Abstract
The objective of this study was to evaluate the cost-effectiveness of 18F-choline PET/multiparametric MRI (mpMRI) versus mpMRI alone for the detection of primary prostate cancer with a Gleason score of greater than or equal to 3 + 4 in men with elevated prostate-specific antigen levels. Methods: A Markov model of prostate cancer onset and progression was used to estimate the health and economic consequences of 18F-choline PET/mpMRI for the detection of primary prostate cancer with a Gleason score of greater than or equal to 3 + 4 in men with elevated prostate-specific antigen levels. Multiple simultaneous hybrid 18F-choline PET/mpMRI strategies were evaluated using Likert or Prostate Imaging Reporting and Data System version 2 (PI-RADSv2) scoring; the first was biopsy for Likert 5 mpMRI lesions or Likert 3–4 lesions with 18F-choline target-to-background ratios of greater than or equal to 1.58, and the second was biopsy for PI-RADSv2 5 mpMRI lesions or PI-RADSv2 3–4 mpMRI lesions with 18F-choline target-to-background ratios of greater than or equal to 1.58. These strategies were compared with universal standard biopsy, mpMRI alone with biopsy only for PI-RADSv2 3–5 lesions, and mpMRI alone with biopsy only for Likert 4–5 lesions. For each mpMRI strategy, either no biopsy or standard biopsy could be performed after negative mpMRI results were obtained. Deaths averted, quality-adjusted life years (QALYs), cost, and incremental cost-effectiveness ratios were estimated for each strategy. Results: When the results of 18F-choline PET/mpMRI were negative, performing a standard biopsy was more expensive and had lower QALYs than performing no biopsy. The best screening strategy among those considered in this study performed hybrid 18F-choline PET/mpMRI with Likert scoring on men with elevated PSA, performed combined biopsy (targeted biopsy and standard 12-core biopsy) for men with positive imaging results, and no biopsy for men with negative imaging results ($22,706/QALY gained relative to mpMRI alone); this strategy reduced the number of biopsies by 35% in comparison to mpMRI alone. When the same policies were compared using PI-RADSv2 instead of Likert scoring, hybrid 18F-choline PET/mpMRI cost $46,867/QALY gained relative to mpMRI alone. In a threshold analysis, the best strategy among those considered remained cost-effective when the sensitivity and specificity of PET/mpMRI and combined biopsy (targeted biopsy and standard 12-core biopsy) were simultaneously reduced by 20 percentage points. Conclusion: 18F-choline PET/mpMRI for the detection of primary prostate cancer with a Gleason score of greater than or equal to 3 + 4 is cost-effective and can reduce the number of unneeded biopsies in comparison to mpMRI alone.
Keywords: PET/MRI, prostate cancer, cost-effectiveness analysis, prostate biopsy, Markov model
Early detection of prostate cancer via prostate-specific antigen (PSA) screening is controversial, in large part because of the poor predictive performance of PSA—the most commonly used biomarker (1). The poor sensitivity and specificity of PSA are associated with unnecessary biopsies, overdiagnosis, and overtreatment (1). As a result, some have called for the elimination of PSA screening (2), whereas others have called for more careful use of PSA screening in a shared decision-making framework (3).
Evidence for the cost-effective use of multiparametric prostate MRI (mpMRI) for the early detection of prostate cancer has developed in recent years (4,5). The benefits of mpMRI over PSA testing alone are due to the higher sensitivity and specificity of mpMRI for clinically important prostate cancer (i.e., Gleason score of ≥3 + 4). However, mpMRI is not perfect. In particular, the specificity of mpMRI for low- and intermediate-risk lesions is relatively low, resulting in negative biopsy results in a large fraction of patients (6,7). If a technique existed to mitigate these false-positive results, it would substantially mitigate biopsy-related costs and complications. However, there is no established method for doing so within an mpMRI-only framework.
There is growing evidence that PET/mpMRI using radiolabeled choline (8) or prostate-specific membrane antigen ligands (9) might address this issue by improving diagnostic accuracy and reducing false-positive findings at mpMRI. A prospective trial studying the addition of 18F-choline PET to mpMRI demonstrated a significant improvement in diagnostic accuracy for the detection of prostate cancer with a Gleason score of greater than or equal to 3 + 4 over mpMRI alone (8,10), with the primary effect being a reduction in false-positive results for mpMRI-identified intermediate-risk lesions. However, combining PET with mpMRI raises the cost of imaging and may negate the incremental cost-effectiveness imparted by the improved diagnosis. Here, we present a cost-effectiveness analysis investigating deaths averted, quality-adjusted life years (QALYs), cost, and incremental cost-effectiveness ratios (ICERs) for standard of care (i.e., universal standard biopsy, mpMRI-directed strategies) versus 18F-choline PET/mpMRI strategies for the early detection of clinically important prostate cancer. The study purpose was to evaluate the cost-effectiveness of 18F-choline PET/mpMRI versus mpMRI alone for the detection of primary prostate cancer with a Gleason score of greater than or equal to 3 + 4 in men with elevated PSA levels.
