Abstract
The potent immune regulatory function of an agonistic B7-H4-Ig fusion protein (B7-H4Ig) has been demonstrated in multiple experimental autoimmune models; however, the identity of a functional B7-H4 receptor remained unknown. The biological activity of B7-H4 is associated with decreased inflammatory CD4+ T cell responses as supported by a correlation between B7-H4–expressing tumor-associated macrophages and Foxp3+ T cells within the tumor microenvironment. Recent data indicate that members of the semaphorin (Sema)/plexin/neuropilin (Nrp) family of proteins both positively and negatively modulate immune cell function. In this study, we show that B7-H4 binds the soluble Sema family member Sema3a. Additionally, B7-H4Ig–induced inhibition of inflammatory CD4+ T cell responses is lost in both Sema3a functional mutant mice and mice lacking Nrp-1 expression in Foxp3+ T cells. These findings indicate that B7-H4Ig binds to Sema3a, which acts as a functional bridge to stimulate an Nrp-1/Plexin A4 heterodimer to form a functional immunoregulatory receptor complex resulting in increased levels of phosphorylated PTEN and enhanced regulatory CD4+ T cell number and function.
Since the initial identification of B7-family member proteins and their roles in positive and negative regulation of immune responses, efforts have continued to identify new B7-family members and their receptors and to determine how these proteins functionally regulate the immune response. Confounding the identification process, each B7-family member receptor interacts with multiple functional ligands, leading to immune cell type–specific modulation of the overall immune response within the inflammatory microenvironment (1). One such example is that cross-linking of CD80 on B cells decreases B cell function and survival (2), whereas cross-linking CD80 on CD4+ T cells induces an increase in inflammatory cytokine secretion and increased CD4+ T cell survival (3). Blockade of costimulatory signals (e.g., with CTLA-4 Ig to block CD28-mediated signal 2 required for the activation of CD4+ T cells) has proven not to be the panacea for autoimmune disease therapy that was originally envisioned. However, mAb blockade of coinhibitory receptors (e.g., CTLA-4 and PD-1) has proven extremely useful for enhancing the immune response in cancer therapy (4, 5). Thus, it is critical to determine if signaling via coinhibitory receptors, such as B7-H4, would be useful in the opposing context for the regulation of autoimmune diseases.
B7-H4/B7-H4 receptor (B7-H4R) interactions negatively regulate inflammatory CD4+ T cells. Overexpression of a membrane-bound form of B7-H4 was found to be anti-inflammatory (6, 7), and lack of B7-H4 expression during both experimental autoimmune encephalomyelitis (EAE) and type-1 diabetes is associated with increased disease severity and decreased numbers of regulatory CD4+ T cells (Tregs) (8). Overexpression of a soluble form of B7-H4 increased disease severity in collagen-induced arthritis, whereas treatment with a B7-H4-Ig fusion protein (B7-H4Ig) that cross-linked the B7-H4R decreased the level of disease severity in collagen-induced arthritis (7), PLP139–151-induced relapsing EAE (R-EAE) in SJL/J mice and MOG35–55-induced chronic EAE (C-EAE) in C57BL/6 mice (9), and type-1 diabetes in NOD mice (10). Further, B7-H4Ig treatment of CD4+ T cell cultures is only inhibitory to the CD4+ T cell responses if B7-H4Ig is plate-bound, bead-bound, or added into cultures containing APCs, live or irradiated, expressing Fc receptors (9, 10). Although the identity of the B7-H4R remained unknown, these findings indicate that membrane-bound B7-H4/B7-H4Ig functions as a receptor agonist. In addition, B7-H4Ig has the unique property of specifically blocking the differentiation of naive mouse and human CD4+ T cells into inflammatory Th1 and Th17 cells while enhancing both the numbers and suppressive function of Tregs (9).
More recently, another family of proteins has also been shown to possess immune modulatory function. First described in axonal guidance, proteins in the semaphorin (Sema), neuropilin (Nrp), and plexin (Plxn) family are expressed in various adult tissues and play important roles in various physiological and pathological processes involving the immune system, organogenesis, vascularization, and some tumors (11). To date, 20 semaphorins have been described (12). Class 1 and 2 semaphorins are expressed only in invertebrates, whereas class 3, 4, 6, and 7 semaphorins are found exclusively in vertebrates, and class 5 semaphorins are expressed in both. All semaphorins have an extracellular Sema domain and, depending on the subclass, contain specific additional sequence motifs in the C terminus. One secreted semaphorin is Sema3a, which is a focus of the present studies. Plexins are signaling receptors for semaphorins that bind directly to plexins, with the exception of class 3 semaphorins (e.g., Sema3a), which binds to Nrp-1 and Nrp-2 and signals through PlxnA4 and other PlxnA proteins (13, 14). Signaling through Nrp-1/PlxnA4 has recently been shown to increase CD4+ Treg number and function and secretion of IL-10 by increasing levels of phosphorylated PTEN to antagonize Akt signaling (15). Nrp-1 has been shown to be the functional receptor for Sema3a on T cells (16, 17), and Sema3a modulates T cell responses by altering actin cytoskeleton reorganization and subsequent TCR distribution via PlxnA4-mediated signaling (16, 18). Additionally, mice deficient in PlxnA4 display exacerbated EAE (18), and mice receiving Nrp1-deficient myelinspecific CD4+ T cells display exacerbated EAE, whereas those receiving T cells overexpressing Nrp-1 are resistant to EAE development (19). Interestingly, these published findings determining the role of Nrp-1/PlxnA4 signaling and function strongly correlate with published data defining the functional mechanism by which hB7-H4Ig modulates both effector CD4+ T cell activity (9, 10) and increased Treg function (9). Therefore, the present studies sought to determine if B7-H4 may bind to Sema3a and thereby induce regulatory signaling through Nrp-1/PlxnA4 complex on Tregs.
The potent immunoregulatory function of B7-H4/B7-H4R engagement has been demonstrated in multiple in vivo and in vitro systems. To facilitate the potential manipulation of this receptor/ ligand pair for the therapy of autoimmune disease and cancer, it is critical to define the functional B7-H4R, which has remained elusive (20). Consistent with the reports detailed above regarding the immunoregulatory properties of Nrp-1/PlxnA4 signaling, we show in this study that hB7-H4Ig binds directly to Sema3a, which acts as a bridge to allow the B7-H4/Sema3a/Nrp-1/PlxnA4 receptor/ligand complex to form and signal to APCs and Tregs via PTEN. Functionally, hB7-H4Ig–induced regulation of EAE correlates with increased numbers and function of Tregs. The present results show that B7-H4Ig regulatory activity is lost in both Sema3a functional mutant mice and mice lacking Nrp-1 expression in Foxp3+ T cells. Therefore, these studies indicate that members of the Sema/Nrp/Plxn family of proteins also function as a receptor complex for hB7-H4Ig.
Materials and Methods
Mice, CD4+ T cell isolation, and culture
Female SJL/J, C57BL/6 (Harlan Labs, Indianapolis, IN); C3H/HeJ, C3H/ HeJ Sema3a functional mutant (The Jackson Laboratory, Bar Harbor, ME); SJL-Foxp3/GFP (bred in-house); Nrp-1fl/fl (wild-type, bred in-house); Nrp-1 conditional knockout; Nrp-1fl/fl×Foxp3Cre/YFP (kindly provided by D. Vignali, University of Pittsburgh, PA); PTEN conditional knockout; and PTENfl/fl×3Foxp3Cre/YFP (kindly provided by H. Chi, St. Jude Children’s Research Hospital) mice were housed under specific pathogen-free conditions in the Northwestern University Center for Comparative Medicine. Naive CD4+ T cells (CD4+ L-selectinhi) were purified using autoMACS Magnetic Bead cell separation technology (Miltenyi Biotec, Auburn, CA) from total lymph node cells isolated from unprimed mice with purity ranging from 98 to 99.9%. For in vitro activation, 5 × 105 naive CD4+ T cells were activated in the presence of plate-bound anti-CD3 (1 μg/ml) and soluble anti-CD28 (1 μg/ml) in the presence of induced Treg-promoting conditions (100 U/ml IL-2, 25 ng/ml TGF-β1, 100 nM retinoic acid). After 3 d of culture, the T cells were collected and reactivated in the presence of plate-bound Control Ig (human IgG1) or human B7-H4Ig (human extracellular domain of B7-H4 plus human IgG1 Fc domain) (hB7-H4Ig) for 3 h. The cells were harvested and isolated, and the concentrations of β-tubulin, total Akt, phosphorylated Akt, total PTEN, and phosphorylated PTEN were determined via multiplex Luminex LiquiChip (Millipore, Billerica, MA).
