Abstract
Background
Proteinase 3-antineutrophil cytoplasmic antibody has been reported to be positive in 5–10% of cases of renal injury complicated by infective endocarditis; however, histological findings have rarely been reported for these cases.
Case presentation
A 71-year-old Japanese man with a history of aortic valve replacement developed rapidly progressive renal dysfunction with gross hematuria and proteinuria. Blood analysis showed a high proteinase 3-antineutrophil cytoplasmic antibody (163 IU/ml) titer. Streptococcus species was detected from two separate blood culture bottles. Transesophageal echocardiography detected mitral valve vegetation. Histological evaluation of renal biopsy specimens showed necrosis and cellular crescents in glomeruli without immune complex deposition. The patient met the modified Duke criteria for definitive infective endocarditis. On the basis of these findings, the patient was diagnosed with proteinase 3-antineutrophil cytoplasmic antibody-positive necrotizing crescentic glomerulonephritis complicated by Streptococcus infective endocarditis. His renal disease improved, and his proteinase 3-antineutrophil cytoplasmic antibody titer normalized with antibiotic monotherapy.
Conclusion
Few case reports have described histological findings of proteinase 3-antineutrophil cytoplasmic antibody-positive renal injury complicated with infective endocarditis. We believe that an accumulation of histological findings and treatments is mandatory for establishment of optimal management for proteinase 3-antineutrophil cytoplasmic antibody-positive renal injury complicated with infective endocarditis.
Keywords: Necrotizing crescentic glomerulonephritis, Infective endocarditis, Proteinase 3-antineutrophil cytoplasmic antibody
Background
Proteinase 3-antineutrophil cytoplasmic antibody (PR3-ANCA) has been reported to be positive in 5–10% of cases of renal injury complicated by infective endocarditis [1]; however, histological findings have rarely been reported for these cases. In addition, the clinical course and optimal treatment have not been fully clarified.
We report a case of a patient with rapidly progressive PR3-ANCA-positive necrotizing crescentic glomerulonephritis complicated by Streptococcus infective endocarditis. The patient’s renal disease improved with antibiotic therapy without any immunosuppressive agents, and his PR3-ANCA titer normalized in accordance with improving infective endocarditis.
Case presentation
Our patient was a 71-year-old Japanese man who had undergone the Bentall procedure and biological aortic valve replacement for the treatment of descending aortic aneurysm and aortic regurgitation at 70 years of age. Thereafter, his renal function had been normal (serum creatinine level, 0.93 mg/dl) without hematuria and proteinuria. Two months before admission, he had appetite loss, malaise, and gross hematuria. One month before admission, he noticed purpura on his lower extremities. A laboratory examination conducted by his primary care physician showed anemia (hemoglobin, 9.2 g/dl), thrombocytopenia (platelet count, 10 × 104/μl), hematuria, and proteinuria. Therefore, he was referred to our hospital for further management.
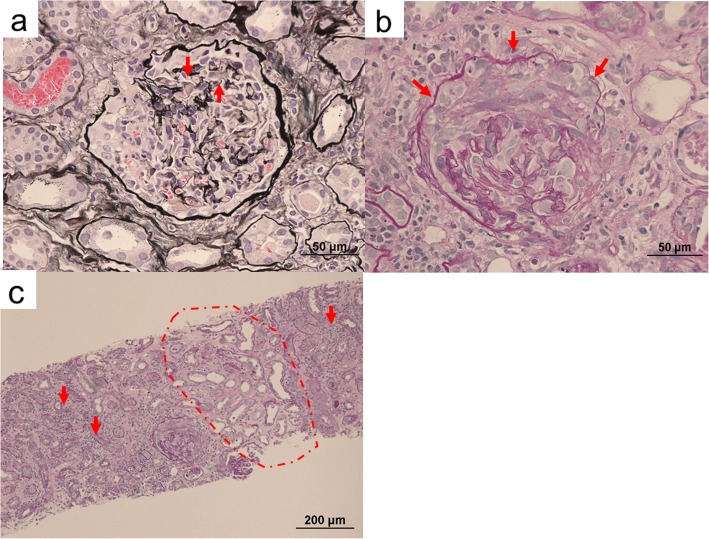