MATERIALS AND METHODS
We extended a previously published partially observable Markov model (4,11) for the early detection of prostate cancer to estimate outcomes from 18F-choline PET/mpMRI used to stratify patients with elevated PSA levels before they receive a biopsy (ClinicalTrials.gov NCT01751737). This model was recently used to estimate the cost-effectiveness of mpMRI alone (4). The extended Markov model (detailed in Supplemental Appendix 1 [supplemental materials are available at http://jnm.snmjournals.org]) includes 5 pretreatment states that are not directly observable, including no prostate cancer, organ-confined prostate cancer (Gleason score <7, =7, or >7), and extraprostatic or lymph node–positive prostate cancer. The model (implemented in C/C++ [3.40-GHz processor; 16 GB of random-access memory]) simulates the onset and progression of prostate cancer from age 40 until the end of life from any cause and has been validated by Barnett et al. (11).
Model Screening Strategies
The screening strategies are defined in Table 1. For each strategy, we used 10,000,000 samples of biopsy-naive men who were screened every 2 y from age 55 to age 69 in accordance with the American Urological Association guideline (3). In strategy 1, a standard biopsy was recommended for all patients with elevated PSA levels (>4 ng/mL). Strategies 2–9 recommended mpMRI with or without 18F-choline PET/mpMRI for patients with elevated PSA levels (>4 ng/mL). In strategies 2 and 6, mpMRI was used alone and combined biopsy (targeted biopsy and standard 12-core biopsy) was performed on all patients with Likert 4–5 lesions on mpMRI. In strategies 3 and 7, mpMRI was used alone and combined biopsy was performed on all patients with Prostate Imaging Reporting and Data System version 2 (PI-RADSv2) 3–5 lesions on mpMRI. In strategies 4 and 8, hybrid 18F-choline PET/mpMRI was used and combined biopsy was performed on all patients with Likert 5 lesions on mpMRI or an elevated 18F-choline target-to-background ratio (TBR) (≥1.58) of Likert 3–4 lesions on mpMRI. In strategies 5 and 9, hybrid 18F-choline PET/mpMRI was used and combined biopsy was performed on all patients with PI-RADSv2 5 lesions on mpMRI or an elevated 18F-choline TBR (≥1.58) in PI-RADSv2 3–4 lesions on mpMRI. Strategy pairs with the same imaging approach varied by the decision to perform standard biopsy (strategies 2–5) or no biopsy (strategies 6–9) after negative imaging results were obtained (Table 1). Our model focuses on initial biopsy decisions; thus, the screening strategy terminates after the patient receives an initial biopsy or 2 negative imaging results. However, the patient continues to make state transitions in the absence of screening until all-other-cause mortality or mortality from prostate cancer after clinical detection.
TABLE 1.
Definitions of Screening Strategies
| Screening strategy designation | Screening strategy description | Definition of positive imaging result | After positive imaging results | After negative imaging results |
| 1 | Standard biopsy | N/A (no imaging) | N/A; always standard biopsy | N/A; always standard biopsy |
| 2 | mpMRI alone (Likert scores) | Likert 4–5 | Combined biopsy | Standard biopsy |
| 3 | mpMRI alone (PI-RADS scores) | PI-RADSv2 3–5 | Combined biopsy | Standard biopsy |
| 4 | 18F-choline PET/mpMRI (Likert scores) | Likert 5 mpMRI lesion or Likert 3–4 mpMRI lesion with 18F-choline TBR of ≥1.58* | Combined biopsy | Standard biopsy |
| 5 | 18F-choline PET/mpMRI (PI-RADS scores) | PI-RADS 5 mpMRI lesion or PI-RADS 3–4 mpMRI lesion with 18F-choline TBR of ≥1.58 | Combined biopsy | Standard biopsy |
| 6 | mpMRI alone (Likert scores) | Likert 4–5 | Combined biopsy | No biopsy |
| 7 | mpMRI alone (PI-RADS scores) | PI-RADS 3–5 | Combined biopsy | No biopsy |
| 8 | 18F-choline PET/mpMRI (Likert scores) | Likert 5 mpMRI lesion or Likert 3–4 mpMRI lesion with 18F-choline TBR of ≥1.58 | Combined biopsy | No biopsy |
| 9 | 18F-choline PET/mpMRI (PI-RADS scores) | PI-RADS 5 mpMRI lesion or PI-RADS 3–4 mpMRI lesion with 18F-choline TBR of ≥1.58 | Combined biopsy | No biopsy |
TBR threshold was defined in study by Piert et al. (8).
N/A = not applicable.
Model Assumptions: Detection of Prostate Cancer
The model is made up of discrete health states that are based on the Gleason score, that are not directly observable, but that can be inferred from PSA and PET/mpMRI subject to published estimates of sensitivity and specificity. In our model, we considered clinically important disease to have any Gleason score of greater than or equal to 3 + 4. For standard biopsy, the results were randomly sampled as either positive or negative for any prostate cancer assuming a sensitivity of 80.0% (2). If the biopsy result was positive, then the probability that the biopsy provided an incorrect grading at diagnosis was based on data reported by Epstein et al. (12). The sensitivity and specificity of combined biopsy (targeted biopsy and standard 12-core biopsy) for clinically important cancer (Gleason score of ≥3 + 4) were considered to be 85.0% and 49.0%, respectively (13). On the basis of Medicare infection rates, 1.1% of biopsies led to hospitalization for postbiopsy infection (14,15).