Human PBMC and human CD14+ monocyte cultures
PBMCs (106 cells per well) from healthy donors (LifeSource, Evanston, IL) or relapsing/remitting multiple sclerosis (MS) patients (Rush University Multiple Sclerosis Clinic, Chicago, IL) were isolated and cultured in the presence of anti-CD3 (clone OKT3; eBioscience, San Jose, CA) or peptide (MBP85–96, or tetanus toxin [TT]830–843; Peptides International [Louisville, KY]) and purified by HPLC (purity of 96–99%) plus Control Ig or hB7-H4Ig (0–10 μg/ml) in the absence or presence of recombinant Nrp-1 (R&D Systems, Minneapolis, MN). Twenty-four hours postculture initiation, the wells were pulsed with 1 μCi of [3H]-TdR, and the cultures were harvested on day 5, and [3H]-TdR incorporation was detected using a TopCount microplate scintillation counter. Results are expressed as the mean cpm of triplicate cultures. For cytokine analysis, replicate wells were harvested on day 5 of culture, and cytokine secretion was determined via multiplex Luminex LiquiChip (Millipore). CD14+ monocytes from healthy human donors (n = 6) were purified via autoMACS Magnetic Bead cell separation technology and cultured in the presence of GM-CSF (20 ng/ml) plus IL-4 or IL-10 (10 ng/ml). In wells that contained plate-bound Control Ig versus hB7-H4Ig (10 g/ml), the morphology of the cells was assessed on day 6 of culture.
Mouse monocyte cultures
Monocytes were magnetically separated (Miltenyi) from the bone marrow of unprimed SJL/J mice. Monocytes were cultured in the presence of complete RMPI medium plus IL-6 (10 ng/ml) in the presence of either platebound Control Ig or B7-H4Ig (10 μg/ml) or in the absence or presence of anti–B7-H4 (clone 6H3), recombinant human Nrp-1, recombinant human PlexnA4, recombinant human Sema3a, or recombinant Nrp-1 plus recombinant Sema3a (10 μg/ml) (R&D Systems). The morphology of the cells was assessed on day 6 of culture.
PLP139–151/CFA, OVA protein/CFA, and MOG35–55/CFA priming
Six- to seven-week-old female SJL/J, C57BL/6, C3H/HeJ, Sema3a functional mutant, Nrp-1fl/fl (wild-type), or Nrp-1 conditional knockout mice were immunized s.c. with 100 μl of an emulsion containing 200 μg of Mycobacterium tuberculosis H37Ra (BD Biosciences, San Jose, CA) and 50–200 μg of PLP139–151, OVA protein, or MOG35–55, respectively, distributed over three sites on the flank. MOG35–55/CFA–primed mice received pertussis toxin on day 0 and day 2 via i.p. injection (200 μl of 1 μg/ml). Mice were treated with PBS, Control Ig, anti-CD80 Fab (Bio X Cell, Lebanon, NH), or hB7-H4Ig either at the time of priming or beginning on day 20. Mice were treated with 100 μg per dose injections 3 times per wk for 2 wk via i.p. injection. Individual animals were observed at the indicated time points, and clinical scores were assessed in a blinded fashion on a 0–5 scale: 0, no abnormality; 1, limp tail; 2, limp tail and hind limb weakness; 3, hind limb paralysis; 4, hind limb paralysis and forelimb weakness; and 5, moribund. The data are reported as the mean daily clinical score.
Delayed type hypersensitivity assay and ex vivo recall
Active C-EAE and whole OVA protein/CFA priming were conducted as described above. On day 11 or day 35 postpriming, mice were assayed for delayed type hypersensitivity (DTH). Mice were anesthetized by inhalation of isoflurane, and the thickness of both ears was measured using a dial thickness gauge. Ten micrograms of PLP139–151 (negative control) and OVA protein in 10 μl of PBS were injected into the left and right ear, respectively. The increase in ear thickness was determined after 24 h. Mice were then sacrificed, and single-cell suspensions of spleens and draining lymph nodes were cultured (0.5 × 106 cells per well) in the presence of medium alone, OVA323–339, PLP139–151, or MOG35–55 (20 μg/ml) in the absence or presence of Control Ig or hB7-H4Ig (0–10 μg/ml). Twenty-four hours postculture initiation, the wells were pulsed with 1 μCi of [3H]-TdR, and the cultures were harvested on day 3. Results are expressed as the mean cpm of triplicate cultures. For cytokine analysis, replicate wells were harvested on day 3 of culture, and the level of cytokine secreted was determined via multiplex Luminex LiquiChip.
Flow cytometry
For cell analysis from primed mice, spleens were dissociated into single-cell suspensions and RBCs were lysed. For the CNS leukocytes, single-cell suspensions were prepared as previously described (21) from spinal cords of individual mice perfused with 20 ml of PBS. For B7-H4Ig binding analysis, cells were collected and treated with Cytochalasin D (Sigma-Aldrich, St. Louis, MO) for 2 h at 37°C. The cells were washed in PBS, stained with LIVE/DEAD Fixable Aqua Dead Cell Stain (Life Technologies, Grand Island, NY), and blocked with anti-CD16/32 (eBioscience) prior to incubating the cells with either directly conjugated Control Ig or hB7-H4Ig. Following incubation with Control Ig or hB7-H4Ig, the cells were washed and then stained with the indicated Abs: anti-CD3 (mouse clone 145–2C11; human clone OKT3), anti-CD4 (mouse clone RM4–5; human clone OKT4), anti-Foxp3 (clone FJK-16s), anti-Helios (clone 22F6) (eBioscience), anti-CD25 (clone PC61) (eBioscience), anti–Nrp-1 (mouse polyclonal sera; human clone no. 446921), anti-PlxnA4 (mouse clone no. 707201; human clone no. 707206), and anti-Sema3a (mouse and human clone no. 215803) (R&D Systems). A total of 106 viable cells were analyzed per individual sample using a BD Canto II cytometer (BD Biosciences), and the data were analyzed using FlowJo version 9.5.2 software (Tree Star, Ashland, OR).
Sema3a and Sema3a/hB7–H4Ig complex ELISA
Active R-EAE in SJL/J mice was conducted as described above. The mice were followed for clinical signs, and beginning at remission from acute disease, mice-were treated with PBS, Control Ig, or hB7-H4Ig (100 μg/dose; 3 times per wk; 2 wk) via i.p. injection. Serum samples were collected 2 h after the first treatment and 2 h after the sixth treatment. The concentration of total serum Sema3a was detected with a Sema3a-specific ELISA (Mybiosource.com, San Diego, CA), and Sema3a/B7-H4Ig complexes were detected via use of the same ELISA plates with an anti–B7-H4 biotin-detecting Ab instead of the anti-Sema3a–detecting Ab. Color development was determined on a SpectraMax Plus microplate reader (Molecular Devices, Menlo Park, CA) at a wavelength of 405 nm.
hB7-H4Ig–binding ELISA
ELISA plates were coated with recombinant human Nrp-1 (R&D Systems) and Sema3a (PeproTech, Rocky Hill, NJ) (10 μg/ml) in 13 PBS for 2 h at 37°C, and the wells were washed and blocked with 1× PBS containing 5% BSA for 1 h at 37°C. For the wells containing Nrp-1 plus Sema3a, Sema3a (10 μg/ml) was incubated in the wells for 1 h at 37˚C, the wells were washed, and Control Ig biotin or hB7-H4Ig biotin (0–10 μg/ml) was incubated overnight at 4°C. The following day, the wells were washed, streptavidin–HRP (1:1000 dilution in PBS) incubated for 1 h at 37°C, and the wells developed with 1-Step Ultra TMB-ELISA Substrate Solution (Life Technologies). Color development was determined on a SpectraMax Plus microplate reader (Molecular Devices, Menlo Park, CA) at a wavelength of 405 nm.
Statistical analyses
Comparisons of the percentage of animals showing clinical disease were analyzed by Χ2 using Fisher exact probability, and two-way ANOVA with a Bonferroni posttest was used to determine statistical differences between mean clinical disease scores. Single comparisons of two means were analyzed by Student t test.
