Abstract
Over the last several decades zebrafish (Danio rerio) has become a major model organism for the study of vertebrate development and physiology. Given this, it may be surprising how little is known about the mechanism that zebrafish use to determine sex. While zebrafish are a gonochoristic species (having two sexes) that do not switch sex as adults, it was appreciated early on that sex ratios obtained from breeding lab domesticated lines were not typically a 1:1 ratio of male and female, suggesting that sex was not determined by a strict chromosomal mechanism. Here we will review the recent progress toward defining the genetic mechanism for sex determination in both wild and domesticated zebrafish.
1. Introduction to zebrafish sexual phenotypes
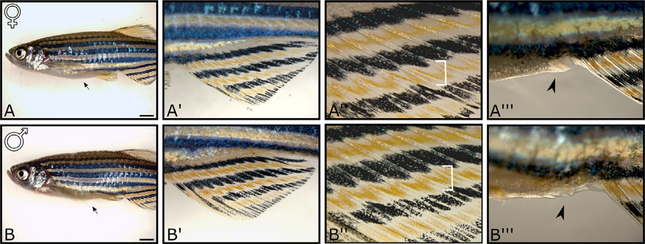
Adult zebrafish males and females can be distinguished based on several sexually dimorphic phenotypes. The three most useful external dimorphisms for identifying the sex of an adult zebrafish are described here. First, because ovaries are significantly larger than testes, females, in general, have a larger abdomen (Fig. 1A and B). A second reliable indicator is the dimorphic color of the anal fin and abdomen. One of the main pigment cell types in zebrafish are the xanthaphores, which form a yellow strip of pigment that lies between the characteristic black pigment stripes formed by melanophores which give zebrafish their name. While xanthaphores are present in both males and females, males produce significantly more yellow pigment than females, and therefore appear more yellow when viewed under the appropriate light. Differences in color are most apparent on the anal fin (Fig. 1A′, B′ and A″, B″). A third and perhaps most reliable distinguishable feature between males and females is the appearance of the genital pore, which is located on the ventral midline of the abdomen just anterior to the anal fin and posterior to the anus (Menke, Spitsbergen, Wolterbeek, & Woutersen, 2011; Fig. 1A‴, B‴). As the name implies, the genital pore is the opening through which the gametes are released. When viewed with the aid of a dissecting microscope, the female genital pore protrudes from the body surface and has characteristic longitudinal ridges (Fig. 1A‴). By contrast, the genital pore of males does not protrude from the body surface and therefore can only be seen when viewed from the ventral surface (Fig. 1B‴). Finally, there are subtler dimorphic structures, such as the presence of mating tubercles on the dorsal surface of the male pectoral fins, but these are more difficult to observe and therefore less commonly used to distinguish between sexes (Kang, Nachtrab, & Poss, 2013).
Fig. 1.
Sexually dimorphic phenotypes that distinguish adult female and male zebrafish. 6-month-old female (A–A‴) and male (B–B‴) zebrafish. Low magnification view shows that females (A) have a larger abdomen (arrow) then males (B). Low magnification view of the anal fins (A′ and B′) shows that females have lighter yellow pigmentation then males. High magnification view of the anal fins (A” and B”) shows that the yellow pigment stripes in females are narrower than those in males (brackets). The genital papilla protrudes from the ventral body surface in females (arrow in A‴), but not in males (arrow in B‴). Scale bar in A and B, 2mM.
2. Wild zebrafish, but not domesticated lines, utilize a ZZ/ZW chromosomal sex determination mechanism
The major domesticated zebrafish lines that are widely used in the laboratory were all derived from fish that were obtained from pet stores. For example, the fish used by George Streisinger to produce the AB line were obtained from a pet store in Albany, Oregon, while those used to derive the TU (Tübingen) line originated from a pet store in Germany (Mullins, Hammerschmidt, Haffter, & Nüsslein-Volhard, 1994; Streisinger, Walker, Dower, Knauber, & Singer, 1981; Wilson et al., 2014). Importantly, because these lines were developed to be used for forward genetic screens, they were initially selected to be free of recessive lethal mutations (Mullins et al., 1994; Streisinger et al., 1981). It is possible that this selection inadvertently affected loci that regulated sex determination.
Early on it was recognized that the ratio of males to females in the domesticated lines could vary widely, suggesting an atypical sex determining mechanism (Streisinger et al., 1981). However, repeated mating of specific pairs of fish from the domesticated AB, TU and Toh lines produced progeny with reproducible sex ratios in a pair-specific manner, providing evidence that genetics plays an influential role in sex determination (Liew et al., 2012).
To further investigate the genetic component of sex determination in zebrafish, three independent groups used genome-wide association (GWA) methodsto identify loci that contributed to sex determination in domesticated lines. While these studies confirmed that no major chromosomal sex-determining locus existed, they each found evidence for a more complex polygenic sex determining system where several unlinked loci each make measurable contributions to sex determination (Anderson et al., 2012; Bradley et al., 2011; Howe et al., 2013; Liew et al., 2012). Importantly, no two groups used the same genetic background for their analysis and as a result, each group identified different contributing loci. These studies therefore suggest that a polygenic system is regulating sex in domesticated lines, but that the particular genes involved vary between the independently selected lines.
While GWA studies of domesticated lines clearly pointed to a strain-specific polygenic system of sex determination, they conflicted with cytogenetic analysis by Sharma, Sharma, and Tripathi (1998) that found evidence that zebrafish collected from the wild possessed heteromorphic chromosomes consistent with a ZZ/ZW chromosomal sex determining system, where the heterogametic ZW animals were females and the homogametic ZZ animals were male. To further investigate this difference Wilson et al. (2014) performed a comparative GWA study using both domesticated lines as well as lines derived from fish collected more recently from the wild. This study included three types of lines: (1) the domesticated lines AB and TU that had been subjected to genetic selection, (2) The EKW (EkkWill) and WIK (Wild India Kolkata), lines that were derived from natural populations but cultured in the lab for many generations without genetic selection, and (3) two strains, NA (Nadia) and CB (Cooch Behar), that were derived from fish more recently collected from wild populations. In accordance to the previous work, this study did not identify any major sex-linked loci in the domesticated lines AB and TU. By contrast, all lines derived from wild-caught fish, regardless of how many generations in the lab, possessed the same significantly sex-liked locus that mapped to the end of the long arm of chromosome 4 (Ch 4q). While the molecular identity of this locus has yet to be determined, these data indicate that wild zebrafish utilize a ZW/ZZ sex determining system, in which female development was associated with the presence of the W chromosome. However, presence of the W chromosome did not guarantee female development as some ZW individuals develop as fertile males. This result suggested that female development requires either the presence of a female promoting factor that localizes to the W chromosome, or that female development can only occur if a single copy of a Z-linked male promoting factor(s) is present (Wilson et al., 2014).
The diversity of loci discovered to contribute to zebrafish sex determination suggests that the genetic mechanism is labile and potentially evolving either toward or away from a more rigid chromosomally-based system in the wild. Although the original analysis by Sharma and colleagues provided evidence for cytogenetically distinct Z and W chromosomes, similar to the mammalian X and Y analysis (Sharma et al., 1998), the molecular analysis of Wilson et al. (2014) did not find significant sequence differences between the Z and W chromosomes, suggesting that any molecular differences between these chromosomes is minor. However, the NA-, WIK- and CB-derived strains originated from fish collected in regions that were 2000km from those used by Sharma et al., 1998 (Mansar Lake, Jammu, India). It is therefore possible that differences between the results of these studies are due to region-specific differences in the karyotypes of zebrafish sex chromosomes (Sharma et al., 1998; Wilson et al., 2014).
3. Overview of zebrafish gonad development and timing of sex determination and differentiation
The primary function of any sex determining mechanism is to specify the sexual characteristic of the somatic gonad cells, which are the major producers of the circulating sex hormones 11-ketotestosterone, the primary male androgen in teleost, and estradiol in females (see, Devlin & Nagahama, 2002 for review). These hormones then instruct the rest of the somatic cells in the body to adopt the sex appropriate appearance and also promote sex appropriate behaviors. Understanding sex determination in zebrafish can therefore be reduced to understanding how cells of the somatic gonad determine their sexual phenotype. While sexual differentiation is likely initiated between 20 and 25 days post-fertilization (dpf ) in zebrafish (see below), sexual maturation, which is defined as the ability to mate, is not complete until zebrafish are 2.5–3months old. Once determined, zebrafish maintain their sex throughout life. Thus, gonad development can be separated into three steps: sex determination, differentiation and maintenance.
Initial studies looking at the timing of sex differentiation in the gonad showed that all zebrafish gonads go through a transient female-like phase during early larval life (approx. 13–25 dpf ), where all larvae produce some early-stage oocytes (Takahashi, 1977). It is therefore likely that sex determination occurs during this period. For animals that were determined as females, the early-stage oocytes they produced continue to mature. By contrast, for animals that were determined as males, their early-stage oocytes undergo apoptosis as the gonads transition from a proto-ovary into a functional testis. Because gonads during this transition period can contain both female- and male-type germ cells, zebrafish have been referred to as juvenile hermaphrodites (Takahashi, 1977). It should be noted that the times listed below are approximate and can vary between studies due to differences in rearing conditions.
3.1. Embryogenesis to 8 dpf
Primordial germ cells (PGC) in zebrafish are the first cell type to be specified in the developing embryo (Yoon, Kawakami, & Hopkins, 1997). PGCs can first be identified based on the localization of several maternally-supplied transcripts that encode germ cell-specific factors, such as Vasa and Nanos3 (Köprunner, Thisse, Thisse, & Raz, 2001; Yoon et al., 1997). During gastrulation the PGCs begin to migrate to the site of the future gonad, reaching this target before the end of the first day of development. One-day old embryos have an average of 30 PGCs that are distributed into two bilateral domains flanking the midline and adjacent to somite 5 (Weidinger et al., 2002; Yoon et al., 1997). While it is not known exactly when somatic gonad precursor cells first arise, somatic cells in close association with germ cells were identified in histological sections as early as 5 dpf (Braat et al., 1999). Between 1 and 8 dpf, germ cell numbers did not appear to increase suggesting that PGCs are quiescent during early larval development (Leerberg, Sano, & Draper, 2017; Tzung et al., 2015). Expression of genes involved in sex determination or differentiation of the gonads prior to 8 dpf have not been reported.