In addition to the detection of prostate cancer through routine screening, the model incorporated the clinical detection of symptomatic prostate cancer. For each patient, we randomly sampled a lead time from an elevated PSA measurement of greater than or equal to 3 ng/mL to a clinical diagnosis of prostate cancer from a distribution developed by Savage et al. (16). If a patient had prostate cancer and a PSA level of greater than or equal to 3 ng/mL (16) and had not yet been diagnosed with prostate cancer in the model during the lead time, then it was assumed that the patient’s cancer was later clinically detected on the basis of symptoms.
Model Assumptions: PSA and mpMRI Sensitivity and Specificity
A published statistical model was used to sample age-dependent and cancer onset–dependent PSA scores (17). mpMRI results were based on either Likert scoring (from 1 to 5) or PI-RADSv2 scoring (from 1 to 5), with an increasing score indicating an increasing likelihood of clinically important cancer. Likert and PI-RADSv2 scores of 1 and 2 were treated as negative. Likert and PI-RADSv2 scores of 3 were treated as low risk, Likert and PI-RADSv2 scores of 4 were treated as intermediate risk, and Likert and PI-RADSv2 scores of 5 were treated as high risk. Likert scoring was tested because it was used in the study of Davenport et al. (10). PI-RADSv2 scoring was tested because it is the current standard of care. The 18F-choline PET/mpMRI results in the model were based on data from a prospective clinical trial of 56 patients who had elevated PSA levels and who underwent 18F-choline PET/mpMRI (10). Hybrid 18F-choline PET/mpMRI was considered to be without gadolinium-containing contrast material (i.e., no dynamic contrast-enhanced imaging). The sensitivities and specificities shown in Table 2 are consistent with other published estimates of the sensitivity and specificity of mpMRI (6,7). For example, the sensitivity and specificity of mpMRI presented in the PROMIS trial, 93% and 41%, respectively (7), fall within the 95% CIs used for the sensitivity analysis in our study (Table 2).
TABLE 2.
Sensitivity and Specificity of mpMRI and 18F-Choline PET/mpMRI Techniques for Detection of Gleason Score ≥3 + 4 Cancers
| Technique | Sensitivity* | Specificity* | Source |
| mpMRI alone (Likert 4–5) | 85.19 (66.27–95.81) | 55.17 (35.69–73.55) | mpMRI base case (Likert 4–5) from Davenport et al. (10) |
| mpMRI alone (PI-RADSv2 3–5) | 92.59 (75.71–99.09) | 58.62 (38.94–76.48) | mpMRI base case (PI-RADSv2 3–5) from Davenport et al. (10) |
| 18F-choline PET/mpMRI (Likert) | 92.59 (75.71–99.09) | 93.10 (77.23–99.15) | Model L1 from Davenport et al. (10) |
| 18F-choline PET/mpMRI (PI-RADSv2) | 88.89 (70.84–97.65) | 93.10 (77.23–99.15) | Model P1 from Davenport et al. (10) |
Reported as percentage, with 95% CI in parentheses.
Model Assumptions: Prostate Cancer Staging and Treatment
Patients with PSA levels of greater than 20 ng/mL or a Gleason score of greater than or equal to 4 + 4 received a bone scan and a CT scan for staging (18,19). Patients who had biopsy-detected prostate cancer with a Gleason score of greater than or equal to 3 + 4 received radical prostatectomy. On the basis of practice patterns reported by Liu et al. (20), 48.5% of patients diagnosed with prostate cancer with a Gleason score of 3 + 3 received active surveillance, whereas the remaining patients received prostatectomy. If prostate cancer was clinically detected in a patient after age 80, then we assumed that the patient received watchful waiting.
Men on active surveillance received an annual PSA test and a biopsy every 2 y and continued to progress through the natural history of the disease. If any biopsy indicated progression in the Gleason score, then the patient received prostatectomy. Similar to past studies (4,11), for men with no indication of progression within 10 y, survival was assumed to be consistent with survival for men with untreated prostate cancer (11). Men treated via prostatectomy had survival consistent with a treated population (21), with the potential for progression to metastatic prostate cancer and prostate cancer mortality. Other-cause mortality was based on estimates from Center for Disease Control and Prevention life tables (22).
Cost-Effectiveness
We estimated the difference in costs and QALYs for each of the 9 screening strategies on the basis of cost and QALY estimates in Table 3 using a third-party payer perspective and assumptions similar to those of previous studies (4,11,23–25). The difference in cost between mpMRI and 18F-choline PET/mpMRI was $449.92 for hybrid PET/mpMRI (Table 3). Because there is not yet a defined reimbursement for PET/mpMRI, we estimated the cost for prostate PET/mpMRI using the Medicare cost for 18F-FDG PET/CT (i.e., $1,414). Applying the cost for 18F-FDG is justified because the production costs for 18F-choline and 18F-FDG are similar. The Medicare cost for prostate mpMRI without gadolinium-containing contrast material is $964.21. Data have indicated that biparametric MRI is not inferior to mpMRI for prostate cancer detection (26). Furthermore, when mpMRI is combined with PET, 18F-choline improves specificity well beyond the limited contribution of dynamic contrast-enhanced imaging (10).