Results
hB7-H4Ig binds Nrp-1 and PlxnA4, and recombinant Nrp-1 blocks B7-H4Ig function
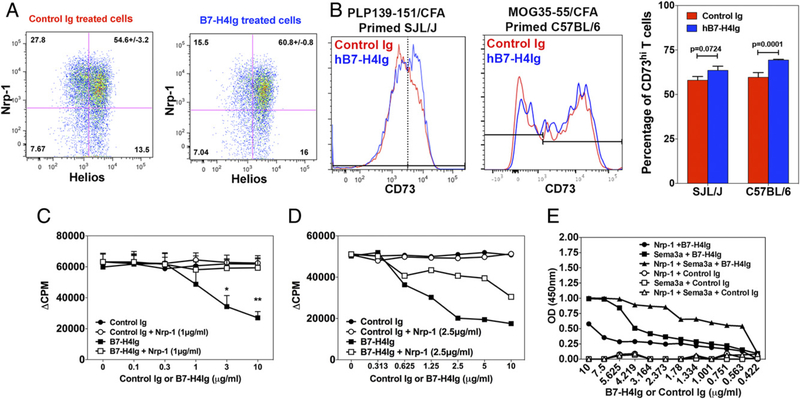
The expression and signaling of Nrp-1 on Tregs has been shown to correlate with and enhance Treg suppressive function (9). To phenotypically address the ability of hB7-H4Ig to modulate Treg function, draining lymph node cells from PLP139–151/CFA–primed SJL/J mice and MOG35–55/CFA–primed C57BL/6 mice were collected on day 8, and total draining lymph node cells were cultured in the presence of PLP139–151 plus Control Ig or hB7-H4Ig. On day 3 of culture, the percentage and phenotype of the Tregs were assessed via flow cytometry. hB7-H4Ig treatment increased the percentage of Nrp-1+/Helioshi Tregs (Fig. 1A). In contrast, the increase in the percentage of CD73hi Tregs does appear to be minimal; however, there was a statistically significant increase in the percentage of CD73hi Tregs in the C57BL/6 MOG35–55 reactivation cultures, but not in the SJL/J PLP139–151 reactivation cultures (Fig. 1B). Because our published and current data show hB7-H4Ig–induced modulation of Treg phenotype (i.e., increased IL-10) suppressor function (9), we sought to determine if a functional connection existed between hB7-H4Ig–induced suppression of CD4+ T cell function and the previously reported function of the Nrp-1/PlxnA4 pathway (15). To do so, the addition of exogenous recombinant Nrp-1 was shown to block the hB7-H4Ig–induced inhibition of T cell proliferation of both human PBMCs from healthy human donors (Fig. 1C) and draining lymph node cells from PLP139–151/CFA–primed SJL/J mice (Fig. 1D). To extend the present and previously published data, we next determined if hB7-H4Ig treatment was able to modulate activation of MS patients’ T cells in vitro. PBMCs isolated from relapsing/remitting MS patients were cultured in the presence of anti-CD3, MBP85–96, or TT830–843 plus Control human IgG1 or hB7-H4Ig for 5 d, and proliferation and secreted cytokine levels were assessed. hB7-H4Ig treatment decreased T cell proliferation and inflammatory cytokine (IFN-γ and IL-17) secretion while increasing the levels of IL-10 and IL-4 (Supplemental Fig. 1) in response to both anti-CD3 and, importantly, to stimulation with MBP85–96 and TT830–843. These results are similar to our previously reported findings using both mouse-and healthy human donor–purified CD4+ T cells cultured in the presence of various activating stimuli in the presence of hB7-H4Ig versus Control Ig (9). Significantly, the addition of recombinant Nrp-1 reversed hB7-H4Ig–induced regulatory function, suggesting a putative correlation between hB7-H4Ig function and Nrp-1 expression.
FIGURE 1.
hB7-H4Ig binds Sema3a. SJL/J mice (n = 5) and C57BL/6 mice (n = 4) were primed with PLP139–151/CFA or MOG35–55/CFA, respectively, and on day 8 postpriming, the draining inguinal lymph nodes were collected. A total of 0.5 × 106 cells per well in a 96-well plate were cultured in the presence of PLP139–151 or MOG35–55 (10 μg/ml) plus Control Ig or hB7-H4Ig (10 μg/ml), and on day 3 of culture, the percentage of Tregs (gated as singlet, live cells, CD3/CD4+, and CD25/Foxp3+) positive for (A) Nrp-1/Helios and (B) CD73 were assessed via FACS. (C) Total PBMCs from healthy human donors (n = 3) were activated in the presence of anti-CD3 (0.5 μg/ml), or (D) draining lymph node cells from PLP139–151/CFA–primed SJL/J mice (n = 3) were activated in the presence of PLP139–151 (10 μg/ml) for 3 d in the presence of Control Ig or hB7-H4Ig (0–10 μg/ml) in the absence or presence of recombinant human Nrp-1 (1 or 2.5 μg/ml), and proliferation was determined via [3H]-TdR incorporation. (E) hB7-H4Ig binding to recombinant human Nrp-1, Sema3a, and Nrp-1 plus Sema3a was assessed via ELISA. All wells were coated singly with recombinant human Nrp-1 or Sema3a, washed, and blocked with PBS containing 5% BSA. For the wells receiving Nrp-1 plus Sema3a, the wells received an additional Sema3a binding step, the wells were washed, and then Control Ig biotin or hB7-H4Ig biotin was added to the wells as indicated. One representative experiment of three is presented. Asterisks indicate a statistically significant difference in proliferation and cytokine production by cells from hB7-H4Ig–treated cultures in comparison with Control Ig. *p < 0.05, **p < 0.01.
hB7-H4Ig binds Sema3a both in vitro and in vivo
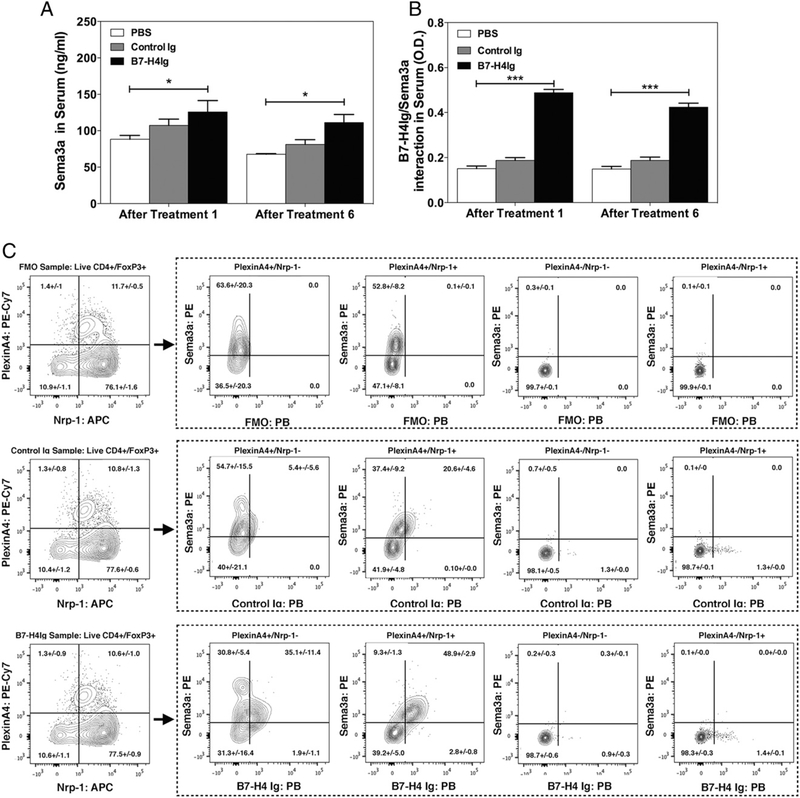
To test the ability of hB7-H4Ig to bind Nrp-1, we performed hB7-H4Ig binding analyses using recombinant human Nrp-1. As shown in Fig. 1E, hB7-H4Ig only mildly interacted directly with Nrp-1. Therefore, we sought to determine if hB7-H4Ig may interact with Nrp-1 via the cobinding of another soluble protein. hB7-H4Ig was found to bind Sema3a in a concentration-dependent manner, and the level binding was increased further if Sema3a was allowed to bind Nrp-1 prior to the incubation with hB7-H4Ig. We next sought to determine if hB7-H4Ig interacts with Sema3a in vivo. SJL/J mice were primed with PLP139–151/CFA and followed for relapsing/remitting EAE disease. On day 20 (disease remission), mice were randomly split into three treatment groups: receiving PBS, Control Ig, and hB7-H4Ig (100 μg/dose; 3 times per wk; 2 wk) via i.p. injection. Serum samples were collected 2 h following both the first and last treatments to assess both the level of total Sema3a as well as the level of Sema3a bound to hB7-H4Ig present in the serum of the mice. hB7-H4Ig treatment significantly increased the level of total Sema3a in serum (Fig. 2A), and more importantly, Sema3a and hB7-H4Ig were found to be interacting within the serum of hB7-H4Ig–treated mice (Fig. 2B). These results show that hB7-H4Ig directly binds to Sema3a both in platebound format as well as in vivo.
