Abstract
Leigh syndrome embodies degenerative disorders with a collection of symptoms secondary to inborn errors of metabolism. Combinations of hypomorphic and loss-of-function alleles in many genes have been shown to result in Leigh syndrome. Interestingly, deficiency for the tricarboxylic acid cycle enzyme succinate dehydrogenase (SDH) can lead to Leigh-like syndrome in some circumstances and to cancer (paraganglioma, renal cell carcinoma, gastrointestinal stromal tumor) in others. In our experiments originally intended to create an inducible whole-body SDH-loss mouse model of tumorigenesis, we generated a condition reminiscent of Leigh-like syndrome that is lethal to mice within 4 wk. Remarkably, as has been shown for other mitochondrial diseases, chronic hypoxia offers substantial protection to mice from this condition after systemic SDH loss, allowing survival in the context of profoundly impaired oxidative metabolism.—Al Khazal, F., Holte, M. N., Bolon, B., White, T. A., LeBrasseur, N., Maher, L. J. III. A conditional mouse model of complex II deficiency manifesting as Leigh-like syndrome.
Keywords: mitochondrial disease, succinate dehydrogenase, familial paraganglioma, hypoxia
Oxidative energy metabolism is responsible for the majority of ATP synthesis in mammals. It is therefore not surprising that mutations affecting the oxidative metabolism machinery are highly deleterious. Because most enzymes of oxidative metabolism reside in mitochondria but are encoded by nuclear genes, it is also not surprising that such deleterious mutations typically manifest as mitochondrial disorders with recessive inheritance. What is perhaps more surprising is that inborn errors of oxidative metabolism often manifest as neurometabolic disorders with symptoms that can appear years after birth (1). Of particular interest is the constellation of more than 30 nuclear and mitochondrial genes whose hypomorphic alleles result in Leigh syndrome in human patients (2). Prominent features of Leigh syndrome include early neurologic deficits and failure to thrive. Subsequent degeneration of the muscular system, sometimes exacerbated by episodic stress, often leads to premature death. Symptoms (loss of appetite, vomiting, irritability, seizure activity) usually are noted between the ages of 3 mo and 2 yr. That many different metabolic errors manifest as Leigh and Leigh-like syndromes points to an underlying commonality in energy insufficiency.
The most prevalent autosomal recessive mutations resulting in Leigh syndrome include defects in the mitochondrially encoded ATP synthase membrane subunit 6 (MT-ATP6) gene [encoding a subunit of the mitochondrial ATP synthase, complex V (3)], nuclear NADH dehydrogenase (ubiquinone) oxidoreductase (NDUF) genes [encoding subunits of NADH:ubiquinone oxidoreductase, complex I (4)], and nuclear surfeit (SURF) genes [encoding subunits of cytochrome c oxidase, complex IV (5)]. French Canadian Leigh syndrome is caused by compound heterozygosity for recessive mutations in the leucine rich pentatricopeptide repeat containing (LRPPRC) gene, encoding a protein that regulates cytoskeletal interactions and whose loss reduces expression of cytochrome c oxidase I.
To date, 4 mouse models of Leigh and Leigh-like syndromes have been described. Mice homozygous for an Ndufs4 mutant allele causing complex I deficiency experience encephalopathy and a lethal breathing disorder (4, 6, 7). Homozygous Surf1 mutant mice experience mitochondrial disease with cytochrome c oxidase deficiency (5). Mice lacking mitochondrial manganese superoxide dismutase accumulate reactive oxygen species and show neurologic phenotypes similar to Leigh syndrome (8). Finally, mice deficient for the mitochondrial membrane protease Presenilins-associated rhomboid-like protein (PARL) suffer complex III defects and severe degeneration (9).
Among the many genes encoding enzymes of oxidative metabolism whose defects cause Leigh-like syndrome are nuclear genes encoding enzymes of the tricarboxylic acid (TCA) cycle (10). Of particular interest here are the nuclear genes encoding the 4 subunits (A, B, C, D) of succinate dehydrogenase (SDH), a TCA cycle enzyme that also functions as complex II of the electron transport chain of the inner mitochondrial membrane. SDH genes are highly conserved through evolution. Inherited combinations of recessive mutations of genes encoding SDH subunits have been reported to result in complex II deficiency and Leigh-like syndrome (10–15). Our interest in SDH mutations lies in a separate and peculiar manifestation of TCA cycle dysfunction (i.e., predisposition to cancer).
It is paradoxical that compound heterozygote combinations of hypomorphic mutant SDH subunit alleles cause Leigh-like syndrome (13), whereas heterozygosity for loss-of-function SDH alleles predisposes to cancers of neuroendocrine cells [paraganglioma (16)], kidney cells [renal cell carcinoma (17)], and the Interstitial Cells of Cajal [gastrointestinal stromal tumor (18)]. The current hypothesis to explain this paradox is that compound heterozygosity for hypomorphic SDH alleles causes systemic SDH deficiency (<50% of normal SDH activity) in all cells of the organism, mimicking other inborn errors of metabolism and resulting in complex II deficiency and Leigh-like syndrome. In contrast, heterozygosity for an allele encoding a loss-of-function SDH subunit confers no phenotype (no haploinsufficiency is observed for 50% of normal SDH gene dose) but predisposes to loss of heterozygosity (LOH) by stochastic events in individual cells. If LOH for an SDH mutation occurs in a susceptible cell at a sensitive time, oncogenesis results in the founder cell if SDH loss is sufficiently profound. Current models for tumorigenesis upon SDH loss invoke succinate as an accumulating oncometabolite (19) that poisons dioxygenase enzymes important for gene expression and epigenetic control in certain cell types (16). It is possible that there is an environment-dependent threshold of succinate accumulation above which oncogenesis occurs in isolated cells suffering SDH loss. In contrast, when SDH activity is reduced in all cells of an organism, typically by compound heterozygosity for SDH hypomorphic alleles, the resulting organismal energy insufficiency may present as complex II deficiency and Leigh-like syndrome.
Although heterozygosity for a loss-of-function SDH subunit allele predisposes to paraganglioma in humans, this predisposition has not been observed in mice (20). Our interest in creating SDH-loss mouse models of familial paraganglioma led us to create mice with conditional succinate dehydrogenase complex subunit C (SDHC) loss in all tissues to enhance the probability of paraganglioma tumorigenesis. This approach to systemic SDH loss has not yet produced a mouse model of familial paraganglioma. However, we report here that inducible systemic loss of the gene encoding the C subunit of SDH is lethal to most mice within 4 wk, and death is accompanied by lactic acidosis and other symptoms. We propose that this outcome represents a new inducible mouse model of a Leigh-like mitochondrial dysfunction syndrome.
Moreover, it has recently been reported that deleterious manifestations of mitochondrial diseases such as Leigh syndrome can be substantially attenuated by hypoxic conditioning (21). This finding invokes the concept that chronic hypoxia induces a cellular stress response that somehow confers resistance to the lethal impacts of reduced oxidative energy metabolism. The mechanism of this induced resistance remains unknown. Intrigued by this notion, we tested effects of chronic hypoxia on the lethal phenotype due to conditional whole-body SDHC loss in the mice described here. Remarkably, we report that chronic hypoxia (10% oxygen) confers substantial protection and durable survival on mice suffering systemic loss of SDHC. This result underscores the power of hypoxic conditioning to remodel physiology or create an environment remarkably favorable to a sustained glycolytic metabolism in animals defective for oxidative metabolism.
