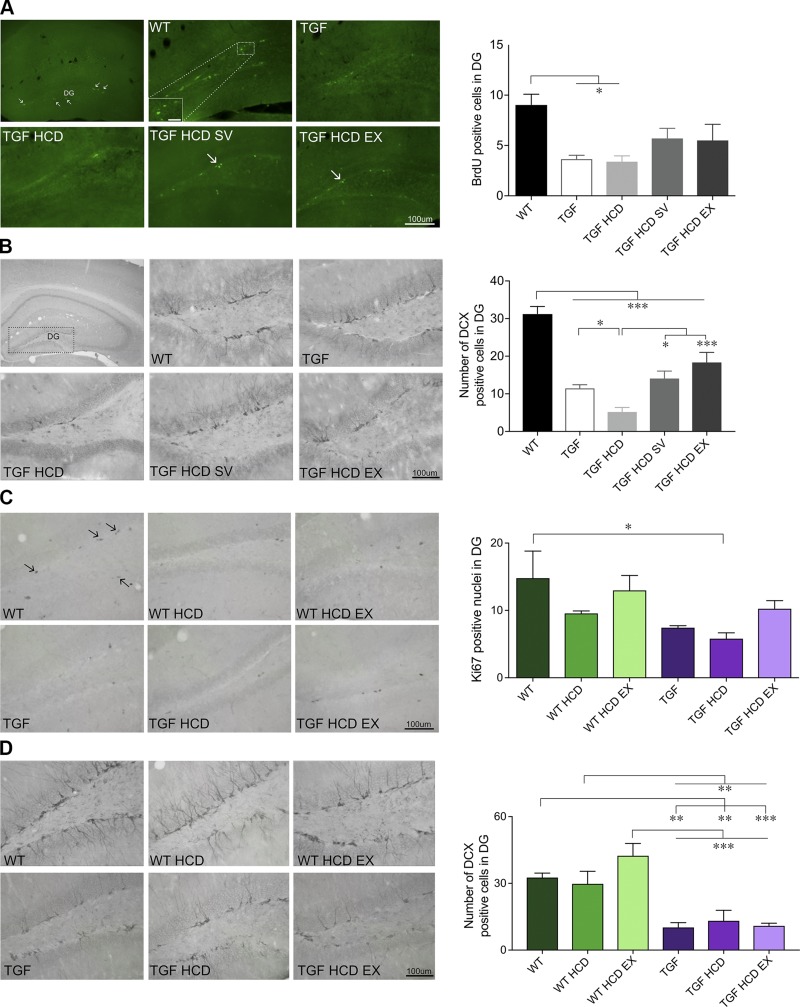
HCD and neurogenesis: effects of SV and EX
Numerous behavioral studies have linked increased hippocampal neurogenesis to improved learning and memory (29, 30); we thus investigated whether altered neurogenesis could play a role in the cognitive impairment of HCD-fed mice and how treatments could modify this response. When looking at the number of newborn cells in the dentate gyrus using bromodeoxyuridine/5-bromo-2'-deoxyuridine (BrdU) (cohort 1) or Ki67 (cohort 2) immunostaining, only TGF or TGF HCD mice were significantly (P < 0.05) different from WT controls after multiple comparisons (Fig. 2A, C), in line with our previous study (12). A main effect of genotype was confirmed in the number of immature DCX-immunopositive neurons in the dentate gyrus in which TGF and TGF HCD mice showed significant decreases compared with WT (12, 31). SV and unlimited EX countered this negative effect of HCD in TGF mice, with significant increases in number of DCX cells after treatments (P < 0.05, P < 0.001, respectively, η2 = 0.40) (Fig. 2A), a benefit not observed in cohort 2 with limited EX (Fig. 2B).
Figure 2.
The number of immature neurons in the dentate gyrus increased with SV and EX. A) In cohort 1, mice injected with BrdU to label newborn cells (small arrows) in the dentate gyrus (DG) of the hippocampus, a significant decrease in BrdU-positive cells was found in TGF and TGF HCD mice, whereas SV and EX groups were in between WT controls and untreated TGF groups, indicating a protective effect. C) Similar results were seen in cohort 2 when proliferating cells were labeled for Ki67. B, D) Staining for immature neurons in the DG revealed that all TGF groups had fewer positively labeled DCX cells, which was further decreased by HCD and protected by SV and EX in cohort 1 (B), but no such effects were seen in mice from cohort 2 with limited EX (D). Scale bars, 100 μm for BrdU (5 μm for inset) and 100 μm for Ki67 and DCX images (n = 4–5 mice/group). *P < 0.05, **P < 0.01, ***P < 0.001.
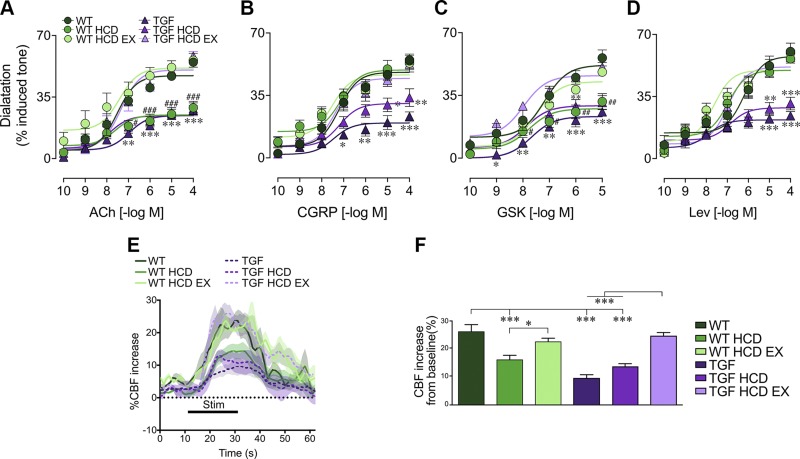
HCD and cerebrovascular function: effects of SV and EX
In a large data-driven analysis, vascular dysregulation in late-onset AD was identified as the earliest detectable biomarker of disease progression (32), and vascular incidents, often due to cardiovascular disease, are seen as causative events in VCID (33). Hence, we investigated whether increased peripheral LDL could trigger central vascular dysfunction and the effects of SV and EX. HCD did not worsen the already impaired vasodilatory capacity and sensory-evoked CBF response of TGF mice in either cohort, as shown here for cohort 2 (Fig. 3). In contrast, HCD had deleterious effects on cerebrovascular function in WT mice, exemplified by reduced endothelium-dependent dilations to ACh and the TRPV4 channel opener GSK1016790A (Fig. 3A, C), and impaired whisker-evoked neurovascular coupling responses (Fig. 3E, F). HCD had no negative effect on smooth muscle cell–dependent vasodilation to CGRP and KATP channel opener Lev (Fig. 3B, D). There was no change in receptor affinity for any of the vasoactive agents tested (Table 2). SV significantly improved or fully normalized impaired dilatory responses (cohort 1, unpublished results), whereas both unlimited (unpublished results) and limited EX (Fig. 3A–D) fully normalized cerebrovascular reactivity in both HCD-fed WT and TGF mice. Additionally, reduced CBF increases to whisker stimulation in the barrel cortex of both WT and TGF mice fed an HCD were restored to WT responses in mice given limited access to wheels (Fig. 3E, F).
