Abstract
Long-chain polyunsaturated fatty acids (LCPUFAs), such as docosahexaenoic acid (DHA, 22:6) and docosapentaenoic acid (DPA, 22:5), have versatile physiologic functions. Studies have suggested that DHA and DPA are beneficial for maintaining sperm quality. However, their mechanisms of action are still unclear because of the poor understanding of DHA/DPA metabolism in the testis. DHA and DPA are mainly stored as LCPUFA-containing phospholipids and support normal spermatogenesis. Long-chain acyl-conenzyme A (CoA) synthetase (ACSL) 6 is an enzyme that preferentially converts LCPUFA into LCPUFA-CoA. Here, we report that ACSL6 knockout (KO) mice display severe male infertility due to attenuated sperm numbers and function. ACSL6 is highly expressed in differentiating spermatids, and ACSL6 KO mice have reduced LCPUFA-containing phospholipids in their spermatids. Delayed sperm release and apoptosis of differentiated spermatids were observed in these mice. The results of this study indicate that ACSL6 contributes to the local accumulation of DHA- and DPA-containing phospholipids in spermatids to support normal spermatogenesis.—Shishikura, K., Kuroha, S., Matsueda, S., Iseki, H., Matsui, T., Inoue, A., Arita, M. Acyl-CoA synthetase 6 regulates long-chain polyunsaturated fatty acid composition of membrane phospholipids in spermatids and supports normal spermatogenic processes in mice.
Keywords: testis, ACSL6, docosahexaenoic acid, docosapentaenoic acid
Long-chain polyunsaturated fatty acids (LCPUFAs) are composed of a 20- or 22-carbon chain length with multiple double bonds (1, 2). LCPUFAs are generally present in the forms of phospholipids and neutral lipids in tissues. The fatty acid composition is different among tissues, which may contribute to normal tissue function and homeostasis (3). However, the physiologic function of LCPUFAs and LCPUFA-containing phospholipids largely remains elusive because of the limited understanding of the molecular mechanisms underlying LCPUFA metabolism in LCPUFA-enriched cells and tissues.
Once taken up into the cytoplasm, free fatty acids are activated to form acyl-coenzyme A (CoA) and are eventually incorporated into phospholipids or neutral lipids. In the testis, docosahexaenoic acid (DHA, 22:6 n-3) and docosapentaenoic acid (DPA, 22:5 n-6) are highly enriched (3, 4). Lysophospholipid acyltransferase 3 (LPAAT3) is abundantly expressed in spermatids and selectively incorporates DHA into phospholipids in the testis (5). LPAAT3 knockout (KO) mice display severe male infertility with abnormal sperm morphology (5). Long-chain acyl-CoA synthetase (ACSL) is an enzyme that synthesizes long-chain acyl-CoA from long-chain fatty acids. ACSLs consist of 5 isoforms (ACSL1 and 3–6); each isoform has a different substrate preference (6). Among the isoforms, ACSL6 preferentially incorporates DHA and related LCPUFAs into phospholipids in vitro (7, 8). Of note, the tissue distribution of ACSL6 gene expression largely overlaps with the relative abundance of DHA-containing phospholipids (9). Recently, Chouinard et al. (10) reported that ACSL6 is involved in the selective incorporation of DHA into brain phospholipids. Here, we report for the first time that ACSL6 is also involved in the selective enrichment of DHA- and DPA-containing membrane phospholipids in differentiating spermatids, which supports normal spermatogenesis in mice.
MATERIALS AND METHODS
Generation of ACSL6 KO mouse strain
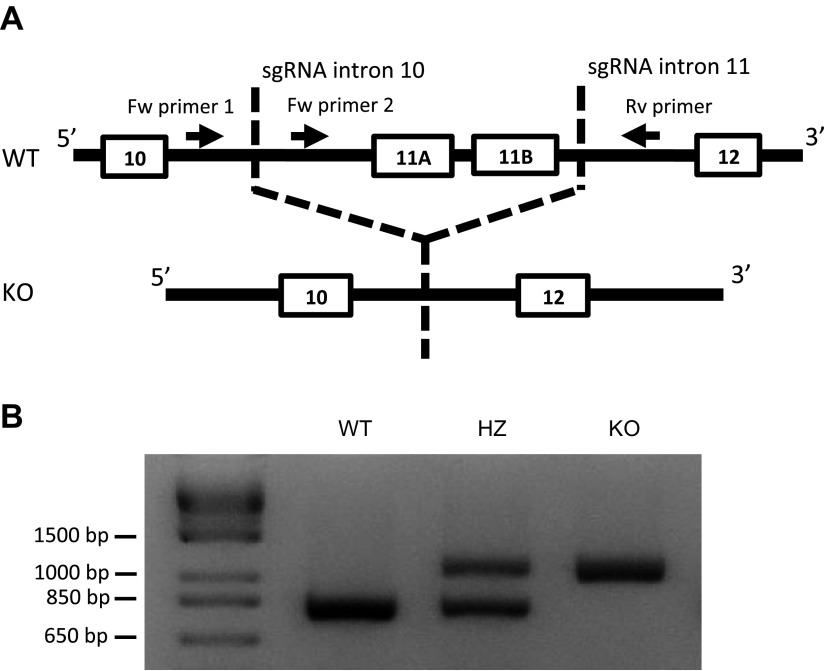
The ACSL6 KO mouse strain was generated using the clustered regularly interspaced short palindromic repeats/CRISPR-associated protein-9 nuclease (CRISPR/Cas9) system. Mouse ACSL6 has 2 gate domains, which are coded by exon 11 of ACSL6 gene and are essential for its catalytic activity (9, 11). We designed 2 single guide RNAs (sgRNAs) that have a common scaffold sequence to delete exon 11 (Fig. 1A). The 2 sgRNAs targeted introns 10 and 11. Cas9 mRNA and 2 sgRNAs were injected into the cytoplasm of C57BL/6J embryos (12). The ACSL6 intron 10 guide sequence was UGUUAGGUCGCAGCGCCAGU(GGG). The ACSL6 intron 11 guide sequence was AGGGAAUCCAUAUAGGACGG(UGG). The scaffold sequence was GUUUUAGAGCUAGAAAUAGCAAGUUAAAAUAAGGCUAGUCCGUUAUCAACUUGAAAAAGUGGCACCGAGUCGGUGCUUU. The protospacer adjacent motif is shown in parenthesis. Genotyping of mice was performed using PCR primer pairs for the wild-type (WT) and deleted sequence of the ACSL6 gene (Fig. 1B). Forward primer 1 was 5′-GCTGGGGTTTAATTTGGCCG-3′. Forward primer 2 was 5′-TGATCCAGGTAAGCCTCCGA-3′. The reverse primer was 5′-TGCCCAGCTGCGTAAAACTA-3′. PCRs were performed from 1 cycle of 3 min at 94°C, followed by 45 cycles of denaturation for 30 s at 94°C, primer annealing for 30 s at 58°C, and primer extension for 75 s at 72°C. In the KO allele, PCR product between primer 1 and reverse primer was well amplified, whereas in the WT allele, the product from primer 1 was not detected because of the short extension time. All animal experiments were approved by the Animal Care and Use Committee of Riken Yokohama Institute and Yokohama City University.