FIGURE 2.
hB7-H4Ig binds Sema3a+ mouse Tregs. The amount of hB7-H4Ig biotin-bound was detected via streptavidin–HRP, and the OD (450 nm) was determined. SJL/J mice (n = 5 per group) were primed with PLP139–151/CFA and treated with PBS, Control Ig, or hB7-H4Ig (100 μg/dose; 3 times per wk; 2 wk) via i.p. injection. At 2 h after the first injection and 2 h after the sixth injection, serum samples were collected, and the concentration of (A) total Sema3a and (B) Sema3a bound to hB7-H4Ig was assessed via ELISA. (C) Total lymph node cells from unprimed SJL-Foxp3/GFP mice (n = 5) were collected, and hB7-H4Ig binding on various Treg populations was assessed. Live CD4+/CD25+/Foxp3-GFP+ single cells were gated into the PlxnA4 versus Nrp-1 contour plots for each of the fluorescence minus one (FMO), Control Ig, and hB7-H4Ig binding FACS samples. The expression of Sema3a versus hB7-H4Ig binding was assessed on the PlxnA4+/Nrp-1−, PlxnA4+/Nrp-1+, PlxnA4−/Nrp-1−, and PlxnA4−/Nrp-1+ populations. One representative experiment of three is presented. Asterisks indicate a statistically significant difference in the level of Sema3a and Sema3a/B7-H4Ig complex present following hB7-H4Ig treatment in comparison with cells cultured in the presence of Control Ig treatment. *p < 0.05, ***p < 0.001.
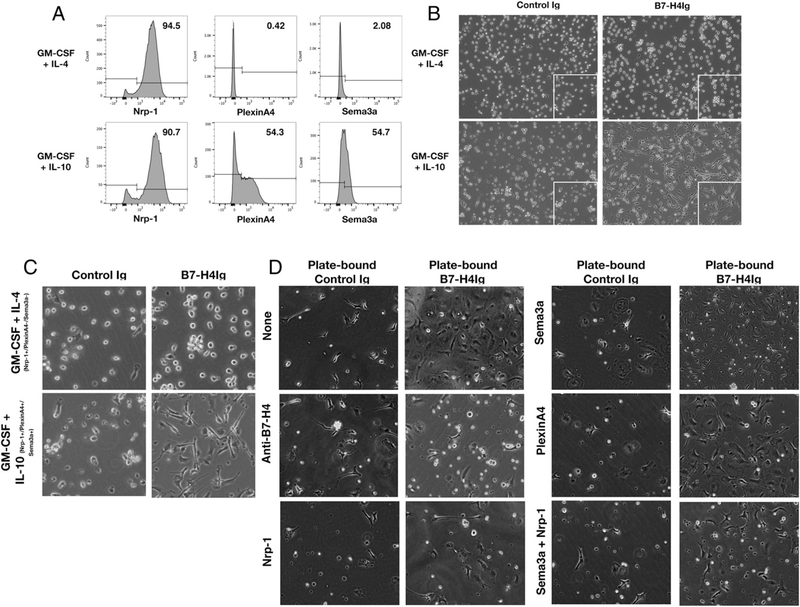
To determine if hB7-H4Ig binding to Tregs correlates with the expression of Sema3a/Nrp-1/PlxnA4, we analyzed binding of hB7-H4Ig to Tregs from SJL-Foxp3/GFP mice. To do so, total lymph node cells from unprimed SJL-Foxp3/GFP mice were assessed for hB7-H4Ig binding via flow cytometry. The present data show that hB7-H4Ig binding to CD4+ Foxp3/GFP+ cells correlates with surface expression of Sema3a/PlxnA4 and Sema3a/Nrp-1/PlxnA4 (Fig. 2B). This finding corroborates the direct binding of hB7-H4Ig to Sema3a as shown in Fig. 1E. Additionally, hB7-H4Ig binding to anti-CD3–activated human CD4+ T cells was also found to correlate with Sema3a and PlxnA4 surface expression (Supplemental Fig. 2). To further assess the functional linkage between hB7-H4Ig and the Sema3a/PlxnA4/Nrp-1 complex, purified human CD14+ monocytes activated with GM-CSF plus IL-4 that express Nrp-1, as compared with monocytes activated with GM-CSF plus IL-10 that express Sema3a, Nrp-1, and PlxnA4, (Fig. 3A) were cultured in the presence of plate-bound Control Ig or hB7-H4Ig. Plate-bound hB7-H4Ig significantly altered the morphology of CD14 monocytes activated with GM-CSF plus IL-10, but not CD14+ monocytes activated with GM-CSF plus IL-4 (Fig. 3B, 3C). Purified SJL/J bone marrow monocytes cultured in the presence of recombinant mouse IL-6 (10 ng/ml) were found to express Sema3a, Nrp-1, and PlxnA4. Similar to the data obtained with human monocytes, plate-bound hB7-H4Ig induced an increase in the number of cells and altered cellular morphology (Fig. 3D). Addition of exogenous anti–B7-H4, anti–Nrp-1, or anti–Sema3a plus Nrp-1 blocked the hB7-H4Ig–induced changes. Taken together, these findings indicate that Sema3a/Nrp-1 forms a functional B7-H4R complex.
FIGURE 3.
Recombinant Nrp-1 blocks hB7-H4Ig–induced monocyte morphology change. Purified CD14+ monocytes from healthy human donors (n = 6) were cultured in the presence of GM-CSF (20 ng/ml) plus IL-4 or IL-10 (10 ng/ml). (A) The levels of Nrp-1, PlxnA4, and Sema3a were assessed on day 6 of culture on the resultant live cells via FACS. (B) The morphology of the cells was assessed on day 6 of culture in wells that contained plate-bound Control Ig versus hB7-H4Ig (10 g/ml). (C) Higher magnification images of the gated region from the micrographs presented in (B) are present. (D) Monocytes were sortpurified from the bone marrow of SJL/J mice and cultured in the presence of IL-6 (10 ng/ml) in the presence of either plate-bound Control Ig or plate-bound hB7-H4Ig plus anti–B7-H4, recombinant human Nrp-1, recombinant human PlxnA4, recombinant human Sema3a, or recombinant human Sema3a plus Nrp-1 (10 μg/ml) as indicated. Micrographs were taken on day 6 of culture (original magnification × 200). One replicate experiment of two is presented.
Treg expression of Nrp-1 and functional Sema3a is required for hB7-H4Ig function in vitro
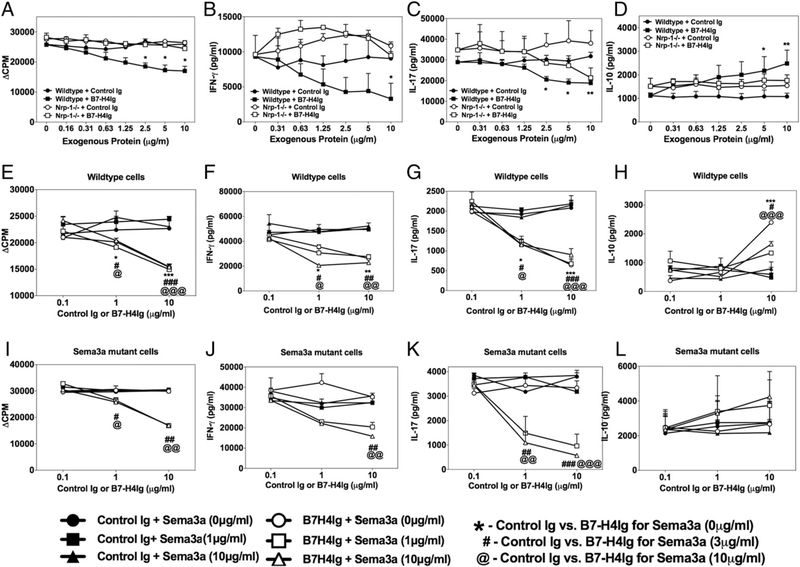
We next tested if the immunoregulatory function of hB7-H4Ig required Nrp-1 expression by Tregs. Splenocytes from mice in which Nrp-1 is conditionally knocked out within Foxp3 cells (Nrp-1fl/fl × Foxp3Cre/YFP) were activated in vitro with anti-CD3 plus various concentrations of Control Ig or hB7-H4Ig. Treg expression of Nrp-1 was required for maximal hB7-H4Ig function, as demonstrated by the inability of hB7-H4Ig to regulate cellular proliferation (Fig. 4A) and secretion of IFN-γ (Fig. 4B) and IL-10 (Fig. 4D) and, to a lesser extent, IL-17 (Fig. 4C). Given that the data presented in Fig. 1E shows that hB7-H4Ig only weakly bound Nrp-1 but more readily bound to Sema3a and Sema3a/Nrp-1 complexes, we next determined if functional Sema3a is required for hB7-H4Ig function in vitro. Splenocytes from Sema3a functional mutant mice (i.e., mice in which a mutation contained within exon 3 of Sema3a allows for the expression of Sema3a while there is a functional loss of Sema3a-induced signaling through Nrp-1) (22, 23) were assessed in vitro in the presence of hB7-H4Ig addition. The present data show that hB7-H4Ig immunoregulatory function was lost in Sema3a functional mutant splenocytes; however, hB7-H4Ig functionality was restored upon addition of recombinant Sema3a to the cultures (Fig. 4E–L). Collectively, these results show that hB7-H4Ig binds Sema3a, and both Treg-expressed Nrp-1 and functional Sema3a are required for functional hB7-H4Ig immunoregulatory function.
