Abstract
Background
The goal of this study was to determine the prognostic factors exclusive for high-grade chondrosarcoma and whether adjuvant radiotherapy could achieve better overall survival (OS) or cancer-specific survival (CSS) for patients with high-grade chondrosarcoma.
Material/Methods
Surveillance, Epidemiology, and End Results (SEER) cancer registry database was utilized to extract the chondrosarcoma cases diagnosed between 1973 and 2014. Among these cases, the histological grades of poorly differentiated (grade 3) and undifferentiated (grade 4) were categorized as high-grade and included in this study. Chondrosarcoma OS and CSS were the primary outcomes in the present study. The log-rank test was performed for univariate analysis, and the Cox regression model was conducted for multivariate analysis.
Results
A total of 743 patients with high-grade chondrosarcoma were identified in this study (430 cases were poorly differentiated tumors, and 313 cases were undifferentiated tumors). Age at diagnosis, pathological grade, histo-type, SEER stage, tumor size and surgical resection were identified as independent predictors in both OS and CSS analysis of high-grade chondrosarcoma. When stratified by histological grade, surgical resection remained the effective treatment. Strikingly, radiotherapy was determined as an independent protective factor in both OS and CSS analysis of undifferentiated (grade 4) dedifferentiated chondrosarcoma, and adjuvant radiotherapy combined surgical resection could improve both the OS and CSS of patients with undifferentiated myxoid and dedifferentiated chondrosarcoma compared with other treatment regimens.
Conclusions
Our study first demonstrated that adjuvant radiotherapy combined surgery could improve the survival of patients with undifferentiated myxoid and dedifferentiated chondrosarcoma. These results encourage the application of adjuvant radiotherapy for patients with high-grade chondrosarcoma and maximize the patients’ outcome.
MeSH Keywords: Chondrosarcoma, Prognosis, Radiotherapy, SEER Program, Survival Analysis
Background
Chondrosarcomas represent a heterogeneous group of malignant neoplasms with diverse histopathological features and are characterized by producing cartilaginous matrix [1]. As the second most common primary malignancy of bone, the annual incidence of chondrosarcoma in the United States is about 1 in 200 000 [2]. The prognosis of chondrosarcoma is tightly associated with the histological grade [2–4]. The low-grade chondrosarcoma with abundant cartilage matrix and poor cellularity, is prone to be localized and shows a good prognosis after surgical resection [5]. However, high-grade tumors show little cartilage matrix and high cellularity, and easily metastasize, leading to dismal prognoses [1,6].
Previous studies have analyzed the prognostic factors and treatment regimens for chondrosarcoma. Bindiganavile et al. demonstrated that histological grade and tumor location could predict the outcome of chondrosarcoma, and the patients with high-grade and axial-location tumors suffered the worst prognosis [7]. Arshi et al. elucidated that age at diagnosis, extent of tumor, surgical resection were independent survival determinants for spinal chondrosarcoma [8]. However, almost all of these studies focused on the entire entity of chondrosarcoma rather than on different histological grades, and to our knowledge, the prognostic study exclusive to high-grade chondrosarcoma has not been reported. Given the significant diversity between the low-and high-grade chondrosarcoma, whether these prognostic factors and treatment strategies can also be applied to high-grade tumors is largely unknown. In addition, due to the few dividing cells, rich cartilage matrix, and poor vascularization, chondrosarcoma is resistant to radiotherapy [9–11]. Although some studies have demonstrated that adjuvant radiotherapy could be beneficial for local control of chondrosarcoma, it failed to achieve better overall survival (OS) or cancer-specific survival (CSS) [12–16]. However, whether high-grade chondrosarcoma, which typically shows higher cellularity and less matrix [1], is sensitive to radiotherapy is also unclear. Therefore, it is meaningful to address the prognostic indicators and effective treatment of high-grade chondrosarcoma, which may be beneficial to optimize the treatment regimen and improve patient survival.
The Surveillance, Epidemiology, and End Results (SEER) cancer registry database is maintained by National Cancer Institute, which routinely records the cancer incidence and survival data from 18 population-based cancer registries. The database doesn’t include the specific chemotherapy data, and the specific information of radiotherapy and surgery, such as the doses of radiotherapy and the margins of surgical resection. In the SEER cancer registry database, the histological grade includes 4 categories: well differentiated (grade 1), moderately differentiated (grade 2), poorly differentiated (grade 3), and undifferentiated (grade 4). As reported in a previous study [17], we categorized poorly differentiated and undifferentiated as high-grade. The method to classify the grade is an assessment of the similarity between the tumor cells and the normal cells of the original organ. The grade 3 tumor (poorly differentiated) has some, or little similarity to the original organ, while the grade 4 (undifferentiated) tumor has no similarity to the original organ [18].
The following questions were addressed in this study: 1) what is the 5 year-OS and CSS for patients with high-grade chondrosarcoma; 2) what are the prognostic factors exclusive for high-grade chondrosarcoma; 3) whether the application of adjuvant radiotherapy can improve the survival of patients with high-grade tumors; and 4) whether the prognostic factors of poorly differentiated and undifferentiated tumors are different due to the biological and clinical differences.
Material and Methods
Patients selection
Due to public access and no unique identification for patients in the SEER database, ethical review was waived after the discussion by the Ethics Committee of Xi’an Jiaotong University (Xi’an, China). The SEER*Stat software (version 8.3.4) was employed to extract the chondrosarcoma cases diagnosed between 1973 and 2014. The following histologic ICD-O-3 codes (International Classification of Diseases for Oncology, Third Edition) were included: code 9220 (chondrosarcoma not otherwise specified), code 9221 (juxtacortical chondrosarcoma), code 9231 (myxoid chondrosarcoma), code 9240 (mesenchymal chondrosarcoma), code 9242 (clear cell chondrosarcoma) and code 9243 (dedifferentiated chondrosarcoma). According to the definition of ICD-0-3: chondrosarcoma, NOS: the most common subtype of chondrosarcoma, which comprises about 75% of the tumors, and is characterized by the chondromyxoid matrix material [19]; myxoid chondrosarcoma: it is characterized by the formation of myxoid stroma, and includes extraskeletal myxoid chondrosarcoma and the myxoid tumor of skull base [20]; mesenchymal chondrosarcoma: a rare subtype of chondrosarcoma, which is characterized by undifferentiated small round cells in addition to the well differentiated hyaline cartilage [21]; clear cell chondrosarcoma: a rare variant of chondrosarcoma, which is histologically characterized by the presence of bland clear cells [22]; dedifferentiated chondrosarcoma: an aggressive subtype of chondrosarcoma, which contains 2 components, a low-grade cartilage sarcoma and a high-grade tumor without cartilage [23]. The classification of these cases was reviewed and confirmed by one senior pathologist in our hospital.
The following criteria were applied to exclude some cases: 1) chondrosarcoma was not the primary tumor; 2) the tumor was diagnosed without histopathology confirmed; 3) the survival time was not clear; 4) the histological grade was well- or moderately-differentiated.
Study variables
Demographic variables, such as age, race, sex, marital status and socioeconomic status (SES), was analyzed in this study. Age was categorized as: <60 years old and ≥60 years old. Race was recoded as white, black and other in the SEER database. Marital status was divided into 4 categories: married, divorced, single, and widowed. Socioeconomic status was created by standard 2000 US Census SES variables as previous studies [24,25] and further categorized as low-SES and high-SES. Tumor-related variables, such as histological grade, tumor location, tumor size, SEER stage, and treatment options, were extracted from the database. The histological grade included 2 categories: poorly differentiated (grade 3) and undifferentiated (grade 4), according to the SEER database. To simplify the analysis, the histo-type was divided into: chondrosarcoma not otherwise specified, myxoid chondrosarcoma, mesenchymal chondrosarcoma, dedifferentiated chondrosarcoma, and other (including juxtacortical chondrosarcoma and clear cell chondrosarcoma). Tumor location was classified as axial (including pelvic bones, sacrum, coccyx, ribs, sternum, and vertebral columns), extremities (including bones of the upper and lower extremities) and other group (including bones of skull, mandible, and other atypical locations) as previous study [24]. As coded in the SEER program, the SEER stage was divided into localized, regional, distant, and unstaged. Tumor size was categorized as: ≤8 cm, >8 cm, and unknown. With regards to surgery and radiotherapy, both were divided into performed and not performed. For the treatment regimen, we included 4 groups: radiotherapy combined surgery, surgery only, radiotherapy only, and no treatment.
