Abstract
Patients with neuroendocrine tumors (NETs) are often treated with somatostatin analogs (SSAs) for control of symptoms and tumor growth. Such therapy could theoretically lead to misinterpretation of somatostatin receptor imaging with 68Ga-DOTATATE PET/CT by interfering with tracer–receptor binding. Guidelines recommend an interval of 3–4 wk between the last dose and imaging. The aim of this study was to evaluate if long-acting (LA) SSA treatment changes the uptake of 68Ga-DOTATATE in patients with NETs. Methods: From 2013 to 2016, 296 patients with, or under evaluation for, NETs were included in this prospective observational study. The effect of LA SSA on tracer uptake was evaluated in 2 main patient populations: those undergoing 68Ga-DOTATATE PET/CT before starting LA SSA treatment and at least once afterward, and those receiving ongoing LA SSA therapy, in whom the effect of the interval between the last dose of LA SSA and the PET/CT exam was analyzed. A third, explorative, analysis was performed to evaluate if clinical disease progression, regression, or stable tumor status changed the uptake of 68Ga-DOTATATE. In the 3 analyses, measurements of SUVmax in normal liver and tumor lesions were compared. Results: The median SUVmax in normal liver was significantly higher before treatment (8.6; interquartile range, 7.4–10.2) than after treatment initiation (6.0; 4.7–8.0) (P < 0.001). No significant changes in SUVmax were seen in tumor lesions after treatment initiation. No significant differences in SUVmax were found in normal liver or tumor lesions dependent on the interval between last dose of LA SSA and PET/CT. Conclusion: Treatment with LA SSA does not change SUVmax in tumor lesions, whereas SUVmax in normal liver is significantly lower after treatment. The findings have implications for interpretation of 68Ga-DOTATATE PET/CT for response assessment after SSA therapy and for guidelines on discontinuation of treatment before PET/CT.
Keywords: 68Ga-DOTATATE, PET/CT, neuroendocrine tumor, somatostatin analogs, somatostatin receptor imaging
Neuroendocrine tumors (NETs) are a heterogeneous group of tumors that are relatively rare but with increasing incidence. The gastrointestinal canal is most commonly affected—mainly small bowel and rectum—followed by the lungs and pancreas (1). Most NETs overexpress somatostatin receptors (2). Somatostatin receptor imaging with 111In-pentetreotide scintigraphy (OctreoScan; Mallinckrodt Medical) has been the accepted method for visualizing NETs for many years but is rapidly being replaced by PET/CT with 68Ga-DOTA-conjugated somatostatin analogs (SSAs) such as 68Ga-DOTATATE, 68Ga-DOTATOC, and 68Ga-DOTANOC, resulting in higher sensitivity, faster procedures, and lower radiation exposure (3–5).
Patients with NETs are often treated with SSAs for control of symptoms and tumor growth (6–8). Analogs of endogenous somatostatin have a short half-life. For more convenient patient administration, long-acting (LA) release versions of SSAs have been developed (9). Theoretically, treatment with LA SSAs could lead to misinterpretation of the somatostatin receptor images by interfering with tracer–receptor binding (5,10). The European Association of Nuclear Medicine procedure guidelines for 68Ga-DOTATATE PET/CT suggest an interval of 3–4 wk after administration of LA SSA to avoid potential somatostatin receptor blockade, although the evidence to support this is scarce (5). Published data rather support an improved tumor-to-background ratio during LA SSA treatment (11–14). An area where there is even less evidence is the clinical significance of changes in 68Ga-DOTATATE uptake and how they relate to tumor progression and regression.
We have conducted a prospective, single-center, observational study aimed at evaluating the effect of LA SSA treatment on 68Ga-DOTATATE uptake in patients with NETs (EudraCT 2012-004313-13). The specific objectives were to evaluate if there is a difference in 68Ga-DOTATATE uptake in normal liver tissue and tumor lesions in patients before and after initiation of treatment with LA SSA; if the interval between the last dose of LA SSA and the 68Ga-DOTATATE PET/CT scan changes the 68Ga-DOTATATE uptake in normal liver tissue and tumor lesions in patients with ongoing LA SSA treatment; and if disease progression, regression, or stable tumor status changes the uptake of 68Ga-DOTATATE.
MATERIALS AND METHODS
Study Population
From 2013 to 2016, 296 patients were enrolled in this prospective, observational clinical trial at Skåne University Hospital, Lund, Sweden. Patients were eligible if they were at least 18 y old, gave written informed consent, and fulfilled either of 2 indications for 68Ga-DOTATATE PET/CT: reevaluation of earlier diagnosed, histologically verified NET with ongoing treatment with LA SSA, or evaluation of suspected NET with likely initiation of LA SSA treatment within a year. The exclusion criteria were pregnancy or breastfeeding, lack of signed informed consent, or age less than 18 y.
Information on the site of the primary tumor, tumor grade, treatment with LA SSA (yes/no, dose, and interval), and indications for 68Ga-DOTATATE PET/CT was registered at inclusion. On the day of the PET/CT exam, the patients reported the number of days since their last LA SSA dose. Subjects received a clinical evaluation from the responsible physician, often within a month after the 68Ga-DOTATATE PET/CT scan, and information about medication and treatment plan was recorded. A new clinical evaluation was performed after the patient had returned for reevaluation with 68Ga-DOTATATE PET/CT, and the disease status was graded as regression, progression, or stable. This overall evaluation was based on clinical examination, tumor markers, and radiology (CT alone) if new lesions were detected or if lesions changed in size compared with the previous examination. Information on changes in degree of uptake in the PET images was not used to determine disease status.