FIGURE 4.
Nrp-1 expression and functional Sema3a are required for hB7-H4Ig function in vitro. (A–D) Splenocytes (0.5 × 106 cells per well in a 96-well plate) from mice in which Nrp-1 is conditionally knocked out within Foxp3+ cells (Nrp-1fl/fl × Foxp3Cre/YFP) versus wild-type mice (n = 2) were cultured in the presence of anti-CD3 (1 mg/ml) plus Control Ig or hB7-H4Ig (0–10 mg/ml) for 3 d, and the level of cellular proliferation was assessed via (A) tritiated thymidine incorporation and the secretion of (B) IFN-γ, (C) IL-17, and (D) IL-10. (E–L) Splenocytes from wild-type C3H/HeJ and C3H/HeJ Sema3a functional mutant mice (n = 5) were cultured in the presence of anti-CD3 (1 μg/ml) plus Control Ig or hB7-H4Ig (0–10 μg/ml) in the absence or presence of exogenous recombinant Sema3a (0, 3, and 10 μg/ml) for 3 d, and the level of cellular proliferation was assessed via (E and I) tritiated thymidine incorporation and the secretion of (F and J) IFN-γ, (G and K) IL-17, and (H and L) IL-10. One replicate experiment of three is presented. Asterisks indicate a statistically significant difference in proliferation and cytokine production by cells from hB7-H4Ig–treated cultures in comparison with cells cultured in the presence of Control Ig. *p < 0.05, **p < 0.01, ***p < 0.001.
Our previously published data showed that hB7-H7Ig treatment increased both the number and function of Tregs (9). Similarly, signaling through Nrp-1/PlxnA4 has been shown to increase the regulatory function of Tregs via an increase in PTEN phosphorylation (15). Thus, to further address the bioactivity of the hypothesized Sema3a/Nrp-1/PlxnA4 B7-H4R complex, we determined if hB7-H4Ig induced the activation of PTEN (15). PLP139–151–sensitized lymph node cells were activated in vitro in the presence of PLP139–151 for 3 d. The cells were collected and recultured for 3 h in the presence of plate-bound Control Ig or hB7-H4Ig. hB7-H4Ig treatment induced a significant increase in the level of phosphorylated PTEN (Ser380) (Supplemental Fig. 3A). In additional experiments, naive CD4+ T cells from SJL-Foxp3/ GFP mice were cultured in the presence of induced Treg, promoting culture conditions, and recultured for 3 h in the presence of plate-bound anti-CD3 (1 μg/ml) and either plate-bound Control Ig or hB7-H4Ig. hB7-H4Ig cross-linking again induced a significant increase in the level of phosphorylated PTEN concomitant with a significant decrease in the level of phospho-Akt (Ser473) (Supplemental Fig. 3B). We next tested if the immunoregulatory function of hB7-H4Ig required PTEN expression by Tregs. To do so, splenocytes from mice in which PTEN is conditionally knocked out within Foxp3+ cells (PTENfl/fl × Foxp3Cre/YFP) (24) were activated in vitro with anti-CD3 plus various concentrations of Control Ig or hB7-H4Ig. Treg expression of PTEN was required for maximal hB7-H4Ig function, as demonstrated by the inability of hB7-H4Ig to regulate cellular proliferation (Supplemental Fig. 3C) and secretion of IFN-γ (Supplemental Fig. 3D), IL-10 (Supplemental Fig. 3E), and IL-17 (Supplemental Fig. 3F). Thus, hB7-H4Ig induces an increase in phosphorylated PTEN, and the loss of PTEN expression by Foxp3+ cells inhibits the ability of hB7-H4Ig to modulate anti-CD3–activated splenocytes.
Treg expression of Nrp-1 and functional Sema3a expression is required for immunoregulatory hB7-H4Ig function in vivo
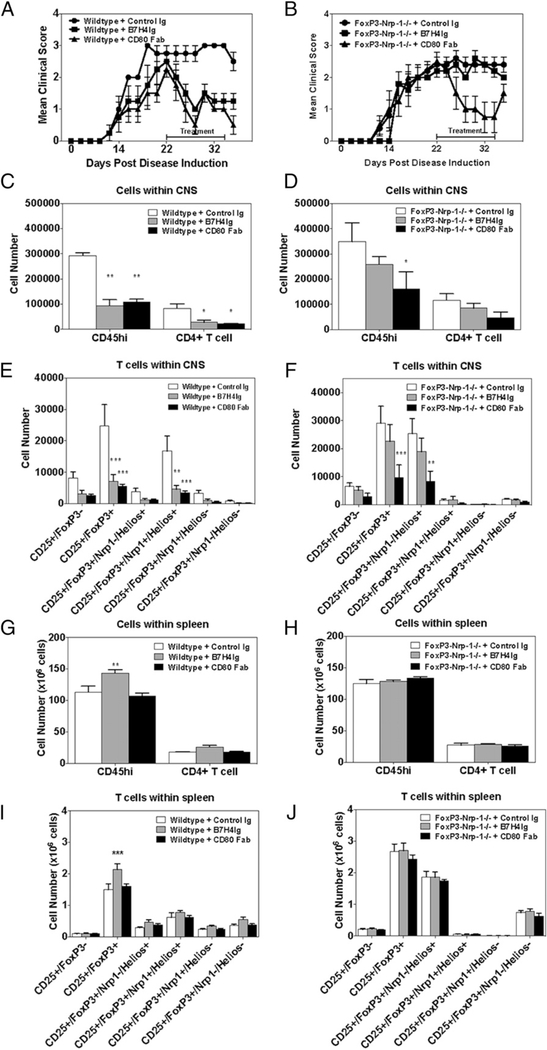
The above findings indicate that Tregs are the major target of hB7-H4Ig functional activity in in vitro splenocyte cultures, and this conclusion is supported by our previous finding that hB7-H4Ig– induced inhibition of Th1 and Th17 cell differentiation is significantly less effective in CD4+ T cell cultures from which Tregs have been depleted (9). To determine if the Sema3a/Nrp-1/PlxnA4 complex is required for hB7-H4Ig function in vivo, we compared the ability of hB7-H4Ig to regulate established MOG35–55/CFA–induced C-EAE in wild-type versus Nrp-1fl/fl × Foxp3Cre/YFP conditional knockout mice. Mice were treated (100 μg/dose; 3 times per wk; 2 wk) with Control Ig, hB7-H4Ig, or anti-CD80 Fab (as a positive control) (3) beginning on day 20 postpriming. As previously reported (3), anti-CD80 Fab treatment significantly decreased disease severity (Fig. 5A, 5B) and the number of CNS-infiltrating immune cells in both wild-type and Nrp-1 conditional knockout mice (Fig. 5C–F). In contrast, hB7-H4Ig treatment was not effective in regulating EAE in mice lacking Nrp-1 expression on Tregs. Additionally, the hB7-H4Ig–induced increase in the number of splenic Tregs was lost in Nrp-1 conditional knockouts (Fig. 5G–J), and the normal hB7-H4Ig induced a decrease in IFN-γ (Supplemental Fig. 4A, 4D) and IL-17 (Supplemental Fig. 4B, 4E), and an increase in IL-10 (Supplemental Fig. 4C, 4F) was also lost. Thus, the expression of Nrp-1 by Tregs is required for hB7-H4Ig– induced regulatory function in vivo.