Statistical analysis
In this study, OS and chondrosarcoma CSS were the primary outcomes. As described in the SEER database, the deaths caused by chondrosarcoma were considered as events for CSS, and the deaths from any cause were considered as events for OS. Descriptive analysis was carried out to assess the distribution of demographic and tumor-related variables. The Kaplan-Meier method was used to estimate the 5-year OS and CSS. The log-rank test was performed for univariate analysis, and the Cox regression model was conducted for multivariate analysis. The aforementioned statistical analyses were performed using SPSS statistics software, version 20 (IBM, SPSS, Inc., Chicago, IL, USA). All P values were 2-sided, and P<0.05 indicated the significant difference.
Results
Baseline patient characteristics
A total of 743 patients with high-grade chondrosarcoma were identified in this study, of which 430 cases were diagnosed as poorly differentiated tumors and 313 cases were classified as undifferentiated tumors. Of these cases with high-grade chondrosarcoma, the majority were found in younger age (57.2%, n=425), white race (87.1%, n=647) and male (57.1%, n=424). The most common SEER stage of high-grade chondrosarcoma was regional (45.1%, n=335), followed by localized (30.1%, n=224) and distant (20.1%, n=149). Most patients were married (58.4%, n=434), followed by single (24.1%, n=179) and divorced (9.0%, n=67). Most patients underwent surgical resection (88.0%, n = 654), while the minority received radiotherapy (28.5%, n=212). The most common treatment regimens were surgery only (64.8%, n=482) and adjuvant radiotherapy combined surgery (23.1%, n=172). Similar distributions of patient demographics and tumor characteristics were found in poorly differentiated tumors and undifferentiated tumors. The baseline characteristics of this study were shown in Table 1.
Table 1.
Baseline demographics and clinicopathological characteristics of patients with high-grade chondrosarcoma in the SEER database.
| Characteristics | Total N=743 |
Poorly differentiated N=430 |
Undifferentiated N=313 |
|---|---|---|---|
| Age (years) at diagnosis | |||
| <60 | 425 (57.2%) | 262 (60.9%) | 163 (52.1%) |
| ≥60 | 318 (42.8%) | 168 (39.1%) | 150 (47.9%) |
| Race | |||
| White | 647 (87.1%) | 379 (88.1%) | 268 (85.6%) |
| Black | 51 (6.9%) | 29 (6.7%) | 22 (7.0%) |
| Other | 45 (6.0%) | 22 (5.2%) | 23 (7.4%) |
| Sex | |||
| Male | 424 (57.1%) | 252 (58.6%) | 172 (55.0%) |
| Female | 319 (42.9%) | 178 (41.4%) | 141 (45.0%) |
| Tumor location | |||
| Axial | 212 (28.5%) | 135 (31.4%) | 77 (24.6%) |
| Extremities | 326 (43.9%) | 175 (40.7%) | 151 (48.2%) |
| Other | 205 (27.6%) | 120 (27.9%) | 85 (27.2%) |
| Histo-type | |||
| Chondrosarcoma, NOS | 417 (56.1%) | 283 (65.8%) | 134 (42.8%) |
| Myxoid | 87 (11.7%) | 50 (11.6%) | 37 (11.8%) |
| Mesenchymal | 72 (9.8%) | 35 (8.2%) | 37 (11.8%) |
| Dedifferentiated | 154 (20.7%) | 52 (12.1%) | 102 (32.5%) |
| Other | 13 (1.7%) | 10 (2.3%) | 3 (1.1%) |
| SEER stage | |||
| Localized | 224 (30.1%) | 138 (32.1%) | 86 (27.5%) |
| Regional | 335 (45.1%) | 200 (46.5%) | 135 (43.1%) |
| Distant | 149 (20.1%) | 66 (15.3%) | 83 (26.5%) |
| Unstaged | 35 (4.7%) | 26 (6.1%) | 9 (2.9%) |
| Tumor size | |||
| ≤8 cm | 268 (36.1%) | 159 (37.0%) | 109 (34.8%) |
| >8 cm | 304 (40.9%) | 171 (39.8%) | 133 (42.5%) |
| Unknown | 171 (23.0%) | 100 (23.2%) | 71 (22.7%) |
| Marital status | |||
| Married | 434 (58.4%) | 255 (59.3%) | 179 (57.2%) |
| Divorced | 67 (9.0%) | 34 (7.9%) | 33 (10.5%) |
| Single | 179 (24.1%) | 117 (27.2%) | 62 (19.8%) |
| Widowed | 63 (8.5%) | 24 (5.6%) | 39 (12.5%) |
| Socioeconomic status | |||
| Low-SES | 380 (51.1%) | 237 (55.1%) | 143 (45.7%) |
| High-SES | 363 (48.9%) | 193 (44.9%) | 170 (54.3%) |
| Surgery | |||
| Performed | 654 (88.0%) | 381 (88.6%) | 273 (87.2%) |
| Not performed | 89 (12.0%) | 49 (11.4%) | 40 (12.8%) |
| Radiotherapy | |||
| Performed | 212 (28.5%) | 124 (28.8%) | 88 (28.1%) |
| Not performed | 531 (71.5%) | 306 (71.2%) | 225 (71.9%) |
| Treatment | |||
| Both | 172 (23.1%) | 99 (23.0%) | 73 (23.3%) |
| Surgery only | 482 (64.8%) | 282 (65.6%) | 200 (63.9%) |
| Radiotherapy only | 40 (5.4%) | 25 (5.8%) | 15 (4.8%) |
| None | 49 (6.7%) | 24 (5.6%) | 25 (8.0%) |
SEER – Surveillance, Epidemiology, and End Results; NOS – not otherwise specified; SES – socioeconomic status.
Survival analysis for patients with high-grade chondrosarcoma
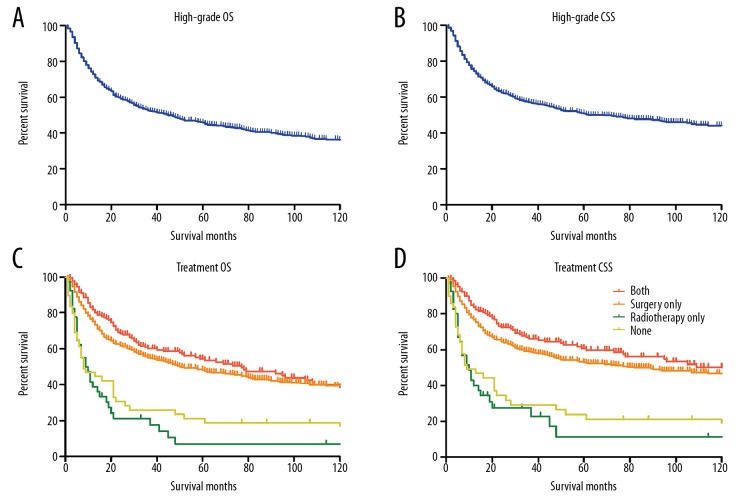
Kaplan-Meier curves (Figure 1A, 1B) showed that the 5-year OS and CSS for patients with high-grade chondrosarcoma were 45.7% and 50.8%, respectively. Adjuvant radiotherapy combined surgery didn’t make a difference on the survival of patients compared with surgery-only group, and surgical resection could significantly improve the outcomes of patients compared with radiotherapy-only or non-treatment groups (OS P<0.00, Figure 1C; CSS P<0.001, Figure 1D). In addition, both the OS and CSS analysis demonstrated that undifferentiated pathological grade, dedifferentiated histo-type, distant SEER stage, larger tumor size, and widowhood were associated with dismal prognoses. The multivariate analysis identified that pathological grade, histo-type, SEER stage, tumor size, and surgical resection were independent predictors for both OS and CSS of high-grade chondrosarcoma, and widowhood was an independent predictor for chondrosarcoma OS. Patients with undifferentiated chondrosarcoma suffered a remarkably higher risk of overall mortality (Hazard ratio [HR] 1.40, 95% confidence interval [CI] 1.14–1.72, P=0.001) and cancer-specific mortality (HR 1.38, 95% CI 1.10–1.73, P=0.005). Similarly, patients without surgical resection also had a dramatical increase in the risk of overall mortality (HR 2.00, 95% CI 1.51–2.66, P<0.001) and cancer-specific mortality (HR 2.06, 95% CI 1.53–2.78, P<0.001). The details of log-rank test and Cox regression were shown in Table 2.
