Summary
Background:
Disruptions in energy homeostasis severely affect reproduction in many organisms and are linked to several reproductive disorders in humans. As a result, understanding the mechanisms that control nutrient accumulation in the oocyte will provide valuable insights into the links between metabolic disease and reproductive dysfunction.
Results:
We show that the steroid hormone ecdysone functions in Drosophila to control lipid metabolism and support oocyte production. First, local EcR-mediated signaling induces a stage-specific accumulation of lipids in stage 10 oocytes. EcR induces lipid accumulation by promoting the activation of the lipogenic transcription factor SREBP and by controlling the expression of the LDL receptor homolog, LpR2. Second, global signaling via the ecdysone receptor, EcR, establishes a female metabolic state and promotes whole body triglyceride and glycogen storage at high levels. EcR acts in the CNS to mediate these effects, in part by promoting higher levels of feeding in females.
Conclusions:
Ecdysone functions at two levels to support reproduction: first by inducing lipid accumulation in the late stages of oocyte development and second by providing a signal that coordinates lipid metabolism in the germline with whole animal lipid homeostasis. Ecdysone regulation allows females to assess the demands of oogenesis and alter their behavior and metabolic state to support the biosynthetic requirements of oocyte production.
Introduction
Reproduction in all systems is heavily influenced by metabolism and nutrition [1, 2]. Several human reproductive disorders have been linked to malnutrition, diabetes, and obesity [3]. Moreover, nutrition influences reproduction in both flies and mammals in very similar ways. For example, fasting leads to decreased fertility as a result of defective oocyte development in both Drosophila and humans [4, 5]. Recent studies in flies, mice, livestock, and human oocytes have shown that lipids accumulate dramatically during oocyte development and are required for oogenesis and the early stages of embryogenesis. [6–10]. However, despite the common need for lipids in oocyte development and reproduction, little is known about the mechanisms controlling oocyte lipid accumulation in any system.
Many key metabolic pathways and lipid regulatory mechanisms are conserved between Drosophila and humans, making the fly an excellent system in which to characterize genetic mechanisms that control lipid metabolism [11]. For example, SREBP is a helix-loop-helix transcription factor that is retained in the ER in the presences of high levels of intracellular lipids. Once lipid levels drop SREBP is transported to the Golgi, cleaved by proteases, and its DNA binding domain moves to the nucleus and induces the expression of many genes involved in lipid synthesis and uptake including the LDL receptor. [12, 13]. Studies of SREBP have focused primarily on its function in the mammalian liver and adipose tissue, however, studies of SREBPs in S. cerevisiae, C.elegans, and Drosophila [14] [15, 16] suggest roles in multiple biological processes. Interestingly, recent studies have shown that the Drosophila LDL receptor, lipophorin receptor 2 (LpR2), is required for lipid uptake in imaginal discs and the ovary (Parra-Peralbo, E., and Culi, J.. 2011), suggesting a possible role for SREBP in germline lipid accumulation.
Nuclear receptors (NRs) are another conserved class of transcription factors that play key roles in numerous aspects of lipid metabolism [17]. NRs are defined by an N-terminal DNA binding domain and a C-terminal ligand-binding domain that senses small lipophilic compounds. Ecdysone receptor, EcR, is a member of the NR1H family of nuclear receptors that includes the mammalian LXR and FXR receptors. LXRs/FXR have well described roles in the regulation of triglyceride metabolism and cholesterol homeostasis [18]. EcR, on the other hand, senses the steroid hormone 20-hydroxyecdysone (20E), and plays an essential role in the regulation of developmental timing [19]. While this family of nuclear receptors has been studied extensively in the liver in mammals, and during embryonic/larval development in Drosophila, relatively little is known about its role in reproduction in any species.
Consistent with a role in reproduction, the EcR ligand, ecdysone, is produced during adulthood primarily in the ovary and accumulates at high levels in females but not males [20–22]. EcR and downstream components of the ecdysone-signaling pathway are genetically required for oocyte development [23–25]. Moreover, a recent study has shown that ecdysone produced from the somatic follicle cells is required for border cell migration during stage 9 of oogenesis [26]. However, the importance of EcR in nutrient storage during late stages of egg development has not been defined.
Our studies define the metabolic demands of oocyte production and the mechanisms that fulfill these demands. Ecdysone signaling functions to induce lipid accumulation during stage 10 of oogenesis by promoting the activation of SREBP. SREBP mediates lipid loading in stage 10 follicles by regulating the expression of the LDLR homolog LpR2 and other genes in the germline. In addition, EcR functions in the central nervous system of Drosophila females to increase female feeding and nutrient storage to provide the building blocks of oocyte development. Our work also establishes Drosophila as a powerful system to investigate the mechanisms that coordinate female metabolism with germline metabolic processes required for oocyte development.
Results
Triglycerides and sterols accumulate in stage 10 follicles
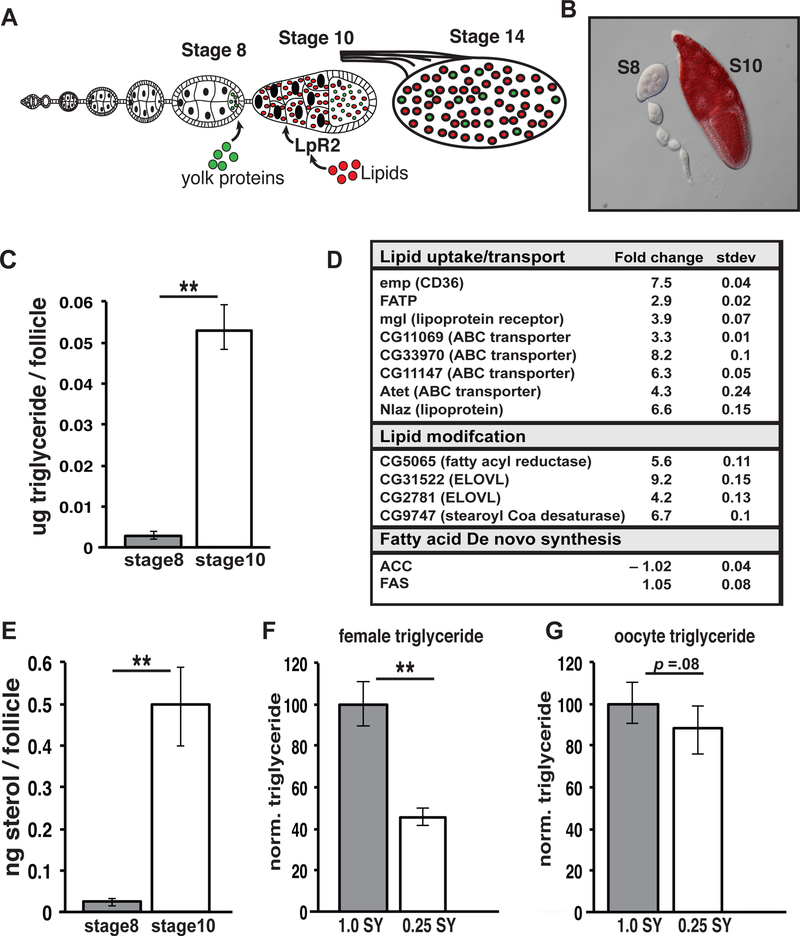
Previous studies have shown that neutral lipids accumulate via LpR2 to high levels in vitellogenic follicles at stage 10 of oogenesis (Figure 1A) [6, 7, 27]. We confirmed that ovaries stained with a neutral lipid-specific dye (Oil Red O) display a dramatic increase in lipid content in stage 10 follicles (Figure 1B). The composition of lipids stored in stage 10 follicles was determined by dissecting mature adult Oregon R females, collecting stage 8 and 10 follicles and measuring both triglycerides and total sterol levels via colorimetric assay and fluorescence assay respectively. We found that stage 10 follicles display a 6-fold increase in triglyceride levels and a significant increase in total sterols, both major forms of stored lipids (Figure 1C,E). Other lipids such as free fatty acids showed no significant rise in stage 10 follicles suggesting that triglycerides and total sterol comprise the major lipid classes stored during stage 10 of oogenesis (Figure S1A).
