Abstract
Interorgan communication mediated by secreted proteins plays a pivotal role in metabolic homeostasis, yet the function of many circulating secretory proteins remains unknown. Here, we describe the function of protease-associated domain-containing 1 (PRADC1), an enigmatic secretory protein widely expressed in humans and mice. In metabolically active tissues (liver, muscle, fat, heart, and kidney), we showed that Pradc1 expression is significantly suppressed by refeeding and reduced in kidney and brown fat in the context of obesity. PRADC1 is dispensable for whole-body metabolism when mice are fed a low-fat diet. However, in obesity induced by high-fat feeding, PRADC1-deficient female mice have reduced weight gain and adiposity despite similar caloric intake. Decreased fat mass is attributed, in part, to increased metabolic rate, physical activity, and energy expenditure in these animals. Reduced adiposity in PRADC1-deficient mice, however, does not improve systemic glucose and lipid metabolism, insulin sensitivity, liver steatosis, or adipose inflammation. Thus, in PRADC1-deficient animals, decreased fat mass and enhanced physical activity are insufficient to confer a healthy metabolic phenotype in the context of an obesogenic diet. Our results shed light on the physiologic function of PRADC1 and the complex regulation of metabolic health.—Rodriguez, S., Stewart, A. N., Lei, X., Cao, X., Little, H. C., Fong, V., Sarver, D. C., Wong, G. W. PRADC1: a novel metabolic-responsive secretory protein that modulates physical activity and adiposity.
Keywords: lipid metabolism, obesity, diabetes, insulin resistance
Regulation of systemic metabolism and energy balance requires integrative mechanisms involving multiple tissues and organs. Secreted hormones mediate interorgan communication necessary to maintain energy homeostasis. Although >3000 human genes encode proteins with a signal peptide for secretion (1–3), and mass spectrometry can detect over 3000 proteins in plasma (3), the function of most of the secretory proteins in circulation remains poorly defined or unknown.
To uncover novel secreted metabolic regulators, we assessed the expression of candidate genes that encode for secretory proteins with unknown functions. We determined the candidate genes whose expression is responsive to metabolic and nutritional cues, suggestive of involvement in metabolic processes. Here, we focus our functional studies on one such gene, protease-associated domain-containing 1 (Pradc1), and show that it encodes a novel secreted regulator of energy metabolism.
PRADC1 (also known as PAP21) is an ∼30-kDa N-glycosylated secretory protein with a protease-associated domain (4). An ortholog encoding PRADC1 is found in Drosophila, worms (Caenorhabditis elegans), zebrafish, Xenopus, mice, and humans. Although its evolutionary conservation across species (4) suggests a basic necessity for the gene, the biologic function of PRADC1 remains elusive. We found that PRADC1 is widely expressed in both human and mouse tissues and that its expression in metabolically active tissues is acutely regulated by food restriction and refeeding while being chronically altered in a diet-induced obese mouse model. Using a genetic loss-of-function mouse model, we demonstrated an important sex-dependent role for PRADC1 in modulating physical activity and adiposity in the context of obesity induced by high-fat feeding. Despite reduced body weight and adiposity, PRADC1-deficient female mice fed a high-fat diet (HFD) did not exhibit an improved metabolic profile compared with wild-type (WT) littermates. Thus, PRADC1-deficient mice appear to have reduced adiposity and enhanced locomotor activity uncoupled from metabolic health. Our mouse model establishes the biologic function of PRADC1, providing a physiologic context to further understand its mechanisms of action.
MATERIALS AND METHODS
Gene expression profiling
Gene expression profiling was performed using the TissueScan (OriGene Technologies, Rockville, MD, USA) human major tissue quantitative PCR array (HMRT303) and mouse tissues from WT C57BL/6J mice. Primers used in real-time PCR analysis were as follows: human PRADC1 forward, 5′-CACGGCTTCCGTATCCATGAT-3′, and reverse, 5′-GGTGAATCTGCTCATACCTTGTG-3′; mouse Pradc1 forward, 5′-TGAGTCCTGGGGATATTCGATAC-3′, and reverse, 5′-CAGCAGGGACGAGGTGAATC-3′. For human PRADC1, all expression data were normalized to glyceraldehyde 3-phosphate dehydrogenase. For mouse Pradc1, all expression data were normalized to β-actin.
Animals
C57BL/6J male mice (8 wk old) were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). Mouse tissues from different brain regions were collected from unfed and refed experiments. For the unfed group, food was removed for 16 h (beginning 10 h into the light cycle), and mice were euthanized 2 h into the light cycle. For the refed group, mice were unfed for 16 h and refed with chow pellets for 3 h before being euthanized. The Pradc1 null mouse strain [Pradc1tm1(KOMP)Vlcg] was generated by Regeneron Pharmaceuticals (Tarrytown, NY, USA). Live mice were generated from Pradc1+/− sperm obtained from the Knockout Mouse Project (KOMP) Repository (www.komp.org). Mouse Pradc1 gene is located on chromosome 6; the gene consists of 3 exons and 2 introns and is 4516 bp. To generate PRADC1-deficient mice, a total of 4095 bp that comprise the entire coding region of the gene (from immediately downstream of the ATG start codon to part of the 3′UTR) were replaced with a targeting cassette containing a β-galactosidase reporter gene, β-galactosidase (LacZ), and a neomycin-resistant gene. Only the 5′ and 3′ UTRs of Pradc1 gene remained after replacement with the cassette. This strategy ensured that knockout (KO) mice were completely devoid of PRADC1 protein. Genotyping primers for the Pradc1 WT allele were as follows: forward, 5′-TCTGGAGGTTCAGCAGGGAC-3′, and reverse, 5′-CCTGGGAGGCAGAGCTAGATTC-3′. The size of the amplified WT PCR product was 388 bp. Primers for the KO allele were as follows: LacZ-forward, 5′-GGTAAACTGGCTCGGATTAGGG-3′, and LacZ-reverse, 5′-TTGACTGTAGCGGCTGATGTTG-3′. The size of the amplified KO PCR product was 211 bp. Pradc1 KO mice were generated on a C57BL/6N genetic background. All mice used in this study were generated by intercrossing Pradc1+/− mice to obtain KO and WT littermates, and at least 2 independent cohorts of mice were used per diet group. Pradc1 KO−/− mice and WT+/+ littermates were housed together in polycarbonate cages on a 12-h light-dark photocycle with ad libitum access to water and food, with no more than 5 adult mice per cage. Mice were fed an HFD (60% kcal derived from fat, D12492; Research Diets, New Brunswick, NJ, USA) or a matched control low-fat diet (LFD; 10% kcal derived from fat, D12450B; Research Diets). Diet was provided at the time of weaning (at 4 wk of age). At termination of the study, animals were unfed for 16 h, except for the HFD-fed Pradc1 KO female mice, which were unfed for 2 h before euthanasia. Tissues were collected, snap frozen in liquid nitrogen, and kept at −80°C until analysis. All animal protocols were submitted to and approved by the Institutional Animal Care and Use Committee of The Johns Hopkins University School of Medicine (MO16M431).
Serum collection and analysis
Blood samples were collected by tail vein bleeding, and serum was isolated using Microvette CB 300 (Sarstedt, Newton, NC, USA) per the manufacturer’s instructions. Serum triglyceride and cholesterol levels were measured using an Infinity kit (Thermo Fisher Scientific, Waltham, MA, USA) per the manufacturer’s instructions. Serum nonesterified free fatty acids (NEFAs) were measured using an NEFA-HR (2) kit (Wako Chemicals, Richmond, VA, USA). Serum β-hydroxybutyrate was measured using a LiquiColor assay (Stanbio, Boerne, TX, USA).
