Abstract
Background
Our population is ageing and in 2050 more than one out of five people will be 60 years or older; 80% of whom will be living in a low-and-middle income country. Living longer does not entail living healthier; however, there is not a widely accepted measure of healthy ageing hampering policy and research. The World Health Organization defines healthy ageing as the process of developing and maintaining functional ability that will enable well-being in older age. We aimed to create a healthy ageing index (HAI) in a subset of six low-and-middle income countries, part of the 10/66 study, by using items of functional ability and intrinsic capacity.
Methods
The study sample included residents 65-years old and over (n = 12,865) from catchment area sites in Cuba, Dominican Republic, Peru, Venezuela, Mexico and Puerto Rico. Items were collected by interviewing participants or key informants between 2003 and 2010. Two-stage factor analysis was employed and we compared one-factor, second-order and bifactor models. The psychometric properties of the index, including reliability, replicability, unidimensionality and concurrent convergent validity as well as measurement invariance per ethnic group and gender were further examined in the best fit model.
Results
The bifactor model displayed superior model fit statistics supporting that a general factor underlies the various items but other subdomain factors are also needed. The HAI indicated excellent reliability (ω = 0.96, ωΗ = 0.84), replicability (H = 0.96), some support for unidimensionality (Explained Common Variance = 0.65) and some concurrent convergent validity with self-rated health. Scalar measurement invariance per ethnic group and gender was supported.
Conclusions
A HAI with excellent psychometric properties was created by using items of functional ability and intrinsic capacity in a subset of six low-and-middle income countries. Further research is needed to explore sub-population differences and to validate this index to other cultural settings.
Electronic supplementary material
The online version of this article (10.1186/s12874-019-0849-y) contains supplementary material, which is available to authorized users.
Keywords: 10/66, Healthy ageing metric, Psychometric properties, Measurement invariance, Bifactor model
Background
Globally, life expectancy has increased by an average of 5 years between 2000 and 2015 [1]. The number of people aged 60 and over is expected to double by 2050 and Latin American countries are to experience the fastest growth over the next 15 years [2]. Risk of disability and noncommunicable chronic diseases increases with age as well [3]. Despite technological and medical advances people are likely to experience multimorbidity in later life, living with multiple chronic conditions [3]. This growing population of frail older people will lead to higher health and societal costs. There is, therefore, an imperative need to examine the ageing process and most importantly the elements that will enable people to live longer and in a healthy way.
Systematic reviews indicate that till now there is neither a unanimous definition nor a standardised metric of healthy ageing and the percentage of healthy or successful agers differs considerably among studies mainly due to lack of common definition and measurement procedures [4, 5]. However, the need for an aggregated metric of health status that would permit valid comparisons among populations and over time is well recognised [6, 7]. Constant monitoring of the health status of older people will enable us to identify key determinants and implement comprehensive healthy ageing policies. According to the latest report of ageing and health from the World Health Organization (WHO), healthy ageing is defined as the process of developing and maintaining the functional ability that will enable well-being in older people [3].
In this study, our key objective is the creation of a healthy ageing index (HAI) based on the WHO conceptual framework in a subset of Latin American countries. We also aim to examine this index for various psychometric properties (i.e. omega reliability coefficients, explained common variance measure of unidimensionality, index H of construct replicability and concurrent convergent validity) and measurement invariance for ethnic groups and gender.
Methods
10/66 study
Data were collected from specific urban and rural catchment areas in Latin America (Cuba, Dominican Republic, Peru, Venezuela, Mexico and Puerto Rico); part of the 10/66 Dementia Research Group (10/66 DRG) survey. 10/66 DRG is a multicentre study on ageing and dementia performed in low-and-middle income countries. Baseline face-to-face interviews of residents 65 years old and over were carried out between 2003 and 2007 in all areas, other than Puerto Rico where baseline data were collected between 2007 and 2010. Catchment areas were selected to be broadly representative of the source community and 2000 target participants per country (with the exemption of 3000 in Cuba) were chosen a priori; response rate was excellent ranging from 80 to 95%. Participant and informant interviews as well as physical examination were part of the 10/66 study protocol. In cases where the participant’s capacity to provide reliable information was in doubt, the information was corroborated by an informant (usually a relative or a caregiver). A more detailed description is available at www.alz.co.uk/1066 and elsewhere [8, 9].
Healthy ageing indicators
Questions measuring health and disability according to the International Classification of Functioning, Disability and Health (ICF) [10] were used as healthy ageing indicators/items to build the index. A total of 26 health questions, which were either self-reported by the participants or provided by key informants, were identified from various questionnaires and operationalised as described below. Difficulties with: household responsibilities, walking a kilometre, washing whole body, getting dressed, carrying out work and everyday activities, making decisions, using the toilet, handling money, finding the right word, completing chores, routine (assessed as: ‘feeling of not coping properly with everyday routine’), sleep (assessed as: ‘trouble with sleep or recent change in pattern’), orientation (assessed as: ‘forgets where he/she is’); hearing and eye problems, change in daily activities, exhaustion (assessed as: ‘gets worn out or exhausted during daytime or evening’) and speed test (assessed by the time in seconds taken to walk 10 m). Finally, cognitive assessment included the following items: instant recall (a 10 word list learning assessed for three times; we considered one value: the maximum number of words among the three trials), delayed recall (assessed as: ‘do you remember the three words I told you a few minutes ago’), long term memory item (correctly remembering the name of a well-known person linked to a historical event), immediate recall (assessed by repeating three words that previously were mentioned by the interviewer), verbal fluency (assessed by the number of animals that the participant could recall in 1 minute), time orientation (day, month, year, season), story recall (repeat a story that just heard from the interviewer), and praxis (fold a piece of paper, following instructions). Most items were categorical and in some cases where a continuous outcome was reported (i.e. instant recall, verbal fluency, speed test, story recall), we divided the whole sample in three groups according to the lower and upper quartiles of each distribution; values below the 25th percentile, between the 25th and 75th and above the 75th indicated high, moderate and low performance, respectively. Higher values indicated worse health outcomes. Table 1 provides more details on items origin (i.e. participant or informant interview; initial questionnaire from the 10/66 interview from which they were extracted).
Table 1.
