Summary
The past decade of research on Drosophila stem cells and niches has provided key insights. Fly stem cells do not occupy a special “state” based on novel “stem cell genes”, but resemble transiently arrested tissue progenitors. Moreover, individual stem cells and downstream progenitors are highly dynamic and dispensable, not tissue bulwarks. Niches, rather than fixed cell lineages, ensure tissue health by holding stem cells and repressing cell differentiation inside, but not outside. We review the five best-understood adult Drosophila stem cells, and argue that the fundamental biology of stem cells and niches is conserved between Drosophila and mice.
Introduction
Stem cells and niches interest us because of their critical role in maintaining adult tissues, as well as the insight they provide into fundamental mechanisms of developmental biology. Any cell that self-renews, whether via single asymmetric divisions or as part of larger group of cells co-regulated over time, can rightly be considered a stem cell. Analyzing the mechanisms that specify self-renewal or differentiation within stem cell lineages is simpler than deciphering tissue development as a whole. Understanding how stem cell niches, spatially specific tissue microenvironments, control stem cell differentiation potentially illuminates how local conditions influence all tissue cells.
It has been slightly more than ten years since a stem cell niche was precisely documented using the Drosophila ovary (Xie and Spradling, 2000). During this period, the study of fly stem cells and niches has greatly expanded, and the knowledge gained has begun to change our views of what stem cells are and how they work (reviewed by Krilly and Xie, 2007; Morrison and Spradling, 2008; Pearson et al 2009; Voog and Jones, 2010). In particular, discovering that individual Drosophila stem cells turn over regularly, compete for niche occupancy and rapidly differentiate when outside their normal milieu has focused attention on the niche. Here we discuss recent studies of the best-understood adult Drosophila stem cells to illuminate the major mechanisms by which they operate. The role of spindle orientation in stem cell biology is reviewed by Morin et al. [2011] in this issue.
Drosophila stem cell niches were made accessible to study by genetic tools that allow individual cells to be lineage labeled, and enable gene function to be disrupted within marked cell clones. Recently, mouse stem cell lineages have been similarly characterized, especially in the testis and small intestine. Whereas stem cell divisions within small, simple Drosophila niches usually produce asymmetric fate outcomes, daughter cells in mammalian tissues often adopt fates stochastically. However, we argue that these differences, like the obsolete distinction between mosaic and regulative embryonic development, are not significant and that the same basic mechanisms govern stem cells in Drosophila and mice.
The female germline stem cell niche- A simple paradigm
All Drosophila niches studied to date function using two key processes- adhesive interactions and asymmetric signaling. Adhesive interactions between stem cells and niche cells primarily determine niche size and occupancy (Fig. 1A). In addition, a sharply asymmetric signaling microenvironment represses stem cell differentiation and promotes stem cell adherence inside the niche, but reduces adhesiveness and stimulates development even one cell diameter outside (Fig. 1A). Little or no role has been found for intrinsic asymmetries during stem cell division in programming differential cell fate outcomes. As far as we know, Drosophila stem cell daughters are epigenetically equivalent, and diverge in fate based on the local signals they receive inside versus outside the niche. However, only a handful of adult stem cell types have been characterized to date, and additional mechanisms may operate in other contexts, such as developing neuroblasts (see Karlsson et al., 2010).
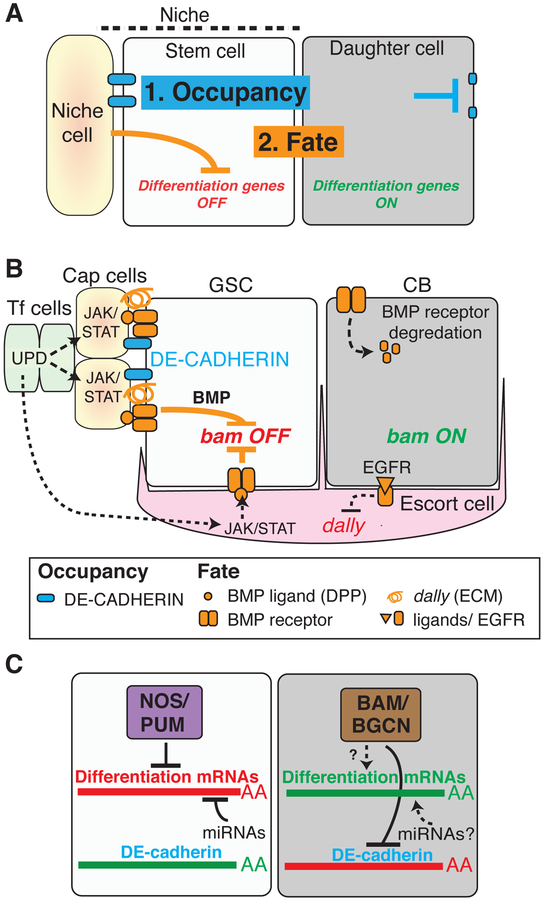
Figure 1. The ovarian germline stem cell niche: a well understood stem cell model system.
(A) A generic niche (dashed line) containing one stem and associated with a niche cell and daughter cell. Two basic niche functions are shown; occupancy (blue) is promoted by stimulating junctions with the niche cell inside, but not outside the niche. Fate regulation (orange) is effected by repressing differentiation genes inside but not outside the niche. (B) The Drosophila ovarian GSC niche contains one GSC and a daughter CB just outside. The niche is generated by terminal filament (Tf), cap, and escort cells. E-cad production is high in the GSC, supporting junctions to the niche. The master switch gene bam is repressed in GSCs by a Jak/STAT to Bmp signaling cascade from the Tf, to cap and escort cells. Bmp reception is turned off in the CB, due to reduced signaling, reduced production of the Bmp co-receptor Dally in response to Egfr signaling, and degradation of the Bmp receptor Tkv. (C) Translational activation of CB differentiation. Differentiating promoting transcripts are repressed in GSC via a Nos/Pum complex and by miRNAs. Bam expression in CB represses E-Cad and Nanos translation, reducing adhesion to the niche and activating differentiation mRNAs.
A prime example, the germline stem cell (GSC) niche located at the tip of each Drosophila ovariole, maintains 2–3 germline stem cells throughout pupal and adult life (Fig. 1B). These simple niches are established by three cooperating somatic cell types, terminal filament cells, cap cells and escort (or inner germarium sheath) cells. Cap cells hold each GSC in place via adherens junctions that prevent them from moving down the ovariole (Song et al., 2002). The terminal filament produces Unpaired (Upd) and related cytokines whose secretion activates Jak/Stat signaling in cap and escort cells and stimulates Bmp ligand production (Lopez-Onieva et al., 2008; Wang et al., 2008). Bmp receptor activation in GSCs prevents transcription of the master differentiation gene bag-of-marbles (bam). GSC divisions are usually asymmetric in outcome not because the daughter cells inherently differ, but because only one is able to remain in the niche. Following an oriented GSC division, the proximal daughter is usually well positioned to retain cap cell adhesion and repression, while the distal daughter has little chance of re-entering the niche. Consequently, bam transcription is activated and it differentiates into a cystoblast (CB), a cell that will give rise to a new germline cyst and ultimately a new follicle.
Since GSC maintenance and daughter differentiation is governed by the local microenvironment, it is not surprising that recent studies have revealed multiple, interlocking mechanisms operating both inside and outside the niche that assure microenvironmental asymmetry. One of the most important involves the extracellular matrix (ECM), which has long been implicated in influencing stem cell activity in mammalian tissues. The ECM surrounding the GSC niche differs from the ECM downstream and actively limits the size of the repressive zone. Dpp and Gbb, the Bmp ligands synthesized by cap cells, carry out long-range patterning in many developing tissues, and would not act over just a one-cell wide region without active restraint. The type IV collagens Viking and Dcg-1 within the ECM can bind Dpp and may limit its diffusion (Wang et al., 2008), but the heparin sulfate glycoprotein Dally plays a major role by stabilizing Dpp and facilitating reception by germ cells (Guo and Wang, 2009; Hayashi et al., 2009). Dally is abundant around the niche but sparse away from the cap cells. Forcing high-level dally expression in all escort cells blocks GSC differentiation, suggesting the low levels of Dally outside the niche are required to downregulate Bmp reception in CBs. Dally and/or other heparin sulfate glycoproteins probably require secondary modifications to function since enzymes involved in this process are also essential for niche function (Hayashi et al., 2009). Thus, the range of the niche signal is limited by the ECM and especially by the localized distribution of Dally.
Intercellular signaling involving escort cells helps maintain the ECM’s asymmetry by reducing Dally production away from the niche. When the transmembrane protease Stet, or its Egf substrate ligands Spitz, Gurken and Keren are mutated in germ cells, or when Egfr or MAP kinase components are disrupted in escort cells, niche signals travel further than normal and block CB differentiation (Schultz et al., 2002; Liu et al., 2011). Stet mutants can be suppressed by mutating dally, indicating that Egfr-mediated signaling in escort cells is required to limit Dally secretion outside of the niche. Loss of Egfr signaling activity causes escort cells to retract the cytoplasmic extensions that normally separate cysts, and likely facilitate escort cell-germ cell communication.
Several other pathways contribute to the sharp decrease in Bmp reception a germ cell experiences upon exit from the niche. The Bmp-induced ubiquitin E3 ligase Smurf mediates one such process (Casaneuva and Ferguson, 2004). The Ser/Thr-protein kinase FUSED, which also functions in hedgehog signaling, acts cell autonomously in conjunction with SMURF to target the Dpp receptor Tkv for degradation in CBs, and is essential for CB differentiation (Narbonne-Reveau et al., 2006; Xia et al., 2010). Fused binds Smurf and Tkv, and may directly phosphorylate Tkv at serine 328. How this pathway is blocked in GSCs remains unclear. Thus, the ability of germ cells to receive the repressive Bmp signal is actively reduced outside the niche by multiple mechanisms.