MATERIALS AND METHODS
Ethical treatment of animals
This study was conducted in accordance with guidance and an experimental plan approved in advance by the Mayo Clinic Institutional Animal Care and Use Committee.
Generation of SDHC conditional knockout mice
SDHC gene trap mouse strain C57BL/6N-SDHCtm1a(EUCOMM)Wtsi/Wtsi was obtained through the European Conditional Mouse Mutagenesis Program, Sanger Center, United Kingdom. Flippase recognition targeted SDHC gene trap floxed (fl) mice were crossed with flippase recombinase (FLP)-expressing mice to excise the Engrailed polyadenylation site, yielding loxP recombination sites flanking exon 4. PCR genotyping primers were LJM-4429 (5′-CT2AGA2CTGATC4TGC3-3′) and LJM-4430 (5′-CACTGC3G2CTCATAT3C-3′). SDHCfl/+ and SDHCfl/fl conditional knockout mice on the R26rtTA SDHCfl/fl tetO-Cre background were created by appropriate breeding, which allowed for disruption of the SDHCfl alleles by Cre recombination between the loxP sites upon doxycycline (dox) treatment. For this purpose, dox (2 mg/ml) was added to drinking water supplemented with 5 mg/ml sucrose as indicated (22). Genotyping for CRE-recombined SDHC was performed using LJM-4429 and LJM-5125 (5′-C2TG2A2CTAGA2T2AT2GATG2ATG-3′.
Mouse husbandry
Mice were housed by sex (n = 5/cage) in filter-capped polycarbonate cages, supplied with commercial pelleted chow (PicoLab Rodent Diet 20; LabDiet, St. Louis, MO, USA) and reverse-osmosis filtered water ad libitum in a room with constant temperature (21 ± 2°C) and humidity (45 ± 10%). Animals were exposed to 12 h of light daily.
PCR analysis of SDHC loss
Genomic DNA was extracted from isolated mouse tissues at the time of death or 3 mo following dox treatment using a DNeasy Blood and Tissue Kit (Qiagen, Germantown, MD, USA). DNA template (100 ng) was then amplified using Choice Taq Blue Master Mix (Thomas Scientific, Swedesboro, NJ, USA) using primers LJM-4429 with LJM-4430 to assess the product from the SDHCfl allele (595 bp) or with LJM-4429 and LJM-5125 to assess the product of the recombinant allele (560 bp). PCR cycling parameters were 15 min at 95°C, 38 cycles of (30 s at 95°C, 90 s at 58°C, 2 min at 72°C), 10 min at 68°C, followed by hold at 4°C.
Western blot analysis of SDH loss
Loss of SDH complex was assessed by Western blotting 25 d post dox treatment. Tissues were collected from the brain frontal lobe, heart ventricles, kidney cortex, and the right posterior liver. Tissue samples (0.5 mg) were lysed in 150 μl ice cold RIPA buffer (Santa Cruz Biotechnology, Dallas, TX, USA) containing 1× protease inhibitor cocktail and 1× phosphatase inhibitors with gentle vortex mixing and ice incubation for 20 min. Samples were then subjected to centrifugation at 15,000 g for 5 min prior to protein quantification with a bicinchoninic acid (BCA) Protein Assay Kit (Pierce, Rockford, IL, USA). Protein extracts were then denatured at 70°C for 10 min after the addition of reducing agent and 4× LDS buffer.
Denatured protein (25 µg) was then loaded onto a NuPage 10% Bis-Tris protein gel in 1× MES-SDS running buffer for electrophoresis at 150 V for 1 h. Gel-to-PVDF membrane transfer was performed following Western Transfer Apparatus instructions (Thermo Fisher Scientific, Waltham, MA, USA) in NuPage 1× transfer buffer containing 20% methanol. Transfer was at 30 V and 245 mA for 90 min at 4°C. Quality of transfer was checked by Ponceau S staining.
The transferred membrane was then exposed to 3% nonfat milk blocking buffer for 1 h at room temperature followed by 3 5-min washes in Tris-buffered saline, 0.1% Tween 20 (TBST). Blocked blots were then cut appropriately to enable staining and detection of the 42 kDa β-actin band and the 32 kDa succinate dehydrogenase (ubiquinone) iron-sulfur subunit, mitochondrial (SDHB) band. Ten milliliters of antibody dilution buffer was made using 7.5 ml TBST, 2.5 ml 4% BSA, and 250 μl 0.5% sodium azide. Rabbit antibodies used were SDHB polyclonal IgG antibody (ab178423, 1:2000 dilution; Abcam, Cambridge, MA, USA) and ACTB polyclonal IgG antibody (A2066, 1:500 dilution; MilliporeSigma, Burlington, MA, USA). Following overnight incubation at 4°C, blots were washed 3 times (5 min each) in TBST before introducing the secondary antibody and incubating them for 1 h at room temperature. Blots were then incubated in ECL 2 Western blot substrate (Pierce) at room temperature for 20 min prior to imaging with a GE Typhoon Fluorimeter (GE Healthcare Life Sciences, Chicago, IL, USA). Digital quantification was done using ImageQuant (GE Healthcare Life Sciences) and ImageJ software (National Institutes of Health, Bethesda, MD, USA), normalizing SDHB signals to β-actin signals after background subtraction.
Lifespan analysis
Mice were fed ad libitum and dox (2 mg/ml) was added to drinking water supplemented with 5 mg/ml sucrose for 1 wk to initiate the experiment. Animals were observed at least daily and euthanized promptly if moribund (weight loss or hunched/lethargic posture).
Hypoxia
Hypoxic housing for mice was in a custom chamber (BioSpherix, Parish, NY, USA), accommodating 12 cages with 5 mice/cage. Sex information is indicated in figure legends. Oxygen was controlled at 10% using a BioSpherix ProOx P360 oxygen single chamber controller. Carbon dioxide was adjusted to ≤1% with a BioSpherix ProCO2 P120 ppm carbon dioxide single chamber controller. This dual regulation system allowed control of oxygen while modulating total gas flow rate to purge carbon dioxide from the chamber to avoid carbon dioxide toxicity. For hypoxia studies, cohorts of SDHCfl/+ and SDHCfl/fl mice were weaned at 21 d, maintained in normal room air or introduced to hypoxia at 25 d, and treated with dox beginning at 30 d. Weights were recorded and moribund animals euthanized as necessary.
TCA cycle metabolite analysis
TCA cycle metabolites were determined by the Mayo Clinic Metabolomics Core Facility. Tissue samples were suspended in 100 µl PBS followed by addition of 20 µl internal standard solution containing [13C]-labeled analytes. Cells were sonicated for 60 s prior to addition of 400 µl chilled methanol/acetonitrile solution to precipitate proteins. After drying the supernatant by centrifugal evaporation, the sample was derivatized with ethoxime and then with 1% N-methyl-N-(t-butyldimethylsilyl)-trifluoroacetamide and 1% t-butyldimethylchlorosilane before analysis on an Agilent 5975C GC/MS instrument (Agilent Technologies, Santa Clara, CA, USA) under electron impact and single ion monitoring conditions. Concentrations of lactic acid (m/z 261.2), fumaric acid (m/z 287.1), succinic acid (m/z 289.1), oxaloacetic acid (m/z 346.2), ketoglutaric acid (m/z 360.2), malic acid (m/z 419.3), cis aconitic acid (m/z 459.3), citric acid (m/z 591.4), isocitric acid (m/z 591.4), and glutamic acid (m/z 432.4) were measured against a 7-point calibration curve after the same derivatization. The concentration of each metabolite was normalized to the number of cells in each sample and to the corresponding metabolite concentration in the control sample.
