Abstract
Adults with comparatively short or long leukocyte telomere length (LTL) typically continue to display comparatively short or long LTL throughout life. This LTL tracking stems from the inability of person-to-person variation in age-dependent LTL shortening during adulthood to offset the wide interindividual LTL variation established prior to adult life. However, LTL tracking in children is unstudied. This study aimed to examine LTL shortening rates and tracking in children and their parents. Longitudinal study in children (n = 67) and their parents (n = 99), whose ages at baseline were 11.4 ± 0.3 and 43.4 ± 0.4 yr, respectively. LTL was measured by Southern blotting at baseline and ∼14 yr thereafter. LTL displayed tracking in both children [intraclass correlation coefficient (ICC) = 0.905, P < 0.001] and their parents (ICC = 0.856, P < 0.001). The children’s rate of LTL shortening was twice that of their parents (40.7 ± 2.5 bp/yr; 20.3 ± 2.1 bp/yr, respectively; P < 0.0001). LTL tracking applies not only to adulthood but also to the second decade of life. Coupled with previous work showing that the interindividual variation in LTL across newborns is as wide as in their parents, these findings support the thesis that the LTL-adult disease connection is principally determined before the second decade of life, perhaps mainly at birth.—Benetos, A., Verhulst, S., Labat, C., Lai, T.-P., Girerd, N., Toupance, S., Zannad, F., Rossignol, P., Aviv, A. Telomere length tracking in children and their parents: implications for adult onset diseases.
Keywords: aging, telomere attrition, leukocytes, terminal restriction fragments
Converging lines of evidence suggest that, as expressed in leukocytes, telomere length (TL) plays a causal role in atherosclerotic cardiovascular disease (CVD) and major cancers. Individuals with comparatively short leukocyte TL (LTL) display propensity for CVD (1, 2), whereas their peers with comparatively long LTL display propensity for major cancers (3, 4). Mendelian randomization of LTL-associated single nucleotide polymorphisms have inferred that these LTL-disease associations are causal (5–8). Learning the determinants of LTL is therefore of fundamental relevance to understanding the role of telomeres in the 2 disease categories that ultimately afflict the majority of contemporary humans.
The individual’s LTL is shaped by LTL dynamics (i.e., LTL at birth and age-dependent LTL shortening thereafter). General features of LTL dynamics during the human life course are now known. First, LTL is highly variable across individuals from birth onwards (9–11). Second, person-to-person variation in the rate of LTL shortening during adulthood (12) is usually insufficient to overcome interindividual variation in LTL that were established prior to adulthood. Therefore, individuals entering adult life display LTL tracking (i.e., individuals with comparatively short or long LTL typically maintain their short or long LTL throughout their remaining life course) (10). Notably, cross-sectional studies indicate a rapid loss of TL in the first decades of life (13, 14). Little is known, however, based on longitudinal studies, about interindividual variation in the rate of LTL shortening and tracking during the first 2 decades of life. To fill this knowledge gap, we studied longitudinal measurements, LTL shortening rates, and tracking in children and their parents.
MATERIALS AND METHODS
The cohort
The Suivi Temporaire Annuel Non-Invasif de la Sante des Lorrains Assures Sociaux (STANISLAS). Study is a single-center, familial longitudinal study comprising 1006 families (4295 participants) from the Nancy region of France (ClinicalTrials.gov identifier: NCT01391442). The study and its goals have been previously described by Ferreira et al. (15). For this telomere project, we identified in the STANISLAS biorepository blood samples, donated between May 1998 and February 2001 (baseline) and samples donated between July 2011 and June 2016 (follow-up) by children, aged <14 yr at baseline, and their parents.
LTL measurements
DNA were obtained from buffy coats (baseline samples) and whole blood (follow-up samples) using a salting-out method as previously described by Miller et al. (16). DNA samples passed an integrity test using a 1% (w/v) agarose gel before LTL measurements performed by Southern blotting of the terminal restriction fragments, as previously described by Kimura et al. (17). Briefly, DNA samples were digested (37°C) overnight with restriction enzymes Hinf I and Rsa I (Roche, Basel, Switzerland). Digested DNA samples and DNA ladders were resolved on 0.5% (wt/vol) agarose gels. After 23 h, the DNA was depurinated, denatured, neutralized, and transferred onto a positively charged nylon membrane (Roche) using a vacuum blotter (Bio-Rad, Hercules, CA, USA). Membranes were hybridized at 65°C with the DIG-labeled telomeric probe, after which the probe was detected by the DIG luminescent procedure (Roche) and exposed on X-ray film. The interassay coefficient of variation for the duplicate measurements (on different gels) was 1.2%, and the intraclass correlation coefficient (ICC) was 0.95 [95% confidence interval (CI): 0.79–1; n = 183].