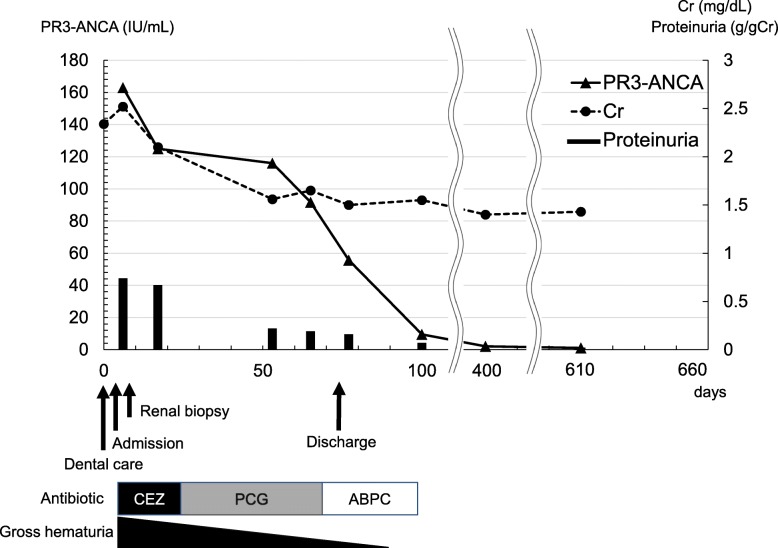
Upon admission, his body temperature was 36.9 °C, and his blood pressure was 120/60 mmHg. Anemia, edema, and symmetrically distributed palpable purpura of the lower extremities were observed. He had no characteristic physical findings of infective endocarditis, such as Osler nodes, Roth spots, and Janeway lesions. Cardiac auscultation revealed 2/6 systolic reflux murmur at the cardiac apex. Blood analysis showed that the patient’s serum creatinine level was elevated at 2.34 mg/dl, and his serum hemoglobin level was reduced at 7.6 g/dl. Urinalysis showed proteinuria at 0.74 g/g Cr and microscopic hematuria. PR3-ANCA level was elevated at 163 IU/ml (normal range, < 10 IU/ml). The patient had negative test results for hepatitis B antigen, hepatitis C antibody, cryoglobulin, antistreptolysin O, antinuclear antibody, immune complex, and myeloperoxidase-ANCA. Serum complement C3 was mildly decreased, whereas C4 was normal. Laboratory data obtained at admission are summarized in Table 1. No abnormalities were found in the patient’s chest x-ray or electrocardiogram. Streptococcus species was detected from two separate blood culture bottles. On the third hospital day, renal biopsy was performed. Histological analysis revealed that 54% (6 of 11) of glomeruli showed partial fibrinoid necrosis with fragmentation of glomerular tufts (Fig. 1a), and 27% (3 of 11) of glomeruli showed cellular crescents (Fig. 1b). No fibrocellular or fibrous crescents and no endocapillary proliferation were found. The mesangium showed no increase in cells or matrix. The tubulointerstitium partially showed neutrophilic and lymphocytic infiltration in the peritubular capillary and atrophy (Fig. 1c). Fibrinoid necrosis was not observed in vessel walls. Immunofluorescence microscopy showed no deposition of immunoglobulins and complement factors. Electron microscopy showed small amounts of nonspecific electron-dense deposits in subendothelial areas and the paramesangial area. At this point, the patient met the modified Duke criteria for definitive infective endocarditis [2] (mitral valve vegetation on echocardiography, two positive blood cultures of Streptococcus species drawn 3 days apart, glomerulonephritis). On the eighth hospital day, transesophageal echocardiography revealed mitral valve vegetation. On the 12th hospital day, spinal magnetic resonance imaging showed pyogenic spondylitis at T7/T8 and L4/L5. On the basis of these findings, the patient was diagnosed with rapidly progressive PR3-ANCA-positive necrotizing crescentic glomerulonephritis complicated by Streptococcus infective endocarditis. Antibiotic therapy including cefazolin and penicillin G followed by oral administration of ampicillin was provided without immunosuppressive agents. Thereafter, his renal disease, endocarditis, and pyogenic spondylitis improved. He was discharged from our center on the 73rd hospital day. He has since received regular outpatient treatment in our department. At 7 months after discharge, his serum creatinine level had decreased to 1.43 mg/dl, his proteinuria had decreased to 0.15 g/g Cr, and his hematuria had decreased to 1.1 red blood cells per high-power field. His PR3-ANCA level had decreased to within the normal range (Fig. 2).
Table 1.
