Abstract
Cytochrome c (Cytc) is a multifunctional protein that operates as an electron carrier in the mitochondrial electron transport chain and plays a key role in apoptosis. We have previously shown that tissue-specific phosphorylations of Cytc in the heart, liver, and kidney play an important role in the regulation of cellular respiration and cell death. Here, we report that Cytc purified from mammalian brain is phosphorylated on S47 and that this phosphorylation is lost during ischemia. We have characterized the functional effects in vitro using phosphorylated Cytc purified from pig brain tissue and a recombinant phosphomimetic mutant (S47E). We crystallized S47E phosphomimetic Cytc at 1.55 Å and suggest that it spatially matches S47-phosphorylated Cytc, making it a good model system. Both S47-phosphorylated and phosphomimetic Cytc showed a lower oxygen consumption rate in reaction with isolated Cytc oxidase, which we propose maintains intermediate mitochondrial membrane potentials under physiologic conditions, thus minimizing production of reactive oxygen species. S47-phosphorylated and phosphomimetic Cytc showed lower caspase-3 activity. Furthermore, phosphomimetic Cytc had decreased cardiolipin peroxidase activity and is more stable in the presence of H2O2. Our data suggest that S47 phosphorylation of Cytc is tissue protective and promotes cell survival in the brain.—Kalpage, H. A., Vaishnav, A., Liu, J., Varughese, A., Wan, J., Turner, A. A., Ji, Q., Zurek, M. P., Kapralov, A. A., Kagan, V. E., Brunzelle, J. S., Recanati, M.-A., Grossman, L. I., Sanderson, T. H., Lee, I., Salomon, A. R., Edwards, B. F. P, Hüttemann, M. Serine-47 phosphorylation of cytochrome c in the mammalian brain regulates cytochrome c oxidase and caspase-3 activity.
Keywords: apoptosis, electron transport chain, ischemia, respiration, signaling
Cytochrome c (Cytc) is a mitochondrial hemeprotein that plays a central role in cellular respiration and apoptosis. Cytc takes part in respiration by transferring electrons from bc1 complex (complex III) to cytochrome c oxidase (complex IV, COX) where oxygen is reduced to water in the electron transport chain (ETC). This is the proposed rate-limiting step of the ETC in intact cells (1, 2), with the free energy released during this reaction being twice as high compared with complexes I and III, making it effectively an irreversible reaction (3). The ETC complexes I, III, and IV pump protons across the mitochondrial inner membrane, generating an electrochemical gradient that is utilized by ATP synthase (complex V) to convert ADP and phosphate to ATP. Cytc is not only an indispensable protein for cellular energy production but also a major signaling molecule for apoptosis. In the presence of an apoptotic signal, Cytc is released from the mitochondria into the cytosol and interacts with the protein apoptotic protease activating factor 1 (Apaf-1) (4). This results in the formation of the apoptosome, which activates the caspase cascade, leading to cell death.
Furthermore, during the early process of apoptosis, Cytc functions as a peroxidase of cardiolipin, a mitochondria-specific lipid that interacts with Cytc (5, 6). About 15–20% of Cytc is tethered to cardiolipin (7, 8). Cytc acts as a catalyst of cardiolipin peroxidation in the presence of reactive oxygen species (ROS) such as H2O2. In apoptotic cells, 60% of cardiolipin is redistributed to the outer mitochondrial membrane, facilitating Cytc release from the mitochondria into the cytosol (9). Cytc is also involved in both ROS production and scavenging, utilizing its ability to accept and donate electrons (10, 11). These multifunctional properties of Cytc identify it as a cellular life and death decision molecule. Consequently, the protein is tightly regulated by allosteric mechanisms (ATP/ADP ratio), tissue-specific isoform expression, and reversible phosphorylations, as reviewed in Kalpage et al. (12).
Reversible phosphorylations of Cytc take place in a highly tissue-specific manner on distinct sites in mammalian liver, heart, and kidney under basal conditions (13–15). We have previously reported that phosphorylation of brain Cytc at Y97 is strongly induced by neuroprotective insulin treatment, but this Tyr phosphorylation is absent under basal conditions (16). In this study, we explored the basal phosphorylation state of brain Cytc and identified a novel phosphorylation site on S47. Interestingly, this phosphorylation is lost under ischemia, which is an oxygen-depleted condition present in pathologies such as ischemic stroke. This modification of Cytc was also identified in a number of high-throughput studies of the human skeletal muscle phosphoproteome (17, 18), but was not functionally characterized.
Most mammals including rodents have a somatic and a testes tissue-specific isoform of Cytc. However, this testes-specific isoform was lost in primates and is only present as a nontranscribed pseudogene in humans (19). The sequence of human Cytc is a mixture of both somatic and testes-specific isoforms of mammals that express the 2 isoforms (19, 20). Cytc, together with its partner COX, is tightly regulated to meet the tissue-specific needs and energy demand of an organism. COX isoforms are known for subunits IV, VIa, VIb, VIIa, and VIII, which are reviewed (21, 22).
Here, we report that Cytc purified from mammalian brain tissue is phosphorylated on S47 under basal conditions. This modification lowers respiration, apoptosis, and cardiolipin-induced peroxidase activity. However, under ischemic conditions, Cytc becomes dephosphorylated, thus reversing all the neuroprotective functions of S47 phosphorylation. Our model proposes that cell signaling mechanisms that target Cytc for phosphorylation control respiration to maintain intermediate mitochondrial membrane potentials (ΔΨm) (23, 24). Such regulation is crucial for higher organisms because ΔΨm is directly related to ROS production. At ΔΨm > 140 mV, ROS production at ETC complexes I and III increases exponentially, whereas mitochondria of resting cells with a low ΔΨm do not produce significant amounts of ROS (25–28). We propose that changes in the phosphorylation state of Cytc in the context of brain ischemia-reperfusion provides a mechanism for brain reperfusion injury through maximal ETC flux, ΔΨm hyperpolarization, and excessive ROS production, exacerbating tissue damage.
MATERIALS AND METHODS
Cytc and COX purification
For Cytc purification, pig brains were obtained from a slaughterhouse (Dunbar, Milan, MI, USA) and snap frozen. Half of the weighed brain tissue was left under ischemic conditions in a tightly sealed bag at 37°C for 1 h. All procedures involving animal tissues were approved by the Wayne State University Institutional Animal Care and Use Committee. The tissue incubated under ischemic conditions and nonischemic tissue were processed separately. Cytc was purified using our established protocol (13–15) with minor modifications: Cytc eluted from the CM52 cellulose column (GE Healthcare) was concentrated under vacuum to 1 ml and further purified by size exclusion using a Sephacryl S-100 (GE Healthcare) gel filtration column. Gel-filtered Cytc was concentrated and buffer exchanged to water using Amicon Ultra-15 10 kDa centrifugal filter units (MilliporeSigma, Burlington, MA, USA).
Regulatory competent COX was purified from pig brain and heart as previously described for cow liver and heart COX under conditions that preserve phosphorylation (29) with minor modifications: additional washing steps with ST buffer (250 mM sucrose, 20 mM Tris, pH 7.4) were performed for the isolation of brain mitochondria because of the large amount of fat present in brain tissue compared with heart. To break the mitochondrial membranes, 20% Triton X-114 (1.5 ml/g protein) was added to brain mitochondria. Extracted COX was loaded onto a DEAE-Sepharose (GE Healthcare) anion-exchange column equilibrated with 125 mM KPi, 0.5% Triton X-100. COX was eluted using a salt gradient (100–700 mM KPi, 0.1% Triton X-100, pH 7.4). COX was further purified by ammonium sulfate precipitation as described in Lee et al. (29).
S47 phosphorylation mapping by mass spectrometry
The purified pig brain Cytc was trypsin digested and enriched for phosphopeptides using the PhosTio Kit (GL Science, Tokyo, Japan). The peptides were eluted into a Linear-Trap Quadrupole Fourier Transform (LTQ-FT) Mass Spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) through C18 reverse-phase gradient column chromatography followed by electrospray ionization as described in Sanderson et al. (16). The tandem mass spectrometry spectra were collected in the positive ion mode and assigned to peptide sequences. Phosphopeptides of interest were manually verified.