Figure 1. Triglycerides and sterols accumulate during stage 10 of oogenesis.
(A) Stages of Drosophila oogenesis showing germline lipid accumulation during stage 10. (B) Ovariole stained with Oil Red O (red). Triglyceride (C) and sterol (E) levels showing increases between stage 8 and stage 10 follicles. (D) RNA expression fold change between stage 8 and stage 10 of the indicated lipid metabolic genes as determined by RNAseq. (F, G) Whole female or stage 14 oocyte triglyceride levels are compared from animals fed on 0.25 SY or 1.0 SY food. TAG levels were normalized to total protein and are displayed as normalized to a wild-type level of 100% . Error bars represent 1xSD. **P<. 005
Since oogenesis in many organisms is heavily influenced by diet, we tested whether nutrient deprivation can alter triglyceride levels in the oocyte. Mature Oregon R flies were fed either a control 1.0SY media or a low nutrient .25SY media for one week. After 7 days we found that the percentage of ovarioles containing stage 10 oocytes was significantly lower on the nutrient-deprived .25 SY media (Figure S1B). Whole body and oocyte triglyceride levels were then assessed by colorimetric assay. As expected, we found that whole body triglyceride levels changed quite dramatically (40–65%) in nutrient-deprived females (Figure 1F). In contrast, the triglyceride content of their stage 14 oocytes was essentially unaffected (0–20%) suggesting that oocyte triglyceride levels are maintained precisely regardless of diet (Figure 1G).
RNA sequencing analysis was performed on stage 8 and 10 follicles from Oregon R females to help define the lipid metabolic mechanisms that mediate lipid accumulation. 662 genes increased and 158 decreased their expression levels at least 2-fold during stage 10. When we compared the 662 transcripts up-regulated at stage 10 to an updated version of a published list of 1,231 metabolic genes [11] [28] we found that 33 predicted metabolic genes increased at stage 10 (Figure S1C). Interestingly, 12 of these genes have predicted functions in lipid metabolism (Figure 1D). These include genes involved with lipid uptake such as the CD36 fatty acid translocase homologue, emp, and the fatty acid transporter, Fatp. Several fatty acid modifying enzymes (CG5065, CG31522, CG2781, CG9747) and genes with putative roles in lipid trafficking and transport (CG11069, CG33970, mgl, CG11147, Atet, and Nlaz) are also induced at stage 10. In contrast, genes involved in de novo fatty acid synthesis are expressed at a constant low level and do not increase at stage 10 (Figure 1D). Surprisingly, LpR2 expression was not up-regulated at stage 10. Conversely, when we examined the 158 genes down-regulated at least 2-fold at stage 10 we found only 14 putative metabolic genes. Among these 14 genes only CG4753 (GPAT-like enzyme) is predicted to have a direct role in lipid metabolism (Figure S1C).
Ecdysone signaling is required for oocyte lipid accumulation
In light of previous work that shows ecdysone signaling is active and required during early and mid-oogenesis [23–25] we wanted to test whether ecdysone signaling acts in stage 10 follicles. We dissected and stained the ovaries from EcRnull/+ and EcRts/EcRnull females with anti-LpR2 antibodies and Nile Red. Compared to EcRnull/+ control ovaries, after 7 days at the non-permissive temperature EcRts/EcRnull ovaries contain 50% fewer stage 10 follicles (Figure S2A). Interestingly, relative to control stage 10 follicles (Figure 2A), EcRts/EcRnull mutant oocytes display a spectrum of lipid accumulation defects as seen by Nile red staining (Figure 2B, C). Approximately 90% of EcRts/EcRnull oocytes display a partial (Figure 2B’) or complete (Figure 2C’) loss of LpR2 expression on germ cell membranes (Figure 2D). Knocking down expression of the EcR target gene Eip75 in the germline, caused similar defects in lipid accumulation and LpR2 expression (Figure S2B,C) but inhibiting several other pathway components (USP, E74, br, and Ftz-F1) did not produce any clear phenotypes, most likely due to the ineffectiveness of these particular RNAi transgenes in the germline. Inactivating ecdysone production using RNAi against the ecdysone biosynthetic enzyme, spook, (Figure S2D–G) also dramatically decreased LpR2 expression in many stage 10 follicles.
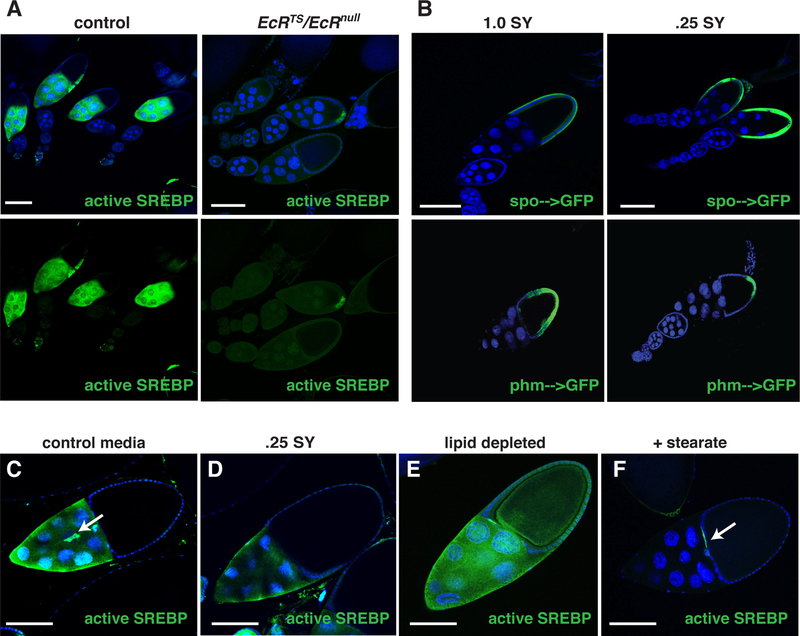
Figure 2. Ecdysone signaling is required for lipid accumulation during vitellogenesis.