3.2. The larval bipotential/undifferentiated gonad (8–20 dpf)
The quiescent phase of early zebrafish gonad development appears to end around 8–10 dpf when, based on cell counts, EdU incorporation and detection of phospho-histone 3, somatic gonad cells, followed by germ cells, begin to proliferate (Leerberg et al., 2017; Tzung et al., 2015). As in most vertebrates, there is evidence that the zebrafish larval gonad is initially bipotential. The expression of many genes known or predicted to be involved in male and female sex determination or differentiation in other vertebrates were detected in zebrafish somatic gonad cells starting around 8 dpf (Leerberg et al., 2017; Rodríguez-Marí et al., 2005). For example, expression of cyp19a1a, which encodes aromatase that converts androgens to estrogen in females was detected in all gonads, even though approximately half of these will eventually develop as testes (Leerberg et al., 2017; Rodríguez-Marí et al., 2005). Similarly, cells expressing the gene encoding the TGF-ß ligand anti-Müllerian hormone, amh, were also detected in all gonads during this time period (Leerberg et al., 2017; Rodríguez-Marí et al., 2005). Importantly, at this stage the cells with male characteristic gene expression were distinct from those with female characteristic gene expression, indicating that early zebrafish gonads are composed of an apparent mosaic of male- and female-type cells (Leerberg et al., 2017).
During the bipotential period, early-stage oocytes (Stage 1a, also called perinuclear-stage oocytes; Selman, Wallace, Sarka, & Qi, 1993) were detected in all gonads starting around 13–14 days post-fertilization, indicating that germ cells initially follow a female-type mode of development regardless of the eventual sex of the animal (Takahashi, 1977; Tzung et al., 2015; Uchida, Yamashita, Kitano, & Iguchi, 2002). Between 13 to ~20 dpf the gonad increases in size due to continued proliferation of both germ cells and somatic gonad cells, though not all gonads appeared to increase in size at the same rate (Leerberg et al., 2017; Tzung et al., 2015).
3.3. Sex-specific differentiation (20–25 dpf)
The first signs of overt sexual differentiation begin around 20–25 dpf when the oocytes that are present in presumptive males begin to undergo apoptosis (Uchida et al., 2002). This period is often referred to as “transitioning” because these gonads are transitioning from an initial ovary-like development to definitive testis development. By contrast, oocytes in animals that will become females continue to mature (Uchida et al., 2002). The transitioning phase of testis development appeared to be over by ~30 dpf, when the majority of oocytes had been cleared from gonads that will differentiate as testes (Uchida et al., 2002). While sex of a fish cannot be reliably assigned based on the external criteria until ~2.5–3 months of age, histological analysis of 30 dpf gonads can reveal the eventual fate of the gonad, and therefore sex of the fish, as ovaries are significantly larger than testes at this time (Takahashi, 1977). Because of this difference in size, it is therefore possible to select for fish of a particular sex at 30 dpf based on the expression levels of various germ cell-expressed transgeneic reporter lines, such as Tg(vas:egfp) and Tg(ziwi:egfp) (Dranow et al., 2016; Krøvel & Olsen, 2002; Wang, Bartfai, Sleptsova-Freidrich, & Orban, 2007).
4. Oocytes are required for primary female sex determination
While the genetic determinants of primary sex determination have yet to be discovered in domesticated or wild zebrafish strains, it has become apparent over the last decade that germ cells, and in particular, oocytes, are a key regulator of gonadal sex determination in domesticated zebrafish. The first key discovery was that ablation of all germ cells during the first day of development resulted in all-male development (Slanchev, Stebler, de la Cueva-Méndez, & Raz, 2005). It was later shown that while these fish were sterile, the somatic cells of their testes expressed the appropriate male-specific genes, suggesting that formation of ovarian somatic cell types, but not testicular cell types, was dependent on germ cells (Siegfried & Nüsslein-Volhard, 2008). Consistent with these findings, mutations that caused germ cell loss during the first several days of development, such as ziwi/piwil1 and nanos3, resulted in animals that developed as phenotypic, sterile males (e.g., Draper, McCallum, & Moens, 2007; Houwing et al., 2007). These studies argued that germ cells were required for female development, but the mechanism by which they promoted female sexual development was not identified.
A critical clue to how germ cells promote female development came from studies of fancl mutants (Rodríguez-Marí et al., 2010). fancl encodes a component of the Fanconi Anemia/BRACA DNA repair pathway and is expressed in early meiotic oocytes, including those that form during the bipotential stage of gonad development. It is likely that Fancl functions to repair Spo11-induced double-strand DNA breaks that are required for the initiation of meiotic recombination. fancl mutants had normal numbers of germ cells at the early larval stage and, like wild-type, all fancl mutants initially produce early-stage oocytes during the bipotential stage. However, unlike wild-type, all fancl mutant oocytes underwent apoptosis during the late larval stage, presumably due to their inability to repair Spo11-induced double strand DNA breaks. By ~30 dpf, all mutant gonads resembled wild-type testes and as adults, all mutants were male. The oocyte loss and sex reversal phenotypes could be rescued by producing fancl;tp53 double mutants, which were unable to activate the Tp53-dependent apoptosis pathway, indicating that oocyte apoptosis was the primary reason why all gonads differentiated as testes. Thus, while previous studies showed the importance of germ cells for female development, this study identified that meiotic oocytes are the key germ cell-stage that promotes ovarian, and thus female, development in domesticated zebrafish. Since this landmark study, other mutants have been described that disrupt oocyte meiosis and/or cause early germ cell loss, and like fancl, result in an all-male phenotype (e.g., zilli, tdrd1, vasa, mps1; Hartung, Forbes, & Marlow, 2014; Houwing, Berezikov, & Ketting, 2008; Huang et al., 2011; Poss, Nechiporuk, Stringer, Lee, & Keating, 2004).
From these studies the general hypothesis emerged that oocytes produce a signal that acts on somatic gonad cells to stabilize the expression of ovary development-promoting genes. Further, it was hypothesized that a threshold amount of this signal was required to stabilize ovarian development and absent this, the somatic gonad upregulates testis-promoting genes. If each oocyte can produce a set amount of signal, then the total amount of signal will be directly correlated to the number of oocytes produced. In turn, the number of oocytes produced by each larva is likely to be proportional to the number of germ cells each animal has at the beginning of the bipotential stage of gonad development. As such, animals with more germ cells are more likely to develop as female. If true, the number of germ cells each animal has at the start of the bipotential stage should not be uniform within a population.
To begin to test this, Tzung and colleagues quantified total germ cell numbers in animals at 7 and 14 dpf, a time before and at the start of the bipotential stage, respectively (Tzung et al., 2015). At 7 dpf, they found that animals had a normal distribution of germ cells, with the average being 26–30 PGCs per animal. By contrast, at 14 dpf, they found that germ cell numbers followed a bimodal distribution, where nearly half of the animals had an average of ~40 PGCs while the other half had an average of ~90 PGCs. As a more direct test of the hypothesis, they experimentally manipulated the numbers of PGCs by injecting dilute amounts of antisense morpholino oligos (MO) targeting the germ cell-localized dead-end (dnd) mRNA into fertilized eggs, as reduction of dnd function leads to loss of PGCs (Weidinger et al., 2003). Consistent with the hypothesis, dnd MO injected animals, but not control animals, had an increase in the number of animals that developed as male. Taken together these results strongly argue that the numbers of germ cells an animal has during the bipotential stage has a strong influence whether an ovary or a testis forms, and thus the eventual sexual fate of the animal. Given the strong evidence that a polygenetic sex determining mechanism influences sex ratios in domesticated zebrafish, it is tempting to speculate that the genes involved function to directly or indirectly regulate PGC proliferation during the early larval stage. It will be important to repeat these experiments in zebrafish strains that have maintained the ancestral ZZ/ZW chromosomal sex determining system to determine if oocyte signaling is also required for female development in these lines.
5. Maintenance of the sexual phenotype
There is growing evidence that sexual determination and differentiation in vertebrates are not irreversible once established, but instead require constant expression of sex-maintenance genes, such as the male- and female-specific transcription factors Dmrt1 and FoxL2, respectively. While mammals do not normally switch sex as adults, it is estimated that 2% of teleost species switch sex at some point during their life and in some species can switch sex multiple times (Avise & Mank, 2009). However, zebrafish do not belong to the 2% of species that switch sex as part of their normal life cycle. It was therefore a surprise that when oocytes were depleted in an adult zebrafish female, either by genetic mutation or chemical ablation, that the fish readily sex reversed to males that, in some cases, were fertile (Dranow, Tucker, & Draper, 2013; Dranow et al., 2016). This was first discovered through analysis of nanos3 mutants, which showed loss of oocytes in adults. Nanos3 is required for germline stem cell maintenance in zebrafish ovaries but is not required for the initial wave of oocytes that are produced during the bipotential stage. This led to a situation in which germline stem cells were normally specified in mutant ovaries such that nanos3 mutants were able to become females (Beer & Draper, 2013; Draper et al., 2007). However, the germline stem cell population was not maintained and all premeiotic germ cells entered meiosis by 40 dpf (Beer & Draper, 2013). Once all eggs were depleted by either spawning or atresia, which occurred between 4 and 5 months post fertilization (mpf), all mutant females sex-reversed to males, as assayed by their appearance and their ability to induce wild-type females to spawn. Not surprisingly, these sex-reversed males were sterile given that all germ cells were lost prior to sex reversal. By contrast, when oocytes were ablated but premeiotic germ cells, including germline stem cells, were left unaffected, the sex reversed males were fertile, likely a result of the germline stem cells switching from producing oocytes to producing functional sperm (Dranow et al., 2016, 2013). These results clearly showed that oocytes play an essential role in both primary sex determinations, as detailed above, and throughout life to maintain the differentiated state of the ovarian somatic cells, which in turn assures continued production of the female sex hormone estradiol.