Figure 3.
HCD worsened endothelial cell–dependent vasodilation and whisker-evoked CBF in WT mice; EX normalized these responses in both genotypes. A, C) HCD impaired endothelium-dependent dilations to ACh and TRPV4 channel opener GSK1016790A (GSK) in WT mice, but did not worsen the already-altered responses in TGF mice; EX restored these responses to WT control levels (n = 3–5/group). B, D) Smooth muscle cell–mediated dilatory responses to CGRP and Lev were only impaired in TGF and TGF HCD mice and fully normalized by EX. E, F) Group mean traces of CBF responses (n = 4–6/group) to 20-s whisker stimulation (E) and quantification (F) for cohort 2 are shown. HCD impaired CBF increases in WT mice, and EX normalized this response in both WT and TGF groups fed an HCD. *P < 0.05, **P < 0.01, ***P < 0.001: differences between TGF groups; #P < 0.05, ##P < 0.01, ###P < 0.001: differences between WT groups; differences between genotypes are not shown, because of significant interactions between treatment condition and genotype.
TABLE 2.
Effects of HCD on cerebrovascular reactivity: effects of limited EX
| Variable | WT | WT HCD | WT HCD EX | TGF | TGF HCD | TGF HCD EX |
|---|---|---|---|---|---|---|
| ACh | ||||||
| EAmax | 54.8 ± 2.4 | 28.9 ± 3.7### | 55.9 ± 3.9 | 27.3 ± 3.0*** | 28.3 ± 3.4*** | 58.4 ± 2.5 |
| pD2 | 7.51 ± 0.24 | 7.60 ± 0.34 | 7.42 ± 0.35 | 6.97 ± 0.21 | 7.84 ± 0.36 | 7.35 ± 0.19 |
| CGRP | ||||||
| EAmax | 54.9 ± 2.5 | 54.4 ± 1.8 | 53.1 ± 5.0 | 21.8 ± 2.3*** | 33.8 ± 5.0** | 52.6 ± 3.2 |
| pD2 | 7.20 ± 0.16 | 7.13 ± 0.22 | 7.53 ± 0.27 | 7.45 ± 0.42 | 7.19 ± 0.29 | 7.36 ± 0.25 |
| GSK | ||||||
| EAmax | 55.9 ± 4.5 | 31.5 ± 4.5## | 48.1 ± 1.5 | 25.8 ± 2.4*** | 31.1 ± 3.8** | 50.9 ± 2.5 |
| pD2 | 7.18 ± 0.24 | 7.77 ± 0.30 | 7.49 ± 0.18 | 7.68 ± 0.32 | 7.88 ± 0.31 | 7.97 ± 0.16 |
| LEV | ||||||
| EAmax | 60.3 ± 1.09 | 56.2 ± 2.4 | 59.5 ± 5.6 | 24.1 ± 2.4*** | 31.0 ± 3.5*** | 57.9 ± 3.3 |
| pD2 | 6.18 ± 0.23 | 6.97 ± 0.21 | 7.65 ± 0.30 | 7.89 ± 0.32 | 6.87 ± 0.35 | 6.95 ± 0.28 |
Data are means ± sem and are expressed as the maximal agonist response (EAmax) and potency [pD2, −log (EC50)] for cohort 2. EAmax is the percentage maximal dilation to ACh, CGRP, and TRPV4 channel opener (GSK1016790A) and KATP channel opener Lev (n = 3–5/group). **P < 0.01, ***P < 0.001 (differences compared with TGF HCD EX); ##P < 0.01, ###P < 0.001 (differences compared with WT controls and WT HCD EX; differences between genotypes are not indicated due to significant interactions between treatment condition and genotype).
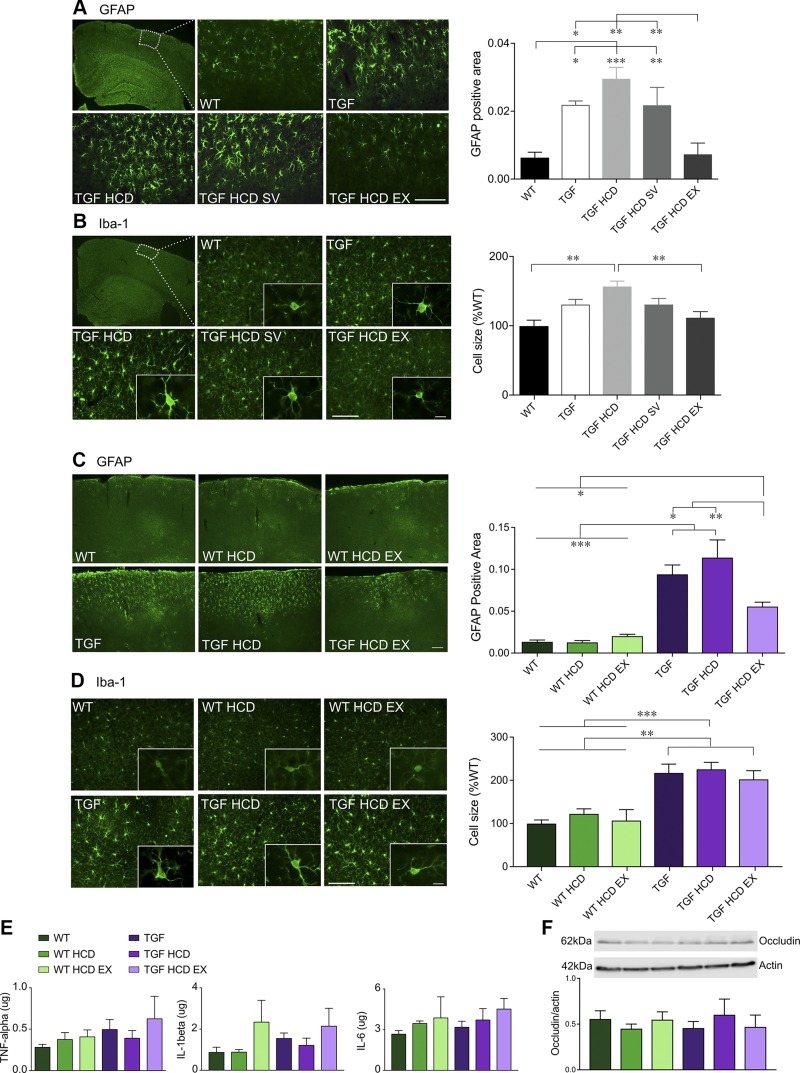
HCD and the neuroinflammatory response: effects of SV and EX
Neuroinflammation in dementia research is a double-edged sword; however, in initial stages of the disease’s manifestation, a heightened inflammatory response in the CNS could initiate and exacerbate cognitive decline, and modulation of this immune response could be a promising therapeutic target (34). In the first cohort, ANOVAs showed significant increases in GFAP- and Iba-1–immunopositive cortical areas in TGF mice compared with WT controls [F(4,18) = 9.65, P < 0.001, η2 = 0.19 for GFAP and F(4,17) = 4.86, P < 0.01, η2 = 0.35 for Iba-1]. The HCD did not significantly increase these indicators of neuroinflammation in TGF mice. However, EX, but not SV, significantly reduced both cortical astrogliosis (Fig. 4A, P < 0.01) and microgliosis to WT levels, as shown here at the single-cell level whereby the reactive phenotype of swollen cell body and processes returned to the cell quiescent phenotype with small soma and thin processes (Fig. 4B, P < 0.01) in TGF mice [F(4,17) = 6.97, η2 = 0.64, P < 0.01]. In cohort 2, there was a main effect of genotype for cortical astrogliosis [F(1,22) = 70.7, η2 = 0.64 P < 0.001] and microgliosis [F(1,22) = 13.94, η2 = 0.36, P < 0.01] (Fig. 4C, D). Limited EX only attenuated astrogliosis in TGF HCD EX mice (P < 0.01 compared with TGF HCD, P < 0.05 compared with TGF controls) (Fig. 4C), but it had no effect on microgliosis, as shown here at the single-cell level (Fig. 4D). In cohort 2, cortical tissues were analyzed for inflammatory cytokines often described as being increased in cerebrospinal fluid of patients with AD and vascular dementia (35), and no differences were found between any of the groups (Fig. 4E), as was also the case for the tight junction protein occludin (Fig. 4F), the latter suggesting no alteration of the BBB due to an HCD.