ELISAs
Serum insulin and fibroblast growth factor 21 (FGF21), adiponectin (EZMADP-60K), and leptin (EZML-82K) levels were measured using Millipore ELISA kits (MilliporeSigma, Burlington, MA, USA). Serum corticosterone was obtained using the Mouse/Rat Corticosterone kit from ALPCO (Salem, NH, USA). Serum T3, T4, and thyroid-stimulating hormone levels were obtained using the Milliplex Map Rat Thyroid Hormone Magnetic Bead Panel (MilliporeSigma). For the multiplex assay, standard curves were generated for each hormone. Serum was diluted according to the manufacturer’s instructions. Samples and standards were analyzed using a Luminex 200 instrument (Luminex, Austin, TX, USA) and XPonent 3.1 software (MilliporeSigma).
Glucose and insulin tolerance test
For glucose tolerance tests, food was removed for 16 h (overnight) before mice were intraperitoneally injected with a dose of 1 g glucose/kilogram body weight diluted in 0.9% sterile filtered saline. Blood glucose was measured at 0, 15, 30, 60, and 120 min after glucose injection using a glucometer (NovaMax Plus, Billerica, MA, USA). For insulin tolerance tests, mice were unfed for 2 h before receiving an intraperitoneal injection of insulin at a dose of 0.75 U/kg body weight (LFD males and females, HFD females) or 1.5 U/kg body weight (HFD males). Blood glucose was measured at 0, 15, 30, 60, and 90 min after insulin injection as described above.
Hepatic lipid extraction
Livers were harvested from the HFD-fed Pradc1 KO female mice following a 2-h food removal. Tissues were collected, flash frozen in liquid nitrogen, and stored at −80°C. Liver tissue (50–100 mg) was homogenized in 500 μl of ice-cold distilled water. A portion of the homogenate (200 μl) was collected for lipid extraction by mixing with 1 ml of 2:1 chloroform:methanol and centrifuged at 313 g for 5 min at 4°C. The chloroform phase was collected and dried under vacuum. Samples were reconstituted in a mixture of 3:1:1 tert-butanol:MeOH:Triton X-100 before triglyceride and cholesterol content was determined using Infinity kits as described above. Samples were normalized to mass content.
Adipose tissue lipolysis
Mice were unfed for 2 h before receiving an intraperitoneal injection of the β3-adrenergic receptor agonist, CL 316243 (Santa Cruz Biotechnology, Dallas, TX, USA), at a dose of 1 mg/kg body weight. Blood glucose was measured with a glucometer before and 15 min postinjection. Blood was collected by the tail vein into Microvette CB 300 capillary tubes before and 15 min postinjection. Serum was isolated and assayed for NEFA (Wako Diagnostics, Mountain View, CA, USA) and glycerol (MilliporeSigma) content following the manufacturer’s instructions.
Body composition analysis
Body composition analyses for fat and lean mass were performed on mice at 24 wk of age using Echo-Magnetic Resonance Imaging (MRI)-100 (Echo Medical Systems, Waco, TX, USA) at The Johns Hopkins University School of Medicine mouse-phenotyping core facility.
Indirect calorimetry
HFD-fed WT and KO female mice at 24 wk of age were assessed daily for body weight changes, food intake (corrected for spillage), physical activity, and whole-body metabolic profiling in the Comprehensive Laboratory Animal Monitoring System (Columbus Instruments, Columbus, OH, USA) as previously described (5). Data were collected for 4 d to confirm that mice were acclimated to the calorimetry chambers (indicated by stable body weights, food intake, and diurnal metabolic patterns), and data were analyzed from the fourth day.
Histology and analysis of crown-like structures of adipose tissue
Gonadal white adipose tissue (gWAT) and inguinal white adipose tissue (iWAT) from WT and Pradc1 KO mice were fixed immediately in 10% formalin at 4°C following dissection. Fixed tissues were embedded in paraffin, sectioned, and stained with hematoxylin and eosin at the Histology Reference Laboratory at The Johns Hopkins University School of Medicine. Images were captured with an Axioplan upright microscope with an Axiocam color charge-coupled device camera (Carl Zeiss GmbH, Oberkochen, Germany). To quantify the crown-like structures (CLSs), an RGB image (1378 × 1091.8 μm) with a set scale of 1000 pixels = 1060 μm was used. CLSs were identified as clusters of macrophages that had infiltrated the adipose tissue and formed ring-like structures. The number of CLSs was manually counted in 5 random fields for each section. Nine mice in each group were sectioned and counted.
Adipocyte cell size analysis
Adipocyte size was measured using MRI Adipocyte Tools’ macro in ImageJ software [National Institutes of Health (NIH), Bethesda, MD, USA] as previously described (6).
Blood and tissue collection
WT and Pradc1 KO mice were unfed for 2 h and anesthetized with isoflurane, and blood was collected through the retro-orbital plexus using capillary tubes (Thermo Fisher Scientific). Tissues were immediately harvested from euthanized mice, flash frozen in liquid nitrogen, and stored at −80°C until further processing.
Real-time quantitative PCR analysis
Total RNA was isolated from tissues using Trizol (Thermo Fisher Scientific) according to the manufacturer’s instructions. Samples were treated with DNAseI (New England Biolabs, Ipswich, MA, USA) to remove genomic DNA contamination and subjected to 3 M (pH 5.2) sodium acetate (Quality Biologic, Gaithersburg, MD, USA) precipitation to purify RNA samples. In brief, sodium acetate was added at one-tenth the volume of the sample, followed by the addition of 2.5 volumes of ice-cold 100% ethanol, and mixed thoroughly. Samples were incubated at −80°C overnight and then centrifuged at 12,000 g for 20 min at 4°C, and the supernatant was decanted. The RNA pellets were washed twice using 1 ml of ice-cold 75% ethanol and centrifuged at 12,000 g at 4°C. A quick spin was performed to remove any traces of residual ethanol in the pellet using a very fine pipette tip. Purified RNA was reverse transcribed using the GoScript Reverse transcription system (Promega, Madison, WI, USA). Real-time quantitative PCR analyses were performed on a CFX Connect system (Bio-Rad, Hercules, CA, USA) using iTaq Universal Sybr Green Supermix (Bio-Rad) per the manufacturer’s directions. Data were normalized to 36B4 (adipose tissue), 18S rRNA (skeletal muscle), and β-actin (liver) and expressed as relative mRNA levels using the ΔΔCt method (7). All of the primers used were previously published (5, 6, 8).
Statistical analysis
Comparisons between 2 groups of data were performed using 2-tailed Student’s t tests with 95% confidence intervals, and ANOVAs were used to make comparisons involving more than 2 groups. Prism 7 (GraphPad Software, La Jolla, CA, USA) was used for statistical analyses, and values were considered to be statistically significant at P < 0.05. All data are presented as means ± se.