(A, B, C) Control and EcRts/EcRnull animals were dissected and ovaries were stained with DAPI (blue), anti-LpR2 antibodies (green), and Nile Red (red). Stained stage 10 egg chambers (follicles) from control n=208 and EcRts/EcRnull n=171 animals were scored (D) as having normal (A’), partial (B’) or absent (C’) LpR2 expression (Arrows point to LpR2 membrane staining). Error bars represent standard deviation. (E, F) Fold change in SCAP, SREBP and the indicated lipid metabolic gene mRNA levels as determined by RNA sequencing of EcRts/EcRnull compared to control stage 10 egg chambers. All error bars represent ± SD. Scale bars = 100um.
To investigate the mechanism of EcR-mediated lipid uptake during oogenesis we conducted RNA sequencing analysis on control and EcRts/EcRnull stage 10 follicles. 397 genes decreased and 67 increased their expression levels at least 2-fold in the EcRts/EcRnull follicles. As expected, many known ecdysone-regulated genes displayed decreased expression following EcR inactivation (Figure S2H). Of the 662 genes that we found to be up-regulated during stage 10, 162 were down-regulated in EcRts/EcRnull follicles, about ten times more than the 16 genes that would be expected by chance, (p=1.70E-178), confirming that many genes up-regulated at this developmental stage are controlled by ecdysone. Significantly, 9 of the 12 lipid metabolic genes up-regulated in stage 10, were down-regulated in EcRts/EcRnull follicles, 30 times more than would be expected by chance (p=9.60E-19). Indeed, these 9 genes (Figure 2F) were the only lipid metabolic genes [11] [28] down-regulated in EcRts/EcRnull follicles. Interestingly, we observed that both SREBP and its activating protein SCAP displayed a modest but significant decrease in expression in EcRts/EcRnull follicles (Figure 2E) suggesting that SREBP signaling may also be hormonally modulated. In contrast, when we examined the 67 genes up-regulated in EcRts/EcRnull follicles we found only 2 genes with predicted metabolic roles , neither of which involves lipid metabolism. These data, clearly connect ecdysone signaling to the changes in lipid metabolic gene expression at stage 10, and suggest that EcR activation induces lipid accumulation by up-regulating genes that mediate lipid modification, storage and trafficking.
SREBP signaling plays a central role in germline lipid accumulation
Consistent with our gene expression data, in a candidate screen of reporters for known metabolic regulatory pathways, we found that a previously characterized reporter of SREBP activity [15] is specifically induced in stage 8–10 follicles. Subsequently, reporter expression declines in late stage 10B and early stage 11 (Figure S3A,C). We investigated the functional role of SREBP in germline lipid accumulation by generating negatively marked germline mutant clones for two null mutant alleles of SREBP [15]. These animals were dissected and stained with anti-GFP antibodies and Nile Red. The majority of follicles lacking a functional SREBP die at stage 8, consistent with previous studies that show lipid storage defects lead to oocyte arrest and death at the onset of vitellogenesis (Figure S3B). Among those SREBP germline mutant follicles that do not die, ~90% show a complete block in germline lipid accumulation (Figure 3B,C and Figure S3D) indicating that SREBP is essential for germline lipid accumulation.
Figure 3. SREBP functions to promote lipid accumulation during vitellogenesis.
Stage 10 follicles from control (A) and SREBP null mutant germ line clones (B, C), stained for DAPI (blue), clonal marker (anti-GFP: green), and Nile Red (red). Ovarioles from control (D) and nos–> SREBP-DBD animals stained with DAPI (blue) and Nile Red (red). Scale bars= 100um.
To determine if SREBP activation is sufficient for oocyte lipid accumulation we expressed an activated version of SREBP (SREBP-DBD) in the germline. While ~85% of the females expressing this activated version of SREBP in the germline contained ovarioles completely lacking germ cells (Figure 3E), 15% of the females contained stage 4–6 follicles that displayed premature lipid accumulation (Figure 3D,F), something that is never seen in control follicles. These data suggests a novel role for SREBP in reproduction by controlling lipid levels in the germline.
SREBP is regulated by Ecdysone signaling and dietary nutrients.
To investigate whether SREBP functions downstream of EcR in the germline, we assessed SREBP reporter activation in EcRts/EcRnull mutant ovarioles and found that EcRts/EcRnull follicles are unable to activate SREBP (Figure 4A and Figure S4A). These data indicate that ecdysone signaling mediates lipid accumulation, at least in part, by promoting SREBP activation. However, there may be other factors that are independent of SREBP that also support lipid accumulation.
Figure 4. SREBP is regulated by Ecdysone signaling and dietary nutrients.
(A) Follicles from control or EcRts/EcRnull animals carrying an SREBP-GFP reporter (green). (B) Follicles from phm–>GFP or spo–>GFP (green) animals fed either a control diet or a 0.25SY low calorie diet for 5 days. A stage 10 follicle from animals carrying an SREBP-GFP reporter (green) raised on a control diet (C), on a 0.25 SY low-calorie diet (D), on a lipid-depleted diet (E), or a 2% stearate supplemented diet (F). Arrows: border cells. Scale bars= 100um.
Given that both nuclear receptors and SREBP are commonly regulated by nutrients, we investigated if SREBP and ecdysone signaling are influenced by changes in diet. Adult flies containing one of two published reporters of ecdysone biosynthetic gene expression, spook-GAL4[22] or phm-GAL4[29] driving expression of UAS-GFP, were fed either a control 1.0SY media or a low nutrient .25SY media. After seven days ovaries were dissected and stained with anti-GFP antibodies. On the control diet, both reporters were strongly expressed in stage 10 follicle cells (Figure 4B). However, in animals fed the nutrient-deprived diet, phm (but not spook) expression was low in stage 10 follicles and was only detected in the posterior follicle cells (Figure 4B; Figure S4C). These observations show that phm, a gene encoding a key cytochrome P450 enzyme in ecdysone biosynthesis, is highly expressed and specifically regulated by diet in stage 10 follicle cells. Furthermore, our data suggest that posterior follicle cells serve as a local source of ecdysone during late stages of oocyte development.