Statistical analysis
Data were analyzed using general linear mixed models in R (18), using the packages lme4 and lmerTest. As random effects, we included family or individual identity. We used the ICC, estimated from models that included age, to estimate the extent to which individuals at different ages differed consistently in LTL from other individuals within their class (children or parents). Age varied between and within individuals because they were sampled twice. To investigate whether LTL shortening in parents and their children (i.e., older and younger individuals) was age dependent, we transformed age at sampling into 2 variables (i.e., the average age at which each individual was sampled and the deviation from the average age). For example, when an individual was sampled at 25 and 35 yr of age, average age = 30 for both samples, whereas Delta age = −5 for the baseline sample, and Delta age = +5 for the follow-up sample. Thus, the slope of Delta age represents the (longitudinal) rate of LTL shortening within individuals, whereas the slope of average age represents the cross-sectional rate of LTL shortening.
To avoid confounding heritability estimates with correlated ages of parents and their children, we used LTL estimates corrected for age by calculating residuals. A midparent LTL estimate was calculated after averaging the residuals of both parents for families, where LTL of both parents was known. These LTL values were transformed to standard normal distributions prior to analysis for parents and children separately.
Female patients have a longer LTL than male patients (19), but capturing this difference requires a larger data set than in the present study (20). Hence, LTL was not corrected for sex in our analyses, but its inclusion made negligible difference in the results.
Comparisons in Table 1 were performed using the Mann-Whitney and χ2 tests, and data are presented as means ± sem.
TABLE 1.
Age and LTL values at baseline and follow-up visits in children and their parents
| Variable | Children | Parents | P |