Healthy Ageing Indicators Origin
| Items/Indicators | Origin | Questionnaire |
|---|---|---|
| Household responsibilities difficulty | Participant | WHO-DAS II |
| Walking a km difficulty | Participant | WHO-DAS II |
| Washing whole body difficulty | Participant | WHO-DAS II |
| Getting dressed difficulty | Participant | WHO-DAS II |
| Carrying out work & everyday activities difficulty | Participant | WHO-DAS II |
| Making decisions difficulty | Informant | CSI’D’-RELSCORE |
| Using the toilet difficulty | Informant | CSI’D’-RELSCORE |
| Handling money difficulty | Informant | CSI’D’-RELSCORE |
| Hearing problem | Participant & informant | Health (including pain and impairments) |
| Eye problem | Participant & informant | Health (including pain and impairments) |
| Finding right word difficulty | Informant | CSI’D’-RELSCORE |
| Change in daily activities | Informant | CSI’D’-RELSCORE |
| Forgets where he/she is | Informant | CSI’D’-RELSCORE |
| Difficulty completing chores | Informant | CSI’D’-RELSCORE |
| Sleep trouble or recent change in pattern | Participant | Mental Health (GMS-version B3) |
| Feeling of not coping properly with everyday routine | Participant | Mental Health (GMS-version B3) |
| Gets worn out or exhausted during daytime or evening | Participant | Mental Health (GMS-version B3) |
| Time in seconds taken to walk 10 m | Clinical examination | neurological assessment (NEUROEX) |
| Learn test | Participant | 10 word list learning |
| Delayed recall | Participant | CSI’D’ |
| Long memory test | Participant | CSI’D’ |
| Immediate recall | Participant | CSI’D’ |
| Verbal fluency | Participant | CSI’D’ |
| Time orientation | Participant | CSI’D’ |
| Praxis-fold a piece of paper | Participant | CSI’D’ |
| Story recall difficulty | Participant | CSI’D’ |
WHO-DAS II World Health Organization. Disabilty Assessment Schedule 2.0; CSI’D’-RELSCORE Community Screening Interview for Dementia-Informant Scale, GMS Geriatric Mental State Interview, NEUROEX Neurological Examination, CSI’D’ Community Screening Interview for Dementia
Data analyses
We used SPSS version 22 for data management and Mplus 7.4 software for any statistical analyses. Mean and variance-adjusted weighted least-squares (WLSMV) estimator, suitable for the analysis of categorical data, polychoric correlations and theta parameterisation -in which residual variances of observed categorical outcome variables are allowed to be parameters in the models- were employed [11]. A pairwise present approach to missing data was used as it is the default in Mplus with WLSMV estimator [12].
Model accuracy was routinely reported by chi-square value with degrees of freedom (df); however, given the sensitivity of chi-square to large sample sizes, we used goodness-of-fit indices to make decisions about the global fit of the models and we inspected discrepancies between predicted and observed correlations to assess local fit [13]. We reported the comparative fit index (CFI) and root mean square error of approximation (RMSEA) with 90% confidence intervals (CI). We considered a model to have an acceptable fit when CFI ≥ 0.90 and RMSEA values close or less than 0.06 [14]. Nested models were compared by using the DIFFTEST command of MPLUS (an adjusted chi-square test when the WLSMV estimator is employed) [11, 13].
Exploratory factor analysis
To identify the appropriate number of factors and the pattern of relationships between items and factors the data-driven methodology of exploratory factor analysis (EFA) was employed [15]. For the EFA, a 30% stratified by gender and country random sample of our initial sample was used and the remaining 70% was used as our validation sample in the confirmatory factor analyses (CFA). Parsimax (oblique) rotation, allowing for factor correlation and for minimum variable complexity, was employed to foster factor interpretability [16]. Factor loadings equal or higher than 0.20, in absolute value term, were considered as factor loading cut-off point. Response categories with less than 4% of the sample were merged with the adjacent higher category to avoid computational issues in the model fitting.
Confirmatory factor analysis
As our objective was to build an index which would represent the multifaceted concept of healthy ageing, CFA framework was employed to identify the best measurement model representing healthy ageing as a single general construct. A second-order model and a bifactor model were considered, recognising the multidimensionality of healthy ageing but also focusing on an overall target construct; a one-factor model was also examined. Multidimensional measurement models without a general factor (i.e. first-order correlated-factors model) were not considered.
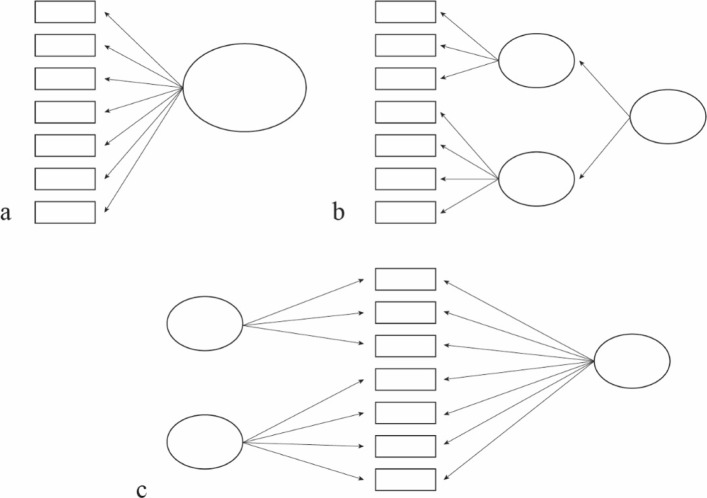
Figure 1 shows a simplified example of the structural difference among a one-factor, a second-order and a bifactor model. The main difference between a bifactor structure and a second-order model is that in the bifactor structure the general factor explains the covariance among all observed items, and not within first-order factors as is the case of the second-order model. Furthermore, a bifactor structure simultaneously allows to have other subdomain factors, which account for the variability not explained by the general factor [17].
Fig. 1.
Graphical representation of a. one factor model; b. second-order; c. bifactor model
Both the second-order and the bifactor model were set up by using the number and the item structure onto first-order and subdomain factors as suggested by the EFA. Hence, the second-order model was constituted by a second-order factor onto which the first-order factors of the EFA were loaded; the bifactor model was constituted by a general factor onto which all items were loaded and a number of orthogonal subdomain factors onto which items were loaded as suggested by the EFA. As a one-factor model and a second-order model are nested within the bifactor model the DIFFTEST command was used for model comparison [11, 18, 19].
Measurement invariance
Since our data were collected from different populations, it was crucial to establish that our latent construct measured the same thing and in the same way across the different ethnic groups and across men and women. We assessed measurement invariance among the six countries and between men and women by performing multi-group confirmatory factor analyses (MGCFA) and creating nested models with increasing parameter constraints [20]. Measurement invariance within the factor analytic framework can be tested by examining the statistical fit of models that differ in the parameters that have been set to equal or not across groups [21]. A lack of invariance could indicate differences in the way a group interprets and replies to a measure. Measurement invariance was assessed in the model that was selected as superior in the fit by the confirmatory factor analyses. The analysis was carried out in three steps:
We checked if our model structure was the same across the different groups, meaning that our suggested model fitted the data well in all six countries and men and women separately.
We assessed configural invariance; a baseline multiple-group model was created in which all factor loadings and thresholds were freely estimated across groups (for identification purposes we set one item loading per factor fixed to 1 -referent indicator-, one threshold per item and one additional threshold for the referent indicator equal across groups, factor means fixed to zero to all groups and residual variances to one to the reference group) [21, 22]. This model constituted our baseline model for subsequent tests.