Laboratory findings upon admission
| Complete blood count and blood chemistry | Value |
|---|---|
| WBC | 13,600/μL |
| Bands | 2% |
| Segments | 82% |
| Eosinophils | 0% |
| Basophils | 0% |
| Lymphocytes | 7% |
| Monocytes | 8% |
| RBC | 323 × 104/μL |
| Hemoglobin | 7.6 g/dL |
| Hematocrit | 29.2% |
| Platelet | 12.0 × 104/μL |
| Total protein | 7.0 g/dL |
| Albumin | 2.6 g/dL |
| AST | 35 IU/L |
| ALT | 23 IU/L |
| CRP | 7.57 mg/dL |
| Na+ | 130 mmol/L |
| K+ | 5.3 mmol/L |
| Cl− | 101 mmol/L |
| Ca2+ | 7.7 mg/dL |
| Phosphate | 3.8 mg/dL |
| BUN | 25 mg/dL |
| Cr | 2.52 mg/dL |
| eGFR | 20.8 ml/minute/1.73 m2 |
| Uric acid | 6.3 mg/dL |
| HbA1c | 6.3% |
| Glucose | 106 mg/dL |
| ASO | 73 IU/mL |
| Hepatitis B antigen | < 0.04 |
| Hepatitis C antibody | < 0.29 |
| IgG | 2692 mg/dL |
| IgA | 340 mg/dL |
| IgM | 350 mg/dL |
| Anti-DNA antibody | < 10 |
| Anti-RNP antibody | Negative |
| Anti-Sm antibody | Negative |
| C3 | 50 mg/dL |
| C4 | 17 mg/dL |
| CH50 | 23.4 U/mL |
| Antinuclear antibody | 160 |
| PR3-ANCA | 163 IU/mL |
| MPO-ANCA | < 1.0 IU/mL |
| Anti-GBM antibody | < 2.0 IU/mL |
| ESR | 86 mm/hour |
| Rheumatoid factor | 86 IU/mL |
| Urinary analysis | |
| RBC | Numerous (dysmorphic)/HPF |
| WBC | 1–4/HPF |
| Protein | 0.74 g/g Cr |
| β2-MG | 12,133 μg/L |
Abbreviations: ALT alanine aminotransferase, ASO antistreptolysin O, AST aspartate aminotransferase, β2-MG β2-microglobulin, BUN blood urea nitrogen, CH50 50% homolytic unit of complement, Cr creatinine, CRP C-reactive protein, C3 complement component 3, C4 complement component 4, eGFR estimated glomerular filtration rate, ESR erythrocyte sedimentation rate, GBM antiglomerular basement membrane antibody, HbA1c hemoglobin A1c, HPF high-power field, Ig immunoglobulin, MPO-ANCA myeloperoxidase antineutrophil cytoplasmic antibody, PR3-ANCA proteinase 3 antineutrophil cytoplasmic antibody, RBC red blood cells, RNP ribonucleoprotein, Sm Smith, WBC white blood cells
Fig. 1.
Renal biopsy findings. a Glomerulus with partial fibrinoid necrosis with fragmentation of glomerular tufts (arrows) (periodic acid-methenamine silver stain; original magnification, 400×). b Glomerulus with cellular crescentic formation (arrows) (periodic acid-Schiff stain; magnification, original magnification, 400×). c Tubulointerstitium with sporadic neutrophil infiltration in the peritubular capillary (arrows) and atrophy (broken line) (periodic acid-Schiff stain; original magnification, 100×)
Fig. 2.
The patient’s clinical course. ABPC Ampicillin, CEZ Cefazolin, Cr Creatinine, PCG Penicillin G, PR3-ANCA Proteinase 3-antineutrophil cytoplasmic antibody
Discussion and conclusions
We report a case of rapidly progressive PR3-ANCA-positive necrotizing crescentic glomerulonephritis complicated by Streptococcus infective endocarditis. The patient’s renal disease improved with antibiotic monotherapy, which led to normalization of PR3-ANCA titer in accordance with improving infective endocarditis.
Renal disease associated with infective endocarditis shows various pathological changes including crescent formation, fibrinoid necrosis, mesangial cell proliferation, and endothelial cell thickening in the glomerulus and tubulointerstitial damage with infiltration of immune cells [3–7]. PR3-ANCA has been reported to be positive in 5–10% of cases of renal disease complicated with infective endocarditis [1]. It is considered that PR3-ANCA may be produced as a result of an immune response against infection by sharing epitopes with cytoplasmic antigens of neutrophils in cases of infective endocarditis [8]. The produced PR3-ANCA is then speculated to contribute to fibrinoid necrosis, crescent formation, and granulomas in the kidney [9]. However, the lack of sufficient histological findings of PR3-ANCA-positive renal diseases complicated by infective endocarditis prevents clarification of detailed pathological changes in the kidney. Although many cases of PR3-ANCA-positive renal disease complicated by infective endocarditis have been reported, including crescentic glomerulonephritis, endocapillary proliferative glomerulonephritis, mesangial proliferative glomerulonephritis, and focal