6. Zebrafish sex determination can be influenced by environmental factors
While it is now clear that zebrafish in the wild utilize a genetic sex determination mechanism, it is also clear that sex ratios in domesticate lines can be greatly influenced by certain environmental factors, such as temperature, pH, oxygen concentration and rearing density. However, because these environmental factors cannot fully explain normal sex ratios, sex of domesticated zebrafish is likely not regulated by a strict environmental sex determining system. Instead, given that all of these factors likely induce stress (e.g., high temperature or high rearing density), and that these factors always increase the proportions of males in the population, it is possible that all of these factors alter sex ratios by indirectly affecting the proliferation of germ cells during the bipotential phase. Regardless, it is useful to review the environmental parameters that are known to influence sex ratios in zebrafish.
6.1. Temperature
Increased temperature during larval development significantly increases the proportion of males. The standard laboratory rearing temperature for zebrafish is 28.5°C (Westerfield, 2007). Uchida et al. (2004) demonstrated that when fish were reared during the critical period of gonad determination (15–20dpf ) at 35 °C, 68.8% of the expected females developed as male. When the rearing temperature was increased to 37 °C, 100% of the expected females developed as male. Indicating that higher temperature can cause female-to-male sex reversal. Although increased temperature caused sex reversal, it also significantly impacted survival. At 37 °C only 54.7% of the individuals survived and no individuals survived at 39 °C. Thus, although temperature may influence gonad development, decreases in survivability at these temperatures suggest that this may be primarily a response to stress.
6.2. Dissolved oxygen content
Similarly, reduced dissolved oxygen content in the water increases the proportion of males. Zebrafish are able to develop normally within a wide range of oxygen concentrations (Padilla & Roth, 2001). Normal water oxygen content at 28.5 °C is 5.8mg/L and rearing individuals at this temperature will cause about 61.9% of fish to develop as male (Shang, Yu, & Wu, 2006). If zebrafish were reared in hypoxic conditions, 0.8mg/L, 74.4% of individuals would develop as male (Shang et al., 2006).
6.3. Food availability
It has often been anecdotally observed that rearing density affects sex ratios in laboratory zebrafish, where high rearing density leads to increased proportions of males. It is likely that food availability is what drives this phenomenon (Lawrence, 2007). Lawrence, Ebersole, and Kesseli (2008) observed that when food availability doubles the proportion of individuals that developed as female significantly increased from 51% to 76% females. Interestingly, a significant interaction between food and the proportion of females was observed in both selected domesticated strain (TU) and Non-selected domesticated strains (WIK) that have a ZZ/ZW karyotype. While not addressed in this study, it is likely in the WIK strain that the increase in females was due to an increased proportion of ZW individuals developing as female. It will be interesting to determine how food availability influences germ cell numbers during the bipotential gonad stage.
7. Genetic analysis of zebrafish sex determining pathway
Studies to identify genes involved in zebrafish sex determination and differentiation have, until recently, been hampered by lack of methods to obtain genetic mutations. Few mutants affecting sex determination or differentiation were identified in any of the major forward genetic screens because these screens were largely focused on identifying recessive lethal mutations that had phenotypes that manifest during the first 5 dpf (Driever et al., 1996; Haffter & Nusslein-Volhard, 1996). In addition, screens for mutations that affect sex determination would be complicated by the highly variable sex ratios obtained in crosses of domesticated zebrafish. However, with the recent advances in genome editing reverse genetic technologies, such as TALENs and CRISPR/Cas9, it is now possible to directly assay the function of candidate genes using loss-of-function mutants. Below we review the current progress that has been made in this area. It should be noted that in all cases reviewed below, the mutants have been produced using one of the domesticated zebrafish strains, not a strain that contains the wild sex chromosomes. In addition, only genes for which there are data available from genetic mutations are present (i.e., genes that have support only from Morpholino-based knockdown experiments are not presented). We will first review the genes that function to promote male sex determination and/or differentiation, and then those that promote female.
8. Male-promoting genes
8.1. Double-sex and Mab-3 related transcription factor 1 (Dmrt1)
The first genes involved in sex determination and differentiation that were found to be widely conserved across animals were orthologs of the Drosophila double-sex and C. elegans mab-3 transcription factors (Raymond et al., 1998). In vertebrates the orthologous genes are referred to as Dmrt’s, for double-sex mab-3 related transcription factors. In mammals Dmrt1 is located on an autosome where it functions downstream of SRY, and as such is not involved in the initiation of sex determination, but instead in sex differentiation. By contrast, in several other species Dmrt orthologs have been identified as the major chromosomal sex determinant. For example, in the Japanese Medaka fish, which utilizes an XX/XY sex determination system, a paralog of dmrt, called dmY, is located on the Y chromosome where it functions to initiate male sex determination, analogous to Sry in mammals (Matsuda et al., 2002). Interestingly, while Dmrt1 is not the male sex determinant in mammals, forced expression of Dmrt1 in XX animals caused femaleto-male sex reversal, indicating that it has the ability to drive male sex determination (Zhao, Svingen, Ng, & Koopman, 2015).
The zebrafish genome contains a single ortholog of dmrt1, and its role in zebrafish sexual development was directly tested using genetic point mutations generated by ENU mutagenesis as well as indel mutations generated using either TALEN or CRISPR/Cas9 genome editing (Lin et al., 2017; Webster et al., 2017). It was found that the majority of dmrt1 mutants developed as females, consistent with the role of Dmrt1 orthologs in promoting male development. Interestingly, a small percentage of dmrt1 mutants developed as phenotypic, though sterile, males, indicating that Dmrt1 is not absolutely required for the development of male secondary sexual characteristics. It is possible that even in the absence of Dmrt1, a threshold level of the female-promoting oocyte signal is still required for female development. If so, then the dmrt1 mutants that developed as males likely produced too few oocytes during the bipotential stage to stabilize female development. That these males were sterile is consistent with the known germ cell-autonomous role of Dmrt1 in mice (Matson et al., 2010; Zhang, Oatley, Bardwell, & Zarkower, 2016). However, it has yet to be determined in zebrafish if dmrt1 is also cell autonomously required for germ cell survival in males.
8.2. Anti-Müllerian Hormone (Amh)
Amh is a member of the Tgf-ß superfamily of growth factors (Cate et al., 1986; Picard, Goulut, Bourrillon, & Josso, 1986). Mammalian early embryos initially form precursors for both the male and female-specific reproductive tracts, called Wolffian and Müllerian ducts, respectively. In mammals, AMH is expressed in fetal Sertoli cells in males and granulosa cells in adult females (Josso et al., 1993; Munsterberg & Lovell-Badge, 1991). Although Amh mutant females developed normally, loss of Amh function in males resulted in persistence of the Müllerian ducts, indicating that AMH functions to inhibit Müllerian duct development (Behringer, Finegold, & Cate, 1994; Belville, Josso, & Picard, 1999). In addition, although Amh mutant testes had Leydig cell hyperplasia, they were able to produce functional sperm (Behringer et al., 1994). Thus, in mammals, AMH is required for aspects of sexual differentiation, but not for sex determination.
By contrast to mammals, in some teleost fish, Amh, or its bone morphogenetic protein (BMP) type 2 receptor, Amhr, has evolved to be the master sex determining gene. For example, in two species of pejerrey a duplicate copy of amh located on the Y chromosome (amhy) appears to be the male sex determinant (Hattori et al., 2012; Yamamoto, Zhang, Sarida, Hattori, & Strüssmann, 2014). In zebrafish, as in mammals, amh is expressed in Sertoli cells in males, and to a lesser extent in granulosa cells that surround stage II and older oocytes in females (Rodríguez-Maríet al., 2005). The role of Amh in zebrafish was investigated using CRISPR/Cas9-induced mutations, and it was found that loss of amh caused a female-biased sex ratio, but that males could develop in the absence of amh function (Lin et al., 2017). Though mutant males had normal fertility at 2.5 mpf, they gradually lost fertility and were sterile by 6 mpf. As mutant animals aged, their abdomens became distended due to gonadal hyperplasia. Based on histology and marker gene analysis, 6 mpf testes contained mostly early stage proliferative germ cells but few meiotic spermatocytes or mature sperm. Though amh is commonly referred to as a male-specific factor, amh is expressed in granulosa cells in the ovary and amh mutant females had similar hyperplasic gonads to those of mutant males. Together these results suggest that in both adult males and females Amh plays a role in limiting germ cell proliferation while promoting gamete maturation (Lin et al., 2017). The phenotypes observed for zebrafish amh mutants appeared similar to those observed in medaka (Oryzias latipes) amhr2 mutants, called hotei (Morinaga et al., 2007), suggesting that the functions of Amh in sex determination and differentiation are conserved in teleost.
Central to defining the role of amh in zebrafish and the degree to which its functions are conserved, will be the identification of its receptors. In mammals Amh signals through the Bmp receptors, composed of a complex between two type 1 and two type 2 receptors (Gouédard et al., 2000; Jamin, Arango, Mishina, Hanks, & Behringer, 2002). Unique to BMP ligands, AMH uses a dedicated Bmp type 2 receptor, called AMHR2, which is conserved in most vertebrates (Visser, 2003). While AMHR2 orthologs have been identified in many teleost species, one has not been identified in the zebrafish genome raising the possibility that zebrafish Amh signaling utilizes a different Bmp type 2 receptor (Pfennig, Standke, & Gutzeit, 2015). In mammals, mutational analysis of the Bmp type 1 receptor BMPR1A provided strong evidence that this is the main Bmp type 1 receptor used by AMH for inhibition of Müllerian duct development, but unlike AMHR2, this receptor was not specific for the AMH ligand (Jamin et al., 2002). Interestingly, a mutation in the zebrafish Bmp type 1 receptor encoded by bmpr1bb, a zebrafish ortholog of mammalian BMPR1B, had a phenotype that was nearly identical to that of amh mutants, raising the possibility that this receptor serves as the Bmp type 1 receptor for Amh in zebrafish (Neumann, Dovey, Chandler, Carbajal, & Amatruda, 2009).