We assessed scalar invariance; all factor loadings and thresholds were constrained to equality across groups (for identification purposes factor means were fixed to zero and residual variances were fixed to one in the first group only). We did not assess metric invariance since in the invariance testing of ordinal items, loadings and thresholds cannot be tested separately [23]. (See Additional file 1 for MPLUS code).
To compare the models, the DIFFTEST command was used; however, the dependence of chi-square statistic on sample size makes it also a non-appropriate indicator for decrement fit evaluation between nested models [13]. For this reason we also examined the change in CFI (ΔCFI) and in the RMSEA (ΔRMSEA) goodness-of-fit. A change in CFI values less than or equal to 0.010 supplemented by a change of less than or equal to 0.015 in RMSEA would provide evidence for not rejecting the hypothesis of measurement invariance [19, 24].
Psychometric coefficients
Omega (ω) and omega hierarchical (ωH) coefficients were calculated as they provide better estimates of measurement precision (reliability) than the traditional Cronbach’s alpha [25]. Omega coefficients estimate the proportion of variance in unit-weighted total score attributable to all sources of common variance and to the general factor within the bifactor framework [26–28]. A high ω value indicates a highly reliable multidimensional composite and a high ωH value (> 0.80) in the bifactor structure indicates that the general factor is the dominant source of systematic variance with subdomain factors having less influence. We also calculated coefficient omega hierarchical subscales (ωΗS) to estimate the strength of influence of subdomain factors. Coefficient ωΗS represents the proportion of reliable systematic variance of a subscale score after partitioning out general factor variability [29]. We also judged the unidimensionality of the index by calculating the Explained Common Variance index (ECV) [17, 30]. Higher values of ECV indicate a strong general factor allowing us to fit a unidimensional model even to multidimensional data. Finally, we checked for construct replicability (i.e. how well a set of items represents a latent variable) with the index H, which provides the proportion of variability in a latent construct explainable by its own indicators [31]. High values of H (> 0.80) indicate a well-defined latent variable which can be considered stable across settings. This index is of high importance in the structural equation model (SEM) framework as it assists in understanding the feasibility of a measurement model [28].
Concurrent convergent validity
To examine the concurrent convergent validity of our index we estimated its association with the self-rated health (SRH) of the participants in the past 30 days. Multiple-indicators multiple-causes model (MIMIC) with latent variables was employed to eliminate any measurement unreliability from our conclusion [32]. Even though the SRH measure is quite subjective, since it is based on individuals’ opinion about their health status, research shows that it has strong predictive validity for mortality in general [33] and in the 10/66 cohort [34]. As a consequence, we estimated the associations of SRH with the healthy ageing latent construct, adjusted for age and sex, in the best fit measurement model.
Results
Sample study characteristics
Descriptive statistics of the study population are provided in Table 2.
Table 2.
Characteristics of the 10/66 Cohort
| Country | Total (%) | Cuba (%) | Dominican Republic (%) | Peru (%) | Venezuela (%) | Mexico (%) | Puerto Rico (%) |
|---|---|---|---|---|---|---|---|
| Total | 12,865 | 2944 | 2011 | 1933 | 1965 | 2003 | 2009 |
| Women | 8288 (64%) | 1913 (65%) | 1325 (66%) | 1183 (61%) | 1252 (64%) | 1268 (63%) | 1347 (67%) |
| Men | 4568 (36%) | 1031 (35%) | 684 (34%) | 750 (39%) | 713 (36%) | 735 (37%) | 655 (33%) |
| Age (years) | |||||||
| 65–69 | 3644 (28%) | 760 (26%) | 533 (27%) | 554 (29%) | 839 (43%) | 544 (27%) | 414 (21%) |
| 70–74 | 3308 (26%) | 789 (27%) | 520 (26%) | 493 (26%) | 469 (24%) | 581 (29%) | 456 (23%) |
| 75–79 | 2689 (21%) | 639 (22%) | 397 (20%) | 399 (21%) | 345 (18%) | 426 (21%) | 483 (24%) |
| 80+ | 3211 (25%) | 749 (25%) | 561 (28%) | 486 (25%) | 308 (16%) | 451 (23%) | 656 (33%) |
| Marital Status | |||||||
| Never married | 1044 (8%) | 275 (9%) | 139 (7%) | 213 (11%) | 189 (10%) | 105 (5%) | 123 (6%) |
| Married/ cohabiting | 5845 (45%) | 1271 (43%) | 586 (29%) | 1092 (56%) | 921 (47%) | 1008 (50%) | 967 (48%) |
| Widowed | 4245 (33%) | 928 (32%) | 806 (40%) | 524 (27%) | 549 (28%) | 766 (38%) | 672 (33%) |
| Divorced/ separated | 1644 (13%) | 462 (16%) | 465 (23%) | 93 (5%) | 261 (13%) | 123 (6%) | 240 (12%) |
| Education | |||||||
| None | 1370 (11%) | 75 (3%) | 392 (19%) | 121 (6%) | 156 (8%) | 554 (28%) | 72 (4%) |
| Some, did not complete primary | 3606 (28%) | 655 (22%) | 1022 (51%) | 231 (12%) | 445 (23%) | 864 (43%) | 389 (19%) |
| Completed primary | 3807 (30%) | 979 (33%) | 370 (18%) | 727 (38%) | 965 (49%) | 351 (18%) | 415 (21%) |
| Completed secondary | 2483 (19%) | 728 (25%) | 135 (7%) | 517 (27%) | 266 (14%) | 124 (6%) | 713 (35%) |
| Tertiary (college) | 1504 (12%) | 499 (17%) | 73 (4%) | 321 (17%) | 93 (5%) | 108 (5%) | 410 (20%) |
| Self-rated health in past 30 days | |||||||
| Very good | 1819 (14%) | 301 (10%) | 272 (14%) | 409 (21%) | 288 (15%) | 392 (20%) | 157 (8%) |
| Good | 5058 (39%) | 1250 (42%) | 699 (35%) | 687 (36%) | 847 (43%) | 639 (32%) | 936 (47%) |
| Moderate | 4958 (39%) | 1113 (38%) | 852 (42%) | 748 (39%) | 697 (35%) | 800 (40%) | 748 (37%) |
| Bad | 775 (6%) | 228 (8%) | 145 (7%) | 66 (3%) | 70 (4%) | 142 (7%) | 124 (6%) |
| Very bad | 182 (1%) | 43 (1%) | 41 (2%) | 15 (1%) | 17 (1%) | 29 (1%) | 37 (2%) |
Exploratory factor analysis
After examination of the eigenvalues (eigenvalues: 10.87, 2.44, 1.57, 1.24, 1.13), goodness-of-fit statistics and interpretability of factor structure, the four factor solution was the best solution (χ2 = 786.05, df = 227, RMSEA = 0.025; 90%CI = 0.023–0.027, CFI = 0.991) [35]. Nevertheless, a strong major factor (indicated by the high ratio of the first two eigenvalues) was also suggested [17]. There were two items (“household responsibilities difficulty”, “carrying out work and everyday activities difficulty”) that seem to be non-congeneric as they exhibited salient loadings (> 0.40) on two factors [15]; and two others (“immediate recall”, “fold a piece of paper”) that showed similar loadings onto two factors. However, we allowed all items to load on one factor according to their highest loading. In the first factor, items of disabilities of daily living loaded and their loadings ranged from 0.496 to 0.888. The second factor included items of general difficulties in everyday life with loadings ranging from 0.607 to 0.749. The third factor comprised items of impairments and mental health with loadings from 0.310 to 0.670. Finally, factor four was a factor of cognition as cognitive items loaded on this (0.243–0.649) (Table 3).