Figure 1.
Construction of ACSL6 KO mice. A) ACSL6 KO mice were produced by CRISPR/Cas9 using sgRNAs targeting introns 10 and 11. A 1-kbp-deletion mutant was produced that lacked 2 copies of exon 11. Using 2 forward (Fw) primers (located 3′ and 5′ of the deletion site in intron 10) and a reverse (Rv) primer (located 3′ of the deletion site in intron 11), we confirmed the deletion of ACSL6 exon 11. B) Genotyping results for ACSL6 WT, HZ, and KO mice. PCR of the WT allele yielded a PCR product from Fw primer 2. PCR of the KO allele amplified a product from Fw primer 1.
Gene expression analysis
Gene expression was measured using real-time quantitative RT-PCR. Total RNA was purified with the RNeasy Mini Kit and DNase I (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Total RNA (organs: 1000 ng, spermatids: 40–100 ng) was used to synthesize cDNA using Prime Script RT Master Mix (Takara Bio, Kyoto, Japan). Quantitative PCR was performed in duplicate using gene-specific primers with the Step One Plus Real-Time PCR system (Thermo Fisher Scientific, Waltham, MA, USA) and TB Green Premix Ex Taq II (Takara Bio) for detection. The primer sequences used were as follows: ACSL6, forward 5′-AAGCAGTCGGAAGAAGTGGAG-3′ and reverse 5′-GGCATCGTCATAGTAATGGGTAAG-3′; doublesex and Mab-3 related transcription factor 1 (DMRT1), forward 5′-TTCTGGCTTGGTCTCCCTCTC-3′ and reverse 5′-TCACTCGTCCTCATCCTCTTCC-3′; A-kinase anchoring protein 3 (AKAP3), forward 5′-CGCAAAGACCTGGAGAAAAG-3′ and reverse 5′-ACCTTTTCGTTGGTCCACTG-3′; protamine 2 (PRM2), forward 5′-CCACAAGAGGCGTCGGTCAT-3′ and reverse 5′-CCTTGGCTCCAGGCAGAATG-3′; and glyceraldehyde 3-phosphate dehydrogenase (GAPDH), forward 5′-TGACAATGAATACGGCTACAGCA-3′ and reverse 5′-CTCCTGTTATTATGGGGGTCTGG-3′. Relative mRNA levels were calculated using the ΔΔCt method and normalized to GAPDH mRNA (13).
Lipid extraction from tissues
Lipids were extracted from tissues or cells using single-phase extraction (14). The homogenates were mixed with MeOH and incubated for 1 h at room temperature. A half volume of CHCl3 was added, and the samples were incubated for 1 h at room temperature. Finally, water was added, and the extracts composed of MeOH:CHCl3:H2O (2:1:0.2; v/v) were obtained. After extraction, samples were centrifuged at 2000 g for 10 min, and the supernatants were collected.
Organelle separation
Homogenated testes were separated by centrifugation at 1000 g for 5 min to remove cell debris. The supernatant was centrifuged at 8000 g for 15 min to isolate mitochondria membrane fraction. The supernatant was further centrifuged at 100,000 g for 60 min to isolate microsome fraction. Post-8000 and -100,000 g pellets were applied to lipidomics analyses. To ensure the successful separation, we determined the presence of organelle marker proteins by immunoblotting. Anti-GM130 antibody (ab169276) and anti-ATP5A antibody (ab14748) were from Abcam (Cambridge, United Kingdom). Polyclonal anti-protein disulphide-isomerase (PDI) antibody (3501) and antilight chain 3B (LC3B) antibody were from Cell Signaling Technology (Danvers, MA, USA) and MilliporeSigma (Burlington, MA, USA), respectively.
Liquid chromatography–mass spectrometry–based lipidomics
Lipidomics was performed as previously described by Aoyagi et al. (15). Briefly, extracted lipids were measured using the Acquity ultra-performance liquid chromatography (LC) (UPLC) system (Waters, Milford, MA, USA) coupled with a quadrupole/time-of-flight mass spectrometry (MS) (TripleTOF 6600; AB Sciex, Framingham, MA, USA). LC separation was performed using a reverse-phase column [Acquity UPLC BEH Peptide C18 (50 × 1.7–mm inner diameter, 2.1-µm particle size; Waters)]. Samples were injected in 1 µl for tissue extracts and 3 µl for cell extracts.
Quantification of lipids
Detected species were annotated and quantified with MS-DIAL using peak height values (16). To calculate the fatty acid composition of the phospholipids, peak heights of individual species were normalized with the sum of the heights of all the detected species and presented as a percentage of the total phospholipid species (5). Phospholipid species in excess of 1% [phosphatidylcholine (PC), phosphatidylserine (PS), phosphatidylinositol (PI)] or 2% [phosphatidylethanolamine (PE)] of the total species are shown in the figures.
High-performance thin-layer chromatography and phospholipid measurement
After extraction by the Bligh and Dyer method, total lipids were applied on high-performance thin-layer chromatography plates (MilliporeSigma) using di-palmitate PC, PE, PS, PI, phosphatidylglycerol (PG), and phosphatidic acid (PA) as standards (17). Plates were developed in chloroform:ethanol:water:triethylamine, 30:35:7:35 (v/v/v/v), as previously described by Fuchs et al. (18). Each phospholipid in silica gel was scraped and extracted with chloroform:methanol, 1:1 (v/v), and the phosphorus content of each fraction was determined after digestion with perchloric acid as previously described by Bartlett et al. (19).
Tissue acyl-CoA synthetase activity
Tissue acyl-CoA synthetase activity was determined as previously described by Narita et al. (20) with slight modifications. After collection of the membrane fraction using buffer (0.25 M sucrose, 20 mM Tris-HCl pH 7.5, 1 mM DTT) with protease inhibitor cocktail (Complete EDTA free; MilliporeSigma), protein concentration was determined with bicinchoninic acid assay. The membrane fraction (2 µg protein) was reacted in 100 µl of reaction buffer (200 mM Tris-HCl pH 7.5, 8 mM MgCl2, 1 mM EDTA, 1 mM NaF, 1 mg/ml bovine serum albumin, 2.5 mM ATP, 0.5 mM CoA, 2.5 µM 16:0-d31, 2.5 µM 20:4-d5, 2.5 µM 22:5, and 2.5 µM 22:6). The reaction was terminated with methanol. Samples were then dried up and reconstituted in methanol:chloroform:water (2:1:0.2, v/v/v). After centrifugation at 2000 g for 10 min, the supernatant was applied to LC-MS.