RESULTS
Expression of Pradc1 transcript in metabolically active tissues is regulated by the nutritional state of the animal
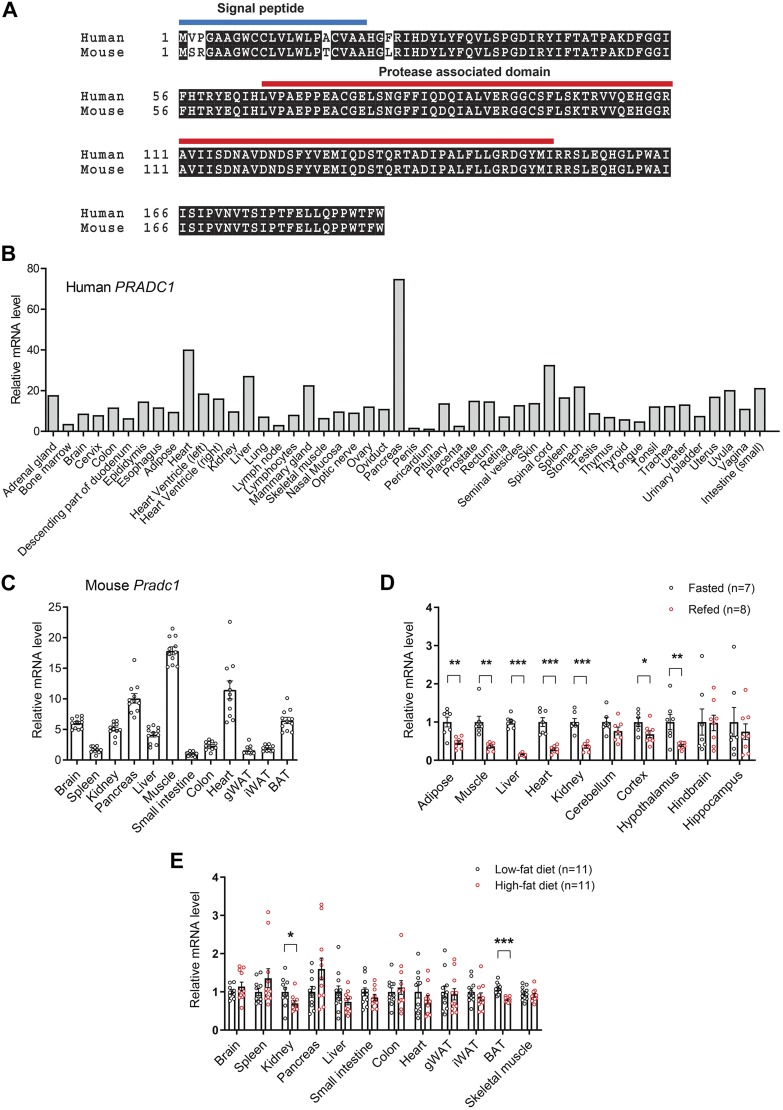
Human and mouse PRADC1 protein are highly conserved (Fig. 1A), differing by only 1 aa in the mature protein (excluding the signal peptide). Both human and mouse PRADC1 were widely expressed among a variety of tissues (Fig. 1B, C). Expression of Pradc1 in metabolically active tissues (liver, muscle, adipose, and heart), as well as different brain regions (cortex and hypothalamus), was acutely regulated by the nutritional state. In the refed state (overnight food restriction followed by a 3-h food intake), we observed a striking suppression of Pradc1 mRNA in different tissues and brain regions of normal WT chow-fed mice (Fig. 1D). In the context of obesity induced by an HFD, the expression of Pradc1 was also reduced in kidney and brown adipose tissue (BAT) relative to mice fed a control LFD (Fig. 1E). These data suggest a potential metabolic function for PRADC1.
Figure 1.
Expression and regulation of Pradc1 in various tissues under different physiologic states. A) Sequence alignment of full-length human (NP_115695) and mouse (NP_001156899) PRADC1 proteins. The predicted signal peptide and protease-associated domain are indicated. B) Expression of PRADC1 in human tissue panel. C) Expression of Pradc1 in mouse tissues (n = 11). D) Real-time PCR analysis of the expression of Pradc1 in different tissues and brain regions from mice subjected to overnight food restriction (unfed group; n = 7) or overnight food restriction followed by 3 h of refeeding (refed group; n = 8). E) Real-time PCR analysis of the expression of Pradc1 in different tissues from mice fed a control LFD (n = 11) or an HFD (n = 11). Expression levels were normalized to β-actin. Data are expressed as means ± sem. *P < 0.05, **P < 0.01, ***P < 0.001.
Loss-of-function mouse model for PRADC1
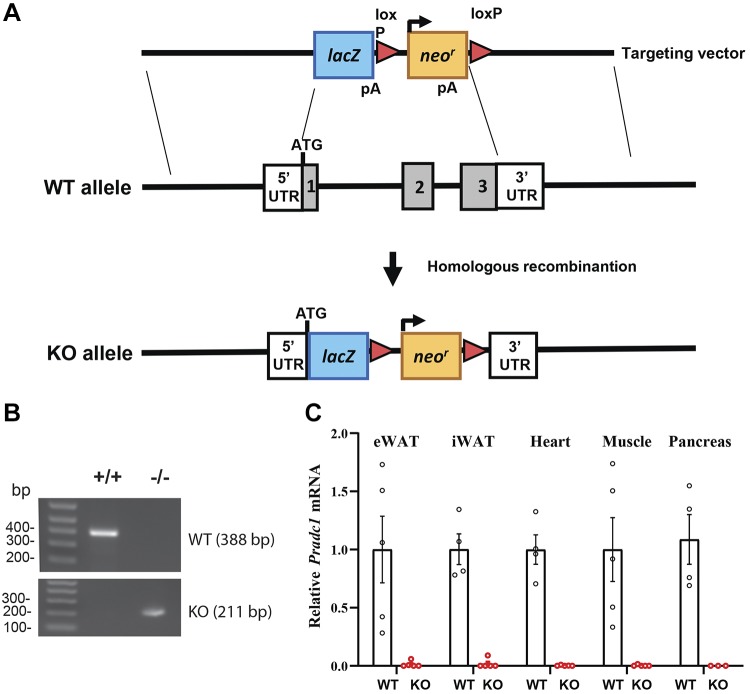
We employed a whole-body KO mouse model to determine the requirement for PRADC1 in metabolic homeostasis. The gene-targeting strategy involved the replacement of the entire protein-coding region of PRADC1 with the LacZ and neomycin-resistance cassette (Fig. 2A). Our PCR analysis confirmed the genotype of the WT and KO mice (Fig. 2B). As expected, in the KO mice, Pradc1 transcript was not detected in the adipose tissue, heart, muscle, or pancreas (Fig. 2C). Pradc1 KO mice of both sexes were born at the expected Mendelian ratio, and the animals appeared normal with no gross developmental abnormalities.
Figure 2.
Generation of Pradc1 KO mice. A) Schematic showing the gene-targeting strategy used to generate Pradc1 KO mice. B) PCR genotyping results showing the successful generation of WT+/+ and homozygous KO−/− mice. C) The absence of Pradc1 mRNA in mouse tissues of KO mice was confirmed by real-time PCR. eWAT, epididymal white adipose tissue; loxP, cleavage sequence for Cre recombinase; Neor, neomycin resistant; pA, polyadenylation signal.
PRADC1 deficiency is dispensable for metabolic homeostasis under basal conditions
When fed a control LFD, WT and Pradc1 KO mice of either sex gained similar weight over time, had no differences in insulin sensitivity as measured by glucose and insulin tolerance tests, and exhibited no differences in fasting blood glucose or serum triglyceride, cholesterol, NEFAs, or β-hydroxybutyrate (Supplemental Figs. S1 and S2). Only LFD-fed Pradc1 KO male mice showed increased fasting NEFA levels compared with WT mice (Supplemental Fig. S1G). These data indicate that PRADC1 is dispensable for glucose and lipid metabolism under the basal condition in which mice were consuming a control LFD. We also observed no differences in organ weights and blood glucose levels between genotypes of either sex (Table 1).
TABLE 1.