8.3. Gonadal soma derived factor (Gdsf)
Gsdf is a new member of the Tgf-ß ligand superfamily that was first identified in the trout testis and that appears to be teleost-specific (Mazurais, Montfort, Delalande, & Le Gac, 2005; Sawatari, Shikina, Takeuchi, & Yoshizaki, 2007). gsdf is expressed in Sertoli cells in the testis and granulosa cells in the ovary (Gautier, Sohm, Joly, Le Gac, & Lareyre, 2011; Sawatari et al., 2007; Shibata et al., 2010). In the Luzon rice fish (Oryzias luzoneseis), Gsdf was identified as the male sex determining gene located on the Y chromosome. While alleles of gsdf were found on both the X and Y chromosome, the Y allele, called gsdfY was expressed at an earlier time-point during development then gsdfX, and early expression of gsdfY in an XX fish led to male development (Myosho et al., 2012). In addition to O. luzoneseis, Gsdf was linked to sex determination in sablefish (Rondeau et al., 2013). In zebrafish, gsdf is expressed in somatic gonadal cells during the bipotential stage and in adults is expressed in granulosa and Sertoli cells (Gautier et al., 2011). gsdf is located on zebrafish chromosome 21, and loss of gsdf function had no apparent effect on sex ratios (Yan et al., 2017). Instead, mutant females, while initially fertile, developed hyperplastic ovaries that had a progressive loss of late-stage oocytes and increase of early stage oocytes, a phenotype that was strikingly similar to amh mutant females. However, unlike amh mutants, which had defects in testis development and spermatogenesis (Lin et al., 2017), gsdf mutant males had enlarged testes relative to wild-type males, but were otherwise fertile (Yan et al., 2017). Thus, while gsdf plays a role in sex determination in some teleost, it does not appear to influence sex determination in domesticated zebrafish, but instead appears to play a similar role to that of Amh in regulating germ cell proliferation and oocyte maturation in the ovary.
8.4. Androgen receptor (Ar)
Androgens are the male-promoting steroid sex hormones that function by binding to the androgen receptor (AR), a member of the nuclear hormone receptor superfamily (Gao, Bohl, & Dalton, 2005). Upon ligand binding, AR regulates sex-specific gene expression. Mutational analysis in mice showed that AR mutant mice formed a testis but were sterile due to a spermatogenesis defect. In addition, AR mutant male mice do not form normal reproductive tracts and had ambiguous or feminized external genitalia (De Gendt et al., 2004; Yeh et al., 2002). Mutant analysis in female mice showed that AR is also required for oocyte maturation, likely through regulating folliculogeneisis (Gill, Jamnongjit, & Hammes, 2004). In adult male zebrafish, AR is expressed in Sertoli cells (de Waal, Wang, Nijenhuis, Schulz, & Bogerd, 2008). While ar is also expressed in the zebrafish ovary, the particular cell type has not been determined (Hossain, Larsson, Scherbak, Olsson, & Orban, 2008). ar mutant zebrafish had a female biased sex ratio relative to wild-type controls, arguing that androgen signaling plays a role in promoting male sexual development (Crowder, Lassiter, & Gorelick, 2018; Yu et al., 2018). In addition, ar mutant males and females had reduced fertility as adults. ar mutant testes had disorganized tubules and did not release sperm during natural mating. However, mature sperm that were capable of fertilizing wild-type eggs were obtained from dissected mutant testes. Mutant ovaries had an increase in early stage oocytes relative to wild-type. In contrast to males, however mutant females were able to release mature eggs during normal mating, though the numbers released were significantly lower than wild-type females (Crowder et al., 2018). Similar to mice, ar mutant females displayed defects in oocyte maturation (Crowder et al., 2018; Yu et al., 2018).
9. Female-promoting genes
9.1. Nr0b1
The zebrafish ortholog of Human DAX1/NR0B1, called nr0b1, encodes an atypical member of the orphan nuclear hormone receptor transcription factor family that is involved in the development of steroidogenic organs (Zhao et al., 2006). In mice, Nr0b1 is expressed in all tissues that constitute the hypothalamus-pituitary-gonad axis (HPG). In mammals NR0B1 is X-linked and duplication of NR0B1 in human males caused male-to-female sex reversal, while mutations in NR0B1 resulted in adrenal hypoplasia congenita syndrome with associated hypogonadotropic hypogonadism (Bardoni et al., 1994; Battistin et al., 2012). In zebrafish, nr0b1 is expressed in the brain and interrenal gland, the fish equivalent of the adrenal gland, during early development (Zhao et al., 2006). Beginning around 13 dpf, nr0b1 is expressed in somatic cells of the early bipotential gonad, and expression persists into adulthood in both the ovaries and testes. Indel mutations were produced using TALENS, and homozygous nr0b1 mutants developed predominantly as males, though some females were identified (Chen, Zhang, Wang, Zhang, & Peng, 2016). Regardless of sex, all mutants were fertile as adults. To investigate why most mutants developed as males, germ cell numbers were quantified during the bipotential phase and it was found that nr0b1 mutants had, on average, 35% to 45% fewer germ cells than wild-type at 14 dpf and 18 dpf, respectively. It was unlikely that the reduction of germ cells in mutants vs. wild-type was due to germ cell apoptosis, as double nr0b1;tp53 mutants, which cannot activate the Tp53-dependent apoptotic pathway, had similar sex ratios and germ cell numbers to those of nrob1 single mutants. Given the known role of germ cells in promoting female sex determination (see above), this likely explains the skewed sex ratios. Finally, examination of gene expression using RT-qPCR showed that at 13 dpf, mutant larvae had significantly decreased expression of the female-specific aromatase-encoding gene cyp19a1a when compared to wild-type larvae, suggesting that nr0b1 functions during the early bipotential stage to promote female development (Chen et al., 2016). Given that loss of cyp19a1a alone did not appear to alter early germ cell proliferation (Dranow et al., 2016) it is possible that nr0b1 independently regulates cyp19a1a expression and germ cell proliferation. Together these results suggest that nr0b1 influences sex determination by regulating both germ cell proliferation and expression of the pro-female cyp19a1a gene during the bipotential stage of gonad development.
9.2. Wnt4a
In mammals, Wnt4 is required for primary female sex determination where it antagonizes the action of the male-promoting FGF9 ligand during the bipotential stage of gonad development (Vainio et al., 1999). Although Wnt4 orthologs have been identified in teleost, including zebrafish, Fgf9 is the only Fgf ortholog not present in teleost genomes. It has therefore remained an open question whether Wnt4 signaling is necessary for sex determination in teleost. As a first test of the role of Wnt signaling in zebrafish sex determination, Sreenivasan et al. (2014) ectopically expressed the Wnt antagonist dikkof1 (dkk1) using a heat shock promoter and found that the majority of zebrafish developed as males. This result strongly argued that canonical Wnt signaling is required for female sex determination in zebrafish (Sreenivasan et al., 2014).
The zebrafish genome contains two Wnt4 orthologs, called wnt4a and wnt4b (Liu, Majumdar, Schauerte, Haffter, & Drummond, 2000; Ungar, Kelly, & Moon, 1995). Though many of the gene duplicates that are present in zebrafish resulted from the teleost-specific whole genome duplication that occurred after this lineage diverged from that of tetrapods, phylogenetic analysis performed by Kossack et al. (2019) argues instead that the duplication event that resulted in wnt4a and wnt4b occurred prior to the divergence of teleost and tetrapods. First, while tetrapod genomes contain a single Wnt4 gene, two Wnt4 genes are present in more basal tetrapods, such as turtles and Coelacanths. Sequence comparisons show that the Wnt4 orthologs group into two clear clades, called Wnt4a and Wnt4b, arguing for a common and more ancient origin. The single mammalian WNT4 groups within the WNT4a clade, arguing that the WNT4b ortholog was lost prior to the emergence of mammals. In zebrafish, expression of wnt4a, but not wnt4b, was detected in gonads during the bipotential phase, after which its expression becomes restricted to somatic cells in the ovary. Thus, the expression pattern of wnt4a in zebrafish is similar to the expression of Wnt4 in mammals (Vainio et al., 1999). Kossack et al. (2019) tested the role of Wnt4a in sex determination using wnt4a point mutants and CRISPR/Cas9-induced indel mutants. They found that while wild-type siblings had normal ratios of males and females (near 50:50), ~95% of wnt4a mutant animals developed as males. The few wnt4a mutants that developed as females appeared to have normal ovarian development. It was proposed that Wnt4a sensitizes the somatic gonad cells to the proposed female promoting oocyte signal that is required during the bipotential stage for stabilizing female development. If so, then only animals that produce the most oocytes during the bipotential stage can develop as females. Thus, Wnt4a appears to promote female sex determination, but is not absolutely required for female development.
In addition to its role in promoting female sex determination, in mammals WNT4 is also required for development of the Müllerian ducts into the fallopian tubes and uterus in females. Though the similarities between reproductive duct development in fish and mammals were unknown, both male and female wnt4a mutants failed to develop functional reproductive ducts (Kossack et al., 2019). This result argues that Wnt4 has an ancient role in promoting reproductive duct development and that the teleost reproductive ducts and the mammalian Müllerian ducts may share a common evolutionary origin.