Table 3.
Exploratory factor analysis standardised loadings-Parsimax Rotation
| Items/Indicators | Factor 1 | Factor 2 | Factor 3 | Factor 4 |
|---|---|---|---|---|
| Household responsibilities difficulty | 0.562 | 0.192 | 0.490 | −0.146 |
| Walking a km difficulty | 0.573 | 0.050 | 0.392 | −0.033 |
| Washing whole body difficulty | 0.851 | 0.003 | 0.106 | 0.182 |
| Getting dressed difficulty | 0.888 | 0.014 | 0.048 | 0.193 |
| Carrying out work & everyday activities difficulty | 0.564 | 0.230 | 0.447 | −0.128 |
| Making decisions difficulty | 0.057 | 0.715 | 0.077 | 0.221 |
| Using the toilet difficulty | 0.496 | 0.301 | 0.109 | 0.341 |
| Handling money difficulty | 0.136 | 0.698 | 0.062 | 0.199 |
| Hearing problem | −0.057 | 0.060 | 0.310 | 0.084 |
| Eye problem | 0.005 | 0.051 | 0.395 | −0.060 |
| Finding right word difficulty | −0.106 | 0.749 | 0.128 | 0.100 |
| Change in daily activities | 0.037 | 0.607 | 0.202 | 0.049 |
| Forgets where he/she is | 0.145 | 0.644 | 0.085 | 0.290 |
| Difficulty completing chores | 0.114 | 0.621 | −0.004 | 0.264 |
| Sleep trouble or recent change in pattern | −0.101 | − 0.198 | 0.624 | 0.050 |
| Feeling of not coping properly with everyday routine | −0.028 | 0.116 | 0.582 | 0.043 |
| Gets worn out or exhausted during daytime or evening | −0.229 | −0.200 | 0.670 | 0.122 |
| Time in seconds taken to walk 10 m | 0.410 | 0.008 | 0.030 | − 0.063 |
| Learn test | 0.074 | 0.113 | 0.054 | 0.601 |
| Delayed recall | 0.130 | 0.002 | 0.072 | 0.401 |
| Long memory test | −0.083 | 0.062 | 0.092 | 0.642 |
| Immediate recall | 0.118 | 0.242 | 0.070 | 0.243 |
| Verbal fluency | 0.149 | 0.126 | 0.031 | 0.556 |
| Time orientation | 0.118 | 0.146 | 0.096 | 0.649 |
| Praxis-fold a piece of paper | 0.130 | 0.202 | 0.038 | 0.266 |
| Story recall difficulty | 0.041 | −0.002 | 0.070 | 0.617 |
Confirmatory factor analysis
Three different CFA models were tested and compared for the construction of the index; a one-factor model, a second-order model and a bifactor model (Table 4). The one-factor model exhibited acceptable fit based on the CFI (0.902), but had a poorer RMSEA fit (0.073). Hence, the one-factor solution did not seem to be appropriate. Both the second-order factor and the bifactor model exhibited good fit (CFI ≥ 0.90 and RMSEA≤0.06) but the bifactor model exhibited higher CFI and lower RMSEA indexes (Bifactor: CFI = 0.972, RMSEA = 0.041; Second-order: CFI = 0.962, RMSEA = 0.045). In addition, the adjusted chi-square test for model comparison supported as superior the bifactor model as its value was significant when compared to the one-factor (χ2 = 5679.77, df = 26, p < 0.001) and the second-order model (χ2 = 1089.78, df = 22, p < 0.001). Local fit assessment revealed that the bifactor model was the one with the fewest (both in number and in amount) discrepancies between predicted and observed correlations (Additional file 2). As a consequence, for the subsequent analyses the bifactor model was employed Fig. 2. (Additional file 3 presents the item loadings onto the general and the subdomain factors).
Table 4.
Fit statistics for confirmatory factor analysis models
| Model | Chi-square | df | CFI | RMSEA | 90%CI | Difftest (chi-square, df) |
|---|---|---|---|---|---|---|
| One factor | 14,497.44 | 299 | 0.902 | 0.073 | 0.072–0.074 | (5679.77, 26)*** |
| Second-order | 5770.29 | 295 | 0.962 | 0.045 | 0.044–0.046 | (1089.78, 22)*** |
| Bifactor | 4327.95 | 273 | 0.972 | 0.041 | 0.040–0.042 | – |
Difftest: an adjusted chi-square difference test; df: degrees of freedom; RMSEA Root Mean Square Error of Approximation, CFI Comparative Fit Index
*** p < 0.001
Fig. 2.