|---|---|---|---|
| Cohort (n) | 67 | 99 | |
| Women (%) | 57 | 53 | 0.59 |
| Age BL (yr) | 11.36 ± 0.28 | 43.41 ± 0.37 | <0.0001 |
| Delta age FU (yr) | 14.02 ± 0.13 | 13.53 ± 0.09 | 0.004 |
| LTL BL (kb) | 8.32 ± 0.08 | 7.42 ± 0.06 | <0.0001 |
| LTL FU (kb) | 7.75 ± 0.08 | 7.15 ± 0.06 | <0.0001 |
| LTL shortening (bp/yr) | 40.7 ± 2.5 | 20.3 ± 2.1 | 0.001 |
Values are means ± sem. BL, baseline; FU, follow-up.
RESULTS
General characteristics of participants are displayed in Table 1, and a breakdown of the 57 families by parents and siblings is provided in Supplemental Fig. S1.
LTL dynamics in children and parents
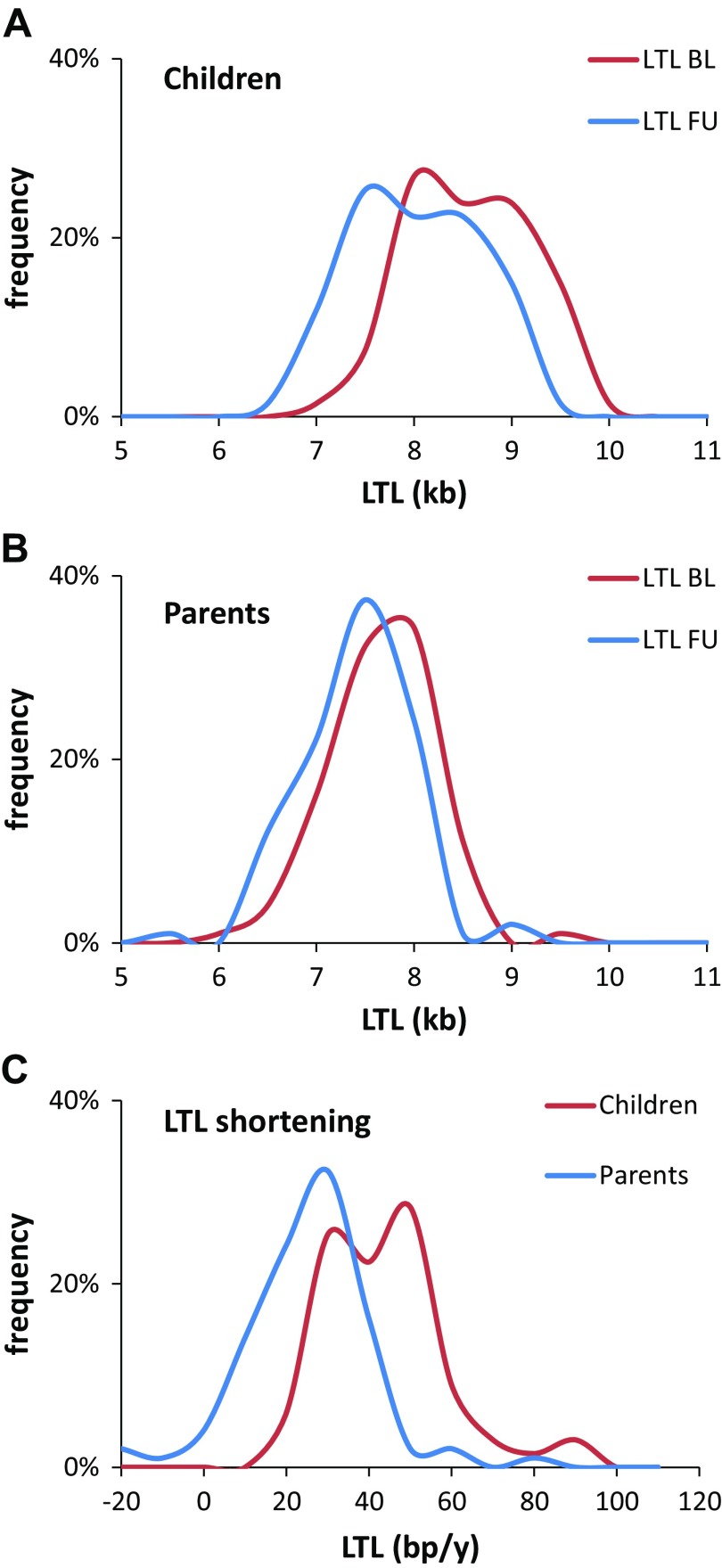
LTL was longer by 906 ± 83 base pairs (bp; t = 10.88, P < 0.001) at baseline and by 622 ± 80 bp (t = 7.75, P < 0.001) at follow-up in children than their parents (Table 1). Figure 1 shows the LTL distribution at baseline and follow-up in children (Fig. 1A) and their parents (Fig. 1B), and LTL shortening in both populations (Fig. 1C). LTL at follow-up was shorter than at baseline by 572 ± 34 bp in the children (t = 16.93, P < 0.001) and by 275 ± 30 in the parents (t = 9.13, P < 0.001). These findings indicate that LTL shortening was faster in children than in parents (children, 40.7 ± 2.5 bp/yr, t = 16.22, P < 0.001; parents, 20.3 ± 2.1 bp/yr, t = 9.47, P < 0.001; interaction, t = 6.21, P < 0.001; Table 1 and Fig. 1C).
Figure 1.
LTL distribution at baseline and follow-up visits in children (A) and their parents (B), and LTL shortening (parents and children) (C). BL, baseline; FU, follow-up.
Tracking
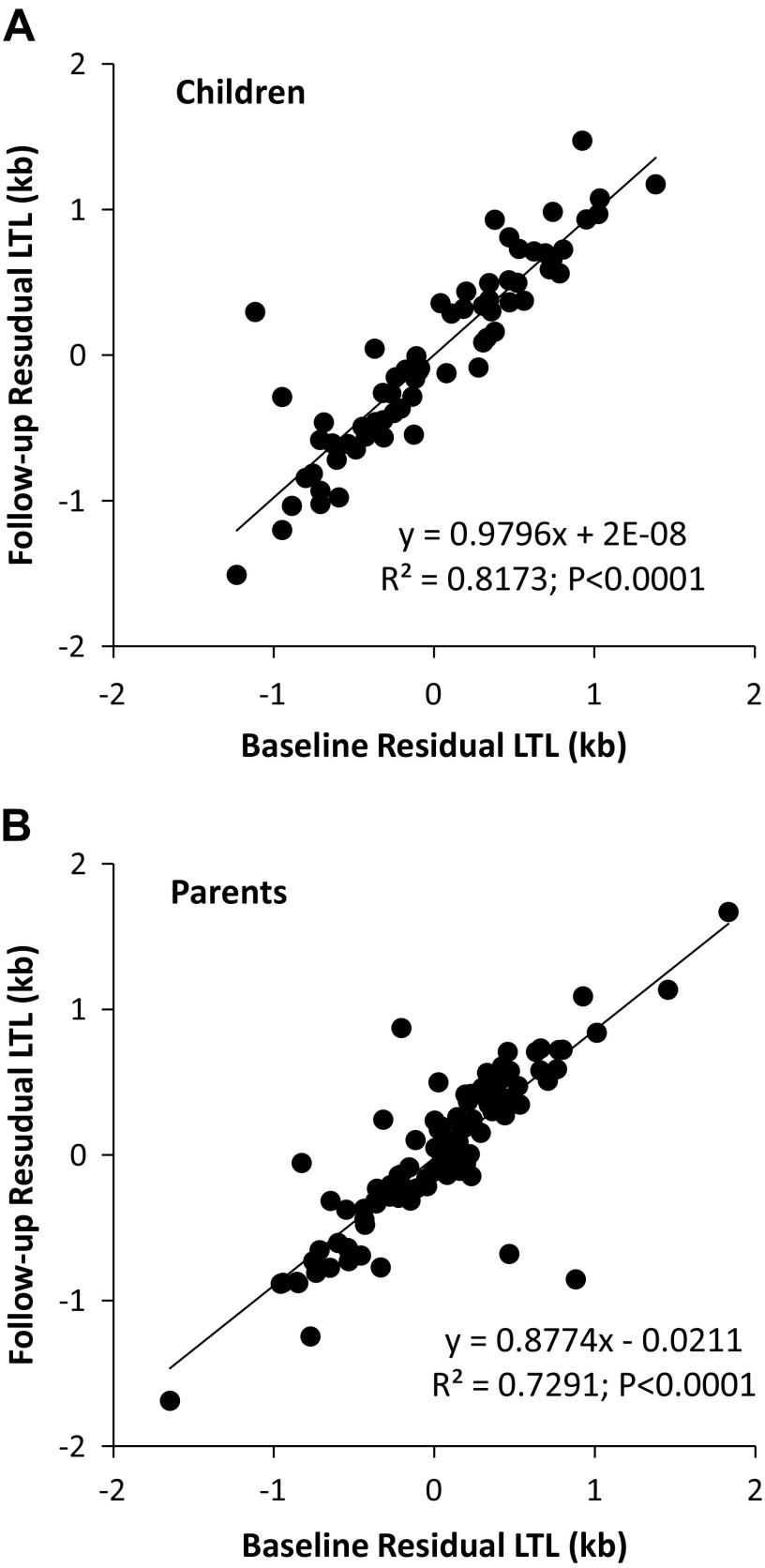
We quantified differences in LTL across individuals within their class (children or parents) at different ages using the ICC estimated from models that included age (Tables 2 and 3). For children, the ICC = 0.905 (95% CI: 0.588–1; P < 0.001; Fig. 2A), whereas for parents the ICC = 0.856 (95% CI: 0.601–1; P < 0.001; Fig. 2B). This denotes high and indistinguishable consistency in tracking of age-dependent LTL between children and parents.