Site-directed mutagenesis
The codon corresponding to S47 of the somatic rodent Cytc cDNA cloned into pLW01 expression vector (30) was mutagenized to a glutamate residue (S47E) as a phosphomimetic replacement and with an alanine residue as an additional control (S47A), which cannot be phosphorylated. These mutants were generated using the QuickChange Lightning Site-Directed Mutagenesis Kit (Agilent Technologies, Santa Clara, CA, USA) according to the manufacturer’s protocol. Briefly, the pLW01 Cytc plasmid was amplified using the following mutagenesis primers. S47A forward: 5′-CCAGGCTGCTGGATTCGCTTACACAGATGCC-3′, S47A reverse: 5′ GGCATCTGTGTAAGCGAATCCAGCAGCCTGG-3′, S47E forward: 5′-CCAGGCTGCTGGATTCGAGTACACAGATGCC-3′, S47E reverse: 5′-GGCATCTGTGTACTCGAATCCAGCAGCCTGG-3′. The parental DNA was digested using DpnI restriction enzyme, and the DNA was transformed into XL10-Gold ultracompetent cells. pLW01 plasmids were purified from individual colonies using the Wizard Plus SV Miniprep Purification System (Promega, Madison, WI, USA) and DNA sequencing was performed to confirm the presence of the desired mutation.
Bacterial overexpression and recombinant protein purification
Following sequence confirmation, the pLW01 plasmid was transformed into C41 (DE3) competent bacterial cells (Lucigen, Middleton, WI, USA). Bacterial colonies were cultured in Terrific Broth (Research Products International, Mount Prospect, IL, USA) medium with 0.1 mg/ml carbenicillin at 37°C and induced with 100 µM isopropyl-β-d-thiogalactoside when an optical density (OD)600 of 1 was reached. The bacterial cells were harvested and lysed, and Cytc was purified, as previously described in Mahapatra et al. (15), with slightly modified ion exchange chromatography conditions for the DE52 anion-exchange (pH 7.4, 4.0 mS/cm conductivity) column and CM52 cation exchange column (pH 6.5, 6.0 mS/cm conductivity). The protein was eluted with a high-salt elution buffer (40 mM KPi, 0.5 M NaCl). Cytc was further purified by size exclusion chromatography and buffer exchanged to water as previously described for Cytc purification from brain tissue.
Analysis of Cytc spectra
Cytc concentration was determined by the spectrophotometric method as previously described in Mahapatra et al. (15). Cytc was oxidized and reduced with K3Fe(CN)6 and Na2S2O4, respectively, and desalted using Sephadex G-25 NAP5 columns (GE Healthcare). The Cytc concentration was calculated using the following equation: (A550red−A550oxi)/19.6 mM/cm × 1 cm (31). Reduced Cytc was used to obtain the spectrum from 220 to 700 nm. Oxidized Cytc (100 µM) was used to obtain the spectrum from 600 to 750 nm to analyze the peak at 695 nm.
COX activity measurements
Purified pig brain COX was diluted to 3 µM, and cardiolipin (MilliporeSigma) was added to a 40:1 cardiolipin: COX molar ratio. The enzyme was dialyzed (MW cutoff: 12,000–14,000 Da) in COX measuring buffer [10 mM K-4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, pH 7.4, 40 mM KCl, 1% Tween 20, supplemented with 0.2 mM ATP] at 4°C overnight to remove bound cholate (15). Oxygen consumption was measured via a Clark-type oxygen electrode (Oxygraph System; Hansatech, Pentney, United Kingdom) at a final concentration of 54.5 nM brain COX and 163 nM heart COX in 220 µl measuring buffer. Ascorbate (20 mM) was added as an electron donor for Cytc. Purified Cytc (5 µM) was injected into the air-tight oxygen electrode. Oxygen consumption was recorded and analyzed using the Oxygraph software (Hansatech). COX activity was expressed as oxygen consumed per minute.
Caspase-3 activity assay
Cytc−/− embryonic fibroblasts (CRL 2613; American Type Culture Collection, Manassas, VA, USA) were cultured in 8 T75 cell culture flasks. The cells were harvested, and a cytosolic extract was prepared as previously described in Mahapatra et al. (15). Enzchek Caspase-3 Assay Kit 2 (Thermo Fisher Scientific) containing the rhodamine 110-linked DEVD tetrapeptide that fluoresces upon cleavage by caspase-3 was used for caspase-3 activity measurements. Fluorescence was measured using a Fluoroskan Ascent FL plate reader (Thermo Fisher Scientific) using 485-nm excitation (14-nm bandwidth) and 527-nm emission (10-nm bandwidth) filters. The final readings were corrected for background fluorescence in the absence of Cytc.
Redox potential measurement
The midpoint redox potential (E°’) was analyzed spectrophotometrically as previously described in Mahapatra et al. (15) using 2,6-dichloroindophenol (DCIP, E°’ = 237 mV) as a reference compound. Data obtained were plotted as log(DCIPox/DCIPred) vs. log(Cytox/Cytred), yielding a linear graph with a slope of nDCIP/nCytc and a y axis intercept of nCytc/59.2(E Cytc – E DCIP). These values were used to calculate the E°’ of Cytc from the Nernst equation.
Heme degradation assay
Oxidized Cytc variants were passed through NAP5 columns to remove the oxidant K3Fe(CN)6 and diluted to a final concentration of 5 µM in 50 mM phosphate buffer (pH 6.1). An excess of 3 mM H2O2 was added and the reduction of 408 nm heme Soret band of Cytc was measured after 60 s as previously described in Liu et al. (32).
Cardiolipin peroxidase activity
Tetralinoleylcardiolipin and Cytc (1 µM) of varying ratios (10:1, 20:1) were incubated for 10 min. Peroxidase activity was measured upon addition of Amplex red (50 µM) and H2O2 (50 µM) (15). Amplex red, when oxidized, is converted to resorufin. Resorufin fluorescence was measured at 535/585 nm (excitation/emission) using a “Fusion R” universal microplate analyzer.
Gel electrophoresis and Western blotting
Purified Cytc was loaded on a 10% acrylamide (37.5:1) Tris-tricine gel, Coomassie stained for 1 h, and destained overnight. For Western blotting, the gel was transferred onto a PVDF membrane using a Bio-Rad trans-blot semidry transfer apparatus (125 mA, 25 V, 1 h). Membranes were blocked with 5% blocking reagent (bovine serum albumin for anti-phospho antibodies and dry milk for the Cytc antibody) for 1 h and incubated with primary antibodies: Cytc mouse monoclonal (BD Pharmingen, San Jose, CA, USA), 1:1000, and Ser phosphoantibody cocktail (4 monoclonal antibodies 1C8, 4A3, 4A9, and 16B4; EMD Biosciences, San Diego, CA, USA), 1:1000. After overnight primary antibody incubation, blots were washed with 1×Tris-buffered saline, 0.1% Tween 20 buffer and incubated with horseradish peroxidase–conjugated secondary mouse IgG (Cytc) and mouse IgM (phosphoserine) antibodies in a 1:5000 dilution for 2 h. The blots were washed with 1× Tris-buffered saline, 0.1% Tween 20 following secondary antibody incubation and visualized with HyGlo chemiluminescent reagent (Denville Scientific, Holliston, MA, USA), using an automatic X-ray film processor, model JP-33.
Phostag gel electrophoresis
A Phostag (100 µM) AAL-107 ligand (Wako Chemicals, Richmond, VA, USA) containing 10% Tris-Tricine gel was cast according to the manufacturer’s protocol. Tissue-purified Cytc from both ischemic and nonischemic conditions was loaded with cow heart Cytc (MilliporeSigma) as an additional control. The Phostag gel was run and immunoblotted with anti-rabbit Cytc antibody (ab18738; Abcam, Cambridge, MA, USA). The Phostag gel was incubated in transfer buffer with 5 µM EDTA for 20 min before transfer to remove Mn2+. The band intensity of phosphorylated and unphosphorylated Cytc was quantified using the density-in-box tool of Quantity One 1-Dimension Analysis Software (Bio-Rad, Hercules, CA, USA), after correction for background.
High-resolution gel for separation of COX subunits
The COX subunits were separated by the Kadenbach SDS-PAGE-urea method (33). The separation gel solution had a final concentration of 16% acrylamide and 7.2 M urea. Purified heart and brain COX (30 µg/lane) were loaded, and the gel was run at 180 V overnight.
Statistical analysis
Experimental data consisting of 3 or more replicates were analyzed for statistical significance for the different experiments by a 2-sided Wilcoxon rank-sum test using MSTAT v.6.1. Data are reported as means ± sd and were considered statistically significant when P < 0.05.