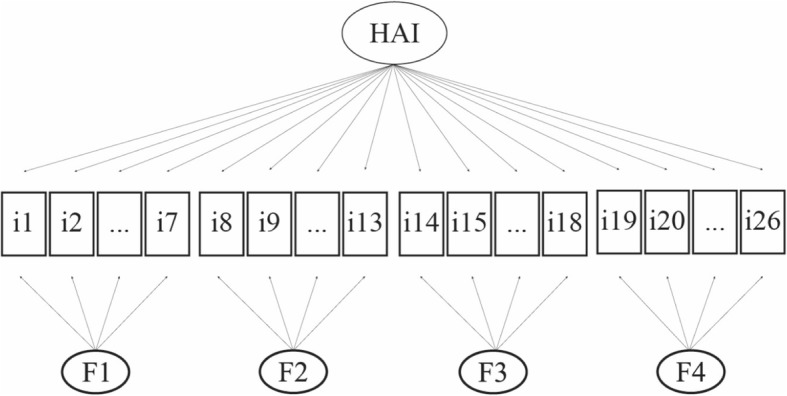
Healthy ageing index bifactor model graphical representation. HAI: Healthy Ageing Index; i1: household responsibilities difficulty; i2: walking a km difficulty; i3: washing whole body difficulty; i4: getting dressed difficulty; i5: carrying out work & everyday activities difficulty; i6: using the toilet difficulty; i7: time in seconds taken to walk 10 m; i8: making decisions difficulty; i9: handling money difficulty; i10: finding right word difficulty; i11: change in daily activities; i12: forgets where he/she is; i13: difficulty completing chores; i14: hearing problem; i15: eye problem; i16: sleep trouble or recent change in pattern; i17: feeling of not coping properly with everyday routine; i18: gets worn out or exhausted during daytime or evening; i19: learn test; i20: delayed recall; i21: long memory test; i22: immediate recall; i23: verbal fluency; i24: time orientation; i25: praxis-fold a piece of paper; i26: story recall difficulty; F1: factor 1; F2: factor 2; F3: factor 3; F4: factor 4
Measurement invariance
Firstly, we checked if the bifactor model fitted well the empirical data from each country. When we run the model in each country the correlation of the items “washing whole body difficulty” and “getting dressed difficulty” for Puerto Rico was close to 1 (r = 0.989); to avoid multicollinearity only one item was kept to the measurement invariance tests. The bifactor model had acceptable fit in each country (RMSEA values range from 0.030 to 0.052; CFI values range from 0.923–0.976) (Table 5). As acceptable fit was established per country we would expect that configural invariance would also be supported. Goodness-of-fit statistics provided evidence for configural invariance (χ2 = 6277.08, df = 1510, RMSEA = 0.046; 90%CI = 0.045–0.047, CFI = 0.958). The hypothesis of scalar invariance (similar loadings and thresholds) was also supported (χ2 = 7668.201, df = 1750, RMSEA = 0.047; 90%CI = 0.046–0.049, CFI = 0.948). In addition, the change of CFI and RMSEA was within the predetermined limits (ΔRMSEA = 0.010, ΔCFI = -0.010).
Table 5.
Model fit for subgroup analyses and for measurement invariance test across countries and gender
| Model | Chi-square | df | RMSEA | 90%CI | CFI | |
|---|---|---|---|---|---|---|
| Country | Cuba | 1188.83 | 260 | 0.042 | 0.039–0.044 | 0.972 |
| Dominican Republic | 1166.40 | 260 | 0.050 | 0.047–0.053 | 0.924 | |
| Peru | 649.06 | 260 | 0.033 | 0.030–0.036 | 0.976 | |
| Venezuela | 1235.53 | 260 | 0.052 | 0.049–0.055 | 0.923 | |
| Mexico | 584.66 | 260 | 0.030 | 0.027–0.033 | 0.964 | |
| Puerto Rico | 1049.99 | 260 | 0.046 | 0.044–0.049 | 0.971 | |
| Configural | 6277.08 | 1510 | 0.046 | 0.045–0.047 | 0.958 | |
| Scalar | 7668.20 | 1750 | 0.047 | 0.046–0.049 | 0.948 | |
| Difftest*** | 1641.51 | 240 | 0.010 | −0.010 | ||
| Gender | Females | 3077.07 | 260 | 0.040 | 0.039–0.042 | 0.964 |
| Males | 1244.39 | 260 | 0.031 | 0.030–0.033 | 0.975 | |
| Configural | 3700.29 | 502 | 0.038 | 0.036–0.039 | 0.967 | |
| Scalar | 4160.82 | 558 | 0.038 | 0.037–0.039 | 0.963 | |
| Difftest*** | 590.81 | 56 | 0.000 | −0.004 | ||
Difftest: an adjusted chi-square difference test; df Degrees of freedom, RMSEA Root Mean Square Error of Approximation, CI Confidence Intervals, CFI Comparative Fit Index
*** p < 0.001
We also assessed the measurement invariance across men and women separately, applying the same steps as above. Goodness-of-fit statistics supported configural invariance (χ2 = 3700.29, df = 502, RMSEA = 0.038; 90%CI = 0.036–0.039, CFI = 0.967) as well as scalar invariance (χ2 = 4160.82, df = 558, RMSEA = 0.038; 90%CI = 0.037–0.039, CFI = 0.963). In addition, the change of CFI and RMSEA was within the predetermined limits (ΔRMSEA = 0.000, ΔCFI = -0.004) (Table 5).
Psychometric coefficients
The general HAI showed excellent reliability (ω = 0.96) and based on our bifactor model, ωH indicated a predominant general factor (ωH = 0.84). A comparison of ωH with ω (0.84/0.96 = 0.88) showed that most of the reliable variance in total scores could be attributed to the general factor. 12% (0.96–0.84) could be attributed to the multidimensionality caused by the subdomain factors and only 4% was estimated to be random error. Omega hierarchical subscale coefficients were very small (ωΗS1 = 0.06, ωΗS2 = 0.02, ωΗS3 = 0.03, ωΗS4 = 0.02), showing that little common variance remained after we accounted for the general factor. ECV was 0.65 also indicating a quite strong general factor accounting for well over half the common variance; however not exceeding the 0.80 benchmark indicated that part of the variance was also explained by the subdomain factors. H value equalled 0.96 indicating that the general factor was a well-defined latent variable [28, 36].
Concurrent convergent validity
The association between the general factor of healthy ageing and the self-rated health measure, adjusted for age and sex, was significant (standardised estimate = 0.373; bootstrap 95%CI: 0.352–0.394, p < 0.001, χ2 = 8238.22, df = 348, RMSEA = 0.050; 90%CI = 0.049–0.051, CFI = 0.922) indicating that a one unit increase in the SRH (deterioration of self-rated health) was associated with a 0.373 standardised score increase in the healthy ageing index (higher values indicate worse health).
Discussion
We showed that a healthy ageing index can be comprised by indicators of intrinsic capacity and functional ability available in different questionnaires. To the best of our knowledge, this the first study creating a healthy ageing index which was tested for various psychometric properties and for measurement invariance. Even though in the literature, there are other successful or healthy ageing indexes [37–39], the novelty of our study lies in the fact that the multifaceted concept of healthy ageing was built by a latent model. Using latent variable modelling to create the healthy ageing index contributes to the creation of a more sensitive measure. Future research on this index will assist in the identification of the most important indicators across the whole range of the latent construct (from the lowest to the highest level of healthy ageing) and of those that are more relevant to people who are in most need. As a consequence, our index will further contribute to person-centered services to the older population.
Regarding the factorial validity of our construct, one-factor model and second-order factor were compared to the bifactor model. We opted for the bifactor structure based on its superior model fit and on its interpretation utility, as there is an ‘inherent statistical bias’ in favour of bifactor models when tested with second-order model [40]. In our study, healthy ageing is conceptualised as the general factor and four subdomain factors, as identified by the EFA. Those subdomain factors are considered common factors as they explain variance above the general construct [41]. A bifactor structure will enable future SEM research to examine the influence of key external covariates both onto the general factor and the subdomain factors; something that is trickier to do in the second-order factor where first- and second-order factors overlap [13].