To assess the effects of dietary lipids on SREBP signaling in the germline, we grew SREBP reporter animals on 1.0SY control media or .25SY nutrient-deprived media for one week, then dissected the ovaries, and stained them with anti-GFP antibody. Animals fed a low-nutrient diet contained ovarioles with modestly reduced germline SREBP activation (Figure 4C,D). When SREBP reporter animals were fed lipid-extracted media SREBP activation was increased in the germline and also activated in the somatic follicle cells (Figure 4E). In contrast, when animals were fed media supplemented with 2% fatty acids the ovarioles showed a complete inhibition of SREBP activation in the germline with only the border cells showing SREBP activation (Figure 4F and Figure S4B). Taken together these data suggest that SREBP senses lipid levels in the germline and directs corresponding changes in lipid uptake and storage, in a similar manner to other tissues. However, additional factors may contribute along with SREBP activation to setting the final levels of lipid accumulation in stage 10 oocytes.
SREBP regulates LpR2 to promote lipid accumulation
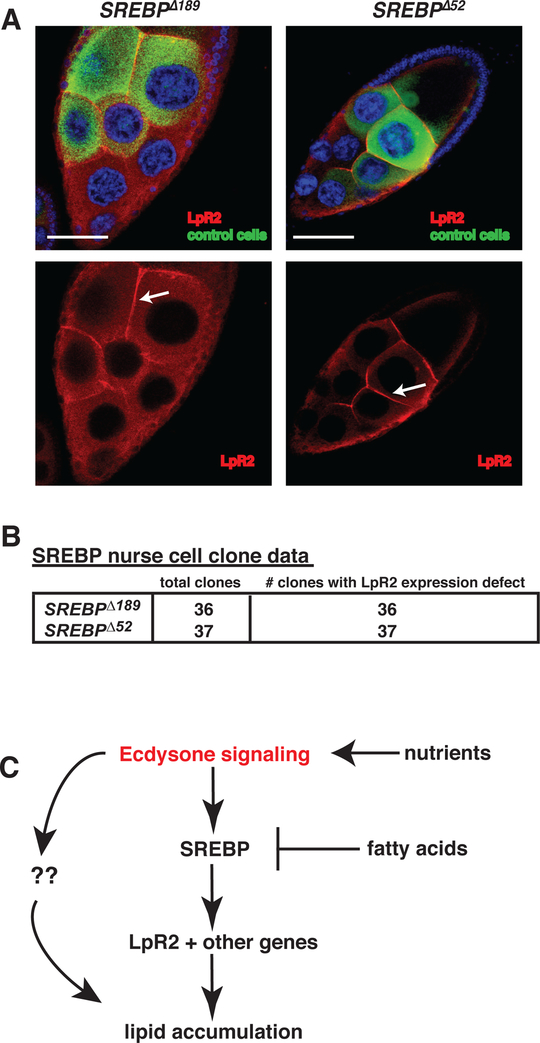
In light of previous studies that show LpR2 is required for lipid uptake in the germline [6] we hypothesized that SREBP regulates the expression of LpR2. To test this idea, we generated negatively marked nurse cell clones mutant for SREBPΛ189 or SREBPΛ52, dissected the ovaries, and stained with anti-GFP antibodies and anti-LpR2 antibodies. Consistent with an essential role for SREBP in adipogenesis we found that nurse cells lacking functional SREBP displayed defective LpR2 expression (Figure 5A and Figure 5B). In addition, by analyzing the genomic sequences surrounding LpR1 and LpR2, we found 5 SRE elements and 6 E-box elements, known binding sites of Drosophila SREBP, contained within introns and intergenic regions (Figure S5A). To determine if SREBP directly binds any of these putative response elements in the 5’ end of the LpR genomic loci we performed chromatin immuno-precipitation assays using antibodies against Drosophila SREBP (3B2) [30] or IgG controls. These experiments indicated that SREBP binds significantly to the SRE element site located near the 5’ end of the large second LpR2 intron. (Figure S5B). These data support a model where ecdysone signaling induces follicle maturation at stage 10 by activating SREBP. SREBP then senses intracellular lipid levels and promotes germline lipid accumulation by controlling LpR2 expression (Figure 5C).
Figure 5. SREBP is required for LpR2 expression.
(A) Stage 10 nurse cells with negatively marked SREBP mutant clones stained for DNA (DAPI: blue), clonal marker (GFP:green), and LpR2 (red). Arrows indicate lpr2 membrane staining. Scale bars = 50 um. (B) summary of nurse cell clones analyzed and their LpR2 membrane expression. (C) Proposed model of germline lipid accumulation.
Females store high levels of whole body triglyceride to support egg production
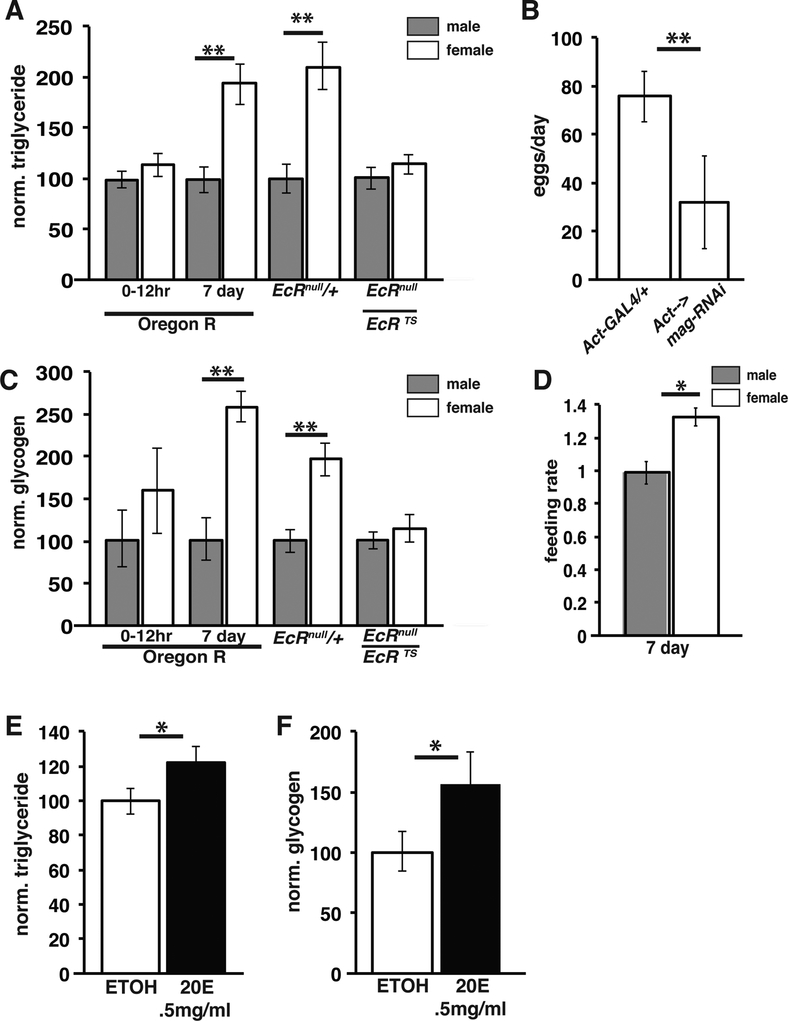
Given that Drosophila females lay roughly 100 nutrient-filled eggs per day, females must specialize their metabolic mechanisms differently than males in order to meet the biosynthetic demands of egg production. To characterize metabolic differences between male and female flies we collected newly eclosed and 7-day old mated animals and measured whole body triglyceride and glycogen levels. While newly eclosed flies show no significant difference between males and females in whole body triglyceride; while mature adult females contain almost twice as much triglyceride as males (Figure 6A). Similarly, females that were allowed to mature for seven days showed pronounced gains in glycogen when compared to newly eclosed females (Figure 6C).