In analysis 1, patients were included if they had 1 68Ga-DOTATATE PET/CT scan performed before starting LA SSA treatment and at least 1 68Ga-DOTATATE PET/CT scan after treatment initiation. If there were multiple examinations, the last PET/CT exam before, and the first after, treatment initiation were chosen to minimize the interval between the two and thereby the possible effect of a change in tumor status. The SUVmax in normal liver tissue and tumors was compared before and after treatment initiation.
In analysis 2, patients who had ongoing treatment with LA SSA and stable disease at clinical evaluation were included. The SUVmax in normal liver tissue and tumors was compared between patients receiving the injection of LA SSA close to the 68Ga-DOTATATE PET/CT exam versus patients with a longer interval between the injection and PET/CT.
In analysis 3, we determined if the clinical evaluation of disease status as regression, progression, or stable disease correlated with a change in SUVmax in tumors. Three subgroups based on disease status were created. Patients were included if they had registered data at the clinical evaluation (regression/stable/progression) in adherence with the 68Ga-DOTATATE PET/CT exam and a previous 68Ga-DOTATATE PET/CT exam for SUVmax comparison.
The study was performed in accordance with the Helsinki Declaration and was approved by the Institutional Review Board at Lund University (2012/657) and the Swedish Medical Products Agency. All patients gave written informed consent before inclusion.
68Ga-DOTATATE PET/CT and Image Analysis
68Ga-DOTATATE was synthesized using established techniques (5,15,16). An activity of 2.5 (±0.2) MBq/kg was injected in a peripheral vein. The amount of injected peptide DOTATATE was held constant in the range of 10–35 μg for all patients, and the average dose was 23 μg per patient. Because of decay of the activity, and because the administered activity depended on patient weight, the amount of peptide varied among patients. Sixty minutes after injection of 68Ga-DOTATATE, a PET/CT scan (from vertex to mid thigh) was performed on a Discovery 690 scanner (GE Healthcare). For most examinations, diagnostic CT with intravenous and oral contrast material was performed concurrently; otherwise, low-dose CT was performed for attenuation correction and anatomic correlation. According to our protocol, low-dose CT was performed if diagnostic CT had been performed 4–6 wk before the PET/CT examination. For low-dose CT, the acquisition was performed in helical mode using 120 kV, 30–160 mA, and a noise index of 45. For diagnostic CT, the acquisition was performed in helical mode using 100 kV, 120–240 mA, and a noise index of 14 for the liver series, whereas 100 kV, 80–450 mA, and a noise index of 40 was used for the neck-thorax-abdomen series. The PET acquisition time was 3 min per bed position. Images were reconstructed using ordered-subset expectation maximization with 3 iterations, 12 subsets, and a 5-mm gaussian postprocessing filter with time-of-flight and point-spread function correction.
Images were analyzed by an experienced nuclear medicine physician on an Extended Brilliance Workspace workstation (Philips Healthcare). The SUVmax of normal liver was measured in a 3-cm region of interest in all scans while avoiding large vessels. In patients having a high tumor burden in the liver, SUVmax in normal liver could not be determined. If multiple tumor lesions were present, the SUVmax of the 5 lesions with the highest SUVmax were registered and their average value calculated (average SUVmax in tumor). The SUVmax for the single hottest lesion (highest SUVmax in tumor) was also registered. The tumor-to-liver SUVmax ratio was calculated from both the highest SUVmax in tumor and the average SUVmax in tumor (highest tumor-to-liver ratio or average tumor-to-liver ratio). The overall location of tumors was registered at every examination, but the size and specific location of the measured tumors were not registered.
Statistical Analysis
Descriptive statistics were used to analyze the data with SPSS software. For the first analysis, a Wilcoxon related-samples rank test was used. A Kruskal–Wallis test was used in the second and third analyses. A P value of less than 0.05 was considered significant.
RESULTS
Of the 296 included patients, 17 were lost to follow-up. For the remaining 279 patients, 530 68Ga-DOTATATE PET/CT examinations were performed, of which 495 had complete data registered. Clinical evaluation was performed by the patient’s physician (oncologist or surgeon) at the follow-up visit, and disease status was registered in 135 of these cases (Table 1). Clinical disease status was missing in 157 of the follow-up examinations.
TABLE 1.
Number of Patients Who Underwent 1–4 68Ga-DOTATATE PET/CT Exams and Number of Patients with Treatment and Registered Disease Status
| Parameter | First exam | Second exam | Third exam | Fourth exam* |
| Total exams with complete data | 262 | 134 | 76 | 23 |
| Number of patients with LA SSA | 108 (41%) | 79 (59%) | 48 (63%) | 20 (87%) |
| Number of patients with no LA SSA | 154 (59%) | 55 (41%) | 28 (37%) | 3 (13%) |
| Number of patients with registered clinical disease status | 59 (23%) | 47 (35%) | 23 (30%) | 6 (26%) |
1 patient had 5 exams.