A translational switch specifies GSC daughters as cystoblasts
The elaborately orchestrated downregulation of Bmp signaling in CBs exerts only one known direct action- the derepression of bam transcription. Consistent with this simple mechanism, Bam production is sufficient to induce GSC differentiation (Ohlstein and McKearin, 1997), through actions exerted primarily at the translational level (Li et al., 2009; Shen et al., 2009; Fig. 1C). In GSCs, differentiation-promoting mRNAs (most of which remain unidentified) are repressed by regulatory complexes containing the conserved germline proteins Nanos (Nos) and Pumilio (Pum), whereas translation of E-cadherin (E-cad) mRNA is high, supporting niche adhesion. Upon induction, Bam associates with the Benign gonial cell neoplasm (Bgcn) and other proteins to lower cap cell adhesion and activate differentiation. The repressive Bam complex acts in part by binding to the 3’ UTRs of E-cad and nanos mRNA. Reducing E-Cad expression helps insure that CBs do not re-associate with cap cells (Jin et al., 2008), while lowering Nos levels de-represses translation of differentiation genes. One mRNA activated in CBs encodes another translational regulator, Brat. Brat functions as a co-repressor with Nos and Pum in the fly embryo, but probably acts with Pum independently of Nos to mediate ovarian germ cell differentiation (Harris et al., 2011). As CB differentiation commences, the translation of myc is reduced to modulate cyst growth, while Mad translation (encoding a SMAD protein) is also downregulated, further enforcing the shutoff of Bmp signaling in CBs. Modeling these interactions shows that the system as a whole can act as a robust, bi-stable switch of GSC to CB fate specification (Kim et al., 2010; Harris et al., 2011).
Multiple non-coding RNAs also modulate the fates of both GSCs and CBs. Abolishing all miRNA activity by mutationally disrupting genes encoding key RISC components including Dicer-I, Ago-1, or Loquacious (Jin and Xie, 2007; Park, et al., 2007; Yang et al., 2007), causes GSCs to differentiate. One important miRNA mediator is Mei-P26, a protein related to Brat whose level is increased in early germ cells by the action of Vasa, which binds to the mei-P26 3’ UTR and stimulates translation (Liu et al., 2009). Both repressive miRNAs acting in GSCs such as bantam, and differentiation-promoting miRNAs acting in CBs, are regulated by Mei-P26 (Page et al., 2000; Neumuller et al., 2008; Yang et al., 2009). Mei-P26 binds to Ago-1 and may influence the RISC complex’s ability to process and degrade miRNA target genes (Neumuller et al., 2008). A distinct miRNA, miR-184, stimulates CB development by translationally repressing yet another Bmp signaling component, the receptor Saxophone (Iovino et al., 2009). A structurally distinct category of small RNA, piRNA, may also participate in the GSC to CB switch, since they have been shown during embryonic development to target relevant mRNAs including nos (Rouget et al., 2010). Several genes implicated in piRNA production and transposon regulation, including Yb and piwi, act in somatic niche cells to maintain normal signaling (Szakmary et al., 2008; Saito et al., 2010; Qi et al., 2011).
If ovarian stem cells operate based on local environmental signals, why do stem cell divisions usually produce asymmetric cell fates? Asymmetry in GSC daughter cell fates is probably an incidental byproduct of the niche’s small size and physical asymmetry. Adhesive cap cells are located only on the proximal side, so distal GSC daughter cells are usually constrained to leave. However, when both daughters do acquire cap cell contact, they both become normal GSCs (Xie and Spradling, 2000), verifying that there are no important intrinsic differences between them. Likewise, external germ cells can re-enter the niche and become GSCs (Kai and Spradling, 2004). GSCs do contain a collection of aggregated organelles known as the spectrosome or fusome that segregates asymmetrically in all adult mitotic germ cell divisions in both sexes, and influences microtubule organization and spindle orientation. The spectrosome/fusome is held together by α-Spectrin and the Adducin-like Hts protein (see Lighthouse et al., 2008). In spectrin or hts mutant flies, the organelle is absent and its components segregate symmetrically, but GSCs are not destabilized. In contrast to its minimal role in the GSC, the spectrosome/fusome is critically important for the downstream events of male and female gametogenesis, including oocyte specification.
The testis stem cell niche: increased size and complexity
Male gametogenesis in Drosophila is typical of diverse species in being supported during adulthood by germline stem cells. In the Drosophila testis, a single large GSC niche, rather than 16–20 small niches in the ovary that each head an ovariole, supplies germ cells for sperm production (Fig. 2A). This larger, more complex niche offers many unique opportunities for understanding stem cell biology. A round cluster of differentiated niche cells known as the hub contacts seven to twelve male GSCs, arrayed around it like spokes. The hub is anchored to the testis apex by integrins and the LASP protein (Lee et al., 2008; Tanentzapf et al., 2007). Two somatic cyst progenitor cells (CySCs), a distinct type of stem cell, surround each GSC, make their own contacts with the hub, and are co-regulated by the niche. GSCs and CySCs must be coordinated since the GSC daughter, the gonialblast (GB), acquires two CySC daughters that provide an external covering as the GB divides synchronously to form a 16-cell cyst that will give rise to 64 sperm. While niche cells in both the male and female derive from the embryonic gonadal mesoderm, the time of niche formation differs between the sexes. Hub cells and CySCs differentiate during embryonic stages, capture GSCs and begin functioning, whereas female niche cell precursors remain undeveloped until late larval stages (Murray et al 2010). Thus, the testis provides an opportunity to study a niche that must control and coordinate multiple stem cells and daughters using mechanisms that may be highly conserved.
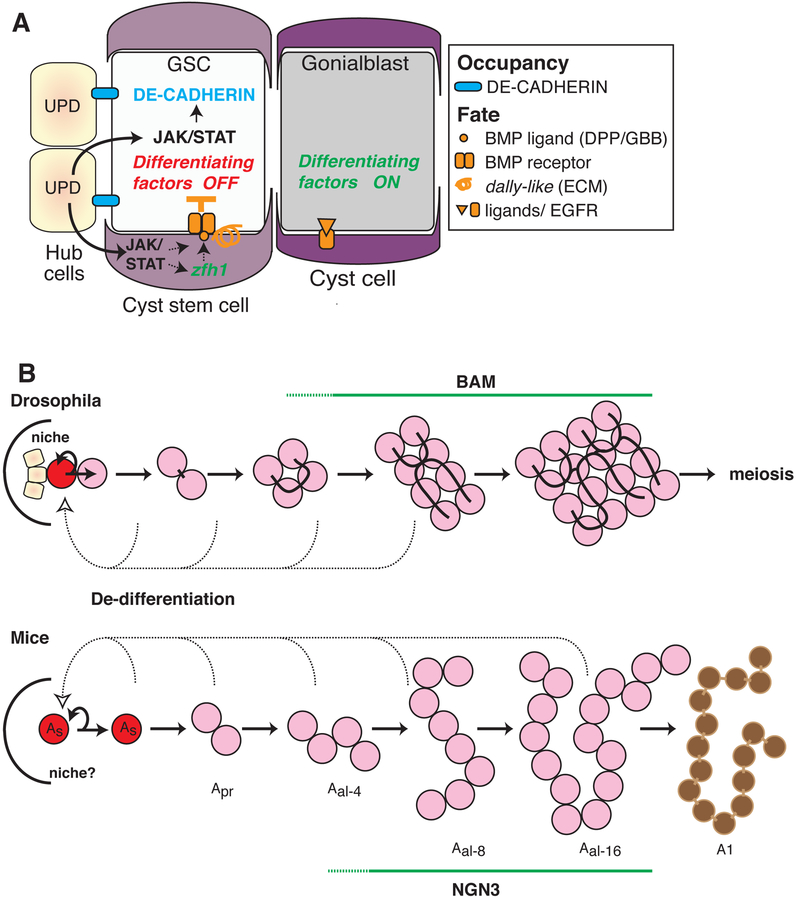
Figure 2. Regulation of male germline stem cell niche and de-differentiation.
(A) The Drosophila testis niche is shown with one GSC and one CySC, and a daughter GB just outside, associated with a cyst cell. Niche occupancy (blue) is promoted Jak/Stat-stimulated E-Cad expression in the GSC. bam is repressed in GSCs by a Jak/STAT to Bmp signaling cascade from the hub to CySC. Bmp reception is turned off in the GB, due to reduced signaling and dally-like expression, which activates bam and currently unknown differentiation genes (?). (B) The growth of germline cysts downstream from the GSC (red) and its niche is diagrammed for both Drosophila and mouse testis (details are uncertain in the case of mice). Differentiation genes such as bam or ngn-3 turn on in a slow and variable manner in both species (dashed line), prior to the 16-cell stage, which leads to meiosis or commitment to mature (A1) spermatogonial fate. Rarely, (dashed lines) cysts can break down and individual cyst cells can re-enter the niche and become GSCs, providing a stem cell reserve.
Despite its greater complexity, the testis niche uses the same general principles and many of the same genetic pathways that operate in the ovary (Fig. 2A). The niche comprises a one-cell wide zone directly adjacent to the hub, since only germ cells directly contacting the hub via adherens junctions self-renew, while daughters that move away begin cyst formation and are wrapped by CySC daughters. Hub cells secrete Upd which initiates a cascade of signal transduction that governs both niche occupancy and self-renewal. Upd signals received directly by GSCs stimulate adhesiveness to the hub (Leatherman and DiNardo, 2008), and prevent CySCs from out-competing GSCs for niche contact (Issigonis et al., 2009; Sheng et al., 2009). Both GSCs (Yamashita et al., 2003) and CySCs (Cheng et al., 2011) orient their spindles to point away from the hub.
Upd signaling received by CySCs mediates the repressive functions of the niche. Jak/Stat activation in CySCs autonomously represses differentiation by stimulating production of the transcriptional repressor ZFH-1, CHINMO and probably other gene products (Leatherman and DiNardo, 2008; Wang et al., 2008; Flaherty et al., 2010). Also in response to Upd, CySCs non-autonomously maintain GSCs as stem cells by secreting Gbb and Dpp, which activate Bmp reception (see Kirilly and Xie, 2007; Wang et al., 2008; Leatherman and DiNardo, 2010). Bmp pathway activation in GSCs represses bam transcription to prevent differentiation. Thus, both male and female GSC differentiation is repressed by a Jak/Stat to Bmp signaling cascade involving two distinct cell types.
The testis niche exhibits unique features, as well. Bmp activation does not block premature GSC differentiation exclusively by repressing bam. Gonialblasts, unlike their cystoblast counterparts, start dividing before bam turns on (see Kirlly and Xie, 2007; Insco et al., 2009; Sheng et al., 2009). Gbb plays a larger role in the male, while Dpp is more potent in the female niche (Kawase et al., 2004). Finally, GSC spindles are precisely oriented by an active mechanism that is maintained by a checkpoint in males (see review Morin et al. in this issue), but are more loosely controlled in females (Deng and Lin, 1997; Morris and Spradling, 2011).