Figure 1.
Kaplan-Meier curves for high-grade chondrosarcoma. (A, B) The overall survival curve (A) and cancer-specific survival curve (B) for high-grade chondrosarcoma. (C, D) The overall survival curves (C) and cancer-specific survival curves (D) for high-grade chondrosarcoma according to treatment regimens.
Table 2.
Overall and cancer-specific survival analysis for patients with high-grade chondrosarcoma in the SEER database.
| Variable | Univariate analysis (log-rank test) | Multivariate analysis (Cox regression) | Univariate analysis (log-rank test) | Multivariate analysis (Cox regression) | ||||
|---|---|---|---|---|---|---|---|---|
| 5-year OS | P | HR (95% CI) | P | 5-year CSS | P | HR (95% CI) | P | |
| Age at diagnosis | <0.001 | <0.001 | ||||||
| <60 | 54.5% | Reference | 57.1% | Reference | ||||
| ≥60 | 33.7% | 1.85 (1.50–2.30) | <0.001 | 40.9% | 1.54 (1.22–1.94) | <0.001 | ||
| Race | 0.401 | 0.675 | ||||||
| White | 45.0% | 50.3% | ||||||
| Black | 46.5% | 51.3% | ||||||
| Other | 51.5% | 53.6% | ||||||
| Sex | 0.135 | 0.198 | ||||||
| Male | 43.1% | 48.5% | ||||||
| Female | 48.8% | 52.5% | ||||||
| Tumor location | 0.004 | 0.001 | ||||||
| Axial | 40.5% | Reference | 45.2% | Reference | ||||
| Extremities | 42.0% | 0.99 (0.79–1.25) | 0.952 | 46.5% | 0.98 (0.77–1.27) | 0.909 | ||
| Other | 55.2% | 0.95 (0.71–1.28) | 0.747 | 63.0% | 0.88 (0.63–1.22) | 0.438 | ||
| Pathological grade | <0.001 | <0.001 | ||||||
| Poorly differentiated | 54.2% | Reference | 60.1% | Reference | ||||
| Undifferentiated | 33.1% | 1.40 (1.14–1.72) | 0.001 | 37.1% | 1.38 (1.10–1.73) | 0.005 | ||
| Histo-type | <0.001 | <0.001 | ||||||
| Chondrosarcoma, NOS | 46.3% | Reference | 52.5% | Reference | ||||
| Myxoid | 49.2% | 0.97 (0.70–1.34) | 0.855 | 54.1% | 0.93 (0.65–1.35) | 0.718 | ||
| Mesenchymal | 57.9% | 0.68 (0.45–1.03) | 0.070 | 61.6% | 0.70 (0.45–1.11) | 0.127 | ||
| Dedifferentiated | 27.3% | 1.33 (1.02–1.72) | 0.032 | 30.4% | 1.46 (1.10–1.92) | 0.007 | ||
| Other | 63.5% | 0.85 (0.31–2.33) | 0.758 | 70.4% | 0.74 (0.23–2.35) | 0.611 | ||
| SEER stage | <0.001 | <0.001 | ||||||
| Localized | 60.7% | Reference | 67.8% | Reference | ||||
| Regional | 47.3% | 1.45 (1.13–1.87) | 0.003 | 52.3% | 1.68 (1.25–2.24) | 0.001 | ||
| Distant | 14.9% | 3.12 (2.34–4.15) | <0.001 | 16.3% | 3.95 (2.87–5.43) | <0.001 | ||
| Unstaged | 56.1% | 0.97 (0.57–1.65) | 0.921 | 69.8% | 0.91 (0.47–1.77) | 0.783 | ||
| Tumor size | <0.001 | <0.001 | ||||||
| ≤8 cm | 59.6% | Reference | 64.3% | Reference | ||||
| >8 cm | 36.5% | 1.42 (1.11–1.81) | 0.006 | 42.0% | 1.45 (1.11–1.90) | 0.007 | ||
| Unknown | 36.7% | 1.37 (1.03–1.83) | 0.030 | 42.2% | 1.38 (1.00–1.90) | 0.049 | ||
| Marital status | <0.001 | <0.001 | ||||||
| Married | 46.4% | Reference | 50.9% | Reference | ||||
| Divorced | 40.3% | 1.17 (0.83–1.65) | 0.358 | 47.5% | 1.12 (0.77–1.64) | 0.551 | ||
| Single | 51.2% | 1.02 (0.79–1.32) | 0.889 | 57.6% | 0.95 (0.72–1.26) | 0.727 | ||
| Widowed | 19.5% | 1.42 (1.04–1.93) | 0.028 | 25.7% | 1.39 (0.99–1.97) | 0.059 | ||
| Socioeconomic status | 0.025 | 0.112 | ||||||
| Low-SES | 41.9% | Reference | 47.7% | |||||
| High-SES | 49.6% | 0.90 (0.74–1.09) | 0.273 | 54.3% | ||||
| Surgery | <0.001 | <0.001 | ||||||
| Performed | 49.8% | Reference | 54.9% | Reference | ||||
| Not performed | 13.9% | 2.00 (1.51–2.66) | <0.001 | 17.6% | 2.06 (1.53–2.78) | <0.001 | ||
| Radiotherapy | 0.928 | 0.573 | ||||||
| Performed | 44.9% | 51.4% | ||||||
| Not performed | 45.7% | 50.1% | ||||||
| Treatment | <0.001 | NA | <0.001 | NA | ||||
| Both | 54.3% | 59.8% | ||||||
| Surgery only | 47.6% | 52.5% | ||||||
| Radiotherapy only | 7.2% | 11.5% | ||||||
| None | 18.8% | 21.1% | ||||||
SEER – Surveillance, Epidemiology, and End Results; OS – overall survival; CSS – cancer-specific survival; HR – hazard ratio; CI – confidence interval; NOS – not otherwise specified; SES – socioeconomic status; NA – the analysis is not available because of the covariance.
Subgroup survival analysis for patients with poorly differentiated chondrosarcoma
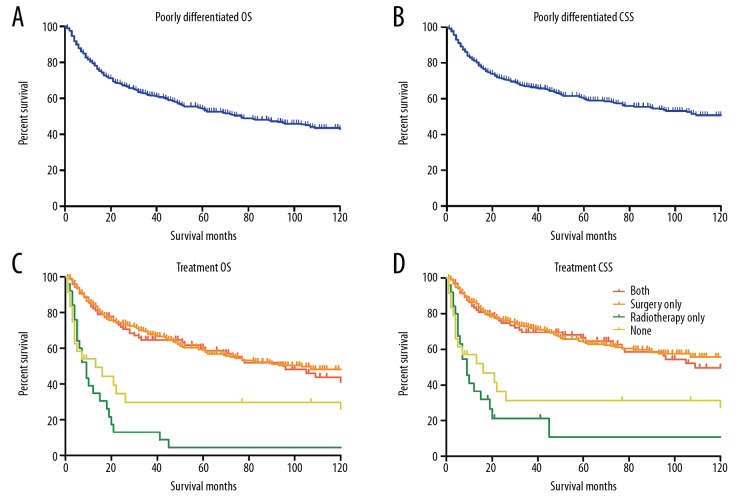
To further determine the therapeutic effects of surgical resection and radiotherapy on poorly differentiated chondrosarcoma, we performed the Kaplan-Meier curves and log-rank test to evaluate the OS and CSS. The Kaplan-Meier curves revealed that the 5-year OS and CSS for patients with poorly differentiated chondrosarcoma were 54.2% and 60.1%, respectively (Figure 2A, 2B). Adjuvant radiotherapy combined surgery didn’t significantly improve the patient outcomes compared with surgery-only group, and surgical resection could dramatically improve the survival rate of patients (OS P<0.001, Figure 2C; CSS P<0.001, Figure 2D). Similar with the survival analysis of high-grade tumors, greater age, dedifferentiated histo-type, distant SEER stage, and larger tumor size were associated with dismal prognoses in both OS and CSS. Widowhood and low-SES were associated with poor outcomes in OS analysis. By multivariate analysis, histo-type, SEER stage, tumor size, and surgical resection were determined as independent prognostic factors for both OS and CSS of poorly differentiated chondrosarcoma. The mortality risk of patients without surgical resection was more than 2 times than that of patients receiving surgical resection in OS (HR 2.58, 95% CI 1.72–3.88, P<0.001) and CSS analysis (HR 2.35, 95% CI 1.55–3.56, P<0.001). The results of survival analysis were shown in Table 3.