Figure 6. Ecdysone signaling promotes triglyceride accumulation in adult females.
Normalized adult triglyceride (A) and glycogen (C) levels from controls of the indicated genotypes and from EcRts/EcRnull mutant animals. (B) Egg laying in control and mag-RNAi expressing animals; n=40; error bar= 1xSD (D) Normalized feeding rate of mature adult males (= 1.0) and females via capillary assay. Triglyceride (E) and glycogen (F) in Oregon R males grown for 4 days on food supplemented with EtOH alone (=1.0) or with 0.5mg/ml 20-hydroxyecdysone . *p<0.005 **p<0.0001.
One possible explanation for the increased triglyceride content of females following eclosion is simply the appearance during this time of mature lipid-rich follicles in their ovaries. Consequently, we compared gonadal triglyceride content to the total triglyceride present in male and females. These experiments showed that the ovaries account for only 5–10% of the total triglyceride present in the female (Figure S6A). This result was confirmed in an independent assay by extracting lipids from ovaries and intact females and measuring total triglyceride content via TLC (Figure S6B). We tested whether the elevated nutrients accumulated by females are required for oocyte production by measuring egg-laying in genetically lean mag-RNAi animals. Previous work has shown that mag-RNAi animals display a dramatic reduction in whole animal triglyceride due to defective dietary triglyceride digestion and uptake [31, 32]. In conjunction with being lean, mag-RNAi animals produced many fewer eggs (Figure 6B). Like wise, we observed reduced egg laying in lsd-2 mutant females, which are lean due to defects in lipid droplet lipolysis in the fat body [33]. These experiments agree with previous studies showing that nutrient restriction and disruptions in lipid metabolism arrest oogenesis and lead to the death of vitellogenic follicles [4, 6] [7].
Previous studies suggested that female flies increase feeding behavior after mating [34]. To investigate whether such a change occurs and whether it is sufficient to explain sexually dimorphic triglyceride contents, we measured the feeding rate of Oregon R animals using a capillary feeding assay (CAFE) (Figure 6D). Consistent with previous studies [35] we found that females feed at a 20–40% higher rate compared to males. These experiments suggest that increased feeding is a primary factor underlying the dimorphic nutrient accumulation by Drosophila females.
Ecdysone functions as a sex hormone to promote female metabolism
Previous studies in many species have shown that steroid hormones produced in the ovary function both locally to promote oogenesis and systemically to support normal reproduction [36, 37] [23]. In Drosophila, the ovary is a primary site of ecdysone production in adults, leading to significantly higher levels of ecdysone in females than males [26, 38, 39]. To investigate whether ecdysone signaling is required for the establishment of female metabolism, we measured triglyceride and glycogen levels in mature EcRnull/+ and EcRts/EcRnull flies. Females with defective EcR function displayed a significant decrease in both whole body triglyceride and glycogen levels (Figure 6A,C). Moreover, triglyceride and glycogen levels were lowered in the EcR mutants to approximately the levels found in males, suggesting that females require EcR-mediated signaling to establish the metabolic differences between the sexes. In contrast, total protein levels were unaffected in EcRts/EcRnull mutant females indicating that EcR specifically affects stored triglyceride and glycogen and without causing an overall change in animal size (Figure S6D).
To investigate whether ecdysone is sufficient to induce nutrient storage, we exposed males to elevated levels of ecdysone. Feeding 0.5mg/ml 20-hydroxy ecdysone to adult Oregon R flies for 4 days caused a significant increase in both triglyceride and glycogen levels in male flies (Figure 6E,F). To determine if ecdysone produced in the ovary is required to establish the female metabolic state we examined whole body triglyceride levels in bamΔ86 mutants, which do not form follicles that produce ecdysone [40]. The bam mutant females contained substantially reduced triglyceride levels, similar to EcRts/EcRnull females, suggesting that ecdysone produced in ovarian follicles is responsible for female nutrient accumulation (Figure S6D).
EcR functions in the CNS to establish female metabolism
To identify the key metabolic tissues responding to ecdysone in the female inhibited EcR using a commonly used EcR dominant-negative (EcR-DN) transgene [41]. To inactivate EcR in particular adult tissues we constructed fly stocks that carry EcR-DN, a tissue-specific GAL4 driver and a temperature sensitive GAL80 transgene, which inhibits GAL4 function at low temperatures. We grew the animals to adulthood at low temperature, switched adults to the restrictive temperature, and after a period of time measured whole body triglyceride and glycogen. Interestingly, when elav-GAL4, GAL80ts was used to drive EcR-DN expression in the adult CNS we found reductions in triglyceride and glycogen levels similar to those in EcRts/EcRnull females (Figure 7A, B) (Figure S7 B&C). Inhibition of EcR in other key metabolic tissues such as the intestine (mex-GAL4) and the fat body (fb-GAL4) yielded no significant changes in triglyceride levels in either sex (Figure S7 A). These data indicate that EcR functions primarily in the CNS to drive the nutrient accumulation in females that is required for oocyte production. To test whether the female-specific metabolic defects that result when EcR function is inhibited in the CNS affect female fertility, we measured egg production in the elav→EcR-DN females. Consistent with a role for EcR in lipid metabolism and oogenesis, elav→EcR-DN females display a dramatic defect in female fertility (Figure 7C). Interestingly, the few stage 10 egg chambers that are produce display normal lipid accumulation and LpR2 expression (Figure S7E) consistent with our previous observation that a reduced number of metabolically normal eggs are produced after dietary restriction. Taken together these data indicate that the CNS plays a major role in establishing metabolic sexual dimorphism and that females require higher levels of stored nutrients for normal egg production.
Figure 7. EcR functions in the central nervous system to promote feeding in females.