Body weight, organ weight, and fed glucose levels in WT and Pradc1 KO male and female mice fed an LFD or HFD
| Parameter | Male |
Female |
||||
|---|---|---|---|---|---|---|
| WT | KO | P | WT | KO | P | |
| LFDa | N = 8 | N = 10 | N = 10–15 | N = 10–13 | ||
| Body weight (g) | 35.97 ± 1.154 | 36.23 ± 1.197 | 0.8804 | 27.83 ± 1.073 | 28.52 ± 1.258 | 0.6778 |
| Gonadal fat mass (g) | 0.83 ± 0.081 | 0.70 ± 0.063 | 0.2145 | 0.68 ± 0.069 | 0.77 ± 0.097 | 0.4386 |
| Gonadal fat mass/body weight | 0.02 ± 0.002 | 0.02 ± 0.002 | 0.261 | 0.02 ± 0.002 | 0.02 ± 0.002 | 0.5576 |
| Inguinal fat mass (g) | 0.68 ± 0.0651 | 0.69 ± 0.069 | 0.8791 | 0.35 ± 0.048 | 0.35 ± 0.049 | 0.9657 |
| Inguinal fat mass/body weight | 0.02 ± 0.001 | 0.02 ± 0.001 | 0.8853 | 0.012 ± 0.001 | 0.01 ± 0.001 | 0.7582 |
| Liver weight (g) | 1.41 ± 0.110 | 1.73 ± 0.234 | 0.271 | 0.96 ± 0.064 | 1.17 ± 0.112 | 0.1094 |
| Liver weight/body weight | 0.04 ± 0.002 | 0.05 ± 0.005 | 0.1877 | 0.03 ± 0.002 | 0.04 ± 0.004 | 0.0928 |
| Kidney (g) | 0.15 ± 0.006 | 0.17 ± 0.006 | 0.0341 | 0.10 ± 0.015 | 0.10 ± 0.011 | 0.9007 |
| Kidney/body weight | 0.004 ± 0.000 | 0.005 ± 0.000 | 0.108 | 0.004 ± 0.000 | 0.004 ± 0.000 | 0.8996 |
| Fed blood glucose (mg/dl) | 134.60 ± 9.371 | 144.2 ± 6.625 | 0.4039 | 112.3 ± 6.854 | 119.3 ± 8.735 | 0.5303 |
| HFDb | N = 10 | N = 6 | N = 9 | N = 12 | ||
| Body weight (g) | 49.9 ± 1.342 | 47.24 ± 2.225 | 0.291 | 55.17 ± 2.214 | 46.94 ± 1.831 | 0.0095 |
| Gonadal fat mass (g) | 0.66 ± 0.066 | 0.52 ± 0.092 | 0.2502 | 3.67 ± 0.234 | 2.94 ± 0.209 | 0.0324 |
| Gonadal fat mass/body weight | 0.01 ± 0.001 | 0.01 ± 0.002 | 0.2964 | 0.07 ± 0.002 | 0.06 ± 0.002 | 0.2382 |
| Inguinal fat mass (g) | 1.25 ± 0.066 | 1.11 ± 0.134 | 0.2925 | 1.54 ± 0.084 | 1.23 ± 0.088 | 0.0205 |
| Inguinal fat mass/body weight | 0.03 ± 0.001 | 0.02 ± 0.002 | 0.3365 | 0.03 ± 0.001 | 0.026 ± 0.002 | 0.3811 |
| Liver weight (g) | 2.73 ± 0.136 | 2.68 ± 0.268 | 0.8766 | 1.51 ± 0.081 | 1.46 ± 0.085 | 0.6713 |
| Liver weight/body weight | 0.05 ± 0.002 | 0.06 ± 0.005 | 0.6674 | 0.03 ± 0.001 | 0.03 ± 0.002 | 0.0761 |
| Kidney (g) | 0.20 ± 0.005 | 0.21 ± 0.014 | 0.4527 | N/A | N/A | N/A |
| Kidney/body weight | 0.004 ± 0.000 | 0.004 ± 0.001 | 0.2239 | N/A | N/A | N/A |
| Fed blood glucose (mg/dl) | 162.2 ± 5.251 | 169.5 ± 15.48 | 0.599 | 178.8 ± 7.59 | 178 ± 5.451 | 0.9327 |
Male = 45 wk; female = 47 wk. bMale = 38 wk; female = 45 wk. N/A, not applicable.
Loss of PRADC1 reduces weight gain in KO female mice fed an HFD by elevating physical activity and energy expenditure
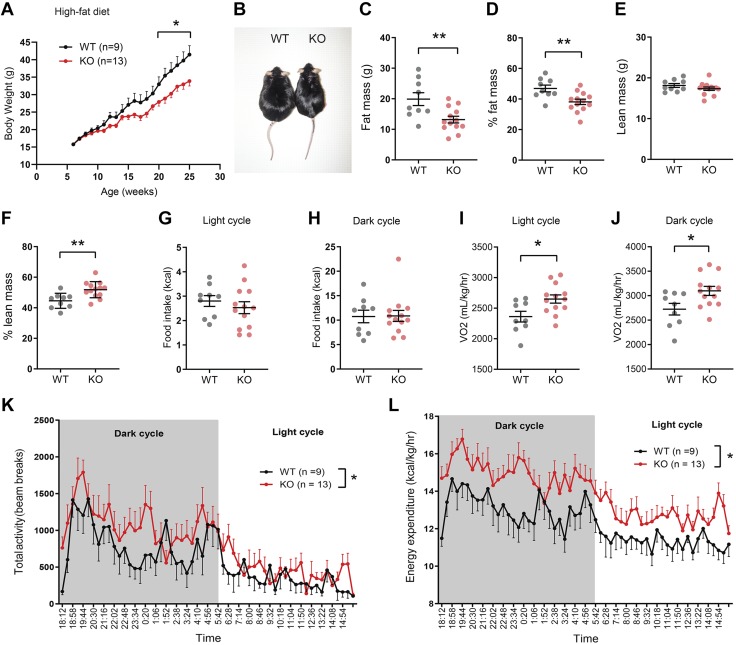
Next, we addressed whether loss of PRADC1 affects the physiologic response to HFD-induced obesity. In male mice fed an HFD, body weight over time, glucose and insulin tolerance tests, and fasting blood glucose and serum triglyceride, cholesterol, NEFAs, and ketone (β-hydroxybutyrate) levels were not significantly different between WT and Pradc1 KO animals (Supplemental Fig. S3 and Table 1). In striking contrast, Pradc1 KO female mice gained significantly less body weight over time when fed an HFD (Fig. 3A, B and Table 1). Reduced body weight was attributed to reduced fat mass (Fig. 3C, D) and not changes in lean mass (Fig. 3E). When normalized to body weight, Pradc1 KO female mice had a higher percentage of lean mass (Fig. 3F). Food intake was not significantly different between WT and Pradc1 KO female mice (Fig. 3G, H). Decreased adiposity seen in the Pradc1 KO mice was due to increased metabolic rate as measured by the rate of oxygen consumption (Fig. 3I, J), enhanced physical activity (Fig. 3K), and enhanced energy expenditure (Fig. 3L). These data indicate that PRADC1 deficiency impacted whole-body energy balance in a sex-dependent manner. Altered thyroid hormones, stress hormone, and FGF21 could potentially increase physical activity and/or energy expenditure in Pradc1 KO female mice. However, serum T3, T4, thyroid-stimulating hormone, corticosterone, and FGF21 levels were not significantly different between WT and Pradc1 KO female mice (Supplemental Fig. S4A–E). Because Pradc1 was expressed in BAT and its expression was reduced in BAT in diet-induced obese mice (Fig. 1), we determined whether there are any changes in uncoupling activity that could lead to greater energy expenditure. Increased BAT thermogenic activity would be reflected in increased body temperature. Measurements of body temperature did not reveal significant differences between genotypes (Supplemental Fig. S4F). The expression of uncoupling protein 1 (ucp1) in BAT and iWAT was in fact lower in the KO mice relative to WT littermates (Supplemental Fig. S4G); thus, BAT activation or browning of iWAT is unlikely to be the mechanism responsible for increased energy expenditure and reduced body weight seen in the HFD-fed KO female mice.
Figure 3.
Reduced adiposity and enhanced physical activity and energy expenditure in Pradc1 KO female mice fed an HFD. A) Body weight gain over time in WT and Pradc1 KO female mice fed an HFD. B) Representative image of WT and KO female mice at 25 wk old. C) Whole-body fat mass of WT and KO female mice as determined by Echo-MRI. D) Percent fat mass relative to body weight. E) Whole-body lean mass of WT and KO female mice as determined by nuclear magnetic resonance. F) Percent lean mass relative to body weight. G, H) Food intake during the light (G) and dark (H) cycle. I, J) Rate of oxygen consumption (Vo2) as a measure of metabolic rate in WT and KO female mice during the light (I) and dark (J) cycle. K) Total physical activity as measured by beam breaks in WT and KO female mice during the light and dark cycle. L) Energy expenditure of WT and KO female mice during the light and dark cycle. Female mice: WT, n = 9; KO, n = 13. Mice were at 25–26 wk of age when indirect calorimetry analyses were performed. Data are expressed as means ± sem. *P < 0.05, **P < 0.01.