Figure 2.
Kaplan-Meier curves for poorly differentiated chondrosarcoma. (A, B) The overall survival curve (A) and cancer-specific survival curve (B) for poorly differentiated chondrosarcoma. (C, D) The overall survival curves (C) and cancer-specific survival curves (D) for poorly differentiated chondrosarcoma according to treatment regimens.
Table 3.
Overall and cancer-specific survival analysis for patients with poorly differentiated chondrosarcoma in the SEER database.
| Variable | Univariate analysis (log-rank test) | Multivariate analysis (Cox regression) | Univariate analysis (log-rank test) | Multivariate analysis (Cox regression) | ||||
|---|---|---|---|---|---|---|---|---|
| 5-year OS | P | HR (95% CI) | P | 5-year CSS | P | HR (95% CI) | P | |
| Age (years) at diagnosis | <0.001 | 0.001 | ||||||
| <60 | 61.3% | Reference | 64.9% | Reference | ||||
| ≥60 | 42.4% | 1.59 (1.16–2.18) | 0.004 | 51.3% | 1.50 (1.07–2.09) | 0.018 | ||
| Race | 0.911 | 0.775 | ||||||
| White | 54.8% | 60.8% | ||||||
| Black | 48.7% | 57.9% | ||||||
| Other | 51.8% | 51.8% | ||||||
| Sex | 0.381 | 0.269 | ||||||
| Male | 52.9% | 58.4% | ||||||
| Female | 56.1% | 61.3% | ||||||
| Tumor location | 0.079 | 0.032 | ||||||
| Axial | 46.0% | 51.8% | Reference | |||||
| Extremities | 54.1% | 58.5% | 0.81 (0.57–1.15) | 0.242 | ||||
| Other | 62.8% | 70.9% | 0.89 (0.57–1.40) | 0.611 | ||||
| Histo-type | <0.001 | <0.001 | ||||||
| Chondrosarcoma, NOS | 53.8% | Reference | 60.2% | Reference | ||||
| Myxoid | 57.9% | 0.99 (0.64–1.54) | 0.953 | 65.8% | 0.92 (0.54–1.57) | 0.749 | ||
| Mesenchymal | 60.4% | 1.27 (0.71–2.25) | 0.423 | 63.1% | 1.35 (0.71–2.56) | 0.357 | ||
| Dedifferentiated | 25.1% | 1.84 (1.22–2.75) | 0.003 | 35.9% | 2.00 (1.30–3.08) | 0.002 | ||
| Other | 71.4% | 0.64 (0.16–2.67) | 0.546 | 85.7% | 0.39 (0.05–2.88) | 0.358 | ||
| SEER stage | <0.001 | <0.001 | ||||||
| Localized | 66.2% | Reference | 75.2% | Reference | ||||
| Regional | 54.4% | 1.36 (0.97–1.91) | 0.082 | 58.9% | 1.84 (1.22–2.78) | 0.004 | ||
| Distant | 18.8% | 3.01 (1.97–4.60) | <0.001 | 21.8% | 4.48 (2.79–7.21) | <0.001 | ||
| Unstaged | 62.9% | 0.78 (0.41–1.51) | 0.461 | 78.5% | 0.76 (0.32–1.78) | 0.525 | ||
| Tumor size | <0.001 | <0.001 | ||||||
| ≤8 cm | 68.8% | Reference | 74.4% | Reference | ||||
| >8 cm | 46.4% | 1.49 (1.06–2.08) | 0.021 | 52.8% | 1.72 (1.16–2.54) | 0.007 | ||
| Unknown | 41.7% | 1.69 (1.12–2.54) | 0.012 | 47.7% | 1.89 (1.20–2.97) | 0.006 | ||
| Marital status | 0.034 | 0.609 | ||||||
| Married | 53.3% | Reference | 58.5% | |||||
| Divorced | 49.1% | 0.91 (0.55–1.53) | 0.849 | 60.9% | ||||
| Single | 56.7% | 0.84 (0.59–1.21) | 0.333 | 62.4% | ||||
| Widowed | 32.1% | 1.30 (0.79–2.15) | 0.299 | 43.4% | ||||
| Socioeconomic status | 0.024 | 0.588 | ||||||
| Low-SES | 49.1% | Reference | 56.6% | |||||
| High-SES | 59.8% | 0.76 (0.57–1.01) | 0.062 | 63.6% | ||||
| Surgery | <0.001 | <0.001 | ||||||
| Performed | 59.1% | Reference | 64.7% | Reference | ||||
| Not performed | 17.3% | 2.58 (1.72–3.88) | <0.001 | 23.3% | 2.35 (1.55–3.56) | <0.001 | ||
| Radiotherapy | 0.055 | 0.086 | ||||||
| Performed | 48.5% | 56.2% | ||||||
| Not performed | 56.6% | 61.6% | ||||||
| Treatment | <0.001 | NA | <0.001 | NA | ||||
| Both | 60.2% | 66.3% | ||||||
| Surgery only | 58.8% | 64.1% | ||||||
| Radiotherapy only | 4.4% | 10.6% | ||||||
| None | 22.2% | 23.4% | ||||||
SEER – Surveillance, Epidemiology, and End Results; OS – overall survival; CSS – cancer-specific survival; HR – hazard ratio; CI – confidence interval; NOS – not otherwise specified; SES – socioeconomic status; NA – the analysis is not available because of the covariance.
Subgroup survival analysis for patients with undifferentiated chondrosarcoma
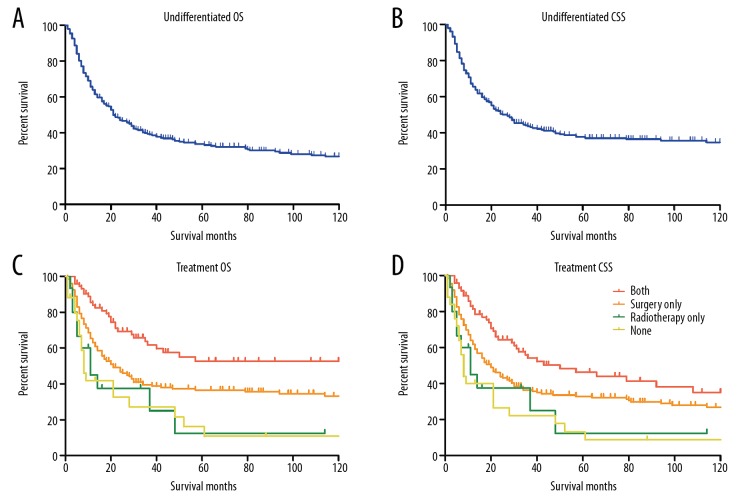
We next evaluated the therapeutic effects of surgical resection and adjuvant radiotherapy on undifferentiated chondrosarcoma by log-rank test and Cox regression analysis. The results showed that the 5-year OS and CSS for patients with undifferentiated chondrosarcoma were 33.1% and 37.1%, respectively (Figure 3A, 3B). Similar with the results of high-grade chondrosarcoma and poorly differentiated chondrosarcoma, surgical resection remained the effective treatment option for patients with undifferentiated chondrosarcoma. Strikingly, adjuvant radiotherapy combined surgery could significantly improve the outcomes of patients with undifferentiated chondrosarcoma in both OS and CSS analysis (OS P<0.001, Figure 3C; CSS P<0.001, Figure 3D), which was in contrast with the results in high-grade chondrosarcoma and poorly differentiated. Besides, greater age, extremities location, dedifferentiated histo-type, distant SEER stage, larger tumor size, and widowhood were associated with dismal prognoses in both OS and CSS. The Cox regression analysis demonstrated that radiotherapy was an independent protective factor for patients with undifferentiated chondrosarcoma in both OS (HR 1.57, 95% CI 1.09–2.27, P=0.017) and CSS (HR 1.66, 95% CI 1.11–2.50, P=0.016). In addition, age at diagnosis, tumor location, histo-type, SEER stage, tumor size, marital status, and surgical resection were also identified as independent prognostic factors for both OS and CSS. The results of survival analysis were shown in Table 4.