TABLE 2.
LTL in relation to age
| Variable | Estimate ± se | df | T | P |
|---|---|---|---|---|
| Children | ||||
| Intercept | 9.290 ± 0.576 | 65.0 | 16.12 | <0.0001 |
| Average age | −0.0681 ± 0.0311 | 65.0 | −2.19 | 0.032 |
| Delta age | −0.0407 ± 0.0024 | 65.0 | −17.09 | <0.0001 |
| Parents | ||||
| Intercept | 8.174 ± 0.716 | 102.46 | 11.41 | <0.0001 |
| Average age | −0.0181 ± 0.0142 | 102.57 | −1.28 | 0.204 |
| Delta age | −0.0203 ± 0.0022 | 98.37 | −9.19 | <0.0001 |
Children (n = 134 observations on 67 individuals). Parents (n = 203 observations on 104 individuals). Average age is the mean age at which individuals were sampled. For each sampling point, Delta age is the difference between average age and age at sampling. Delta age yields the estimate for longitudinal effects of age on LTL. Average age is not significant for parents, but is retained in the model for consistency.
TABLE 3.
LTL in relation to age: random effect and variance
| Random effect | Variance |
|---|---|
| Children | |
| Individual identity | 0.361 |
| Residual | 0.038 |
| Parents | |
| Individual identity | 0.268 |
| Residual | 0.045 |
Figure 2.
Relationships between LTL measured at baseline (BL) and follow-up (FU) in children (A) and their parents (B).
Heritability of LTL
The regression of the children LTL on midparent LTL, including family identity as random effect, yielded an estimated narrow sense heritability of 0.35 ± 0.13 (P = 0.01; Tables 4). Regression on paternal LTL yielded an estimate of 0.80 (i.e., 2 times the coefficient in Table 5), whereas an estimate based on regression on maternal LTL yielded an estimate of 0.35 (Table 4). Only a minority of the families had more than 1 child in these analyses (see sample sizes in Supplemental Fig. S1); hence, we attach little value to the variance explained by family identity as random effect (exclusion of family identity had little effect on the estimates). In addition, LTL correlation between parents and children observed in this study (see also Supplemental Fig. S2) might also stem from shared environment as well as heritability. However, LTL was poorly correlated between parents (P = 0.36), suggesting a negligible environmental effect on LTL, at least in the parents (Supplemental Fig. S3).
TABLE 4.
LTL inheritance: explanatory variable
| Variable | Estimate ± se | df | T | P |
|---|---|---|---|---|
| Intercept | −0.050 ± 0.133 | 45.78 | 0.38 | 0.73 |
| Midparent LTL | 0.349 ± 0.134 | 46.15 | 2.61 | 0.012 |
| Paternal LTL | ||||
| Intercept | −0.055 ± 0.128 | 44.16 | 0.43 | 0.67 |
| Paternal LTL | 0.399 ± 0.132 | 44.97 | 3.02 | 0.004 |
| Maternal LTL | ||||
| Intercept | −0.061 ± 0.130 | 51.48 | 0.47 | 0.64 |
| Maternal LTL | 0.177 ± 0.128 | 53.43 | 1.38 | 0.17 |
Regression of LTL of children on parental LTL. LTL was corrected for age and transformed to a standard normal distribution for parents and children separately. Children LTL regressed on midparent LTL (n = 56 children from 48 families). Children LTL regressed on paternal LTL (n = 58 children from 50 families). Children LTL regressed on maternal LTL (n = 64 children from 54 families).
TABLE 5.