TABLE 3.
Costs and Annual Disutilities for Health States Considered in Our Cost-Effectiveness Analysis
| Intervention | Unit costs in USD ($) | Source | Annual disutility (range) |
| PSA screening | 33.86 | Medicare | |
| mpMRI | 964.21 | Medicare | |
| Hybrid 18F-choline PET/mpMRI | 1,414.13 | Medicare | |
| Standard prostate biopsy* | 2,953.67 | Medicare | |
| Combined prostate biopsy† | 3,018.35 | Medicare | |
| Post-biopsy infection-related hospitalization | 6,361.31 | (36) (15) | |
| Staging | 1,059.28 | Medicare | |
| Active surveillance – standard biopsy (per year)‡ | 1,642.58 | Medicare | |
| Active surveillance – combined biopsy (per year)‡ | 1,674.92 | Medicare | |
| Prostatectomy | 15,752.37 | (23) | |
| Distant-stage initial treatment | 17,831.29 | (24) | |
| Distant-stage management (per year) | 2,500.65 | (24) | |
| Other cause of death | 5,975.15 | (37) | |
| Prostate cancer death (age <65) | 103,884.24 | (37) | |
| Prostate cancer death (age ≥65) | 69,256.16 | (37) | |
| Health state | |||
| PSA screening | (25) | 0.00019 (0.0–0.00019) | |
| PET/mpMRI | (28) (25) | 0.00077 (0.00038–0.0012) | |
| Biopsy | (25) | 0.00577 (0.00346–0.0075) | |
| Postbiopsy infection | (27) (25) | 0.0161 (0.00969–0.0291) | |
| Diagnosis | (25) | 0.0167 (0.0125–0.0208) | |
| Prostatectomy | (25) | 0.247 (0.0917–0.323) | |
| Postprostatectomy recovery | (25) | 0.05 (0.0–0.07) | |
| Active surveillance | (25) | 0.03 (0.0–0.15) | |
| Palliative therapy | (25) | 0.4 (0.14–0.76) | |
| Terminal illness | (25) | 0.3 (0.3–0.38) |
Includes professional, technical, and facility fees; pathology costs; and office visit.
Includes professional, technical, and facility fees; pathology costs; office visit; and 3-dimensional reconstruction.
Assumed to include an annual office visit, annual PSA test, and biopsy every two years
The postrecovery period for prostatectomy was assumed to last 9 y (25). Li et al. reported the disutility for hospitalization because of postbiopsy infection to be 0.28 (27), which we assumed lasted for 3 wk (25). Grann et al. reported the disutility for mpMRI to be 0.04 (28), which we assumed lasted for 1 wk (25).
Future costs and QALYs were discounted to net present value using an annual discount rate of 3% (29). We removed dominated strategies (i.e., strategies that were more expensive or less effective than another strategy) as well as strategies ruled out by extended dominance (i.e., strategies that had higher ICERs than a combination of 2 or more effective strategies) (29). The ICERs of the efficient policies were calculated as the incremental costs divided by the incremental health gains in comparison to the next most effective strategy. Consistent with standard cost-effectiveness analysis procedures, we considered the strategy to be cost-effective if the ICER was less than $100,000/QALY (30).
Sensitivity Analysis
A 1-way sensitivity analysis was performed on the net costs per QALY gained relative to no screening for the best screening strategy among those considered in this study (deemed the “best” strategy). The ranges used for the QALY disutilities are shown in Table 3. Cost estimates and other-cause mortality rates (22) were varied by ±20%. The sensitivity and specificity of mpMRI and PET/mpMRI results were varied using the 95% CIs reported in Table 2. The annual metastasis rate for patients with undiagnosed prostate cancer was varied within the 95% CI reported by Johansson et al. (31). Finally, we varied the annual prostate cancer incidence rate within the 95% CI reported by Haas et al. (2).
A threshold analysis was performed on the sensitivity and specificity of 18F-choline PET/mpMRI and combined biopsy for the “best” strategy. During the threshold analysis, we simultaneously reduced the sensitivity and specificity of hybrid PET/mpMRI and combined biopsy until it was no longer cost-effective to use hybrid 18F-choline PET/mpMRI for screening.