Measurement invariance, which is fundamental to comparing data among different populations and over time [42] especially when self-reported questionnaires have been employed, was also examined. Our index exhibited excellent measurement invariance properties (configural and scalar invariance) both across ethnic groups and gender, making it possible to meaningfully compare the healthy ageing level of these subpopulations in future research. Furthermore, to assess the concurrent convergent validity of this index with other health measures, we checked its association with the SRH measure adjusted for age and sex to limit any potential moderating effect [43]. The association was moderately strong providing some evidence of our index concurrent convergent validity.
As recommended when a bifactor model is used, we also calculated psychometrically informative bifactor-derived statistics [28, 44]. We calculated ω and ωΗ which indicated that a strong percentage of total score variance is attributable to a single general factor. Hence, we can conclude that raw scores can essentially be assumed as indicators of the healthy ageing general factor and are not affected by the multidimensionality of the four subdomain factors. A strong general factor was also indicated from the ECV value (ECV = 0.65), but as it is less than 0.80 subscale scores should also be considered. However, as the ωΗS reliability subscale estimates are low (ωΗS1 = 0.06, ωΗS2 = 0.02, ωΗS3 = 0.03, ωΗS4 = 0.02) once we account for the general factor, subscale scores have limited added value [45]. We concluded that despite the multidimensionality of the healthy ageing construct, raw scores of the general factor can be interpreted as an essentially unidimensional concept of healthy ageing. Regarding H index, its high value (H = 0.96) provided support that our general factor is a well-defined latent construct appropriate to be used in future SEM research.
A potential limitation of this study is that only selected catchment urban and rural areas of the countries involved were considered. As a consequence, the generalisability of the findings beyond the specific study sites could have been affected. Moreover, the baseline sample included only people 65 years old and over. Thus, it is possible that our results may not be generalisable to a younger sample.
In addition, in our study we included data from Latin America only, even though 10/66 survey has collected data to catchment areas of China and India as well; the reason behind this decision was that we wanted to initially create a common metric of healthy ageing and examine its properties to a multi-country setting but still culturally and geographically homogeneous sample. In addition, knowing that the Chinese and Indian centres collected data by using English language whereas the Hispanic centres of Latin America used Spanish language also contributed to our selecting of a sub-sample of the whole 10/66 cohort. Future research should focus on the creation of this index to all countries participated in the 10/66 cohort and to the follow-up survey dataset.
Another limitation of this study is that some could argue that our cut-off point considered for factor loadings (±0.20) is not stringent enough [46, 47] or that there were indicators with substantial cross-loadings. As a consequence, these problematic items should not be included in the index. However, for content validity purposes we did not exclude any of the initially employed indicators as all questions seem to be representative and meaningful for measuring health status in an older population [13]. Moreover, to capture various domains of health and create an index representing health status at later years we considered as many indicators as possible from different questionnaires, but with no overlapping [48].
Finally, we assessed the concurrent convergent validity of our index by examining its association with a subjective measurement of health; the self-rated health. Future research should focus on the predictive validity of our index by comparing it with the mortality outcome, which is a more objective measure of an individual’s general health [49] or other indexes related with adverse health outcomes in older people, for instance the frailty index [50].
Conclusions
There is an emerging need of further empirical work on the scope, construct development and validity of a common healthy ageing metric. Our findings showed that a healthy ageing index with excellent psychometric and measurement invariance properties can be created in a subset of six low-and-middle income countries. As the challenge of global population ageing is constantly growing, especially in low-and-middle income countries [2], replication of our index to other cultural settings and to longitudinal designs will contribute to a more comprehensive understanding of the ageing process. Future research will allow us to validly explore subpopulation differences and key-determinants that could offer new strategies for policy interventions.
Additional files
Mplus code.
Residual differences (differences between predicted and observed correlations) for the one factor, the second-order and the bifactor models to assess local fit assessment.
Standardised item loadings for the bifactor model.
Acknowledgements
ATHLOS (Ageing Trajectories of Health: Longitudinal Opportunities and Synergies) project, funded by the European Union’s Horizon 2020 Research and Innovation Programme under grant agreement number 635316.
CD would like to thank Dr. Silia Vitoratou BRC Lecturer in Psychometrics and Measurement in the Biostatistics and Health Informatics Department, King’s College London, for her comments in the first draft of this manuscript.
Funding
The 10/66 Dementia Research Group’s research has been funded by the Wellcome Trust Health Consequences of Population Change Programme (GR066133 – Prevalence phase in Cuba and Brazil; GR080002- Incidence phase in Peru, Mexico, Cuba, Dominican Republic, Venezuela and China), the World Health Organization (India, Dominican Republic and China), the US Alzheimer’s Association (IIRG – 04 – 1286 - Peru, Mexico and Argentina), and FONDACIT (Venezuela). On-going data collection and analysis is supported by the European Research Council (ERC-2013-ADG 340755 LIFE2YEARS1066).
This work is also supported by the ATHLOS (Ageing Trajectories of Health: Longitudinal Opportunities and Synergies) project, funded by the European Union’s Horizon 2020 Research and Innovation Programme under grant agreement number 635316.
A. M. P. was supported by the MRC MR/K021907/1.
Availability of data and materials
The 10/66 Dementia Research Group dataset is available upon request via the official site of the study: https://www.alz.co.uk/1066. All data generated in this study are available from the corresponding author on reasonable request.
Abbreviations
- CFA
Confirmatory Factor Analysis
- CFI
Comparative Fit Index
- CI
Confidence Intervals
- df
Degrees of freedom
- DRG
Dementia Research Group
- ECV
Explained Common Variance
- EFA
Exploratory Factor Analysis
- HAI
Healthy Ageing Index
- ICF
International Classification of Functioning, Disability and Health
- MGCFA
Multi-Group Confirmatory Factor Analysis
- MIMIC
Multiple-Indicators Multiple-Causes model
- RMSEA
Root Mean Square Error of Approximation
- SEM
Structural Equation Model
- SRH
Self-Rated Health
- WHO
World Health Organization
- WLSMV
Mean and Variance-adjusted Weighted Least-Squares
- ω
Omega
- ωH
Omega Hierarchical
- ωΗS
Omega Hierarchical Subscales
Authors’ contributions
CD planned the study, performed all statistical analyses and wrote the paper. KCC conceived the idea of bifactor model, assisted in the statistical analyses and contributed to the writing of this paper. AK supervised the data analysis and contributed to writing the paper. FFC assisted in the statistical analyses and contributed to paper writing. MP helped to plan the study, provided insight to the 10/66 cohort and contributed to writing the paper. AMP planned the study, supervised the data analysis, provided insight to the 10/66 cohort and contributed to writing the paper. All authors reviewed the draft versions of this manuscript, read and approved the final version of it.