Serum biomarker analysis
Blood serum was collected at 28–35 d post dox treatment via cardiac puncture in serum gel blood collection microtubes (SAI Infusion Technologies, Lake Villa, IL, USA). Commercial ELISA kits were used to measure serum insulin (80-INSMSU-E01; Alpco, Salem, NH, USA) and corticosterone (55-CORMS-E01; Alpco). Colorimetric assay kits were used to measure lactate (K627-100; BioVision, Milpitas, CA, USA), succinate (K649; BioVision) and creatine kinase (CK) (K777; BioVision). Manufacturer protocols were followed directly without modifications. Glucose levels were measured from tail vein blood using a TrueTrack blood glucose meter (Trividia Health, Fort Lauderdale, FL, USA).
Body composition
Experimental and control mice of relevant genotypes were treated with dox. Lean mass and fat mass of individual mice were measured 3 wk after dox treatment by quantitative NMR using an EchoMRI analyzer (Houston, TX, USA).
Treadmill performance
Physical function was characterized 2 wk after dox treatment by measuring running time, distance, and total work to exhaustion using a motorized treadmill. Mice were first acclimated to the treadmill for 3 consecutive days in 5-min sessions at a speed of 10 m/min at a 5% grade. The next day, animals ran on the treadmill at an initial speed of 10 m/min and a grade of 5% for 2 min, and each subsequent 2 min the speed was increased by 2 m/min until the mice were exhausted. Exhaustion was defined as the inability of the mouse to remain on the treadmill despite a mild electrical shock stimulus and mechanical prodding. Running time was recorded, and running distance (a function of time and speed of the treadmill), work [the product of body weight (kg), gravity (9.81 m/s2), vertical speed [m/s × cos(angle)], and time were calculated.
Forelimb grip strength measurement
An Ametek Chatillon DFE2-002 device (Berwyn, PA, USA) was used to determine forelimb grip strength of animals 21 d post dox. Briefly, each mouse was lifted toward a metal bar allowing establishment of a symmetric, tight grip with the front paws. Mice were then gently pulled away from the bar until the grip was broken. The test was repeated 3 times per animal and results averaged for each animal and per genotype.
Accelerating rotarod test
A rotarod test was used to assess motor coordination in animals 7, 14, and 21 d following dox treatment. Caged mice were tail-marked using nontoxic colored ink then left to acclimate in the testing room for 30 min prior to testing. The procedure consisted of 3 testing trials separated by 30 min intertrial intervals with no prior training. The rotarod testing apparatus (Mouse Rota-Rod 47600; Ugo Basile, Gemonio, Italy) was used in accelerating mode to move from 5 to 40 rpm in 300 s. Mice were then placed on the apparatus lanes facing forward before initiation of testing. Latency to fall from the accelerating rotarod was recorded in second (up to a maximum of 500 s) either automatically as mice triggered a sensor upon falling or manually following the completion of a full passive rotation. Data were averaged for each subject and genotype.
Open field test
Locomotor activity and exploratory behavior were assessed using an open field test on 3 consecutive days at 7 and 25 d post dox. Caged mice were allowed to acclimate to the testing room for 30 min prior to test start. Meanwhile, 8 open field chambers (ENV-510; Med Associates, San Diego, CA, USA) were sprayed with 70% ethanol to clean the sensor surface and eliminate scent clues. Following acclimation, mice were placed into the chamber with minimal handling stress. The testing room was inaccessible during the 1-h testing session to eliminated external stimuli. Med Associates’ Activity Monitor 5 Monitoring Software was used to collect and analyze the data.
Comprehensive Lab Animal Monitoring System analysis
In a subset of 4 mice per group 3 wk after dox treatment, habitual ambulatory activity, oxygen consumption (Vo2), and carbon dioxide production (Vco2) of individual mice were monitored over a 24-h period with ad libitum feeding using a Comprehensive Laboratory Animal Monitoring System (CLAMS) equipped with photocells (Oxymax Open Circuit Calorimeter System; Columbus Instruments, Columbus, OH, USA). Ambulatory activity was summed and analyzed for light and dark periods. Vo2 and Vco2 values were used to calculate respiratory exchange ratio (RER), and Vo2 and RER values were used to determine energy expenditure (kcal/kg/h).
Pathology analysis
At necropsy, animals were euthanized with carbon dioxide, perfused with 1% PBS, and then preserved with 10% neutral buffered formalin. Selected organs were removed and fixed by immersion in neutral buffered formalin containing methanol as a stabilizing agent for at least 48 h prior to transfer (by overnight shipping in fresh fixative) to the histology facility. Brains (23) and other organs (24–26) were trimmed according to recognized schemes, after which all samples were embedded in paraffin. Serial sections of brain were stained with hematoxylin and eosin to assess general tissue architecture. Sections were evaluated using a Nikon E600 brightfield light microscope (Nikon, Tokyo, Japan) by an American College of Veterinary Pathologists board-certified veterinary pathologist.
RESULTS
Generation of SDHC-loss mice
We are interested in creating mouse models of SDH-loss familial paraganglioma (20). For this purpose, we exploited a null gene trap allele (Fig. 1A, top) that can be manipulated by Flp recombinase to revert the null allele to a functional conditional allele where coding exon 4 is flanked by loxP sites (Fig. 1A, middle). Expression of Cre recombinase drives exon deletion to create a mRNA with frameshift and premature stop codon (Fig. 1A, bottom). This strategy was applied to the mouse sdhc gene (Fig. 1B, top) to generate a functional fl allele (Fig. 1B, middle) that is knocked out by expression of Cre recombinase (Fig. 1B, bottom). Our previous study (20) and a study by other researchers (27) had revealed that, unlike humans, mice heterozygous for a null SDH allele are not predisposed to paraganglioma. We considered it possible that the rate of LOH limited paraganglioma tumorigenicity. We therefore generated conditional SDHC mice with 1 or 2 copies of a fl allele in animals carrying Cre recombinase under the control of the ubiquitously expressed reverse tet activator system, triggered by dox. We wished to test the hypothesis that SDHCfl/fl animals might survive SDHC gene loss and develop paraganglioma, whereas SDHCfl/+ animals would, like humans, show no SDH haploinsufficiency and serve as controls. This design allowed all animals to be treated with dox and express Cre recombinase, eliminating concerns that these variables could have untoward effects. Thee triggering of whole-body SDHC loss does not predispose to paraganglioma but rather creates a conditional model of Leigh-like syndrome.
Figure 1.
Generation of SDHC conditional knockout allele. A) Generic schematic showing Flp recombinase conversion of an inactivated gene trap allele to create a fl conditional allele, subject to knockout by exon deletion after dox treatment of mice carrying a Cre recombinase gene driven by bacterial tetracycline response elements in the presence of the R26rtTA reverse tet transactivator protein. B) Details of mouse SDHC engineered alleles relevant to SDHC knockout allele creation, including diagnostic PCR primers and genotyping PCR product sizes.