LTL inheritance: random effect and variance
| Random effect | Variance |
|---|---|
| Midparent LTL | |
| Family identity | 0.529 |
| Residual | 0.356 |
| Paternal LTL | |
| Family identity | 0.492 |
| Residual | 0.362 |
| Maternal LTL | |
| Family identity | 0.608 |
| Residual | 0.346 |
DISCUSSION
The key findings of this longitudinal evaluation of LTL dynamics in children and their parents are as follows: 1) the rate of age-dependent LTL shortening is much faster during the second decade than in adulthood (from third decade onwards), and 2) LTL tracking is a phenomenon that applies not only to adults but also to children during their second decade of life. In addition, we confirmed LTL heritability, observed in previous studies (21, 22), but the small sample size might explain lower heritability found in this study than observed previously. For the same reasons, there was no detectable heritability of LTL shortening in this study (unpublished results).
Given the age range of children participating in this study, we do not know at present whether the LTL tracking phenomenon also covers the first decade of life, but we consider this likely. The implications of our findings are considerable because they suggest that LTL trajectory during adulthood is largely determined before the second decade of life (i.e., at birth and during the first decade of life). Moreover, based on cross-sectional data derived from our previous study of LTL in newborns and their parents (9), our work using skeletal muscle TL as a reference of early life TL (11, 23) and longitudinal data on children and parents in this study, it is clear that LTL shortening during the first decade is much faster than during the second decade of life (details are elaborated under Supplemental Data). Such findings confirm theoretical considerations of LTL dynamics due the expansion of the hematopoietic system during somatic growth (24), which jointly with empirical data indicate that LTL shortening during the first decade of life is inversely related to age and amounts to ∼1 kb. This body of population-based telomere research underscores the importance of birth LTL and LTL shortening during the first decade as determinants of LTL throughout the life course.
Coupled with recent Mendelian randomization analyses that infer a causal role of LTL in CVD and cancer (5–8), the LTL tracking phenomenon provides further impetus to understand intrinsic mechanisms that determine the length of human telomeres in the newborn and the impact of nonheritable parameters on LTL dynamics throughout the life course but particularly in utero and early extrauterine life. This is relevant, given that TL dynamics in the hematopoietic system, as expressed in LTL dynamics, and not, for instance, skeletal muscle TL, explains the role of telomeres in atherosclerotic CVD (25).
Finally, the concept that absolute LTL is a biomarker (bioclock) of human biologic aging has dominated telomere population research as no other concept has, and it remains stubbornly popular despite overwhelming evidence that suggests otherwise (9–11, 25–27). Classically, a bioclock is a marker reflecting the loss of function or substance with passing time. The extension of the LTL tracking from adulthood to childhood and possibly to birth, coupled with wide interindividual variation in LTL in newborns (9), provide the strongest evidence refuting this concept. Indeed, even if LTL shows a progressive attrition with age, LTL absolute value at a given age reflects essentially TL at birth and TL attrition during the first decade and much less TL loss after childhood. Longitudinal studies that determine the rate of age-dependent LTL attrition might provide insight into biologic pathways linked to aging, but such information is rudimentary at present. In conclusion, the ramifications of LTL tracking from the second decade onward are sweeping because they point to a lifelong influence of LTL at birth and perhaps early childhood on disease risk during adulthood. It is imperative, therefore, that intensive research is undertaken to understand the root causes of the interindividual LTL variation at birth and the first decade of life.
ACKNOWLEDGMENTS
This work was supported by the regional project Contrats de Plan État-Région (CPER)–Innovations Technologiques, Modélisation et Médecine Personnalisée (ITM2P) 2015–2020, the French PIA Project Lorraine Université d’Excellence (ANR-15-IDEX-04-LUE), and the Investments for the Future Program under Grant ANR-15-RHU-0004. The Suivi Temporaire Annuel Non-Invasif de la Sante des Lorrains Assures Sociaux (STANISLAS) study was sponsored by Nancy Centre Hospitalier Régional Universitaire (CHRU), Université de Lorraine. Abraham Aviv research was supported by U.S. National Institutes of Health (NIH) Grants R01HL116446 and R01HL13840 (National Heart, Lung, and Blood Institute), R01HD071180 (Eunice Kennedy Shriver National Institute of Child Health and Human Development), and Norwegian Institute of Public Health Grants 262700 and 262043. The STANISLAS cohort study is declared on ClinicalTrials.gov under the identifier NCT01391442. The authors declare no conflicts of interest.