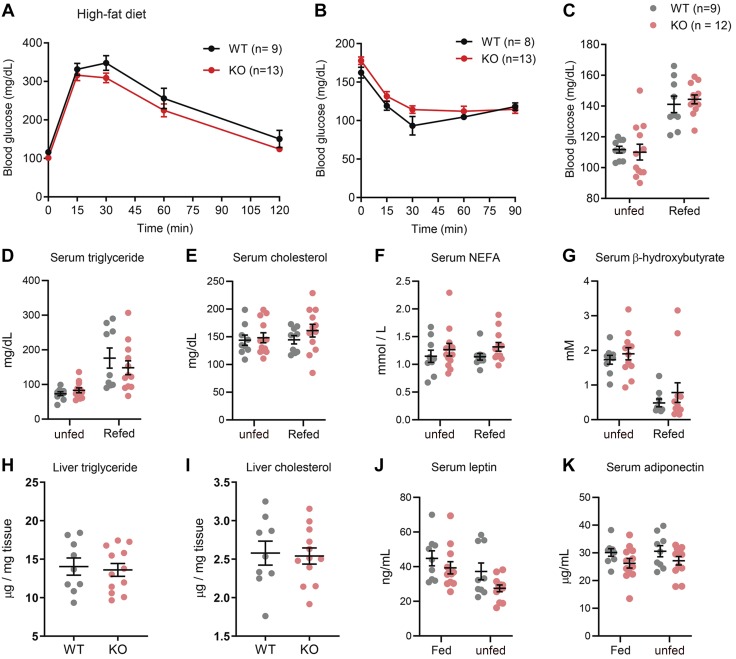
Reduced body weight and adiposity in Pradc1 KO female mice did not alter whole-body glucose or lipid metabolism
Given that Pradc1 KO female mice had significantly reduced body weight and adiposity and increased physical activity and energy expenditure, we reasoned that they might have improved metabolic profiles. Contrary to expectation, insulin sensitivity as measured by glucose and insulin tolerance tests, as well as food restriction and refed blood glucose, and serum triglyceride, cholesterol, NEFA, and ketone (β-hydroxybutyrate) levels were not significantly different between HFD-fed WT and Pradc1 KO female mice (Fig. 4A–G). Hepatic triglyceride and cholesterol levels were also not significantly different between WT and KO female mice (Fig. 4H, I). We also measured the circulating levels of 2 major adipocyte-derived adipokines, leptin and adiponectin. Serum levels of leptin and adiponectin in both the fed and unfed states were not significantly different between genotypes (Fig. 4J, K).
Figure 4.
Glucose and lipid metabolism in WT and Pradc1 KO female mice fed an HFD. A, B) Glucose tolerance (A) and insulin tolerance (B) tests in WT (n = 8–9) and KO (n = 13) female mice at 18 and 19 wk of age. C–G) Overnight unfed and refed blood glucose (C), serum triglyceride (D), cholesterol (E), NEFAs (F), and β-hydroxybutyrate (G) in WT (n = 9) and KO (n = 12) female mice. H, I) Liver triglyceride (H) and cholesterol (I) levels in WT (n = 9) and KO (n = 12) female mice. J, K) Serum leptin (J) and adiponectin (K) levels in fed and overnight unfed WT (n = 9) and KO (n = 12) female mice. Animals were at 43–45 wk of age when serum metabolite and adipokines were measured. Data are expressed as means ± sem.
Impact of PRADC1 deficiency on adipose tissue histology, function, and gene expression in female mice
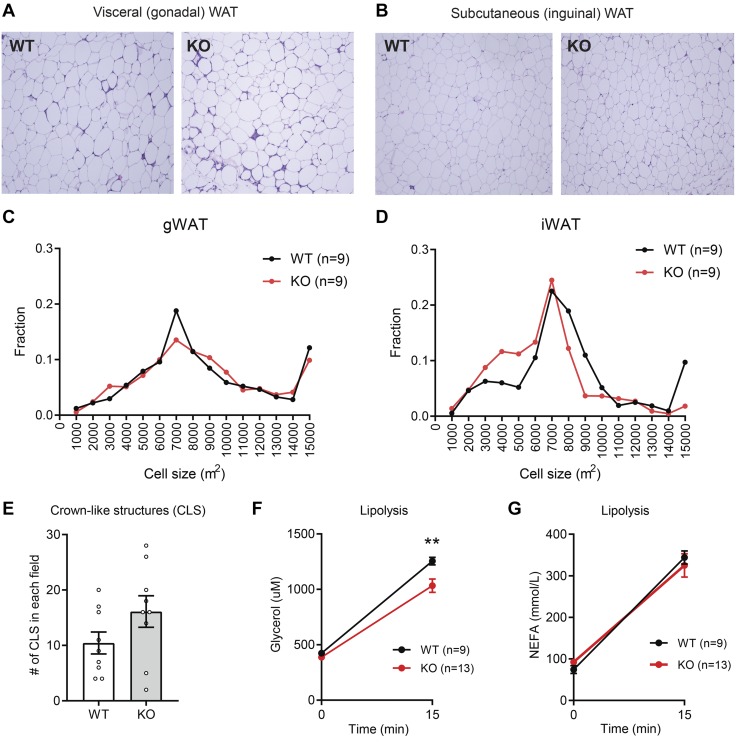
It is known that HFD-induced obesity leads to adipocyte hypertrophy and macrophage infiltration (9–11). Because Pradc1 KO female mice had reduced adiposity, we therefore determined the impact of PRADC1 deficiency on adipose tissue structure and function. Histologic analysis of visceral (gonadal) and subcutaneous (inguinal) fat depots between HFD-fed WT and Pradc1 KO female mice revealed comparable adipocyte cell size and no significant difference in the number of CLSs that marked apoptotic adipocytes surrounded by infiltrating macrophages (Fig. 5A–E). We next determined whether there were any functional differences in fat mobilization in adipose tissue between WT and KO mice in response to β3-adrenergic receptor agonist (CL 316243) stimulation. The extent of adipose tissue lipolysis, as judged by glycerol release into plasma, was reduced in Pradc1 KO female mice (Fig. 5F). Serum levels of NEFAs were not significantly different between genotypes in response to CL 316243 stimulation (Fig. 5G). The expression of fatty acid synthesis, triglyceride synthesis, and fat oxidation genes were largely unchanged in WT and Pradc1 KO female mice (Table 2). Seven out of the 15 inflammation-related genes profiled by real-time PCR were altered between WT and KO female mice in a depot-specific manner (Table 2). Specifically, the expression of Tnf-α, F4/80, and Il-10 was reduced in the visceral fat depot of KO female mice relative to WT controls. In the subcutaneous fat depot, the expression of Tnf-α, monocyte chemoattractant protein-1 (Mcp-1), C-C motif chemokine ligand (Ccl) 3, Ccl4, F4/80, and Cd68 was decreased in KO female mice compared with WT controls. Reduced inflammatory gene expression in the white adipose tissue, however, did not appear to affect serum adipokine (adiponectin and leptin) levels or whole-body lipid profiles and glucose metabolism (Fig. 4). Genes involved in ER stress (e.g., activating transcription factor 4), oxidative stress (e.g., NADH quinone dehydrogenase 1), and fibrosis (e.g., Tgf-β) were also down-regulated in the visceral or subcutaneous fat depot of Pradc1 KO female mice relative to WT littermates.
Figure 5.
Impact of PRADC1 deficiency on adipocyte size, CLSs, and adipose lipolysis in female mice fed an HFD. A, B) Representative histology (hematoxylin and eosin stained) of visceral (gWAT; A) and subcutaneous (iWAT; B) white adipose tissue of WT and KO female mice at 45 wk of age. Original magnification, ×100. C, D) Quantification of adipocyte cell size in gWAT (C) and iWAT (D) of WT (n = 9) and KO (n = 9) female mice. E) Quantification of CLSs in gWAT of WT (n = 9) and KO (n = 9) female mice. F, G) Serum glycerol (F) and NEFA (G) levels in 31-wk-old WT (n = 9) and KO (n = 13) female mice injected with a β3-adrenergic receptor agonist (CL 316243) to induce adipose lipolysis. Data are expressed as means ± sem. **P < 0.01.