Ethics approval and consent to participate
The study protocol, the consent procedures, including the witnessed consent procedure, and all studies were approved by the King’s College London research ethics committee and all local ethical committees: the Memory, Depression Institute and Risk Diseases (IMEDER) Ethics Committee (Peru); Finlay Albarran Medical Faculty of Havana Medical University Ethical Committee (Cuba); Hospital Universitario de Caracas Ethics Committee (Venezuela); Consejo Nacional de Bioética y Salud (CONABIOS, Dominican Republic); Instituto Nacional de Neurología y Neurocirugía Ethics Committee (Mexico); Oficina para la Protección de Participantes Humanos en Investigación (OPPHI, Puerto Rico).
All participation has been on the basis of individual informed signed consent. Persons with dementia who lacked capacity for consent were recruited on the basis of a next of kin signed agreement. The study information sheet was read to the older person, and if at that stage or subsequently they seemed to show distress or dissent from participation they were not included regardless of whether assent by next of kin had been provided. The next of kin was informed of this together with the reason for withdrawal from the study. Illiterate persons were read the information sheet and consent form and asked for their verbal consent. An independent, literate witness would then sign their attestation that this process had occurred and that verbal consent had been provided.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Christina Daskalopoulou, Email: christina.daskalopoulou@kcl.ac.uk.
Kia-Chong Chua, Email: kia-chong.chua@kcl.ac.uk.
Artemis Koukounari, Email: Artemis.Koukounari@lshtm.ac.uk.
Francisco Félix Caballero, Email: felix.caballero@uam.es.
Martin Prince, Email: martin.prince@kcl.ac.uk.
A. Matthew Prina, Email: matthew.prina@kcl.ac.uk.
References
- 1.World Health Organization. World health statistics 2016: monitoring health for the SDGs, sustainable development goals. In: World Health Organization. Geneva: World Health Organization; 2016.
- 2.United Nations . World population ageing 2015. New York: United Nations, Department of Economic and Social Affairs, Population Division; 2015. [Google Scholar]
- 3.World Health Organization. World report on ageing and health. In: World Health Organization. Luxembourg: World Health Organization; 2015.
- 4.Depp CA, Jeste DV. Definitions and predictors of successful aging: a comprehensive review of larger quantitative studies. Am J Geriatr Psychiatry. 2006;14(1):6–20. doi: 10.1097/01.JGP.0000192501.03069.bc. [DOI] [PubMed] [Google Scholar]
- 5.Daskalopoulou C, Stubbs B, Kralj C, Koukounari A, Prince M, Prina AM. Physical activity and healthy ageing: a systematic review and meta-analysis of longitudinal cohort studies. Ageing Res Rev. 2017;38:6–17. doi: 10.1016/j.arr.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Cieza A, Oberhauser C, Bickenbach J, Jones RN, Ustun TB, Kostanjsek N, et al. The English are healthier than the Americans: really? Int J Epidemiol. 2015;44(1):229–238. doi: 10.1093/ije/dyu182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caballero FF, Soulis G, Engchuan W, Sanchez-Niubo A, Arndt H, Ayuso-Mateos JL, et al. Advanced analytical methodologies for measuring healthy ageing and its determinants, using factor analysis and machine learning techniques: the ATHLOS project. Sci Rep. 2017;7:43955. doi: 10.1038/srep43955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prince M, Ferri CP, Acosta D, Albanese E, Arizaga R, Dewey M, et al. The protocols for the 10/66 dementia research group population-based research programme. BMC Public Health. 2007;7:165. doi: 10.1186/1471-2458-7-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prina AM, Acosta D, Acosta I, Guerra M, Huang Y, Jotheeswaran AT, et al. Cohort profile: the 10/66 study. Int J Epidemiol. 2017;46(2):406–406i. doi: 10.1093/ije/dyw056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization . How to use the ICF: a practical manual for using the international classification of functioning, disability and health (ICF). Exposure draft for comment. Geneva: World Health Organization; 2013. [Google Scholar]
- 11.Muthén LK, Muthén BO. Mplus User’s guide. 7. Los Angeles: Muthén & Muthén; 1998. [Google Scholar]
- 12.Asparouhov T, Muthén B. Weighted least squares estimation with missing data. Mplus technical appendix. Los Angeles: Muthén & Muthén; 2010. [Google Scholar]
- 13.Kline RB. Principles and practice of structural equation modeling. 4. New York London: The Guilford Press; 2016. [Google Scholar]
- 14.Hair JF, Black WC, Babin BJ, Anderson RE. Multivariate data analysis. 7th ed. USA: Pearson; 2010.
- 15.Brown TA. Confirmatory factor analysis for applied research. 2. New York London: The Guilford Press; 2015. [Google Scholar]
- 16.Sass DA, Schmitt TA. A comparative investigation of rotation criteria within exploratory factor analysis. Multivar Behav Res. 2010;45(1):73–103. doi: 10.1080/00273170903504810. [DOI] [PubMed] [Google Scholar]
- 17.Reise SP, Moore TM, Haviland MG. Bifactor models and rotations: exploring the extent to which multidimensional data yield univocal scale scores. J Pers Assess. 2010;92(6):544–559. doi: 10.1080/00223891.2010.496477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yung Y-F, Thissen D, McLeod LD. On the relationship between the higher-order factor model and the hierarchical factor model. Psychometrika. 1999;64(2):113–128. doi: 10.1007/BF02294531. [DOI] [Google Scholar]
- 19.Chen FF. Sensitivity of goodness of fit indexes to lack of measurement invariance. Struct Equ Model Multidiscip J. 2007;14(3):464–504. doi: 10.1080/10705510701301834. [DOI] [Google Scholar]
- 20.Vandenberg RJ, Lance CE. A review and synthesis of the measurement invariance literature: suggestions, practices, and recommendations for organizational research. Organ Res Methods. 2000;3(1):4–70. doi: 10.1177/109442810031002. [DOI] [Google Scholar]
- 21.Millsap RE, Yun-Tein J. Assessing factorial invariance in ordered-categorical measures. Multivar Behav Res. 2004;39(3):479–515. doi: 10.1207/S15327906MBR3903_4. [DOI] [Google Scholar]
- 22.Koukounari A, Pickles A, Hill J, Sharp H. Psychometric properties of the parent-infant caregiving touch scale. Front Psychol. 2015;6(1887):1887. doi: 10.3389/fpsyg.2015.01887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Asparouhov T, Muthén B. Latent variable analysis with categorical outcomes: multiple-group and growth modeling in Mplus. Los Angeles: Muthén & Muthén; 2002. [Google Scholar]
- 24.Cheung GW, Rensvold RB. Evaluating goodness-of-fit indexes for testing measurement invariance. Struct Equ Model Multidiscip J. 2002;9(2):233–255. doi: 10.1207/S15328007SEM0902_5. [DOI] [Google Scholar]
- 25.Dunn TJ, Baguley T, Brunsden V. From alpha to omega: a practical solution to the pervasive problem of internal consistency estimation. Br J Psychol. 2014;105(3):399–412. doi: 10.1111/bjop.12046. [DOI] [PubMed] [Google Scholar]
- 26.Zinbarg RE, Revelle W, Yovel I, Li W. Cronbach’s α, Revelle’s β, and Mcdonald’s ω H : their relations with each other and two alternative conceptualizations of reliability. Psychometrika. 2005;70(1):123–133. doi: 10.1007/s11336-003-0974-7. [DOI] [Google Scholar]
- 27.Zinbarg RE, Yovel I, Revelle W, McDonald RP. Estimating generalizability to a latent variable common to all of a Scale's indicators: a comparison of estimators for ωh. Appl Psychol Measur. 2006;30(2):121–144. doi: 10.1177/0146621605278814. [DOI] [Google Scholar]
- 28.Rodriguez A, Reise SP, Haviland MG. Applying Bifactor statistical indices in the evaluation of psychological measures. J Pers Assess. 2016;98(3):223–237. doi: 10.1080/00223891.2015.1089249. [DOI] [PubMed] [Google Scholar]
- 29.Reise SP, Bonifay WE, Haviland MG. Scoring and modeling psychological measures in the presence of multidimensionality. J Pers Assess. 2013;95(2):129–140. doi: 10.1080/00223891.2012.725437. [DOI] [PubMed] [Google Scholar]
- 30.Ten Berge JMF, Sočan G. The greatest lower bound to the reliability of a test and the hypothesis of unidimensionality. Psychometrika. 2004;69(4):613–625. doi: 10.1007/BF02289858. [DOI] [Google Scholar]
- 31.Hancock GR, Mueller RO. Rethinking construct reliability within latent variable systems. In: Cudeck R, Jöreskog KG, editors. Structural equation modeling present and future a festschrift in honor of Karl Joreskog. USA: Scientific Software International; 2001. p. 195–216.