segmental glomerulonephritis, only three cases showed necrotizing crescentic glomerulonephritis complicated by infective endocarditis [10–34] (Table 2). Regarding treatment for PR3-ANCA-positive renal disease complicated by infective endocarditis, previous studies suggested antibiotic monotherapy for patients with low PR3-ANCA titers (< 25 IU/ml) and combination therapy with immunosuppressive agents, including steroids for patients with high PR3-ANCA titers (> 50 IU/ml), when the patients’ condition does not improve with antibiotic monotherapy [22, 35]. The three previous cases of PR3-ANCA-positive necrotizing crescentic glomerulonephritis showed various PR3-ANCA titers (2.96, > 8.0, and 85 IU/ml) and were treated with immunosuppressive agents such as corticosteroids in addition to antibiotics. Among those three cases, the renal disease resolved completely in two patients but progressed to end-stage renal disease in the other (Table 2). The other types of PR3-ANCA-positive renal disease complicated by infective endocarditis also showed various PR3-ANCA titers (3–359 IU/ml) and were treated with antibiotics with or without immunosuppressive agents (Table 2). Regarding treatment outcomes, most renal diseases recovered, except for one patient with crescentic glomerulonephritis with high PR3-ANCA titers (247 IU/ml) and one patient with mesangial proliferative glomerulonephritis with high PR3-ANCA titers (143 IU/ml), both of whom died (Table 2). In our patient, necrotizing crescentic glomerulonephritis improved with antibiotic monotherapy, and PR3-ANCA titer normalized in accordance with improving infective endocarditis; however, PR3-ANCA titer was highly elevated at 163 IU/ml. The results of our patient’s case suggest that antibiotic monotherapy can be effective even if the PR3-ANCA titer is considerably high in PR3-ANCA-positive necrotizing crescentic glomerulonephritis complicated by infective endocarditis. However, caution is needed with the use of immunosuppressive agents because they may exacerbate bacteremia and infective endocarditis. Furthermore, a greater accumulation of cases with histological evidence is needed to investigate optimal treatments for PR3-ANCA-positive renal disease complicated with infective endocarditis.
Table 2.
Case reports of PR3-ANCA-positive renal injury complicated by infective endocarditis
| Age (years)/sex | Renal biopsy histology (IF/EM) | PR3-ANCA (IU/mL) | Microbe detected | Past medical history | Treatments | Outcome | Reference |
|---|---|---|---|---|---|---|---|
| 54/M |
Focal necrotizing crescentic GN (negative/no deposits) |
2.96 | Streptococcus mutans | MVP | Piperacillin, tazobactam, cyclophosphamide, corticosteroids | Complete recovery | [10] |
| 59/M |
Focal necrotizing crescentic GN (negative/no deposits) |
> 8.0 | Enterococcus faecalis | Hepatitis B and C | Pulse methylprednisolone ⇒ prednisolone | Hemodialysis | [11] |
| 67/M |
Focal necrotizing crescentic GN (IgA+, IgM+, IgG+, C3+, C1q+/mesangial and subendothelial dense deposits) |
85 | Gemella sanguinis | No mention | Ceftriaxone, gentamicin, methylprednisolone | Temporary hemodialysis ⇒ complete recovery | [12] |
| 6/M |
Crescentic GN (no mention) |
Positive | Bartonella henselae | CHD | Doxycycline, rifampicin | No mention | [13] |
| 12/F |
Crescentic GN (C3+/subendothelial dense deposits) |
Positive | Gemella morbillorum | Nothing | Penicillin, gentamicin, steroids | Complete recovery | [14] |
| 14/M |
Crescentic GN (no mention) |
Positive | Bartonella henselae | CHD | Doxycycline | No mention | [13] |
| 18/F |
Crescentic GN (IgG[1+], IgM[3+], C3[2+], C1q[2+]/subendothelial dense deposits) |
32 | Bartonella henselae | Tetralogy of Fallot, SSS, and complete heart block | Antibiotic therapy, methylprednisolone | Complete recovery | [15] |
| 24/M |
Crescentic GN (C3+/mesangial and subendothelial dense deposits) |
14 | α-Hemolytic Streptococcus | VSD | Cefotaxime, prednisolone | No renal dysfunction | [16] |
| 26/F |
Crescentic GN (IgM+ C3+/no mention) |
160 | Streptococcus viridans | ASD, recent dental Tx | Penicillin G, tobramycin | Recovery | [17] |
| 36/M |
Crescentic GN (negative/no mention) |