TABLE 2.
Real-time PCR analysis of the expression of metabolic, inflammatory, and oxidative stress genes in visceral and subcutaneous fat depots of WT and Pradc1 KO female mice fed an HFD
| Visceral gWAT |
s.c. iWAT |
||||||
|---|---|---|---|---|---|---|---|
| Gene | Gene name | WT | KO | P | WT | KO | P |
| Fatty acid synthesis | N = 8 | N = 8 | N = 8 | N = 8 | |||
| Fasn | Fatty acid synthase | 1.00 ± 0.26 | 1.28 ± 0.41 | 0.574 | 1.00 ± 0.31 | 0.58 ± 0.19 | 0.3044 |
| Acc1 | Acetyl-CoA carboxylase | 1.00 ± 0.22 | 0.74 ± 0.16 | 0.385 | 1.00 ± 0.16 | 0.89 ± 0.32 | 0.768 |
| Scd1 | Stearoyl-CoA desaturase-1 | 1.00 ± 0.16 | 0.92 ± 0.26 | 0.816 | 1.00 ± 0.09 | 0.62 ± 0.10* | 0.022 |
| Srebp-1c | Sterol regulatory element-binding transcription factor 1C | 1.00 ± 0.11 | 1.01 ± 0.22 | 0.946 | 1.00 ± 0.34 | 0.54 ± 0.16 | 0.291 |
| Ppar-γ | Peroxisome proliferator activated receptor γ | 1.00 ± 0.16 | 0.74 ± 0.13 | 0.247 | 1.00 ± 0.20 | 0.55 ± 0.19 | 0.146 |
| Triglyceride synthesis | |||||||
| Gpat1 | Glycerol-3-phosphate acyltransferase 1 | 1.00 ± 0.11 | 1.08 ± 0.12 | 0.635 | 1.04 ± 0.08 | 0.82 ± 0.18 | 0.545 |
| Gpat2 | Glycerol-3-phosphate acyltransferase 2 | 1.00 ± 0.20 | 0.68 ± 0.16 | 0.256 | N/A | N/A | |
| Agpat1 | 1-Acyl-glycerol-3-phosphate acyltransferase 1 | 1.00 ± 0.08 | 0.89 ± 0.14 | 0.534 | 1.00 ± 0.08 | 1.47 ± 0.38 | 0.260 |
| Agpat2 | 1-Acyl-glycerol-3-phosphate acyltransferase 2 | 1.00 ± 0.20 | 1.13 ± 0.26 | 0.698 | 1.00 ± 0.22 | 0.41 ± 0.05* | 0.046 |
| Dgat1 | Diacylglycerol O-Acyltransferase 1 | 1.00 ± 0.08 | 0.89 ± 0.16 | 0.586 | 1.00 ± 0.15 | 0.71 ± 0.24 | 0.337 |
| Dgat2 | Diacylglycerol O-Acyltransferase 2 | 1.00 ± 0.12 | 1.16 ± 0.26 | 0.560 | 1.00 ± 0.19 | 0.42 ± 0.07* | 0.032 |
| Lipolysis | |||||||
| Atgl | Adipose triglyceride lipase | 1.00 ± 0.17 | 0.91 ± 0.09 | 0.663 | 1.00 ± 0.14 | 0.90 ± 0.31 | 0.776 |
| Hsl | Hormone-sensitive lipase | 1.00 ± 0.17 | 0.78 ± 0.16 | 0.389 | 1.00 ± 0.13 | 0.72 ± 0.22 | 0.317 |
| Fat oxidation | |||||||
| Ppar-α | Peroxisome proliferator activated receptor α | 1.00 ± 0.26 | 0.87 ± 0.15 | 0.708 | 1.00 ± 0.16 | 0.84 ± 0.31 | 0.672 |
| Cpt-1a | Carnitine palmitoyltransferase 1A | 1.00 ± 0.12 | 0.75 ± 0.14 | 0.205 | 1.00 ± 0.16 | 0.59 ± 0.17 | 0.112 |
| Acox1 | Acyl-CoA oxidase 1 | 1.00 ± 0.14 | 0.90 ± 0.11 | 0.600 | 1.00 ± 0.19 | 0.72 ± 0.31 | 0.479 |
| Lcad | Long chain acyl-CoA dehydrogenase | 1.00 ± 0.08 | 0.69 ± 0.12 | 0.061 | 1.00 ± 0.12 | 0.88 ± 0.33 | 0.757 |
| Mcad | Medium chain acyl-CoA dehydrogenase | 1.00 ± 0.16 | 0.78 ± 0.19 | 0.415 | 1.00 ± 0.13 | 0.89 ± 0.30 | 0.766 |
| Hadha | Trifunctional enzyme subunit α | 1.00 ± 0.11 | 0.58 ± 0.07* | 0.010 | 1.00 ± 0.13 | 0.82 ± 0.27 | 0.562 |
| Inflammation | |||||||
| Tnf-α | Tumor necrosis factor α | 1.00 ± 0.11 | 0.58 ± 0.09* | 0.013 | 1.00 ± 0.16 | 0.51 ± 0.05* | 0.021 |
| IL-1β | Interleukin 1-β | 1.00 ± 0.15 | 1.38 ± 0.40 | 0.397 | 1.00 ± 0.15 | 3.34 ± 1.11 | 0.073 |
| IL-6 | Interleukin 6 | 1.00 ± 0.17 | 0.78 ± 0.13 | 0.352 | 1.00 ± 0.32 | 0.87 ± 0.23 | 0.767 |
| Mcp-1 | Monocyte chemoattractant protein 1 | 1.00 ± 0.15 | 1.11 ± 0.28 | 0.719 | 1.00 ± 0.12 | 0.55 ± 0.05** | 0.005 |
| Ccr2 | C-C chemokine receptor type 2 | 1.00 ± 0.12 | 1.16 ± 0.27 | 0.598 | 1.00 ± 0.13 | 1.00 ± 0.20 | 0.980 |
| Ccl3 | C-C Motif chemokine ligand 3 | 1.00 ± 0.12 | 0.65 ± 0.12 | 0.072 | 1.00 ± 0.12 | 0.37 ± 0.03*** | 0.001 |
| Ccl4 | C-C Motif chemokine ligand 4 | 1.00 ± 0.10 | 0.92 ± 0.14 | 0.687 | 1.00 ± 0.16 | 0.51 ± 0.05* | 0.021 |
| Nos2 | Nitric oxide synthase 2 | 1.00 ± 0.23 | 1.19 ± 0.33 | 0.647 | 1.00 ± 0.15 | 0.81 ± 0.26 | 0.550 |
| F4/80 | EGF-like module-containing mucin-like hormone receptor-like 1 | 1.00 ± 0.11 | 0.62 ± 0.08* | 0.025 | 1.00 ± 0.09 | 0.55 ± 0.07** | 0.002 |
| Mgl2 | Macrophage galactose N-acetyl-galactosamine-specific lectin 2 | 1.00 ± 0.19 | 1.20 ± 0.27 | 0.554 | 1.00 ± 0.13 | 1.37 ± 0.33 | 0.307 |
| Cd206 | Cluster of differentiation 206 | 1.00 ± 0.17 | 1.57 ± 0.35 | 0.171 | 1.00 ± 0.14 | 0.62 ± 0.11 | 0.055 |
| IL-10 | Interleukin 10 | 1.00 ± 0.07 | 0.63 ± 0.11* | 0.019 | 1.00 ± 0.11 | 1.18 ± 0.24 | 0.491 |
| Arg1 | Arginase 1 | 1.00 ± 0.22 | 0.82 ± 0.17 | 0.556 | 1.00 ± 0.13 | 0.85 ± 0.24 | 0.646 |
| Cd68 | Cluster of differentiation 68 | 1.00 ± 0.12 | 0.66 ± 0.13 | 0.100 | 1.00 ± 0.16 | 0.46 ± 0.11* | 0.016 |
| Retnl | Resistin-like | 1.00 ± 0.44 | 1.70 ± 0.46 | 0.295 | 1.00 ± 0.13 | 1.21 ± 0.41 | 0.641 |
| Fibrosis | |||||||
| Tgf-β | Tranforming growth factor β | 1.00 ± 0.13 | 0.44 ± 0.08** | 0.004 | 1.00 ± 0.14 | 0.58 ± 0.16 | 0.081 |
| Col3a1 | Collagen type III alpha 1 chain | 1.00 ± 0.19 | 0.80 ± 0.14 | 0.4414 | 1.00 ± 0.16 | 0.60 ± 0.17 | 0.112 |
| Col6a1 | Collagen type VI alpha 1 chain | 1.00 ± 0.10 | 0.99 ± 0.11 | 0.988 | 1.00 ± 0.10 | 0.78 ± 0.20 | 0.391 |
| Hif-1α | Hypoxia-inducible factor 1 α | 1.00 ± 0.08 | 1.04 ± 0.11 | 0.763 | 1.00 ± 0.24 | 0.81 ± 0.27 | 0.617 |
| ER stress | |||||||
| Xbp-1s | X-box binding protein 1 (spliced) | 1.00 ± 0.08 | 0.83 ± 0.13 | 0.329 | 1.00 ± 0.13 | 0.74 ± 0.13 | 0.201 |
| Xbp-1t | X-box binding Protein 1 (unspliced) | 1.00 ± 0.07 | 0.81 ± 0.08 | 0.115 | 1.00 ± 0.07 | 0.90 ± 0.07 | 0.393 |
| Chop | C/EBP homologous protein | 1.00 ± 0.05 | 0.87 ± 0.07 | 0.212 | 1.00 ± 0.18 | 0.59 ± 0.13 | 0.098 |
| Atf4 | Activating transcription factor 4 | 1.00 ± 0.06 | 0.78 ± 0.06* | 0.037 | 1.00 ± 0.06 | 0.81 ± 0.10 | 0.167 |
| Oxidative stress | |||||||
| Sod1 | Superoxide dismutase 1 | 1.00 ± 0.14 | 0.97 ± 0.12 | 0.892 | 1.00 ± 0.08 | 0.94 ± 0.11 | 0.740 |
| Sod2 | Superoxide dismutase 2 | 1.00 ± 0.10 | 1.06 ± 0.12 | 0.713 | 1.00 ± 0.15 | 0.80 ± 0.18 | 0.468 |