Figure 4.
Neuroinflammatory responses in the cortex were not exacerbated by HCD. A, B) Activation of cortical astrocytes and microglia as quantified with GFAP (A) and Iba-1 (B) immunostaining was significantly reduced by unlimited EX but not by SV (cohort 1). C–F) When EX was limited (cohort 2), significant decreases were observed in cortical GFAP (C) but not in Iba-1 (D). E, F) HCD did not exacerbate these pathologies that are already present in TGF mice in either cohort. IL levels of TNF-α, IL-1β, and IL-6 measured by ELISA were not different between groups (E), and no evidence of BBB damage was found when quantifying the tight junction protein occludin by Western blot (F) (n = 4–5 mice/group). Scale bars, 100 μm for GFAP and Iba-1 (10 μm, inset). *P < 0.05, **P < 0.01, ***P < 0.001.
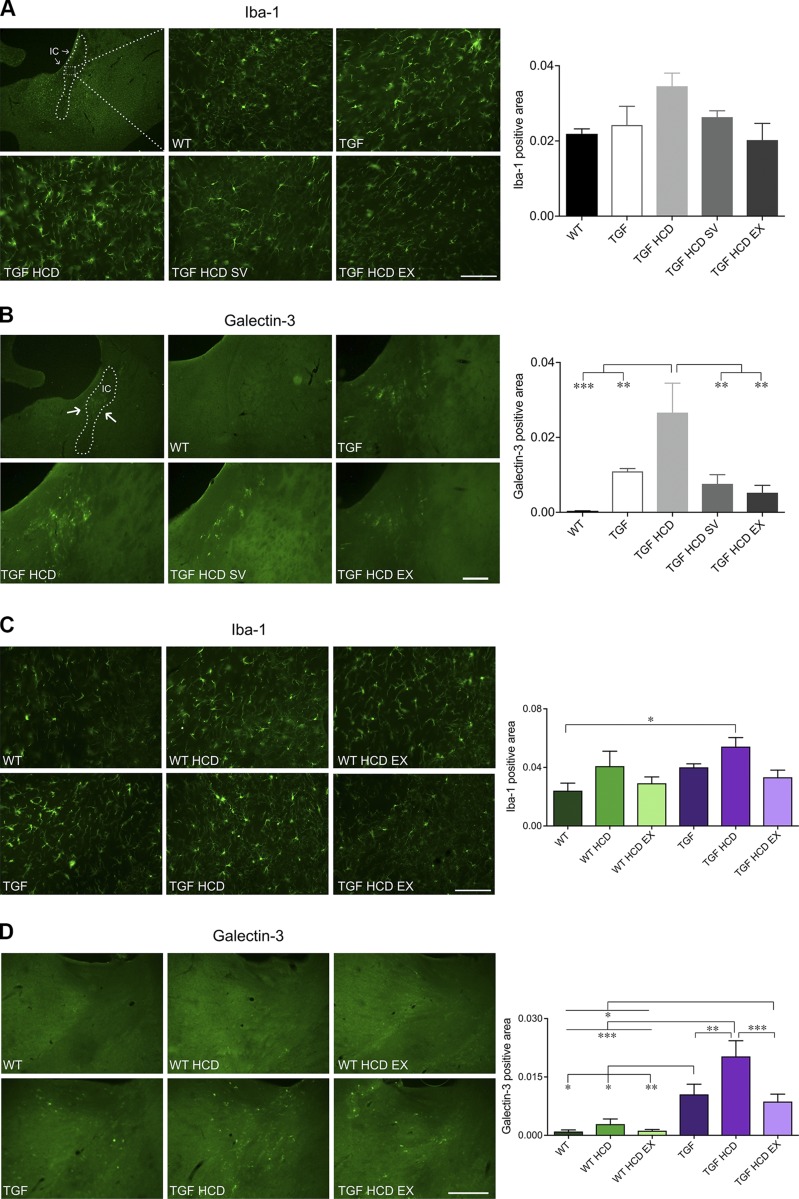
HCD and WM microglia: effects of SV and EX
Alterations in WM can affect the efficiency by which information is processed and transmitted between neuronal networks (36), and so we investigated neuroinflammatory changes in WM tracts, particularly in the internal capsule. Although the apparent increase in Iba-1–positive WM area in TGF mice fed an HCD in cohort 1 did not reach significance (P = 0.07), overall ANOVA in cohort 2 showed a significant main effect for genotype [F(2,21) = 4.54, P < 0.05, η2 = 0.13] and treatment conditions [F(2,21) = 4.13, η2 = 0.24, P < 0.05], and post hoc analysis revealed a significant difference only between the WT control group and HCD-fed TGF mice (P < 0.05, Fig. 5A, C). When looking at Gal-3, a member of the galectin family of β-galactoside binding lectins present in microglia and macrophages that respond to proinflammatory stimuli and phagocytose myelin debris (37), we found significant main effects in both cohorts, indicating that Gal-3–positive microglial cells were more abundant in WM tracts in TGF mice compared with WT controls. What is more, we found that this up-regulation was further increased in TGF mice fed an HCD in both cohorts, as shown by multiple comparisons (P < 0.01) (Fig. 5B, D). In TGF HCD mice with preserved cognitive function following SV or both unlimited and limited EX, this highly characteristic WM phenotype disappeared, and the Gal-3–positive area was reduced to levels comparable to those of control TGF mice (Fig. 5B, D).
Figure 5.