RESULTS
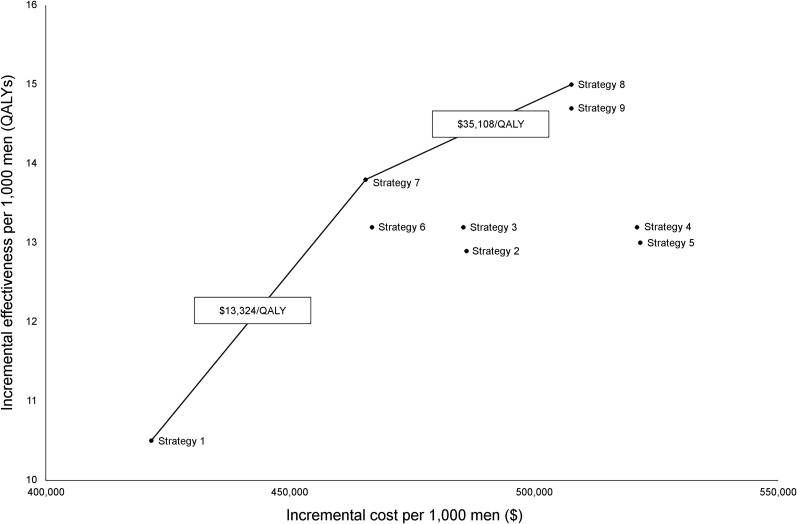
Figure 1 shows the incremental health benefits and costs per 1000 men for the alternative screening strategies relative to no screening. Points are labeled with the screening strategy. Lines connecting points representing 2 efficient screening strategies indicate the ICER. The “best” strategy was strategy 8 (hybrid 18F-choline PET/mpMRI with Likert scoring), with an ICER of $35,108/QALY and with $22,706/QALY gained relative to strategy 6 (mpMRI alone with biopsy for Likert 4–5 lesions). When the same policies were compared using PI-RADSv2 (strategies 7 and 9), hybrid 18F-choline PET/mpMRI cost $46,867/QALY gained relative to mpMRI alone when biopsy was performed for PI-RADS 3–5.
FIGURE 1.
Incremental health benefits and costs associated with alternative strategies relative to no screening. Costs and QALYs are discounted at rate of 3%. Each point is labeled with screening strategy (defined in Table 1). Lines connecting points representing 2 efficient screening strategies indicate ICER.
Strategies in which standard biopsy was performed after a negative imaging result (strategies 2–5) were dominated (i.e., were more expensive while resulting in fewer QALYs) by strategies in which no biopsy was performed after a negative imaging result (strategies 6–9). This finding suggests that performing a biopsy after a negative imaging result is more expensive and does not provide aggregate health benefits than performing no biopsy, despite the fact that imaging does not have 100% sensitivity for clinically important prostate cancer.
Table 4 shows the prostate cancer deaths averted, life-years gained, QALYs gained relative to no screening, and the number of biopsies performed for each strategy. The “best” strategy (strategy 8: hybrid 18F-choline PET/mpMRI with Likert scoring) maximized the number of QALYs gained while substantially reducing the number of needed biopsies in comparison to screening with PSA alone (strategy 1, by 44%) or mpMRI alone (strategy 6; by 35%).
TABLE 4.
Predicted Effects for Various Screening Strategies per 1,000 Men
| Screening strategy | Prostate cancer deaths averted* | Life-years gained* | QALYs gained* (95% confidence interval) | Number of biopsies |
| No screening | (reference) | (reference) | (reference) | 0.0 |
| Strategy 1 | 4.7 | 57.8 | 46.9 (45.9–47.9) | 151.0 |
| Strategy 2 | 5.6 | 69.0 | 57.0 (55.9–58.0) | 151.0 |
| Strategy 3 | 5.7 | 69.8 | 57.7 (56.6–58.8) | 151.0 |
| Strategy 4 | 5.6 | 68.6 | 56.9 (55.8–58.0) | 151.0 |
| Strategy 5 | 5.6 | 68.1 | 56.4 (55.4–57.5) | 151.0 |
| Strategy 6 | 5.6 | 68.6 | 57.3 (56.3–58.4) | 128.3 |
| Strategy 7 | 5.8 | 70.5 | 59.3 (58.2–60.3) | 127.3 |
| Strategy 8 | 5.7 | 69.4 | 60.4 (59.4–61.4) | 83.9 |
| Strategy 9 | 5.6 | 68.3 | 59.4 (58.4–60.4) | 83.2 |
Compared with no screening.
Screening strategies are defined in Table 1. Effects are shown without discount.
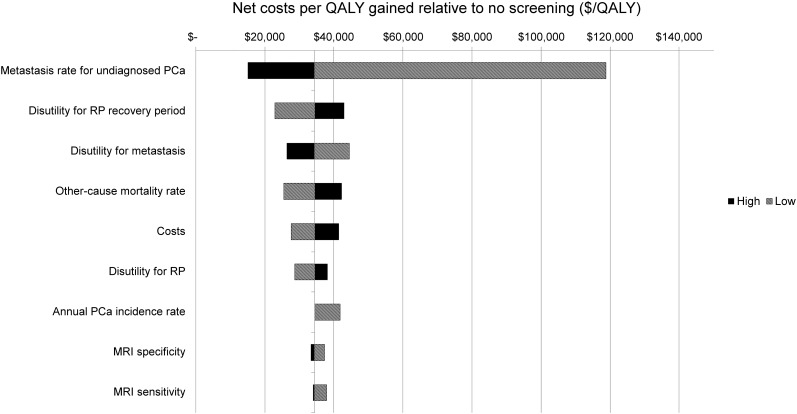
Figure 2 shows a 1-way sensitivity analysis of the net costs per QALY gained for strategy 8 relative to no screening. All model parameters were evaluated, but Figure 2 shows only the parameters for which the net costs varied by at least $3,500/QALY. The 3 model parameters that had the greatest impact were metastasis rate for undiagnosed prostate cancer, annual QALY disutility for the 9-y postprostatectomy recovery period, and annual QALY disutility for living with metastatic disease. In the sensitivity analysis, the only parameter estimate with a cost per QALY gained over $100,000 relative to no screening was a substantially lower risk of metastasis in comparison to the base case, suggesting that our results are robust for most patients and cost-effective under a willingness-to-pay threshold of $100,000/QALY. A threshold analysis showed that strategy 8 remained cost-effective when sensitivity and specificity for clinically important prostate cancer assessed by hybrid 18F-choline PET/mpMRI and combined biopsy were all simultaneously reduced by 20 percentage points. Specifically, it was still cost-effective when the sensitivity and specificity of hybrid PET/mpMRI were greater than or equal to 72.6% and greater than or equal to 73.1%, respectively, and when the sensitivity and specificity of combined biopsy were greater than or equal to 65.0% and greater than or equal to 29.0%, respectively. Furthermore, analysis for strategy 8 with all parameters at their minimum or respective maximum values resulted in a range of net costs per QALY from $32,754 for the low values to $39,749 for the high values. Thus, our main conclusions still hold under these extreme cases.