9.3. Cyp19a1a, Aromatase
Regardless of what genes functions as upstream regulators of sex determination in zebrafish, one of the key downstream steps must be to activate that production of the sex-appropriate hormone: the androgen 11-ketotestosterone in males, and estrogen in females. Estrogen is the key female sex hormone that regulates secondary sex differentiation in mammals, and pharmacological manipulation of estrogen production in zebrafish indicated it plays a key role in sex determination and/or differentiation (Fenske, Maack, Schäfers, & Segner, 2005; Guiguen, Fostier, Piferrer, & Chang, 2010; Uchida, Yamashita, Kitano, & Iguchi, 2004). The final step in estrogen production is catalyzed by Cyp19a1 aromatase in mammals, which is expressed in ovarian granulosa cells. The zebrafish genome contains two ohnologs of Cyp19a1, called cyp19a1a and cyp19a1b, which are expressed in the ovary and brain, respectively (Chiang, Yan, et al., 2001). Two cell types are required for estrogen production in mammals: androgens produced by theca cells are converted to estrogen by CYP19A1/Aromatase, which is expressed in granulosa cells. By contrast, in zebrafish, cyp19a1a is expressed in both theca cells and granulosa cells, but its expression in granulosa cells is restricted to those that surround oocytes that have advanced past mid-Stage II (Dranow et al., 2016; Rodríguez-Marí et al., 2005). Similar expression had been reported in medaka suggesting that, in contrast to mammals, both theca and granulosa cells are capable of estrogen production in teleost (Nakamura, Kurokawa, Asakawa, Shimizu, & Tanaka, 2009).
Loss-of-function mutations in both cyp19a1a and cyp19a1b have been produced via TALEN and CRISPR/Cas9 (Dranow et al., 2016; Yin et al., 2017). While cyp19a1b mutants had no discernable defects in the brain or gonad, all cyp19a1a mutants developed as fertile males. Analysis of the developing mutant gonads showed that cyp19a1a mutants produced oocytes during the bipotential stage of gonad development, like wild-type animals, but that these oocytes underwent apoptosis as animals transitioned to spermatogenesis. By contrast to mammals, where estrogen is required for maturation of sperm in males (Robertson et al., 1999), zebrafish cyp19a1a mutant males appeared fully fertile (Dranow et al., 2016; Yin et al., 2017).
Interestingly, while mature sperm were not evident in wild-type zebrafish gonads until ~60 dpf, mature sperm were found in some cyp19a1a mutant gonads as early as 35 dpf (Dranow et al., 2016). This later result suggests that during normal development, cyp19a1a activity during the bipotential phase delays the time when overt testis differentiation can occur in individuals that will become male. To date, cyp19a1a mutants have the most penetrant early sex reversal phenotype, with 100% of prospective female animals being fully sex-reversed by 40 dpf. This result suggests that cyp19a1a plays an early role in female gonad function.
10. Genes required for maintenance of female sex differentiation
10.1. FoxL2a and FoxL2b
In mammals, the transcription factor FoxL2 is expressed in granulosa cells and loss of FoxL2 in adults results in female-to-male sex reversal of the somatic gonad (Uhlenhaut et al., 2009). FOXL2 is proposed to form a mutually antagonistic interaction with the male promoting transcription factor DMRT1. Both FOXL2 and DMRT1 are continually expressed in adult gonads and if the function of either gene is lost postnatally, the other gene is upregulated in somatic gonad cells resulting in partial sex reversal of the gonad (Matson et al., 2011; Uhlenhaut et al., 2009). Thus, the sexual phenotype of the somatic gonad must be actively maintained by the function of these factors.
Zebrafish have two ohnologs of Foxl2, called foxl2a and foxl2b, which were derived during the teleost-specific whole genome duplication (Yang, Wang, Li, & Gui, 2017). As in mice, foxl2a and foxl2b appeared to be expressed only in ovarian granulosa cells. Granulosa cells that surrounded early stage oocytes expressed the highest levels of foxl2a (stage I–II; Selman et al., 1993), while foxl2b was expressed in follicle cells around all oocytes (Stage I–IV). The functions of foxl2a and foxl2b were tested using TALEN-induced Indel (Yang et al., 2017). Single mutants for foxl2a had normal sex ratios. foxl2a mutant ovaries were indistinguishable from wild-type controls up to 150 dpf. However, by 180 dpf, mutant ovaries showed signs of premature ovarian failure (POF) as they contained no oocytes more mature than stage I. Thus, foxl2a is not required for initial oogenesis, but instead is required for maintaining oogenesis. foxl2b mutant ovaries followed a similar developmental trajectory to that of foxl2a mutants, undergoing POF by 150 dpf. However, in contrast to foxl2a mutants, following POF, the ovaries of foxl2b mutant underwent a partial sex reversal around 270 dpf, and contained regions of germ cells that were undergoing spermatogenesis. Finally, foxl2a;foxl2b double mutants had a more severe defect in maintaining female sex determination then foxl2b single mutants. At 35 dpf, foxl2a;foxl2b double mutants appeared to have normal sex ratios based on the morphology of the gonads. However, between 40 and 60 dpf, the oocytes in double mutants degraded and were replaced by spermatogenetic germ cells, indicating that these ovaries had undergone a more complete ovary-to-testis conversion. Analysis of gene expression during this time showed a correlation between sex reversal and upregulation of the male-specific factors dmrt1 and sox9a. Together these results argue that, similar to the role of FOXL2 in mammals, zebrafish Foxl2a and Foxl2b function to maintain the female sex differentiated fate of somatic ovarian cells after primary sex determination (Yang et al., 2017).
10.2. Bone morphogenetic protein 15 (Bmp15)
During the juvenile and adult stages, oocytes continue to play a key role by producing a factor(s) that is necessary to maintain ovary fate. Bmp15 is a TGF-ß ligand that is expressed in all oocytes beginning at Stage Ib (Clelland et al., 2006; Dranow et al., 2016). In mice, Bmp15 mutants had only slightly reduced fertility but were otherwise normal, while mutations in the close ortholog growth and differentiation factor 9 (Gdf9) resulted in arrested development of pre-antral follicles(Donget al.,1996; Yan et al., 2001). When Gdf9 heterozygous mutations were introduced in a Bmp15 background, female mice were sterile (Yan et al., 2001). This suggests that Gdf9 and Bmp15 synergize to promote oocyte maturation. It has been proposed that metabolic failure causes the phenotype observed in Bmp15 and Gdf9 mutants (Su et al., 2007). For example, in normal development, oocytes down regulated the expression of enzymes such as those required for glycolysis. Instead, the oocyte-produced Bmp15 and Gdf9 ligands instruct the overlying granulosa cells to upregulate expression of genes encoding glycolytic enzymes. The granulosa cells in turn provide pyruvate to the oocyte via gap junctions that form between these cells. It was proposed that this assures coordinated growth between oocytes and granulosa cells (Su et al., 2007).
In zebrafish, bmp15 and gdf9 are also expressed in oocytes. In contrast to mammals, however, mutational analysis has showed that loss of bmp15, but not gdf9, resulted in a failure of oocytes to progress past early stage II of oogenesis. Once bmp15 mutant oocytes had reached this stage (~50 dpf ), they underwent apoptosis and were replaced by spermatogenic germ cells. By 90 dpf, the mutant ovaries had fully sex reversed to fertile testes. As such, Bmp 15 mutants had a phenotype that was identical to ablation of oocytes using pharmacogenetic methods (Dranow et al., 2013), which identifies Bmp15 as a probable oocyte-produced factor that maintains female sex differentiation in adults. Because loss of bmp15 did not appear to affect premeiotic germ cells, the sex-reversed males were fertile. In contrast to bmp15 mutants, gdf9 mutants had no apparent defect in gonad development (Dranow et al., 2016).
11. Loose ends
The studies so far reviewed make it clear that many of the genes involved in the regulatory network that controls sex determination and differentiation in mammals are conserved in zebrafish. However, in some important cases, the role of a conserved gene in zebrafish has yet to be established. For example, there is ample evidence that SOX9 is a critical factor in mammalian sex determination, and one of the two zebrafish ohnologs of Sox9, called sox9a, is expressed in somatic gonad cells during the bipotential phase and in adult Sertoli cells of the testes, consistent with a role in male sex determination (Chiang, Pai, et al., 2001; Rodríguez-Marí et al., 2005). However, sox9a mutants are embryonic lethal due to defects in cartilage development (Yan et al., 2002). By contrast, sox9b is not expressed in the bipotential gonad or the adult testis, but instead is expressed in oocytes in the adult ovary (Rodríguez-Marí et al., 2005). Mutations in sox9b were viable but had defects in the development of the hepatopancreatic duct (Delous et al., 2012; Manfroid et al., 2012). Although sox9b mutant fish can survive to adulthood, these studies did not report the sex of mutant adults. Thus, it remains to be determined if sox9a and/or sox9b have roles in sex determination in zebrafish.
Another key player in mammalian sex determination is the FGF9 ligand. During the bipotential phase of mammalian gonad development, FGF9 is expressed in somatic gonadal cells in both males and females. If SRY is present and activates SOX9 expression, FGF9 expression is potentiated by SOX9 and positive feedback with FGFR2, leading to male development. Loss of Fgf9 function caused male-to-female sex reversal (Colvin, Green, Schmahl, Capel, & Ornitz, 2001; Schmahl, Kim, Colvin, Ornitz, & Capel, 2004). While zebrafish have orthologs of Sox9 and Fgfr2, Fgf9 is the only Fgf ortholog that appears to be absent from all teleost genomes. It remains unclear if another Fgf ligand in teleost plays an analogous role to FGF9 during sex determination.
12. Concluding remarks
The development of genome editing tools over the past decade have led to rapid advancements in our understanding of the genetic regulation of sex determination, differentiation and maintenance in teleost. Studies in zebrafish, described here, as well as studies in other model and non-model teleost have firmly established that vertebrates share a core genetic regulatory module for sex determination and differentiation and that specific genes or gene duplicates within this module can evolve to become the major genetic determinant of sex (e.g., dmy in Medaka or amhrY in pejerrey; Hattori et al., 2012; Matsuda et al., 2002; Yamamoto et al., 2014). While the majority of zebrafish studies described above were conducted in derived domesticated lines that have lost the natural sex determining locus identified in wild zebrafish, this has not prevented the identification of many of the major genes that likely function downstream of the natural sex determinant. Once the natural determinant is identified, it will then be possible to build a unified genetic model of sex determination in zebrafish. Regardless, the progress to date has established zebrafish as a significant model for understanding the genetic regulation of sex determination and differentiation. In addition, the discovery that zebrafish can undergo complete functional sex reversion as adults has established zebrafish as a model for investigating genes that function to maintain a stable sexual phenotype during adult life.