For all patients in the study, the small intestine/right colon was the most common primary tumor localization (138 patients, 49%), followed by the pancreas (54 patients, 19%). Tumor grade 1 was the most common (163 patients, 58%). The mean age at inclusion was 63 y (±11.7 y), and 142 patients were female (51%). Further details can be found in Table 2.
TABLE 2.
Patient Characteristics and PET/CT Details
| Characteristic | Total | Analysis 1 | Analysis 2 | Analysis 3 |
| Total patients | 279 | 19 | 37 | 41 |
| Age (y) | 63.0 ± 11.7 | 65.7 ± 8.2 | 63.7 ± 9.4 | 64.4 ± 8.8 |
| Female | 142 (51%) | 12 | 17 | 21 |
| Tumor location | ||||
| Small intestine/right colon | 138 (49%) | 10 | 26 | 19 |
| Pancreas | 54 (19%) | 4 | 7 | 10 |
| Unknown | 24 (9%) | 1 | 1 | |
| Lungs | 15 (5%) | 1 | 1 | 6 |
| Appendix | 13 (5%) | 1 | 1 | |
| Ovarian | 7 (3%) | 2 | 1 | 2 |
| Left colon/rectum | 6 (2%) | 1 | 1 | |
| Stomach/duodenum | 6 (2%) | 1 | 1 | |
| Thymus | 4 (1%) | |||
| Other | 19 (7%) | |||
| Tumor grade | ||||
| G1 | 163 (58%) | 12 | 20 | 20 |
| G2 | 88 (32%) | 6 | 17 | 20 |
| G3 | 4 (1%) | |||
| NA | 24 (9%) | 1 | 1 | |
| Patients with… | ||||
| No treatment | ||||
| At inclusion | 159 (57%) | |||
| At first exam | 19 | 14 | ||
| At second exam | 9 | |||
| Octreotide treatment | 30 | |||
| At inclusion | 81 (29%) | |||
| At first exam | 22 | |||
| At second exam | 8 | 23 | ||
| Lanreotide treatment | 7 | |||
| At inclusion | 36 (13%) | |||
| At first exam | 5 | |||
| At second exam | 11 | 9 | ||
| Pasireotide treatment | ||||
| At inclusion | 3 (1%) | |||
| Activity (MBq/kg) | 2.5 ± 0.2 | |||
| At inclusion | 2.5 ± 0.2 | |||
| At first exam | 2.5 ± 0.3 | 2.5 ± 0.2 | ||
| At second exam | 2.6 ± 0.1 | 2.5 ± 0.2 | ||
| Accumulation time (min) | 62 ± 5 | |||
| At inclusion | 62 ± 5 | |||
| At first exam | 63 ± 4 | 62 ± 4 | ||
| At second exam | 61 ± 3 | 61 ± 5 |
NA = not applicable.
Qualitative data are expressed as numbers followed by percentages in parentheses; continuous data are expressed as mean ± SD.
Effect of LA SSA Treatment Initiation on SUVmax
Of the 159 patients who did not have LA SSA at inclusion, 26 later initiated treatment with LA SSA. Of those 26, 3 were excluded because of conflicting data on when they received the first dose of LA SSA, 3 were excluded because they received chemotherapy (possible confounder), and 1 was excluded because no tumor was found at the first examination (performed after surgery). The remaining 19 patients had no treatment with LA SSA on the first examination and later received LA SSA for at least 3 mo before the follow-up 68Ga-DOTATATE PET/CT exam; these were included in analysis 1.
There was no statistically significant difference in the injected activity of 68Ga-DOTATATE between the first and the second examinations (P = 0.33), nor was there any significant difference in accumulation time (P = 0.17) (Table 2).
The median time between the 2 PET/CT scans was 202 d (interquartile range, 151–308 d). At the time of the second PET/CT exam, the mean interval between injection with LA SSA and the examination was 14.7 d (SD, 8.8; range, 3–30). Eleven patients were treated with lanreotide, 120 mg (9 patients) or 90 mg (2 patients), every 4 wk; 1 patient had an interval of 3 wk. Eight patients had treatment with octreotide, 30 mg (4 patients) or 20 mg (4 patients), with an interval of 4 wk; 1 patient had an interval of 3 wk.