359 | Bartonella henselae | No mention | Prednisolone, intravenous cyclophosphamide ⇒ azathioprine, MMF, prednisolone, CV surgery | Complete recovery | [18] |
| 43/M |
Crescentic GN (negative/no mention) |
Positive | Bartonella henselae | Infective endocarditis (blood culture was negative) | Cyclophosphamide, prednisoloneCV surgery | No mention | [19] |
| 46/M |
Crescentic GN (C3+ C1q+/no mention) |
25 | Negative | No mention | Amoxicillin, gentamicin, penicillin | Complete recovery | [20] |
| 47/M |
Crescentic GN (IgM+, IgA+, C3+, C1q+/mesangial and subendothelial dense deposits) |
160 | Bartonella henselae | Cat scratch disease | Doxycycline, rifampicin 6 weeks | Recovery | [21] |
| 50/M |
Crescentic GN (negative/not performed) |
247 | Streptococcus oralis | Nothing | Steroid therapy ⇒ ampicillin, gentamicin, vancomycin | Death | [22] |
| 54/M |
Crescentic GN (IgM+, C3+/no deposits) |
3 | Streptococcus mutans | No mention | Ampicillin ⇒ vancomycin, corticosteroids, cyclophosphamide | Recovery | [23] |
| 55/M |
Crescentic GN (C3[2+], IgA+/no deposits) |
> 8.0 | Bartonella henselae, Bartonella quintana | Depression | Vancomycin, cefepime ⇒ doxycycline, rifampicin, methylprednisolone | Recovery | [24] |
| 67/M |
Crescentic GN (IgM+, C3+, C1q+/no mention) |
41 | Bartonella henselae | Thoracic aortic aneurysm repair | Rifampicin, doxycycline, methylprednisolone | Temporary hemodialysis ⇒ recovery | [25] |
| 72/F |
Crescentic GN (no mention) |
Positive | Aggregatibacter aphrophilus | No mention | Vancomycin, ceftriaxone | Temporary hemodialysis ⇒ recovery | [26] |
| 42/M |
Diffuse endocapillary proliferative GN (C3+/subendothelial dense deposits) |
21.3 | Staphylococcus aureus | Nothing | Cefazolin | Recovery | [27] |
| 68/M |
Diffuse endocapillary proliferative GN and crescentic GN (IgG[2+], IgM[3+], C3[3+], C1q[2+]/subendothelial dense deposits) |
102 | Negative | Schistosomiasis | Cefoperazone, tazobactam | Complete recovery | [28] |
| 78/F |
Endocapillary proliferative GN (IgM+, C3+, C1q+/no mention) |
30 | Bartonella henselae | Hypertension | Doxycycline | Complete recovery | [29] |
| 48/M |
Mesangial proliferative GN (C3+/no mention) |
12 | Negative | Alcoholism, DM | Amoxicillin, gentamicin | Complete recovery | [20] |
| 57/M |
Mesangial proliferative GN (IgG+, IgM+, C3+ /no mention) |
45 | Negative | Nothing | Corticosteroids ⇒ ampicillin, ceftriaxone, gentamicin, vancomycin | Recovery | [30] |
| 74/M |
Mesangial proliferative GN (IgG+, C1q+/not performed) |
> 100 | Bartonella henselae, Bartonella quintana | IHD, pacemaker, DM, pulmonary embolus | Antibiotic therapy | Recovery | [31] |
| 78/M |
Mesangial proliferative GN (IgM+, C3+, IgA+, C1q+/subendothelial dense deposits) |
143 | Enterococcus faecalis | Coronary artery bypass surgery | Antibiotic therapy | Death | [32] |
| 57/M |
FSGS (IgM+, C3+/paramesangial dense deposits) |
40 | Negative | DM, AVR, aortic aneurysm | Pulse methylprednisolone ⇒ vancomycin, gentamicin, rifampicin | Plasmapheresis ⇒ recovery | [33] |
| 64/M |
FSGS and mild interstitial inflammation (no mention) |
60 | Negative | Insidious mild renal dysfunction | Ceftriaxone, doxycycline | Complete recovery | [34] |
Abbreviations: ASD atrial septal defect, AVR aortic valve replacement, CHD chronic heart disease, C3 complement component 3, CV cardiovascular, DM diabetes mellitus, EM electron microscopy, F female, FSGS focal segmental glomerulonephritis, GN glomerular nephritis, IF immunofluorescence, IHD ischemic heart disease, Ig immunoglobulin, LM light microscopy, M male, MMF mycophenolate mofetil, MVP mitral valve prolapse, PR3-ANCA proteinase 3 antineutrophil cytoplasmic antibody, SSS sick sinus syndrome, Tx treatment, VSD ventricular septal defect
In conclusion, we describe a case of a patient with PR3-ANCA-positive necrotizing crescentic glomerulonephritis complicated by infective endocarditis. His renal disease was improved with antibiotic agents, and his PR3-ANCA titer normalized in accordance with improving infective endocarditis.
Acknowledgements
We thank Christina Croney, Ph.D., of Edanz Group (www.edanzediting.com/ac) for editing a draft of the manuscript.