Cytc crystallization
S47E Cytc was purified and crystallized using the same procedures employed successfully for 3 previous Cytc structures (15). Briefly, it was gel-filtered on an S-100 column, concentrated to 15 mg/ml or higher in water, and oxidized with 5 mM K3Fe(CN)6 [Protein Data Bank (PDB): FC6] prior to crystallization. Crystals were grown by vapor diffusion after mixing 1 μl of protein solution with 1 μl of precipitant solution and equilibrating the drop against 0.5 ml of the precipitant at room temperature. The respective precipitant solutions are listed in Supplemental Table S1. The single crystal was soaked for 10 min in a cryo-protectant solution (Supplemental Table S1) before flash freezing in liquid nitrogen. Diffraction data were collected at the Life Sciences Collaborative Access Team facility (Advanced Photon Source sector 21; Argonne National Laboratory, Lemont, IL, USA). The data were integrated using XDS (34) in AutoProc (35). The structure was solved, rebuilt, refined, and analyzed using the Phenix (36) suite of programs (AutoMR, AutoBuild, Refine, Composite.Omit). The beginning model for AutoMR was chain-A of wild-type (WT) Cytc (5C0Za). The 4 FC6 molecules were located in anomalous diffraction difference maps, and their occupancies were refined in Phenix. The final structure was optimized with PDB-REDO (37), which also refined the heme links to Cys14, Cys17, His18, and Met80 against standard bond values. This final step improved Rwork by 2.2%, Rfree by 1.7%, and the real space correlation coefficient for the electron density of 115/416 residues (none were worse).
Molecular dynamics
Molecular dynamics were performed with Yasara v.17-12-24 (38) using its conservative “slow” protocol, recommended defaults for all parameters, and the most recent force field, Amber 2014 (39). The starting structures for the molecular dynamics calculations on the S47 mutants were prepared from chain 5C0Za. Root mean square fluctuation and root mean square deviation (RMSD) plots were generated with Excel using data imported from Yasara. The “omit” electron density plots were assembled with PyMOL [The PyMOL Molecular Graphics System, v.2.2.3 (2018) Schrödinger, New York, NY, USA] using the simple “composite.omit” electron density calculated by Phenix. The final assembly of all figures was done in Photoshop (Adobe, San Jose, CA, USA).
RESULTS
Brain Cytc is S47 phosphorylated and becomes dephosphorylated during ischemia
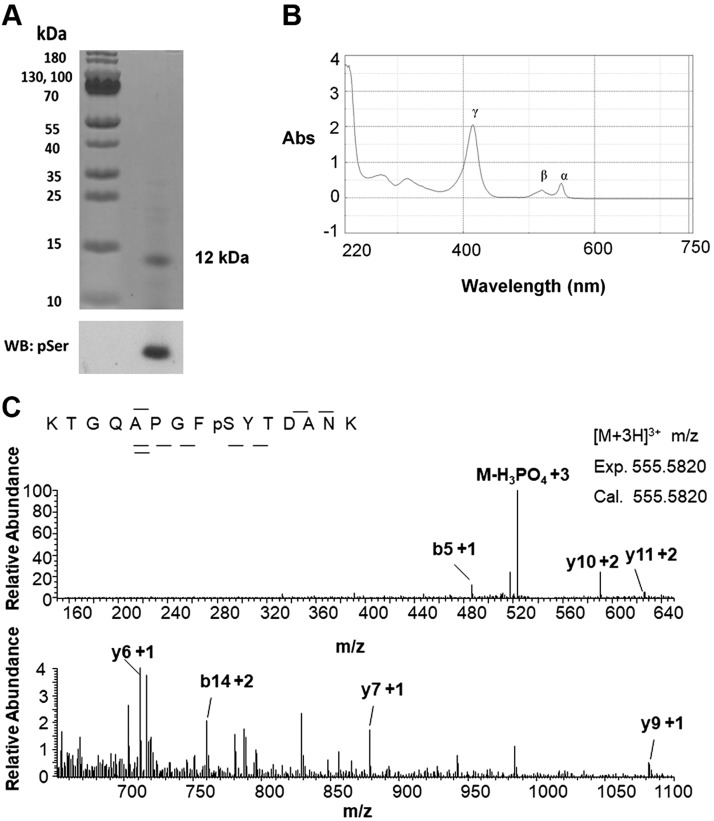
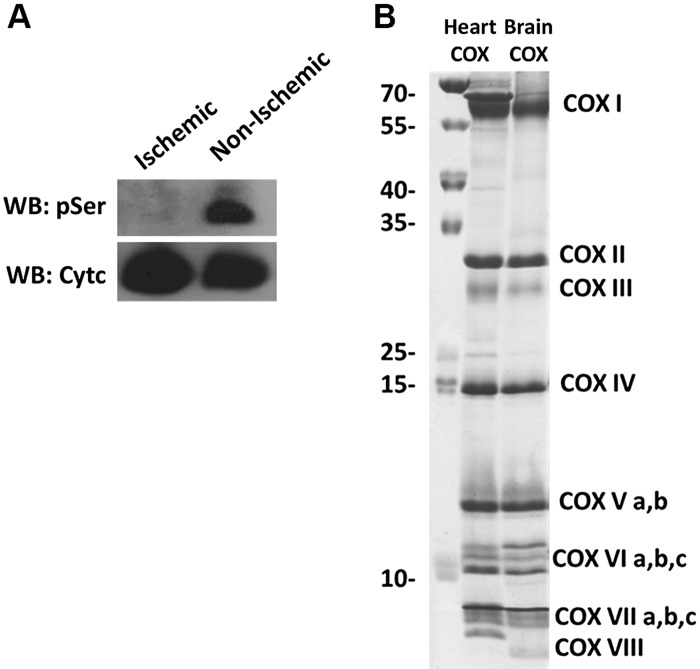
Previously, our lab identified distinct phosphorylation sites of Cytc purified from the heart, liver, and kidney under basal conditions. However, the basal phosphorylation state of Cytc in the brain was not known. Cytc purified from pig brain tissue was found by Western analysis to be Ser phosphorylated (Fig. 1A). The purified protein was properly folded and functional based on the presence of its characteristic α, β, and γ peaks (40) in the reduced absorption spectrum of Cytc (Fig. 1B). Through mass spectrometry, we showed that the protein was phosphorylated at S47 (Fig. 1C), the only Ser residue present in porcine and rodent somatic Cytc. This Ser phosphorylation was completely lost when Cytc was isolated from brain tissue that was left ischemic for 1 h (Fig. 2A).
Figure 1.
Cytc purified from porcine brain tissue is phosphorylated at S47 under basal conditions. A) Purified porcine brain Cytc is Ser phosphorylated. Top, Coomassie gel; bottom, anti-phosphoserine (pSer) Western blot (WB). B) The reduced absorption spectrum of porcine brain Cytc (13 µM) showing its characteristic α (550–558), β (521–527), and γ (415–423) peaks. C) nano–liquid chromatography electrospray ionization tandem mass spectrometric spectrum of KTGQAPGFpSYTDANK indicates that porcine brain Cytc is phosphorylated on S47. The phosphorylation site was unambiguously revealed by fragment ions y6 and y7. Abs, absorbance.
Figure 2.
Cytc purified from ischemic porcine brain tissue is dephosphorylated. A) Purified porcine brain Cytc from ischemic tissue (lane 1) and normal, nonischemic tissue (lane 2). Top, immunoblot with phosphoserine (pSer) antibodies; bottom, immunoblot with a Cytc antibody. B) COX purified from porcine heart (lane 1) and brain (lane 2) resolved on a high-resolution SDS-PAGE-urea gel showing all 13 tightly bound subunits of COX. WB, Western blot.
Cytc S47 phosphorylation inhibits respiration
To analyze the effect of this post-translational modification in reaction with COX, the corresponding COX enzyme was purified from porcine brain and from heart tissue as a control. These proteins were resolved in a high-resolution urea-polyacrylamide gel (33) to separate the 13 tightly bound subunits of COX (Fig. 2B).
The COX brain and heart enzymes differ by 3 tissue-specific isoforms of subunits VIa, VIIa, and VIII, which run at slightly different positions, with the heart COX containing the heart isoforms and the brain COX containing the liver-type isoforms (41). Using Cytc purified from nonischemic (phosphorylated) and ischemic (dephosphorylated) brain tissue, oxygen consumption with the matching COX isozyme from pig brain was measured with a Clark-type oxygen electrode. The phosphorylated (nonischemic) form resulted in partial inhibition of respiration by 48% compared with dephosphorylated (ischemic) Cytc (Table 1). This suggests that S47-phosphorylated (phospho-S47) Cytc may regulate ETC electron flux to maintain healthy intermediate ΔΨm levels under physiologic conditions (see Discussion).
TABLE 1.
COX activity and Caspase-3 activity of Cytc purified from pig brain tissue (ischemic/nonischemic)
| Type | Brain COX activity (min) | Caspase-3 activity (%) |
|---|---|---|
| Dephosphorylated brain Cytc (ischemic) | 283.5 ± 13.3 (100% ± 4.7)a | 100 ± 4.3 |
| Phosphorylated brain Cytc (nonischemic) | 147.5 ± 19.6* (52.0% ± 6.9)a | 37.9 ± 16.1* |
Percentage of WT.
P < 0.05 compared with dephosphorylated Cytc.