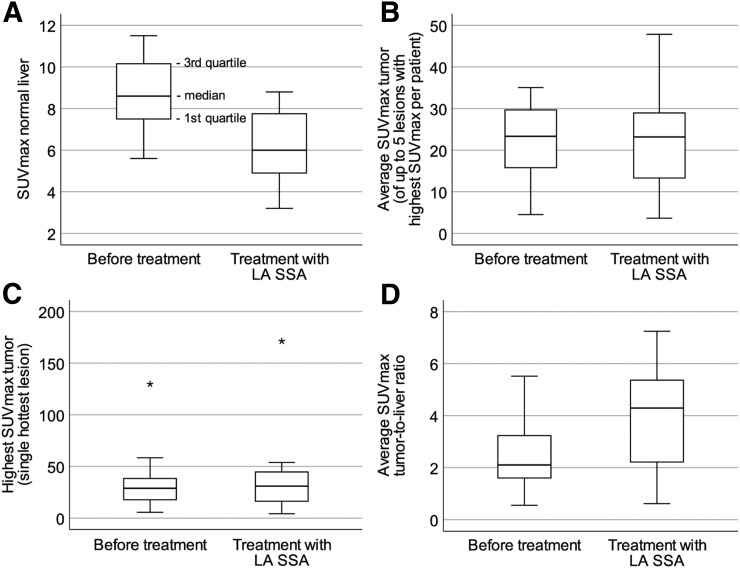
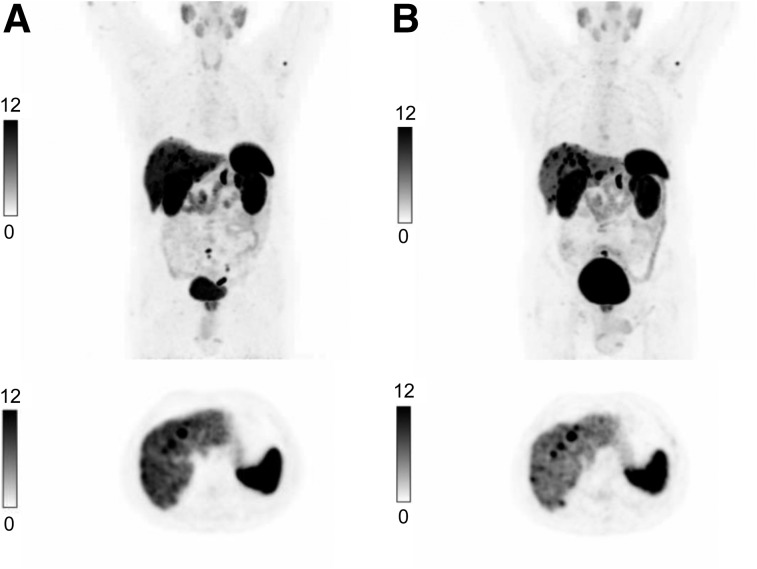
Table 3 shows that the median SUVmax in normal liver decreased significantly after treatment initiation. Neither the median highest SUVmax in tumor nor the average SUVmax in tumor changed significantly after treatment initiation. The median ratio of highest tumor-to-liver ratio and average tumor-to-liver ratio differed significantly before and after treatment (Fig. 1). Figure 2 shows that the tumor-to-liver ratio was higher after treatment initiation with LA SSA and that the tumor lesions in liver were better visualized.
TABLE 3.
Overview of SUVmax in Normal Liver and Tumor Lesions Before and After Treatment Initiation with LA SSA
| Measurement | Before | After | P |
| Number of assessed metastases | 73 | 72 | |
| SUVmax in normal liver | 8.6 (7.4–10.2) | 6.0 (4.7–8.0) | <0.001 |
| Highest SUVmax in tumor | 28.8 (17.3–40.7) | 30.9 (15.1–48.0) | 0.15 |
| Average SUVmax in tumor | 23.3 (15.3–30.9) | 23.2 (12.4–29.4) | 0.33 |
| Highest tumor-to-liver ratio | 2.6 (1.9–5.5) | 5.6 (3.2–8.1) | 0.008 |
| Average tumor-to-liver ratio | 2.1 (1.6–3.3) | 4.3 (1.9–5.7) | 0.01 |
Data are median followed by interquartile range in parentheses. P < 0.05 on Wilcoxon related-samples rank test was considered statistically significant.
FIGURE 1.
Box plots of uptake before and after initiation of LA SSA treatment: SUVmax in normal liver (P < 0.001) (A), average SUVmax in tumor (P = 0.33) (B), highest SUVmax in tumor (P = 0.15) (C), and average SUVmax tumor-to-liver ratio (P = 0.01) (D). P < 0.05 on Wilcoxon rank testing was considered statistically significant.
FIGURE 2.
Examination of same patient with 68Ga-DOTATATE/PET/CT before (A) and after (B) treatment with LA SSA. At top are coronal maximum-intensity projections of PET images; at bottom, images at same axial plane as PET images.
SUVmax in Relationship to Interval of LA SSA Before Imaging
There were 37 patients with ongoing treatment with LA SSA who were evaluated as having stable disease based on clinical evaluation, tumor markers, and radiology (CT alone). Patient characteristics and imaging details are shown in Table 2 (analysis 2).
The mean interval between injection of LA SSA and 68Ga-DOTATATE PET/CT was 14.4 d (range, 1–31 d). Patients were divided into 4 subgroups depending on the number of days since injection with LA SSA: 0–7 d (7 patients), 8–14 d (13 patients), 15–21 d (9 patients), and more than 21 d (8 patients).