Abbreviations
- ABPC
Ampicillin
- ALT
Alanine aminotransferase
- ASD
Atrial septal defect
- ASO
Antistreptolysin O
- AST
Aspartate aminotransferase
- AVR
Aortic valve replacement
- β2-MG
β2-Microglobulin
- BUN
Blood urea nitrogen
- C3
Complement component 3
- C4
Complement component 4
- CEZ
Cefazolin
- CH50
50% Homolytic unit of complement
- CHD
Chronic heart disease
- Cr
Creatinine
- CRP
C-reactive protein
- CV
Cardiovascular
- DM
Diabetes mellitus
- eGFR
Estimated glomerular filtration rate
- EM
Electron microscopy
- ESR
Erythrocyte sedimentation rate
- F
Female
- FSGS
Focal segmental glomerulonephritis
- GBM
Antiglomerular basement membrane antibody
- GN
Glomerular nephritis
- HbA1c
Hemoglobin A1c
- HPF
High-power field
- IF
Immunofluorescence
- Ig
Immunoglobulin
- IHD
Ischemic heart disease
- LM
Light microscopy
- M
Male
- MMF
Mycophenolate mofetil
- MPO-ANCA
Myeloperoxidase antineutrophil cytoplasmic antibody
- MVP
Mitral valve prolapse
- PCG
Penicillin G
- PR3-ANCA
Proteinase 3-antineutrophil cytoplasmic antibody
- RBC
Red blood cells
- RNP
Ribonucleoprotein
- Sm
Smith
- SSS
Sick sinus syndrome
- Tx
Treatment
- VSD
Ventricular septal defect
- WBC
White blood cells
Authors’ contributions
KY and YK wrote the manuscript. KH and YM supervised the study. YU, MH, and YM undertook histological analysis. All authors participated in patient care. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Approval of the institutional ethics committee was not required, because this is a case report without any experimental trial.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Katsunori Yanai and Yoshio Kaku are equal first authors.
Contributor Information
Katsunori Yanai, Email: yanai03310751@yahoo.co.jp.
Yoshio Kaku, Email: kwaynan@gmail.com.
Keiji Hirai, Phone: +81-48-647-2111, Email: keijihirai@kfy.biglobe.ne.jp.
Shohei Kaneko, Email: shohei.sasurai@gmail.com.
Saori Minato, Email: saorim0404@gmail.com.
Yuko Mutsuyoshi, Email: mutsuyoshiyuko@yahoo.co.jp.
Hiroki Ishii, Email: hi.ro.ki.i.99@gmail.com.
Taisuke Kitano, Email: lovbrace@yahoo.co.jp.
Mitsutoshi Shindo, Email: mitsu_shin@hotmail.com.
Haruhisa Miyazawa, Email: hal1300.m@gmail.com.
Kiyonori Ito, Email: kiyonori.ito@gmail.com.
Yuichiro Ueda, Email: mini_bouz_butterfly@yahoo.co.jp.
Masahiro Hiruta, Email: masahiru@jichi.ac.jp.
Susumu Ookawara, Email: su-ooka@hb.tp1.jp.
Yoshihiko Ueda, Email: yoshi@dokkyomed.ac.jp.
Yoshiyuki Morishita, Phone: +81-48-647-2111, Email: ymori@jichi.ac.jp.
References
- 1.Boils CL, Nasr SH, Walker PD, Couser WG, Larsen CP. Update on endocarditis-associated glomerulonephritis. Kidney Int. 2015;87:1241–1249. doi: 10.1038/ki.2014.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pierce D, Calkins BC, Thornton K. Infectious endocarditis: diagnosis and treatment. Am Fam Physician. 2012;85:981–986. [PubMed] [Google Scholar]
- 3.Nasr SH, Radhakrishnan J, D’Agati VD. Bacterial infection-related glomerulonephritis in adults. Kidney Int. 2013;83:792–803. doi: 10.1038/ki.2012.407. [DOI] [PubMed] [Google Scholar]
- 4.Kannan S, Mattoo TK. Diffuse crescentic glomerulonephritis in bacterial endocarditis. Pediatr Nephrol. 2001;16:423–428. doi: 10.1007/s004670000550. [DOI] [PubMed] [Google Scholar]
- 5.Toth T. Crescentic involved glomerulonephritis in infective endocarditis. Int Urol Nephrol. 1990;22:77–88. doi: 10.1007/BF02550440. [DOI] [PubMed] [Google Scholar]
- 6.Orfila C, Lepert JC, Modesto A, Goudable C, Suc JM. Rapidly progressive glomerulonephritis associated with bacterial endocarditis: efficacy of antibiotic therapy alone. Am J Nephrol. 1993;13:218–222. doi: 10.1159/000168622. [DOI] [PubMed] [Google Scholar]
- 7.Neugarten J, Gallo GR, Baldwin DS. Glomerulonephritis in bacterial endocarditis. Am J Kidney Dis. 1984;3:371–379. doi: 10.1016/S0272-6386(84)80086-4. [DOI] [PubMed] [Google Scholar]
- 8.Collazos J, Diaz F, Mayo J, Martinez E. Infectious endocarditis, vasculitis, and glomerulonephritis. Clin Infect Dis. 1999;28:1342–1343. doi: 10.1086/517800. [DOI] [PubMed] [Google Scholar]