Acyl-CoA measurement
Acyl-CoAs were measured with Acquity UPLC system coupled with a triple-quadropole MS (Qtrap 6500; AB Sciex). LC separation was performed using a reverse-phase metal-free column [YMC-triart C18 (50 × 2.1–mm inner diameter, 1.9-µm particle size; YMC, Kyoto, Japan)] with a gradient elution of mobile phase A [methanol:acetonitrile:water (1:1:3, v/v/v) containing 50 mM ammonium acetate, 500 nM EDTA, and 0.025% ammonium hydroxide] and mobile phase B (100% isopropanol containing 50 mM ammonium acetate, 500 nM EDTA, and 0.025% ammonium hydroxide). Column temperature was set at 40°C, and flow rate was 0.25 ml/min. For the separation, gradient elution started at 100% A, and after 1 min of hold time, B was increased linearly to 50% at 4 min and 95% at 6 min. Eluted acyl-CoAs were detected with multiple reaction monitoring (MRM) mode by using neutral loss of 3-phosphate-adenosine-5-diphosphate moiety (21). Acyl-CoAs were quantified by measuring the metabolite peak area as a ratio to the area of internal standard. The internal standard was 17:0 CoA (Avanti, Alabaster, AL, USA).
Single testicular cell sorting
After harvesting the testis, the tunica albuginea was removed and digested with 1 mg/ml collagenase type I (Wako, Osaka, Japan) in DMEM for 10 min at 37°C. The digested samples were centrifuged at 350 g at 20°C for 5 min. The pellets were treated with 0.25% trypsin (Wako) and DNase Type I (Wako) in PBS at 37°C for 10 min. The reaction was neutralized with 10% fetal bovine serum/DMEM. A single-cell suspension was obtained by passage of the cells through a 40-µm cell strainer. Testicular single cells were stained with 2.5 µg/ml Hoechst 33342 (Dojindo Laboratories, Kumamoto, Japan) in 10% fetal bovine serum/DMEM at 33°C for 60 min. After centrifugation at 350 g for 5 min at 4°C, cells were stained with 1 µg/ml propidium iodide. Stained cells were analyzed and sorted using the BD fluorescence-activated cell sorting Aria flow cytometer (BD Biosciences, San Jose, CA, USA). To obtain highly purified spermatids, we gated cells using a combination of 2 methods (22, 23). The gating is shown in Supplemental Fig. S2.
Isolation of sperm from the cauda epididymis
Sperm were isolated from the cauda epididymis by cutting and gentle squeezing and then incubated in human tubal fluid (HTF) medium (Merck GmbH, Darmstadt, Germany) at 37°C for 1 h. After the incubation, the sperm were fixed with 2% paraformaldehyde and counted using a cell counting plate (Watson, San Diego, CA, USA).
Morphologic analysis of epididymal sperm
Fixed sperm were smeared on glass slides and dried at room temperature for 2 h. The samples were washed with PBS and stained with hematoxylin and eosin (HE). Images were obtained by fluorescence microscopy BZ-X710 (Keyence, Osaka, Japan). Sperm that had a tail and nucleus were counted. Sperm that did not have a sharp craw-like head were defined as abnormal. Cell debris was ruled out during counting.
Oocyte collection and in vitro fertilization
MII-stage oocytes were collected from 8-wk-old Institute of Cancer (ICR) females superovulated with 7.5 I.U. Pregnant mare serum gonadotropin/human chorionic gonadotropin (PMSG/hCG) (ASKA Animal Health, Tokyo, Japan). Sperm were obtained from the caudal epididymis of adult ACSL6 heterozygous (HZ) or KO males in 200 μl HTF medium supplemented with 10 mg/ml bovine serum albumin (MilliporeSigma). Spermatozoa capacitation was attained by 1 h of incubation in HTF medium. For some experiments, the zona pellucida (ZP) was removed from oocytes by a brief treatment with Acidic Tyrode’s solution (Merck). A constant concentration of sperm was used for in vitro fertilization (IVF) (4.5–7.0 × 105/ml for ZP-intact IVF). The fertilization ratio was determined 6 h after IVF by observing pronuclei and the second polar bodies. Zygotes were cultured in potassium simplex optimized medium (KSOM) (Merck) in a humidified atmosphere with 5% CO2/95% air at 37.8°C.
Intracytoplasmic sperm injection
MII-stage oocytes were collected from 8-wk-old B6D2F1/J (BDF1) females superovulated with 7.5 I.U. PMSG and hCG. Cumulus cells were dissociated from the MII oocytes by treatment with M2 medium containing hyaluronidase (Merck), and the oocytes were incubated in KSOM medium. Sperm collection and capacitation were performed in HTF as described above. After cutting the sperm tails in 10% polyvinylpyrrolidone (PVP) solution (Irvine Scientific, Santa Ana, CA, USA) using an impact-driven piezomicromanipulator, sperm heads were injected into MII oocytes. The oocytes were then transferred back to KSOM medium.
Histologic analysis
The testes were fixed with Davidson’s fixative for 24 h at room temperature and embedded in paraffin. Samples were cut and stained with HE. TUNEL staining was performed using the In Situ Cell Death Detection Kit (MilliporeSigma) according to the manufacturer’s instructions. Images were obtained using the Leica TCS SP8 Laser Scanning Confocal Microscope (Leica, Wetzlar, Germany).
Field emission scanning electron microscopy
Perfusion fixation was performed through the left ventricle with 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4). The testes or epididymis were then immersed in the same fixative for 2 h at 4°C. After rinsing 3 times with 0.1 M phosphate buffer, the samples were postfixed with 2% osmium tetroxide (Taab, Aldermaston, United Kingdom) for 1 h at room temperature and then dehydrated for 20 min each with 50, 70, 80, 90, and 95% ethanol and 3 changes of 100% ethanol. After dehydration, tissues were incubated with 100% propylene oxide (2 changes for 20 min each) and then treated with 1:1 propylene oxide–Taab Epon 812 (Taab) mixture overnight at room temperature. Tissues were incubated with 1:2 propylene oxide–Taab Epon 812 mixture for 3 h and then transferred to Epon 812 for 4 h at room temperature. The samples were hardened at 60°C for 2 d in a Cryo Dish (Shoei Works, Tokyo, Japan). After trimming, 3 × 4–mm sections were cut horizontally with a histo diamond knife (Diatome, Hatfield, PA, USA) using an Ultracut UCT Microtome (Leica) at 0.15-µm thickness. The sections were placed on slide glasses and then stained with Toluidine blue (Wako). Images were collected using the BZ-X710 (Keyence). The stained sections were further stained with 0.4% uranium acetate and Reynold’s solution. After drying, they were coated with osmium using an HPC-1SW vacuum evaporator (Shinku Device, Mito, Japan). Electron microscopic images were obtained using the SU8220 field emission scanning electron microscope (Hitachi High Technologies Corp., Tokyo, Japan) with a YAG-BSE detector (5 kV).