Triggered sdhc gene knockout in mouse tissues
Mice suffer rapid whole-body sdhc gene loss that is triggered when dox is introduced into the drinking water for 7 d. Representative tail biopsies from 32 mice demonstrate substantial sdhc rearrangement with diagnostic PCR patterns revealing genotype (Fig. 2A). Wild-type animals show no Cre-dependent gene rearrangement (Fig. 2A, lanes 1, 12, 24). Recombination is Cre-dependent (Fig. 2A, lanes 6, 7, 17–20, 28, 30), and heterozygotes demonstrate both rearranged and unrearranged alleles as expected (Fig. 2A, lanes 4, 5, 9, 11, 14, 15, 22, 25, 31).
Figure 2.
Conditional knockout of SDHC in mice. A) Genotyping results for tail DNA biopsies from 32 representative mice of the indicated genotypes 1 wk after dox treatment. Male mice: 478, 763–765. Female mice: 479, 480, 677, 766, 767, 776. B) Time and tissue dependence of SDHC knockout. Tail and muscle biopsy genotyping results from 8 representative mice (groups of 4 lanes) of the indicated genotypes, determined at weaning or at the indicated times relative to 7-d dox treatment. Male mice: 484, 488, 489, 492. Female mice: 482, 483, 493, 494. C. Tissue-dependent sdhc gene rearrangement for 2 female mice of the indicated genotypes. Rearrangement of the SDHCfl allele is compared for biopsies of tail and the indicated tissues at the indicated times relative to dox treatment.
The rapidity and durability of induced sdhc gene loss is demonstrated by sequential biopsies (tail or muscle) at weaning and 7 or 21 d after 1 wk of dox treatment. Data for 8 representative animals are shown in Fig. 2B. Rearrangement of sdhcfl alleles required Cre recombinase, as before (Fig. 2B, lanes 5–8 and 17–20). Interestingly, rearrangement of fl alleles in tail at 21 d exceeded that in quadriceps at the same time (Fig. 2B, compares lanes 11–12, 15–15, 23–24, 27–28, 31–32). Examination of multiple tissues from an additional pair of mice 21 d after conclusion of the 7-d dox treatment confirmed strong rearrangement in tail, adrenal gland (the adrenal medulla is rich in neuroendocrine cells), intestine, and muscle (Fig. 2C). Because the probability of rearranging both sdhcfl alleles in a single cell is perhaps the square of the probability of rearranging either allele, the data in Fig. 2 suggest that a significant fraction of cells in mouse tissues have become null for SDHC at 21 d.
Metabolite and blood marker analysis in SDHC-loss mice
We performed metabolite analysis of muscle biopsies from mice of 3 genotypes. Results from 11 female animals sampled 3 wk after dox treatment are shown in Fig. 3. SDHCfl/fl animals expressing Cre recombinase after dox treatment showed statistically significant decreases in TCA cycle metabolites citrate, isocitrate, and 2-ketoglutarate. Perhaps surprisingly, succinate levels were not elevated in muscle, but given that some other TCA cycle metabolites were reduced, this may reflect a relative accumulation of succinate in the tissue. Importantly, the succinate:2-ketoglutarate ratio, crucial for determining activity of dioxygenase enzymes (28), was greatly increased in tissues of dox-treated sdhcfl/fl mice, suggesting dioxygenase inhibition (Fig. 3). We also analyzed common blood serum markers in groups of 3 mice following a 4-h unfed period (Fig. 4). Animals showed weight loss (Fig. 4A). Serum glucose and insulin were found to be significantly lower in experimental SDH-loss animals compared to controls at 28 d following dox treatment (Fig. 4B, C), suggesting failed glucose metabolism. Whereas muscle lactate levels were increased (Fig. 3), serum lactate levels were not increased in dox-treated sdhcfl/fl mice (Fig. 4D). Corticosteroid levels were unchanged across genotypes, suggesting preserved adrenal function (Fig. 4E). In contrast, serum CK was significantly elevated in experimental animals (Fig. 4F), indicative of muscle damage. Interestingly, succinate levels were significantly elevated in the serum, of dox-treated sdhcfl/fl mice (Fig. 4G), consistent with systemic SDHC loss with succinate accumulation.
Figure 3.
Metabolite analysis from quadriceps muscle for 2–4-mo-old female mice of the indicated genotypes, where F indicates the sdhcfl allele, + indicates the wild-type SDHC allele, C indicates a chromosome encoding Cre recombinase under tet response element control, and x indicates the corresponding chromosome without CRE. All animals expressed the R26rtTA reverse tet transactivator protein. The indicated comparisons were found to be statistically significant by 1-way ANOVA followed by Tukey’s test for multiple comparisons (α = 0.05). Numbers of animals of each genotype: FFxx (3), F + Cx (3), FFCx (5).
Figure 4.
Serum metabolic profiles of female mice. Animals were weighed (A) and terminally bled 28–35 d post dox. Tail vein blood was used to monitor glucose (B), whereas purified serum was used to measure serum concentrations of glucose (B), insulin (C), lactate (D), corticosteroid (E), CK (F), and succinate (G). Statistical analysis was conducted using Student’s t tests, and only P values below 0.05 are shown. Error bars represent means ± sem. Data represent determinations for 6 experimental and control samples.
Behavioral and physical characteristics of SDHC-loss animals
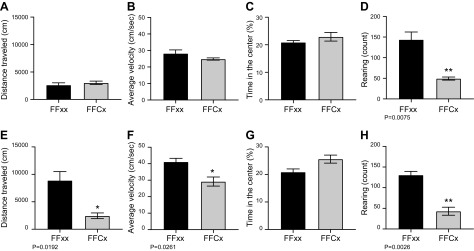
We used a conventional open field test to assess the level of locomotor activity, anxiety, and exploratory behavior in groups of 6 experimental and control animals 7 and 25 d following dox treatment. As expected based on casual observation, SDH-loss mice exhibited significant decline in spontaneous motor and exploratory activity level as they became moribund on approach to humane end point at ∼25 d post dox (Fig. 5). Interestingly, decreased rearing activity was observed even shortly after dox, unlike other open field parameters. An accelerating rotarod test was used to measure grip and balance on the moving apparatus. SDH-loss animals showed progressive decline in rotarod performance following dox treatment compared to controls (Fig. 6A). Finally, a forelimb grip test was employed to further evaluate muscle force performance for groups of 3 SDH-loss mice and controls 25 d following termination of dox treatment. Experimental mice demonstrated more than 50% reduction in forelimb grip strength (Fig. 6B).
Figure 5.
Impaired locomotor activity and exploration behavior in female conditional SDHC knockout mice. Experimental (n = 6) and control (n = 6) mice underwent 3 sessions of hour-long open field testing at 7- and 25-d post dox to assess the progression of locomotor loss. Distance traveled (A, E) and average velocity (B, F) were used to assess spontaneous movement, whereas percentage of time spent in the center (C, G) and rearing activity (D, H) were used to assess stress and exploration behavior. Open field track maps were analyzed in Med Associates’ Activity Monitor 5 software. Prism was used to perform statistical analysis by Student’s t test. Error bars represent means ± sem (P < 0.05 are shown).
Figure 6.