The somatic cells that wrap around the GSCs and downstream germ cells in the two sexes are maintained differently. The most anterior escort cells surround GSCs in the female niche and resemble CySCs both morphologically, and in their responses to niche-generated Upd signals and germ cell-derived Egf signals. However, they do not function as active stem cells, but remain in place as germ cells pass from one escort cell to the next (Morris and Spradling, 2011). In contrast, CySCs not only divide to produce cyst cells, but under some circumstances CySC daughters can differentiate into hub cells (Voog et al., 2008; DiNardo et al 2011). Hub replenishment may help maintain niche function, but has no known female analog.
How male GSCs are maintained by Bmp and possibly other signals and how their differentiation outside the niche is controlled are still poorly understood. Translational regulation under the control of Nanos and Pumilio is important during the embryonic development of male as well as female germ cells (Asaoka-Taguchi et al., 1999). Yet, in Drosophila after the male niche forms and spermatogenesis commences, Nanos and Pumilio are no longer required, although they continue to be expressed. Whether Bam and Bgcn control the translation of stored mRNAs during the male differentiation program is unknown. A threshold level of Bam is needed to terminate cystocyte divisions (Insco et al., 2009), and minimal levels have to be maintained while cysts are growing to prevent their premature breakdown (Pek et al., 2010). Both the RNA-binding protein HOW and miR-7, whose levels are controlled by Malestrom, can repress bam mRNA translation and are important for maintaining Bam levels (Pek et al., 2010; Monk et al., 2010). However, the genes that control the bam-independent aspects of the GSC/gonialblast switch have yet to be described.
The male niche generates a spatially limited repressive microenvironment; germ cells initiate the gonialblast program shortly after losing contact with the hub and by the time they have moved one cell diameter away. As in females, the ECM helps control the diffusion and presentation of Gbb and Dpp, but male GSC maintenance requires the related protein Dally-like, instead of Dally (Hayashi et al., 2009). Germline signaling via STET-processed Egf ligands activates Egfr/Map kinase signaling in cyst cells that is required for them to extend membranes around GBs and for their normal differentiation (Sarkar et al., 2007). It is not clear if this involves ECM modification, however. Smurf acting in concert with Fused does not play the same role in downregulating Bmp signaling in males that it does in female. Males do not require fused for fertility, but expressing constitutively-active TKV using the bam promoter causes male sterilily (Xia et al., 2010). This same construct has no effect on female fertility, presumably because of rapid Smurf/Fused-mediated Tkv turnover Due to the lack of this pathway for downregulating Bmp reception, the Bmp gradient may be shallower in the male compared to the female niche region, which might explain why bam turns on more slowly in males relative to the cyst divisions.
The tightly regulated spindle orientation characteristic of the testis niche (Yamashita et al., 2003), which helps ensure divisional asymmetry for both GSCs and CySCs, does not necessarily contradict the idea that local environmental differences specify daughter cell fates. Neither the spectrosome/fusome, nor any other GSC cellular component is known whose asymmetric segregation is essential for stem cell maintenance or GB specification. Indeed, as in the case of females, downstream cyst cells can regain hub contact under a variety of circumstances, become GSCs and function normally in the niche microenvironment (Brawley and Matunis, 2004; Sheng et al., 2009). Perhaps the highly regulated system of spindle orientation in the testis niche is needed to maintain its larger size and complexity. Divisional orientation, supported by the spindle checkpoint, may function primarily to preserve the regular interdigitated arrangement of GSCs and CySCs around the hub, not to specify cell fates. Off-axis GSC divisions would reduce GSC-CySC contact, which might perturb GSC regulation, since repressive Bmp signals are transmitted via CySCs. Altered organization of the stem cells around the hub might also prevent GBs from acquiring the two CySC daughters needed to found a new cyst.
The Follicle Stem Cell niche- Interaction at a distance
The niche that supports production of the epithelial follicle cell layer surrounding developing ovarian follicle differs in several ways from the GSC niches. Instead of being associated with a readily identified, differentiated cell population, the follicle stem cell (FSC) niche and its single resident stem cell are not morphologically distinctive and were only discovered through lineage analysis (Margolis et al., 1995). No genes have thus far been found that are expressed differentially in the FSCs and its immediate daughter. Although fate specification in the FSC lineage depends on Notch, Hedgehog (Hh), Wingless (Wf), Jak/Stat and other signals (see Huynh and St. Johnston, 2004; Krilly and Xie, 2007; Nystul and Spradling, 2010), the precise origin and timing of these signals has proved difficult to pinpoint early in the lineage. Despite these differences, the FSC niche supports cell production as efficiently and reliably as the GSC niche throughout life.
The anatomy of the FSC niche and its lineage has been clarified recently (Nystul and Spradling, 2007; Morris and Spradling, 2011). Posterior escort cells contact FSCs, and may organize a cellular FSC niche on each lateral germarium surface. About half the time, FSC daughters become cross-migrating cells (Cmcs) that move to the opposite side of the germarium, where they occasionally replace the resident stem cell. Successful cross-migration and stem cell replacement are dependent on a Delta-mediated Notch signal received by the migrating cell from germ cells. The presence of Cmcs in variable locations, expressing genes indistinguishable from FSCs, has been a source of confusion regarding FSC number. The situation provides one of many examples where cellular behavior rather than gene expression provides the only reliable means to identify stem cells.
The genetic requirements of the FSC niche argue that it operates in a basically similar manner as other Drosophila niches. E-Cad and β-Catenin are essential to niche function, suggesting that adherens junctions tether FSCs in the niche (Song and Xie, 2002). Hypomorphic mutations of CycE that reduce kinase activity cause preferential loss of FSCs, and this defect can be suppressed by upregulating E-cad (Wang and Kalderon, 2009). These results suggest that FSCs cycling below a minimum rate detach from the niche. In vivo, however, even in the absence of sufficient nutrition to make an egg, FSCs slow down but are not lost (Drummon-Barbosa and Spradling, 2001). ECM components such as Laminin A and integrins may also contribute to FSC function (O’Reilly et al., 2008). However, the effects of disrupting integrin function in FSCs may be secondary to general changes in germarium struture that result from the loss of integrin function in downstream follicle cells.
How differentiation is repressed in the niche is not understood, but FSCs require Hh, Wg, and Dpp signaling to be maintained (reviewed in Krilly and Xie, 2007). These signals may be sent from a relatively distant source, the GSC niche, because terminal filament and cap cells express pathway ligands much more strongly than escort cells. FSC division responds to the level of Hh, whose reception is mediated by Patched and the co-receptor Boi (Hartman et al 2010; Yan et al 2010; Zheng et al 2010). Boi production in anterior cells sequesters Hh away from the FSCs, thereby limiting FSC proliferation (Hartman et al., 2010). If FSCs do respond to distant signaling sources, the lack of sharp gene expression differences between the FSC and its daughters might be due to the shallow ligand gradients expected from such an arrangement.
Dynamic stem cells undergo replacement and compete for niche occupancy
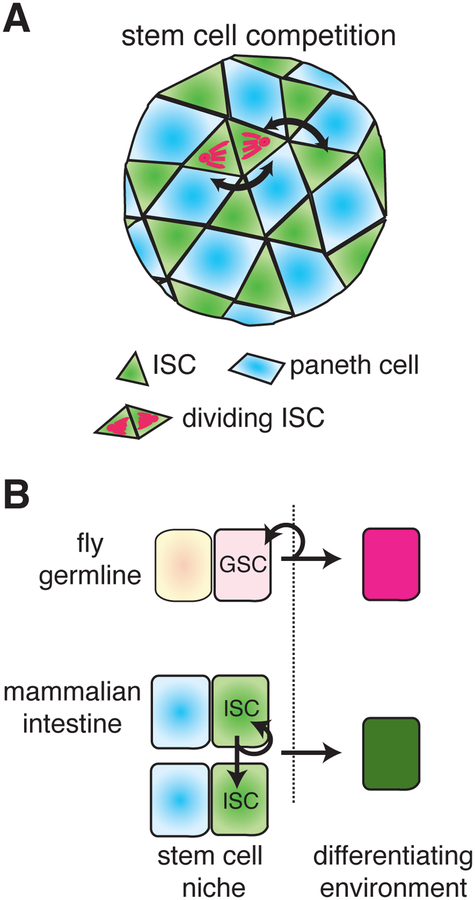
One of the most important and initially surprising general findings from the study of Drosophila stem cells is their regular turnover and replacement. Initially, the observed turnover of marked stem cells was assumed to result from cell loss (Margolis and Spradling, 1995). Eventually, the realization that the departing cells were being replaced provided proof for the stem cell niche (Xie and Spradling, 2000). Three of the four well-studied Drosophila stem cell types that reside in niches undergo regular replacement by other competent progenitors. In the ovarian GSC niche, half of the stem cells are replaced every two to three weeks by daughters of neighboring stem cells (Xie and Spradling, 2000). In the testis, GSCs in flies more than two weeks old are replaced even more frequently, mostly by cells derived from the breakdown of two-, four- and eight-cell cysts (Brawley and Matunis, 2004; Cheng et al., 2008 Sheng et al., 2009; Fig. 2B). FSCs are replaced every two to three weeks by FSC daughters (Cmcs) that have migrated across the germarium (Nystul and Spradling, 2007). The net result is that very few of the initial stem cells that populate a tissue’s niches remain throughout adult life. Stem cell replacement by the daughters of other nearby stem cells causes the size and shape of marked stem cell clones to continue changing long after the time required for a cell to transit the entire lineage. The movement, growth and extinction of cell patches with different genotypes driven by somatic mutation and stem cell replacement represents a distinct process of dynamic somatic mosacism that is likely to be widespread, but whose biological significance remains little explored.
Replacement potentially places each stem cell in competition with every other stem cell that lies within reach of its replacement-competent daughters. However, it has yet to be decisively demonstrated that competition between stem cell genotypes serves a biological purpose. Which of two wild type GSCs or FSCs gets replaced appears to be random (Xie and Spradling, 2000; Nystul and Spradling, 2007). Equivalently, one could say that the two GSCs (or FSCs) undergo “neutral competition.” However, if one stem cell is wild type and one is mutant, then competition might maintain stem cell quality by ensuring that weaker cells are expelled (Nystul and Spradling, 2007; Jin et al., 2008; Johnston, 2009; Rhiner et al., 2009). GSCs with reduced levels of E-Cad (Jin et a. 2008) or with reduced Myc expression (Rhiner et al., 2009) are lost preferentially. A potential disadvantage of maintaining stem cell quality by cell competition is that stem cells might acquire selfish mutations that promote an ability to replace wild type stem cells. This is exactly what was observed when E-Cad or Myc levels were increased above normal., Whether competition occurs in wild type tissues between spontaneously arising genotypes and whether such changes impact aging and cancer development remains to be addressed.