Mitochondrial membrane potential
For the mitochondrial membrane potential (MMP) measurement, sperms from epididymis were stained with 2.5 µg/ml Hoechst 33342 for 20 min at 33°C and then incubated for 10 min in 5 µg/µl JC-1 (Thermo Fisher Scientific) with or without 100 µM carbonyl cyanide m-chlorophenyl hydrazone (CCCP; MilliporeSigma). After staining with Ghost dye Red 780 (Tonbo Bioscience, San Diego, CA, USA), cells were analyzed with fluorescence-activated cell sorting Canto II (BD Biosciences) as previously described by Agnihotri et al. (24).
Measurement of reproductive hormones
Serum follicle stimulating hormone (FSH), LH, and testosterone were quantified using ELISA kits according to the manufacturers’ instructions: Testosterone Parameter Assay Kit KGE010 (R&D Systems, Minneapolis, MN, USA); LH ELISA Kit CEA441Mu (Cloud-Clone, Houston, TX, USA); FSH ELISA Kit CEA830Mu (Cloud-Clone).
RESULTS
Male infertility in ACSL6 KO mice
The ACSL6 KO mouse strain was established using the CRISPR/Cas9 system (Fig. 1). The crossing of ACSL6 HZ mice yielded ACSL6 WT, HZ, and KO mice almost at a Mendelian ratio (79:157:65). Although each genotype grew normally, we found that mature ACSL6 KO males (8 wk of age) were completely infertile and failed to produce offspring when mated to 8-wk-old WT females for 12 wk (Table 1).
TABLE 1.
Male infertility of ACSL6 KO mice
| Pairs (male × female) | Litter | Litter size |
|---|---|---|
| WT × WT (n = 9) | 23 | 129 |
| HZ × WT (n = 9) | 23 | 142 |
| KO × WT (n = 10) | 0 | 0 |
Decreased levels of DHA and DPA phospholipids in spermatids
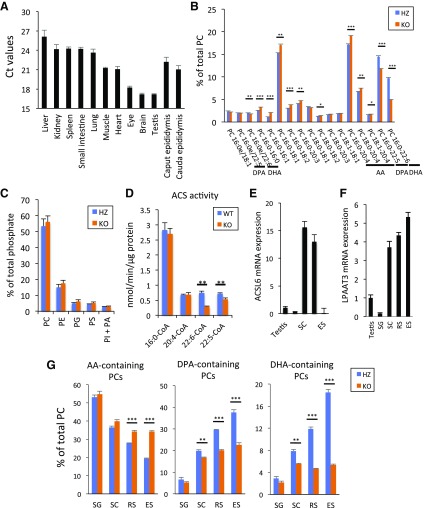
ACSL6 is abundantly expressed in brain, testis, eyes, heart, and skeletal muscle (Fig. 2A). As reported previously (25, 26), DHA- and DPA-containing phospholipids are abundant in the testis. We found that these phospholipid species were significantly reduced in ACSL6 KO mice. In contrast, arachidonic acid (AA, 20:4)-containing PC was significantly increased in ACSL6 KO mice without changing the amount of each phospholipid species (Fig. 2B, C). The total acyl-CoA synthetase activity was significantly reduced by 60% for DHA and by 25% for DPA, whereas no change was observed for palmitate and AA in ACSL6 KO testis (Fig. 2D). These results indicate that ACSL6 is involved in the selective incorporation of DHA and DPA into the phospholipids of the testis via the DHA- and DPA-CoA production (Supplemental Fig. S1). Lipidomic analysis revealed that the changes in fatty acid species in phospholipids were similar among different membrane fractions (Supplemental Fig. S2). To determine the ACSL6 localization in the testes, we isolated different stages of germ cells from the adult mouse testis using flow cytometry (Supplemental Fig. S3). The cell fractions contained diploid spermatogonia, tetraploid spermatocyte (SC), haploid round spermatid (RS), and elongated spermatid (ES) (27). Compared with the average testicular ACSL6 gene expression, SC and RS showed higher ACSL6 mRNA expression (Fig. 2E). This gene expression pattern was similar to that of LPAAT3 expression in SC, RS, and ES (Fig. 2F). DPA- and DHA-containing PCs were significantly increased during spermatid differentiation, whereas the levels of AA-containing PCs were reduced. Differentiating spermatids from ACSL6 KO mice had reduced levels of DPA- and DHA-containing PCs compared with HZ mice (Fig. 2G).
Figure 2.
ACSL6 produces DHA phospholipid in spermatids. A) ACSL6 expression was determined by RT-PCR from total RNA extracted from different tissues. B) Percentage of total PC in ACSL6 HZ and KO testes. Only phospholipids present in excess of 1% are shown. C) Abundance of major phospholipid species in ACSL6 HZ and KO testes. Data are presented as means ± sem (n = 3). D) Enzymatic activity of WT and KO testes. Data are presented as means ± sem (n = 5). E, F) mRNA expression of ACSL6 (E) and LPAAT3 (F) in isolated spermatids compared with that of the whole testis. mRNA levels were normalized to GAPDH. G) Percentage of AA (20:4)-, DHA (22:6)-, and DPA (22:5)-containing PC in isolated spermatids. SG, spermatogonia. Data are presented as means ± sem (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001 (unpaired Student’s t test).