- 9.Hilhorst M, Shirai T, Berry G, Goronzy JJ, Weyand CM. T cell-macrophage interactions and granuloma formation in vasculitis. Front Immunol. 2014;5:432. doi: 10.3389/fimmu.2014.00432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Konstantinov KN, Harris AA, Hartshorne MF, Tzamaloukas AH. Symptomatic anti-neutrophil cytoplasmic antibody-positive disease complicating subacute bacterial endocarditis: to treat or not to treat? Case Rep Nephrol Urol. 2012;2:25–32. doi: 10.1159/000339409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cervi A, Kelly D, Alexopoulou I, Khalidi N. ANCA-associated pauci-immune glomerulonephritis in a patient with bacterial endocarditis: a challenging clinical dilemma. Clin Nephrol Case Stud. 2017;5:32–37. doi: 10.5414/CNCS109076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rousseau-Gagnon M, Riopel J, Desjardins A, Garceau D, Agharazii M, Desmeules S. Gemella sanguinis endocarditis with c-ANCA/anti-PR-3-associated immune complex necrotizing glomerulonephritis with a ‘full-house’ pattern on immunofluorescence microscopy. Clin Kidney J. 2013;6:300–304. doi: 10.1093/ckj/sft030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ouellette CP, Joshi S, Texter K, Jaggi P. Multiorgan involvement confounding the diagnosis of Bartonella henselae infective endocarditis in children with congenital heart disease. Pediatr Infect Dis J. 2017;36:516–520. doi: 10.1097/INF.0000000000001510. [DOI] [PubMed] [Google Scholar]
- 14.Kumar G, Al Ali AS, Gulzar Bhatti N. Rare bacteria infecting the heart and affecting the kidney of a young child. Case Rep Nephrol Dial. 2017;7:138–143. doi: 10.1159/000484474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khalighi MA, Nguyen S, Wiedeman JA, Palma Diaz MF. Bartonella endocarditis-associated glomerulonephritis: a case report and review of the literature. Am J Kidney Dis. 2014;63:1060–1065. doi: 10.1053/j.ajkd.2013.10.058. [DOI] [PubMed] [Google Scholar]
- 16.Fukuda M, Motokawa M, Usami T, Oikawa T, Morozumi K, Yoshida A, Kimura G. PR3-ANCA-positive crescentic necrotizing glomerulonephritis accompanied by isolated pulmonic valve infective endocarditis, with reference to previous reports of renal pathology. Clin Nephrol. 2006;66:202–209. doi: 10.5414/CNP66202. [DOI] [PubMed] [Google Scholar]
- 17.Wagner J, Andrassy K, Ritz E. Is vasculitis in subacute bacterial endocarditis associated with ANCA? Lancet. 1991;337:799–800. doi: 10.1016/0140-6736(91)91427-V. [DOI] [PubMed] [Google Scholar]
- 18.Shah SH, Grahame-Clarke C, Ross CN. Touch not the cat bot a glove: ANCA-positive pauci-immune necrotizing glomerulonephritis secondary to Bartonella henselae. Clin Kidney J. 2014;7:179–181. doi: 10.1093/ckj/sft165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vikram HR, Bacani AK, DeValeria PA, Cunningham SA, Cockerill FR., 3rd Bivalvular Bartonella henselae prosthetic valve endocarditis. J Clin Microbiol. 2007;45:4081–4084. doi: 10.1128/JCM.01095-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Subra JF, Michelet C, Laporte J, Carrere F, Reboul P, Cartier F, Saint-Andre JP, Chevailler A. The presence of cytoplasmic antineutrophil cytoplasmic antibodies (C-ANCA) in the course of subacute bacterial endocarditis with glomerular involvement, coincidence or association? Clin Nephrol. 1998;49:15–18. [PubMed] [Google Scholar]
- 21.Vercellone J, Cohen L, Mansuri S, Zhang PL, Kellerman PS. Bartonella endocarditis mimicking crescentic glomerulonephritis with PR3-ANCA positivity. Case Rep Nephrol. 2018;2018:9607582. doi: 10.1155/2018/9607582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kishimoto N, Mori Y, Yamahara H, Kijima Y, Nose A, Uchiyama-Tanaka Y, Tokoro T, Nagata T, Umeda Y, Takahashi N, et al. Cytoplasmic antineutrophil cytoplasmic antibody positive pauci-immune glomerulonephritis associated with infectious endocarditis. Clin Nephrol. 2006;66:447–454. doi: 10.5414/CNP66447. [DOI] [PubMed] [Google Scholar]