Chromatin regulation of stem cells and differentiation
Proper chromatin organization is critically important for stem cells to maintain their full differentiation potential despite ongoing proliferation and environmental fluctuations. Epigenetic states must remain flexible enough to support differentiation along multiple pathways, but maintain sufficient programming to interpret rather general tissue signals so as to produce appropriate cell types. The chromatin requirements for repressing bam transcription in GSCs provide a relevant focus for understanding stem cell chromatin programming. Iswi, a component of the NURF nucleosome remodeling complex, is specifically needed (Xi and Xie, 2005). Steroid hormone signaling can modulate this requirement (Ables and Drummond-Barbosa, 2010). Repression of bam has also been reported to depend on an interaction between SMADs and the lamin-like nuclear membrane protein Otefin (Jiang et al., 2008). Otefin mutant ovaries lose GSCs suggesting that bam cannot be repressed sufficiently without this interaction. Otefin has been proposed to tether bam to the nuclear periphery, but moving SMAD-bound bam to the nuclear margin by an independent mechanism did not rescue Otefin mutants (Sui and Yang, 2011). NURF is also required in male GSCs (Cherry and Matunis, 2010).
Several other genes encode chromatin factors that contribute to stem cell maintenance. The histone lysine methyltransferase Setdb1, which generates repressive histone H3 lysine 9 methylation, is required to maintain GSCs, and its expression relative to the related methyltransferase Su(var)3–9 changes during early germ cell development (Clough et al., 2007; Yoon et al., 2008). Scrawny, an H2B Ubiquitin protease that acts autonomously within stem cells, likely dampens premature gene activation to help maintain GSCs, FSCs, and intestinal stem cells (Buszczak et al., 2009). Niche cells also require chromatin-modifying proteins. The histone demethylase Lsd1 functions within escort cells to limit niche size in the ovary (Eliazer et al, 2011). The piRNA pathway in niche cells may not just suppress transposon damage, but may also actively program chromatin and gene activity (Yin and Lin, 2007). Enhanced GSC loss caused by piRNA pathway mutations in niche cells can be partially suppressed by mutating corto, which encodes a chromatin repressor protein (Smulders-Srinivasan et al., 2010). It is still challenging to identify the direct targets of all these chromatin-modifying proteins and to distinguish chromatin regulation that is unique to stem cell function from general requirements for differentiation or gene expression.
Chromatin also plays critical but incompletely understood roles during lineage differentiation downstream from the stem cell. The FSC lineage shows particular promise as a system for systematically analyzing the chromatin changes involved in stem cell differentiation. An assay based on Gal4/UAS variegation allows changes in epigenetic stability to be measured at each step in the main body cell lineage and to be dissected genetically (Skora and Spradling, 2010). Consistent with gain-of-function assays in embryos, epigenetic instability in the FSC lineage is very high in early progenitors, but stabilizes 80-fold prior to the completion of cell division. Some specific genes likely to be involved in modulating FSC chromatin have been identified genetically. The nucleosome remodeling protein Dom, rather than Iswi, functions in this tissue (Xi and Xie, 2005). Mutating Psc and Su(z)2, Polycomb group genes that are involved in interpreting spatial patterning, disrupts differentiation downstream from the FSC (Li et al., 2010).
Intestinal stem cells and their niche
The Drosophila posterior midgut contains 800–1,000 active intestinal stem cells (ISCs) that replenish the abundant enterocytes (ECs) and rare enteroendocrine (ee) cells of the gut every one to two weeks (Fig. 3A). The abundance, simple lineage, and high activity of ISCs makes the fly midgut an exceptionally attractive system for studying stem cell regulation (reviewed in Casali and Batlle, 2009; Chatterjee and Ip, 2009; Pitsouli et al., 2009; Karpowicz and Perrimon, 2010; Hou, 2010). However, it is still unclear if ISCs are located in niches similar to those described for reproductive tissue stem cells. ISCs are arrayed irregularly across the basement membrane of the midgut, surrounded on all sides by ECs, and associated with no special cell or morphological structure that might organize a niche. Muscle layers located beneath the basement membrane ensheath the gut with circular and longitudinal fibers. Wg is expressed in some intestinal muscles, and was proposed to generate an ISC niche (Lin et al., 2008). However, Wg signal reception in ISCs may not be required for stem cell maintenance, but rather to tune their proliferation rate. Clones expressing a constitutively active ®−catenin are hyperplasic, but do not block differentiation (Lee et al., 2009).
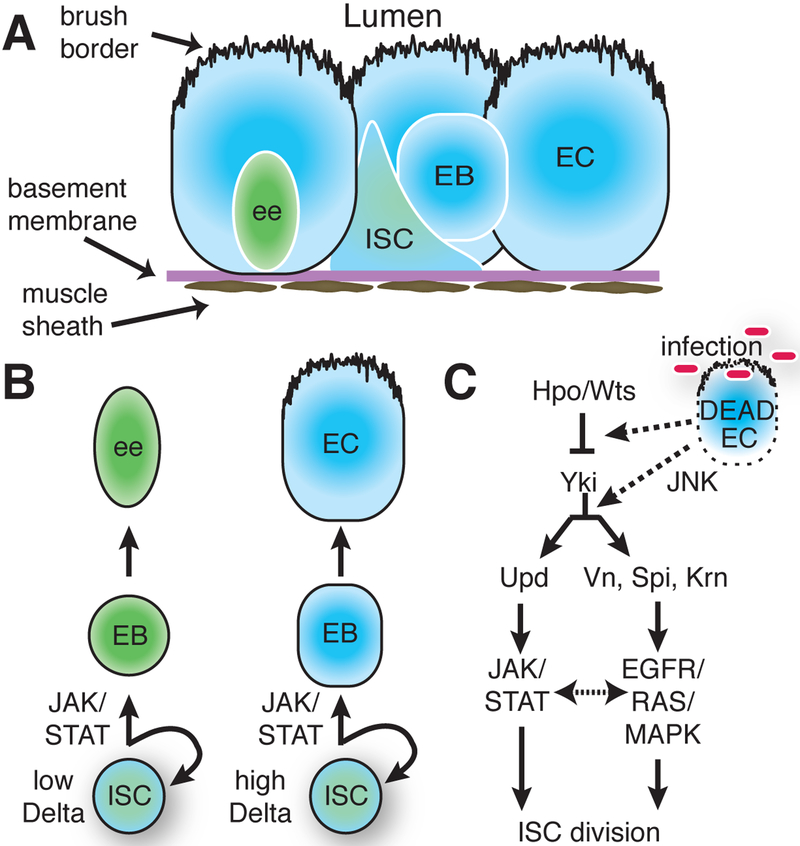
Figure 3. The responsive Drosophila intestinal stem cell lineage.
(A) Diagram showing the Drosophila intestinal stem cell (ISC) in its location on the basement membrane, near a recent daughter enteroblast (EB), several mature enterocytes (EC, blue) and a rare enteroendocrine cells (ee, green). (B) The level of the Notch ligand Delta in the ISC acts with the Jak/Stat pathway to specifiy EC v ee fates. (C) Stress, infection, and/or damage to the intestinal epithelium trigger signaling through, Hpo/Wts, Jnk, Jak/Stat, and gfr/Ras/Mapk to modulate the ISC based proliferative response.
ISC niches might be fixed but cryptic, like FSC niches, or they might form around each ISC anywhere on the basement membrane. There is reason to suspect that ISCs might be able to generate their own niche. During development ISC precursors within midgut “imaginal islands” (Jiang and Edgar, 2009) are maintained by 1–2 island cells that differentiate early (Mathur et al., 2010). Developing progenitors produce Egfr ligands that act as their own mitogens (Jiang and Edgar, 2009). CySCs provide another precedent for a stem cell contributing to its own niche, since they are able to generate replacement hub cells (Voog et al., 2008). Recently, mouse Paneth cells have been clearly shown to carry out exactly this function for ISCs in the intestinal crypt (Sato et al., 2011; Fig. 4A).
Figure 4. The mouse intestinal stem cell niche.
(A) Diagram of a cross section of a mouse intestinal crypt showing stem cellsss (ISC, green) and Paneth cells (blue) (adapted from Snippert et al., 2010). Oriented ISC division generates competition to maximize ISC-Paneth cell contacts between the ISC daughters and neighboring stem cells. One stem cell is forced out of the niche by “neutral competition” and differentiates. (B) Diagram showing similarity between the Drosophila GSC niche (upper drawing) and the mouse ISC niche (lower drawing). Stem cells in both niches are retained by competition for adhesion to niche cells. In the small Drosophila niche, following GSC division the distal daughter usually loses competition for niche contact, exits and differentiations, generating asymmetric daughter cell fates. In the larger mouse niche, one of the recently divided ISC daughters or another adjacent ISC, loses competition for niche contact, exits the niche and differentiates, generating symmetric or asymmetric cell fates. The practical effect of both systems for cell production is similar or identical.
ISCs also play an active role in specifying the fate of their daughters. The choice between an EC or ee outcome depends on Notch signaling between the stem cell and its daughter, the enteroblast (EB), shortly after division. An ISC that expresses high levels of the Notch ligand Delta will signal strongly and induce EC differentiation, whereas ISCs with low Delta expression will trigger differentiation toward an ee fate (Ohlstein and Spradling, 2007; Bardin et al; 2010; Takashima et al., 2011; Fig. 3B). The direct targets of Notch signaling that mediate differentiation and fate choice are poorly understood. In addition, the Jak/Stat pathway coordinates with Notch to regulate EB differentiation. Stat is expressed in ISCs and EBs and is required for differentiation, even when Notch signaling is activated (Beebe et al., 2010).
Stem cells respond to environmental cues
Drosophila stem cells do not just function autonomously in their niche, but are exquisitely sensitive to conditions that impinge on the anticipated need for cell production (reviewed in Drummond-Barbosa, 2008). For example, GSC niches support fourfold higher levels of stem cell activity and the ovary produces many more eggs per unit time, when nutrients are readily available, and environmental conditions for embryo development are favorable. ISCs respond to nutrition, stress and infection. In several tissues, stem cell activity declines with age (Boyle et al., 2007; Pan et al., 2007; Zhao et al., 2008; reviewed in Wang and Jones, 2010).