A subset of microglia in WM was up-regulated by HCD and completely normalized by SV and EX. A, C) Iba-1–positive microglial area was quantified in the internal capsule (IC). In both cohorts, there was a tendency for an increase in microgliosis in HCD-fed mice and for SV and EX to prevent this response. B, D) In contrast, Gal-3–positive microglial area was selectively and significantly increased in TGF HCD mice in both cohort 1 (B) and cohort 2 (D). SV and EX, whether unlimited (B) or limited (D), fully countered this HCD-induced up-regulation of Gal-3 in TGF mice. Scale bars: 100 μm (Iba-1 images), 250 μm (Gal-3 images) (n = 4–5 mice/group). *P < 0.05, **P < 0.01, ***P < 0.001.
DISCUSSION
Our main findings are that an HCD negatively impacted cognitive performance in both WT and TGF mice when looking at the overall ANOVAs and that both SV and EX exerted cognitive benefits, with EX (unlimited or limited) significantly preventing cognitive decline in HCD-fed TGF mice. Moreover, HCD did not worsen the existing cerebrovascular dysfunction in adult TGF mice but induced impairments in WT mice, all of which were restored by SV or EX interventions. Our results further indicate that a commonality between SV and EX that potentially underlays their benefits is their shared ability to restore endothelial function and reduce WM-associated inflammation.
SV and EX prevented the negative influence of HCD on object recognition memory
In the NOR test, which involves use of executive functions relevant to VCID (38, 39), we found a damaging effect in both cohorts of HCD in TGF mice. Albeit not significant after multiple comparisons, this deleterious effect of HCD is in line with our recent study reporting increased susceptibility to cognitive decline (12) and another study reporting accelerated pathology in TGF mice exposed to a comorbid disease (40). Most impressive in the NOR test was the robust and powerful therapeutic benefits of both unlimited and limited EX on cognitive dysfunction in hypercholesterolemic TGF mice. This is in accordance with studies showing cognitive benefits in healthy individuals that exercise (41, 42) and EX reducing the rate of cognitive decline in older at-risk individuals (43–46) and in mouse models of dementia (47, 48). Similarly, the apparent recovery potential of SV against the HCD-induced deficit in the NOR test in TGF mice corroborated SV’s benefits on spatial memory in HCD-fed TGF mice (12) and in other animal models of cognitive impairment (21, 49, 50), as well as in reducing the risk of developing dementia in longitudinal and epidemiologic human studies (15, 51–53). It is difficult to pinpoint the precise mechanisms of action of EX, as it has multifaceted effects (18), but comparing its benefits with those of SV in HCD-fed TGF mice may shed some light on possible protective mechanisms.
Neurogenic effects of SV and EX
Neuronal loss in the hippocampus, particularly in the CA1 region, has been documented in several animal models of dementia (54) and in patients with VCID (1, 55). Neurogenesis is viewed as beneficial for learning and memory, as new neurons can be integrated into existing circuits and provide the potential for more efficient connections to form. SV possesses neurogenic properties (56), and unlimited EX has been shown to protect synaptic plasticity in the hippocampus and increase brain-derived neurotrophic factor (57, 58). Here, beneficial effects of SV and unlimited EX on neurogenesis were observed when looking at the number of immature neurons, an effect not seen in mice receiving limited access to running wheels (cohort 2), an intervention that also resulted in preserved cognitive function. Our findings, aside from showing that cognitive benefits can be achieved with different regimens of EX, confirm that neurogenesis is highly dependent on EX intensity or total distance run (59). From these observations, we conclude that benefits of SV and EX on cognition cannot be solely attributed to increased neurogenesis and that other mechanisms are at play.
Impaired endothelial cell function restored by SV and EX
Our current findings substantiate our recent ones showing that HCD fails to worsen the impaired cerebrovascular function in TGF mice but elicits endothelial alterations in WT mice (12). We found exclusively impaired dilatory responses to endothelium-located muscarinic ACh receptors and TRPV4 channels, together with reduced CBF responses evoked by whisker stimulation in HCD-fed WT mice. Although often associated with cardiovascular disease (60), chronically high levels of circulating cholesterol can impact the cerebrovasculature through various mechanisms including reduced baseline CBF and endothelial cell damage through oxidative stress (61). Additionally, elevated levels of circulating oxysterols, notably 27-hydroxycholesterol (62), which is capable of crossing the BBB (63), could have detrimental effects on brain function. Two separate studies investigating hypercholesterolemia in mice reported compromised cerebrovascular integrity with thickened basement membranes and decreased microvascular density and vessel length (64, 65), likely impairing vasodilatory function and whisker-evoked CBF responses, which are highly dependent on intact endothelial cell function (66), and affected in WT HCD-fed mice. This could also disrupt the tight communication between neurons, astrocytes, and brain vasculature required for neurovascular coupling (67).
We found that cerebrovascular reactivity and whisker-evoked neurovascular coupling responses were preserved in WT and TGF mice given the opportunity to exercise and confirmed the benefits of SV reported in TGF (7) and TGF HCD mice (12). It has been widely reported that EX improves endothelium-dependent vasodilation in the periphery, and although more work on the cerebral endothelium is required (68), some groups have reported improved cerebrovascular reactivity following EX training intervention (69, 70). There is also evidence that the cerebral endothelium may be central to relaying the multitudinous benefits of EX in the context of dementia (18).
Likewise, SV protected endothelial-mediated ACh and TRPV4 channel cerebral dilatations and reinstated whisker-evoked CBF responses in HCD-fed mice, as previously documented in an AD mouse model (21) and in TGF mice (7). Additional studies have shown that pleotropic effects of SV include increasing NO bioavailability and eNOS activity (71), restoring ACh-, CGRP-, TRPV4-, and KATP channel-mediated dilation (10, 12, 21) and acting as an antioxidant and immunomodulator (12). All of these can improve endothelial function. Furthermore, SV and EX effectively reduced WM neuroinflammation, in line with SV’s ability to improve cerebrovascular reactivity in patients with postischemic stroke, mainly in regions of damaged WM (12).
SV and EX impact on neuroinflammation in gray matter and WM
HCD did not worsen the already-elevated gray matter astrogliosis or microgliosis in the cerebral cortex of TGF mice, nor did it elicit gray matter inflammation in WT mice fed an HCD. More importantly, despite the ability of EX to reduce cortical gray matter inflammation, treatment with SV did not alter this feature, yet both had positive effects on cognition. These observations indicate that reduction of astro- and microglial inflammatory markers in the cortex are not essential in protecting against cognitive decline. However, not tested in the present study is the possibility that SV and EX alleviate neuroinflammation surrounding the vasculature, allowing for restored endothelial cell function and maintenance of vascular niches important for neurogenesis. There is conflicting evidence regarding HCD’s ability to affect dementia severity via entry of cytokines across the BBB (72, 73). We found no effect of HCD or limited EX on brain levels of TNF-α, IL-1β, or IL-6, nor in occludin, an important BBB tight junction protein, supporting local neuroinflammation due to activation of resident microglial cells. Another possible explanation for HCD not negatively impacting gray matter could relate to its higher metabolic reserve compared with WM (74), in which we observed the strongest increase in inflammatory response.