- 23.Zeledon JI, McKelvey RL, Servilla KS, Hofinger D, Konstantinov KN, Kellie S, Sun Y, Massie LW, Hartshorne MF, Tzamaloukas AH. Glomerulonephritis causing acute renal failure during the course of bacterial infections: histological varieties, potential pathogenetic pathways and treatment. Int Urol Nephrol. 2008;40:461–470. doi: 10.1007/s11255-007-9323-6. [DOI] [PubMed] [Google Scholar]
- 24.Raybould JE, Raybould AL, Morales MK, Zaheer M, Lipkowitz MS, Timpone JG, Kumar PN. Bartonella endocarditis and pauci-immune glomerulonephritis: a case report and review of the literature. Infect Dis Clin Pract (Baltim Md) 2016;24:254–260. doi: 10.1097/IPC.0000000000000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Haare HS, Wilmes D, Aydin S, Clerckx C, Labriola L. Necrotizing ANCA-positive glomerulonephritis secondary to culture-negative endocarditis. Case Rep Nephrol. 2015;2015:649763. doi: 10.1155/2015/649763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirano K, Tokui T, Inagaki M, Fujii T, Maze Y, Toyoshima H. Aggregatibacter aphrophilus infective endocarditis confirmed by broad-range PCR diagnosis: a case report. Int J Surg Case Rep. 2017;31:150–153. doi: 10.1016/j.ijscr.2017.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawamorita Y, Fujigaki Y, Imase A, Arai S, Tamura Y, Tanemoto M, Uozaki H, Yamaguchi Y, Uchida S. Successful treatment of infectious endocarditis associated glomerulonephritis mimicking C3 glomerulonephritis in a case with no previous cardiac disease. Case Rep Nephrol. 2014;2014:569047. doi: 10.1155/2014/569047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peng H, Chen WF, Wu C, Chen YR, Peng B, Paudel SD, Lou TQ. Culture-negative subacute bacterial endocarditis masquerades as granulomatosis with polyangiitis (Wegener’s granulomatosis) involving both the kidney and lung. BMC Nephrol. 2012;13:174. doi: 10.1186/1471-2369-13-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salvado C, Mekinian A, Rouvier P, Poignard P, Pham I, Fain O. Rapidly progressive crescentic glomerulonephritis and aneurism with antineutrophil cytoplasmic antibody: Bartonella henselae endocarditis. Presse Med. 2013;42:1060–1061. doi: 10.1016/j.lpm.2012.07.031. [DOI] [PubMed] [Google Scholar]
- 30.Reza Ardalan M, Trillini M. Infective endocarditis mimics ANCA associated glomerulonephritis. Caspian J Intern Med. 2012;3:496–499. [PMC free article] [PubMed] [Google Scholar]
- 31.Forbes SH, Robert SC, Martin JE, Rajakariar R. Quiz page January 2012: Acute kidney injury with hematuria, a positive ANCA test, and low levels of complement. Am J Kidney Dis. 2012;59:A28–A31. doi: 10.1053/j.ajkd.2011.06.032. [DOI] [PubMed] [Google Scholar]
- 32.McAdoo SP, Densem C, Salama A, Pusey CD. Bacterial endocarditis associated with proteinase 3 anti-neutrophil cytoplasm antibody. NDT Plus. 2011;4:208–210. doi: 10.1093/ndtplus/sfr030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mohandes S, Satoskar A, Hebert L, Ayoub I. Bacterial endocarditis manifesting as autoimmune pulmonary renal syndrome: ANCA-associated lung hemorrhage and pauci-immune crescentic glomerulonephritis. Clin Nephrol. 2018;90:431–433. doi: 10.5414/CN109495. [DOI] [PubMed] [Google Scholar]
- 34.Sugiyama H, Sahara M, Imai Y, Ono M, Okamoto K, Kikuchi K, Nagai R. Infective endocarditis by Bartonella quintana masquerading as antineutrophil cytoplasmic antibody-associated small vessel vasculitis. Cardiology. 2009;114:208–211. doi: 10.1159/000228645. [DOI] [PubMed] [Google Scholar]
- 35.Haseyama T, Imai H, Komatsuda A, Hamai K, Ohtani H, Kibira S, Miura AB. Proteinase-3-antineutrophil cytoplasmic antibody (PR3-ANCA) positive crescentic glomerulonephritis in a patient with Down’s syndrome and infectious endocarditis. Nephrol Dial Transplant. 1998;13:2142–2146. doi: 10.1093/ndt/13.8.2142. [DOI] [PubMed] [Google Scholar]