Loss of coordination and muscle strength in female SDHC knockout mice. A) Latency to fall from the rotarod apparatus is reported for experimental (n = 3) and control animals (n = 3) 7, 4, and 21 d following 1 wk of dox treatment in normal O2 conditions. Data are presented as the means ± sem. Statistical analysis was performed using 1-way ANOVA corrected by Bonferroni posttest. *P < 0.0320, ***P = 0.0006. B) Forelimb strength was measured in 6 experimental and 6 control females using digital force gauge 25 d following dox treatment. Analysis was done by Student’s t test, and error bars represent means ± sem.
We then undertook physiologic testing of groups of 5 mice of different genotypes. Automated CLAMS analysis of animal physiology was undertaken for animals 2–4 mo of age, 2–3 wk after dox treatment. Several physiologic parameters showed statistically significant changes 3 wk after dox treatment. Results for female mice are shown in Fig. 7. Conditional SDHC-loss mice showed increased fat body mass, reduced resting, active and total energy expenditure, reduced total activity, and reduced ambulation both day and night.
Figure 7.
Physiologic testing. Female mice of the indicated genotypes were subjected to CLAMS analysis 2 wk after conclusion of 1-wk treatment with dox. The indicated characteristics were statistically significant (α = 0.05). Genotype abbreviations are as in Fig. 3. Characteristics not found to have statistically significant differences include rearing activity, food intake, RER, and treadmill endurance. The fat body mass, resting energy expenditure (EE), active EE, and total EE comparisons were found to be statistically significant by paired Student’s t test (α = 0.05). Total activity and ambulation analysis was by 2-way ANOVA with Tukey’s test over genotype and time. Animal numbers were F + Cx (5), FFCx (5).
Mice suffering conditional whole-body SDHC loss showed only subtle changes in behavior, weight, and physiology over the first 3 wk following the 1-wk dox treatment. However, at about 4 wk, most SDH-loss mice suddenly declined in health, necessitating euthanasia. This health crisis was preceded by a brief period of weight loss (Fig. 8), although feeding behavior appeared normal. For mice of both sexes, there was no statistically significant difference in treadmill endurance (2 wk after dox treatment) or in food intake, RER, or rearing activity (3 wk after dox treatment; unpublished results).
Figure 8.
Weight data for female mice of different genotypes in room air. Female mice of the indicated genotypes were treated with dox in drinking water for 1 wk, and then weights were determined at the indicated times for surviving animals. Genotype abbreviations are as in Fig. 3.
Pathology
Detailed histopathology analysis was undertaken to understand the cause of death and to compare with classic Leigh syndrome. Remarkably, this careful analysis revealed no tissue pathology in any tested organs when comparing moribund SDH-loss animals with healthy control animals at the point that euthanasia of SDH-loss animals was necessary (Supplemental Fig. S1). We interpret these histopathology results to indicate that decline and death in SDH-loss animals occurs due to rapid and acute chemical or biochemical changes that are lethal prior to triggering detectable tissue pathology in major organs. This result is consistent with this unique conditional model where sudden loss of SDH activity occurs in many tissues of young adult mice, unlike human patients with Leigh syndrome who express sustained but inadequate levels of relevant metabolic enzymes and often show neurodegeneration.
SDHC-loss mouse lifespan and effects of hypoxic conditioning
As shown in Fig. 9A, B, only rare animals survived past 5 wk after dox triggering of systemic SDHC loss. Interestingly, some very rare survivors lived at least 100 d after dox treatment despite evidence of strong SDHC gene loss in multiple tissues. These animals showed no signs of paraganglioma tumorigenesis.
Figure 9.
A, B) Lifespan after systemic SDHC knockout for male mice (A) or female mice (B) of the indicated genotypes. Animals were maintained for the indicated number of days after 1-wk dox treatment in normoxia or 10% oxygen, as indicated. Genotype abbreviations are as in Fig. 3. C) PCR evidence of SDHC gene rearrangement at the indicated times in the indicated tissues (B, H, K, Li, Lu, T correspond to brain, heart, kidney, liver, lung, and tail, respectively). Control samples below horizontal bar were obtained from animals with wild-type (WT) SDHC, or mouse embryonic fibroblasts homozygous for the fl form of SDHC before (fl/fl) or after [knockout (KO)] dox treatment in vitro. D) Western blot evidence of SDH protein loss (using SDHB as marker) at the indicated times in the indicated tissues and oxygen conditions. Male mice: 210–212, 214, 215, 238, 241, 244. Female mice: 200, 201, 216, 239, 240, 242, 243, 245. E) Quantitation of data from (D). Error bars show means ± sd Analysis was done using ANOVA followed by Tukey’s correction for multiple comparisons. ***P = 0.0003 (liver, 10% O2) or ***P = 0.0002 (liver, 21% O2); ****P ≤ 0.0001.
A recent report (21) suggests that chronic hypoxic signaling can be protective for conditions involving respiratory energy defects, exemplified by a previous mouse model of Leigh syndrome due to loss of the Ndufs4 gene encoding NADH:ubiquinone oxidoreductase subunit S4. The basis for this protective effect of hypoxia remains unknown. We therefore compared the lifespans of SDHC-loss mice maintained in room air (normoxia), or at 10% oxygen (hypoxia). Data are shown in Fig. 9A, B. These data represent 74 male and 76 female mice. The results were striking. Whereas sdhcfl/+ control animals survived haploid SDHC loss induced by dox treatment in both normoxia and hypoxia, as expected, most sdhcfl/fl animals died 4 wk after SDHC loss induced by dox treatment in normoxia. In contrast, more than 50% of these homozygous SDHC-loss animals demonstrated prolonged survival after dox treatment in hypoxia. Genetic and Western blot analysis representing 8 male and 8 female mice (Fig. 9C, D) confirmed substantial and comparable SDH loss in multiple tissues under both oxygen levels. For Western blotting, we took advantage of the fact that SDHB protein is lost when any SDH subunit (e.g., SDHC) is depleted (29). Importantly, SDH loss is comparable independent of oxygen level, indicating that the protective effect of hypoxia is not due to inhibition of SDH loss (Fig. 9E).
DISCUSSION
The first signs of classic Leigh syndrome in human patients are often seen during early infancy. Adult-onset cases of the syndrome are less common, typically not as progressive, and sometimes appear with uncharacteristic symptoms and diagnostic parameters, such as the absence of acidosis and normal brain pathology (30–33). Existing mouse models of Leigh and Leigh-like syndrome, most notably Ndufs4-knockout, target activity of mitochondrial complex I during early development, resulting in progressive ataxic neurodegenerative symptoms that resemble human Leigh phenotype, leading to postnatal fatality in 50 d (34). Interestingly, deletion of Ndufs4 in adult mice via conditional Cre recombinase system produces milder, gliosis-like behavioral and neuromuscular symptoms. These mice do not improve on hypoxic treatment (4). Here we describe a conditional mouse model targeting mitochondrial complex II after weaning age, resulting in metabolic deficiency–related symptoms resembling those of adult-onset Leigh syndrome. SDHC-deficient animals used in this study show gradual loss of muscle tone and strength, coupled with locomotor hypoactivity, growth retardation, and mortality within a month following SDHC deletion. The animals also display low levels of serum insulin and glucose associated with metabolic chain defects (35) and elevated CK activity linked to mitochondrial myopathy (36). Whereas serum lactate is relatively normal, lactate elevation is detected in skeletal muscles. Elevation of lactate in cerebrospinal fluid, thought to be a reliable measurement of metabolic acidosis in humans, was not tested here (37). Given the predominance of muscle tissue in mediating blood metabolite levels, enhanced muscle glycolytic utilization could explain lowered blood glucose levels and enhanced lactate levels in muscle. Although unchanged serum corticosteroid levels suggested preserved adrenal function, there is a trend toward increased corticosteroid production in SDHC-loss animals, consistent with elevated stress. Serum creatine levels were increased upon SDHC loss, consistent with muscle damage. Interestingly, serum succinate levels were strikingly increased upon SDHC loss, consistent with release of accumulated succinate from tissues. Potential pathologic effects of elevated serum succinate are not known.