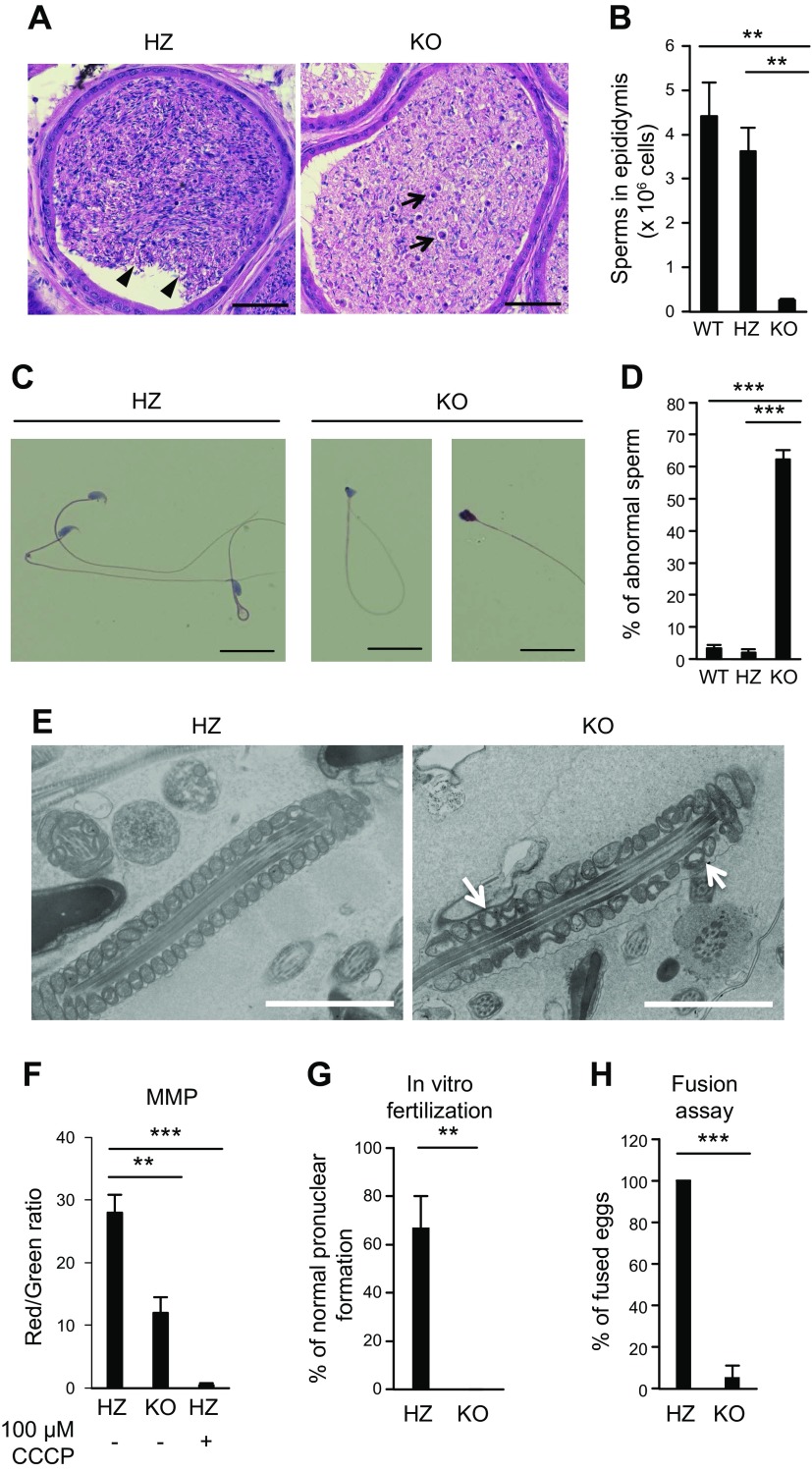
Decreased sperm number and function in ACSL6 KO mice
Sperm is stored in the cauda epididymis. Thus, we examined the number and function of sperm from the cauda epididymis of ACSL6 HZ and KO mice to determine male fertility (28). HE staining revealed that the cauda epididymis of ACSL6 KO mice contained abnormal sperm and cell debris (Fig. 3A). Consistent with the histologic findings, the number of sperm isolated from the cauda epididymis of ACSL6 KO mice was much lower than that of the HZ mice (Fig. 3B). A closer examination of the sperm morphology demonstrated that the majority of the ACSL6 KO sperm were abnormally shaped and had irregularly sized heads (Fig. 3C, D). MMP was measured by a fluorescent dye, JC-1, and we found that MMP was decreased (−50%) in the KO sperm, as shown in Fig. 3F. Also, electron microscopy identified uneven and void mitochondria in the KO sperm, as shown Fig. 3E. To examine the fertility of the ACSL6 KO sperm, we performed IVF using WT oocytes. We found that the oocytes could not be fertilized by the KO sperm even though the KO and HZ sperm were used at the same concentration (Fig. 3G). To examine whether the ACSL6 KO sperm were unable to fuse with oocytes, we removed the ZP from oocytes prior to IVF. In contrast with the HZ sperm that showed a 100% fusion ratio, only 5% of the KO sperm could fuse with the oocytes, indicating that the fusion capacity of ACSL6 KO sperm was severely defective (Fig. 3H). To address the developmental potential of embryos derived from ACSL6 KO sperm, we performed intracytoplasmic sperm injection (ICSI). We assessed the pronuclear formation ratio and the cleavage ratio at 6 and 24 h after ICSI, respectively, and found that the pronuclear formation ratio in oocytes injected with ACSL6 KO sperm was smaller than that of the HZ sperm (Table 2). In addition, the cleavage ratio in the KO group was smaller than that of the HZ group (Table 2). These results indicate that not only the fertilization capacity but also the developmental capacity of ACSL6 sperm are diminished, which could account for male infertility observed with ACSL6 KO mice.
Figure 3.
The function and quantity of ACSL6 KO sperm were disordered. A) Representative images of HE staining of the ACSL6 HZ and KO cauda epididymis. Arrowheads indicate normal sperm with tails and heads. Arrows point to cell debris for the KO mice. B) The numbers of WT, HZ, and KO sperm from the cauda epididymis. Data are presented as means ± sem (n = 4). C) HE staining of HZ and KO sperm. D) Percentage of abnormal sperm from WT, HZ, and KO mice. Data are presented as means ± sem (n = 4). E) FE-SEM images of ACSL6 HZ and KO epididymis. The arrows show the mitochondria devoid of content. F) MMP measured by flow cytometry. G, H) Successful IVF with (G) or without (H) the ZP. Scale bars, 2 µm. Data are presented as means ± sem (n = 3). **P < 0.01, ***P < 0.001 [Tukey’s honestly significant difference (HSD) test (B, D, F); unpaired Student’s t test (G, H)].
TABLE 2.
Fertilization and cleavage ratios after ICSI for HZ and KO sperm
| Genotype | Pronuclear formation (%) | Cleavage (%) |
|---|---|---|
| HZ | 33/42 (78.6) | 32/42 (76.2) |
| KO | 33/67 (49.3) | 22/67 (32.8) |
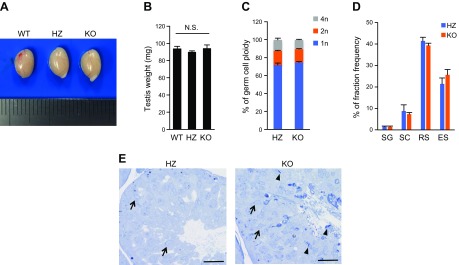
Disordered sperm release and ES apoptosis
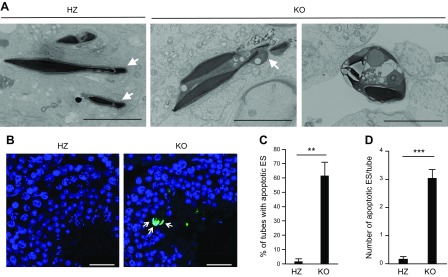
Abnormal spermatogenesis was attributed to disordered sperm release in the testes of LPAAT3 KO mice (5). To determine the stage of sperm release, we used toluidine blue staining to identify ESs in the ACSL6 KO testis. Although there were no significant changes in testis weight or testicular cell populations, many ESs were observed in the stage IX seminiferous tubules (Fig. 4). These results indicate that sperm release was disordered in ACSL6 KO mice, without a change in development or cell differentiation. Field emission scanning electron microscopy (FE-SEM) of stage IX seminiferous tubules revealed abnormally shaped acrosomes in the ESs, which was similar to the phenotype observed in LPAAT3 KO mice (Fig. 5A). However, many vesicular structures containing ESs were uniquely observed in the ACSL6 KO mouse–derived ESs (Fig. 5A). Because this structure was reminiscent of ES apoptosis, we performed TUNEL staining (29, 30). Notably, more TUNEL-positive ESs were observed in ACSL6 KO mice compared with HZ mice (Fig. 5B–D). In contrast, we found that the serum concentrations of sex hormones (e.g., FSH, LH, and testosterone), which regulate ES cell survival and sperm release (31), were not significantly different between HZ and KO mice (Table 3).