FIGURE 5.
Treg-expressed Nrp-1 is required for hB7-H4Ig function in vivo. (A and B) Wild-type C57BL/6 mice and Nrp-1fl/fl × Foxp3Cre/YFP mice (n = 5 per group) were primed with MOG35–55/CFA and treated beginning on day 20 with Control Ig, hB7-H4Ig, or anti-CD80 Fab (100 μg/dose; 3 times per wk; 2 wk). The mice were followed for disease, and the data are presented as the mean clinical score. (C–J) On day 35 postinduction of disease, the CNS and spleens from each mouse were collected, and (C, D, G, and H) the number of total CD45hi and CD4+ T cells were enumerated via FACS, as well as (E, F, I, and J) the number of Tregs that were positive or negative for Nrp-1 and Helios. One replicate experiment of two is presented. Asterisks indicate a statistically significant difference in the cellular populations between the Control Ig–treated mice as compared with the mice receiving either hB7-H4Ig or anti-CD80 Fab treatment. *p < 0.05, **p < 0.01, ***p < 0.001.
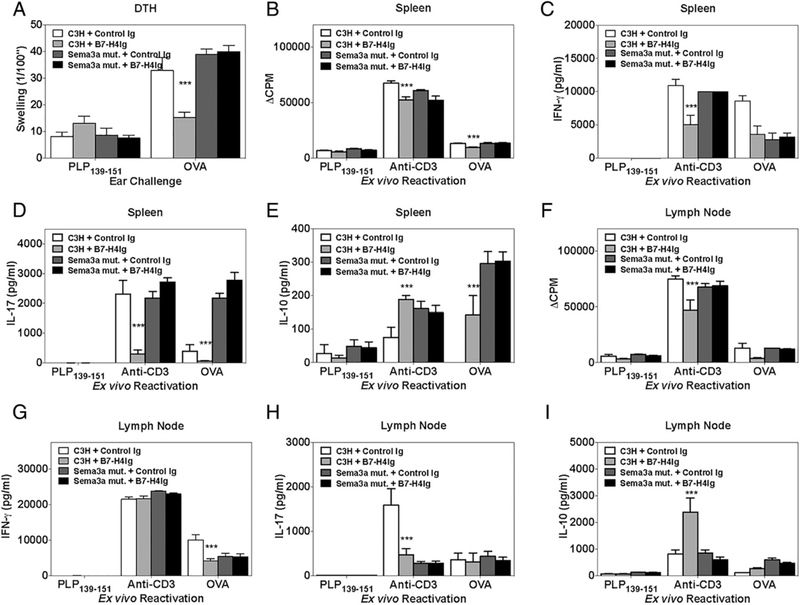
It has been previously reported that a Sema3a Ig fusion protein, which would allow for Nrp-1/PlxnA4 cross-linking similar to our proposed hB7-H4Ig/Sema3a complex, downregulates T cell activation (17, 25–27). Therefore, we tested the ability of hB7-H4Ig treatment to modulate CD4+ T cell responses in vivo in the absence of functional Sema3a. To do so, wild-type and Sema3a functional mutant C3H/HeJ mice were primed with OVA protein/ CFA and treated with Control Ig or hB7-H4Ig (100 μg/dose; 3 times per wk; 2 wk) beginning on the day of priming. Treatment with hB7-H4Ig decreased both in vivo OVA-specific DTH responses (Fig. 6A) as well as ex vivo recall responses in wildtype C3H/HeJ mice (Fig. 6B–I); however, this immunoregulatory function of hB7-H4Ig was lost in Sema3a functional mutant mice. Therefore, the in vivo expression of functional Sema3a is required for hB7-H4Ig to modulate OVA/CFA–induced responses in vivo.
FIGURE 6.
Functional Sema3a is required for hB7-H4Ig function in vivo. Wild-type and Sema3a functional mutant C3H/HeJ mice (n = 5) were primed with OVA/CFA and treated with Control Ig or hB7-H4Ig (100 μg/dose; 3 times per wk; 2 wk) beginning on the day of priming. On day 11 postpriming, (A) the in vivo DTH responses to challenge with OVA protein, and ex vivo recall responses of splenocytes and draining lymph node cells (0.5 × 106 cells per well in a 96-well plate) to PLP139–151 (10 μg/ml), anti-CD3 (1 μg/ml), and OVA protein (10 μg/ml) were determined. (B and F) Two replicate sets of wells were set up such that one was collected for cytokine production, and the second set was pulsed with 1 mCi of tritiated thymidine at 24 h, and both cytokine wells and proliferation wells were collected at 72 h postculture setup. (C and G) IFN-γ, (D and H) IL-17, and (E and I) IL-10 production was assessed at 72 h. One representative experiment of two is presented. Asterisks indicate a statistically significant difference in proliferation and cytokine production by cells from hB7-H4Ig–treated mice in comparison with cells collected from Control Ig–treated mice. ***p < 0.001.
Discussion
FunctionalstudiesusingB7-H4transfectantsandimmobilizedhB7-H4Ig fusion proteins demonstrate that B7-H4 signals inhibit TCR-mediated CD4+ and CD8+ T proliferation, cell cycle progression, and IL-2 production. Further support of the inhibitory role of B7-H4 comes from studies in which the blockade of B7-H4 by a blocking mAb against B7-H4 promoted alloreactive CTL activity in a parental to F1 graft-versus-host disease model (6). Additionally, a B7-H4–blocking mAb has been shown to enhance disease progression in an EAE model of MS (28). In agreement with these data, B7-H4 knockout mice develop severe autoimmunity inversely correlated to the levels of B7-H4 expression (8). The broad and inducible expression of B7-H4, together with functional studies, suggests that B7-H4 downregulates immune responses in peripheral tissues and that B7-H4 promotes T cell tolerance. Although the ability of B7-H4 signals to both increase the number and function of Tregs (9), the loss of B7-H4 expression does not block Treg development and function altogether (29). Similarly, signaling through Nrp-1/PlxnA4 has been shown to enhance Treg function while not being strictly required for Treg development and function (15). Therefore, these two pathways have both been shown to increase Treg function/ activity while not being directly required for Treg development.
We previously showed that the immunoregulatory activity of hB7-H4Ig on inflammatory CD4+ T cell responses is mediated both by direct inhibition of effector CD4+ T cells and via increasing the number and function of Tregs (9). Although the utility of hB7-H4Ig–mediated immunoregulatory function has been demonstrated in multiple experimental autoimmune models, the identity of a functional B7-H4R has remained unknown (30). The current data show that hB7-H4Ig binds Sema3a (Fig. 1E), a soluble Sema family member, and that hB7-H4Ig binding to CD4+ T cells and hB7-H4Ig–induced alterations in both mouse and human monocyte morphology is associated with Sema3a/PlxnA4/Nrp-1 coexpression (Fig. 2D, Supplemental Fig. 2) and a significant increase in the level of phosphorylated PTEN concomitant with a significant decrease in the level of phospho-Akt (Supplemental Fig. 3). Critically, following hB7-H4Ig treatment of SJL/J mice during PLP139–151/CFA– induced R-EAE, Sema3a was found bound to the fusion protein (Fig. 2B). Together, these findings show that hB7-H4Ig directly interacts with Sema3a, and that the results from the in vitro hB7-H4Ig/Sema3a binding analyses are not an artifact of the recombinant Sema3a protein. Additionally, the functionality of hB7-H4Ig treatment is lost in Sema3a functional mutant mice (Fig. 6) and in mice lacking Nrp-1 or PTEN expression in Foxp3+ Tregs, correlating with the hB7-H4Ig–induced increase in the number and function of Tregs within the spleen and CNS of EAE mice (Figs. 5, Supplemental Figs. 3C–F, 4).