Unlike classic early-onset Leigh syndrome in human patients where neurodegeneration is common, pathology analysis of the present SDHC-loss mouse model revealed normal brain and adrenal functions and no other tissue abnormalities at time of death. This suggests that acute metabolic defects such as hypoglycemia drive rapid decline after SDH loss, resulting in death prior to detectable tissue damage except for elevated serum CK. There is evidence of lactic acidosis in muscle tissue but not in blood. This is unsurprising because blood lactate levels are considered less reliably elevated, especially in adult Leigh syndrome (30, 38). Given that conditional SDHC loss has been shown to provoke immune dysfunction in spleen (39), we also confirmed that mice do not succumb to infectious disease.
In summary, the conditional mouse SDHC-loss model reported here has features in common with Leigh-like syndrome, including muscle lactic acidosis, hypoglycemia, circulating insulin decrease, weight loss, blood CK increase, decreased muscle function, decline in activity, and, most importantly, protection from death by hypoxic conditioning. Because this mouse model shares some, but not all, classic characteristics of Leigh syndrome (40, 41), it is reasonable to describe the model as Leigh-like and to consider the model as representing a complex II–deficient mitochondrial disease more generally, with aspects of Leigh syndrome.
Although the work reported here is directly relevant to systemic metabolic disorders that typify Leigh-like syndrome, this study began as an experimental approach to a conditional mouse model of familial paraganglioma, a rare neuroendocrine cancer in which SDH genes serve as tumor suppressors (16). It is important to consider the distinct contexts of SDH loss in a metabolic disorder such as Leigh syndrome vs. paraganglioma. SDH gene disruption is embryonic lethal in mammals. The basis for tumor suppression by SDH in neuroendocrine cells is unknown, but succinate accumulation is thought to have a fundamental role. A mouse model of paraganglioma is desirable, but to date, mice heterozygous for SDH loss alleles are not, unlike humans, predisposed to paraganglioma (20), and strategies to provoke LOH have not been effective. Targeting complete conditional SDH-loss to specific tissues and developmental stages has been our general strategy for initiating paraganglioma in mice. We first created the systemic SDHC-loss model described here, inducible by dox, to monitor tumorigenesis after global loss of SDHC in young mice. We describe how this model induces profound SDHC loss across multiple tissues, and rather than initiating paraganglioma in neuroendocrine tissues, it results in an acute condition reminiscent of Leigh syndrome. Lethality commonly ensues after 4 wk. This short survival window does not allow for paraganglioma tumorigenesis. As described here, induced SDHC-loss mice do, however, provide a novel model of Leigh-like syndrome. Of particular interest in these conditional SDHC-loss mice are suppression of tissue 2-ketoglutarate and other TCA cycle intermediates, more than doubling the succinate:2-ketoglutarate ratio, and the sharp increase in lactate production in skeletal muscle, reminiscent of Leigh syndrome.
We were impressed by the previous report that chronic hypoxia can suppress deleterious effects of mitochondrial diseases such as Leigh syndrome (21). A genome-wide screen showed that loss of Von Hippel-Lindau factor was protective in a cell model of mitochondrial disease, implicating a hypoxic response as the active suppressor of disease (21). It remains unknown whether hypoxia induces a stress response that facilitates glycolytic metabolism or if the hypoxic environment simply makes glycolytic metabolism tolerable. This surprising observation led us to test our SDH-loss conditional Leigh syndrome model in mice maintained in chronic hypoxia (10% oxygen). We report that, as in previous models of mitochondrial disease, chronic hypoxia suppresses deleterious effects of SDH loss, allowing survival of mice that systemically lack SDH. As shown in Fig. 6, hypoxia does not alter SDH gene rearrangement or SDH protein loss. Protection from death in our new Leigh syndrome mouse model is a remarkable result for 2 reasons. First, hypoxic protection emphasizes the power of chronic hypoxia to alter an organismal response to a major metabolic insult. The mechanism of hypoxic suppression of deleterious effects due to mitochondrial dysfunction is unknown. Hypoxia inducible factors might play a role. For example, systemic energy deficit due to sudden loss of mitochondrial oxidative metabolism upon SDH loss may drive lethality in the current mouse model. Conditioning of mouse physiology by hypoxia-induced changes in gene expression may remodel metabolism toward anaerobic glycolysis, making animals more resilient to induced mitochondrial dysfunction. It will be interesting to determine in future studies whether chronic activation of hypoxia inducible factors by cobalt chloride administration could mimic this protective effect. Remarkably, hypoxic protection demonstrates the ability of a mammal to survive transition to a fundamentally glycolytic systemic metabolic state. The metabolism of SDH-loss mice enjoying long-term survival in hypoxia is the subject of future research. In additional, these surviving mice create the opportunity to monitor tumorigenesis in the context of SDH loss across many tissues.
It is interesting to consider the paradoxical interplay between hypoxia and SDH loss in the contexts of Leigh-like syndrome and cancer. Organism-wide SDH deficiency due to Mendelian inheritance of hypomorphic and loss-of-function SDH alleles can lead to complex II deficiency and Leigh-like syndrome if SDH expression is below some threshold. Chronic hypoxia is protective, with an unknown stress response shifting this threshold and decreasing the probability of Leigh-like syndrome, as shown here. In contrast, loss of SDH in individual neuroendocrine cells can occur by LOH in cells heterozygous for loss-of-function SDH alleles. In this case, paraganglioma tumorigenesis in humans can be triggered by the resulting metabolic derangement including succinate accumulation. In this context, chronic hypoxia may exacerbate tumorigenesis (28, 42, 43).
Finally, while providing a new conditional mouse model of complex II deficiency and Leigh-like syndromes, the long-term survival of SDH-loss mice under protective hypoxic conditions creates another potential model. SDH loss in susceptible neuroendocrine cells drives paraganglioma in humans. The ability to generate mice that can survive with profound SDH loss affecting many tissues will allow these animals to be monitored over time for paraganglioma tumorigenesis.
ACKNOWLEDGMENTS
The authors acknowledge the European Conditional Mouse Mutagenesis Program (Wellcome Sanger Institute, Hinxton, United Kingdom) for providing the SDHC gene trap mouse line. The technical assistance of Jenny Pattengill and the Mayo Clinic Metabolomics Core Facility and the Mayo Clinic Mouse Histology Core Facility is acknowledged. This work was supported by generous funding from the Paradifference Foundation, U.S. National Institutes of Health (NIH) National Cancer Institute Grant RO1 CA166025 (to L. J. M), and by the Robert and Arlene Kogod Center on Aging (Mayo Clinic). Funding of the Mayo Clinic Metabolomics Resource Core was by NIH National Institute of Diabetes and Digestive and Kidney Diseases Grant U24DK100469, and originated from the NIH Director’s Common Fund. The authors declare no conflicts of interest.