Triglyceride (A) and glycogen (B) in mature adult controls (UAS-EcR-DN/+) or in animals with CNS-driven (Elav-GAL4, GAL80ts) UAS-EcR-DN. (C) Egg laying/day by females from (UAS-EcR-DN/+) and (Elav-GAL4, GAL80ts) UAS-EcR-DN) animals, N=40; error bars= 1x SD. (D,E) Feeding rate via capillary assay for the indicated genotypes, normalized to control males = 1.0. (F) Model for the role of ecdysone in metabolic sexual dimorphism. *p<0.005 **p<0.0001.
Ecdysone signaling may act in the CNS to promote nutrient accumulation by regulating feeding behavior. To test this possibility we measured the feeding rate by a capillary feeding assay using individual EcRnull/+ or EcRts/EcRnull flies and found that EcR mutant females have a significantly reduced feeding rate while males are largely unaffected (Figure 7D). To assess if EcR function in the CNS affects feeding behavior, we inhibited EcR function using EcR-DN specifically in the adult CNS and measured feeding rate by capillary assay. Consistent with the EcR mutant, inhibiting ecdysone signaling in the adult CNS significantly lowers the feeding rate in females, while males show only a modest decrease (Figure 7E; Figure S7D). Importantly, inhibiting ecdysone signaling abolished the sexually dimorphic feeding behavior observed in normal flies. Overall, these data suggest that elevated levels of ecdysone in the female signal through EcR in the CNS to promote an increase in female feeding. This increase in female feeding increases the uptake and storage of nutrients such as triglyceride and glycogen and helps drive the differences in metabolism between the sexes (Figure 7F).
Discussion
Uptake and storage drives lipid accumulation in oocytes
Studies in numerous species have described lipid accumulation during the later stages of oocyte maturation, however, the precise composition of those lipids and the mechanism that promotes storage are poorly understood. Here we show that in Drosophila, triglycerides and sterols accumulate between stage 8 and 10 of oogenesis. These same stored lipids are utilized during embryogenesis for energy and biosynthetic purposes [8, 10]. The oocyte’s triglyceride and sterol depot can be broken down for ATP or interconverted to other lipid classes such as phospholipids, sphingolipids, ceramides, and steroid hormones. Ultimately, this strategy allows the female to provide stored energy and the necessary lipid building blocks to the oocyte without having to load all of the individual lipid species needed during embryogenesis. Since the mature oocyte’s lipid levels were not affected by diet our data also suggests that steroid signaling and SREBP act as a homeostatic mechanism during oogenesis to ensure that the an optimal level of lipids are loaded into each oocyte despite a wide range of exogenous nutrient availability.
Ecdysone promotes oocyte lipid accumulation via SREBP and LpR2
EcR is closely related to mammalian LXR receptors; these proteins show 40% identity and 56% similarity in their DNA binding domains. Furthermore, EcR and LXR both heterodimerize with the RXR/USP family of nuclear receptors and bind the DR4 LXR response element (AGGTCAnnnAGGTCA) [42, 43]. EcR mediates oocyte lipid accumulation, in large part by promoting the expression and activation of SREBP, a protein that in mammals regulates lipid uptake and synthesis in the liver [13, 15]. Mammalian LXR receptors also promote lipid accumulation and storage by inducing SREBP expression in the liver [18]. Also consistent with mammalian data [12], we found that SREBP controls lipid uptake and accumulation in the Drosophila germline, in part, by regulating the LDL receptor homolog LpR2. These data suggest that SREBP supports reproduction by acting as a nutrient responsive regulator of oocyte lipid accumulation.
While LXR, SREBP, and LDLR are best known for their roles in the liver, it remains unclear whether these genes act in the same manner in tissues such as the germline. In species like C.elegans, which never evolved liver or adipose tissue, these genes display a significant degree of sequence conservation. Interestingly, studies of mutants in the C.elegans NR1H receptor (daf-12) and the SREBP homolog (sbp-1) display significant defects in fertility and lipid storage [14, 44–46]. How daf-12 and sbp-1 function during oogenesis to promote fertility, however, has never been described. Moreover, limited studies reveal that LXRαβ double knockout mice display a 50% reduction in brood size and a significant defect in FSH-stimulated oocyte maturation [47]. Taken together these studies suggest that NR1H receptors and SREBP have conserved roles supporting normal reproduction. Our data suggests that the sterility associated with disrupting LXR or SREBP function in multiple systems arises from the roles these factors play in regulating metabolism in the germline. In fact, our data suggests that LXRs, SREBP, and LDLR play an ancient evolutionarily conserved role in promoting lipid accumulation in developing oocytes.
Ecdysone is a sex hormone that controls reproduction and female metabolism.
Estrogen, one of the primary female sex hormones in vertebrates, plays a wide variety of roles in female reproduction. In particular, estrogen receptor (ER) α and β are both required in the ovary for normal oocyte development [48] and ovulation [49]. In light of the roles for EcR in female metabolism and oocyte development, we propose that ecdysone signaling in the adult fly is analogous to estrogen signaling in mammals. Our data suggests that regulation of SREBP may be a conserved target of both ERs and LXRs. In fact, studies in mammals suggest that ER and LXR function together in adipose tissue to control target genes such as SREBP-1c; however, the mechanism by which this occurs is not at all clear [50]. These observations, in conjunction with the similar defects in stimulated oocyte maturation and ovulation seen between LXR double mutant mice [47] and ER double mutant mice[49] [36] mice, suggest that ERs and LXRs may function together to promote oocyte production. Consistent with this idea, we suggest that Drosophila represents an ancestral system for how nuclear receptors coordinate metabolism and reproduction.
In conjunction with controlling reproduction, sex hormones function to establish many secondary sex traits in mammals. The evidence presented here indicates that 20-hydroxyecdysone fulfills a similar role in Drosophila to establish metabolic sexual dimorphism. EcR mutant females lack the elevation of feeding and nutrient storage seen in controls and display metabolite levels similar to those seen in male animals. These findings are reminiscent of those in mice where the estrogen receptor functions in the hypothalamus to control responsiveness to leptin and suppress feeding, reduce weight gain, and regulate the distribution of fat storage [37]. It is interesting to note that EcR promotes feeding and triglyceride storage whereas ER seems to antagonize feeding and lipid storage. This difference between EcR and ER may derive from the very different reproductive strategies used by mice and Drosophila. While flies store all of the nutrients required for embryogenesis in the oocyte and produce many offspring, mammals produce relatively few progeny and provide the vast majority of the nutrients needed during embryogenesis through placental connections. Interestingly, mouse LXRs have also been shown to display sex-specific effects in the regulation of cholesterol metabolism [51] although sexually dimorphic roles for these receptors remain unclear.