Accordingly, in contrast to gray matter, we found dramatically increased Gal-3–positive microglial cells in WM tracts in HCD-fed TGF mice and a reduction by both SV and EX, supporting the important relationship between WM pathology and cognition. Of interest, lower cognitive performance, reduced CBF, and greater WM hyperintensity load and brightness in MRI images indicative of WM lesions have been shown in patients with hypercholesterolemic and high visit-to-visit LDL variability, independently of mean LDL levels (75). WM hyperintensities detected in MRI and WM lesions identified histopathologically with inflammatory and myelin debris phagocyting markers like Gal-3 have been correlated with severity of cognitive impairment in aging humans, aged nonhuman primates and rodents, and mouse models of demyelinating diseases (37, 76–79). WM hyperintensities are clinically relevant, as they can be predictive of future mild cognitive impairment (80) and clinical severity of AD (81). Serum levels of Gal-3 showed strong correlations with scores on the Mini-Mental Status Examination, in which lower Mini-Mental Status Examination scores were predictive of higher serum Gal-3, with a stark difference between individuals with mild cognitive impairment and those with diagnosed AD (82). Although the presence of Gal-3 is indicative of injury, its role is also associated with protective and regenerative properties; however, in a pathologic context, microglial cells can become aberrantly phagocytic (83). Our Gal-3 findings in TGF mice fed an HCD support these associations, such that the incidence of cognitive impairment coincides with the appearance of increased Gal-3 staining in WM regions. However, the lack of Gal-3 staining in the WM of WT HCD mice, who displayed trends of cognitive deficits on the NOR test, would suggest that this pathology may be dependent on or precipitated by preexisting vascular impairments, as found in TGF mice. The beneficial effects of our treatments on WM inflammation in TGF HCD mice are supported by previous reports of improved WM integrity following EX training programs (84) or SV treatment (12, 85), which seems to relate to preserved cognitive abilities. It is also interesting to note that mice initially fed a cuprizone diet that causes demyelination, recover cognitive abilities when taken off the diet (86), which may be attributable to a shift in the role of Gal-3 during remyelination (79).
CONCLUSIONS
Our findings demonstrate that hypercholesterolemia can initiate endothelial-related cerebrovascular dysfunction in WT mice and induce cognitive deficits and that, in the context of a chronically compromised cerebral circulation, lead to loss of WM integrity associated with cognitive decline. Our results further indicate that the course of cognitive decline can be altered by concomitant SV treatment and, most importantly, not only by unlimited but also by a translationally relevant, moderate regimen of physical EX limited to 2–3 km daily, 5 d/wk. The findings suggest that middle-aged individuals at risk of dementia could greatly benefit from brain-penetrant statins if lipid-lowering medication is required or daily moderate levels of aerobic EX regimens are used. Our findings also point to WM pathology and endothelial cell dysfunction as possible starting points of what ultimately leads to cognitive decline.
ACKNOWLEDGMENTS
The authors thank Dr. L. Mucke and the J. David Gladstone Institutes for providing the TGF transgenic mice for breeding (Gladstone Institute of Neurological Disease and Department of Neurology, University of California–San Francisco San Francisco, CA, USA). This study was supported by grants from the Canadian Institutes of Health Research (CIHR; Grant MOP-126001 to E.H.), the Alzheimer Society of Canada, the Canadian Vascular Network (CVN), and the Canadian Consortium on Neurodegeneration in Aging (CCNA), and studentships (to L.J.T.) from CIHR, Alzheimer Society of Canada, Fonds de Recherche du Québec–Santé, and Healthy Brains for Healthy Lives. The authors declare no conflicts of interest.
Glossary
- ACh
acetylcholine
- AD
Alzheimer’s disease
- BBB
blood-brain barrier
- CBF
cerebral blood flow
- BrdU
bromodeoxyuridine/5-bromo-2'-deoxyuridine
- CGRP
calcitonin gene-related peptide
- DCX
doublecortin
- EAmax
maximal response
- EX
aerobic physical exercise
- Gal-3
galectin-3
- GFAP
glial fibrillary acidic protein
- HCD
high-cholesterol diet
- Iba-1
ionized calcium-binding adaptor molecule 1
- KATP
ATP-sensitive potassium
- LDF
laser Doppler flowmetry
- Lev
levcromakalim
- MWM
Morris water maze
- NOR
novel object recognition
- pD2
−log EC50 value
- SV
simvastatin
- TRPV4
transient receptor potential cation channel subfamily V member 4
- VCID
vascular cognitive impairment and dementia
- WM
white matter
- WT
wild type
AUTHOR CONTRIBUTIONS
L. J. Trigiani, X.-K. Tong, and E. Hamel designed research; L. J. Trigiani performed research; L. J. Trigiani and J. Royea analyzed data; and L. J. Trigiani and E. Hamel wrote the manuscript.
REFERENCES
- 1.Iadecola C. (2013) The pathobiology of vascular dementia. Neuron 80, 844–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiesmann M., Kiliaan A. J., Claassen J. A. (2013) Vascular aspects of cognitive impairment and dementia. J. Cereb. Blood Flow Metab. 33, 1696–1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zlokovic B. V. (2011) Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 12, 723–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grammas P., Ovase R. (2002) Cerebrovascular transforming growth factor-β contributes to inflammation in the Alzheimer’s disease brain. Am. J. Pathol. 160, 1583–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malaguarnera L., Motta M., Di Rosa M., Anzaldi M., Malaguarnera M. (2006) Interleukin-18 and transforming growth factor-beta 1 plasma levels in Alzheimer’s disease and vascular dementia. Neuropathology 26, 307–312 [DOI] [PubMed] [Google Scholar]
- 6.Tarkowski E., Issa R., Sjögren M., Wallin A., Blennow K., Tarkowski A., Kumar P. (2002) Increased intrathecal levels of the angiogenic factors VEGF and TGF-β in Alzheimer’s disease and vascular dementia. Neurobiol. Aging 23, 237–243 [DOI] [PubMed] [Google Scholar]
- 7.Tong X. K., Hamel E. (2015) Simvastatin restored vascular reactivity, endothelial function and reduced string vessel pathology in a mouse model of cerebrovascular disease. J. Cereb. Blood Flow Metab. 35, 512–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaertner R. F., Wyss-Coray T., Von Euw D., Lesné S., Vivien D., Lacombe P. (2005) Reduced brain tissue perfusion in TGF-beta 1 transgenic mice showing Alzheimer’s disease-like cerebrovascular abnormalities. Neurobiol. Dis. 19, 38–46 [DOI] [PubMed] [Google Scholar]