Figure 4.
Sperm release was delayed in ACSL6 KO mice. A, B) The appearance (A) and weight (B) of testes from WT, HZ, and KO mice. N.S., not significant. Data are presented as means ± sem (n = 4). C) Germ cell ploidy in HZ and KO mice. Data are presented as means ± sem (n = 3). D) Fraction frequency of isolated germ cells. Data are presented as means ± sem (n = 3). E) Toluidine blue staining of stage IX seminiferous tubules from ACSL6 HZ and KO mice. The arrows indicate the start of RS elongation, which is characteristic of stage IX tubules (46). The arrowheads point to the ESs. Scale bars, 25 µm. B) Tukey’s honestly significant difference. C, D) No significant change was detected; unpaired Student’s t test.
Figure 5.
Apoptosis of ESs in ACSL6 KO mice. A) FE-SEM images of ACSL6 HZ and KO testes. The arrows show the acrosomes. Scale bars, 4 µm. B) TUNEL staining of ACSL6 HZ and KO seminiferous tubules. Nuclei are stained blue (Hoechst). Apoptotic cells are green (TUNEL). The arrows indicate ES undergoing apoptosis. Scale bars, 30 µm. C, D) The percentage of tubes with apoptotic ES (C) and the number of apoptotic ES per tubules (D). Data are presented as means ± sem (n = 3). **P < 0.01, ***P < 0.001 (unpaired Student’s t test).
TABLE 3.
Serum sex hormone levels in ACSL6 HZ and KO mice
| Hormone | HZ | KO |
|---|---|---|
| FSH (ng/ml) | 24.34 ± 1.34 | 23.10 ± 3.19 |
| LH (ng/ml) | 5.24 ± 1.08 | 6.23 ± 1.70 |
| Testosterone (ng/ml) | 15.59 ± 0.90 | 17.0 ± 2.14 |
Data are presented as means ± sem (n = 4). No significant changes were detected; unpaired Student’s t test.
DISCUSSION
In this study, we demonstrated that ACSL6 is involved in the selective enrichment of LCPUFA-containing membrane phospholipids in differentiating spermatids, which supports normal spermatogenesis. Based on human and animal studies, it has been reported that dietary intake of DHA correlates well with increased sperm number and function (32). Conversely, decreased testicular DPA levels are correlated with male infertility (4). DHA and DPA are highly enriched in the testis and spermatozoa in the forms of membrane phospholipids, suggesting those phospholipid species have some important roles in maintaining normal testicular function (4, 32, 33). These data are consistent with the finding that LPAAT3 KO mice have a defect in selective DHA incorporation into phospholipids and also display severe male infertility with abnormal sperm morphology (5). ACSL6 KO and LPAAT3 KO mice have a similar phenotype: decreased sperm fertilization and number and unreleased spermatids. However, remarkably, ES apoptosis was observed in ACSL6 KO but not LPAAT3 KO mice. This difference could stem from the type of decreased LCPUFA-containing phospholipids. In ACSL6 KO mice, DHA- and DPA-containing phospholipids were partially reduced (∼50% for DHA, ∼20% for DPA), whereas DHA- but not DPA-containing phospholipids were dramatically reduced in LPAAT3 KO mice. The precise function of DHA- and DPA-containing phospholipids in spermatogenesis is still poorly understood. Nevertheless, in the previous report on male infertility in LPAAT3 KO mice, it was discussed that membrane flexibility increased by LCPUFA-containing phospholipids may support the endocytosis of excess cytoplasm at the late stage of spermatogenesis (5).
ACSL family enzymes catalyze a wide range of fatty acids to form acyl-CoAs, with some substrate preferences (6, 34–36). Lipidomic profiling of ACSL6 KO mice revealed significant reductions of DHA (22:6)- and DPA (22:5)-containing phospholipids with a compensatory increase in other polyunsaturated fatty acid (PUFA)-containing species, such as AA (20:4), in differentiating spermatids. This type of change is not observed in the KO mice for other ACSL family members (10, 37–41), suggesting that ACSL6 has selectivity for chain length and double bonds in PUFA structures in vivo. Indeed, the total ACS activity was significantly reduced for DHA and DPA, whereas no change was observed for palmitate and AA in ACSL6 KO testis. Because ACSL6 and LPAAT3 are highly coexpressed in spermatids, there might be some functional coupling of these enzymes to efficiently and selectively incorporate LCPUFA into membrane phospholipids in vivo.
Delta-6 fatty acid desaturase KO mice fed an AIN93G diet exhibit spermatogenic arrest between stages RS and ES that is accompanied by decreased LCPUFA levels in the testis (42, 43). In addition, elongation of very-long-chain fatty acids protein 2 KO mice display complete arrest of spermatogenesis with reduced very-long-chain PUFA levels in the testis (44). Ceramide synthase 3 is an enzyme expressed in SCs that generates ultra-long polyunsaturated ceramides; ceramide synthase 3 KO mice display spermatogenic arrest at RS (45). These results clearly indicate the critical role of LCPUFAs in normal spermatogenesis, but further investigation is required to identify which LCPUFA-containing species are important for conferring a spermatoprotective effect in vivo.
In summary, we identified ACSL6 as a critical enzyme that enriches LCPUFA-containing phospholipids in the testis and is essential for normal male fertility by regulating proper spermatogenesis.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
This study was supported by the Japan Society for the Promotion of Science (JSPS) Grants-in-Aid for Scientific Research (KAKENHI; JP15H05897, 15H05898, and 15H04648 to M.A.), and Riken Junior Research Associate Program (to K.S.). The authors thank T. Ogawa (Yokohama City University Association of Medical Science) for constructive comments on the research, and K. Toyooka (Science Technology Platform Division, Center for Sustainable Resource, Riken) for technical support for the FE-SEM analyses, and all the members of the M.A. laboratory and the Laboratory for Developmental Genetics (Riken Center for Integrative Medical Sciences) for technical support for the histological analysis. The authors also thank the staff at BioMed Proofreading, LLC (Cleveland, OH, USA) for help with the English translation of the manuscript. The authors declare no conflicts of interest.