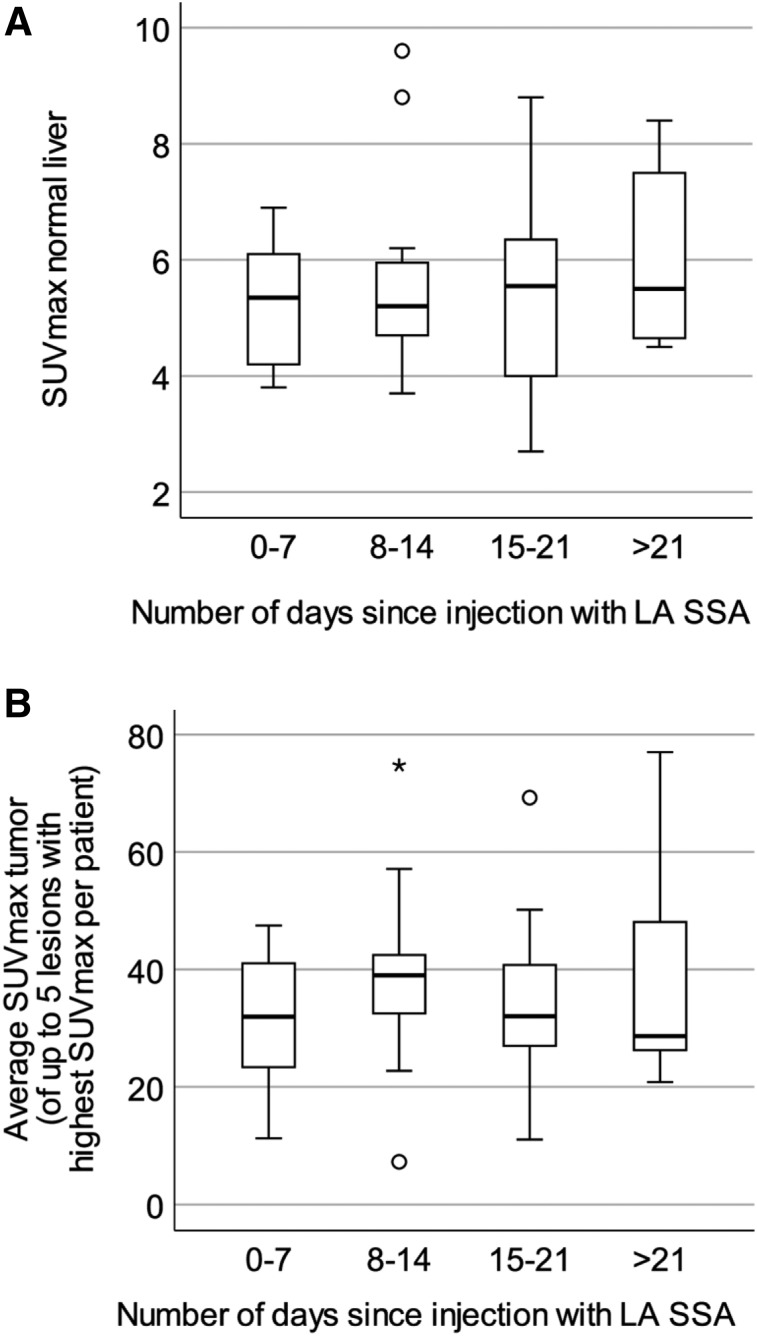
No significant difference was found among the 4 subgroups dependent on number of days since injection with LA SSA and SUVmax in normal liver (P = 0.88), highest SUVmax in tumor (P = 0.75), or average SUVmax in tumor (P = 0.68) (Fig. 3), nor were any significant differences found when comparing the highest tumor-to-liver ratio or average tumor-to-liver ratio among the subgroups.
FIGURE 3.
Box plots of uptake at various intervals since initiation of last dose of LA SSA: SUVmax in normal liver (P = 0.88) (A) and average SUVmax in tumor (P = 0.68) (B). P < 0.05 on Kruskal–Wallis testing was considered statistically significant. No significant differences dependent on time since injection were found.
Change in SUVmax Depending on Disease Status
In analysis 3, we created 3 subgroups based on whether disease status was evaluated as regression, stable disease, or progression (Table 2).
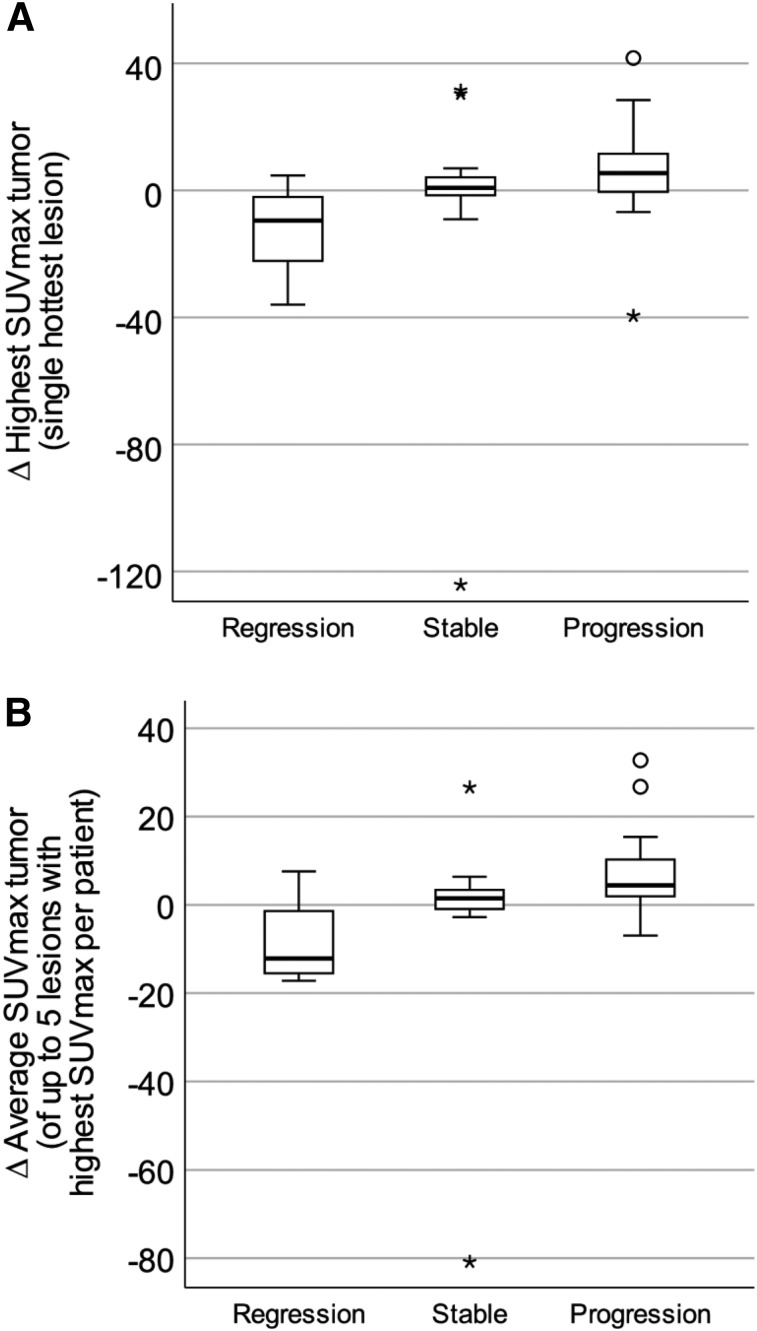
Seven patients were included in the group with regression, 20 patients in the group with stable disease, and 14 patients in the group with progression. The median time between the 2 examinations was 333 d (interquartile range, 240–410 d). Changes in SUVmax in tumor between the 2 PET/CT scans (change in highest SUVmax in tumor and change in average SUVmax in tumor) are shown in Table 4 and Figure 4. Statistically significant differences were found between the groups. The group with regression had a small but significant decrease in uptake, the group with stable disease had only a small change in uptake, and the group with progressive disease had a small increase in uptake.
TABLE 4.
Differences in SUVmax Depending on Change in Clinical Disease Status
| Measurement | Regression | Stable | Progression | P |
| Number of patients | 7 | 20 | 14 | |
| Change in highest SUVmax in tumor | −9.5 (−31.1 to −0.2) | 0.8 (−1.5 to 4.2) | 5.5 (−0.5 to 13.1) | 0.022 |
| Change in average SUVmax in tumor | −12.2 (−16.2 to −0.7) | 1.5 (−1.0 to 3.6) | 4.4 (1.7 to 11.5) | 0.004 |
Data are median followed by interquartile range in parentheses. P < 0.05 on Kruskal–Wallis testing was considered statistically significant.
FIGURE 4.
Box plots of differences (∆) in highest SUVmax in tumor (P = 0.022) (A) and in average SUVmax in tumor (P = 0.004) (B) related to disease status at second examination with 68Ga-DOTATATE/PET/CT. P < 0.05 on Kruskal–Wallis testing was considered statistically significant. Small significant differences were found.
DISCUSSION