FIGURE 2.
Tornado diagram of 1-way sensitivity analysis of net costs per QALY gained of screening strategy 8 relative to no screening. Costs and QALYs are discounted at rate of 3%. PCa = prostate cancer; RP = radical prostatectomy.
DISCUSSION
We found that hybrid 18F-choline PET/mpMRI is more cost-effective than mpMRI alone for the detection of primary prostate cancer with a Gleason score of greater than or equal to 3 + 4 in men with elevated PSA levels. The “best” strategy involved combined biopsy (targeted biopsy and standard 12-core biopsy) for Likert 5 mpMRI lesions as well as for Likert 3–4 mpMRI lesions with an elevated 18F-choline TBR of greater than or equal to 1.58. When imaging results were negative, no biopsy was performed. The ICER for this strategy was $35,108/QALY, which is well below the commonly used willingness-to-pay threshold of $100,000/QALY. An additional sensitivity analysis showed that these results were robust in the presence of variations in all model parameters.
mpMRI was previously shown to be cost-effective for the early detection of clinically important prostate cancer (4,5,32). Although hybrid 18F-choline PET/mpMRI is more expensive than mpMRI alone (by $449.92, according to Medicare data), the increased accuracy of hybrid 18F-choline PET/mpMRI leads to better stratification of who should receive biopsy, allowing unneeded biopsies to be avoided in approximately 35% of cases in comparison to mpMRI alone. This factor contributes sufficient health benefits in terms of QALYs to justify the added cost based on standard estimates of willingness to pay in the United States. Prostate biopsies are stressful experiences that carry small but important risks of temporary and permanent harm (e.g., hospitalization and death due to sepsis).
18F-choline PET was previously explored for primary prostate cancer detection, with mixed results (33). Although the sensitivity generally has been reported to be high (73%–100%), the specificity has been shown to be limited (43%–86%). The reason is that, although there is increased uptake in clinically important cancer, there is also false-positive uptake in benign conditions, such as benign prostatic hyperplasia and prostatitis. Therefore, using 18F-choline PET alone for primary prostate cancer detection is not advised. However, when data from mpMRI are used for lesion selection and data from 18F-choline PET are used to stratify the risk of observed lesions, the diagnostic accuracy substantially improves (8,10)—likely because common confounders can be more readily identified with mpMRI. This approach enables the sensitivity of 18F-choline PET to be harnessed without the harm of its comparatively lower specificity. Although we explored 18F-choline in the present study, prostate-specific membrane antigen–based tracers may outperform 18F-choline in a hybrid PET/mpMRI context (9). Further studies are needed to determine whether that is the case.
The present study has limitations based on assumptions made during the modeling process. The sensitivity and specificity of 18F-choline PET/mpMRI were based on a prospective trial of 56 subjects who had 90 mpMRI-identified lesions and who underwent hybrid 18F-choline PET/mpMRI. Although these data were the best available for the 18F-choline PET/mpMRI patient population, the sample size was small. We accounted for this in our sensitivity analysis. The sensitivity and specificity of mpMRI were consistent with other published estimates based on large trials (6,7), and those estimates were included in our sensitivity analysis. A standard approach (for U.S. studies) of using Medicare reimbursements for cost estimates was applied; however, these costs are specific to the United States and vary depending on the payer. Sensitivity and threshold analyses demonstrated that our conclusions were not sensitive to diagnostic accuracy or cost estimates.
An additional limitation involved the inconsistent definitions of clinically important prostate cancer in the literature. For example, Siddiqui et al. (13) defined clinically important cancer to be cancer with a high-volume Gleason score of 3 + 4 or a Gleason score of greater than or equal to 4 + 3, whereas our model assumed it to be cancer with any Gleason score of greater than or equal to 3 + 4. Finally, prostatectomy was the only curative treatment included in our model. Although prostatectomy is the most common curative treatment, patients undergoing radiation therapy have similar health outcomes (34).