Dietary conditions directly impact stem cell division rate both through the insulin signaling pathway and by alternative routes (Hsu et al., 2008; LaFever et al., 2010; Amcheslavsky et al., 2009; Biteau et al., 2010; McLeod et al., 2010; Ueishi et al., 2009). For example, in the ovary, both INR and TOR activity are required to prevent GSCs from arresting in G2 of the cell cycle. Drosophila insulin-like peptides (Dilps) not only bind to receptors expressed on GSCs, but also modulate how cap cells interact with GSCs (Hsu and Drummond-Barbosa, 2009) and respond to Notch signaling (Hsu and Drummond-Barbosa, 2011). Even under laboratory conditions, where food is relatively plentiful, a significant fraction of GSCs pause in G2 of the cell cycle. In addition to InR and Tor-mediated signals, arrest and restart in G2 may require effete-mediated degradation of CycA, a process that can affect GSC maintenance (Chen et al., 2009). Release from arrest can be recapitulated by the addition of insulin to germaria developing in vitro (Morris and Spradling, 2011).
The intestinal epithelium, unlike the germline, is in constant contact with the external environment due to the ingestion of nutrients. Even in the absence of stress, the gut hosts a community of commensual microorganisms that interact extensively with ECs and participate in setting the basal level of cell turnover (Buchon et al., 2009). Therefore, it is not surprising that ISCs are highly responsive to external influences and much has been learned about how they are regulated when the intestine is exposed to pathogens or toxic agents that can damage the gut epithelium (Apidianakis et al., 2009; Biteau et al., 2008; Buchon et al., 2009; 2010; Chatterjee and Ip, 2009; Cronin et al., 2009; Karpowicz et al., 2010; Jiang et al., 2009; 2011; Ren et al., 2010; Shaw et al., 2010; Staley and Irvine, 2010).
Various stimuli that damage ECs stimulate the production and release of Jak/Stat (Upd2 and Upd3) and Egfr (Vn, Spi and Krn) ligands. In fact, the EC seems to be the major source for these stimulatory cytokines, since genetically inhibiting these factors with a EC-specific Gal4 can block accelerated stem cell division under conditions of stress. Clonal or tissue-wide activation of Jak/Stat and/or Egfr pathways in ISCs mimics the effect of tissue injury by accelerating ISC division, while impairing either pathway blocks ISC’s ability to respond. There is crosstalk between Jak/Stat and Egfr pathway. Blocking Ras signaling in clones expressing Upd reduces ISC proliferation and likewise loss of STAT can reduce clone size when division is stimulated with the Egfr ligand VN (Jiang et al., 2011). Depending on the type and severity of injury, cytokine production by ECs can also be induced by the Jnk and Hippo/Warts pathways leading to further modulation of the ISC division rate (Fig. 3C).
Do mouse and Drosophila stem cells and niches use similar regulatory strategies?
The basic mechanisms of metazoan development and gene regulation arose prior to the divergence of the major phyla and have been conserved in evolution. However, mammalian tissues are much larger than Drosophila tissues, contain many more stem cells, live much longer, and contend with potentially different challenges resulting from the organisms’ different ecological lifestyles. Studies of mammalian stem cells have been intensively pursued due to their medical importance, and have spanned a wide range of species and tissues (reviewed in Barker et al., 2010). Despite this, the technical difficulty of mapping mammalian stem cells and niches at the level of individual cells in vivo has made direct comparisons with Drosophila difficult (see Morrison and Spradling, 2008). Recently, genetically controlled lineage tracing in the mouse epidermis, testis, small intestine and several other tissues has started to overcome this problem (Clayton et al., 2007; Yoshida et al., 2007; Barker et al., 2007; Hsu et al. 2011). Moreover, a niche in the murine small intestine has now been characterized in detail (Sato et al., 2011). Whether these findings reveal fundamental differences in the operation of mammalian and Drosophila stem cell lineages and niches is a subject of current interest (Nakagawa et al., 2010; Snippert et al., 2010; Klein et al., 2010).
The mouse testis and stem cell replacement
The mouse testis houses thousands of spermatogonial stem cells (here called GSCs) along the basal layer of the seminiferous tubules that can be assayed by lineage marking or transplantation (reviewed in Oatley and Brinster, 2008; Yoshida, 2010). Mouse spermatogonia, like those in Drosophila and many other species, develop from single cells (As) into germline cysts interconnected by intercellular bridges via synchronous divisions (Fig. 2B). This suggests that the single cells represent stem cells and gonialblasts, while the interconnected cells form a developmental sequence. Clusters of up to 16 or 32 cells appear morphologically “undifferentiated,” while cells in larger cysts are thought to have initiated a differentiation process leading to the onset of meiosis 5–6 divisions later (see Chiarini-Garcia and Russell, 2001).
Whether discrete niches support mouse GSCs as in Drosophila remains controversial. Somatic Sertoli cells contact As cells and clustered spermatogonia and may serve niche-like functions possibly analogous to the roles played by Drosophila somatic cyst cells or escort cells. GSCs require the Tgf-β family member Gdnf produced by Sertoli cells and received by germline Gfra1/cRet-1 receptors to maintain self-renewal. The conserved Nanos2 gene is preferentially expressed from As cells to small cysts, and is essential for GSC maintenance (Sada et al., 2009). Germ cell production is thought to respond to nutritional conditions via a pathway involving mTORC1 and the transcription factor Plzf that feeds back on Gdnf reception (Hobbs et al., 2010). Despite the evolutionary conservation revealed by these shared features, the existence of a murine male GSC niche remains unclear. No cellular structure analogous to the Drosophila hub appears to be present. Instead, regions of the basal layer near interstitial cells and blood vessels may provide niche function (Yoshida et al., 2007; but see Oatley et al., 2011). Knockdown experiments failed to detect a requirement for either Stat3 or E-Cad in GSCs assayed by transplantation (Kanatsu-Shinohara et al., 2008; Oatley et al., 2010). Until more is learned about the existence and nature of a male niche, it remains difficult to compare this aspect of mouse and Drosophila stem cell biology.
However, one very striking similarity has been documented between cell replenishment in Drosophila and mouse testes. This is the dynamic character of the early germ cells (Fig. 2B). Mouse cysts can lose their interconnections and form new As cells (Nakagawa et al., 2010). Cyst breakdown also takes place in Drosophila, especially in older animals and the released germ cells can re-enter the niche and function as GSCs (Brawley and Matunis, 2004; Cheng et al., 2008; Sheng et al., 2009). Lineage tracing and transplantation experiments suggest that cyst breakdown is a source of replacement stem cells in mice as well (Nakagawa et al., 2007). Long-term lineage studies indicate that cysts break down at an easily detectable rate (Klein et al., 2010). Shortly before, during or after cyst breakdown, germ cells turn off differentiation-associated genes such as ngn3, and induce self-renewal-associated genes such as nanos2 and GFRa1. This might explain why some As cells and short cysts express ngn3, while some longer cysts express genes associated with self renewal (Suzuki et al. 2009; Nakagawa et al., 2010). Despite the existence of cyst breakdown and dedifferentiation, most mouse cysts probably do not experience such events or else cyst cell numbers would not usually correspond to powers of two.
The heterogeneity of gene expression within cysts and the observation that small cysts or even single As cells can begin to differentiate directly as A1 cells (Nakagawa et al., 2010) has called into question for some the view of early cysts as a fundamental development sequence. However, the fact that fragmented cysts can start to development may not require such a conclusion. Drosophila female cysts with half the normal number of cells can still form fertile gametes, and even cysts with just a few cells, such as those produced by hts mutants, support extensive differentiation (but not fertility). Consequently, a conservative interpretation would be that in both Drosophila and mice, cysts usually differentiate without interruption, a few break down and supply replacement stem cells, and in the process some single former cyst cells and cyst fragments with discordant gene expression develop extensively, but eventually arrest and die.
The mouse intestinal stem cell niche
Recently, the mammalian small intestine has emerged as one of the best characterized mammalian stem cell niches (reviewed in Barker et al., 2010; Fig. 4A). Paneth cells, an ISC-derived cell type with anti-microbial function, are found at the base of the crypt interdigitated between about 14 ISCs, where they help generate and maintain an ISC niche (Sato et al., 2011). Paneth cells adhere to ISCs and express Egf, Tgfα, Wnt3 and Dll4, factors needed to maintain ISC activity in culture (Sato et al., 2009). Niche signals probably induce ISCs to express Lgr5, a WNT-inducible orphan G protein-coupled receptor that is dispensable for ISC function, but which has been widely used as an ISC marker (Barker et al., 2007; Barker and Clevers, 2010). After ISC daughters lose contact with Paneth cells, they turn off Lgr5 and become transit-amplifying cells, which continue to divide and differentiate while moving up the crypt wall.
The similarities between the mouse intestinal niche and those studied previously in the fly are striking. The ISC niche depends on a specific cell, the Paneth cell, to which ISCs attach and which sends signals that repress differentiation, exactly as in Drosophila niches. Reducing the number of Paneth cells in a crypt, reduces the number of ISCs, but they remain attached to the Paneth cells that remain (Sato et al., 2011), exactly as when cap cell or hub cell number is decreased. When crypts are lineage-labeled with a single Lgr5+ cell, 1–6 months later some have become fully labeled (Barker et al., 2007), indicating that ISCs at the crypt base are regularly replaced by other ISCs or their daughters. Indeed, the mechanism of ISC maintenance is proposed to be competition for Paneth cell contact (Sato et al., 2011; Fig. 4B), just as Drosophila GSCs compete for cap cell contact (Jin et al., 2008).
Interestingly, the fates of ISC daughters following division are usually symmetric, rather than asymmetric as in the case of the well-studied Drosophila niches (Snippert et al., 2010). Following an oriented division (Quyn et al., 2010), the recently divided ISCs and their nearby neighbors undergo “neutral competition”, probably to maximize ISC-Paneth cell contact, and soon one cell is ejected from the niche (Fig. 4A arrows, 4B). Therefore, the net effect of ISC division, just as in the Drosophila niches, is to produce a cell that renews the crypt stem cell pool and a cell that differentiates. This system of orientated division followed by neutral competition probably helps in some way not currently understood to maintain 1) ISC and Paneth cell number and position; 2) the ability to orient subsequent ISC divisions, and 3) the ability to direct exiting cells upward.
While “neutral competition” might seem different from asymmetric division, this is not the case when the daughters of stem cell division are intrinsically equal. Which equivalent cell remains and which exits the niche after each stem cell division will have little if any functional consequence as long as robust niche mechanisms guarantee that both events remain coupled. In this respect, stem cell biology appears to be like other aspects of embryonic and tissue development. Patterning in these systems depends on cellular interactions, not lineage; reproducible lineages usually result from small systems with few cells and many constrains that just generate reproducible intercellular interactions.