Glossary
- AA
arachidonic acid
- ACSL
long-chain acyl-coenzyme A (CoA) synthetase
- CRISPR/Cas9
clustered regularly interspaced short palindromic repeats/CRISPR-associated protein-9 nuclease
- DHA
docosahexaenoic acid
- DPA
docosapentaenoic acid
- ES
elongated spermatid
- FE-SEM
field emission scanning electron microscopy
- FSH
follicle stimulating hormone
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- HE
hematoxylin and eosin
- HZ
heterozygous
- ICSI
intracytoplasmic sperm injection
- IVF
in vitro fertilization
- KO
knockout
- KSOM
potassium simplex optimized medium
- LC
liquid chromatography
- LCPUFA
long-chain PUFA
- LPAAT3
lysophospholipid acyltransferase 3
- MMP
mitochondrial membrane potential
- MS
mass spectrometry
- PC
phosphatidylcholine
- PUFA
polyunsaturated fatty acid
- RS
round spermatid
- SC
spermatocyte
- sgRNA
single guide RNA
- UPLC
ultra-performance LC
- WT
wild type
- ZP
zona pellucida
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
K. Shishikura and M. Arita designed research; K. Shishikura, S. Kuroha, H. Iseki, T. Matsui, and A. Inoue performed research and analyzed data; S. Matsueda contributed to development of the knockout mice; and K. Shishikura and M. Arita wrote the manuscript.
REFERENCES
- 1.Spector A. A., Kim H. Y. (2015) Discovery of essential fatty acids. J. Lipid Res. 56, 11–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lands B. (2017) Highly unsaturated fatty acids (HUFA) mediate and monitor food’s impact on health. Prostaglandins Other Lipid Mediat. 133, 4–10 [DOI] [PubMed] [Google Scholar]
- 3.Hishikawa D., Valentine W. J., Iizuka-Hishikawa Y., Shindou H., Shimizu T. (2017) Metabolism and functions of docosahexaenoic acid-containing membrane glycerophospholipids. FEBS Lett. 591, 2730–2744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zanetti S. R., Maldonado E. N., Aveldaño M. I. (2007) Doxorubicin affects testicular lipids with long-chain (C18-C22) and very long-chain (C24-C32) polyunsaturated fatty acids. Cancer Res. 67, 6973–6980 [DOI] [PubMed] [Google Scholar]
- 5.Iizuka-Hishikawa Y., Hishikawa D., Sasaki J., Takubo K., Goto M., Nagata K., Nakanishi H., Shindou H., Okamura T., Ito C., Toshimori K., Sasaki T., Shimizu T. (2017) Lysophosphatidic acid acyltransferase 3 tunes the membrane status of germ cells by incorporating docosahexaenoic acid during spermatogenesis. J. Biol. Chem. 292, 12065–12076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mashek D. G., Li L. O., Coleman R. A. (2007) Long-chain acyl-CoA synthetases and fatty acid channeling. Future Lipidol. 2, 465–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marszalek J. R., Kitidis C., Dirusso C. C., Lodish H. F. (2005) Long-chain acyl-CoA synthetase 6 preferentially promotes DHA metabolism. J. Biol. Chem. 280, 10817–10826 [DOI] [PubMed] [Google Scholar]
- 8.Iijima H., Fujino T., Minekura H., Suzuki H., Kang M. J., Yamamoto T. (1996) Biochemical studies of two rat acyl-CoA synthetases, ACS1 and ACS2. Eur. J. Biochem. 242, 186–190 [DOI] [PubMed] [Google Scholar]
- 9.Lee E. J., Kim H. C., Cho Y. Y., Byun S. J., Lim J. M., Ryoo Z. Y. (2005) Alternative promotion of the mouse acyl-CoA synthetase 6 (mAcsl6) gene mediates the expression of multiple transcripts with 5′-end heterogeneity: genetic organization of mAcsl6 variants. Biochem. Biophys. Res. Commun. 327, 84–93 [DOI] [PubMed] [Google Scholar]
- 10.Chouinard-Watkins R., Bazinet R. P. (2018) ACSL6 is critical for maintaining brain DHA levels. Proc. Natl. Acad. Sci. USA 115, 12343–12345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soupene E., Dinh N. P., Siliakus M., Kuypers F. A. (2010) Activity of the acyl-CoA synthetase ACSL6 isoforms: role of the fatty acid Gate-domains. BMC Biochem. 11, 18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horii T., Arai Y., Yamazaki M., Morita S., Kimura M., Itoh M., Abe Y., Hatada I. (2014) Validation of microinjection methods for generating knockout mice by CRISPR/Cas-mediated genome engineering. Sci. Rep. 4, 4513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmittgen T. D., Livak K. J. (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 3, 1101–1108 [DOI] [PubMed] [Google Scholar]
- 14.Ikeda K. (2015) Mass spectrometric analysis of phospholipids by target discovery approach. In Bioactive Lipid Mediators (Yokomizo T., and Murakami M., eds.), pp. 349–356, Springer, Tokyo, Japan [Google Scholar]
- 15.Aoyagi R., Ikeda K., Isobe Y., Arita M. (2017) Comprehensive analyses of oxidized phospholipids using a measured MS/MS spectra library. J. Lipid Res. 58, 2229–2237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsugawa H., Cajka T., Kind T., Ma Y., Higgins B., Ikeda K., Kanazawa M., VanderGheynst J., Fiehn O., Arita M. (2015) MS-DIAL: data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 12, 523–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bligh E. G., Dyer W. J. (1959) A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917 [DOI] [PubMed] [Google Scholar]
- 18.Fuchs B., Schiller J., Süss R., Schürenberg M., Suckau D. (2007) A direct and simple method of coupling matrix-assisted laser desorption and ionization time-of-flight mass spectrometry (MALDI-TOF MS) to thin-layer chromatography (TLC) for the analysis of phospholipids from egg yolk. Anal. Bioanal. Chem. 389, 827–834 [DOI] [PubMed] [Google Scholar]
- 19.Bartlett G. R. (1959) Phosphorus assay in column chromatography. J. Biol. Chem. 234, 466–468 [PubMed] [Google Scholar]
- 20.Narita T., Naganuma T., Sase Y., Kihara A. (2016) Long-chain bases of sphingolipids are transported into cells via the acyl-CoA synthetases. Sci. Rep. 6, 25469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Q., Zhang S., Berthiaume J. M., Simons B., Zhang G. F. (2014) Novel approach in LC-MS/MS using MRM to generate a full profile of acyl-CoAs: discovery of acyl-dephospho-CoAs. J. Lipid Res. 55, 592–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mays-Hoopes L. L., Bolen J., Riggs A. D., Singer-Sam J. (1995) Preparation of spermatogonia, spermatocytes, and round spermatids for analysis of gene expression using fluorescence-activated cell sorting. Biol. Reprod. 53, 1003–1011 [DOI] [PubMed] [Google Scholar]