| Cox2 | Cyclooxygenase 2 | 1.00 ± 0.21 | 1.05 ± 0.24 | 0.860 | 1.00 ± 0.18 | 0.86 ± 0.22 | 0.636 |
| Nox4 | NADPH oxidase 4 | 1.00 ± 0.12 | 0.94 ± 0.10 | 0.762 | 1.00 ± 0.11 | 0.87 ± 0.16 | 0.539 |
| Nqo1 | NAD(P)H quinone dehydrogenase 1 | 1.00 ± 0.21 | 0.50 ± 0.13 | 0.077 | 1.00 ± 0.10 | 0.51 ± 0.05** | 0.001 |
*P < 0.05, **P < 0.01, ***P < 0.001. N/A, not applicable.
DISCUSSION
PRADC1, as a novel metabolic regulator and secretory protein, is significantly suppressed in metabolically active tissues (liver, adipose, skeletal muscle, kidney, and heart), as well as in different brain regions (hypothalamus and cortex), during the refeeding phase following overnight food restriction. The striking nutritional regulation of Pradc1 expression suggests a metabolic function for this protein. We used a genetic loss-of-function mouse model to determine whether PRADC1 is required for maintaining energy homeostasis.
Under nonstressed basal conditions when mice were fed a control LFD, PRADC1 was dispensable for metabolic control. None of the major metabolic parameters (body weight, insulin sensitivity, fasting glucose, or serum lipid profiles) were significantly different between genotypes of either sex. However, significant sex-dependent phenotypes were revealed when Pradc1 KO mice were challenged with an HFD to induce obesity and metabolic dysfunction, underscoring the importance of sex as a significant biologic variable in the physiologic response to high caloric intake (12–14). Obese male Pradc1 KO mice consuming an HFD appeared indistinguishable from WT littermates in whole-body glucose and lipid metabolism. In striking contrast, female Pradc1 KO mice gained significantly less body weight and fat mass relative to WT littermates when fed an HFD. Reduced adiposity is not due to any differences in food intake; rather, we attribute it, at least in part, to increased physical activity and energy expenditure in Pradc1 KO animals. We ruled out changes in thyroid or stress hormones, or circulating FGF21 levels, as the possible cause of elevated physical activity and energy expenditure; none of these were significantly different between genotypes. Increased BAT thermogenic activity could result in greater energy dissipation; however, the body temperature was not significantly different between genotypes. Expression of uncoupling protein 1 was, in fact, lower in BAT and iWAT of Pradc1 KO mice compared with WT controls, thus ruling out uncoupling or “browning” of iWAT as a possible contributor to increased energy expenditure and lower adiposity seen in the KO animals. Sympathetic input via the adrenergic pathway can promote lipolysis in WAT and BAT, as well as BAT activation (15, 16). Loss of PRADC1 could potentially lead to greater lipid turnover in adipose tissue. In fact, we observed reduced lipolysis in Pradc1 KO female mice in response to a β3-adrenergic receptor agonist (CL 316243) stimulation compared with WT controls. Thus, our data point to increased physical activity as a likely contributor to increased energy expenditure in Pradc1 KO female mice fed an HFD.
Excess adiposity is tightly linked to metabolic dysfunction (17). In the obese state, adipose tissue undergoes significant remodeling that impairs its functions; these include low-grade inflammation due to immune cell infiltration (9–11) and fibrosis (18). Because of elevated physical activity and marked reduction in body weight (∼15%) between genotypes, we expected to see significant improvements in glucose and lipid metabolism in the female Pradc1 KO mice relative to WT littermates. Surprisingly, the metabolic profiles (insulin sensitivity, fasting blood glucose and serum lipid profiles, extent of hepatic steatosis, and adipose tissue inflammation) were largely indistinguishable between WT and Pradc1 KO female mice. It appears that in this mouse model, physical activity and adiposity are uncoupled from metabolic health and that enhanced locomotor activity and reduced fat mass are insufficient to improve local inflammatory milieu within the adipose tissue, as well as systemic glucose and lipid metabolism in the context of an obesogenic diet. The integrative nature of energy balance and whole-body metabolism entails complex regulatory mechanisms that remain poorly understood. Although elevated physical activity generally confers significant health benefits in humans and animal models (19), there are subsets of humans in which regular exercise appears to have only limited improvements in metabolic health (20). Increased adiposity due to obesity is known to be a major risk and contributing factor to impaired insulin action and metabolic health (17); conversely, reducing fat mass through exercise or surgical interventions often significantly improves insulin sensitivity and systemic glucose and lipid profiles (21–23). Thus, the uncoupling of physical activity and fat mass from systemic metabolic health seen in the Pradc1 KO female mice sheds lights on the complex regulation of energy balance.
In summary, our effort to identity novel metabolically responsive secretory proteins led to the functional characterization of PRADC1. Future studies will help address whether the combination of phenotypes we observed in KO female mice are primarily due to the lack of PRADC1’s action in peripheral tissues and/or brain where it is expressed. In light of how little is known about this evolutionarily conserved protein, our studies lay the groundwork and provide a context to further interrogate PRADC1’s function and mechanisms of action.