Conclusions
More than a decade of studying Drosophila stem cells and niches has provided new insights into how cell production is maintained in adult tissues. Well-characterized stem cells reside in highly asymmetric microenvironments capable of holding stem cells in place, controlling their proliferation and repressing their differentiation. The number of niches and their size is tightly regulated, perhaps because of their potential to support excessive cell production. Consequently, niches are associated with robust, self-reinforcing mechanisms to ensure that on average, half the daughters of stem cell division emerge from the niche into conditions conducive to differentiation. The methods used to reinforce functional asymmetry are diverse, and will provide insights into the even greater mechanistic sophistication throughout metazoan tissues. Another decade analyzing Drosophila niches will be even more information than the last.
In contrast, close examination has scraped some of the misplaced luster off stem cells themselves. So far, only extrinsic mechanisms of cell fate specification have been uncovered, and unique biological mechanisms shared among different stem cells have not been identified. The master controls of cell production reside within the niche, and in the overall organization of the system, rather than in a special cell population. Early progenitors play a previously unappreciated role, based on their ability to infrequently “reverse” course, compete to re-enter available niches and regain stem cell function. At present one can only speculate that the entire system of niches, reversible differentiation, competition and replacement evolved to maintain order among potentially unruly somatic cell populations as they deal with growth, stress, aging, mutation and disease. While a few details will differ, the basic strategies described here appear to be used by stem cells throughout diverse organisms, including Drosophila and mice. Thus, further study of stem cell biology promises new insights into the most fundamental mechanisms that govern the metazoan genome.
ACKNOWLEDGEMENTS
We thank the Sprading lab for comments. V.L.. is a fellow of the Jane Coffin Childs Memorial Fund; D. F., L. M, and ACS are supported by HHMI.
Footnotes
Competing interests statement
The authors declare that they have no competing financial interests.
References
- Ables ET, and Drummond-Barbosa D (2010). The steroid hormone ecdysone functions with intrinsic chromatin remodeling factors to control female germline stem cells in Drosophila. Cell Stem Cell 7, 581–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amcheslavsky A, Jiang J, and Ip YT (2009). Tissue damage-induced intestinal stem cell division in Drosophila. Cell Stem Cell 4, 49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apidianakis Y, Pitsouli C, Perrimon N, and Rahme L (2009). Synergy between bacterial infection and genetic predisposition in intestinal dysplasia. Proc. Natl. Acad. Sci. USA 106, 20883–20888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asaoka-Taguchi M, Yamada M, Nakamura A, Hanyu K, and Kobayashi S (1999). Maternal Pumilio acts together with Nanos in germline development in Drosophila embryos. Nat Cell Biol. 1, 431–7. [DOI] [PubMed] [Google Scholar]
- Bardin AJ, Perdigoto CN, Southall TD, Brand AH, and Schweisguth F (2010). Transcriptional control of stem cell maintenance in the Drosophila intestine. Development 137, 705–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, and Clevers H (2007). Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–7. [DOI] [PubMed] [Google Scholar]
- Barker N, and Clevers H (2010). Leucine-rich repeat-containing G-protein-coupled receptors as markers of adult stem cells. Gastroenterology 138, 1681–96. [DOI] [PubMed] [Google Scholar]
- Barker N, Bartfeld S, and Clevers H (2010). Tissue-resident adult stem cell populations of rapidly self-renewing organs. Cell Stem Cell 7, 656–70. [DOI] [PubMed] [Google Scholar]
- Beebe K, Lee WC, and Micchelli CA (2010). JAK/STAT signaling coordinates stem cell proliferation and multilineage differentiation in the Drosophila intestinal stem cell lineage. Dev Biol. 338, 28–37. [DOI] [PubMed] [Google Scholar]
- Biteau B, Hochmuth CE, and Jasper H (2008). JNK activity in somatic stem cells causes loss of tissue homeostasis in the aging Drosophila gut. Cell Stem Cell 3, 442–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle M, Wong C, Rocha M, and Jones DL (2007). Decline in self-renewal factors contributes to aging of the stem cell niche. Cell Stem Cell 1, 458–469. [DOI] [PubMed] [Google Scholar]
- Brawley C, and Matunis E (2004). Regeneration of male germline stem cells by spermatogonial dedifferentiation in vivo. Science 304, 1331–1334. [DOI] [PubMed] [Google Scholar]
- Buchon N, Broderick NA, Chakrabarti S, and Lemaitre B (2009). Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Genes Dev. 23, 2333–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchon N, Broderick NA, Kuraishi T, and Lemaitre B (2010). Drosophila Egfr pathway coordinates stem cell proliferation and gut remodeling following infection. BMC Biol. 8, 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buszczak M, Paterno S, and Spradling AC (2009). Drosophila stem cells share a common requirement for the histone H2B ubiquitin protease Scrawny. Science 323, 248–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarini-Garcia H, Russell LD. (2001). High-resolution light microscopic characterization of mouse spermatogonia. Biol Reprod. 65, 1170–8. [DOI] [PubMed] [Google Scholar]
- Casali A, and Batlle E (2009). Intestinal stem cells in mammals and Drosophila. Cell Stem Cell. 4, 124–7. [DOI] [PubMed] [Google Scholar]
- Casanueva MO, and Ferguson EL (2004). Germline stem cell number in the Drosophila ovary is regulated by redundant mechanisms that control Dpp signaling. Development 131, 1881–90. [DOI] [PubMed] [Google Scholar]
- Chatterjee M, and Ip YT (2009). Pathogenic stimulation of intestinal stem cell response in Drosophila. J Cell Physiol 220, 664–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Wang Q, Huang H, Xia L, Jiang X, Kan L, Sun Q, and Chen D (2009). Effete-mediated degradation of Cyclin A is essential for the maintenance of germline stem cells in Drosophila. Development 136, 4133–42. [DOI] [PubMed] [Google Scholar]
- Cheng J, Türkel N, Hemati N, Fuller MT, Hunt AJ, and Yamashita YM (2008). Centrosome misorientation reduces stem cell division during ageing. Nature 456, 599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Tiyaboonchai A, Yamashita YM, and Hunt AJ (2011). Asymmetric division of cyst stem cells in Drosophila testis is ensured by anaphase spindle repositioning. Development 138, 831–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry CM, and Matunis EL (2010). Epigenetic regulation of stem cell maintenance in the Drosophila testis via the nucleosome-remodeling factor NURF. Cell Stem Cell 6, 557–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton E, Doupe DP, Klein AM, Winton DJ, Simons BD, and Jones PH (2007). A single type of progenitor cell maintains normal epidermis. Nature 446, 185–189. [DOI] [PubMed] [Google Scholar]
- Clough E, Moon W, Wang S, Smith K, and Hazelrigg T (2007) Histone methylation is required for oogenesis in Drosophila. Development 134, 157–165. [DOI] [PubMed] [Google Scholar]
- Cronin SJ, Nehme NT, Limmer S, Liegeois S, Pospisilik JA, Schramek D, Leibbrandt A, Simoes Rde M, Gruber S, Puc U, et al. , (2009). Genome-wide RNAi screen identifies genes involved in intestinal pathogenic bacterial infection. Science 325, 340–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, and Lin H (1997). Spectrosomes and fusomes anchor mitotic spindles during asymmetric germ cell divisions and facilitate the formation of a polarized microtubule array for oocyte specification in Drosophila. Dev. Biol 189, 79–94. [DOI] [PubMed] [Google Scholar]
- Dinardo S, Okegbe T, Wingert L, Freilich S, and Terry N (2011). lines and bowl affect the specification of cyst stem cells and niche cells in the Drosophila testis. Development 138, 1687–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond-Barbosa D, and Spradling AC (2001). Stem cells and their progeny respond to nutritional changes during Drosophila oogenesis. Dev. Biol 231, 265–278. [DOI] [PubMed] [Google Scholar]
- Drummond-Barbosa D (2008). Stem cells, their niches and the systemic environment: An aging network. Genetics 180, 1787–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliazer S, Shalaby NA, and Buszczak M (2011). Loss of lysine-specific demethylase 1 non-autonomously causes stem cell tumors in the Drosophila ovary. Proc. Natl. Acad. Sci. USA 108, 7064–7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan CM and Spradling AC (2010). Counterfeiting the family jewels. Cell Stem Cell 6, 405–406. [DOI] [PubMed] [Google Scholar]
- Flaherty MS, Salis P, Evans CJ, Ekas LA, Marouf A, Zavadil J, Banerjee U, and Bach EA (2010). chinmo is a functional effector of the JAK/STAT pathway that regulates eye development, tumor formation, and stem cell self-renewal in Drosophila. Dev. Cell 20, 556–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, and Wang Z (2009). The glypican Dally is required in the niche for the maintenance of germline stem cells and short-range Bmp signaling in the Drosophila ovary. Development 136, 3627–35. [DOI] [PubMed] [Google Scholar]
- Harris RE, Pargett M, Sutcliffe C, Umulis D, and Ashe HL (2011). Brat promotes stem cell differentiation via control of a bistable switch that restricts Bmp signaling. Dev Cell. 20, 72–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman TR, Zinshteyn D, Schofield HK, Nicolas E, Okada A, and O’Reilly AM (2010). Drosophila Boi limits Hedgehog levels to suppress follicle stem cell proliferation. J Cell Biol. 191, 943–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, Kobayashi S, and Nakato H (2009). Drosophila glypicans regulate the germline stem cell niche. J Cell Biol. 187, 473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs RM, Seandel M, Falciatori I, Rafii S, and Pandolfi PP (2010). Plzf regulates germline progenitor self-renewal by opposing mTORC1. Cell 142, 468–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou SX (2010). Intestinal stem cell asymmetric division in the Drosophila posterior midgut. J Cell Physiol. 224, 581–584. [DOI] [PubMed] [Google Scholar]
- Hsu HJ, LaFever L, and Drummond-Barbosa D (2008). Diet controls normal and tumorous germline stem cells via insulin-dependent and -independent mechanisms in Drosophila. Dev Biol. 313, 700–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu HJ, and Drummond-Barbosa D (2009). Insulin levels control female germline stem cell maintenance via the niche in Drosophila. Proc. Natl. Acad. Sci. USA 106, 1117–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu HJ, and Drummond-Barbosa D (2011). Insulin signals control the competence of the Drosophila female germline stem cell niche to respond to Notch ligands. Dev. Biol 350, 290–300. [DOI] [PubMed] [Google Scholar]