Our finding that hB7-H4Ig functionally interacts with and signals through a multiprotein complex has been recently reported for another B7-family member protein, PD-L2, which interacts with repulsion guidance molecule b (RGMb) (31). The identification of this additional receptor for PD-L2 supports the use of nonclassical receptors and receptor complexes for B7-family member proteins, such as the Sema3a/Nrp-1/PlxnA4 complex for binding B7-H4 described in this article. In the case of PD-L2:RGMb interaction, these proteins are thought to form a protein complex that interacts with the other proteins within a multimeric protein complex (i.e., bone morphogenetic protein [BMP] and neogenin) which serve as the signaling component of the PD-L2:RGMb/ BMP/neogenin complex (31). These two findings serve to illustrate alternatives to the previously defined one-to-one protein interaction for B7-family member proteins with associated functional receptors. These findings also further illustrate the multifunctionality of B7-family member proteins. For example, although PD-L1 and PD-L2 have a shared receptor (i.e., PD-1) there are functional differences induced by the two different ligands. These differences may be due to the higher affinity of PDL2 for PD-1 as compared with PD-L1 and/or PD-L1 interacting with CD80 versus PD-L2 interacting with RGMb/BMP/neogenin. To further confound the study of B7-family member proteins, the resultant functionality of a specific B7-family member protein or specific-Ab treatment may also be dependent upon the immune cell type targeted (2, 3, 32). Therefore, multiple aspects must be considered when studying B7-family member proteins, such as B7-H4 and its potential receptors.
In addition to effects on Tregs, receptor ligation by the immune modulatory molecules, such as B7-H4, have been suggested to directly alter the phenotype of CD4+ T cells (6, 33). Coculture of CD4+ T cells with IL-10/TGF-β–treated macrophages expressing B7-H4 decreased CD4+ T cell proliferation and increased the number of Foxp3+ CD4+ T cells (34). Published data show that hB7-H4Ig binds to CD4+ T cells in an activation-dependent manner, indicating that B7-H4 may function as a coinhibitory (negative costimulatory) molecule for CD4+ T cells (6, 8, 9). This is supported by our previous findings showing that hB7-H4Ig can downregulate IL-17 and IFN-γ production of mouse T cells in the absence of Tregs (9). Similarly, B7-H4 expression by tumor cells is a putative mechanism by which these cells evade antitumor immune responses. In the majority of breast and ovarian cancers, B7-H4 mRNA is expressed at ~2-fold or greater than the level expressed within normal tissue (35) and has a role in pulmonary metastases, and B7-H4 protein is present in half of early stage and two thirds of late stage ovarian tumors (36–38). The expression of B7-H4 within the tumor microenvironment either by the tumor cells and/or by infiltrating monocytes is hypothesized to promote immune evasion. In support of this hypothesis, we showed that hB7-H4Ig treatment of mice during EAE increased the number and function of Tregs (9). Considered with the fact that signaling via Nrp-1/PlxnA4 increases the stability of Tregs (15), expression of B7-H4 on either the tumor cells and/or tumor-infiltrating leukocytes would potentially increase the number, function, and stability of tumor-associated Tregs.
To our knowledge, our findings are the first to show that a B7-family member protein interacts with the Sema/Nrp/Plxn family of proteins. Although the majority of B7-family member proteins interact with CD28-like proteins, there is precedence for a B7-family member protein to interact with non-CD28–like proteins (i.e., B7-H6 interacts with NKp30) (39). A commonality among CD28-like proteins, NKp30, and Sema3a is that each of these proteins contains a canonical Ig protein domain (40–42). In the present circumstance, there is the varied biological activity of Sema3 proteins to consider. Although Sema3a binds to Nrp-1, it has also been reported to bind to PlxnA4, PlxnA1, and PlxnA2 (13, 14). In fact, we show that hB7-H4Ig can bind to activated T cells expressing Sema3a/PlxnA4 in the absence of Nrp-1, similar to a previous report showing Sema3E/PlxnD1 signaling did not require neuropilins (43). Therefore, further studies are required to determine if B7-H4 may also interact with other members of the Sema/Nrp/Plxn family of proteins.
In summary, the characterization of a functional B7-H4R, as identified in these studies, has extended our knowledge about the potential roles that Sema3a/Nrp-1/PlxnA4 may play in controlling both inflammatory immune responses and in regulating tumor cell growth. These findings further suggest that targeting the B7-H4/ Sema3a/Nrp-1/PlxnA4 pathway may provide important therapeutic benefits both in autoimmune disease following ligation of the B7-H4R using agonistic infusion of hB7-H4Ig and in cancer following blockade of B7-H4 or the B7-H4R with an antagonistic therapeutic such as blocking mAbs.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Multiple Sclerosis Society (RG 4624A10/1) and Amplimmune, Inc.
Abbreviations used in this article:
- B7-H4Ig
B7-H4-Ig fusion protein
- B7-H4R
B7-H4 receptor
- BMP
bone morphogenetic protein
- C-EAE
chronic EAE
- DTH
delayed type hypersensitivity
- EAE
experimental autoimmune encephalomyelitis
- hB7-H4Ig
human B7-H4Ig (human extracellular domain of B7-H4 plus human IgG1 Fc domain)
- MS
multiple sclerosis
- Nrp
neuropilin
- Plxn
plexin
- R-EAE
relapsing EAE
- RGMb
repulsion guidance molecule b
- Sema
semaphorin
- Treg
regulatory CD4+ T cell
- TT
tetanus toxin
Footnotes
Disclosures
The authors have no financial conflicts of interest.
The online version of this article contains supplemental material.
References
- 1.Greenwald RJ, Freeman GJ, and Sharpe AH. 2005. The B7 family revisited. Annu. Rev. Immunol 23: 515–548. [DOI] [PubMed] [Google Scholar]
- 2.Suvas S, Singh V, Sahdev S, Vohra H, and Agrewala JN. 2002. Distinct roleof CD80 and CD86 in the regulation of the activation of B cell and B cell lymphoma. J. Biol. Chem 277: 7766–7775. [DOI] [PubMed] [Google Scholar]
- 3.Podojil JR, Kohm AP, and Miller SD. 2006. CD4+ T cell expressed CD80 regulates central nervous system effector function and survival during experimental autoimmune encephalomyelitis. J. Immunol 177: 2948–2958. [DOI] [PubMed] [Google Scholar]
- 4.Curran MA, Montalvo W, Yagita H, and Allison JP. 2010. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory Tand myeloid cells within B16 melanoma tumors. Proc. Natl. Acad. Sci. USA 107: 4275–4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gubin MM, Zhang X, Schuster H, Caron E, Ward JP, Noguchi T, Ivanova Y, Hundal J, Arthur CD, Krebber WJ, et al. 2014. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature 515: 577–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sica GL, Choi IH, Zhu G, Tamada K, Wang SD, Tamura H, Chapoval AI, Flies DB, Bajorath J, and Chen L. 2003. B7-H4, a molecule of the B7 family, negatively regulates T cell immunity. Immunity 18: 849–861. [DOI] [PubMed] [Google Scholar]
- 7.Azuma T, Zhu G, Xu H, Rietz AC, Drake CG, Matteson EL, andL. Chen. 2009. Potential role of decoy B7-H4 in the pathogenesis of rheumatoid arthritis: a mouse model informed by clinical data. PLoS Med. 6: e1000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei J, Loke P, Zang X, and Allison JP. 2011. Tissue-specific expressionof B7x protects from CD4 T cell-mediated autoimmunity. J. Exp. Med 208: 1683–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Podojil JR, Liu LN, Marshall SA, Chiang MY, Goings GE, Chen L, Langermann S, and Miller SD. 2013. B7-H4Ig inhibits mouse and human T-cell function and treats EAE via IL-10/Treg-dependent mechanisms. J. Autoimmun 44: 71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee IF, Wang X, Hao J, Akhoundsadegh N, Chen L, Liu L, Langermann S, Ou D, and Warnock GL. 2013. B7-H4.Ig inhibits the development of type 1 diabetes by regulating Th17 cells in NOD mice. Cell. Immunol 282: 1–8. [DOI] [PubMed] [Google Scholar]