Cytc S47 phosphorylation inhibits apoptosis
The effect of Cytc S47 phosphorylation on its second important function, apoptosis, was determined as a measure of caspase-3 activity. Caspase-3 is an executioner caspase that acts as a terminal enzyme in the caspase cascade. Caspase-3 activity was measured using cytosolic extracts from Cytc knockout mouse embryonic fibroblasts in combination with the artificial substrate DEVD-Rhodamine110, which fluoresces upon cleavage by active caspase-3. The apoptotic activity was significantly lower, by 62%, in the presence of phospho-S47 nonischemic Cytc, as compared with dephosphorylated (ischemic) Cytc (Table 1). In order to determine the proportion of Cytc that is phosphorylated in the brain, the phosphorylated protein was separated from the unphosphorylated protein on a Phostag gel (Supplemental Fig. S1). Based on densitometry analysis, we report that about 35% of the protein was phosphorylated, which is likely an underestimation because of the delay in time extracting the organ that causes the tissue to become ischemic and to lose Cytc phosphorylation (see Discussion).
S47E phosphomimetic Cytc as a model for S47 phosphorylation
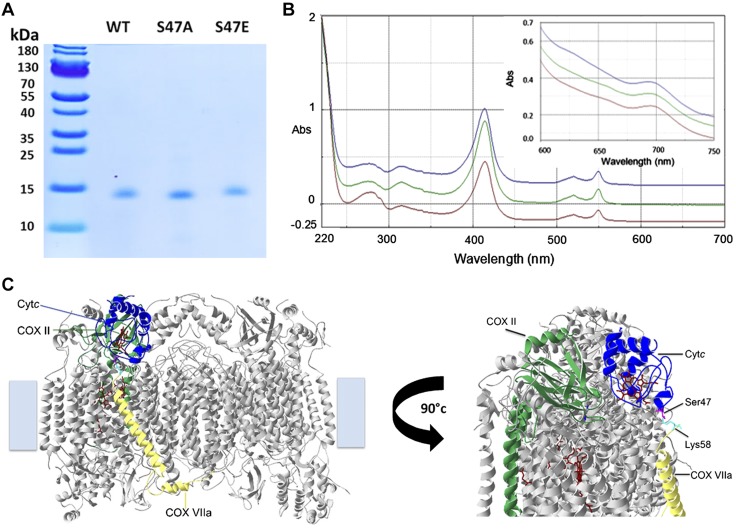
To further functionally characterize the role of S47 phosphorylation, a recombinant phosphomimetic Cytc mutant, S47E, was generated. Using a phosphomimetic mutant instead of in vivo phosphorylated Cytc has the advantage that the modification cannot be lost during functional assays, especially those that require extended incubation times that could otherwise lead to hydrolysis of the phosphate group. In addition, essentially unlimited amounts can be generated by bacterial overexpression. The S47E mutant was purified along with recombinant WT and S47A Cytc, a variant that cannot be phosphorylated, using a bacterial expression system (Fig. 3A). The reduced and oxidized absorption spectra of these recombinant proteins were analyzed in order to confirm that the proteins were properly folded (Fig. 3B). The presence of the weak 695 nm peak of the oxidized spectra indicates the proper hexacoordinate structure of the heme iron (Fig. 3B, inset) (42). To effectively function as an electron carrier in the ETC, Cytc must have a redox potential value between complex III and complex IV. The redox potential value for WT Cytc was 272 ± 2 mV, which is similar to the values of 270 ± 1 mV and 256 ± 12 mV for S47A and S47E Cytc, respectively (Table 2). These values lie within the literature values of mammalian Cytc redox potential in the range of 220–270 mV (43, 44), suggesting that they can effectively participate in electron transfer.
Figure 3.
Characterization of recombinant WT, S47A, and S47E Cytc. A) Coomassie gel of bacterially overexpressed and purified recombinant WT (lane 1), S47A (lane 2), and S47E (lane 3) Cytc indicates that the proteins were purified to homogeneity. B) Reduced Cytc (5 µM) spectra (220–700 nm) and oxidized Cytc (100 µM) spectra (600–700 nm, inset) of recombinant WT (blue), S47A (red), and S47E (green) indicate correct folding and functionality of the proteins. C) Docking model of Cytc and COX (45, 60). A COX residue within a distance of 3 Å from residue 47 (magenta) is highlighted (Lys58 of COX subunit VIIa, cyan). Abs, absorbance.
TABLE 2.
Functional studies of WT, S47A, and S47E Cytc
| Cytc variant | Brain COX activity (min) | Heart COX activity (min) | Caspase-3 activity (%) | Heme degradation (%) | Redox potential (mV) |
|---|---|---|---|---|---|
| WT | 233.2 ± 15.1 (100% ± 6.5)a | 98.1 ± 13.5 (100% ± 13.8)a | 100 ± 1.9 | 100 ± 4.0 | 272 ± 2 |
| S47A | 192.3 ± 15.4* (82.5% ± 6.6)a | 65.4 ± 14.6* (66.7% ± 14.9)a | 38.0 ± 6.8* | 99.5 ± 8.6 | 270 ± 1 |
| S47E | 106.6 ± 15.6* (45.7% ± 6.7)a | 49.9 ± 2.7* (50.9% ± 2.8)a | 35.5 ± 4.0* | 67.5 ± 3.9* | 256 ± 12 |
Percentage of WT.
P < 0.05 compared with WT.
Based on a published docking model of Cytc and COX (45), S47 was suggested to interact with COX subunit VIIa (Fig. 3C). Brain and heart COX differ in their tissue-specific isoforms of subunits VIa, VIIa, and VIII (Fig. 2B). Therefore, COX activity with the Cytc mutants was measured with both brain COX containing subunit VIIaL (liver-type) and heart COX containing subunit VIIaH (heart-type) to reveal potential kinetic differences of the 2 isozymes.
The COX activity in the presence of 5 µM phosphomimetic (S47E) Cytc in reaction with pig brain COX was 54% lower compared with COX activity in the presence of 5 µM WT Cytc (Table 2). These data suggest that S47E replacement is a good mimetic for in vivo S47 phosphorylation (Table 1). The S47A mutant resulted in COX activity values between the phosphomimetic and the WT, suggesting that this substitution does not optimally represent unphosphorylated WT Cytc. The apparent Km of Cytc in the reaction with brain COX was 1.5, 1.4, and 1.5 µM for WT, S47A, and S47E Cytc, respectively. With heart COX, phosphomimetic Cytc resulted in partially inhibited respiration to an extent comparable with brain COX, suggesting that the tissue-specific isoforms of COX subunit VII in heart and brain do not modulate the effect of Cytc S47 phosphorylation. The apparent Km of Cytc in the reaction with heart COX was 1.5, 1.2, and 1.5 µM for WT, S47A, and S47E Cytc, respectively. However, COX activity of heart COX was 2.5 times lower than COX activity of brain COX (Table 2), which is consistent with other reports in the literature suggesting that liver-type COX has a higher turnover compared with heart-type COX, as reviewed in ref. (22).
In the presence of the phosphomimetic S47E replacement, caspase-3 activity was significantly inhibited (by 65%) compared with WT Cytc (Table 2). The S47A mutant also resulted in significantly lower caspase-3 activity, suggesting that the hydroxyl group of the S47 residue is important for apoptotic activity and that any modification of this residue may affect the interaction of Cytc with Apaf-1. In fact, the Apaf-1 Cytc docking model (46) revealed that S47 is located in the Apaf-1 binding domain of Cytc (Supplemental Fig. S2).
With increased ROS levels, Cytc undergoes oxidative modifications such as sulfoxidation, which causes Cytc to lose its function (47). This loss in function, upon addition of 3 mM H2O2, was monitored by a decrease in heme absorption of Cytc at 408 nm. S47E Cytc was about 30% more resistant to heme degradation by excess H2O2 compared with both WT and S47A Cytc, suggesting that S47E Cytc is more resistant to oxidative stress (Table 2).
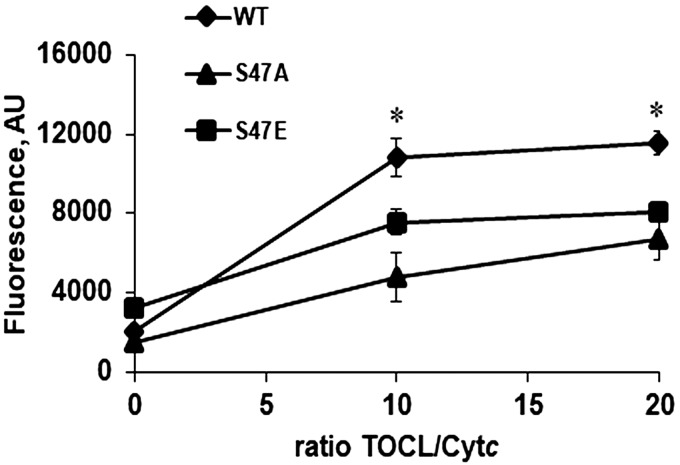
As discussed in the Introduction, cardiolipin peroxidase activity is a proapoptotic function of Cytc. Cardiolipin peroxidase activity was measured in the presence of cardiolipin, Cytc, H2O2, and Amplex red as the fluorescence indicator of cardiolipin oxidation. In a 10:1 and 20:1 ratio of cardiolipin:Cytc, WT Cytc has the highest peroxidase activity compared with the S47A and S47E mutants (Fig. 4), further supporting the antiapoptotic function of the phosphorylated protein.