Acknowledgments
This work was supported by funding from the National Institute of Environmental Health Sciences (T32 ES-0070599 to M.E.K.) and the National Science Foundation (IOS-1456737 to B.W.D.).
References
- Anderson JL, Marí A, Braasch I, Amores A, Hohenlohe P, Batzel P, et al. (2012). Multiple sex-associated regions and a putative sex chromosome in zebrafish revealed by RAD mapping and population genomics. PLoS One, 7, e40701 10.1371/journal.pone.0040701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avise JC, & Mank JE (2009). Evolutionary perspectives on hermaphroditism in fishes. Sexual Development, 3, 152–163. 10.1159/000223079. [DOI] [PubMed] [Google Scholar]
- Bardoni B, Zanaria E, Guioli S, Floridia G, Worley KC, Tonini G, et al. (1994). A dosage sensitive locus at chromosome Xp21 is involved in male to female sex reversal. Nature Genetics, 7, 497–501. [DOI] [PubMed] [Google Scholar]
- Battistin C, Menezes Filho HC, Domenice S, Nishi MY, Della Manna T, Kuperman H, et al. (2012). A novel DAX1/NR0B1 mutation in a patient with adrenal hypoplasia congenita and hypogonadotropic hypogonadism. Arquivos Brasileiros de Endocrinologia e Metabologia, 56, 496–500. [DOI] [PubMed] [Google Scholar]
- Beer RL, & Draper BW (2013). nanos3 maintains germline stem cells and expression of the conserved germline stem cell gene nanos2 in the zebrafish ovary. Developmental Biology, 374, 308–318. [DOI] [PubMed] [Google Scholar]
- Behringer RR, Finegold MJ, & Cate RL (1994). Müllerian-inhibiting substance function during mammalian sexual development. Cell, 79, 415–425. 10.1016/0092-8674(94)90251-8. [DOI] [PubMed] [Google Scholar]
- Belville C, Josso N, & Picard JY (1999). Persistence of Müllerian derivatives in males. American Journal of Medical Genetics Part C Seminars in Medical Genetics, 89, 218–223. . [DOI] [PubMed] [Google Scholar]
- Braat AK, Zandbergen T, Van De Water S, Goos HJTH, & Zivkovic D (1999). Characterization of zebrafish primordial germ cells: Morphology and early distribution of vasa RNA. Developmental Dynamics, 216, 153–167. . [DOI] [PubMed] [Google Scholar]
- Bradley KM, Breyer JP, Melville DB, Broman KW, Knapik EW, & Smith JR (2011). An SNP-based linkage map for zebrafish reveals sex determination loci. G3 Bethesda, 1, 3–9. 10.1534/g3.111.000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cate RL, Mattaliano RJ, Hession C, Tizard R, Farber NM, Cheung A, et al. (1986). Isolation of the bovine and human genes for Müllerian inhibiting substance and expression of the human gene in animal cells. Cell, 45, 685–698. 10.1016/0092-8674(86)90783-X. [DOI] [PubMed] [Google Scholar]
- Chen S, Zhang H, Wang F, Zhang W, & Peng G (2016). nr0b1 (DAX1) mutation in zebrafish causes female-to-male sex reversal through abnormal gonadal proliferation and differentiation. Molecular and Cellular Endocrinology, 433, 105–116. 10.1016/j.mce.2016.06.005. [DOI] [PubMed] [Google Scholar]
- Chiang EF, Pai CI, Wyatt M, Yan YL, Postlethwait J, & Chung B (2001). Two sox9 genes on duplicated zebrafish chromosomes: Expression of similar transcription activators in distinct sites. Developmental Biology, 231, 149–163. 10.1006/dbio.2000.0129. [DOI] [PubMed] [Google Scholar]
- Chiang EF, Yan YL, Tong SK, Hsiao PH, Guiguen Y, Postlethwait J, et al. (2001). Characterization of duplicated zebrafish cyp19 genes. The Journal of Experimental Zoology, 290, 709–714. [DOI] [PubMed] [Google Scholar]
- Clelland E, Kohli G, Campbell RK, Sharma S, Shimasaki S, & Peng C (2006). Bone morphogenetic protein-15 in the zebrafish ovary: Complementary deoxyribonucleic acid cloning, genomic organization, tissue distribution, and role in oocyte maturation. Endocrinology, 147, 201–209. 10.1210/en.2005-1017. [DOI] [PubMed] [Google Scholar]
- Colvin JS, Green RP, Schmahl J, Capel B, & Ornitz DM (2001). Male-to-female sex reversal in mice lacking fibroblast growth factor 9. Cell, 104, 875–889. 10.1016/S0092-8674(01)00284-7. [DOI] [PubMed] [Google Scholar]
- Crowder CM, Lassiter CS, & Gorelick DA (2018). Nuclear androgen receptor regulates testes organization and oocyte maturation in zebrafish. Endocrinology, 159, 980–993. 10.1210/en.2017-00617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gendt K, Swinnen JV, Saunders PTK, Schoonjans L, Dewerchin M, Devos A, et al. (2004). A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proceedings of the National Academy of Sciences, 101, 1327–1332. 10.1073/pnas.0308114100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal PP, Wang DS, Nijenhuis WA, Schulz RW, & Bogerd J (2008). Functional characterization and expression analysis of the androgen receptor in zebrafish (Danio rerio) testis. Reproduction, 136, 225–234. 10.1530/REP-08-0055. [DOI] [PubMed] [Google Scholar]
- Delous M, Yin C, Shin D, Ninov N, Debrito Carten J, Pan L, et al. (2012). Sox9B is a key regulator of Pancreaticobiliary ductal system development. PLoS Genetics, 8, 1–16. 10.1371/journal.pgen.1002754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin RH, & Nagahama Y (2002). Sex determination and sex differentiation in fish: An overview of genetic, physiological, and environmental influences. Aquaculture, 208, 191–364. 10.1016/S0044-8486(02)00057-1. [DOI] [Google Scholar]
- Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, & Matzuk MM (1996). Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature, 383, 531–535. 10.1038/383531a0. [DOI] [PubMed] [Google Scholar]
- Dranow DB, Hu K, Bird AM, Lawry ST, Adams MT, Sanchez A, et al. (2016). Bmp15 is an oocyte-produced signal required for maintenance of the adult female sexual phenotype in zebrafish. PLoS Genetics, 12, e1006323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dranow DB, Tucker RP, & Draper BW (2013). Germ cells are required to maintain a stable sexual phenotype in adult zebrafish. Developmental Biology, 376, 43–50. [DOI] [PubMed] [Google Scholar]
- Draper BW, McCallum CM, & Moens CB (2007). nanos1 is required to maintain oocyte production in adult zebrafish. Developmental Biology, 305, 589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driever W, Schier AF, Neuhauss SCF, Malicki J, Stemple DL, Stainier DYR, et al. (1996). A genetic screen for mutations affecting embryogenesis in zebrafish. Development, 123, 37–46. [DOI] [PubMed] [Google Scholar]
- Fenske M, Maack G, Sch afers C, & Segner H (2005). An environmentally relevant concentration of estrogen induces arrest of male gonad development in zebrafish, Danio rerio. Environmental Toxicology and Chemistry, 24, 1088–1098. 10.1897/04-096R1.1. [DOI] [PubMed] [Google Scholar]
- Gao W, Bohl CE, & Dalton JT (2005). Chemistry and structural biology of androgen receptor. Chemical Reviews, 105, 3352–3370. 10.1021/cr020456u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier A, Sohm F, Joly J-S, Le Gac F, & Lareyre J-J (2011). The proximal promoter region of the zebrafish gsdf gene is sufficient to mimic the spatio-temporal expression pattern of the endogenous gene in Sertoli and granulosa cells. Biology of Reproduction, 85, 1240–1251. 10.1095/biolreprod.111.091892. [DOI] [PubMed] [Google Scholar]
- Gill A, Jamnongjit M, & Hammes SR (2004). Androgens promote maturation and signaling in mouse oocytes independent of transcription: A release of inhibition model for mammalian oocyte meiosis. Molecular Endocrinology, 18, 97–104. 10.1210/me.2003-0326. [DOI] [PubMed] [Google Scholar]
- Gouédard L, Chen YG, Thevenet L, Racine C, Borie S, Lamarre I, et al. (2000). Engagement of bone morphogenetic protein type IB receptor and Smad1 signaling by anti-Mu llerian hormone and its type II receptor. The Journal of Biological Chemistry, 275, 27973–27978. [DOI] [PubMed] [Google Scholar]
- Guiguen Y, Fostier A, Piferrer F, & Chang C-FF (2010). Ovarian aromatase and estrogens: A pivotal role for gonadal sex differentiation and sex change in fish. General andComparativeEndocrinology,165,352–366. 10.1016/j.ygcen.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Haffter P, & Nusslein-Volhard C (1996). Large scale genetics in a small vertebrate, the zebrafish. The International Journal of Developmental Biology, 40, 221–227. [PubMed] [Google Scholar]
- Hartung O, Forbes MM, & Marlow FL (2014). Zebrafish vasa is required for germ-cell differentiation and maintenance. Molecular Reproduction and Development, 81, 946–961. 10.1002/mrd.22414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori RS, Murai Y, Oura M, Masuda S, Majhi SK, Sakamoto T, et al. (2012). A Y-linked anti-Müllerian hormone duplication takes over a critical role in sex determination. Proceedings of the National Academy of Sciences of the United States of America, 109, 2955–2959. 10.1073/pnas.1018392109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain MS, Larsson A, Scherbak N, Olsson P-E, & Orban L (2008). Zebrafish androgen receptor: Isolation, molecular, and biochemical characterization1. Biology of Reproduction, 78, 361–369. 10.1095/biolreprod.107.062018. [DOI] [PubMed] [Google Scholar]