The Centers for Medicare & Medicaid Services have not defined specific reimbursement codes for hybrid PET/mpMRI. Effectively, the reimbursement for PET/mpMRI procedures in the United States is the same as that for PET/CT (35). Because 18F-choline is not a U.S. Food and Drug Administration–approved radiopharmaceutical, we estimated radiotracer reimbursement on the basis of 18F-FDG. As a result, the cost for hybrid 18F-choline PET/mpMRI was estimated on the basis of the cost for hybrid 18F-FDG PET/CT and not on the sum of the costs for separately performed mpMRI and PET/CT. Additionally, the hybrid imaging approach assumed that the mpMRI part of PET/mpMRI did not include dynamic contrast-enhanced imaging. This assumption was considered justifiable because prior work showed that the incremental benefit of dynamic contrast-enhanced imaging is low (26).
CONCLUSION
18F-choline PET/mpMRI for the early detection of primary prostate cancer with a Gleason score of greater than or equal to 3 + 4 appears to be cost-effective and can reduce the number of unneeded biopsies in comparison to mpMRI alone. Screening strategies that involved performing a biopsy after negative imaging were more expensive and resulted in fewer QALYs than strategies that involved performing no biopsy after screening. Regardless of the particular scoring system used (PI-RADSv2 or Likert), strategies involving hybrid 18F-choline PET/mpMRI for patients with elevated PSA levels, in which a combined biopsy is performed for all high-risk mpMRI targets as well as all low- or intermediate-risk mpMRI targets with18F-choline TBRs of greater than or equal to 1.58, and no biopsy otherwise were cost-effective. These findings were robust in sensitivity and threshold analyses.
DISCLOSURE
This work was supported by the National Science Foundation (grant CMMI 0844511 to Brian T. Denton; grant DGE 1256260 to Christine L. Barnett), the U.S. Department of Defense (grant PC110389), and NIH/NCI (grants P01CA87634 and P50CA069568). No other potential conflict of interest relevant to this article was reported.
Supplementary Material
KEY POINTS
QUESTION: Given the improved accuracy of 18F-choline PET/mpMRI over mpMRI alone for the detection of prostate cancer with a Gleason score of greater than or equal to 3 + 4 in men with elevated prostate-specific antigen levels, is hybrid PET/mpMRI cost-effective?
PERTINENT FINDINGS: The analysis showed that hybrid 18F-choline PET/mpMRI was cost-effective in comparison to mpMRI alone or standard biopsy without imaging, largely by reducing the number of unneeded biopsies and treatments.
IMPLICATIONS FOR PATIENT CARE: Within the current health-care environment of the United States, hybrid 18F-choline PET/mpMRI offers important health benefits for patients with significant prostate cancer.
REFERENCES
- 1.U.S. Preventive Services Task Force. Final recommendation statement. Prostate cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening1. Accessed on September 19, 2019.
- 2.Haas GP, Delongchamps NB, Jones RF, et al. Needle biopsies on autopsy prostates: sensitivity of cancer detection based on true prevalence. J Natl Cancer Inst. 2007;99:1484–1489. [DOI] [PubMed] [Google Scholar]
- 3.Carter HB, Albertsen PC, Barry MJ, et al. Early detection of prostate cancer: AUA guideline. J Urol. 2013;190:419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnett CL, Davenport MS, Montgomery JS, Wei JT, Montie JE, Denton BT. Cost-effectiveness of magnetic resonance imaging and targeted fusion biopsy for early detection of prostate cancer. BJU Int. 2018;122:50–58. [DOI] [PubMed] [Google Scholar]
- 5.Pahwa S, Schiltz NK, Ponsky LE, Lu Z, Griswold MA, Gulani V. Cost-effectiveness of MR imaging-guided strategies for detection of prostate cancer in biopsy-naive men. Radiology. 2017;285:157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kasivisvanathan V, Rannikko AS, Borghi M, et al. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med. 2018;378:1767–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmed HU, El-Shater Bosaily A, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet. 2017;389:815–822. [DOI] [PubMed] [Google Scholar]
- 8.Piert M, Montgomery J, Kunju LP, et al. 18F-choline PET/MRI: the additional value of PET for MRI-guided transrectal prostate biopsies. J Nucl Med. 2016;57:1065–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bailey J, Piert M. Performance of 68Ga-PSMA PET/CT for prostate cancer management at initial staging and time of biochemical recurrence. Curr Urol Rep. 2017;18:84. [DOI] [PubMed] [Google Scholar]
- 10.Davenport MS, Montgomery JS, Kunju LP, et al. 18F-choline PET/mpMRI for detection of clinically significant prostate cancer: Part 1. Improved risk stratification for MRI-guided transrectal prostate biopsies. J Nucl Med. August 16, 2019. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barnett CL, Tomlins SA, Underwood DJ, et al. Two-stage biomarker protocols for improving the precision of early detection of prostate cancer. Med Decis Making. 2017;37:815–826. [DOI] [PubMed] [Google Scholar]
- 12.Epstein JI, Feng Z, Trock BJ, Pierorazio PM. Upgrading and downgrading of prostate cancer from biopsy to radical prostatectomy: incidence and predictive factors using the modified Gleason grading system and factoring in tertiary grades. Eur Urol. 2012;61:1019–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siddiqui MM, Rais-Bahrami S, Turkbey B, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA. 2015;313:390–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loeb S, Carter HB, Berndt SI, Ricker W, Schaeffer EM. Complications after prostate biopsy: data from SEER-Medicare. J Urol. 2011;186:1830–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzalez CM, Averch T, Boyd LA, et al. AUA/SUNA white paper on the incidence, prevention and treatment of complications related to prostate needle biopsy. https://www.suna.org/resources/pnbWhitePaper.pdf. Accessed August 20, 2019.