We found significantly lower uptake of 68Ga-DOTATATE in normal liver after treatment initiation with LA SSA and no significant change in SUVmax in tumors, resulting in an increase in the tumor-to-liver ratio. We did not find the interval from last LA SSA injection to imaging to have any effect on SUVmax in liver or tumor lesions. These results agree with previously published retrospective data. Haug et al. showed a significantly lower uptake of 68Ga-DOTATATE in liver and spleen in patients treated with SSAs than in patients without treatment, but they found no significant differences in the uptake of 68Ga-DOTATATE in tumors (13). Ayati et al. and Cherk et al. compared the uptake of 68Ga-DOTATATE in normal tissues such as liver, spleen, and thyroid and in tumors before and after treatment initiation with LA SSA, with results similar to ours (11,12).
A plausible explanation for these results is the effect of the amount of circulating cold peptide at the time of the PET examination, as both the initiation of LA SSA and the time interval from injection affect this parameter (17). Velikyan et al. showed, in an experimental patient setting, that the highest tumor–to–normal-tissue ratio occurred when 50 μg of octreotide were administered before the 68Ga-DOTATOC PET/CT exam (18). In another, more recently published, prospective study, the effect of LA SSA on the uptake of 68Ga-DOTATATE was examined 1 d before and 1 d after injection of lanreotide, with no evidence of decreased tumor uptake but an improved tumor-to-liver ratio (14).
The reason for the observed decreased uptake in normal liver, leading to an increased tumor-to-liver ratio, is not clear but could be the different receptor internalization and expression kinetics in tumor versus normal tissue (19). Internalization of somatostatin receptor after treatment with SSAs may be paralleled by upregulation of expression of somatostatin receptor in tumor during the steady state of LA SSA. Differences in receptor density could also lead to a lower threshold for receptor saturation in normal tissues than in tumor.
During treatment with LA SSA, with administration every fourth week, a steady state is likely reached after approximately 3 mo although the pharmacokinetic profiles of octreotide and lanreotide are different (17). The uptake of 68Ga-DOTATATE in tumor lesions, however, may be a more dynamic process affected by several factors such as amount of injected activity and peptide, distribution volume, spatial resolution of the detector system, accumulation time, lesion size, and receptor density. It has also been shown that receptor expression might be underestimated for SUVs of more than 25, with the correlation with receptor density not being linear for higher SUVs. Instead, net uptake rate better correlated with receptor density but requires dynamic PET/CT examinations (20). All these factors could contribute to the observed effect on the tumor-to-liver ratio and the lack of effect of injection-to-imaging interval.