- Houwing S, Berezikov E, & Ketting RF (2008). Zili is required for germ cell differentiation and meiosis in zebrafish. The EMBO Journal, 27, 2702–2711. 10.1038/emboj.2008.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houwing S, Kamminga LM, Berezikov E, Cronembold D, Girard A, Van Den Elst H, et al. (2007). A role for Piwi and piRNAs in germ cell maintenance and transposon silencing in zebrafish. Cell, 129, 69–82. [DOI] [PubMed] [Google Scholar]
- Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, et al. (2013). The zebrafish reference genome sequence and its relationship to the human genome. Nature, 496, 498–503. 10.1038/nature12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HY, Houwing S, Kaaij LJT, Meppelink A, Redl S, Gauci S, et al. (2011). Tdrd1 acts as a molecular scaffold for Piwi proteins and piRNA targets in zebrafish. The EMBO Journal, 30, 3298–3308. 10.1038/emboj.2011.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamin SP, Arango NA, Mishina Y, Hanks MC, & Behringer RR (2002). Requirement of Bmpr1a of Müllerian duct regression during male sexual development. Nature Genetics, 32, 408–410. https://doi.org/https://www.nature.com/articles/ng1003z. [DOI] [PubMed] [Google Scholar]
- Josso N, Lamarre I, Picard JY, Berta P, Davies N, Morichon N, et al. (1993). Anti-Müllerian hormone in early human development. Early Human Development, 33, 91–99. 10.1016/0378-3782(93)90204-8. [DOI] [PubMed] [Google Scholar]
- Kang J, Nachtrab G, & Poss KD (2013). Local Dkk1 crosstalk from breeding ornaments impedes regeneration of injured male zebrafish fins. Developmental Cell, 27, 19–31. 10.1016/j.devcel.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprunner M, Thisse C, Thisse B, & Raz E (2001). A zebrafish nanos-related gene is essential for the development of primordial germ cells. Genes & Development, 15, 2877–2885. 10.1101/gad.212401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kossack ME, High SK, Hopton RE, Yan Y-L, Postlethwait JH, & Draper BW (2019). Female sex development and reproductive duct formation depend on Wnt4a in zebrafish. Genetics, 211, 219–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krøvel AV, & Olsen LC (2002). Expression of a vas::EGFP transgene in primordial germ cells of the zebrafish. Mechanisms of Development, 116, 141–150. 10.1016/S0925-4773(02)00154-5. [DOI] [PubMed] [Google Scholar]
- Lawrence C (2007). The husbandry of zebrafish (Danio rerio): A review. Aquaculture, 269, 1–20. 10.1016/j.aquaculture.2007.04.077. [DOI] [Google Scholar]
- Lawrence C, Ebersole JP, & Kesseli RV (2008). Rapid growth and out-crossing promote female development in zebrafish (Danio rerio). Environmental Biology of Fishes, 81, 239–246. 10.1007/s10641-007-9195-8. [DOI] [Google Scholar]
- Leerberg DM, Sano K, & Draper BW (2017). Fibroblast growth factor signaling is required for early somatic gonad development in zebrafish. PLoS Genetics, 13, 1–28. 10.1371/journal.pgen.1006993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew WC, Bartfai R, Lim Z, Sreenivasan R, Siegfried KR, & Orban L (2012). Polygenic sex determination system in zebrafish. PLoS One, 7, e343971 10.1371/journal.pone.0034397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Q, Mei J, Li Z, Zhang X, Zhou L, & Gui J (2017). Distinct and cooperative roles of amh and dmrt1 in self-renewal and differentiation of male germ cells in zebrafish. Genetics, 207, 1007–1022. 10.1534/genetics.117.300274/-/DC1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A, Majumdar A, Schauerte HE, Haffter P, & Drummond IA (2000). Zebrafish wnt4b expression in the floor plate is altered in sonic hedgehog and gli-2 mutants. Mechanisms of Development, 91, 409–413. 10.1016/S0925-4773(99)00308-1. [DOI] [PubMed] [Google Scholar]
- Manfroid I, Ghaye A, Naye F, Detry N, Palm S, Pan L, et al. (2012). Zebrafish sox9b is crucial for hepatopancreatic duct development and pancreatic endocrine cell regeneration. Developmental Biology, 366, 268–278. 10.1016/j.ydbio.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson CK, Murphy MW, Griswold MD, Yoshida S, Bardwell VJ, & Zarkower D (2010). The mammalian doublesex homolog DMRT1 is a transcriptional gatekeeper that controls the mitosis versus meiosis decision in male germ cells. Developmental Cell, 19, 612–624. 10.1016/j.devcel.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson CK, Murphy MW, Sarver AL, Griswold MD, Bardwell VJ, & Zarkower D (2011). DMRT1 prevents female reprogramming in the postnatal mammalian testis. Nature, 476, 101–105. 10.1038/nature10239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda M, Nagahama Y, Shinomiya A, Sato T, Matsuda C, Kobayashi T, et al. (2002). DMY is a Y-specific DM-domain gene required for male development in the medaka fish. Nature, 417, 559–563. 10.1038/nature751. [DOI] [PubMed] [Google Scholar]
- Mazurais D, Montfort J, Delalande C, & Le Gac F (2005). Transcriptional analysis of testis maturation using trout cDNA macroarrays. General and Comparative Endocrinology, 142, 143–154. 10.1016/j.ygcen.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Menke AL, Spitsbergen JM, Wolterbeek APM, & Woutersen RA (2011). Normal anatomy and histology of the adult zebrafish. Toxicologic Pathology, 39, 759–775. 10.1177/0192623311409597. [DOI] [PubMed] [Google Scholar]
- Morinaga C, Saito D, Nakamura S, Sasaki T, Asakawa S, Shimizu N, et al. (2007). The hotei mutation of medaka in the anti-Müllerian hormone receptor causes the dysregulation of germ cell and sexual development. Proceedings of the National Academy of Sciences, 104, 9691–9696. 10.1073/pnas.0611379104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins MC, Hammerschmidt M, Haffter P, & Nusslein-Volhard C (1994). Large-scale mutagenesis in the zebrafish: In search of genes controlling development in a vertebrate. Current Biology, 4, 189–202. 10.1016/S0960-9822(00)00048-8. [DOI] [PubMed] [Google Scholar]
- Munsterberg A, & Lovell-Badge R (1991). Expression of the mouse anti-Müllerian hormone gene suggests a role in both male and female sexual differentiation. Development, 113, 613–624. 10.1177/0146167211402216. [DOI] [PubMed] [Google Scholar]
- Myosho T, Otake H, Masuyama H, Matsuda M, Kuroki Y, Fujiyama A, et al. (2012). Tracing the emergence of a novel sex-determining gene in medaka, Oryzias luzonensis. Genetics, 191, 163–170. 10.1534/genetics.111.137497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S, Kurokawa H, Asakawa S, Shimizu N, & Tanaka M (2009). Two distinct types of theca cells in the medaka gonad: Germ cell-dependent maintenance of cyp19a1-expressing theca cells. Developmental Dynamics, 238, 2652–2657. [DOI] [PubMed] [Google Scholar]
- Neumann JC, Dovey JS, Chandler GL, Carbajal L, & Amatruda JF (2009). Identification of a heritable model of testicular germ cell tumor in the zebrafish. Zebrafish, 6, 319–327. 10.1089/zeb.2009.0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla PA, & Roth MB (2001). Oxygen deprivation causes suspended animation in the zebrafish embryo. Proceedings of the National Academy of Sciences of the United States of America, 98 7331 LP–7335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfennig F, Standke A, & Gutzeit HO (2015). The role of Amh signaling in teleost fish— Multiple functions not restricted to the gonads. General and Comparative Endocrinology, 223, 87–107. 10.1016/j.ygcen.2015.09.025. [DOI] [PubMed] [Google Scholar]
- Picard JY, Goulut C, Bourrillon R, & Josso N (1986). Biochemical analysis of bovine testicular anti-Müllerian hormone. FEBS Letters, 195, 73–76. [DOI] [PubMed] [Google Scholar]
- Poss KD, Nechiporuk A, Stringer KF, Lee C, & Keating MT (2004). Germ cell aneuploidy in zebrafish with mutations in the mitotic checkpoint gene mps1. Genes & Development, 18, 1527–1532. 10.1101/gad.1182604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond CS, Shamu CE, Shen MM, Seifert KJ, Hirsch B, Hodgkin J, et al. (1998). Evidence for evolutionary conservation of sex-determining genes. Nature, 391, 691–695. 10.1111/lre.12210. [DOI] [PubMed] [Google Scholar]
- Robertson KM, O’Donnell L, Jones ME, Meachem SJ, Boon WC, Fisher CR, et al. (1999). Impairment of spermatogenesis in mice lacking a functional aromatase (cyp 19) gene. Proceedings of the National Academy of Sciences of the United States of America, 96, 7986–7991. 10.1073/pnas.96.14.7986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Marí A, Cañestro C, Bre Miller RA, Nguyen-Johnson A, Asakawa K, Kawakami K, et al. (2010). Sex reversal in zebrafish fancl mutants is caused by Tp53-mediated germ cell apoptosis. PLoS Genetics, 6, 1–14. 10.1371/journal.pgen.1001034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Marí A, Yan Y-L, BreMiller RA, Wilson C, Cañestro C, & Postlethwait JH (2005). Characterization and expression pattern of zebrafish anti-Müllerian hormone (amh) relative to sox9a, sox9b, and cyp19a1a, during gonad development. Gene Expression Patterns, 5, 655–667. 10.1016/j.modgep.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Rondeau EB, Messmer AM, Sanderson DS, Jantzen SG, von Schalburg KR, Minkley DR, et al. (2013). Genomics of sablefish (Anoplopoma fimbria): Expressed genes, mitochondrial phylogeny, linkage map and identification of genetic sex markers. BMC Genomics, 14, 452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawatari E, Shikina S, Takeuchi T, & Yoshizaki G (2007). A novel transforming growth factor-β superfamily member expressed in gonadal somatic cells enhances primordial germ cell and spermatogonial proliferation in rainbow trout (Oncorhynchus mykiss). Developmental Biology, 301, 266–275. 10.1016/j.ydbio.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Schmahl J, Kim Y, Colvin JS, Ornitz DM, & Capel B (2004). Fgf9 induces proliferation and nuclear localization of FGFR2 in Sertoli precursors during male sex determination. Development, 131, 3627–3636. 10.1242/dev.01239. [DOI] [PubMed] [Google Scholar]