- 16.Savage CJ, Lilja H, Cronin AM, Ulmert D, Vickers AJ. Empirical estimates of the lead time distribution for prostate cancer based on two independent representative cohorts of men not subject to prostate-specific antigen screening. Cancer Epidemiol Biomarkers Prev. 2010;19:1201–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gulati R, Inoue L, Katcher J, Hazelton W, Etzioni R. Calibrating disease progression models using population data: a critical precursor to policy development in cancer control. Biostatistics. 2010;11:707–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merdan S, Womble PR, Miller DC, et al. Toward better use of bone scans among men with early-stage prostate cancer. Urology. 2014;84:793–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Risko R, Merdan S, Womble PR, et al. Clinical predictors and recommendations for staging computed tomography scan among men with prostate cancer. Urology. 2014;84:1329–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu J, Womble PR, Merdan S, et al. Factors influencing selection of active surveillance for localized prostate cancer. Urology. 2015;86:901–905. [DOI] [PubMed] [Google Scholar]
- 21.Roehl KA, Han M, Ramos CG, Antenor JAV, Catalona WJ. Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3,478 consecutive patients: long-term results. J Urol. 2004;172:910–914. [DOI] [PubMed] [Google Scholar]
- 22.Arias E. United States life tables, 2006. Natl Vital Stat Rep. 2010;58:1–40. [PubMed] [Google Scholar]
- 23.Aizer AA, Gu X, Chen MH, et al. Cost implications and complications of overtreatment of low-risk prostate cancer in the United States. J Natl Compr Canc Netw. 2015;13:61–68. [DOI] [PubMed] [Google Scholar]
- 24.Roth JA, Gulati R, Gore JL, Cooperberg MR, Etzioni R. Economic analysis of prostate-specific antigen screening and selective treatment strategies. JAMA Oncol. 2016;2:890–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heijnsdijk EA, Wever EM, Auvinen A, et al. Quality-of-life effects of prostate-specific antigen screening. N Engl J Med. 2012;367:595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuhl CK, Bruhn R, Krämer N, Nebelung S, Heidenreich A, Schrading S. Abbreviated biparametric prostate MR imaging in men with elevated prostate-specific antigen. Radiology. 2017;285:493–505. [DOI] [PubMed] [Google Scholar]
- 27.Li CK, Tong BCY, You JHS. Cost-effectiveness of culture-guided antimicrobial prophylaxis for the prevention of infections after prostate biopsy. Int J Infect Dis. 2016;43:7–12. [DOI] [PubMed] [Google Scholar]
- 28.Grann VR, Patel PR, Jacobson JS, et al. Comparative effectiveness of screening and prevention strategies among BRCA1/2-affected mutation carriers. Breast Cancer Res Treat. 2011;125:837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shepard DS. Cost‐Effectiveness in Health and Medicine. New York, NY: Oxford University Press; 1996. [Google Scholar]
- 30.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness: the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371:796–797. [DOI] [PubMed] [Google Scholar]
- 31.Johansson JE, Andren O, Andersson SO, et al. Natural history of early, localized prostate cancer. JAMA. 2004;291:2713–2719. [DOI] [PubMed] [Google Scholar]
- 32.Venderink W, Govers TM, de Rooij M, Futterer JJ, Sedelaar JPM. Cost-effectiveness comparison of imaging-guided prostate biopsy techniques: systematic transrectal ultrasound, direct in-bore MRI, and image fusion. AJR. 2017;208:1058–1063. [DOI] [PubMed] [Google Scholar]
- 33.Schwarzenböck S, Souvatzoglou M, Krause BJ. Choline PET and PET/CT in primary diagnosis and staging of prostate cancer. Theranostics. 2012;2:318–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamdy FC, Donovan JL, Lane JA, et al. 10-Year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375:1415–1424. [DOI] [PubMed] [Google Scholar]
- 35.Centers for Medicare & Medicaid Services. Decision memo for positron emission tomography (FDG) for solid tumors (CAG-00181R4). CMS.gov website. https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=263&NcaName=Positron+Emission+Tomography+(FDG)+for+Solid+Tumors&TimeFrame=7&DocType=All&bc=AQAAIAAACAAAAA%3d%3d&. Accessed August 20, 2019.
- 36.Adibi M, Pearle MS, Lotan Y. Cost-effectiveness of standard vs intensive antibiotic regimens for transrectal ultrasonography (TRUS)-guided prostate biopsy prophylaxis. BJU Int. 2012;110:E86–E91. [DOI] [PubMed] [Google Scholar]
- 37.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 2011;103:117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.