Glossary
- CI
confidence interval
- CVD
cardiovascular disease
- ICC
intraclass correlation coefficient
- LTL
leukocyte telomere length
- TL
telomere length
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
A. Benetos, S. Toupance, F. Zannad, P. Rossignol, and A. Aviv designed research; T.-P. Lai, N. Girerd, and S. Toupance performed research; T.-P. Lai contributed new reagents or analytic tools; S. Verhulst and C. Labat analyzed data; A. Benetos and A. Aviv drafted the initial manuscript, and reviewed and revised the paper; S. Verhulst, C. Labat, T.-P. Lai, N. Girerd,S. Toupance, F. Zannad, and P. Rossignol revised the paper for important intellectual content; and all authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work were appropriately investigated and resolved.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Haycock P. C., Heydon E. E., Kaptoge S., Butterworth A. S., Thompson A., Willeit P. (2014) Leucocyte telomere length and risk of cardiovascular disease: systematic review and meta-analysis. BMJ 349, g4227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.D’Mello M. J., Ross S. A., Briel M., Anand S. S., Gerstein H., Paré G. (2015) Association between shortened leukocyte telomere length and cardiometabolic outcomes: systematic review and meta-analysis. Circ. Cardiovasc. Genet. 8, 82–90 [DOI] [PubMed] [Google Scholar]
- 3.Caini S., Raimondi S., Johansson H., De Giorgi V., Zanna I., Palli D., Gandini S. (2015) Telomere length and the risk of cutaneous melanoma and non-melanoma skin cancer: a review of the literature and meta-analysis. J. Dermatol. Sci. 80, 168–174 [DOI] [PubMed] [Google Scholar]
- 4.Seow W. J., Cawthon R. M., Purdue M. P., Hu W., Gao Y. T., Huang W. Y., Weinstein S. J., Ji B. T., Virtamo J., Hosgood H. D., III, Bassig B. A., Shu X. O., Cai Q., Xiang Y. B., Min S., Chow W. H., Berndt S. I., Kim C., Lim U., Albanes D., Caporaso N. E., Chanock S., Zheng W., Rothman N., Lan Q. (2014) Telomere length in white blood cell DNA and lung cancer: a pooled analysis of three prospective cohorts. Cancer Res. 74, 4090–4098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhan Y., Karlsson I. K., Karlsson R., Tillander A., Reynolds C. A., Pedersen N. L., Hägg S. (2017) Exploring the causal pathway from telomere length to coronary heart disease: a network mendelian randomization study. Circ. Res. 121, 214–219 [DOI] [PubMed] [Google Scholar]
- 6.Codd V., Nelson C. P., Albrecht E., Mangino M., Deelen J., Buxton J. L., Hottenga J. J., Fischer K., Esko T., Surakka I., Broer L., Nyholt D. R., Mateo Leach I., Salo P., Hägg S., Matthews M. K., Palmen J., Norata G. D., O’Reilly P. F., Saleheen D., Amin N., Balmforth A. J., Beekman M., de Boer R. A., Böhringer S., Braund P. S., Burton P. R., de Craen A. J., Denniff M., Dong Y., Douroudis K., Dubinina E., Eriksson J. G., Garlaschelli K., Guo D., Hartikainen A. L., Henders A. K., Houwing-Duistermaat J. J., Kananen L., Karssen L. C., Kettunen J., Klopp N., Lagou V., van Leeuwen E. M., Madden P. A., Mägi R., Magnusson P. K., Männistö S., McCarthy M. I., Medland S. E., Mihailov E., Montgomery G. W., Oostra B. A., Palotie A., Peters A., Pollard H., Pouta A., Prokopenko I., Ripatti S., Salomaa V., Suchiman H. E., Valdes A. M., Verweij N., Viñuela A., Wang X., Wichmann H. E., Widen E., Willemsen G., Wright M. J., Xia K., Xiao X., van Veldhuisen D. J., Catapano A. L., Tobin M. D., Hall A. S., Blakemore A. I., van Gilst W. H., Zhu H., Erdmann J., Reilly M. P., Kathiresan S., Schunkert H., Talmud P. J., Pedersen N. L., Perola M., Ouwehand W., Kaprio J., Martin N. G., van Duijn C. M., Hovatta I., Gieger C., Metspalu A., Boomsma D. I., Jarvelin M. R., Slagboom P. E., Thompson J. R., Spector T. D., van der Harst P., Samani N. J.; CARDIoGRAM Consortium (2013) Identification of seven loci affecting mean telomere length and their association with disease. Nat. Genet. 