Figure 3.
Kaplan-Meier curves for undifferentiated chondrosarcoma. (A, B) The overall survival curve (A) and cancer-specific survival curve (B) for undifferentiated chondrosarcoma. (C, D) The overall survival curves (C) and cancer-specific survival curves (D) for undifferentiated chondrosarcoma according to treatment regimens.
Table 4.
Overall and cancer-specific survival analysis for patients with undifferentiated chondrosarcoma in the SEER database.
| Variable | Univariate analysis (log-rank test) | Multivariate analysis (Cox regression) | Univariate analysis (log-rank test) | Multivariate analysis (Cox regression) | ||||
|---|---|---|---|---|---|---|---|---|
| 5-year OS | P | HR (95% CI) | P | 5-year CSS | P | HR (95% CI) | P | |
| Age (years) at diagnosis | <0.001 | <0.001 | ||||||
| <60 | 43.4% | Reference | 44.6% | Reference | ||||
| ≥60 | 21.6% | 2.16 (1.58–2.97) | <0.001 | 28.3% | 1.75 (1.25–2.45) | 0.001 | ||
| Race | 0.037 | 0.089 | ||||||
| White | 30.6% | Reference | 34.8% | |||||
| Black | 43.3% | 0.80 (0.43–1.49) | 0.485 | 43.3% | ||||
| Other | 50.6% | 0.44 (0.23–0.84) | 0.012 | 54.8% | ||||
| Sex | 0.098 | 0.321 | ||||||
| Male | 28.3% | 33.1% | ||||||
| Female | 38.7% | 41.3% | ||||||
| Tumor location | 0.003 | 0.001 | ||||||
| Axial | 31.6% | Reference | 35.0% | Reference | ||||
| Extremities | 25.7% | 1.14 (0.80–1.62) | 0.474 | 29.9% | 1.13 (0.77–1.64) | 0.543 | ||
| Other | 45.6% | 1.08 (0.67–1.73) | 0.763 | 51.5% | 0.89 (0.53–1.51) | 0.679 | ||
| Histo-type | 0.007 | 0.008 | ||||||
| Chondrosarcoma, NOS | 29.5% | Reference | 36.2% | Reference | ||||
| Myxoid | 37.5% | 1.03 (0.63–1.67) | 0.912 | 39.5% | 1.11 (0.65–1.88) | 0.712 | ||
| Mesenchymal | 55.5% | 0.52 (0.29–0.92) | 0.026 | 60.3% | 0.52 (0.27–1.01) | 0.053 | ||
| Dedifferentiated | 24.0% | 1.07 (0.77–1.50) | 0.681 | 27.8% | 1.08 (0.75–1.55) | 0.680 | ||
| Other | NA* | 1.41 (0.33–6.05) | 0.647 | NA* | 1.61 (0.37–7.02) | 0.527 | ||
| SEER stage | <0.001 | <0.001 | ||||||
| Regional | 37.2% | 1.66 (1.13–2.43) | 0.010 | 41.8% | 1.54 (1.00–2.36) | 0.048 | ||
| Distant | 11.6% | 3.41 (2.25–5.16) | <0.001 | 12.1% | 3.66 (2.35–5.72) | <0.001 | ||
| Unstaged | 33.3% | 1.23 (0.42–3.59) | 0.711 | 38.9% | 1.18 (0.35–3.99) | 0.791 | ||
| Tumor size | 0.001 | 0.001 | ||||||
| ≤8 cm | 46.0% | Reference | 50.8% | Reference | ||||
| >8 cm | 23.2% | 1.29 (0.89–1.86) | 0.179 | 26.8% | 1.28 (0.87–1.89) | 0.219 | ||
| Unknown | 29.9% | 1.17 (0.76–1.79) | 0.471 | 34.6% | 0.96 (0.59–1.54) | 0.850 | ||
| Marital status | 0.001 | 0.001 | ||||||
| Married | 35.8% | Reference | 39.3% | Reference | ||||
| Divorced | 32.1% | 1.17 (0.71–1.91) | 0.538 | 33.1% | 1.21 (0.72–2.03) | 0.465 | ||
| Single | 38.3% | 1.12 (0.74–1.68) | 0.591 | 48.0% | 0.98 (0.62–1.54) | 0.922 | ||
| Widowed | 10.7% | 1.54 (1.01–2.34) | 0.045 | 13.1% | 1.65 (1.06–2.57) | 0.027 | ||
| Socioeconomic status | 0.559 | 0.584 | ||||||
| Low-SES | 31.8% | 35.2% | ||||||
| High-SES | 35.0% | 39.6% | ||||||
| Surgery | 0.003 | 0.001 | ||||||
| Performed | 36.1% | Reference | 40.9% | Reference | ||||
| Not performed | 10.8% | 1.84 (1.17–2.89) | 0.008 | 12.4% | 1.90 (1.19–3.04) | 0.008 | ||
| Radiotherapy | 0.015 | 0.004 | ||||||
| Performed | 41.1% | Reference | 46.2% | Reference | ||||
| Not performed | 30.0% | 1.57 (1.09–2.27) | 0.017 | 33.4% | 1.66 (1.11–2.50) | 0.016 | ||
| Treatment | <0.001 | NA# | <0.001 | NA# | ||||
| Both | 46.3% | 52.8% | ||||||
| Surgery only | 32.3% | 36.5% | ||||||
| Radiotherapy only | 12.5% | 12.5% | ||||||
| None | 8.3% | 10.9% | ||||||
SEER – Surveillance, Epidemiology, and End Results; OS – overall survival; CSS – cancer-specific survival; HR – hazard ratio; CI – confidence interval; NOS – not otherwise specified; SES – socioeconomic status;
NA* – the analysis is not available because of the small sample size;
NA# – the analysis is not available because of the covariance.
Subgroup survival analysis for patients with different histo-types of undifferentiated chondrosarcoma
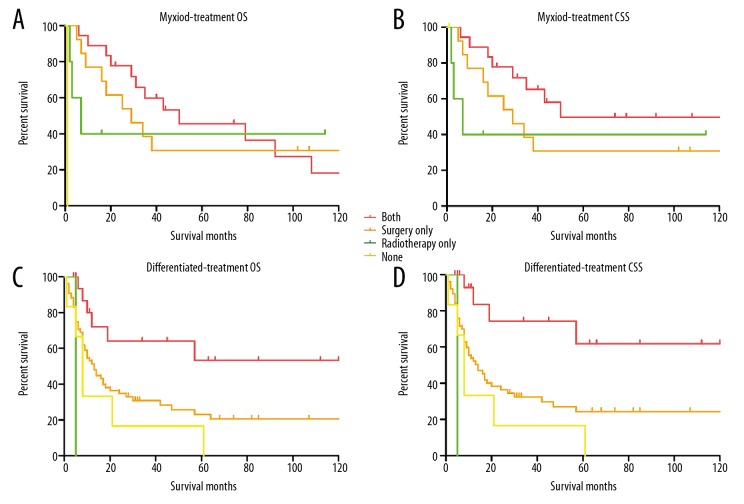
To explore which histo-type of undifferentiated chondrosarcoma was sensitive to the adjuvant radiotherapy, the log-rank test and Cox regression analysis were performed. The log-rank test of undifferentiated myoxid chondrosarcoma showed both the 5-year OS and CSS of patients with adjuvant radiotherapy were much higher than that of patients without radiotherapy, although the differences were not significant (Figure 4A, 4B). The univariate analysis of undifferentiated dedifferentiated chondrosarcoma demonstrated that the adjuvant radiotherapy could remarkably benefit the OS and CSS (OS P=0.015, Figure 4C; CSS P=0.007, Figure 4D), and the Cox regression analysis demonstrated that radiotherapy was an independent protective factor for patients in both OS (HR 2.64, 95% CI 1.17–5.95, P=0.019) and CSS (HR 3.56, 95% CI 1.39–9.15, P=0.008) analysis. While the survival analysis showed that the adjuvant radiotherapy did not benefit the survival of patients with undifferentiated chondrosarcoma (NOS) and mesenchymal chondrosarcoma. Together, these results determined that the adjuvant radiotherapy combined with surgery could benefit the patients with undifferentiated myxoid and dedifferentiated chondrosarcoma rather than those with undifferentiated chondrosarcoma (NOS) and mesenchymal chondrosarcoma. The results of survival analysis were shown in Table 5.