- 11.Roth L, Koncina E, Satkauskas S, Crémel G, Aunis D, and Bagnard D. 2009. The many faces of semaphorins: from development to pathology. Cell. Mol. Life Sci 66: 649–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kolodkin AL, Matthes DJ, O’Connor TP, Patel NH, Admon A, Bentley D, and Goodman CS. 1992. Fasciclin IV: sequence, expression, and function during growth cone guidance in the grasshopper embryo. Neuron 9: 831–845. [DOI] [PubMed] [Google Scholar]
- 13.He Z, and Tessier-Lavigne M. 1997. Neuropilin is a receptor for the axonal chemorepellent Semaphorin III. Cell 90: 739–751. [DOI] [PubMed] [Google Scholar]
- 14.Casazza A, Laoui D, Wenes M, Rizzolio S, Bassani N, Mambretti M, Deschoemaeker S, Van Ginderachter JA, Tamagnone L, and Mazzone M. 2013. Impeding macrophage entry into hypoxic tumor areas by Sema3A/Nrp1 signaling blockade inhibits angiogenesis and restores antitumor immunity. Cancer Cell 24: 695–709. [DOI] [PubMed] [Google Scholar]
- 15.Delgoffe GM, Woo SR, Turnis ME, Gravano DM, Guy C, Overacre AE, Bettini ML, Vogel P, Finkelstein D, Bonnevier J, et al. 2013. Stability and function of regulatory T cells is maintained by a neuropilin-1semaphorin-4a axis. Nature 501: 252–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lepelletier Y, Moura IC, Hadj-Slimane R, Renand A, Fiorentino S, Baude C, Shirvan A, Barzilai A, and Hermine O. 2006. Immunosuppressive role of semaphorin-3A on T cell proliferation is mediated by inhibition of actin cytoskeleton reorganization. Eur. J. Immunol 36: 1782–1793. [DOI] [PubMed] [Google Scholar]
- 17.Catalano A, Caprari P, Moretti S, Faronato M, Tamagnone L, and Procopio A. 2006. Semaphorin-3A is expressed by tumor cells and alters T-cell signal transduction and function. Blood 107: 3321–3329. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto M, Suzuki K, Okuno T, Ogata T, Takegahara N, Takamatsu H, Mizui M, Taniguchi M, Chédotal A, Suto F, et al. 2008. Plexin-A4 negatively regulates T lymphocyte responses. Int. Immunol 20: 413–420. [DOI] [PubMed] [Google Scholar]
- 19.Solomon BD, Mueller C, Chae WJ, Alabanza LM, and Bynoe MS. 2011. Neuropilin-1 attenuates autoreactivity in experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 108: 2040–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watanabe N, Gavrieli M, Sedy JR, Yang J, Fallarino F, Loftin SK, Hurchla MA, Zimmerman N, Sim J, Zang X, et al. 2003. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat. Immunol 4: 670–679. [DOI] [PubMed] [Google Scholar]
- 21.McMahon EJ, Bailey SL, Castenada CV, Waldner H, and Miller SD.2005. Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nat. Med 11: 335–339. [DOI] [PubMed] [Google Scholar]
- 22.Catalano A. 2010. The neuroimmune semaphorin-3A reduces inflammation and progression of experimental autoimmune arthritis. J. Immunol 185: 6373–6383. [DOI] [PubMed] [Google Scholar]
- 23.Merte J, Wang Q, Vander Kooi CW, Sarsfield S, Leahy DJ, Kolodkin AL, and Ginty DD. 2010. A forward genetic screen in mice identifies Sema3A (K108N), which binds to neuropilin-1 but cannot signal. J. Neurosci 30: 5767–5775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shrestha S, Yang K, Guy C, Vogel P, Neale G, and Chi H. 2015. Treg cellsrequire the phosphatase PTEN to restrain TH1 and TFH cell responses. Nat. Immunol 16: 178–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia F, Lepelletier Y, Smaniotto S, Hadj-Slimane R, Dardenne M, Hermine O, and Savino W. 2012. Inhibitory effect of semaphorin-3A, a known axon guidance molecule, in the human thymocyte migration induced by CXCL12. J. Leukoc. Biol 91: 7–13. [DOI] [PubMed] [Google Scholar]
- 26.Okuno T, Nakatsuji Y, and Kumanogoh A. 2011. The role of immune semaphorins in multiple sclerosis. FEBS Lett. 585: 3829–3835. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka J, Tanaka H, Mizuki N, Nomura E, Ito N, Nomura N, Yamane M, Hida T, Goshima Y, Hatano H, and Nakagawa H. 2015. Semaphorin 3A controls allergic and inflammatory responses in experimental allergic conjunctivitis. Int. J. Ophthalmol 8: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prasad DV, Richards S, Mai XM, and Dong C. 2003. B7S1, a novel B7 family member that negatively regulates T cell activation. Immunity 18: 863–873. [DOI] [PubMed] [Google Scholar]
- 29.Zang X, Loke P, Kim J, Murphy K, Waitz R, and Allison JP. 2003. B7x: awidely expressed B7 family member that inhibits T cell activation. Proc. Natl. Acad. Sci. USA 100: 10388–10392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith JB, Stashwick C, and Powell DJ Jr. 2014. B7-H4 as a potential target for immunotherapy for gynecologic cancers: a closer look. Gynecol. Oncol 134: 181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiao Y, Yu S, Zhu B, Bedoret D, Bu X, Francisco LM, Hua P, Duke-Cohan JS, Umetsu DT, Sharpe AH, et al. 2014. RGMb is a novel binding partner for PD-L2 and its engagement with PD-L2 promotes respiratory tolerance. J. Exp. Med 211: 943–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keir ME, Francisco LM, and Sharpe AH. 2007. PD-1 and its ligands in T-cell immunity. Curr. Opin. Immunol 19: 309–314. [DOI] [PubMed] [Google Scholar]
- 33.Choi IH, Zhu G, Sica GL, Strome SE, Cheville JC, Lau JS, Zhu Y, Flies DB, Tamada K, and Chen L. 2003. Genomic organization and expression analysis of B7-H4, an immune inhibitory molecule of the B7 family. J. Immunol 171: 4650–4654. [DOI] [PubMed] [Google Scholar]
- 34.Cao Q, Wang Y, Zheng D, Sun Y, Wang Y, Lee VW, Zheng G, Tan TK, Ince J, Alexander SI, and Harris DC. 2010. IL-10/TGF-beta-modified macrophages induce regulatory T cells and protect against adriamycin nephrosis. J. Am. Soc. Nephrol 21: 933–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salceda S, Tang T, Kmet M, Munteanu A, Ghosh M, Macina R, Liu W, Pilkington G, and Papkoff J. 2005. The immunomodulatory protein B7-H4 is overexpressed in breast and ovarian cancers and promotes epithelial cell transformation. Exp. Cell Res 306: 128–141. [DOI] [PubMed] [Google Scholar]
- 36.Simon I, Katsaros D, Rigault de la Longrais I, Massobrio M, Scorilas A, Kim NW Sarno MJ, Wolfert RL, and Diamandis EP. 2007. B7-H4 is over-expressed in early-stage ovarian cancer and is independent of CA125 expression. Gynecol. Oncol 106: 334–341. [DOI] [PubMed] [Google Scholar]
- 37.Qian Y, Shen L, Cheng L, Wu Z, and Yao H. 2011. B7-H4 expression invarious tumors determined using a novel developed monoclonal antibody. Clin. Exp. Med 11: 163–170. [DOI] [PubMed] [Google Scholar]
- 38.Abadi YM, Jeon H, Ohaegbulam KC, Scandiuzzi L, Ghosh K, Hofmeyer KA, Lee JS, Ray A, Gravekamp C, and Zang X. 2013. Host b7x promotes pulmonary metastasis of breast cancer. J. Immunol 190: 3806–3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matta J, Baratin M, Chiche L, Forel JM, Cognet C, Thomas G, Farnarier C, Piperoglou C, Papazian L, Chaussabel D, et al. 2013. Induction of B7-H6, a ligand for the natural killer cell-activating receptor NKp30, in inflammatory conditions. Blood 122: 394–404. [DOI] [PubMed] [Google Scholar]
- 40.Eickholt BJ, Morrow R, Walsh FS, and Doherty P. 1997. Structural featuresof collapsin required for biological activity and distribution of binding sites in the developing chick. Mol. Cell. Neurosci 9: 358–371. [DOI] [PubMed] [Google Scholar]
- 41.Harper K, Balzano C, Rouvier E, Mattéi MG, Luciani MF, and Golstein P. 1991. CTLA-4 and CD28 activated lymphocyte molecules are closely related in both mouse and human as to sequence, message expression, gene structure, and chromosomal location. J. Immunol 147: 1037–1044. [PubMed] [Google Scholar]
- 42.Li Y, Wang Q, and Mariuzza RA. 2011. Structure of the human activatingnatural cytotoxicity receptor NKp30 bound to its tumor cell ligand B7-H6. J. Exp. Med 208: 703–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gu C, Yoshida Y, Livet J, Reimert DV, Mann F, Merte J, Henderson CE, Jessell TM, Kolodkin AL, and Ginty DD. 2005. Semaphorin 3E and plexinD1 control vascular pattern independently of neuropilins. Science 307: 265–268. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.