- Hsu YC, Pasolli HA, and Fuchs E (2011). Dynamics between stem cells, niche, and progeny in the hair follicle. Cell 144, 92–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh J-R, and St. Johnston D, (2004). The origin of asymmetry: early polarisation of the Drosophila germline cyst and ooctye. Cur. Biol 14, R438–R449. [DOI] [PubMed] [Google Scholar]
- Iovino N, Pane A, and Gaul U (2009). miR-184 has multiple roles in Drosophila female germline development. Dev. Cell 17, 123–133. [DOI] [PubMed] [Google Scholar]
- Insco ML, Leon A, Tam CH, McKearin DM, and Fuller MT (2010). Accumulation of a differentiation regulator specifies transit amplifying division number in an adult stem cell lineage. Proc. Natl. Acad. Sci. USA 106, 22311–22316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issigonis M, Tulina N, de Cuevas M, Brawley C, Sandler L, and Matunis E (2009). JAK–STAT signal inhibition regulates competition in the Drosophila testis stem cell niche. Science 326, 153–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Xia L, Chen D, Yang Y, Huang H, Yang L, Zhao Q, Shen L, Wang J, Chen D (2008). Otefin, a nuclear membrane protein, determines the fate of germline stem cells in Drosophila via interaction with Smad complexes. Dev Cell. 14, 494–506. [DOI] [PubMed] [Google Scholar]
- Jiang H, and Edgar BA (2009). Egfr signaling regulates the proliferation of Drosophila adult midgut progenitors. Development 136, 483–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Patel PH, Kohlmaier A, Grenley MO, McEwen DG, and Edgar BA (2009). Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell 137, 1343–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Grenley MO, Bravo MJ, Blumhagen RZ, and Edgar BA. (2011). Egfr/Ras/MAPK signaling mediates adult midgut epithelial homeostasis and regeneration in Drosophila. Cell Stem Cell. 8, 84–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z, Kirilly D, Weng C, Kawase E, Song X, Smith S, Schwartz J, and Xie T (2008). Differentiation-defective stem cells outcompete normal stem cells for niche occupancy in the Drosophila ovary. Cell Stem Cell 10, 39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z and Xie T (2007). Dcr-1 maintains Drosophila ovarian stem cells. Current Biology 17, 539–44. [DOI] [PubMed] [Google Scholar]
- Johnston LA (2009). Competitive interactions between cells: death, growth, and geography. Science 324, 1679–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai T, and Spradling AC (2004). Differentiating germ cells can revert into functional stem cells in Drosophila melanogaster ovaries. Nature 428, 564–569. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Takehashi M, Takashima S, Lee J, Morimoto H, Chuma S, Raducanu A, Nakatsuji N, Fässler R, and Shinohara T (2008). Homing of mouse spermatogonial stem cells to germline niche depends on beta1-integrin. Cell Stem Cell 3, 533–42. [DOI] [PubMed] [Google Scholar]
- Karlsson D, Baumgardt M, Thor S (2010). Segment-specific neuronal subtype specification by the integration of anteroposterior and temporal cues. PLoS Biol. 8, e1000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpowicz P, Perez J, and Perrimon N (2010). The Hippo tumor suppressor pathway regulates intestinal stem cell regeneration. Development 137, 4135–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawase E, Wong MD, Ding BC, and Xie T (2004). Gbb/Bmp signaling is essential for maintaining germline stem cells and for repressing bam transcription in the Drosophila testis. Development 131, 1365–1375. [DOI] [PubMed] [Google Scholar]
- Kim JY, Lee YC, and Kim C (2010). Direct inhibition of Pumilo activity by Bam and Bgcn in Drosophila germ line stem cell differentiation. J Biol Chem. 285, 4741–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirilly D and Xie T (2007). The Drosophila ovary: an active stem cell community. Cell Res. 17, 15–25. [DOI] [PubMed] [Google Scholar]
- Klein AM, Nakagawa T, Ichikawa R, Yoshida S, and Simons BD (2010). Mouse germ line stem cells undergo rapid and stochastic turnover. Cell Stem Cell 7, 214–24. [DOI] [PubMed] [Google Scholar]
- LaFever L, Feoktistov A, Hsu HJ, and Drummond-Barbosa D (2010). Specific roles of Target of rapamycin in the control of stem cells and their progeny in the Drosophila ovary. Development 137, 2117–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leatherman JL, and Dinardo S (2008). Zfh-1 controls somatic cell self-renewal in the Drosophila testis and nonautonomously influences germline stem cell self-renewal., Cell Stem Cell 3, 44–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leatherman JL, and Dinardo S (2010). Germline self-renewal requires cyst stem cells and stat regulates niche adhesion in Drosophila testes. Nature Cell Biol. 12, 806–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Zhou L, Kim J, Kalbfleisch S, and Schock F (2008). Lasp anchors the Drosophila male stem cell niche and mediates spermatid individualization. Mech. Dev 125, 768–776 [DOI] [PubMed] [Google Scholar]
- Lee WC, Beebe K, Sudmeier L, and Micchelli CA (2009). Adenomatous polyposis coli regulates Drosophila intestinal stem cell proliferation. Development 136, 2255–2264. [DOI] [PubMed] [Google Scholar]
- Li X, Han Y, and Xi R (2010). Polycomb group genes Psc and Su(z)2 restrict follicle stem cell self-renewal and extrusion by controlling canonical and noncanonical Wnt signaling. Genes Dev. 24, 933–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Minor NT, Park JK, McKearin DM, and Maines JZ (2009). Bam and Bgcn antagonize Nanos-dependent germ-line stem cell maintenance. Proc. Natl. Acad. Sci. USA 106, 9304–9309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lighthouse D, Buszczak M and Spradling AC (2008) New components of the Drosophila fusome suggest it plays novel roles in signaling and transport. Dev. Biol 217, 59–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin G, Xu N, and Xi R (2008). Paracrine Wingless signalling controls self-renewal of Drosophila intestinal stem cells. Nature 455, 1119–1123. [DOI] [PubMed] [Google Scholar]
- Liu N, Han H, and Lasko P (2009). Vasa promotes Drosophila germline stem cell differentiation by activating mei-P26 translation by directly interacting with a (U)-rich motif in its 3’ UTR. Genes and Dev. 23, 2742–2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Lim TM, and Cai Y (2011). The Drosophila female germline stem cell lineage acts to spatially restrict Dpp function within the niche. Sci Signal 3, ra57. [DOI] [PubMed] [Google Scholar]
- López-Oneiva L, Fernandez-Minan A, and Gonzalez-Reyes A (2008). Jak/Stat signalling in niche support cells regulates dpp transcription to contol germline stem cell maintenance in the Drosophila ovary. Development 135, 533–540. [DOI] [PubMed] [Google Scholar]
- Margolis J and Spradling AC (1995). Identification and behavior of epithelial stem cells in the Drosophila ovary. Development 121, 3797–3807. [DOI] [PubMed] [Google Scholar]
- Mathur D, Bost A, Driver I, and Ohlstein B (2010). A transient niche regulates the specification of Drosophila intestinal stem cells. Science 327, 210–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod CJ, Wang L, Wong C, and Jones DL (2010). Stem cell dynamics in response to nutrient availabililty. Curr. Biol 20, 2100–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk AC, Siddall NA, Volk T, Fraser B, Quinn LM, McLaughlin EA, and Hime GR (2010). HOW is required for stem cell maintenance in the Drosophila testis and for the onset of transit-amplifying divisions. Cell Stem Cell 6, 348–60. [DOI] [PubMed] [Google Scholar]
- Morris L, and Spradling AC (2011). Long-term live imaging provides new insight into stem cell regulation and germline-soma coordination in the Drosophila ovary. Development 138, 2207–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison S, and Spradling AC (2008). Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell 132, 598–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray SM, Yang SY, and Van Doren M (2010). Germ cell sex determination: a collaboration between soma and germline. Curr. Opin. Cell Biol 22, 722–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Nabeshima Y, and Yoshida S (2007). Functional identification of the actual and potential stem cell compartments in mouse spermatogenesis. Dev. Cell 12, 195–206. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Sharma M, Nabeshima Y, Braun RE, and Yoshida S (2010). Functional hierarchy and reversibility within the murine spermatogenic stem cell compartment. Science 328, 62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narbonne-Reveau K, Besse F, Lamour-Isnard C, Busson D and Pret A-M (2006). fused regulates germline cyst mitosis and differentiation during Drosophila oogenesis. Mech. Dev 123, 197–209. [DOI] [PubMed] [Google Scholar]
- Neumüller RA, Betschinger J, Fischer A, Bushati N, Poernbacher I, Mechtler K, Cohen SM, and Knoblich JA (2008). Mei-P26 regulates microRNAs and cell growth in the Drosophila ovarian stem cell lineage. Nature 454, 241–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nystul T, and Spradling AC (2007). An epithelial niche in the Drosophila ovary undergoes long range stem cell replacement. Cell Stem Cell 1, 277–285. [DOI] [PubMed] [Google Scholar]
- Nystul T and Spradling A, (2010) Regulation of epithelial cell replacement and follicle formation in the Drosophila ovary. Genetics 184, 503–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oatley MJ, Racicot KE, and Oatley JM (2011). Sertoli cells dictate spermatogonial stem cell niches in the mouse testis. Biol Reprod. 84, 639–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oatley JM, Kaucher AV, Avarbock MR, and Brinster RL (2010). Regulation of mouse spermatogonial stem cell differentiation by STAT3 signaling. Biol Reprod. 83, 427–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oatley JM, and Brinster RL (2008). Regulation of spermatogonial stem cell self-renewal in mammals. Annu. Rev. Cell Dev. Biol 24, 263–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlstein B, and McKearin D. (1997). Ectopic expression of the Drosophila Bam protein eliminates oogenic germline stem cells. Development 124, 3651–62. [DOI] [PubMed] [Google Scholar]
- Ohlstein B, and Spradling AC (2007). Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential Notch signaling. Science 315, 988–992. [DOI] [PubMed] [Google Scholar]