- 32.Joreskog KG, Goldberger AS. Estimation of a model with multiple indicators and multiple causes of a single latent variable. J Am Stat Assoc. 1975;70(351):631–639. doi: 10.2307/2285946. [DOI] [Google Scholar]
- 33.Idler EL, Benyamini Y. Self-rated health and mortality: a review of twenty-seven community studies. J Health Soc Behav. 1997;38(1):21–37. doi: 10.2307/2955359. [DOI] [PubMed] [Google Scholar]
- 34.Falk Hanna, Skoog Ingmar, Johansson Lena, Guerchet Maëlenn, Mayston Rosie, Hörder Helena, Prince Martin, Prina A Matthew. Self-rated health and its association with mortality in older adults in China, India and Latin America—a 10/66 Dementia Research Group study. Age and Ageing. 2017;46(6):932–939. doi: 10.1093/ageing/afx126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang Y, Xia Y. On the number of factors to retain in exploratory factor analysis for ordered categorical data. Behav Res Methods. 2015;47(3):756–772. doi: 10.3758/s13428-014-0499-2. [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez A, Reise SP, Haviland MG. Evaluating bifactor models: calculating and interpreting statistical indices. Psychol Methods. 2016;21(2):137–150. doi: 10.1037/met0000045. [DOI] [PubMed] [Google Scholar]
- 37.Cosco TD, Stephan BC, Brayne C. Validation of an a priori, index model of successful aging in a population-based cohort study: the successful aging index. Int Psychogeriatr. 2015;27(12):1971–1977. doi: 10.1017/S1041610215000708. [DOI] [PubMed] [Google Scholar]
- 38.Young Y, Fan MY, Parrish JM, Frick KD. Validation of a novel successful aging construct. J Am Med Dir Assoc. 2009;10(5):314–322. doi: 10.1016/j.jamda.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 39.Tyrovolas S, Haro JM, Mariolis A, Piscopo S, Valacchi G, Tsakountakis N, et al. Successful aging, dietary habits and health status of elderly individuals: a k-dimensional approach within the multi-national MEDIS study. Exp Gerontol. 2014;60:57–63. doi: 10.1016/j.exger.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 40.Murray AL, Johnson W. The limitations of model fit in comparing the bi-factor versus higher-order models of human cognitive ability structure. Intelligence. 2013;41(5):407–422. doi: 10.1016/j.intell.2013.06.004. [DOI] [Google Scholar]
- 41.Chen FF, West SG, Sousa KH. A comparison of Bifactor and second-order models of quality of life. Multivar Behav Res. 2006;41(2):189–225. doi: 10.1207/s15327906mbr4102_5. [DOI] [PubMed] [Google Scholar]
- 42.Salomon JA, Mathers CD, Chatterji S, Sadana R, Ästàn TB, Murray CJL. Quantifying individual levels of health: definitions, concepts and measurement issues. In: CJL M, Evans DB, editors. Health systems performance assessment: debates, methods and empiricism. Geneva: World Health Organization; 2003. pp. 705–713. [Google Scholar]
- 43.Zajacova A, Woo H. Examination of age variations in the predictive validity of self-rated health. J Gerontol Ser B. 2016;71(3):551–557. doi: 10.1093/geronb/gbv050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bonifay W, Lane SP, Reise SP. Three concerns with applying a Bifactor model as a structure of psychopathology. Clin Psychol Sci. 2017;5(1):184–186. doi: 10.1177/2167702616657069. [DOI] [Google Scholar]
- 45.Reise SP. Invited paper: the rediscovery of Bifactor measurement models. Multivar Behav Res. 2012;47(5):667–696. doi: 10.1080/00273171.2012.715555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Costello AB, Osborne J. Best practices in exploratory factor analysis: four recommendations for getting the most from your analysis. Pract Assess Res Eval. 2005;10(7):173–178. [Google Scholar]
- 47.Tabachnick BG, Fidell LS. Using multivariate statistics. Boston: Allyn and Bacon; 2001. [Google Scholar]
- 48.Salomon JA, Mathers CD, Chatterji S, Sadana R, Ästàn TB, CJL M. Quantifying individual levels of health: definitions, concepts and measurement issues. In: CJL M, Evans DB, editors. Health systems performance assessment: debates, methods and empiricism. Geneva: World Health Organization; 2003. pp. 301–318. [Google Scholar]
- 49.Quesnel-Vallée A. Self-rated health: caught in the crossfire of the quest for ‘true’ health? Int J Epidemiol. 2007;36(6):1161–1164. doi: 10.1093/ije/dym236. [DOI] [PubMed] [Google Scholar]
- 50.At J, Bryce R, Prina M, Acosta D, Ferri CP, Guerra M, et al. Frailty and the prediction of dependence and mortality in low- and middle-income countries: a 10/66 population-based cohort study. BMC Med. 2015;13:138. doi: 10.1186/s12916-015-0378-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mplus code.
Residual differences (differences between predicted and observed correlations) for the one factor, the second-order and the bifactor models to assess local fit assessment.
Standardised item loadings for the bifactor model.
Data Availability Statement
The 10/66 Dementia Research Group dataset is available upon request via the official site of the study: https://www.alz.co.uk/1066. All data generated in this study are available from the corresponding author on reasonable request.