45, 422–427e1–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haycock P. C., Burgess S., Nounu A., Zheng J., Okoli G. N., Bowden J., Wade K. H., Timpson N. J., Evans D. M., Willeit P., Aviv A., Gaunt T. R., Hemani G., Mangino M., Ellis H. P., Kurian K. M., Pooley K. A., Eeles R. A., Lee J. E., Fang S., Chen W. V., Law M. H., Bowdler L. M., Iles M. M., Yang Q., Worrall B. B., Markus H., Hung R. J., Amos C. I., Spurdle A. B., Thompson D. J., O’Mara T. A., Wolpin B., Amundadottir L., Stolzenberg-Solomon R., Trichopoulou A., Onland-Moret N. C., Lund E., Duell E. J., Canzian F., Severi G., Overvad K., Gunter M. J., Tumino R., Svenson U., van Rij A., Baas A. F., Bown M. J., Samani N. J., van t’Hof F. N. G., Tromp G., Jones G. T., Kuivaniemi H., Elmore J. R., Johansson M., Mckay J., Scelo G., Carreras-Torres R., Gaborieau V., Brennan P., Bracci P. M., Neale R. E., Olson S. H., Gallinger S., Li D., Petersen G. M., Risch H. A., Klein A. P., Han J., Abnet C. C., Freedman N. D., Taylor P. R., Maris J. M., Aben K. K., Kiemeney L. A., Vermeulen S. H., Wiencke J. K., Walsh K. M., Wrensch M., Rice T., Turnbull C., Litchfield K., Paternoster L., Standl M., Abecasis G. R., SanGiovanni J. P., Li Y., Mijatovic V., Sapkota Y., Low S. K., Zondervan K. T., Montgomery G. W., Nyholt D. R., van Heel D. A., Hunt K., Arking D. E., Ashar F. N., Sotoodehnia N., Woo D., Rosand J., Comeau M. E., Brown W. M., Silverman E. K., Hokanson J. E., Cho M. H., Hui J., Ferreira M. A., Thompson P. J., Morrison A. C., Felix J. F., Smith N. L., Christiano A. M., Petukhova L., Betz R. C., Fan X., Zhang X., Zhu C., Langefeld C. D., Thompson S. D., Wang F., Lin X., Schwartz D. A., Fingerlin T., Rotter J. I., Cotch M. F., Jensen R. A., Munz M., Dommisch H., Schaefer A. S., Han F., Ollila H. M., Hillary R. P., Albagha O., Ralston S. H., Zeng C., Zheng W., Shu X. O., Reis A., Uebe S., Hüffmeier U., Kawamura Y., Otowa T., Sasaki T., Hibberd M. L., Davila S., Xie G., Siminovitch K., Bei J. X., Zeng Y. X., Försti A., Chen B., Landi S., Franke A., Fischer A., Ellinghaus D., Flores C., Noth I., Ma S. F., Foo J. N., Liu J., Kim J. W., Cox D. G., Delattre O., Mirabeau O., Skibola C. F., Tang C. S., Garcia-Barcelo M., Chang K. P., Su W. H., Chang Y. S., Martin N. G., Gordon S., Wade T. D., Lee C., Kubo M., Cha P. C., Nakamura Y., Levy D., Kimura M., Hwang S. J., Hunt S., Spector T., Soranzo N., Manichaikul A. W., Barr R. G., Kahali B., Speliotes E., Yerges-Armstrong L. M., Cheng C. Y., Jonas J. B., Wong T. Y., Fogh I., Lin K., Powell J. F., Rice K., Relton C. L., Martin R. M., Davey Smith G.; Telomeres Mendelian Randomization Collaboration (2017) Association between telomere length and risk of cancer and non-neoplastic diseases: A mendelian randomization study. JAMA Oncol. 3, 636–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iles M. M., Bishop D. T., Taylor J. C., Hayward N. K., Brossard M., Cust A. E., Dunning A. M., Lee J. E., Moses E. K., Akslen L. A., Andresen P. A., Avril M. F., Azizi E., Scarrà G. B., Brown K. M., Dębniak T., Elder D. E., Friedman E., Ghiorzo P., Gillanders E. M., Goldstein A. M., Gruis N. A., Hansson J., Harland M., Helsing P., Hočevar M., Höiom V., Ingvar C., Kanetsky P. A., Landi M. T., Lang J., Lathrop G. M., Lubiński J., Mackie R. M., Martin N. G., Molven A., Montgomery G. W., Novaković S., Olsson H., Puig S., Puig-Butille J. A., Radford-Smith G. L., Randerson-Moor J., van der Stoep N., van Doorn R., Whiteman D. C., MacGregor S., Pooley K. A., Ward S. V., Mann G. J., Amos C. I., Pharoah P. D., Demenais F., Law M. H., Newton Bishop J. A., Barrett J. H.; AMFS Investigators ; IBD Investigators ; QMEGA and QTWIN Investigators ; SDH Study Group ; GenoMEL Consortium (2014) The effect on melanoma risk of genes previously associated with telomere length. J. Natl. Cancer Inst. 106, dju267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Factor-Litvak P., Susser E., Kezios K., McKeague I., Kark J. D., Hoffman M., Kimura M., Wapner R., Aviv A. (2016) Leukocyte telomere length in newborns: implications for the role of telomeres in human disease. Pediatrics 137, e20153927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benetos A., Kark J. D., Susser E., Kimura M., Sinnreich R., Chen W., Steenstrup T., Christensen K., Herbig U., von Bornemann Hjelmborg J., Srinivasan S. R., Berenson G. S., Labat C., Aviv A. (2013) Tracking and fixed ranking of leukocyte telomere length across the adult life course. Aging Cell 12, 615–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daniali L., Benetos A., Susser E., Kark J. D., Labat C., Kimura M., Desai K., Granick M., Aviv A. (2013) Telomeres shorten at equivalent rates in somatic tissues of adults. Nat. Commun. 