Figure 4.
Cardiolipin peroxidase activity. Cardiolipin peroxidase activity is significantly lower with S47A and S47E Cytc at 10:1 and 20:1 tetralinoleylcardiolipin (TOCL):Cytc ratios compared with the WT.
Crystal structure of S47E Cytc and molecular dynamics simulations
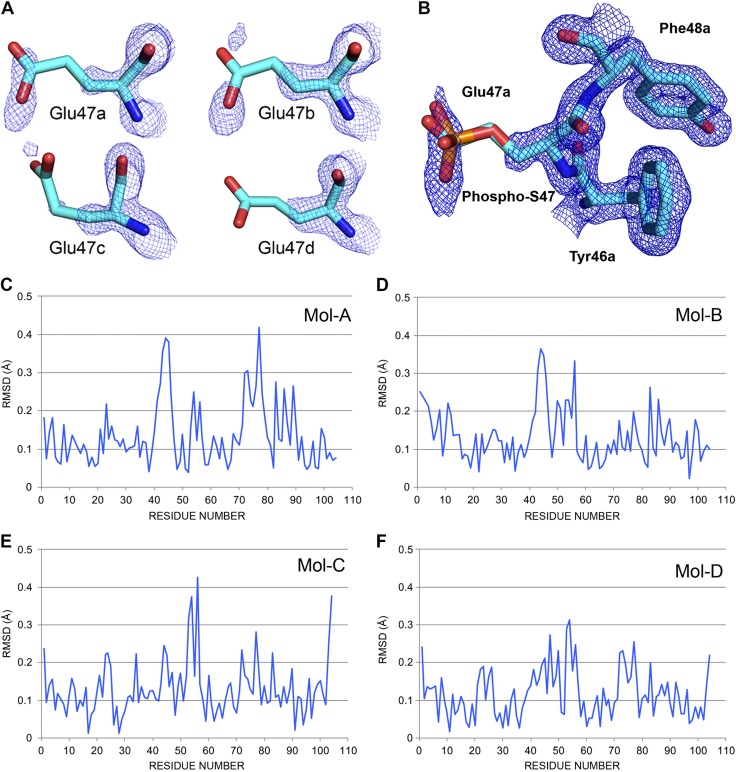
To gain a better understanding of the structure-function relationships of Cytc when the S47 residue is modified, we determined the crystal structure of S47E phosphomimetic Cytc at 1.55 Å (Supplemental Table S1) and performed molecular dynamics simulations. The crystal structure of S47E (6N1O.pdb) frozen at 100 K is similar to the WT Cytc structure (5C0Z.pdb) (Fig. 5A). Phospho-S47 was superposed onto the Glu-47 residue of the S47E crystal structure (Fig. 5B) to demonstrate that S47E and phospho-S47 spatially arrange in a similar manner and that S47E is a good phosphomimetic model. Figure 5C–F shows the RMSD of the 4 molecules A, B, C, and D of the S47E Cytc tetramer (6N1O.pdb) relative to the corresponding molecules A, B, C, and D of the WT Cytc tetramer (5C0Z.pdb). Increased RMSD was observed around the S47 residue; however, this was variable across the 4 molecules possibly because the S47E crystal structure was disordered with broken omit density especially in molecules C and D.
Figure 5.
S47E crystal structure at 1.55 Å. A) Omit electron density for the S47E residues. The unbiased electron density calculated by Phenix.composite.omit and contoured at 1.0 RMSD with PyMOL is shown for the Glu-47 residues in the 4 Cytc chains in the asymmetric unit of 6N1O.pdb. Cytc S47E has 8 glutamate residues for a total of 32 in the 6N1O tetramer. They are on the surface, and most are mobile and disordered with broken omit density for the side chain atoms. If one counts the number of carboxyl group atoms (carbon δ, oxygen ε1. and oxygen ε2) in density at 1.0 RMSD in this figure then the numbers are E47a (3), E47b (1), E47c (0), and E47d (1). Applying this simple analysis to the entire crystal structure gives scores (maximum = 24) of 20, 17, 13, and 18, for chains A, B, C, and D, respectively. B) Phosphomimetic Glu-47. The 2Fo-Fc electron density calculated by Coot (https://www2.mrc-lmb.cam.ac.uk/personal/pemsley/coot/web/docs/coot.html) from the 6N10 structure and data are shown for Phe-46a, Glu-47a, and Tyr-48a contoured at 1 RMSD (PyMOL). There are no residues within 3.5 Å of the Glu-47a side chain. The equivalent phospho-S47 sidechain is superposed onto Glu-47a to show the structural match. The phosphorus atom is orange. S47 was modeled by mutating Glu-47a back to a Ser with YASARA and then attaching a phosphate group with Coot. C–F) The RMSD figures comparing the 4 molecules A, B, C, and D of S47E (6N10.pdb) and WT (5C0Z.pdb) in the 2 crystal structures.
We performed molecular dynamics calculations to evaluate the possible effects of the mutations on the structrue of Cytc in solution. WT Cytc (5C0Z.pdb) and S47A, S47E, and phospho-S47 Cytc modeled in silico from WT Cytc (5C0Z.pdb) were run for 300 ns and divided into three 100-ns blocks. The first block was considered a preequilibrium period. The RMSD of the second block from 100 to 200 ns and third block from 200 to 300 ns were plotted for the Cα position on each residue (Supplemental Fig. S3A–D). The molecular dynamics runs suggest that there is no major perturbation in the overall structure at Cytc position 47 in S47A, S47E, and phospho-S47 molecules. However, the negative classic γ turn containing residues K27, T28, and G29 shows the highest RMSD, suggesting that this is the most unstable loop of Cytc. Superposed WT Cytc with S47A and S47E Cytc shows that S47A (red) and S47E (cyan) Cytc superpose well on top of each other, whereas the WT (blue) Cytc structure is more deviated, especially around the loop containing T28 (Supplemental Fig. S3E).
DISCUSSION
Even though Cytc is a well-studied protein and has been known for over a century, its regulation through cell signaling and post-translational modifications was only recently reported. This was made possible by improved protein purification protocols developed to preserve post-translational modifications and thus the physiologic regulatory properties of mitochondrial proteins including Cytc and COX (13, 29). We previously identified tissue-specific phosphorylation sites of Cytc in the heart (13), liver (14), and kidney (15). We also reported a phosphorylation site in the brain (Y97) that was induced by insulin treatment (16), but absent under basal conditions. Therefore, the basal phosphorylation state of Cytc in the brain was unknown until this report. The brain is an organ that heavily depends on oxidative phosphorylation. In adult humans, the brain accounts for only about 2% of the body weight, yet it consumes 20% of the oxygen we breathe (48), making it the most energy expensive organ of the body.
Here, we show that Cytc purified from pig brain is phosphorylated on residue S47. Cytc is 95% conserved between human and pig, making the latter a good experimental model because of its close proteome resemblance to humans (49). In addition, we also mapped the same modification on immunoprecipitated Cytc from rat brain tissue (data not shown), suggesting that it is the common regulatory post-translational modification present in the mammalian brain. To date, no studies have identified Cytc S47 phosphorylation in the human brain, but S47 phosphorylation of Cytc was mapped in 2 high-throughput phosphoproteomics studies from human skeletal muscle, suggesting that S47 phosphorylation is present in humans under normal physiologic conditions (17, 50). However, no functional follow-up studies were performed, and it is unclear if a meaningful fraction of the Cytc protein pool contains this modification in skeletal muscle.
The Phos-tag gel data indicate that about 35% of the brain Cytc pool was S47-phosphorylated, suggesting that this post-translational modification is biologically relevant. It should be noted that the observed fraction of phosphorylated Cytc is likely an underestimation because of the time it takes to open the pig skull and snap freeze the brains, a period in which the tissue is ischemic and may lose some of this phosphorylation, which we know is completely lost after 1 h of ischemia. Thus, to overcome the challenge with regard to variability in phosphorylation of tissue-purified Cytc, we used recombinant phosphomimetic (S47E) Cytc for functional analyses. The phosphomimetic form behaved in a similar way to in vivo phosphorylated Cytc in both COX activity and caspase-3 activity assays. In addition, the crystal structure of S47E Cytc, obtained at a resolution of 1.55 Å, shows that it spatially arranges similarly to modeled phospho-S47 Cytc (Fig. 5B), further supporting that S47E Cytc serves as a good phosphomimetic model.