- O’Reilly AM, Lee HH, and Simon MA (2008). Integrins control the positioning and proliferation of follicle stem cells in the Drosophila ovary. J. Cell Biol 182, 801–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page SL, McKim KS, Deneen B, Van Hook TL, and Hawley RS (2000). Genetic studies of mei-P26 reveal a link between the processes that control germ cell proliferation in both sexes and those that control meiotic exchange in Drosophila. Genetics 155, 1757–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan L, Chen S, Weng C, Call G, Zhu D, Tang H, Zhang N, and Xie T (2007). Stem cell aging is controlled both intrinsically and extrinsically in the Drosophila ovary. Cell Stem Cell 1, 470–478. [DOI] [PubMed] [Google Scholar]
- Park JK, Liu X, Strauss TJ, McKearin DM, and Liu Q (2007) The miRNA pathway intrinsically controls self-renenwal of Drosophila germline stem cells. Current Biology 17, 533–538. [DOI] [PubMed] [Google Scholar]
- Pearson J, Lopez-Onieva L, Rojas-Rios P, Gonzalez-Reyes A (2009). Recent advances in Drosophila stem cell biology. Int. J. Dev. Biol 53, 1329–1339. [DOI] [PubMed] [Google Scholar]
- Pek JW, Lim AK, and Kai T (2009). Drosophila maelstrom ensures proper germline stem cell lineage differentiation by repressing microRNA-7. Dev. Cell 3, 417–424. [DOI] [PubMed] [Google Scholar]
- Pitsouli C, Apidianakis Y, and Perrimon N (2009). Homeostasis in infected epithelia: stem cells take the lead. Cell Host Microbe 6, 301–307. [DOI] [PubMed] [Google Scholar]
- Qi H, Watanabe T, Ku HY, Liu N, Zhong M, and Lin H (2011). The Yb body, a major site for Piwi-associated RNA biogenesis and a gateway for Piwi expression and transport to the nucleus in somatic cells. J Biol. Chem 286, 3789–3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quyn AJ, Appleton PL, Carey FA, Steele RJ, Barker N, Clevers H, Ridgway RA, Sansom OJ, and Näthke IS (2010). Spindle orientation bias in gut epithelial stem cell compartments is lost in precancerous tissue. Cell Stem Cell. 6, 175–181. [DOI] [PubMed] [Google Scholar]
- Ren F, Wang B, Yue T, Yun EY, Ip YT, and Jiang J (2010). Hippo signaling regulates Drosophila intestine stem cell proliferation through multiple pathways. Proc. Natl. Acad. Sci. USA 107, 21064–21069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhiner C, Díaz B, Portela M, Poyatos JF, Fernández-Ruiz I, López-Gay JM, Gerlitz O, and Moreno E (2009). Persistent competition among stem cells and their daughters in the Drosophila ovary germline niche. Development 136, 995–1006. [DOI] [PubMed] [Google Scholar]
- Rouget C, Papin C, Boureux A, Meunier AC, Franco B, Robine N, Lai EC, Pelisson A, and Simonelig M (2010). Maternal mRNA deadenylation and decay by the piRNA pathway in the early Drosophila embryo. Nature 467, 1128–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sada A, Suzuki A, Suzuki H, and Saga Y (2009). The RNA-binding protein Nanos2 Is required to maintain murine spermatogonial stem cells. Science 325, 1394–1398. [DOI] [PubMed] [Google Scholar]
- Sarkar A, Parikh N, Hearn SA, Fuller MT, Tazuke SI, and Schulz C (2007). Antagonistic roles of Rac and Rho in organizing the germ cell microenvironment. Curr Biol. 17, 1253–1258. [DOI] [PubMed] [Google Scholar]
- Saito K, Ishizu H, Komai M, Kotani H, Kawamura Y, Nishida KM, Siomi H, and Siomi MC (2010). Roles for the Yb body components Armitage and Yb in primary piRNA biogenesis in Drosophila. Genes Dev. 24, 2493–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, and Clevers H (2011). Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469, 415–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, and Clevers H (2009). Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262–265. [DOI] [PubMed] [Google Scholar]
- Schultz C, Wood CG, Jones DL, Tazuke SI and Fuller MT (2002). Signaling from germ cells mediated by the rhomboid homolog stet organizes encapsulation by somatic support cells. Development 129, 4523–34. [DOI] [PubMed] [Google Scholar]
- Shaw RL, Kohlmaier A, Polesello C, Veelken C, Edgar BA, and Tapon N (2010). The Hippo pathway regulates intestinal stem cell proliferation during Drosophila adult midgut regeneration. Development 137, 4147–4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen R, Weng C, Yu J, and Xie T (2009). eIF4A controls germline stem cell self-renewal by directly inhibiting Bam function in the Drosophila ovary. Proc. Natl. Acad. Sci 106, 11623–11628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng XR, Brawley CM and Matunis EL (2009). Dedifferentiating spermatogonia outcompete somatic stem cells for niche occupancy in the Drosophila testis. Cell Stem Cell 5, 191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skora AD, and Spradling AC (2010). Epigenetic stability increases extensively during Drosophila follicle stem cell differentiation. Proc. Natl. Acad. Sci. USA 107, 7389–7394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smulders-Srinivasan TK, Szakmary A, and Lin H (2010) A Drosophila chromatin factor interacts with the Piwi-interacting RNA mechanism in niche cells to regulate germline stem cell self-renewal., Genetics 186, 573–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snippert HJ, van der Flier LG, Sato T, van Es JH, van den Born M, Kroon-Veenboer C, Barker N, Klein AM, van Rheenen J, Simons BD, and Clevers H (2010). Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell 143, 134–144. [DOI] [PubMed] [Google Scholar]
- Song X, and Xie T (2002). DE-cadherin-mediated cell adhesion is essential for maintaining somatic stem cells in the Drosophila ovary. Proc. Natl. Acad. Sci. USA 99, 14813–14818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Zhu CH, Doan C, and Xie T (2002). Germline stem cells anchored by adherens junctions in the Drosophila ovary niches. Science 296, 1855–1857. [DOI] [PubMed] [Google Scholar]
- Staley BK, and Irvine KD (2010). Warts and Yorkie mediate intestinal regeneration by influencing stem cell proliferation. Curr Biol. 20, 1580–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui L, and Yang Y (2011). Distinct effects of nuclear membrane localization on gene transcription silencing in Drosophila S2 cells and germ cells. J. Genet. Genomics 38, 55–61. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Sada A, Yoshida S & Saga Y 2009. The heterogeneity of spermatogonia is revealed by their topology and expression of marker proteins including the germ cell-specific proteins Nanos2 and Nanos3. Dev. Biol 336, 222–231. [DOI] [PubMed] [Google Scholar]
- Szakmary A, Reedy M, Qi H, and Lin H (2008). The Yb protein defines a novel organelle and regulates male germline stem cell self-renewal in Drosophila melanogaster. J. Cell Biol 185, 613–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima S, Adams KL, Ortiz PA, Ying CT, Moridzadeh R, Younossi-Hartenstein A, and Hartenstein V (2011). Development of the Drosophila entero-endocrine lineage and its specification by the Notch signaling pathway. Dev Biol. 353, 161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanentzapf G, Davenport D, Godt D, and Brown NH (2007) Integrin-dependent anchoring of a stem-cell niche. Nature Cell Biol. 12, 1413–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueishi S, Shimizu H,. and Inoue Y (2009). Male germline stem cell division and spermatocyte growth require insulin signaling in Drosophila. Cell Struct. Funct 34, 61–69. [DOI] [PubMed] [Google Scholar]
- Voog J, D’Alterio C, and Jones DL (2008). Multipotent somatic stem cells contribute to the stem cell niche in the Drosophila testis. Nature 454, 1132–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voog J, and Jones DL Stem cells and the niche: a dynamic duo. Cell Stem Cell 6, 103–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, and Jones DL (2010). The effects of aging on stem cell behavior in Drosophila. Exp Gerontol. 46, 340–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Li Z, and Cai Y (2008). The JAK/STAT pathway positively regulates Dpp signaling in the Drosophila germline stem cell niche. J Cell Biol 180, 721–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Harris RE, Bayston LJ, and Ashe HL (2008). Type IV collagens regulate Bmp signaling in Drosophila. Nature. 455, 72–77. [DOI] [PubMed] [Google Scholar]
- Wang ZA, and Kalderon D (2009). Cyclin E-dependent protein kinase activity regulates niche retention of Drosophila ovarian follicle stem cells. Proc. Natl. Acad. Sci. USA 106, 21701–21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi R, and Xie T (2005). Stem cell self-renewal controlled by chromatin remodeling factors. Science 310, 1487–1489. [DOI] [PubMed] [Google Scholar]
- Xia L, Jia S, Huang S, Wang H, Zhu Y, Mu Y, Kan L, Zheng W, Wu D, Li X, Sun Q, Meng A, and Chen D (2010). The Fused/Smurf complex controls the fate of Drosophila germline stem cells by generating a gradient Bmp response. Cell 143, 978–990. [DOI] [PubMed] [Google Scholar]
- Xie T, and Spradling AC (2000). A niche maintaining germ line stem cells in the Drosophila ovary. Science 290, 328–330. [DOI] [PubMed] [Google Scholar]
- Yan D, Wu Y, Yang Y, Belenkaya TY, Tang X, and Lin H (2010). The cellsurface proteins Dally-like and Ihog differentially regulate Hedgehog signaling strength and range during development. Development 137, 2033–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Chen D, Duan R, Xia L, Wang J, Qurashi A, Jin P,. and Chen D (2007). Argonaute 1 regulates the fate of germline stem cells in Drosophila. Development 134, 4265–4672. [DOI] [PubMed] [Google Scholar]
- Yamashita YM, Jones DL, and Fuller MT (2003). Orientation of asymmetric stem cell division by the APC tumor suppressor and centrosome. Science 301, 1547–1550. [DOI] [PubMed] [Google Scholar]
- Yang Y, Xu S, Xia L, Wang J, Wen S, Jin P, and Chen D (2009). The Bantam microRNA is associated with Drosophila Fragile X Mental Retardation Protein and Regulates the Fate of Germline Stem Cells. PLoS Genet. 5, e1000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H, and Lin H. (2007). An epigenetic activation role of Piwi and a Piwi-associated piRNA in Drosophila melanogaster. Nature 450, 304–8. [DOI] [PubMed] [Google Scholar]
- Yoon J, Lee KS, Park JS, Yu K, Paik SG, and Kang YK (2008). dSETDB1 and SU(VAR)3–9 sequentially function during germline-stem cell differentiation in Drosophila melanogaster. PLoS One 3, e2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S (2010). Stem cells in mammalian spermatogenesis. Dev Growth Differ. 52, 311–317. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Sukeno M, and Nabeshima Y (2007). A vasculature-associated niche for undifferentiated spermatogonia in the mouse testis. Science 317, 1722–1726. [DOI] [PubMed] [Google Scholar]
- Zhao R, and Xi R (2010). Stem cell competition for niche occupancy: emerging themes and mechanisms. Stem Cell Rev. 6, 345–350. [DOI] [PubMed] [Google Scholar]
- Zheng X, Mann RK, Sever N, and Beachy PA. (2010). Genetic and biochemical definition of the Hedgehog receptor. Genes Dev. 24, 57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]