Figure 4.
Kaplan-Meier curves for undifferentiated myxoid and dedifferentiated chondrosarcoma. (A, B) The overall survival curves (A) and cancer-specific survival curves (B) for undifferentiated myxoid chondrosarcoma according to treatment regimens. (C, D) The overall survival curves (C) and cancer-specific survival curves (D) for undifferentiated dedifferentiated chondrosarcoma according to treatment regimens.
Table 5.
Subgroup survival analysis for patients with different histo-types of undifferentiated chondrosarcoma in the SEER database.
| Histo-type | Variable | Univariate analysis (log-rank test) | Multivariate analysis (Cox regression) | Univariate analysis (log-rank test) | Multivariate analysis (Cox regression) | ||||
|---|---|---|---|---|---|---|---|---|---|
| 5-year OS | P | HR (95% CI) | P | 5-year CSS | P | HR (95% CI) | P | ||
| Chondrosarco-ma, NOS | Radiotherapy | 0.642 | 0.709 | ||||||
| Performed | 27.8% | 32.0% | |||||||
| Not performed | 31.0% | 37.1% | |||||||
| Treatment | 0.064 | 0.085 | |||||||
| Both | 27.9% | 40.8% | |||||||
| Surgery only | 34.9% | 39.1% | |||||||
| Radiotherapy only | 1.0% | 1.4% | |||||||
| None | 8.0% | 11.5% | |||||||
| Myxoid | Radiotherapy | 0.608 | 0.211 | ||||||
| Performed | 42.7% | 52.9% | |||||||
| Not performed | 28.6% | 28.6% | |||||||
| Treatment | 0.000 | 0.000 | |||||||
| Both | 45.6% | 58.0% | |||||||
| Surgery only | 30.8% | 30.8% | |||||||
| Radiotherapy only | NA* | NA* | |||||||
| None | NA* | NA* | |||||||
| Mesenchymal | Radiotherapy | 0.934 | 0.883 | ||||||
| Performed | 53.7% | 59.7% | |||||||
| Not performed | 56.7% | 60.2% | |||||||
| Treatment | 0.703 | 0.380 | |||||||
| Both | 60.9% | 69.6% | |||||||
| Surgery only | 61.4% | 65.1% | |||||||
| Radiotherapy only | 1.0% | 1.0% | |||||||
| None | 33.3% | 33.3% | |||||||
| Dedifferentiated | Radiotherapy | 0.020 | 0.005 | ||||||
| Performed | 50.2% | Reference | 58.3% | Reference | |||||
| Not performed | 20.6% | 2.64 (1.17–5.95) | 0.019 | 21.5% | 3.56 (1.39–9.15) | 0.008 | |||
| Treatment | 0.015 | NA# | 0.007 | NA# | |||||
| Both | 53.3% | 61.9% | |||||||
| Surgery only | 23.6% | 24.3% | |||||||
| Radiotherapy only | NA* | NA* | |||||||
| None | NA* | NA* | |||||||
SEER – Surveillance, Epidemiology, and End Results; OS – overall survival; CSS – cancer-specific survival; HR – hazard ratio; CI – confidence interval; NOS – not otherwise specified;
NA* – the analysis is not available because of the small sample size;
NA# – the analysis is not available because of the covariance.
Discussion
Since most prognostic studies focus on the entire entity of chondrosarcoma [2,26–28], the prognostic factors and optimal treatment for high-grade chondrosarcoma remain poorly understood. Given the significant diversity between different histopathology grades and the dismal prognosis of high-grade chondrosarcomas, prognostic studies specifically for high-grade tumors are imperative. In this study, we demonstrated that age at diagnosis, pathological grade, histo-type, SEER stage, tumor size and surgical resection were independent prognostic factors in OS and CSS analysis for the entire high-grade tumor group. While radiotherapy was identified as a protective factor in both OS and CSS analysis of patients with undifferentiated (grade 4) dedifferentiated chondrosarcoma, and adjuvant radiotherapy combined with surgery could improve both the OS and CSS of patients with undifferentiated (grade 4) myxoid and dedifferentiated chondrosarcoma, which encourage the application of adjuvant radiotherapy for patients with undifferentiated chondrosarcoma.
With an 88.5% CSS at 5 years [24], the low-grade chondrosarcoma is often considered as an indolent cancer. However, the survival of patients with high-grade chondrosarcoma, especially the undifferentiated chondrosarcoma was unfavorable. In this study, the 5-year OS and CSS for poorly-differentiated chondrosarcoma were 54.2% and 60.1%, respectively, and the OS and CSS for undifferentiated chondrosarcoma were 33.1% and 37.1%, respectively, emphasizing the clinical difference between the poorly-differentiated and undifferentiated tumor. In consistent with our survival data, Fiorenza et al. found that the 5-year OS rate of grade-2 chondrosarcoma was 53%, and the survival rate of grade-3 tumor was 38% [29]. Similarly, the study by Giuffrida et al. reported a 37% 5-year disease-specific survival rate of high-grade chondrosarcoma [2]. However, a single institutional study including 31 patients with high-grade chondrosarcoma revealed that the 5-year disease-specific survival was 82.5% [7], which was much higher than our study and other studies. The reason for the huge gap may be attributed to the small sample size in this single institutional study.
It seems that almost all previous studies on chondrosarcoma elucidated that radiotherapy and chemotherapy are not beneficial to the patients’ outcome, and the surgical resection is the only effective treatment for patients with chondrosarcoma [30–32]. In the present study, we also found that surgical resection could benefit the OS and CSS of patients with high-grade chondrosarcoma. However, our study firstly demonstrated that the adjuvant radiotherapy combined with surgery could benefit both OS and CSS of patients with undifferentiated myxoid and dedifferentiated chondrosarcoma compared with other treatment regimens. Similar to our findings, a large-scale SEER study by Koshy et al. analyzed 6960 cases of high-grade soft tissue sarcomas, which included 154 cases of osseous and chondromatous neoplasms, suggesting that the radiotherapy was associated with improved 3-year OS [33]. Some studies have shown radiotherapy could benefit the local tumor control and reduce the recurrence rate, but these studies failed to show the advantage of radiotherapy on survival. Drilon et al. identified 73 cases of localized myxoid chondrosarcoma and found that the patients with adjuvant radiotherapy exhibited a lower incidence of distant metastasis [20]. Kawaguchi et al. retrospectively reviewed 43 cases of mesenchymal chondrosarcoma, which has higher malignant biological behavior than other histo-types and enhanced the effect of adjuvant radiotherapy on local tumor control, but no benefit on OS and disease-free survival was identified [13]. Similarly, a systematic review by Xu et al. revealed that adjuvant radiotherapy may reduce local recurrence of mesenchymal chondrosarcoma but cannot improve the OS [12]. The study of Holliday et al. included 19 patients with spinal chordoma and chondrosarcoma, noting that early postoperative adjuvant radiotherapy may contribute to 2-year local control [14]. Together, these findings encourage the application of adjuvant radiotherapy in the treatment of high-grade chondrosarcoma, especially the undifferentiated myxoid and dedifferentiated chondrosarcoma.