Glossary
- CK
creatine kinase
- CLAMS
Comprehensive Lab Animal Monitoring System
- dox
doxycycline
- fl
floxed
- FLP
flippase recombinase
- LOH
loss of heterozygosity
- NDUF
NADH dehydrogenase (ubiquinone) oxidoreductase
- RER
respiratory exchange ratio
- SDH
succinate dehydrogenase
- SDHB
succinate dehydrogenase (ubiquinone) iron-sulfur subunit, mitochondrial
- SDHC
succinate dehydrogenase complex subunit C
- TBST
Tris-buffered saline, 0.1% Tween 20
- TCA
tricarboxylic acid
- Surf
surfeit
- Vco2
carbon dioxide production
- Vo2
oxygen consumption
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
L. J. Maher and N. LeBrasseur designed research; F. Al Khazal and M. N. Holte performed research; F. Al Khazal, M. N. Holte, B. Bolon, T. A. White, N. LeBrasseur, and L. J. Maher analyzed data; and L. J. Maher, F. Al Khazal, and N. LeBrasseur wrote the manuscript.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Finsterer J. (2006) Central nervous system manifestations of mitochondrial disorders. Acta Neurol. Scand. 114, 217–238 [DOI] [PubMed] [Google Scholar]
- 2.Baertling F., Rodenburg R. J., Schaper J., Smeitink J. A., Koopman W. J. H., Mayatepek E., Morava E., Distelmaier F. (2014) A guide to diagnosis and treatment of Leigh syndrome. J. Neurol. Neurosurg. Psychiatry 85, 257–265 [DOI] [PubMed] [Google Scholar]
- 3.Moslemi A. R., Darin N., Tulinius M., Oldfors A., Holme E. (2005) Two new mutations in the MTATP6 gene associated with Leigh syndrome. Neuropediatrics 36, 314–318 [DOI] [PubMed] [Google Scholar]
- 4.Quintana A., Kruse S. E., Kapur R. P., Sanz E., Palmiter R. D. (2010) Complex I deficiency due to loss of Ndufs4 in the brain results in progressive encephalopathy resembling Leigh syndrome. Proc. Natl. Acad. Sci. USA 107, 10996–11001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agostino A., Invernizzi F., Tiveron C., Fagiolari G., Prelle A., Lamantea E., Giavazzi A., Battaglia G., Tatangelo L., Tiranti V., Zeviani M. (2003) Constitutive knockout of Surf1 is associated with high embryonic lethality, mitochondrial disease and cytochrome c oxidase deficiency in mice. Hum. Mol. Genet. 12, 399–413 [DOI] [PubMed] [Google Scholar]
- 6.Quintana A., Zanella S., Koch H., Kruse S. E., Lee D., Ramirez J. M., Palmiter R. D. (2012) Fatal breathing dysfunction in a mouse model of Leigh syndrome. J. Clin. Invest. 122, 2359–2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Haas R., Russel F. G., Smeitink J. A. (2016) Gait analysis in a mouse model resembling Leigh disease. Behav. Brain Res. 296, 191–198 [DOI] [PubMed] [Google Scholar]
- 8.Melov S., Schneider J. A., Day B. J., Hinerfeld D., Coskun P., Mirra S. S., Crapo J. D., Wallace D. C. (1998) A novel neurological phenotype in mice lacking mitochondrial manganese superoxide dismutase. Nat. Genet. 18, 159–163 [DOI] [PubMed] [Google Scholar]
- 9.Spinazzi M., Radaelli E., Horré K., Arranz A. M., Gounko N. V., Agostinis P., Maia T. M., Impens F., Morais V. A., Lopez-Lluch G., Serneels L., Navas P., De Strooper B. (2019) PARL deficiency in mouse causes Complex III defects, coenzyme Q depletion, and Leigh-like syndrome. Proc. Natl. Acad. Sci. USA 116, 277–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rustin P., Bourgeron T., Parfait B., Chretien D., Munnich A., Rötig A. (1997) Inborn errors of the Krebs cycle: a group of unusual mitochondrial diseases in human. Biochim. Biophys. Acta 1361, 185–197 [DOI] [PubMed] [Google Scholar]
- 11.Hoekstra A. S., Bayley J.-P. (2013) The role of complex II in disease. Biochim. Biophys. Acta 1827, 543–551 [DOI] [PubMed] [Google Scholar]
- 12.Birch-Machin M. A., Taylor R. W., Cochran B., Ackrell B. A. C., Turnbull D. M. (2000) Late-onset optic atrophy, ataxia, and myopathy associated with a mutation of a complex II gene. Ann. Neurol. 48, 330–335 [PubMed] [Google Scholar]
- 13.Alston C. L., Davison J. E., Meloni F., van der Westhuizen F. H., He L., Hornig-Do H.-T., Peet A. C., Gissen P., Goffrini P., Ferrero I., Wassmer E., McFarland R., Taylor R. W. (2012) Recessive germline SDHA and SDHB mutations causing leukodystrophy and isolated mitochondrial complex II deficiency. J. Med. Genet. 49, 569–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horváth R., Abicht A., Holinski-Feder E., Laner A., Gempel K., Prokisch H., Lochmüller H., Klopstock T., Jaksch M. (2006) Leigh syndrome caused by mutations in the flavoprotein (Fp) subunit of succinate dehydrogenase (SDHA). J. Neurol. Neurosurg. Psychiatry 77, 74–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackson C. B., Nuoffer J.-M., Hahn D., Prokisch H., Haberberger B., Gautschi M., Häberli A., Gallati S., Schaller A. (2014) Mutations in SDHD lead to autosomal recessive encephalomyopathy and isolated mitochondrial complex II deficiency. J. Med. Genet. 51, 170–175 [DOI] [PubMed] [Google Scholar]
- 16.Her Y. F., Maher L. J., III (2015) Succinate dehydrogenase loss in familial paraganglioma: biochemistry, genetics, and epigenetics. Int. J. Endocrinol. 2015, 296167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williamson S. R., Eble J. N., Amin M. B., Gupta N. S., Smith S. C., Sholl L. M., Montironi R., Hirsch M. S., Hornick J. L. (2015) Succinate dehydrogenase-deficient renal cell carcinoma: detailed characterization of 11 tumors defining a unique subtype of renal cell carcinoma. Mod. Pathol. 28, 80–94 [DOI] [PubMed] [Google Scholar]
- 18.Wang Y. M., Gu M. L., Ji F. (2015) Succinate dehydrogenase-deficient gastrointestinal stromal tumors. World J. Gastroenterol. 21, 2303–2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Selak M. A., Armour S. M., MacKenzie E. D., Boulahbel H., Watson D. G., Mansfield K. D., Pan Y., Simon M. C., Thompson C. B., Gottlieb E. (2005) Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell 7, 77–85 [DOI] [PubMed] [Google Scholar]
- 20.Maher L. J., Smith E. H., Rueter E. M., Becker N. A., Bida J. P., Nelson-Holte M., Paloma J. I. P., Garcia-Flores P., Lopez-Barneo J., van Deursen J. (2011) Mouse models of human familial paraganglioma. In Pheochromocytoma - A New View of the Old Problem (Martin J. F., ed.), pp. 25-46, Intech, Rijeka, Croatia: [Google Scholar]