- Selman K, Wallace RA, Sarka A, & Qi X (1993). Stages of oocyte development in the zebrafish, Brachydanio rerio. Journal of Morphology, 218, 203–224. 10.1002/jmor.1052180209. [DOI] [PubMed] [Google Scholar]
- Shang EHH, Yu RMK, & Wu RSS (2006). Hypoxia affects sex differentiation and development leading to a male-dominated population in zebrafish (Danio rerio). Environmental Science & Technology, 40, 3118–3122. 10.1021/es0522579. [DOI] [PubMed] [Google Scholar]
- Sharma KK, Sharma OP, & Tripathi NK (1998). Female hetero-gamety in Danio rerio (Cypriniformes: Cyprinidae). Proceedings of the National Academy of Sciences India Section B, Biological Sciences, 68(B), 123–126. [Google Scholar]
- Shibata Y, Paul-Prasanth B, Suzuki A, Usami T, Nakamoto M, Matsuda M, et al. (2010). Expression of gonadal soma derived factor (Gsdf ) is spatially and temporally correlated with early testicular differentiation in medaka. Gene Expression Patterns, 10, 283–289. 10.1016/j.gep.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Siegfried KR, & Nüsslein-Volhard C (2008). Germ line control of female sex determination in zebrafish. Developmental Biology, 324, 277–287. 10.1016/j.ydbio.2008.09.025. [DOI] [PubMed] [Google Scholar]
- Slanchev K, Stebler J, de la Cueva-Mendez G, & Raz E (2005). Development without germ cells: The role of the germ line in zebrafish sex differentiation. Proceedings of the National Academy of Sciences of the United States of America, 102, 4074–4079. 10.1073/pnas.0407475102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreenivasan R, Jiang J, Wang X, Bártfai R, Kwan HY, Christoffels A, et al. (2014). Gonad differentiation in zebrafish is regulated by the canonical Wnt signaling pathway. Biology of Reproduction, 90(1–10), 45 10.1095/biolreprod.113.110874. [DOI] [PubMed] [Google Scholar]
- Streisinger G, Walker C, Dower N, Knauber D, & Singer F (1981). Production of clones of homozygous diploid zebra fish (Brachydanio rerio). Nature, 291, 293–296. [DOI] [PubMed] [Google Scholar]
- Su Y-Q, Sugiura K, Wigglesworth K, O’Brien MJ, Affourtit JP, Pangas SA, et al. (2007). Oocyte regulation of metabolic cooperativity between mouse cumulus cells and oocytes: BMP15 and GDF9 control cholesterol biosynthesis in cumulus cells. Development, 135, 111–121. 10.1242/dev.009068. [DOI] [PubMed] [Google Scholar]
- Takahashi H (1977). Juvenile hermaphroditism in the zebrafish Brachydanio rerio. Bulletin of the Faculty of Fisheries Hokkaido University, 28, 57–65. [Google Scholar]
- Tzung K-W, Goto R, Saju JM, Sreenivasan R, Saito T, Arai K, et al. (2015). Early depletion of primordial germ cells in zebrafish promotes testis formation. Stem Cell Reports, 4, 61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida D, Yamashita M, Kitano T, & Iguchi T (2002). Oocyte apoptosis during the transition from ovary-like tissue to testes during sex differentiation of juvenile zebrafish. The Journal of Experimental Biology, 205, 711–718. [DOI] [PubMed] [Google Scholar]
- Uchida D, Yamashita M, Kitano T, & Iguchi T (2004). An aromatase inhibitor or high water temperature induce oocyte apoptosis and depletion of P450 aromatase activity in the gonads of genetic female zebrafish during sex-reversal. Comparative Biochemistry and Physiology, Part A: Molecular & Integrative Physiology, 137, 11–20. 10.1016/S1095-6433(03)00178-8. [DOI] [PubMed] [Google Scholar]
- Uhlenhaut NH, Jakob S, Anlag K, Eisenberger T, Sekido R, Kress J, et al. (2009). Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation. Cell, 139, 1130–1142. [DOI] [PubMed] [Google Scholar]
- Ungar AR, Kelly GM, & Moon RT (1995). Wnt4 affects morphogenesis when misexpressed in the zebrafish embryo. Mechanisms of Development, 52, 153–164. 10.1016/0925-4773(95)00386-F. [DOI] [PubMed] [Google Scholar]
- Vainio S, Heikkila M, Kispert A, Chin N, McMahon AP, Heikkil a M, et al. (1999). Female development in mammals is regulated by Wnt-4 signalling. Nature, 397, 405–409. [DOI] [PubMed] [Google Scholar]
- Visser JA (2003). AMH signaling: From receptor to target gene. Molecular and Cellular Endocrinology, 211, 65–73. 10.1016/j.mce.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Wang XG, Bartfai R, Sleptsova-Freidrich I, & Orban L (2007). The timing and extent of “juvenile ovary” phase are highly variable during zebrafish testis differentiation. Journal of Fish Biology, 70, 33–44. 10.1111/j.1095-8649.2007.01363.x. [DOI] [Google Scholar]
- Webster KA, Schach U, Ordaz A, Steinfeld JS, Draper BW, & Siegfried KR (2017). Dmrt1 is necessary for male sexual development in zebrafish. Developmental Biology, 422, 33–46. 10.1016/j.ydbio.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidinger G, Stebler J, Slanchev K, Dumstrei K, Wise C, Lovell-Badge R, et al. (2003). Dead end, a novel vertebrate germ plasm component, is required for zebrafish primordial germ cell migration and survival. Current Biology, 13, 1429–1434. 10.1016/S0960-9822(03)00537-2. [DOI] [PubMed] [Google Scholar]
- Weidinger G, Wolke U, Köprunner M, Thisse C, Thisse B, & Raz E (2002). Regulation of zebrafish primordial germ cell migration by attraction towards an intermediate target. Development, 129, 25–36. [DOI] [PubMed] [Google Scholar]
- Westerfield M (2007). In Westerfield M (Ed.), The zebrafish book: A guide for the laboratory use of zebrafish Danio (Brachydanio) rerio (5th ed). Eugene, OR: University of Oregon Press. [Google Scholar]
- Wilson CA, High SK, McCluskey BM, Amores A, Yan YL, Titus TA, et al. (2014). Wild sex in zebrafish: Loss of the natural sex determinant in domesticated strains. Genetics, 198, 1291–1308. 10.1534/genetics.114.169284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Zhang Y, Sarida M, Hattori RS, & Strussmann CA (2014). Coexistence of genotypic and temperature-dependent sex determination in pejerrey Odontesthes bonariensis. PLoS One, 9, 1–8. 10.1371/journal.pone.0102574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y-L, Desvignes T, Bremiller R, Wilson C, Dillon D, High S, et al. (2017). Gonadal soma controls ovarian follicle proliferation through Gsdf in zebrafish. Developmental Dynamics, 246, 925–945. 10.1002/dvdy.24579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y-L, Miller CT, Nissen RM, Singer A, Liu D, Kirn A, et al. (2002). A zebrafish sox9 gene required for cartilage morphogenesis. Development, 129, 5065–5079. [DOI] [PubMed] [Google Scholar]
- Yan C, Wang P, DeMayo J, DeMayo FJ, Elvin JA, Carino C, et al. (2001). Synergistic roles of bone morphogenetic protein 15 and growth differentiation factor 9 in ovarian function. Molecular Endocrinology, 15, 854–866. 10.1210/mend.15.6.0662. [DOI] [PubMed] [Google Scholar]
- Yang Y-J, Wang Y, Li Z, & Gui J-F (2017). Sequential, divergent and cooperative requirements of Foxl2a and Foxl2b in ovary development and maintenance of zebrafish. Genetics, 205, 1551–1572. 10.1534/genetics.116.199133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh S, Tsai M-Y, Xu Q, Mu X-M, Lardy H, Huang K-E, et al. (2002). Generation and characterization of androgen receptor knockout (ARKO) mice: An in vivo model for the study of androgen functions in selective tissues. PNAS, 99, 13498–13503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Tang H, Liu Y, Chen Y, Li G, Liu X, et al. (2017). Targeted disruption of aromatase reveals dual functions of cyp19a1a during sex differentiation in zebrafish. Endocrinology, 158, 3030–3041. 10.1210/en.2016-1865. [DOI] [PubMed] [Google Scholar]
- Yoon C, Kawakami K, & Hopkins N (1997). Zebrafish vasa homologue RNA is localized to the cleavage planes of 2- and 4-cell-stage embryos and is expressed in the primordial germ cells. Development, 124, 3157–3165. [DOI] [PubMed] [Google Scholar]
- Yu G, Zhang D, Liu W, Wang J, Liu X, & Zhou C (2018). Zebrafish androgen receptor is required for spermatogenesis and maintenance of ovarian function. Oncotarget, 9, 24320–24334. 10.18632/oncotarget.24407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Oatley J, Bardwell VJ, & Zarkower D (2016). DMRT1 is required for mouse Spermatogonial stem cell maintenance and replenishment. PLoS Genetics, 12, 1–18. 10.1371/journal.pgen.1006293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Svingen T, Ng ET, & Koopman P (2015). Female-to-male sex reversal in mice caused by transgenic overexpression of Dmrt1. Development, 142, 1083–1088. 10.1242/dev.122184. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Yang Z, Phelan JK, Wheeler DA, Lin S, & McCabe ERB (2006). Zebrafish dax 1 is required for development of the Interrenal organ, the adrenal cortex equivalent. Molecular Endocrinology, 20, 2630–2640. 10.1210/me.2005-0445. [DOI] [PubMed] [Google Scholar]