- 9.Nicolakakis N., Aboulkassim T., Aliaga A., Tong X. K., Rosa-Neto P., Hamel E. (2011) Intact memory in TGF-β1 transgenic mice featuring chronic cerebrovascular deficit: recovery with pioglitazone. J. Cereb. Blood Flow Metab. 31, 200–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tong X. K., Hamel E. (2015) Simvastatin improves adult hippocampal neuronal maturation by up-regulating the Wnt/β-catenin pathway in a mouse model of AD. BRAIN and BRAIN PET Conference, Vancouver, BC, Canada [Google Scholar]
- 11.Lifshitz V., Weiss R., Benromano T., Kfir E., Blumenfeld-Katzir T., Tempel-Brami C., Assaf Y., Xia W., Wyss-Coray T., Weiner H. L., Frenkel D. (2012) Immunotherapy of cerebrovascular amyloidosis in a transgenic mouse model. Neurobiol Aging 33, 432.e1–432.e13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tong X.-K., Trigiani L. J., Hamel E. (2019) High cholesterol triggers white matter alterations and cognitive deficits in a mouse model of cerebrovascular disease: benefits of simvastatin. Cell Death Dis. 10, 89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dougherty R. J., Ellingson L. D., Schultz S. A., Boots E. A., Meyer J. D., Lindheimer J. B., Van Riper S., Stegner A. J., Edwards D. F., Oh J. M., Koscik R. L., Dowling M. N., Gallagher C. L., Carlsson C. M., Rowley H. A., Bendlin B. B., Asthana S., Hermann B. P., Sager M. A., Johnson S. C., Okonkwo O. C., Cook D. B. (2016) Meeting physical activity recommendations may be protective against temporal lobe atrophy in older adults at risk for Alzheimer’s disease. Alzheimers Dement. (Amst.) 4, 14–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hörder H., Johansson L., Guo X., Grimby G., Kern S., Östling S., Skoog I. (2018) Midlife cardiovascular fitness and dementia: a 44-year longitudinal population study in women. Neurology 90, e1298–e1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jick H., Zornberg G. L., Jick S. S., Seshadri S., Drachman D. A. (2000) Statins and the risk of dementia. Lancet 356, 1627–1631 [DOI] [PubMed] [Google Scholar]
- 16.Whitmer R. A., Sidney S., Selby J., Johnston S. C., Yaffe K. (2005) Midlife cardiovascular risk factors and risk of dementia in late life. Neurology 64, 277–281 [DOI] [PubMed] [Google Scholar]
- 17.Patten A. R., Yau S. Y., Fontaine C. J., Meconi A., Wortman R. C., Christie B. R. (2015) The benefits of exercise on structural and functional plasticity in the rodent hippocampus of different disease models. Brain Plast. 1, 97–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trigiani L. J., Hamel E. (2017) An endothelial link between the benefits of physical exercise in dementia. J. Cereb. Blood Flow Metab. 37, 2649–2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haag M. D., Hofman A., Koudstaal P. J., Stricker B. H., Breteler M. M. (2009) Statins are associated with a reduced risk of Alzheimer disease regardless of lipophilicity. The Rotterdam study. J. Neurol. Neurosurg. Psychiatry 80, 13–17 [DOI] [PubMed] [Google Scholar]
- 20.Zissimopoulos J. M., Barthold D., Brinton R. D., Joyce G. (2017) Sex and race differences in the association between statin use and the incidence of Alzheimer disease. JAMA Neurol. 74, 225–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tong X. K., Lecrux C., Rosa-Neto P., Hamel E. (2012) Age-dependent rescue by simvastatin of Alzheimer’s disease cerebrovascular and memory deficits. J. Neurosci. 32, 4705–4715; erratum: 7766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li L., Cao D., Kim H., Lester R., Fukuchi K. (2006) Simvastatin enhances learning and memory independent of amyloid load in mice. Ann. Neurol. 60, 729–739 [DOI] [PubMed] [Google Scholar]
- 23.Wyss-Coray T., Lin C., Sanan D. A., Mucke L., Masliah E. (2000) Chronic overproduction of transforming growth factor-beta1 by astrocytes promotes Alzheimer’s disease-like microvascular degeneration in transgenic mice. Am. J. Pathol. 156, 139–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deipolyi A. R., Fang S., Palop J. J., Yu G. Q., Wang X., Mucke L. (2008) Altered navigational strategy use and visuospatial deficits in hAPP transgenic mice. Neurobiol. Aging 29, 253–266 [DOI] [PubMed] [Google Scholar]
- 25.Tong X. K., Nicolakakis N., Kocharyan A., Hamel E. (2005) Vascular remodeling versus amyloid beta-induced oxidative stress in the cerebrovascular dysfunctions associated with Alzheimer’s disease. J. Neurosci. 25, 11165–11174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anstey K. J., Lipnicki D. M., Low L.-F. (2008) Cholesterol as a risk factor for dementia and cognitive decline: a systematic review of prospective studies with meta-analysis. Am. J. Geriatr. Psychiatry 16, 343–354 [DOI] [PubMed] [Google Scholar]
- 27.Notkola I.-L., Sulkava R., Pekkanen J., Erkinjuntti T., Ehnholm C., Kivinen P., Tuomilehto J., Nissinen A. (1998) Serum total cholesterol, apolipoprotein E epsilon 4 allele, and Alzheimer’s disease. Neuroepidemiology 17, 14–20 [DOI] [PubMed] [Google Scholar]
- 28.Zambón D., Quintana M., Mata P., Alonso R., Benavent J., Cruz-Sánchez F., Gich J., Pocoví M., Civeira F., Capurro S., Bachman D., Sambamurti K., Nicholas J., Pappolla M. A. (2010) Higher incidence of mild cognitive impairment in familial hypercholesterolemia. Am. J. Med. 123, 267–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deng W., Aimone J. B., Gage F. H. (2010) New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat. Rev. Neurosci. 11, 339–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kitabatake Y., Sailor K. A., Ming G. L., Song H. (2007) Adult neurogenesis and hippocampal memory function: new cells, more plasticity, new memories? Neurosurg. Clin. N. Am. 18, 105–113, x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buckwalter M. S., Yamane M., Coleman B. S., Ormerod B. K., Chin J. T., Palmer T., Wyss-Coray T. (2006) Chronically increased transforming growth factor-β1 strongly inhibits hippocampal neurogenesis in aged mice. Am. J. Pathol. 169, 154–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iturria-Medina Y., Sotero R. C., Toussaint P. J., Mateos-Pérez J. M., Evans A. C.; Alzheimer’s Disease Neuroimaging Initiative (2016) Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis. Nat. Commun. 7, 11934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shabir O., Berwick J., Francis S. E. (2018) Neurovascular dysfunction in vascular dementia, Alzheimer’s and atherosclerosis. BMC Neurosci. 19, 62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cuello A. C. (2017) Early and late CNS inflammation in Alzheimer’s disease: two extremes of a continuum? Trends Pharmacol. Sci. 38, 956–966 [DOI] [PubMed] [Google Scholar]
- 35.Tarkowski E., Liljeroth A. M., Minthon L., Tarkowski A., Wallin A., Blennow K. (2003) Cerebral pattern of pro- and anti-inflammatory cytokines in dementias. Brain Res. Bull. 61, 255–260 [DOI] [PubMed] [Google Scholar]