The S47 residue belongs to the ΩNY-loop (foldon V) comprising residues 40–57, which were previously reported to have the highest unfolding capacity (51, 52). Furthermore, this is the loop that contains the only 2 disease-related mutations reported for Cytc (G41S, Y48H) that cause thrombocytopenia in humans (53). This may further explain the substantial functional changes in respiration and apoptosis caused by single point mutations in this evolutionarily optimized protein. Surprisingly, RMSD analysis did not show a significant perturbation in the foldon V region of Cytc containing S47 when phosphorylated or replaced with alanine or glutamate. However, significant changes in RMSD were observed around the T28 residue when S47 was modified. In contrast to most studies that report S47-containing ΩNY-loop as the most unstable region of Cytc, our molecular dynamics runs have suggested the T28-containing loop to be the most unstable portion of the protein. This loop is referred to as the “negative classic” γ turn of the protein comprising residues 27–29, which has been shown to be very important for the stability of Cytc sidechains (54). Furthermore, we and others have previously reported that T28 is an important regulatory site of Cytc that controls the ETC function (15, 55).
In several in vitro assays the control mutant, S47A Cytc, behaved more like the phosphomimetic protein or showed a behavior somewhere between the two. This finding suggests that this specific site on Cytc (i.e., the presence of the Ser residue) is essential for maximal ETC electron flux and apoptotic activity. Even though alanine is the closest neutral replacement for a Ser residue, it lacks a polar group to mimic the hydroxyl group. We and others have observed effects deviating from the WT in some functional analysis with an alanine replacement in Cytc (55). Recently we reported similar behavior for another alanine mutant, T28A Cytc (15). Superposition of WT, S47A, and S47E after 300 ns of molecular dynamics shows similar arrangement of S47A and S47E Cytc. This may explain the similar functional effects observed for these 2 mutants in caspase-3 activity and cardiolipin peroxidation.
Interestingly, alanine substitution is found in position 47 across several different species such as Drosophila, fungi, algae, arthropods, and bacteria (56), indicating that an alanine replacement is evolutionarily permissible at this site. In fact, the presence of an alanine may be beneficial for these species since S47A demonstrates increased protective functions as observed with the phosphorylated protein, such as lower caspase-3 and cardiolipin peroxidase activities. The negatively charged amino acid aspartate, which is an alternative phosphomimetic replacement, was also found at this site in species such as snails, Caenorhabditis elegans, and nematodes (56). However, based on our crystallographic analysis, including the length of the phosphorylated side chain together with our functional data, we conclude that glutamate and not aspartate is the better phosphomimetic replacement to study Ser phosphorylation. This is also suggested by the fact that, despite S47D Cytc showing lower caspase and cardiolipin peroxidase activities similar to S47E Cytc, it behaves in an opposite manner in reaction with COX compared with in vivo phosphorylated or S47E Cytc, leading to about a 70% increase in respiration (55). In contrast, the glutamate phosphomimetic reflects the activity seen with the in vivo phosphorylated protein.
COX along with Cytc are evolutionarily adapted to meet tissue-specific energy needs of different organs. One adaptive mechanism is the expression of tissue-specific isoforms, such as the liver-type and heart and skeletal muscle-type specific isoforms in COX subunits VIa, VIIa, and VIII (22). Heart-type COX is specific to the heart and skeletal muscle tissue, whereas the liver-type is expressed in most other tissues (57, 58). The liver-type isoforms are expressed in tissues such as the liver and brain that have specialized functions and not such a high capacity for mitochondria compared with heart and skeletal muscle. Therefore, liver-type COX has a higher basal activity compared with heart-type COX (22). However, the in vitro COX activity experimental setup used for these measurements do not take into account the ΔΨm and supercomplex formation of the ETC complexes, which are important features of intact mitochondria. Therefore, in order to better represent the physiologic in vivo situation, COX activity should be measured in live cells or tissues. Because COX subunit VIIa was suggested to interact with S47 of Cytc based on a computational docking model (45), we asked whether the effect of the Cytc S47 modification on COX activity may be further modulated by the presence of the liver- vs. the heart-type isoform of subunit VIIa. Surprisingly, both heart and brain COX showed a similar level of inhibition of respiration with phosphomimetic Cytc compared with WT, suggesting that interaction of Cytc with subunit VIIa does not alter the respiration rate in a tissue-specific manner.
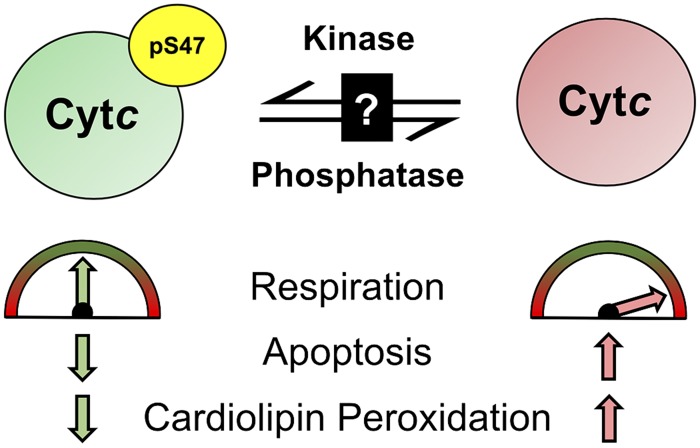
In summary, this is the first study reporting the basal phosphorylation state of Cytc in the mammalian brain and its functional characterization using both in vivo phosphorylated and phosphomimetic (S47E) Cytc. Our results suggest that this modification is cytoprotective in terms of maintaining optimal intermediate mitochondrial respiration rates by partially inhibiting COX activity (Fig. 6). We propose that the loss of this post-translational modification during cellular stress, such as during brain ischemia (Fig. 2A), would cause pathologically high respiration rates during reperfusion that would lead to ΔΨm hyperpolarization and a ROS burst, promoting cardiolipin peroxidation and triggering apoptosis (24). Lower caspase-3 and cardiolipin peroxidase activities provide another safeguard mechanism from apoptosis when Cytc is phosphorylated. These mechanisms should be further investigated in an in vivo model. In addition, the kinase and phosphatase involved in this signaling pathway are yet to be discovered. A small number of kinases and phosphatases with localization to brain mitochondria has been reported (59), further supporting tissue-specific phosphorylation of mitochondrial proteins. We propose that a better understanding of Cytc regulation in the brain may facilitate the development of a Cytc-targeted neuroprotective therapy to improve debilitating pathologies such as ischemic stroke when Cytc becomes dephosphorylated.
Figure 6.
S47 phosphorylation (pS47) of Cytc by an unknown kinase leads to intermediate healthy respiration, lower apoptosis, and lower cardiolipin peroxidase activity. Cytc gets dephosphorylated under ischemia, which leads to pathologically high respiration rates, increased apoptosis, and increased cardiolipin peroxidase activity.
ACKNOWLEDGMENTS
This work was supported by the U.S. National Institutes of Health (NIH) National Institute of General Medical Sciences Grants R01 GM116807 and R01 GM113908, NIH National Institute of Neurological Disorders and Stroke Grants R01 NS091242 and R42 NS105238, and by the Office of the Assistant Secretary of Defense for Health Affairs through the Peer Reviewed Medical Research Program under Award W81XWH-16-1-0175. Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the funding agencies including the Department of Defense or the NIH. This research used resources of the Advanced Photon Source, a U.S. Department of Energy Office of Science User Facility operated for the Department of Energy Office of Science by Argonne National Laboratory under Contract DE-AC02- 06CH11357. Use of the Life Sciences Collaborative Access Team (LS-CAT) Sector 21 was supported by the Michigan Economic Development Corp., the Michigan Technology Tri-Corridor (Grant 085P1000817), and Wayne State University Office of the Vice-President for Research. The authors declare no conflicts of interest.