4, 1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steenstrup T., Hjelmborg J. V., Kark J. D., Christensen K., Aviv A. (2013) The telomere lengthening conundrum--artifact or biology? Nucleic Acids Res. 41, e131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frenck R. W., Jr., Blackburn E. H., Shannon K. M. (1998) The rate of telomere sequence loss in human leukocytes varies with age. Proc. Natl. Acad. Sci. USA 95, 5607–5610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aubert G., Baerlocher G. M., Vulto I., Poon S. S., Lansdorp P. M. (2012) Collapse of telomere homeostasis in hematopoietic cells caused by heterozygous mutations in telomerase genes. PLoS Genet. 8, e1002696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferreira J. P., Girerd N., Bozec E., Mercklé L., Pizard A., Bouali S., Eby E., Leroy C., Machu J. L., Boivin J. M., Lamiral Z., Rossignol P., Zannad F. (2018) Cohort Profile: rationale and design of the fourth visit of the STANISLAS cohort: a familial longitudinal population-based cohort from the Nancy region of France. Int. J. Epidemiol. 47, 395–395j [DOI] [PubMed] [Google Scholar]
- 16.Miller S. A., Dykes D. D., Polesky H. F. (1988) A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 16, 1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kimura M., Stone R. C., Hunt S. C., Skurnick J., Lu X., Cao X., Harley C. B., Aviv A. (2010) Measurement of telomere length by the Southern blot analysis of terminal restriction fragment lengths. Nat. Protoc. 5, 1596–1607 [DOI] [PubMed] [Google Scholar]
- 18.R Core Team (2016) A Language and Environment for Statistical Computing, R Foundation for Statistical Computing, Vienna, Austria [Google Scholar]
- 19.Gardner M., Bann D., Wiley L., Cooper R., Hardy R., Nitsch D., Martin-Ruiz C., Shiels P., Sayer A. A., Barbieri M., Bekaert S., Bischoff C., Brooks-Wilson A., Chen W., Cooper C., Christensen K., De Meyer T., Deary I., Der G., Diez Roux A., Fitzpatrick A., Hajat A., Halaschek-Wiener J., Harris S., Hunt S. C., Jagger C., Jeon H. S., Kaplan R., Kimura M., Lansdorp P., Li C., Maeda T., Mangino M., Nawrot T. S., Nilsson P., Nordfjall K., Paolisso G., Ren F., Riabowol K., Robertson T., Roos G., Staessen J. A., Spector T., Tang N., Unryn B., van der Harst P., Woo J., Xing C., Yadegarfar M. E., Park J. Y., Young N., Kuh D., von Zglinicki T., Ben-Shlomo Y.; Halcyon Study Team (2014) Gender and telomere length: systematic review and meta-analysis. Exp. Gerontol. 51, 15–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verhulst S., Susser E., Factor-Litvak P. R., Simons M. J., Benetos A., Steenstrup T., Kark J. D., Aviv A. (2015) Commentary: the reliability of telomere length measurements. Int. J. Epidemiol. 44, 1683–1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hjelmborg J. B., Dalgård C., Möller S., Steenstrup T., Kimura M., Christensen K., Kyvik K. O., Aviv A. (2015) The heritability of leucocyte telomere length dynamics. J. Med. Genet. 52, 297–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slagboom P. E., Droog S., Boomsma D. I. (1994) Genetic determination of telomere size in humans: a twin study of three age groups. Am. J. Hum. Genet. 55, 876–882 [PMC free article] [PubMed] [Google Scholar]
- 23.Benetos A., Toupance S., Gautier S., Labat C., Kimura M., Rossi P. M., Settembre N., Hubert J., Frimat L., Bertrand B., Boufi M., Flecher X., Sadoul N., Eschwege P., Kessler M., Tzanetakou I. P., Doulamis I. P., Konstantopoulos P., Tzani A., Korou M., Gkogkos A., Perreas K., Menenakos E., Samanidis G., Vasiloglou-Gkanis M., Kark J. D., Malikov S., Verhulst S., Aviv A. (2018) Short leukocyte telomere length precedes clinical expression of atherosclerosis: the blood-and-muscle model. Circ. Res. 122, 616–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sidorov I., Kimura M., Yashin A., Aviv A. (2009) Leukocyte telomere dynamics and human hematopoietic stem cell kinetics during somatic growth. Exp. Hematol. 37, 514–524 [DOI] [PubMed] [Google Scholar]
- 25.Hunt S. C., Kark J. D., Aviv A. (2015) Association between shortened leukocyte telomere length and cardio-metabolic outcomes. Circ. Cardiovasc. Genet. 8, 4–7 [DOI] [PubMed] [Google Scholar]
- 26.Aviv A., Shay J. W. (2018) Reflections on telomere dynamics and ageing-related diseases in humans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 373, 20160436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boonekamp J. J., Simons M. J., Hemerik L., Verhulst S. (2013) Telomere length behaves as biomarker of somatic redundancy rather than biological age. Aging Cell 12, 330–332 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.