- 21.Jain I. H., Zazzeron L., Goli R., Alexa K., Schatzman-Bone S., Dhillon H., Goldberger O., Peng J., Shalem O., Sanjana N. E., Zhang F., Goessling W., Zapol W. M., Mootha V. K. (2016) Hypoxia as a therapy for mitochondrial disease. Science 352, 54–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cawthorne C., Swindell R., Stratford I. J., Dive C., Welman A. (2007) Comparison of doxycycline delivery methods for Tet-inducible gene expression in a subcutaneous xenograft model. J. Biomol. Tech. 18, 120–123 [PMC free article] [PubMed] [Google Scholar]
- 23.Bolon B., Garman R. H., Pardo I. D., Jensen K., Sills R. C., Roulois A., Radovsky A., Bradley A., Andrews-Jones L., Butt M., Gumprecht L. (2013) STP position paper: recommended practices for sampling and processing the nervous system (brain, spinal cord, nerve, and eye) during nonclinical general toxicity studies. Toxicol. Pathol. 41, 1028–1048 [DOI] [PubMed] [Google Scholar]
- 24.Kittel B., Ruehl-Fehlert C., Morawietz G., Klapwijk J., Elwell M. R., Lenz B., O’Sullivan M. G., Roth D. R., Wadsworth P. F.; RITA Group ; NACAD Group (2004) Revised guides for organ sampling and trimming in rats and mice--part 2. A joint publication of the RITA and NACAD groups. Exp. Toxicol. Pathol. 55, 413–431 [DOI] [PubMed] [Google Scholar]
- 25.Morawietz G., Ruehl-Fehlert C., Kittel B., Bube A., Keane K., Halm S., Heuser A., Hellmann J.; RITA Group ; NACAD Group (2004) Revised guides for organ sampling and trimming in rats and mice--part 3. A joint publication of the RITA and NACAD groups. Exp. Toxicol. Pathol. 55, 433–449 [DOI] [PubMed] [Google Scholar]
- 26.Ruehl-Fehlert C., Kittel B., Morawietz G., Deslex P., Keenan C., Mahrt C. R., Nolte T., Robinson M., Stuart B. P., Deschl U.; RITA GroupNACAD Group (2003) Revised guides for organ sampling and trimming in rats and mice--part 1. Exp. Toxicol. Pathol. 55, 91–106 [PubMed] [Google Scholar]
- 27.Piruat J. I., Millán-Uclés A. (2014) Genetically modeled mice with mutations in mitochondrial metabolic enzymes for the study of cancer. Front. Oncol. 4, 200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peters J. P., Her Y. F., Maher L. J., III (2015) Modeling dioxygenase enzyme kinetics in familial paraganglioma. Biol. Open 4, 1281–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gill A. J., Benn D. E., Chou A., Clarkson A., Muljono A., Meyer-Rochow G. Y., Richardson A. L., Sidhu S. B., Robinson B. G., Clifton-Bligh R. J. (2010) Immunohistochemistry for SDHB triages genetic testing of SDHB, SDHC, and SDHD in paraganglioma-pheochromocytoma syndromes. Hum. Pathol. 41, 805–814 [DOI] [PubMed] [Google Scholar]
- 30.Jabeen S. A., Sandeep G., Mridula K. R., Meena A. K., Borgohain R., Sundaram C. (2016) Adult-onset Leigh’s disease: a rare entity. Ann. Indian Acad. Neurol. 19, 140–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phadke R. (2017) Myopathology of adult and paediatric mitochondrial diseases. J. Clin. Med. 6, 64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oh E. H., Chae S.-H., Cho J.-W., Baik S. K., Choi S.-Y., Choi K.-D., Choi J.-H. (2017) Fatigable ptosis as an initial presentation of adult-onset Leigh syndrome. Neurology 89, 1754 [DOI] [PubMed] [Google Scholar]
- 33.Pal G., Goldman J. (2013) An atypical case presentation of adult-onset Leigh’s disease (P07.206). Neurology 80, P07.206 [Google Scholar]
- 34.Kruse S. E., Watt W. C., Marcinek D. J., Kapur R. P., Schenkman K. A., Palmiter R. D. (2008) Mice with mitochondrial complex I deficiency develop a fatal encephalomyopathy. Cell Metab. 7, 312–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mochel F., Slama A., Touati G., Desguerre I., Giurgea I., Rabier D., Brivet M., Rustin P., Saudubray J.-M., DeLonlay P. (2005) Respiratory chain defects may present only with hypoglycemia. J. Clin. Endocrinol. Metab. 90, 3780–3785 [DOI] [PubMed] [Google Scholar]
- 36.Barić I., Fumić K., Petković Ramadža D., Sperl W., Zimmermann F. A., Muačević-Katanec D., Mitrović Z., Pažanin L., Cvitanović Šojat L., Kekez T., Reiner Z., Mayr J. A. (2013) Mitochondrial myopathy associated with a novel 5522G>A mutation in the mitochondrial tRNA(Trp) gene. Eur. J. Hum. Genet. 21, 871–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruhoy I. S., Saneto R. P. (2014) The genetics of Leigh syndrome and its implications for clinical practice and risk management. Appl. Clin. Genet. 7, 221–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wesolowska M., Gorman G. S., Alston C. L., Pajak A., Pyle A., He L., Griffin H., Chinnery P. F., Miller J. A. L., Schaefer A. M., Taylor R. W., Lightowlers R. N., Chrzanowska-Lightowlers Z. M. (2015) Adult onset Leigh syndrome in the intensive care setting: a novel presentation of a C12orf65 related mitochondrial disease. J. Neuromuscul. Dis. 2, 409–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bailis W., Shyer J. A., Zhao J., Canaveras J. C. G., Al Khazal F. J., Qu R., Steach H. R., Bielecki P., Khan O., Jackson R., Kluger Y., Maher L. J., III, Rabinowitz J., Craft J., Flavell R. A. (2019) Distinct modes of mitochondrial metabolism uncouple T cell differentiation and function. Nature 571, 403–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rahman S., Blok R. B., Dahl H. H., Danks D. M., Kirby D. M., Chow C. W., Christodoulou J., Thorburn D. R. (1996) Leigh syndrome: clinical features and biochemical and DNA abnormalities. Ann. Neurol. 39, 343–351 [DOI] [PubMed] [Google Scholar]
- 41.Sakushima K., Tsuji-Akimoto S., Niino M., Saitoh S., Yabe I., Sasaki H. (2011) Adult Leigh disease without failure to thrive. Neurologist 17, 222–227 [DOI] [PubMed] [Google Scholar]
- 42.Astrom K., Cohen J. E., Willett-Brozick J. E., Aston C. E., Baysal B. E. (2003) Altitude is a phenotypic modifier in hereditary paraganglioma type 1: evidence for an oxygen-sensing defect. Hum. Genet. 113, 228–237 [DOI] [PubMed] [Google Scholar]
- 43.Her Y. F., Nelson-Holte M., Maher L. J., III (2015) Oxygen concentration controls epigenetic effects in models of familial paraganglioma. PLoS One 10, e0127471 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.