Glossary
- ΔΨm
mitochondrial membrane potential
- Apaf-1
apoptotic protease activating factor 1
- COX
cytochrome c oxidase
- Cytc
cytochrome c
- DCIP
2,6-dichloroindophenol
- E°’
midpoint redox potential
- ETC
electron transport chain
- PDB
Protein Data Bank
- RMSD
root mean square deviation
- ROS
reactive oxygen species
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
H. A. Kalpage, J. Liu, A. Varughese, J. Wan, A. A. Turner, Q. Ji, M. P. Zurek, A. A. Kapralov, I. Lee, and M. Hüttemann designed and performed experiments; H. A. Kalpage and M. Hüttemann wrote the manuscript; A. Vaishnav, J. S. Brunzelle, and B. F. P. Edwards determined the crystal structures; V. E. Kagan, L. I. Grossman, T. H. Sanderson, A. R. Salomon, B. F. P. Edwards, and M. Hüttemann assisted with the experimental design and supervised the project; and all authors edited and approved the final version of the manuscript.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Piccoli C., Scrima R., Boffoli D., Capitanio N. (2006) Control by cytochrome c oxidase of the cellular oxidative phosphorylation system depends on the mitochondrial energy state. Biochem. J. 396, 573–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dalmonte M. E., Forte E., Genova M. L., Giuffrè A., Sarti P., Lenaz G. (2009) Control of respiration by cytochrome c oxidase in intact cells: role of the membrane potential. J. Biol. Chem. 284, 32331–32335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hinkle P. C., Kumar M. A., Resetar A., Harris D. L. (1991) Mechanistic stoichiometry of mitochondrial oxidative phosphorylation. Biochemistry 30, 3576–3582 [DOI] [PubMed] [Google Scholar]
- 4.Hüttemann M., Pecina P., Rainbolt M., Sanderson T. H., Kagan V. E., Samavati L., Doan J. W., Lee I. (2011) The multiple functions of cytochrome c and their regulation in life and death decisions of the mammalian cell: from respiration to apoptosis. Mitochondrion 11, 369–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kagan V. E., Tyurin V. A., Jiang J., Tyurina Y. Y., Ritov V. B., Amoscato A. A., Osipov A. N., Belikova N. A., Kapralov A. A., Kini V., Vlasova I. I., Zhao Q., Zou M., Di P., Svistunenko D. A., Kurnikov I. V., Borisenko G. G. (2005) Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat. Chem. Biol. 1, 223–232 [DOI] [PubMed] [Google Scholar]
- 6.Kagan V. E., Bayir H. A., Belikova N. A., Kapralov O., Tyurina Y. Y., Tyurin V. A., Jiang J., Stoyanovsky D. A., Wipf P., Kochanek P. M., Greenberger J. S., Pitt B., Shvedova A. A., Borisenko G. (2009) Cytochrome c/cardiolipin relations in mitochondria: a kiss of death. Free Radic. Biol. Med. 46, 1439–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schlame M., Rua D., Greenberg M. L. (2000) The biosynthesis and functional role of cardiolipin. Prog. Lipid Res. 39, 257–288 [DOI] [PubMed] [Google Scholar]
- 8.Kagan V. E., Borisenko G. G., Tyurina Y. Y., Tyurin V. A., Jiang J., Potapovich A. I., Kini V., Amoscato A. A., Fujii Y. (2004) Oxidative lipidomics of apoptosis: redox catalytic interactions of cytochrome c with cardiolipin and phosphatidylserine. Free Radic. Biol. Med. 37, 1963–1985 [DOI] [PubMed] [Google Scholar]
- 9.Kagan V. E., Tyurina Y. Y., Bayir H., Chu C. T., Kapralov A. A., Vlasova I. I., Belikova N. A., Tyurin V. A., Amoscato A., Epperly M., Greenberger J., Dekosky S., Shvedova A. A., Jiang J. (2006) The “pro-apoptotic genies” get out of mitochondria: oxidative lipidomics and redox activity of cytochrome c/cardiolipin complexes. Chem. Biol. Interact. 163, 15–28 [DOI] [PubMed] [Google Scholar]
- 10.Giorgio M., Migliaccio E., Orsini F., Paolucci D., Moroni M., Contursi C., Pelliccia G., Luzi L., Minucci S., Marcaccio M., Pinton P., Rizzuto R., Bernardi P., Paolucci F., Pelicci P. G. (2005) Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell 122, 221–233 [DOI] [PubMed] [Google Scholar]
- 11.Pereverzev M. O., Vygodina T. V., Konstantinov A. A., Skulachev V. P. (2003) Cytochrome c, an ideal antioxidant. Biochem. Soc. Trans. 31, 1312–1315 [DOI] [PubMed] [Google Scholar]
- 12.Kalpage H. A., Bazylianska V., Recanati M. A., Fite A., Liu J., Wan J., Mantena N., Malek M. H., Podgorski I., Heath E. I., Vaishnav A., Edwards B. F., Grossman L. I., Sanderson T. H., Lee I., Hüttemann M. (2019) Tissue-specific regulation of cytochrome c by post-translational modifications: respiration, the mitochondrial membrane potential, ROS, and apoptosis. FASEB J. 33, 1540–1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee I., Salomon A. R., Yu K., Doan J. W., Grossman L. I., Hüttemann M. (2006) New prospects for an old enzyme: mammalian cytochrome c is tyrosine-phosphorylated in vivo. Biochemistry 45, 9121–9128 [DOI] [PubMed] [Google Scholar]
- 14.Yu H., Lee I., Salomon A. R., Yu K., Hüttemann M. (2008) Mammalian liver cytochrome c is tyrosine-48 phosphorylated in vivo, inhibiting mitochondrial respiration. Biochim. Biophys. Acta 1777, 1066–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahapatra G., Varughese A., Ji Q., Lee I., Liu J., Vaishnav A., Sinkler C., Kapralov A. A., Moraes C. T., Sanderson T. H., Stemmler T. L., Grossman L. I., Kagan V. E., Brunzelle J. S., Salomon A. R., Edwards B. F., Hüttemann M. (2017) Phosphorylation of cytochrome c threonine 28 regulates electron transport chain activity in kidney: implications for AMP kinase. J. Biol. Chem. 292, 64–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanderson T. H., Mahapatra G., Pecina P., Ji Q., Yu K., Sinkler C., Varughese A., Kumar R., Bukowski M. J., Tousignant R. N., Salomon A. R., Lee I., Hüttemann M. (2013) Cytochrome C is tyrosine 97 phosphorylated by neuroprotective insulin treatment. PLoS One 8, e78627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao X., León I. R., Bak S., Mogensen M., Wrzesinski K., Højlund K., Jensen O. N. (2011) Phosphoproteome analysis of functional mitochondria isolated from resting human muscle reveals extensive phosphorylation of inner membrane protein complexes and enzymes. Mol. Cell Proteomics 10, M110.000299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffman N. J., Parker B. L., Chaudhuri R., Fisher-Wellman K. H., Kleinert M., Humphrey S. J., Yang P., Holliday M., Trefely S., Fazakerley D. J., Stöckli J., Burchfield J. G., Jensen T. E., Jothi R., Kiens B., Wojtaszewski J. F., Richter E. A., James D. E. (2015) Global phosphoproteomic analysis of human skeletal muscle reveals a network of exercise-regulated kinases and AMPK substrates. Cell Metab. 22, 922–935; erratum: 948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pierron D., Opazo J. C., Heiske M., Papper Z., Uddin M., Chand G., Wildman D. E., Romero R., Goodman M., Grossman L. I. (2011) Silencing, positive selection and parallel evolution: busy history of primate cytochromes C. PLoS One 6, e26269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hüttemann M., Jaradat S., Grossman L. I. (2003) Cytochrome c oxidase of mammals contains a testes-specific isoform of subunit VIb--the counterpart to testes-specific cytochrome c? Mol. Reprod. Dev. 66, 8–16 [DOI] [PubMed] [Google Scholar]
- 21.Pierron D., Wildman D. E., Hüttemann M., Letellier T., Grossman L. I. (2012) Evolution of the couple cytochrome c and cytochrome c oxidase in primates. Adv. Exp. Med. Biol. 748, 185–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sinkler C. A., Kalpage H., Shay J., Lee I., Malek M. H., Grossman L. I., Hüttemann M. (2017) Tissue- and condition-specific isoforms of mammalian cytochrome c oxidase subunits: from function to human disease. Oxid. Med. Cell. Longev. 2017, 1534056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Helling S., Hüttemann M., Ramzan R., Kim S. H., Lee I., Müller T., Langenfeld E., Meyer H. E., Kadenbach B., Vogt S., Marcus K. (2012) Multiple phosphorylations of cytochrome c oxidase and their functions. Proteomics 12, 950–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanderson T. H., Reynolds C. A., Kumar R., Przyklenk K., Hüttemann M. (2013) Molecular mechanisms of ischemia-reperfusion injury in brain: pivotal role of the mitochondrial membrane potential in reactive oxygen species generation. Mol. Neurobiol. 47, 9–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu S. S. (1999) Cooperation of a “reactive oxygen cycle” with the Q cycle and the proton cycle in the respiratory chain--superoxide generating and cycling mechanisms in mitochondria. J. Bioenerg. Biomembr. 31, 367–376 [DOI] [PubMed] [Google Scholar]