The third analysis was conducted to examine if a change in clinical disease status alters the uptake of 68Ga-DOTATATE in tumors. Gabriel et al. observed a decrease in SUV in responders to peptide receptor radionuclide therapy (PRRT) compared with patients with stable or progressive disease (21), and Haug et al. showed that patients with decreased uptake after the first cycle of PRRT had a longer time to progression and a greater improvement in symptoms (22). They also found that a change in SUV tumor-to-spleen ratio was more accurate in predicting outcome than a change in SUVmax in tumors. In the current study, we found small but significant changes in SUVmax in tumor lesions characterized as being in progression, stable disease, or regression that, together with previous reports, support changes in SUVmax as a valid marker for changes in disease status. However, there was a wide range in SUVmax with relatively few patients. Changes in SUVmax could also be influenced by other factors, including the amount of injected activity of 68Ga-DOTATATE, accumulation time, elimination, total tumor burden, treatment with chemotherapy, PRRT, and dedifferentiation of tumor. We are still far from understanding how to weight each of these factors when interpreting changes in SUVmax in tumors.
To our knowledge, this is the first prospective observational study to evaluate the effect of uptake of 68Ga-DOTATATE before and after initiation of treatment with LA SSA. Our results, in combination with similar results from previous studies, might impact the procedure guidelines for 68Ga-DOTATATE PET/CT, which suggest an interval of 3–4 wk after administration of LA SSA to avoid potential somatostatin receptor blockade (5). It would seem more relevant to not discontinue treatment with LA SSA, as it has good effects on both symptoms and tumor growth and improves tumor-to-liver ratio, thereby increasing the detectability of hepatic metastases.
The increased tumor-to-liver ratio seen after initiation of LA SSA treatment is also relevant in relation to treatment with PRRT. The side effects of PRRT might be lower if uptake of 177Lu in normal tissue decreases. Perhaps LA SSA can potentiate the effect of PRRT to tumors and reduce adverse effects on normal tissue, thereby further improving the therapeutic index. More research in this field is needed.
This work should be viewed in the light of some limitations. First, there were relatively few patients in the first analysis and a long interval (median, 202 d) between the 2 scans, during which a possible decrease in SUVmax in tumor might be masked by disease progression. Other potential errors could be a difference in the hottest lesion measured between the 2 examinations and that some lesions might have been removed by surgery. In the second and third analyses, there was a big loss of data regarding clinical disease status at follow-up. A study with more patients would improve the power.
CONCLUSION
There was no evidence of a change in SUVmax in tumor lesions after treatment initiation with LA SSA, but the SUVmax in normal liver was significantly lower during treatment. These findings have implications for the interpretation of 68Ga-DOTATATE PET/CT for response assessment after SSA therapy and for guidelines regarding discontinuation of treatment before 68Ga-DOTATATE PET/CT scans. Detection of lesions might improve as the tumor-to-liver ratio increases during LA SSA therapy. In addition, small but significant changes were found in the uptake of 68Ga-DOTATATE when disease status changed, supporting the role of this PET modality in evaluating the response to different therapies.
DISCLOSURE
This work was made possible by research grants from the Knut and Alice Wallenberg Foundation, the Swedish Federal Government under the ALF agreement, and Region Skåne. The funders of the study were not involved in study design, data collection, data interpretation, writing of the report, or the decision to submit the paper for publication. Anna Sundlöv has received consultancy fees from Novartis, Ipsen, Bayer, and Spago Nanomedical. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Does uptake of 68Ga-DOTATATE by normal liver tissue and tumor lesions differ before and after initiation of treatment with LA SSA or dependent on interval between last dose of LA SSA and 68Ga-DOTATATE PET/CT?
PERTINENT FINDINGS: In this prospective, observational clinical trial, 296 patients were enrolled and subgroup analyses were performed. There was no evidence of a change in SUVmax in tumor lesions after initiation of LA SSA treatment, but the SUVmax in normal liver was significantly lower during treatment.
IMPLICATIONS FOR PATIENT CARE: These findings have implications for using 68Ga-DOTATATE PET/CT to assess response to SSA therapy and for creating guidelines on discontinuing treatment before 68Ga-DOTATATE PET/CT scans.