Strong connections have been established between nutrition, hormone production, and reproduction in a wide variety of organisms. In humans, syndromes in which these connections become altered, such as polycystic ovary syndrome, are common causes of subfertility [3]. A fundamental understanding of the complex relationships between energy metabolism and reproduction would be of inestimable value in guiding more accurate diagnosis and treatment, but progress has been slow[37]. To that end our studies indicate that Drosophila oogenesis provides a powerful genetic system for advancing such an understanding.
Experimental procedures
Metabolic assays
Mature adult ovaries were dissected and follicles from stages 8 and 10 were collected (200/sample). Adult whole body samples were collected from 5 males and 5 females per sample. TAG, glycogen, protein, and cholesterol were measured as described in Sieber et al 2009 and Sieber et al 2011[31, 32]. Each experiment utilized 5–12 replicate samples and each experiment was repeated at least 3 times.
Supplementary Material
Acknowledgements
We thank Michael O’Connor, Michael Brown, and Joaquin Culi for generously sharing fly stocks and antibodies. We also thank the University of Utah GC/MS core facility and James Cox for their assistance in analyzing our GC/MS samples. We are grateful to Haiyang Chen, Erin Zeituni, Rebecca Obniski, and Joseph Tran for helpful discussion and comments during the preparation of this manuscript. A.C.S. is supported by HHMI and the Carnegie Institution for Science and M.H.S. is funded by the Jane Coffin Child Research Fund.
Footnotes
Conflict of interest: The authors declare no conflict of interest.
References
- 1.Shahjahan M, Kitahashi T, and Parhar IS (2014). Central Pathways Integrating Metabolism and Reproduction in Teleosts. Front Endocrinol (Lausanne) 5, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roland AV, and Moenter SM (2014). Reproductive neuroendocrine dysfunction in polycystic ovary syndrome: Insight from animal models. Front Neuroendocrinol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orbetzova MM, Kamenov ZA, Kolarov GB, Orbetzova VT, Genchev GD, Genov NS, and Zacharieva SZ (2003). Metabolic disturbances in women with polycystic ovary syndrome. Folia Med (Plovdiv) 45, 12–20. [PubMed] [Google Scholar]
- 4.Drummond-Barbosa D, and Spradling AC (2001). Stem cells and their progeny respond to nutritional changes during Drosophila oogenesis. Dev Biol 231, 265–278. [DOI] [PubMed] [Google Scholar]
- 5.Yan J, Zhou B, Yang J, Tai P, Chen X, Zhang H, Zhang M, and Xia G (2008). Glucose can reverse the effects of acute fasting on mouse ovulation and oocyte maturation. Reprod Fertil Dev 20, 703–712. [DOI] [PubMed] [Google Scholar]
- 6.Parra-Peralbo E, and Culi J (2011). Drosophila lipophorin receptors mediate the uptake of neutral lipids in oocytes and imaginal disc cells by an endocytosis-independent mechanism. PLoS Genet 7, e1001297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buszczak M, Lu X, Segraves WA, Chang TY, and Cooley L (2002). Mutations in the midway gene disrupt a Drosophila acyl coenzyme A: diacylglycerol acyltransferase. Genetics 160, 1511–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tennessen JM, Bertagnolli NM, Evans J, Sieber MH, Cox J, and Thummel CS (2014). Coordinated Metabolic Transitions During Drosophila Embryogenesis and the Onset of Aerobic Glycolysis. G3 (Bethesda). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wood BR, Chernenko T, Matthaus C, Diem M, Chong C, Bernhard U, Jene C, Brandli AA, McNaughton D, Tobin MJ, et al. (2008). Shedding new light on the molecular architecture of oocytes using a combination of synchrotron Fourier transform-infrared and Raman spectroscopic mapping. Anal Chem 80, 9065–9072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunning KR, Cashman K, Russell DL, Thompson JG, Norman RJ, and Robker RL (2010). Beta-oxidation is essential for mouse oocyte developmental competence and early embryo development. Biol Reprod 83, 909–918. [DOI] [PubMed] [Google Scholar]
- 11.Baker KD, and Thummel CS (2007). Diabetic larvae and obese flies-emerging studies of metabolism in Drosophila. Cell Metab 6, 257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yokoyama C, Wang X, Briggs MR, Admon A, Wu J, Hua X, Goldstein JL, and Brown MS (1993). SREBP-1, a basic-helix-loop-helix-leucine zipper protein that controls transcription of the low density lipoprotein receptor gene. Cell 75, 187–197. [PubMed] [Google Scholar]
- 13.Desvergne B, Michalik L, and Wahli W (2006). Transcriptional regulation of metabolism. Physiol Rev 86, 465–514. [DOI] [PubMed] [Google Scholar]
- 14.Walker AK, Jacobs RL, Watts JL, Rottiers V, Jiang K, Finnegan DM, Shioda T, Hansen M, Yang F, Niebergall LJ, et al. (2011). A conserved SREBP-1/phosphatidylcholine feedback circuit regulates lipogenesis in metazoans. Cell 147, 840–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kunte AS, Matthews KA, and Rawson RB (2006). Fatty acid auxotrophy in Drosophila larvae lacking SREBP. Cell Metab 3, 439–448. [DOI] [PubMed] [Google Scholar]
- 16.Bien CM, and Espenshade PJ (2010). Sterol regulatory element binding proteins in fungi: hypoxic transcription factors linked to pathogenesis. Eukaryot Cell 9, 352–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beaven SW, and Tontonoz P (2006). Nuclear receptors in lipid metabolism: targeting the heart of dyslipidemia. Annu Rev Med 57, 313–329. [DOI] [PubMed] [Google Scholar]
- 18.Kalaany NY, and Mangelsdorf DJ (2006). LXRS and FXR: the yin and yang of cholesterol and fat metabolism. Annu Rev Physiol 68, 159–191. [DOI] [PubMed] [Google Scholar]
- 19.Thummel CS (2001). Molecular mechanisms of developmental timing in C. elegans and Drosophila. Dev Cell 1, 453–465. [DOI] [PubMed] [Google Scholar]
- 20.Bownes M, and Smith T (1984). Ecdysteroids in adult males and females of Drosophila Melanogaster. J Insect Physiol 30, 823–830. [Google Scholar]
- 21.Birnbaum MJ, Kelly TJ, Woods CW, and Imberski RB (1984). Hormonal regulation of ovarian ecdysteroid production in the autogenous mosquito, Aedes atropalpus. Gen Comp Endocrinol 56, 9–18. [DOI] [PubMed] [Google Scholar]
- 22.Ono H, Rewitz KF, Shinoda T, Itoyama K, Petryk A, Rybczynski R, Jarcho M, Warren JT, Marques G, Shimell MJ, et al. (2006). Spook and Spookier code for stage-specific components of the ecdysone biosynthetic pathway in Diptera. Dev Biol 298, 555–570. [DOI] [PubMed] [Google Scholar]