- 26.Korshunov S. S., Skulachev V. P., Starkov A. A. (1997) High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 416, 15–18 [DOI] [PubMed] [Google Scholar]
- 27.Hüttemann M., Lee I., Pecinova A., Pecina P., Przyklenk K., Doan J. W. (2008) Regulation of oxidative phosphorylation, the mitochondrial membrane potential, and their role in human disease. J. Bioenerg. Biomembr. 40, 445–456 [DOI] [PubMed] [Google Scholar]
- 28.Rottenberg H., Covian R., Trumpower B. L. (2009) Membrane potential greatly enhances superoxide generation by the cytochrome bc1 complex reconstituted into phospholipid vesicles. J. Biol. Chem. 284, 19203–19210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee I., Salomon A. R., Yu K., Samavati L., Pecina P., Pecinova A., Hüttemann M. (2009) Isolation of regulatory-competent, phosphorylated cytochrome C oxidase. Methods Enzymol. 457, 193–210 [DOI] [PubMed] [Google Scholar]
- 30.Pecina P., Borisenko G. G., Belikova N. A., Tyurina Y. Y., Pecinova A., Lee I., Samhan-Arias A. K., Przyklenk K., Kagan V. E., Hüttemann M. (2010) Phosphomimetic substitution of cytochrome C tyrosine 48 decreases respiration and binding to cardiolipin and abolishes ability to trigger downstream caspase activation. Biochemistry 49, 6705–6714 [DOI] [PubMed] [Google Scholar]
- 31.Margoliash E., Frohwirt N. (1959) Spectrum of horse-heart cytochrome c. Biochem. J. 71, 570–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Z., Lin H., Ye S., Liu Q. Y., Meng Z., Zhang C. M., Xia Y., Margoliash E., Rao Z., Liu X. J. (2006) Remarkably high activities of testicular cytochrome c in destroying reactive oxygen species and in triggering apoptosis. Proc. Natl. Acad. Sci. USA 103, 8965–8970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kadenbach B., Jarausch J., Hartmann R., Merle P. (1983) Separation of mammalian cytochrome c oxidase into 13 polypeptides by a sodium dodecyl sulfate-gel electrophoretic procedure. Anal. Biochem. 129, 517–521 [DOI] [PubMed] [Google Scholar]
- 34.Kabsch W. (2010) Xds. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vonrhein C., Flensburg C., Keller P., Sharff A., Smart O., Paciorek W., Womack T., Bricogne G. (2011) Data processing and analysis with the autoPROC toolbox. Acta Crystallogr. D Biol. Crystallogr. 67, 293–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adams P. D., Afonine P. V., Bunkóczi G., Chen V. B., Davis I. W., Echols N., Headd J. J., Hung L. W., Kapral G. J., Grosse-Kunstleve R. W., McCoy A. J., Moriarty N. W., Oeffner R., Read R. J., Richardson D. C., Richardson J. S., Terwilliger T. C., Zwart P. H. (2010) PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joosten R. P., Long F., Murshudov G. N., Perrakis A. (2014) The PDB_REDO server for macromolecular structure model optimization. IUCrJ 1, 213–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krieger E., Vriend G. (2015) New ways to boost molecular dynamics simulations. J. Comput. Chem. 36, 996–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hornak V., Abel R., Okur A., Strockbine B., Roitberg A., Simmerling C. (2006) Comparison of multiple Amber force fields and development of improved protein backbone parameters. Proteins 65, 712–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Michel B., Bosshard H. R. (1984) Spectroscopic analysis of the interaction between cytochrome c and cytochrome c oxidase. J. Biol. Chem. 259, 10085–10091 [PubMed] [Google Scholar]
- 41.Kuhn-Nentwig L., Kadenbach B. (1985) Isolation and properties of cytochrome c oxidase from rat liver and quantification of immunological differences between isozymes from various rat tissues with subunit-specific antisera. Eur. J. Biochem. 149, 147–158 [DOI] [PubMed] [Google Scholar]
- 42.Dickerson R., Timkovich R. (1975) Cytochrome c. In The Enzymes (Boyer P. D., ed.), Vol. 11, pp. 397–472, Academic Press, New York [Google Scholar]
- 43.Cammack R. (1995) Redox states and potentials. In Bioenergetics - A Practical Approach (Brown G. C., Cooper C. E., eds.), pp. 93–95, IRL Press, Oxford, United Kingdom [Google Scholar]
- 44.Nicholls D. G., Ferguson S. J. (1992) Bioenergetics 2, Academic Press Limited, London, San Diego [Google Scholar]
- 45.Roberts V. A., Pique M. E. (1999) Definition of the interaction domain for cytochrome c on cytochrome c oxidase. III. Prediction of the docked complex by a complete, systematic search. J. Biol. Chem. 274, 38051–38060 [DOI] [PubMed] [Google Scholar]
- 46.Cheng T. C., Hong C., Akey I. V., Yuan S., Akey C. W. (2016) A near atomic structure of the active human apoptosome. eLife 5, e17755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hannibal L., Tomasina F., Capdevila D. A., Demicheli V., Tórtora V., Alvarez-Paggi D., Jemmerson R., Murgida D. H., Radi R. (2016) Alternative conformations of cytochrome c: structure, function, and detection. Biochemistry 55, 407–428 [DOI] [PubMed] [Google Scholar]
- 48.Raichle M. E., Gusnard D. A. (2002) Appraising the brain’s energy budget. Proc. Natl. Acad. Sci. USA 99, 10237–10239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bassols A., Costa C., Eckersall P. D., Osada J., Sabrià J., Tibau J. (2014) The pig as an animal model for human pathologies: a proteomics perspective. Proteomics Clin. Appl. 8, 715–731 [DOI] [PubMed] [Google Scholar]
- 50.Højlund K., Bowen B. P., Hwang H., Flynn C. R., Madireddy L., Geetha T., Langlais P., Meyer C., Mandarino L. J., Yi Z. (2009) In vivo phosphoproteome of human skeletal muscle revealed by phosphopeptide enrichment and HPLC-ESI-MS/MS. J. Proteome Res. 8, 4954–4965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maity H., Maity M., Englander S. W. (2004) How cytochrome c folds, and why: submolecular foldon units and their stepwise sequential stabilization. J. Mol. Biol. 343, 223–233 [DOI] [PubMed] [Google Scholar]
- 52.Karsisiotis A. I., Deacon O. M., Wilson M. T., Macdonald C., Blumenschein T. M., Moore G. R., Worrall J. A. (2016) Increased dynamics in the 40-57 Ω-loop of the G41S variant of human cytochrome c promote its pro-apoptotic conformation. Sci. Rep. 6, 30447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deacon O. M., Svistunenko D. A., Moore G. R., Wilson M. T., Worrall J. A. R. (2018) Naturally occurring disease-related mutations in the 40-57 Ω-Loop of human cytochrome c control triggering of the alkaline isomerization. Biochemistry 57, 4276–4288 [DOI] [PubMed] [Google Scholar]
- 54.Sanishvili R., Volz K. W., Westbrook E. M., Margoliash E. (1995) The low ionic strength crystal structure of horse cytochrome c at 2.1 A resolution and comparison with its high ionic strength counterpart. Structure 3, 707–716 [DOI] [PubMed] [Google Scholar]
- 55.Guerra-Castellano A., Díaz-Moreno I., Velázquez-Campoy A., De la Rosa M. A., Díaz-Quintana A. (2016) Structural and functional characterization of phosphomimetic mutants of cytochrome c at threonine 28 and serine 47. Biochim. Biophys. Acta 1857, 387–395 [DOI] [PubMed] [Google Scholar]
- 56.Zaidi S., Hassan M. I., Islam A., Ahmad F. (2014) The role of key residues in structure, function, and stability of cytochrome-c. Cell. Mol. Life Sci. 71, 229–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hüttemann M., Mühlenbein N., Schmidt T. R., Grossman L. I., Kadenbach B. (2000) Isolation and sequence of the human cytochrome c oxidase subunit VIIaL gene. Biochim. Biophys. Acta 1492, 252–258 [DOI] [PubMed] [Google Scholar]
- 58.Jaradat S. A., Ko M. S., Grossman L. I. (1998) Tissue-specific expression and mapping of the Cox7ah gene in mouse. Genomics 49, 363–370 [DOI] [PubMed] [Google Scholar]
- 59.Arachiche A., Augereau O., Decossas M., Pertuiset C., Gontier E., Letellier T., Dachary-Prigent J. (2008) Localization of PTP-1B, SHP-2, and Src exclusively in rat brain mitochondria and functional consequences. J. Biol. Chem. 283, 24406–24411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tsukihara T., Aoyama H., Yamashita E., Tomizaki T., Yamaguchi H., Shinzawa-Itoh K., Nakashima R., Yaono R., Yoshikawa S. (1996) The whole structure of the 13-subunit oxidized cytochrome c oxidase at 2.8 A. Science 272, 1136–1144 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.