- 36.Fields R. D. (2010) Neuroscience. Change in the brain’s white matter. Science 330, 768–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Venkatesan C., Chrzaszcz M., Choi N., Wainwright M. S. (2010) Chronic upregulation of activated microglia immunoreactive for galectin-3/Mac-2 and nerve growth factor following diffuse axonal injury. J. Neuroinflammation 7, 32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Graham N. L., Emery T., Hodges J. R. (2004) Distinctive cognitive profiles in Alzheimer’s disease and subcortical vascular dementia. J. Neurol. Neurosurg. Psychiatry 75, 61–71 [PMC free article] [PubMed] [Google Scholar]
- 39.DeVito L. M., Eichenbaum H. (2010) Distinct contributions of the hippocampus and medial prefrontal cortex to the “what-where-when” components of episodic-like memory in mice. Behav. Brain Res. 215, 318–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Endo F., Komine O., Fujimori-Tonou N., Katsuno M., Jin S., Watanabe S., Sobue G., Dezawa M., Wyss-Coray T., Yamanaka K. (2015) Astrocyte-derived TGF-β1 accelerates disease progression in ALS mice by interfering with the neuroprotective functions of microglia and T cells. Cell Rep. 11, 592–604 [DOI] [PubMed] [Google Scholar]
- 41.Gomez-Pinilla F., Hillman C. (2013) The influence of exercise on cognitive abilities. Compr. Physiol. 3, 403–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bherer L., Erickson K. I., Liu-Ambrose T. (2013) A review of the effects of physical activity and exercise on cognitive and brain functions in older adults. J. Aging Res. 2013, 657508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Larson E. B., Wang L., Bowen J. D., McCormick W. C., Teri L., Crane P., Kukull W. (2006) Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann. Intern. Med. 144, 73–81 [DOI] [PubMed] [Google Scholar]
- 44.Ahlskog J. E., Geda Y. E., Graff-Radford N. R., Petersen R. C. (2011) Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clin. Proc. 86, 876–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chapman S. B., Aslan S., Spence J. S., Defina L. F., Keebler M. W., Didehbani N., Lu H. (2013) Shorter term aerobic exercise improves brain, cognition, and cardiovascular fitness in aging. Front. Aging Neurosci. 5, 75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ngandu T., Lehtisalo J., Solomon A., Levälahti E., Ahtiluoto S., Antikainen R., Bäckman L., Hänninen T., Jula A., Laatikainen T., Lindström J., Mangialasche F., Paajanen T., Pajala S., Peltonen M., Rauramaa R., Stigsdotter-Neely A., Strandberg T., Tuomilehto J., Soininen H., Kivipelto M. (2015) A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet 385, 2255–2263 [DOI] [PubMed] [Google Scholar]
- 47.García-Mesa Y., López-Ramos J. C., Giménez-Llort L., Revilla S., Guerra R., Gruart A., Laferla F. M., Cristòfol R., Delgado-García J. M., Sanfeliu C. (2011) Physical exercise protects against Alzheimer’s disease in 3xTg-AD mice. J. Alzheimers Dis. 24, 421–454 [DOI] [PubMed] [Google Scholar]
- 48.Belarbi K., Burnouf S., Fernandez-Gomez F. J., Laurent C., Lestavel S., Figeac M., Sultan A., Troquier L., Leboucher A., Caillierez R., Grosjean M. E., Demeyer D., Obriot H., Brion I., Barbot B., Galas M. C., Staels B., Humez S., Sergeant N., Schraen-Maschke S., Muhr-Tailleux A., Hamdane M., Buée L., Blum D. (2011) Beneficial effects of exercise in a transgenic mouse model of Alzheimer’s disease-like tau pathology. Neurobiol. Dis. 43, 486–494 [DOI] [PubMed] [Google Scholar]
- 49.Dalla Y., Singh N., Jaggi A. S., Singh D. (2010) Memory restorative role of statins in experimental dementia: an evidence of their cholesterol dependent and independent actions. Pharmacol. Rep. 62, 784–796 [DOI] [PubMed] [Google Scholar]
- 50.Yamamoto N., Fujii Y., Kasahara R., Tanida M., Ohora K., Ono Y., Suzuki K., Sobue K. (2016) Simvastatin and atorvastatin facilitates amyloid β-protein degradation in extracellular spaces by increasing neprilysin secretion from astrocytes through activation of MAPK/Erk1/2 pathways. Glia 64, 952–962 [DOI] [PubMed] [Google Scholar]
- 51.Dufouil C., Richard F., Fiévet N., Dartigues J. F., Ritchie K., Tzourio C., Amouyel P., Alpérovitch A. (2005) APOE genotype, cholesterol level, lipid-lowering treatment, and dementia: the Three-City study. Neurology 64, 1531–1538 [DOI] [PubMed] [Google Scholar]
- 52.Vuorinen M., Solomon A., Rovio S., Nieminen L., Kåreholt I., Tuomilehto J., Soininen H., Kivipelto M. (2011) Changes in vascular risk factors from midlife to late life and white matter lesions: a 20-year follow-up study. Dement. Geriatr. Cogn. Disord. 31, 119–125 [DOI] [PubMed] [Google Scholar]
- 53.Bettermann K., Arnold A. M., Williamson J., Rapp S., Sink K., Toole J. F., Carlson M. C., Yasar S., Dekosky S., Burke G. L. (2012) Statins, risk of dementia, and cognitive function: secondary analysis of the ginkgo evaluation of memory study. J. Stroke Cerebrovasc. Dis. 21, 436–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Padurariu M., Ciobica A., Mavroudis I., Fotiou D., Baloyannis S. (2012) Hippocampal neuronal loss in the CA1 and CA3 areas of Alzheimer’s disease patients. Psychiatr. Danub. 24, 152–158 [PubMed] [Google Scholar]
- 55.Kril J. J., Patel S., Harding A. J., Halliday G. M. (2002) Patients with vascular dementia due to microvascular pathology have significant hippocampal neuronal loss. J. Neurol. Neurosurg. Psychiatry 72, 747–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Holmberg E., Nordstrom T., Gross M., Kluge B., Zhang S. X., Doolen S. (2006) Simvastatin promotes neurite outgrowth in the presence of inhibitory molecules found in central nervous system injury. J. Neurotrauma 23, 1366–1378 [DOI] [PubMed] [Google Scholar]
- 57.Dao A. T., Zagaar M. A., Alkadhi K. A. (2015) Moderate treadmill exercise protects synaptic plasticity of the dentate gyrus and related signaling cascade in a rat model of Alzheimer’s disease. Mol. Neurobiol. 52, 1067–1076; erratum: 55, 901 [DOI] [PubMed] [Google Scholar]
- 58.De Assis G. G., de Almondes K. M. (2017) Exercise-dependent BDNF as a modulatory factor for the executive processing of individuals in course of cognitive decline. A systematic review. Front. Psychol. 8, 584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Diederich K., Bastl A., Wersching H., Teuber A., Strecker J. K., Schmidt A., Minnerup J., Schäbitz W. R. (2017) Effects of different exercise strategies and intensities on memory performance and neurogenesis. Front. Behav. Neurosci. 11, 47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stapleton P. A., Goodwill A. G., James M. E., Brock R. W., Frisbee J. C. (2010) Hypercholesterolemia and microvascular dysfunction: interventional strategies. J. Inflamm. (Lond.) 7, 54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kitayama J., Faraci F. M., Lentz S. R., Heistad D. D. (2007) Cerebral vascular dysfunction during hypercholesterolemia. Stroke 38, 2136–2141 [DOI] [PubMed] [Google Scholar]