- 23.Buszczak M, Freeman MR, Carlson JR, Bender M, Cooley L, and Segraves WA (1999). Ecdysone response genes govern egg chamber development during mid-oogenesis in Drosophila. Development 126, 4581–4589. [DOI] [PubMed] [Google Scholar]
- 24.Carney GE, and Bender M (2000). The Drosophila ecdysone receptor (EcR) gene is required maternally for normal oogenesis. Genetics 154, 1203–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ables ET, and Drummond-Barbosa D (2010). The steroid hormone ecdysone functions with intrinsic chromatin remodeling factors to control female germline stem cells in Drosophila. Cell Stem Cell 7, 581–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Domanitskaya E, Anllo L, and Schupbach T (2014). Phantom, a cytochrome P450 enzyme essential for ecdysone biosynthesis, plays a critical role in the control of border cell migration in Drosophila. Dev Biol 386, 408–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teixeira L, Rabouille C, Rorth P, Ephrussi A, and Vanzo NF (2003). Drosophila Perilipin/ADRP homologue Lsd2 regulates lipid metabolism. Mech Dev 120, 1071–1081. [DOI] [PubMed] [Google Scholar]
- 28.Tennessen JM, Barry WE, Cox J, and Thummel CS (2014). Methods for studying metabolism in Drosophila. Methods 68, 105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park D, Shafer OT, Shepherd SP, Suh H, Trigg JS, and Taghert PH (2008). The Drosophila basic helix-loop-helix protein DIMMED directly activates PHM, a gene encoding a neuropeptide-amidating enzyme. Mol Cell Biol 28, 410–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dobrosotskaya IY, Seegmiller AC, Brown MS, Goldstein JL, and Rawson RB (2002). Regulation of SREBP processing and membrane lipid production by phospholipids in Drosophila. Science 296, 879–883. [DOI] [PubMed] [Google Scholar]
- 31.Sieber MH, and Thummel CS (2009). The DHR96 nuclear receptor controls triacylglycerol homeostasis in Drosophila. Cell Metab 10, 481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sieber MH, and Thummel CS (2012). Coordination of triacylglycerol and cholesterol homeostasis by DHR96 and the Drosophila LipA homolog magro. Cell Metab 15, 122–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gronke S, Beller M, Fellert S, Ramakrishnan H, Jackle H, and Kuhnlein RP (2003). Control of fat storage by a Drosophila PAT domain protein. Curr Biol 13, 603–606. [DOI] [PubMed] [Google Scholar]
- 34.Barnes AI, Wigby S, Boone JM, Partridge L, and Chapman T (2008). Feeding, fecundity and lifespan in female Drosophila melanogaster. Proc Biol Sci 275, 1675–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deshpande SA, Carvalho GB, Amador A, Phillips AM, Hoxha S, Lizotte KJ, and Ja WW (2014). Quantifying Drosophila food intake: comparative analysis of current methodology. Nat Methods 11, 535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fisher CR, Graves KH, Parlow AF, and Simpson ER (1998). Characterization of mice deficient in aromatase (ArKO) because of targeted disruption of the cyp19 gene. Proc Natl Acad Sci U S A 95, 6965–6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown LM, Gent L, Davis K, and Clegg DJ (2010). Metabolic impact of sex hormones on obesity. Brain Res 1350, 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harshman LG, Loeb AM, and Johnson BA (1999). Ecdysteroid titers in mated and unmated Drosophila melanogaster females. J Insect Physiol 45, 571–577. [DOI] [PubMed] [Google Scholar]
- 39.Tu MP, Yin CM, and Tatar M (2002). Impaired ovarian ecdysone synthesis of Drosophila melanogaster insulin receptor mutants. Aging Cell 1, 158–160. [DOI] [PubMed] [Google Scholar]
- 40.McKearin DM, and Spradling AC (1990). bag-of-marbles: a Drosophila gene required to initiate both male and female gametogenesis. Genes Dev 4, 2242–2251. [DOI] [PubMed] [Google Scholar]
- 41.Wu Q, and Brown MR (2006). Signaling and function of insulin-like peptides in insects. Annu Rev Entomol 51, 1–24. [DOI] [PubMed] [Google Scholar]
- 42.Horner MA, Chen T, and Thummel CS (1995). Ecdysteroid regulation and DNA binding properties of Drosophila nuclear hormone receptor superfamily members. Dev Biol 168, 490–502. [DOI] [PubMed] [Google Scholar]
- 43.Quack M, Frank C, and Carlberg C (2002). Differential nuclear receptor signalling from DR4-type response elements. J Cell Biochem 86, 601–612. [DOI] [PubMed] [Google Scholar]
- 44.Larsen PL, Albert PS, and Riddle DL (1995). Genes that regulate both development and longevity in Caenorhabditis elegans. Genetics 139, 1567–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang F, Vought BW, Satterlee JS, Walker AK, Jim Sun ZY, Watts JL, DeBeaumont R, Saito RM, Hyberts SG, Yang S, et al. (2006). An ARC/Mediator subunit required for SREBP control of cholesterol and lipid homeostasis. Nature 442, 700–704. [DOI] [PubMed] [Google Scholar]
- 46.Ludewig AH, Kober-Eisermann C, Weitzel C, Bethke A, Neubert K, Gerisch B, Hutter H, and Antebi A (2004). A novel nuclear receptor/coregulator complex controls C. elegans lipid metabolism, larval development, and aging. Genes Dev 18, 2120–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Steffensen KR, Robertson K, Gustafsson JA, and Andersen CY (2006). Reduced fertility and inability of oocytes to resume meiosis in mice deficient of the Lxr genes. Mol Cell Endocrinol 256, 9–16. [DOI] [PubMed] [Google Scholar]
- 48.Couse JF, Hewitt SC, Bunch DO, Sar M, Walker VR, Davis BJ, and Korach KS (1999). Postnatal sex reversal of the ovaries in mice lacking estrogen receptors alpha and beta. Science 286, 2328–2331. [DOI] [PubMed] [Google Scholar]
- 49.Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, and Smithies O (1998). Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci U S A 95, 15677–15682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Han SI, Komatsu Y, Murayama A, Steffensen KR, Nakagawa Y, Nakajima Y, Suzuki M, Oie S, Parini P, Vedin LL, et al. (2014). Estrogen receptor ligands ameliorate fatty liver through a nonclassical estrogen receptor/Liver X receptor pathway in mice. Hepatology 59, 1791–1802. [DOI] [PubMed] [Google Scholar]
- 51.Joseph SB, McKilligin E, Pei L, Watson MA, Collins AR, Laffitte BA, Chen M, Noh G, Goodman J, Hagger GN, et al. (2002). Synthetic LXR ligand inhibits the development of atherosclerosis in mice. Proc Natl Acad Sci U S A 99, 7604–7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.