REFERENCES
- 1.Dasari A, Shen C, Halperin D, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017;3:1335–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reubi JC. Peptide receptors as molecular targets for cancer diagnosis and therapy. Endocr Rev. 2003;24:389–427. [DOI] [PubMed] [Google Scholar]
- 3.Barrio M, Czernin J, Fanti S, et al. The impact of somatostatin receptor-directed PET/CT on the management of patients with neuroendocrine tumor: a systematic review and meta-analysis. J Nucl Med. 2017;58:756–761. [DOI] [PubMed] [Google Scholar]
- 4.Deppen SA, Blume J, Bobbey AJ, et al. 68Ga-DOTATATE compared with 111In-DTPA-octreotide and conventional imaging for pulmonary and gastroenteropancreatic neuroendocrine tumors: a systematic review and meta-analysis. J Nucl Med. 2016;57:872–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bozkurt MF, Virgolini I, Balogova S, et al. Guideline for PET/CT imaging of neuroendocrine neoplasms with 68Ga-DOTA-conjugated somatostatin receptor targeting peptides and 18F-DOPA. Eur J Nucl Med Mol Imaging. 2017;44:1588–1601. [DOI] [PubMed] [Google Scholar]
- 6.Vinik AI, Wolin EM, Liyanage N, Gomez-Panzani E, Fisher GA. Evaluation of lanreotide depot/autogel efficacy and safety as a carcinoid syndrome treatment (ELECT): a randomized, double-blind, placebo-controlled trial. Endocr Pract. 2016;22:1068–1080. [DOI] [PubMed] [Google Scholar]
- 7.Caplin ME, Pavel M, Cwikla JB, et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371:224–233. [DOI] [PubMed] [Google Scholar]
- 8.Rinke A, Wittenberg M, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors (PROMID): results of long-term survival. Neuroendocrinology. 2017;104:26–32. [DOI] [PubMed] [Google Scholar]
- 9.Modlin IM, Pavel M, Kidd M, Gustafsson BI. Review article: somatostatin analogues in the treatment of gastroenteropancreatic neuroendocrine (carcinoid) tumours. Aliment Pharmacol Ther. 2010;31:169–188. [DOI] [PubMed] [Google Scholar]
- 10.Kwekkeboom DJ, Kam BL, van Essen M, et al. Somatostatin-receptor-based imaging and therapy of gastroenteropancreatic neuroendocrine tumors. Endocr Relat Cancer. 2010;17:R53–R73. [DOI] [PubMed] [Google Scholar]
- 11.Cherk MH, Kong G, Hicks RJ, Hofman MS. Changes in biodistribution on 68Ga-DOTA-octreotate PET/CT after long acting somatostatin analogue therapy in neuroendocrine tumour patients may result in pseudoprogression. Cancer Imaging. 2018;18:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ayati N, Lee ST, Zakavi R, et al. Long-acting somatostatin analog therapy differentially alters 68Ga-DOTATATE uptake in normal tissues compared with primary tumors and metastatic lesions. J Nucl Med. 2018;59:223–227. [DOI] [PubMed] [Google Scholar]
- 13.Haug AR, Rominger A, Mustafa M, et al. Treatment with octreotide does not reduce tumor uptake of 68Ga-DOTATATE as measured by PET/CT in patients with neuroendocrine tumors. J Nucl Med. 2011;52:1679–1683. [DOI] [PubMed] [Google Scholar]
- 14.Aalbersberg EA, de Wit-van der Veen BJ, Versleijen MWJ, et al. Influence of lanreotide on uptake of 68Ga-DOTATATE in patients with neuroendocrine tumours: a prospective intra-patient evaluation. Eur J Nucl Med Mol Imaging. 2019;46:696–703. [DOI] [PubMed] [Google Scholar]
- 15.Zhernosekov KP, Filosofov DV, Baum RP, et al. Processing of generator-produced 68Ga for medical application. J Nucl Med. 2007;48:1741–1748. [DOI] [PubMed] [Google Scholar]
- 16.Mueller D, Klette I, Baum RP, Gottschaldt M, Schultz MK, Breeman WA. Simplified NaCl based 68Ga concentration and labeling procedure for rapid synthesis of 68Ga radiopharmaceuticals in high radiochemical purity. Bioconjug Chem. 2012;23:1712–1717. [DOI] [PubMed] [Google Scholar]
- 17.Astruc B, Marbach P, Bouterfa H, et al. Long-acting octreotide and prolonged-release lanreotide formulations have different pharmacokinetic profiles. J Clin Pharmacol. 2005;45:836–844. [DOI] [PubMed] [Google Scholar]
- 18.Velikyan I, Sundin A, Eriksson B, et al. In vivo binding of [68Ga]-DOTATOC to somatostatin receptors in neuroendocrine tumours: impact of peptide mass. Nucl Med Biol. 2010;37:265–275. [DOI] [PubMed] [Google Scholar]
- 19.Reubi JC, Waser B, Cescato R, Gloor B, Stettler C, Christ E. Internalized somatostatin receptor subtype 2 in neuroendocrine tumors of octreotide-treated patients. J Clin Endocrinol Metab. 2010;95:2343–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Velikyan I, Sundin A, Sorensen J, et al. Quantitative and qualitative intrapatient comparison of 68Ga-DOTATOC and 68Ga-DOTATATE: net uptake rate for accurate quantification. J Nucl Med. 2014;55:204–210. [DOI] [PubMed] [Google Scholar]
- 21.Gabriel M, Oberauer A, Dobrozemsky G, et al. 68Ga-DOTA-Tyr3-octreotide PET for assessing response to somatostatin-receptor-mediated radionuclide therapy. J Nucl Med. 2009;50:1427–1434. [DOI] [PubMed] [Google Scholar]
- 22.Haug AR, Auernhammer CJ, Wangler B, et al. 68Ga-DOTATATE PET/CT for the early prediction of response to somatostatin receptor-mediated radionuclide therapy in patients with well-differentiated neuroendocrine tumors. J Nucl Med. 2010;51:1349–1356. [DOI] [PubMed] [Google Scholar]