- 62.Heverin M., Maioli S., Pham T., Mateos L., Camporesi E., Ali Z., Winblad B., Cedazo-Minguez A., Björkhem I. (2015) 27-hydroxycholesterol mediates negative effects of dietary cholesterol on cognition in mice. Behav. Brain Res. 278, 356–359 [DOI] [PubMed] [Google Scholar]
- 63.Martín M. G., Pfrieger F., Dotti C. G. (2014) Cholesterol in brain disease: sometimes determinant and frequently implicated. EMBO Rep. 15, 1036–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Franciosi S., Gama Sosa M. A., English D. F., Oler E., Oung T., Janssen W. G., De Gasperi R., Schmeidler J., Dickstein D. L., Schmitz C., Gandy S., Hof P. R., Buxbaum J. D., Elder G. A. (2009) Novel cerebrovascular pathology in mice fed a high cholesterol diet. Mol. Neurodegener. 4, 42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hohsfield L. A., Daschil N., Orädd G., Strömberg I., Humpel C. (2014) Vascular pathology of 20-month-old hypercholesterolemia mice in comparison to triple-transgenic and APPSwDI Alzheimer’s disease mouse models. Mol. Cell. Neurosci. 63, 83–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen B. R., Kozberg M. G., Bouchard M. B., Shaik M. A., Hillman E. M. (2014) A critical role for the vascular endothelium in functional neurovascular coupling in the brain. J. Am. Heart Assoc. 3, e000787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Czuba E., Steliga A., Lietzau G., Kowiański P. (2017) Cholesterol as a modifying agent of the neurovascular unit structure and function under physiological and pathological conditions. Metab. Brain Dis. 32, 935–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barnes J. N., Corkery A. T. (2018) Exercise improves vascular function, but does this translate to the brain? Brain Plast. 4, 65–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brugniaux J. V., Marley C. J., Hodson D. A., New K. J., Bailey D. M. (2014) Acute exercise stress reveals cerebrovascular benefits associated with moderate gains in cardiorespiratory fitness. J. Cereb. Blood Flow Metab. 34, 1873–1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Murrell C. J., Cotter J. D., Thomas K. N., Lucas S. J., Williams M. J., Ainslie P. N. (2013) Cerebral blood flow and cerebrovascular reactivity at rest and during sub-maximal exercise: effect of age and 12-week exercise training. Age (Dordr.) 35, 905–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McFarland A. J., Anoopkumar-Dukie S., Arora D. S., Grant G. D., McDermott C. M., Perkins A. V., Davey A. K. (2014) Molecular mechanisms underlying the effects of statins in the central nervous system. Int. J. Mol. Sci. 15, 20607–20637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schreurs B. G. (2010) The effects of cholesterol on learning and memory. Neurosci. Biobehav. Rev. 34, 1366–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Angelopoulos P., Agouridaki H., Vaiopoulos H., Siskou E., Doutsou K., Costa V., Baloyiannis S. I. (2008) Cytokines in Alzheimer’s disease and vascular dementia. Int. J. Neurosci. 118, 1659–1672 [DOI] [PubMed] [Google Scholar]
- 74.Harris J. J., Attwell D. (2012) The energetics of CNS white matter. J. Neurosci. 32, 356–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Smit R. A., Trompet S., Sabayan B., le Cessie S., van der Grond J., van Buchem M. A., de Craen A. J., Jukema J. W. (2016) Higher visit-to-visit low-density lipoprotein cholesterol variability is associated with lower cognitive performance, lower cerebral blood flow, and greater white matter hyperintensity load in older Subjects. Circulation 134, 212–221 [DOI] [PubMed] [Google Scholar]
- 76.Prins N. D., Scheltens P. (2015) White matter hyperintensities, cognitive impairment and dementia: an update. Nat. Rev. Neurol. 11, 157–165 [DOI] [PubMed] [Google Scholar]
- 77.Shobin E., Bowley M. P., Estrada L. I., Heyworth N. C., Orczykowski M. E., Eldridge S. A., Calderazzo S. M., Mortazavi F., Moore T. L., Rosene D. L. (2017) Microglia activation and phagocytosis: relationship with aging and cognitive impairment in the rhesus monkey. Geroscience 39, 199–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Raj D., Yin Z., Breur M., Doorduin J., Holtman I. R., Olah M., Mantingh-Otter I. J., Van Dam D., De Deyn P. P., den Dunnen W., Eggen B. J. L., Amor S., Boddeke E. (2017) Increased white matter inflammation in aging- and Alzheimer’s disease brain. Front. Mol. Neurosci. 10, 206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hillis J. M., Davies J., Mundim M. V., Al-Dalahmah O., Szele F. G. (2016) Cuprizone demyelination induces a unique inflammatory response in the subventricular zone. J. Neuroinflammation 13, 190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Boyle P. A., Yu L., Fleischman D. A., Leurgans S., Yang J., Wilson R. S., Schneider J. A., Arvanitakis Z., Arfanakis K., Bennett D. A. (2016) White matter hyperintensities, incident mild cognitive impairment, and cognitive decline in old age. Ann. Clin. Transl. Neurol. 3, 791–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kantarci K., Murray M. E., Schwarz C. G., Reid R. I., Przybelski S. A., Lesnick T., Zuk S. M., Raman M. R., Senjem M. L., Gunter J. L., Boeve B. F., Knopman D. S., Parisi J. E., Petersen R. C., Jack C. R., Jr., Dickson D. W. (2017) White-matter integrity on DTI and the pathologic staging of Alzheimer’s disease. Neurobiol. Aging 56, 172–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang X., Zhang S., Lin F., Chu W., Yue S. (2015) Elevated galectin-3 levels in the serum of patients with Alzheimer’s disease. Am. J. Alzheimers Dis. Other Demen. 30, 729–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rahimian R., Béland L. C., Kriz J. (2018) Galectin-3: mediator of microglia responses in injured brain. Drug Discov. Today 23, 375–381 [DOI] [PubMed] [Google Scholar]
- 84.Voss M. W., Heo S., Prakash R. S., Erickson K. I., Alves H., Chaddock L., Szabo A. N., Mailey E. L., Wójcicki T. R., White S. M., Gothe N., McAuley E., Sutton B. P., Kramer A. F. (2013) The influence of aerobic fitness on cerebral white matter integrity and cognitive function in older adults: results of a one-year exercise intervention. Hum. Brain Mapp. 34, 2972–2985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stein A., Stroobants S., Gieselmann V., D’Hooge R., Matzner U. (2015) Anti-inflammatory therapy with simvastatin improves neuroinflammation and CNS function in a mouse model of metachromatic leukodystrophy. Mol. Ther. 23, 1160–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sun Z. Y., Gu H. S., Chen X., Zhang L., Li X. M., Zhang J. W., Li L. (2017) A novel flavanone derivative ameliorates cuprizone-induced behavioral changes and white matter pathology in the brain of mice. Psychiatry Res. 257, 249–259 [DOI] [PubMed] [Google Scholar]