- 23.Bastos H., Lassalle B., Chicheportiche A., Riou L., Testart J., Allemand I., Fouchet P. (2005) Flow cytometric characterization of viable meiotic and postmeiotic cells by Hoechst 33342 in mouse spermatogenesis. Cytometry A 65, 40–49 [DOI] [PubMed] [Google Scholar]
- 24.Agnihotri S. K., Agrawal A. K., Hakim B. A., Vishwakarma A. L., Narender T., Sachan R., Sachdev M. (2016) Mitochondrial membrane potential (MMP) regulates sperm motility. In Vitro Cell. Dev. Biol. Anim. 52, 953–960 [DOI] [PubMed] [Google Scholar]
- 25.Suh M., Merrells K. J., Dick A., Taylor C. G. (2011) Testes of obese rats are highly responsive to n-3 long-chain fatty acids. Br. J. Nutr. 106, 1005–1012 [DOI] [PubMed] [Google Scholar]
- 26.Lin D. S., Connor W. E., Wolf D. P., Neuringer M., Hachey D. L. (1993) Unique lipids of primate spermatozoa: desmosterol and docosahexaenoic acid. J. Lipid Res. 34, 491–499 [PubMed] [Google Scholar]
- 27.Griswold M. D. (2016) Spermatogenesis: the commitment to meiosis. Physiol. Rev. 96, 1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar N., Singh A. K. (2015) Trends of male factor infertility, an important cause of infertility: a review of literature. J. Hum. Reprod. Sci. 8, 191–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Y., Wang H., Qi N., Wu H., Xiong W., Ma J., Lu Q., Han D. (2009) Functions of TAM RTKs in regulating spermatogenesis and male fertility in mice. Reproduction 138, 655–666 [DOI] [PubMed] [Google Scholar]
- 30.Rainey M. A., George M., Ying G., Akakura R., Burgess D. J., Siefker E., Bargar T., Doglio L., Crawford S. E., Todd G. L., Govindarajan V., Hess R. A., Band V., Naramura M., Band H. (2010) The endocytic recycling regulator EHD1 is essential for spermatogenesis and male fertility in mice. BMC Dev. Biol. 10, 37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruwanpura S. M., McLachlan R. I., Meachem S. J. (2010) Hormonal regulation of male germ cell development. J. Endocrinol. 205, 117–131 [DOI] [PubMed] [Google Scholar]
- 32.Esmaeili V., Shahverdi A. H., Moghadasian M. H., Alizadeh A. R. (2015) Dietary fatty acids affect semen quality: a review. Andrology 3, 450–461 [DOI] [PubMed] [Google Scholar]
- 33.Lenzi A., Picardo M., Gandini L., Dondero F. (1996) Lipids of the sperm plasma membrane: from polyunsaturated fatty acids considered as markers of sperm function to possible scavenger therapy. Hum. Reprod. Update 2, 246–256 [DOI] [PubMed] [Google Scholar]
- 34.Fujino T., Kang M. J., Suzuki H., Iijima H., Yamamoto T. (1996) Molecular characterization and expression of rat acyl-CoA synthetase 3. J. Biol. Chem. 271, 16748–16752 [DOI] [PubMed] [Google Scholar]
- 35.Oikawa E., Iijima H., Suzuki T., Sasano H., Sato H., Kamataki A., Nagura H., Kang M. J., Fujino T., Suzuki H., Yamamoto T. T. (1998) A novel acyl-CoA synthetase, ACS5, expressed in intestinal epithelial cells and proliferating preadipocytes. J. Biochem. 124, 679–685 [DOI] [PubMed] [Google Scholar]
- 36.Kang M. J., Fujino T., Sasano H., Minekura H., Yabuki N., Nagura H., Iijima H., Yamamoto T. T. (1997) A novel arachidonate-preferring acyl-CoA synthetase is present in steroidogenic cells of the rat adrenal, ovary, and testis. Proc. Natl. Acad. Sci. USA 94, 2880–2884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li L. O., Grevengoed T. J., Paul D. S., Ilkayeva O., Koves T. R., Pascual F., Newgard C. B., Muoio D. M., Coleman R. A. (2015) Compartmentalized acyl-CoA metabolism in skeletal muscle regulates systemic glucose homeostasis. Diabetes 64, 23–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Killion E. A., Reeves A. R., El Azzouny M. A., Yan Q. W., Surujon D., Griffin J. D., Bowman T. A., Wang C., Matthan N. R., Klett E. L., Kong D., Newman J. W., Han X., Lee M. J., Coleman R. A., Greenberg A. S. (2018) A role for long-chain acyl-CoA synthetase-4 (ACSL4) in diet-induced phospholipid remodeling and obesity-associated adipocyte dysfunction. Mol. Metab. 9, 43–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meller N., Morgan M. E., Wong W. P., Altemus J. B., Sehayek E. (2013) Targeting of Acyl-CoA synthetase 5 decreases jejunal fatty acid activation with no effect on dietary long-chain fatty acid absorption. Lipids Health Dis. 12, 88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ellis J. M., Li L. O., Wu P. C., Koves T. R., Ilkayeva O., Stevens R. D., Watkins S. M., Muoio D. M., Coleman R. A. (2010) Adipose acyl-CoA synthetase-1 directs fatty acids toward beta-oxidation and is required for cold thermogenesis. Cell Metab. 12, 53–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ellis J. M., Mentock S. M., Depetrillo M. A., Koves T. R., Sen S., Watkins S. M., Muoio D. M., Cline G. W., Taegtmeyer H., Shulman G. I., Willis M. S., Coleman R. A. (2011) Mouse cardiac acyl coenzyme a synthetase 1 deficiency impairs fatty acid oxidation and induces cardiac hypertrophy. Mol. Cell. Biol. 31, 1252–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roqueta-Rivera M., Stroud C. K., Haschek W. M., Akare S. J., Segre M., Brush R. S., Agbaga M. P., Anderson R. E., Hess R. A., Nakamura M. T. (2010) Docosahexaenoic acid supplementation fully restores fertility and spermatogenesis in male delta-6 desaturase-null mice. J. Lipid Res. 51, 360–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roqueta-Rivera M., Abbott T. L., Sivaguru M., Hess R. A., Nakamura M. T. (2011) Deficiency in the omega-3 fatty acid pathway results in failure of acrosome biogenesis in mice. Biol. Reprod. 85, 721–732 [DOI] [PubMed] [Google Scholar]
- 44.Zadravec D., Tvrdik P., Guillou H., Haslam R., Kobayashi T., Napier J. A., Capecchi M. R., Jacobsson A. (2011) ELOVL2 controls the level of n-6 28:5 and 30:5 fatty acids in testis, a prerequisite for male fertility and sperm maturation in mice. J. Lipid Res. 52, 245–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rabionet M., Bayerle A., Jennemann R., Heid H., Fuchser J., Marsching C., Porubsky S., Bolenz C., Guillou F., Gröne H. J., Gorgas K., Sandhoff R. (2015) Male meiotic cytokinesis requires ceramide synthase 3-dependent sphingolipids with unique membrane anchors. Hum. Mol. Genet. 24, 4792–4808 [DOI] [PubMed] [Google Scholar]
- 46.Russell D. L., Ettlin A. R., Sinha P. A., Clegg D. E. (1993) Histological and histopathological evaluation of the testis. Int. J. Androl. 16, 83 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.