Previous studies on chondrosarcoma have shown that anatomical location, tumor size, and stage can predict patient outcomes [34–37]. Similar to these studies, we demonstrated that tumor size and tumor stage were independent prognostic factors for high-grade tumors in OS and CSS analyses. However, we failed to determine the anatomical location as an independent prognostic factor of high-grade chondrosarcoma, indicating the different nature of high-grade tumors. Additionally, the present study showed that older age was associated with a significant worse survival in high-grade chondrosarcoma group and other 2 subgroups. Consistent with our findings, Giuffrida et al. reported that an age larger than 50 years was associated with a significant worse OS [2]. Our previous study also determined age as an independent prognostic factor in CSS analysis [24]. Together, these results reinforced the role of age in the management of chondrosarcoma. With regard to the marital status, the present study determined that widowhood was an independent risk factor in the subgroup of undifferentiated chondrosarcoma but not in the subgroup of poorly differentiated chondrosarcoma, suggesting the spousal support [38] and psychosocial factors [39–41] play more important roles in higher malignant tumors. The spousal support could increase the frequency of medical screening and the adherence to the treatment [38,42]. Besides, the widowhood status poses psychosocial stress on the surviving companions. The study by van Grootheest et al. revealed that the widowed people exhibited a high level of depression for a long time [40]. It is well known that the psychosocial disorders exert adverse effects on the immune and endocrine systems and contribute to the dismal survival [43]. Therefore, the widowhood patients with undifferentiated chondrosarcoma need more psychiatric intervention to improve the patients’ outcome.
The surgical margins play a critical role in the control of the tumor local recurrence. Stevenson et al. demonstrated that the surgical margins determined the local recurrence in all grades of chondrosarcoma [44]. Besides, our study determined that the adjuvant radiotherapy could benefit the patients with undifferentiated chondrosarcoma, while the radiation doses are also important for the treatment regimen [45]. Due to the inherent characteristics of the SEER database, this study couldn’t analyze the impact of surgical margins and radiotherapy doses on patients’ survival. Moreover, chemotherapy may benefit the outcomes of patients with mesenchymal and dedifferentiated chondrosarcoma, but not other subtypes such as the conventional chondrosarcoma [46]. In our study, we didn’t include the chemotherapy due to the lack of the specific data in the database, which may lead to incomplete analysis of the optimal treatment regimen for patients with chondrosarcoma. Finally, the 4-grade system in the SEER database is not commonly used for chondrosarcoma, thus the application of our results may be compromised. Although with these limitations, our study was the first large series to investigate the effect of adjuvant radiotherapy on high-grade chondrosarcoma, and has observed the survival benefit on patients with undifferentiated myxoid and dedifferentiated tumors, which may enhance the confidence of applying adjuvant radiotherapy on high-grade chondrosarcoma, and maximize the patients’ outcome.
Conclusions
Our study first demonstrated that radiotherapy was an independent protective factor in both OS and CSS for undifferentiated (grade 4) dedifferentiated chondrosarcoma, and adjuvant radiotherapy combined with surgery can improve both the OS and CSS of patients with undifferentiated (grade 4) myxoid and dedifferentiated chondrosarcoma. In addition, we determined that age at diagnosis, pathological grade, histo-type, SEER stage, tumor size, and surgical resection were independent prognostic factors in OS and CSS analysis for the entire high-grade tumor group. Our findings encourage the application of adjuvant radiotherapy for patients with high-grade chondrosarcoma, especially the undifferentiated myxoid and dedifferentiated chondrosarcoma.
Footnotes
Source of support: This study was funded by National Natural Science Foundation of China (81571209)
Ethical Statement
The SEER program database is publicly available and contains no unique identification for patients such as name, date of birth, or Social Security number. As such, a formal ethical review was waived after the discussion by the Ethics Committee of Xi’an Jiaotong University (Xi’an, China).
Conflicts of interest
None.
References
- 1.Chen JC, Fong YC, Tang CH. Novel strategies for the treatment of chondrosarcomas: Targeting integrins. Biomed Res Int. 2013;2013 doi: 10.1155/2013/396839. 396839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giuffrida AY, Burgueno JE, Koniaris LG, et al. Chondrosarcoma in the United States (1973 to 2003): An analysis of 2890 cases from the SEER database. J Bone Joint Surg Am. 2009;91(5):1063–72. doi: 10.2106/JBJS.H.00416. [DOI] [PubMed] [Google Scholar]
- 3.van Oosterwijk JG, Anninga JK, Gelderblom H, et al. Update on targets and novel treatment options for high-grade osteosarcoma and chondrosarcoma. Hematol Oncol Clin North Am. 2013;27(5):1021–48. doi: 10.1016/j.hoc.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 4.Douis H, Singh L, Saifuddin A. MRI differentiation of low-grade from high-grade appendicular chondrosarcoma. Eur Radiol. 2014;24(1):232–40. doi: 10.1007/s00330-013-3003-y. [DOI] [PubMed] [Google Scholar]
- 5.Bjornsson J, McLeod RA, Unni KK, et al. Primary chondrosarcoma of long bones and limb girdles. Cancer. 1998;83(10):2105–19. [PubMed] [Google Scholar]
- 6.Evans HL, Ayala AG, Romsdahl MM. Prognostic factors in chondrosarcoma of bone: A clinicopathologic analysis with emphasis on histologic grading. Cancer. 1977;40(2):818–31. doi: 10.1002/1097-0142(197708)40:2<818::aid-cncr2820400234>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 7.Bindiganavile S, Han I, Yun JY, Kim HS. Long-term outcome of chondrosarcoma: A single institutional experience. Cancer Res Treat. 2015;47(4):897–903. doi: 10.4143/crt.2014.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arshi A, Sharim J, Park DY, et al. Chondrosarcoma of the osseous spine: an analysis of epidemiology, patient outcomes, and prognostic factors using the SEER registry from 1973 to 2012. Spine (Phila Pa 1976) 2017;42(9):644–52. doi: 10.1097/BRS.0000000000001870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gelderblom H, Hogendoorn PC, Dijkstra SD, et al. The clinical approach towards chondrosarcoma. Oncologist. 2008;13(3):320–29. doi: 10.1634/theoncologist.2007-0237. [DOI] [PubMed] [Google Scholar]
- 10.Bovee JV, Cleton-Jansen AM, Taminiau AH, Hogendoorn PC. Emerging pathways in the development of chondrosarcoma of bone and implications for targeted treatment. Lancet Oncol. 2005;6(8):599–607. doi: 10.1016/S1470-2045(05)70282-5. [DOI] [PubMed] [Google Scholar]
- 11.Delaney TF, Kepka L, Goldberg SI, et al. Radiation therapy for control of soft-tissue sarcomas resected with positive margins. Int J Radiat Oncol Biol Phys. 2007;67(5):1460–69. doi: 10.1016/j.ijrobp.2006.11.035. [DOI] [PubMed] [Google Scholar]
- 12.Xu J, Li D, Xie L, et al. Mesenchymal chondrosarcoma of bone and soft tissue: A systematic review of 107 patients in the past 20 years. PLoS One. 2015;10(4):e0122216. doi: 10.1371/journal.pone.0122216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawaguchi S, Weiss I, Lin PP, et al. Radiation therapy is associated with fewer recurrences in mesenchymal chondrosarcoma. Clin Orthop Relat Res. 2014;472(3):856–64. doi: 10.1007/s11999-013-3064-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holliday EB, Mitra HS, Somerson JS, et al. Postoperative proton therapy for chordomas and chondrosarcomas of the spine: Adjuvant versus salvage radiation therapy. Spine (Phila Pa 1976) 2015;40(8):544–49. doi: 10.1097/BRS.0000000000000804. [DOI] [PubMed] [Google Scholar]