ACKNOWLEDGMENTS
The authors thank Susan Aja (Department of Neuroscience and Center for Metabolism and Obesity Research, Johns Hopkins University) for help with indirect calorimetry and Andrew Wolfe for help with multiplex quantification of thyroid hormones. This work was supported by a grant from the U.S. National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases (DK084171 to G.W.W.). S.R. was supported by a postdoctoral fellowship from the American Diabetes Association (1-18-PMF-022). A.N.S. was supported by a predoctoral fellowship from the Ford Foundation. The authors declare no conflicts of interest.
Glossary
- BAT
brown adipose tissue
- Ccl
C-C motif chemokine ligand
- CLS
crown-like structure
- FGF21
fibroblast growth factor 21
- gWAT
gonadal white adipose tissue
- HFD
high-fat diet
- iWAT
inguinal white adipose tissue
- KO
knockout
- LacZ
β-galactosidase
- LFD
low-fat diet
- MRI
magnetic resonance imaging
- NEFA
nonesterified free fatty acid
- PRADC1
protease-associated domain-containing 1
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
S. Rodriguez and G. W. Wong contributed to the experimental design; S. Rodriguez, A. N. Stewart, X. Lei, X. Cao, H. C. Little, V. Fong, and D. C. Sarver performed the experiments; S. Rodriguez, A. N. Stewart, and G. W. Wong analyzed and interpreted the data; and S. Rodriguez and G. W. Wong wrote the paper.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Clark H. F., Gurney A. L., Abaya E., Baker K., Baldwin D., Brush J., Chen J., Chow B., Chui C., Crowley C., Currell B., Deuel B., Dowd P., Eaton D., Foster J., Grimaldi C., Gu Q., Hass P. E., Heldens S., Huang A., Kim H. S., Klimowski L., Jin Y., Johnson S., Lee J., Lewis L., Liao D., Mark M., Robbie E., Sanchez C., Schoenfeld J., Seshagiri S., Simmons L., Singh J., Smith V., Stinson J., Vagts A., Vandlen R., Watanabe C., Wieand D., Woods K., Xie M. H., Yansura D., Yi S., Yu G., Yuan J., Zhang M., Zhang Z., Goddard A., Wood W. I., Godowski P., Gray A. (2003) The secreted protein discovery initiative (SPDI), a large-scale effort to identify novel human secreted and transmembrane proteins: a bioinformatics assessment. Genome Res. 13, 2265–2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uhlén M., Fagerberg L., Hallström B. M., Lindskog C., Oksvold P., Mardinoglu A., Sivertsson Å., Kampf C., Sjöstedt E., Asplund A., Olsson I., Edlund K., Lundberg E., Navani S., Szigyarto C. A., Odeberg J., Djureinovic D., Takanen J. O., Hober S., Alm T., Edqvist P. H., Berling H., Tegel H., Mulder J., Rockberg J., Nilsson P., Schwenk J. M., Hamsten M., von Feilitzen K., Forsberg M., Persson L., Johansson F., Zwahlen M., von Heijne G., Nielsen J., Pontén F. (2015) Proteomics. Tissue-based map of the human proteome. Science 347, 1260419 [DOI] [PubMed] [Google Scholar]
- 3.Nanjappa V., Thomas J. K., Marimuthu A., Muthusamy B., Radhakrishnan A., Sharma R., Ahmad Khan A., Balakrishnan L., Sahasrabuddhe N. A., Kumar S., Jhaveri B. N., Sheth K. V., Kumar Khatana R., Shaw P. G., Srikanth S. M., Mathur P. P., Shankar S., Nagaraja D., Christopher R., Mathivanan S., Raju R., Sirdeshmukh R., Chatterjee A., Simpson R. J., Harsha H. C., Pandey A., Prasad T. S. (2014) Plasma Proteome Database as a resource for proteomics research: 2014 update. Nucleic Acids Res. 42, D959–D965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou Y. B., Liu F., Zhu Z. D., Zhu H., Zhang X., Wang Z. Q., Liu J. H., Han Z. G. (2004) N-glycosylation is required for efficient secretion of a novel human secreted glycoprotein, hPAP21. FEBS Lett. 576, 401–407 [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez S., Lei X., Petersen P. S., Tan S. Y., Little H. C., Wong G. W. (2016) Loss of CTRP1 disrupts glucose and lipid homeostasis. Am. J. Physiol. Endocrinol. Metab. 311, E678–E697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lei X., Seldin M. M., Little H. C., Choy N., Klonisch T., Wong G. W. (2017) C1q/TNF-related protein 6 (CTRP6) links obesity to adipose tissue inflammation and insulin resistance. J. Biol. Chem. 292, 14836–14850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Δ Δ C(T)) Method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 8.Petersen P. S., Lei X., Wolf R. M., Rodriguez S., Tan S. Y., Little H. C., Schweitzer M. A., Magnuson T. H., Steele K. E., Wong G. W. (2017) CTRP7 deletion attenuates obesity-linked glucose intolerance, adipose tissue inflammation, and hepatic stress. Am. J. Physiol. Endocrinol. Metab. 312, E309–E325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harman-Boehm I., Blüher M., Redel H., Sion-Vardy N., Ovadia S., Avinoach E., Shai I., Klöting N., Stumvoll M., Bashan N., Rudich A. (2007) Macrophage infiltration into omental versus subcutaneous fat across different populations: effect of regional adiposity and the comorbidities of obesity. J. Clin. Endocrinol. Metab. 92, 2240–2247 [DOI] [PubMed] [Google Scholar]
- 10.Weisberg S. P., McCann D., Desai M., Rosenbaum M., Leibel R. L., Ferrante A. W., Jr (2003) Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112, 1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu H., Barnes G. T., Yang Q., Tan G., Yang D., Chou C. J., Sole J., Nichols A., Ross J. S., Tartaglia L. A., Chen H. (2003) Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 112, 1821–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mauvais-Jarvis F. (2015) Sex differences in metabolic homeostasis, diabetes, and obesity. Biol. Sex Differ. 6, 14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palmer B. F., Clegg D. J. (2015) The sexual dimorphism of obesity. Mol. Cell. Endocrinol. 402, 113–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varlamov O., Bethea C. L., Roberts C. T., Jr (2015) Sex-specific differences in lipid and glucose metabolism. Front. Endocrinol. (Lausanne) 5, 241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duncan R. E., Ahmadian M., Jaworski K., Sarkadi-Nagy E., Sul H. S. (2007) Regulation of lipolysis in adipocytes. Annu. Rev. Nutr. 27, 79–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cannon B., Nedergaard J. (2004) Brown adipose tissue: function and physiological significance. Physiol. Rev. 84, 277–359 [DOI] [PubMed] [Google Scholar]
- 17.Rosen E. D., Spiegelman B. M. (2014) What we talk about when we talk about fat. Cell 156, 20–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun K., Tordjman J., Clément K., Scherer P. E. (2013) Fibrosis and adipose tissue dysfunction. Cell Metab. 18, 470–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hawley J. A., Hargreaves M., Joyner M. J., Zierath J. R. (2014) Integrative biology of exercise. Cell 159, 738–749 [DOI] [PubMed] [Google Scholar]
- 20.Pandey A., Swift D. L., McGuire D. K., Ayers C. R., Neeland I. J., Blair S. N., Johannsen N., Earnest C. P., Berry J. D., Church T. S. (2015) Metabolic effects of exercise training among fitness-nonresponsive patients with type 2 diabetes: the HART-D study. Diabetes Care 38, 1494–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poirier P., Giles T. D., Bray G. A., Hong Y., Stern J. S., Pi-Sunyer F. X., Eckel R. H., American Heart Association Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism (2006) Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 113, 898–918 [DOI] [PubMed] [Google Scholar]
- 22.Vitola B. E., Deivanayagam S., Stein R. I., Mohammed B. S., Magkos F., Kirk E. P., Klein S. (2009) Weight loss reduces liver fat and improves hepatic and skeletal muscle insulin sensitivity in obese adolescents. Obesity (Silver Spring) 17, 1744–1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bradley D., Magkos F., Klein S. (2012) Effects of bariatric surgery on glucose homeostasis and type 2 diabetes. Gastroenterology 143, 897–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.