- 15.Outani H, Hamada K, Imura Y, et al. Comparison of clinical and functional outcome between surgical treatment and carbon ion radiotherapy for pelvic chondrosarcoma. Int J Clin Oncol. 2016;21(1):186–93. doi: 10.1007/s10147-015-0870-z. [DOI] [PubMed] [Google Scholar]
- 16.McNaney D, Lindberg RD, Ayala AG, et al. Fifteen year radiotherapy experience with chondrosarcoma of bone. Int J Radiat Oncol Biol Phys. 1982;8(2):187–90. doi: 10.1016/0360-3016(82)90512-0. [DOI] [PubMed] [Google Scholar]
- 17.Chiu MS, Verma V, Bennion NR, et al. Comparison of outcomes between rectal squamous cell carcinoma and adenocarcinoma. Cancer Med. 2016;5(12):3394–402. doi: 10.1002/cam4.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donghi R, Longoni A, Pilotti S, et al. Gene p53 mutations are restricted to poorly differentiated and undifferentiated carcinomas of the thyroid gland. J Clin Invest. 1993;91(4):1753–60. doi: 10.1172/JCI116385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bus MPA, Campanacci DA, Albergo JI, et al. Conventional primary central chondrosarcoma of the pelvis: Prognostic factors and outcome of surgical treatment in 162 patients. J Bone Joint Surg Am. 2018;100(4):316–25. doi: 10.2106/JBJS.17.00105. [DOI] [PubMed] [Google Scholar]
- 20.Drilon AD, Popat S, Bhuchar G, et al. Extraskeletal myxoid chondrosarcoma: A retrospective review from 2 referral centers emphasizing long-term outcomes with surgery and chemotherapy. Cancer. 2008;113(12):3364–71. doi: 10.1002/cncr.23978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El Beaino M, Roszik J, Livingston JA, et al. Mesenchymal chondrosarcoma: A review with emphasis on its fusion-driven biology. Curr Oncol Rep. 2018;20(5):37. doi: 10.1007/s11912-018-0668-z. [DOI] [PubMed] [Google Scholar]
- 22.Jiang XS, Pantanowitz L, Bui MM, et al. Clear cell chondrosarcoma: Cytologic findings in six cases. Diagn Cytopathol. 2014;42(9):784–91. doi: 10.1002/dc.23043. [DOI] [PubMed] [Google Scholar]
- 23.Liu C, Xi Y, Li M, et al. Dedifferentiated chondrosarcoma: Radiological features, prognostic factors and survival statistics in 23 patients. PLoS One. 2017;12(3):e0173665. doi: 10.1371/journal.pone.0173665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao Z, Ren F, Song H, et al. Marital status and survival of patients with chondrosarcoma: a population-based analysis. Med Sci Monit. 2018;24:6638–48. doi: 10.12659/MSM.911673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun M, Abdollah F, Liberman D, et al. Racial disparities and socioeconomic status in men diagnosed with testicular germ cell tumors: A survival analysis. Cancer. 2011;117(18):4277–85. doi: 10.1002/cncr.25969. [DOI] [PubMed] [Google Scholar]
- 26.Andreou D, Ruppin S, Fehlberg S, et al. Survival and prognostic factors in chondrosarcoma: results in 115 patients with long-term follow-up. Acta Orthop. 2011;82(6):749–55. doi: 10.3109/17453674.2011.636668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stuckey RM, Marco RA. Chondrosarcoma of the mobile spine and sacrum. Sarcoma. 2011;2011 doi: 10.1155/2011/274281. 274281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duchman KR, Lynch CF, Buckwalter JA, Miller BJ. Estimated cause-specific survival continues to improve over time in patients with chondrosarcoma. Clin Orthop Relat Res. 2014;472(8):2516–25. doi: 10.1007/s11999-014-3600-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fiorenza F, Abudu A, Grimer RJ, et al. Risk factors for survival and local control in chondrosarcoma of bone. J Bone Joint Surg Br. 2002;84(1):93–99. doi: 10.1302/0301-620x.84b1.11942. [DOI] [PubMed] [Google Scholar]
- 30.van Maldegem AM, Gelderblom H, Palmerini E, et al. Outcome of advanced, unresectable conventional central chondrosarcoma. Cancer. 2014;120(20):3159–64. doi: 10.1002/cncr.28845. [DOI] [PubMed] [Google Scholar]
- 31.Lee FY, Mankin HJ, Fondren G, et al. Chondrosarcoma of bone: An assessment of outcome. J Bone Joint Surg Am. 1999;81(3):326–38. doi: 10.2106/00004623-199903000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Grimer RJ, Gosheger G, Taminiau A, et al. Dedifferentiated chondrosarcoma: Prognostic factors and outcome from a European group. Eur J Cancer. 2007;43(14):2060–65. doi: 10.1016/j.ejca.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 33.Koshy M, Rich SE, Mohiuddin MM. Improved survival with radiation therapy in high-grade soft tissue sarcomas of the extremities: A SEER analysis. Int J Radiat Oncol Biol Phys. 2010;77(1):203–9. doi: 10.1016/j.ijrobp.2009.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fromm J, Klein A, Baur-Melnyk A, et al. Survival and prognostic factors in conventional central chondrosarcoma. BMC Cancer. 2018;18(1):849. doi: 10.1186/s12885-018-4741-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ellis MA, Gerry DR, Byrd JK. Head and neck chondrosarcomas: Analysis of the Surveillance, Epidemiology, and End Results database. Head Neck. 2016;38(9):1359–66. doi: 10.1002/hed.24434. [DOI] [PubMed] [Google Scholar]
- 36.Puri A, Shah M, Agarwal MG, et al. Chondrosarcoma of bone: does the size of the tumor, the presence of a pathologic fracture, or prior intervention have an impact on local control and survival? J Cancer Res Ther. 2009;5(1):14–19. doi: 10.4103/0973-1482.44362. [DOI] [PubMed] [Google Scholar]
- 37.Schneiderman BA, Kliethermes SA, Nystrom LM. Survival in mesenchymal chondrosarcoma varies based on age and tumor location: A survival analysis of the seer database. Clin Orthop Relat Res. 2017;475(3):799–805. doi: 10.1007/s11999-016-4779-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iwashyna TJ, Christakis NA. Marriage, widowhood, and health-care use. Soc Sci Med. 2003;57(11):2137–47. doi: 10.1016/s0277-9536(02)00546-4. [DOI] [PubMed] [Google Scholar]
- 39.Goodwin JS, Zhang DD, Ostir GV. Effect of depression on diagnosis, treatment, and survival of older women with breast cancer. J Am Geriatr Soc. 2004;52(1):106–11. doi: 10.1111/j.1532-5415.2004.52018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Grootheest DS, Beekman AT, Broese van Groenou MI, Deeg DJ. Sex differences in depression after widowhood. Do men suffer more? Soc Psychiatry Psychiatr Epidemiol. 1999;34(7):391–98. doi: 10.1007/s001270050160. [DOI] [PubMed] [Google Scholar]
- 41.Goodwin JS, Hunt WC, Key CR, Samet JM. The effect of marital status on stage, treatment, and survival of cancer patients. JAMA. 1987;258(21):3125–30. [PubMed] [Google Scholar]
- 42.Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(6):514–23. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garssen B, Goodkin K. On the role of immunological factors as mediators between psychosocial factors and cancer progression. Psychiatry Res. 1999;85(1):51–61. doi: 10.1016/s0165-1781(99)00008-6. [DOI] [PubMed] [Google Scholar]
- 44.Stevenson JD, Laitinen MK, Parry MC, et al. The role of surgical margins in chondrosarcoma. Eur J Surg Oncol. 2018;44(9):1412–18. doi: 10.1016/j.ejso.2018.05.033. [DOI] [PubMed] [Google Scholar]
- 45.De Amorim Bernstein K, DeLaney T. Chordomas and chondrosarcomas-the role of radiation therapy. J Surg Oncol. 2016;114(5):564–69. doi: 10.1002/jso.24368. [DOI] [PubMed] [Google Scholar]
- 46.Italiano A, Mir O, Cioffi A, et al. Advanced chondrosarcomas: Role of chemotherapy and survival. Ann Oncol. 2013;24(11):2916–22. doi: 10.1093/annonc/mdt374. [DOI] [PMC free article] [PubMed] [Google Scholar]