Abstract
The C-terminal end segment of troponin subunit I (TnI) is a structure highly conserved among the three muscle type-specific isoforms and across vertebrate species. Partial deletion or point mutation in this segment impairs cardiac muscle relaxation. In the present study, we characterized the C-terminal 27 amino acid peptide of human cardiac TnI (HcTnI-C27) for its role in modulating muscle contractility. Biologically or chemically synthesized HcTnI-C27 peptide retains an epitope structure in physiological solutions similarly to that in intact TnI as recognized by an anti-TnI C-terminus monoclonal antibody (mAb TnI-1). Protein binding studies found that HcTnI-C27 retains the binding affinity for tropomyosin as previously shown with intact cardiac TnI. A restrictive cardiomyopathy mutation R192H in this segment abolishes the bindings to mAb TnI-1 and tropomyosin, demonstrating a pathogenic loss of function. Contractility studies using skinned muscle preparations demonstrated that addition of HcTnI-C27 peptide reduces the Ca2+-sensitivity of myofibrils without decreasing maximum force production. The results indicate that the C-terminal end segment of TnI is a regulatory element of troponin, which retains the native configuration in the form of free peptide to confer an effect on myofilament Ca2+-desensitization. Without negative inotropic impact, this short peptide may be developed into a novel reagent to selectively facilitate cardiac muscle relaxation at the activated state as a potential treatment for heart failure.
Keywords: Troponin I, C-terminal end segment, myofilament Ca2+-desensitization, cardiac muscle, diastolic function, peptide drug
1. Introduction
Muscle contraction is vital in animal mobility and heart function. Skeletal and cardiac muscles are striated muscles in which contraction is generated by the interaction of sarcomeric thick and thin filaments in the crossbridge ATPase cycle [1, 2]. The thick filaments are mainly composed of the motor protein myosin, while the thin filaments are composed of actin and the regulatory proteins tropomyosin and troponin [1, 3]. The troponin complex contains three protein subunits: The calcium-binding subunit (troponin C, TnC), the inhibitory subunit (troponin I, TnI), and the tropomyosin-binding subunit (troponin T, TnT) [4]. The contraction of myocytes is initiated by the rise of cytosolic Ca2+ that binds TnC and induces a series of allosteric changes in troponin and the thin filament to allow myosin heads to bind actin, which activates myosin ATPase and crossbridge cycling to generate power strokes [1, 5]. Subsequent decline of cytosolic Ca2+ results in dissociation of Ca2+ from troponin to return the thin filament to the inhibitory state, detachment of myosin heads from the thin filament, and relaxation of the myocyte [1, 6].
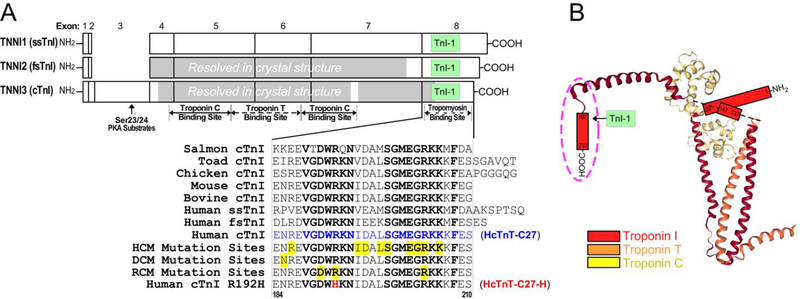
The primary function of troponin as a Ca2+-regulated brake in the sarcomere involves the key function of TnI that is responsible for the inhibition of myosin ATPase and muscle relaxation [7]. Encoded by homologous genes, three muscle fiber type-specific isoforms of TnI have evolved in vertebrates. With the exception that cardiac TnI has a unique N-terminal extension, the structures of cardiac, fast and slow skeletal muscle TnI isoforms are largely conserved [8, 9]. The C-terminal end segment of TnI is encoded by a conserved exon with highly conserved amino acid sequences in the three TnI isoforms and across vertebrate species [9] (Figure 1).
Figure 1: The highly conserved C-terminal end segment of TnI.
(A) Linear structures of the three isoforms of human TnI are aligned to show their exon organizations, major functional sites and the location of the mAb TnI-1 epitope. The regions resolved in the crystal structure of human cardiac troponin and chicken fast skeletal muscle troponin are shaded. The highly conserved amino acid sequences of the exon 8-encoded C-terminal end segment of cardiac (cTnI), slow skeletal muscle (ssTnI) and fast skeletal muscle (fsTnI) isoforms from representative vertebrate species are shown by the alignment. The conserved residues are bolded and known cardiomyopathic mutation sites are highlighted in yellow, among which the RCM mutation R192H is shown in red. (B) The crystal structure of cardiac troponin complex is depicted (PDB ID: 1J1E) to illustrate the position of TnI C-terminal end segment (in the dash oval) among the unresolved regions.
Mutations in the C-terminal end segment of cardiac TnI are associated with cardiomyopathies [10–15], the majority of which presents clinically with diastolic dysfunction (i.e., hypertrophic cardiomyopathy, HCM, and restrictive cardiomyopathy, RCM). An extensively studied RCM mutation, R192H (Figure 1) has been shown to cause severe diastolic dysfunction of the heart [16]. The heart of transgenic mice expressing C-terminal 19 amino acid-deleted cardiac TnI also demonstrated severely impaired diastolic function [17]. To explore the physiologic function of the C-terminal end segment of TnI as well as the molecular mechanism underlying the phenotype of the myopathic mutations, we have previously demonstrated that this segment is a Ca2+-regulated structural and functional domain of the troponin complex with a saturable binding to tropomyosin in low Ca2+ state [18], indicating a role in the inhibitory activity of TnI during muscle relaxation. This segment has also been implicated as a mobile domain that is able to dock to the actin thin filament in a Ca2+-dependent manner [19].
The C-terminal end segment of TnI was not resolved in the static crystallographic structures of both cardiac [20] and skeletal muscle [21] troponin complexes, potentially due to its allosteric nature. On the other hand, the C-terminal end segment of TnI forms a conserved epitope structure that is recognized by a monoclonal antibody (mAb) TnI-1 (Figure 1) [22]. Consistent with its binding to tropomyosin when residing in the troponin complex, this epitope is an exposed structure for affinity chromatographic isolation [23] or immunoprecipitation of the entire troponin complex [24]. Further supporting the functional importance of the C-terminal end segment of TnI, the single amino acid substitution RCM mutation R192H abolishes the epitope recognized by mAb TnI-1 [25].
Despite the medical importance and decades of extensive research, the fundamental function of troponin as a Ca2+-regulated molecular brake at the center of striated muscle contraction and relaxation remains not fully understood. In the present study, we characterized the C-terminal 27 amino acid peptide of human cardiac TnI (HcTnI-C27) for its function in modulating muscle contractility. HcTnI-C27 peptide demonstrates an epitope structure in physiological solution similar to that in intact TnI and retains the binding affinity for tropomyosin. Treatment of skinned muscle preparations with free HcTnI-C27 peptide reduced myofilament Ca2+-sensitivity without decreasing the maximum force production. The results indicate that HcTnI-C27 is a regulatory element of troponin and functions in the form of free peptide to produce myofilament Ca2+-desensitization with a potential value in the treatment of heart failure.
2. Materials and Methods
2.1. Cloning and Bacterial Expression of HcTnI-C27 and R192H Mutant HcTnI-C27-H
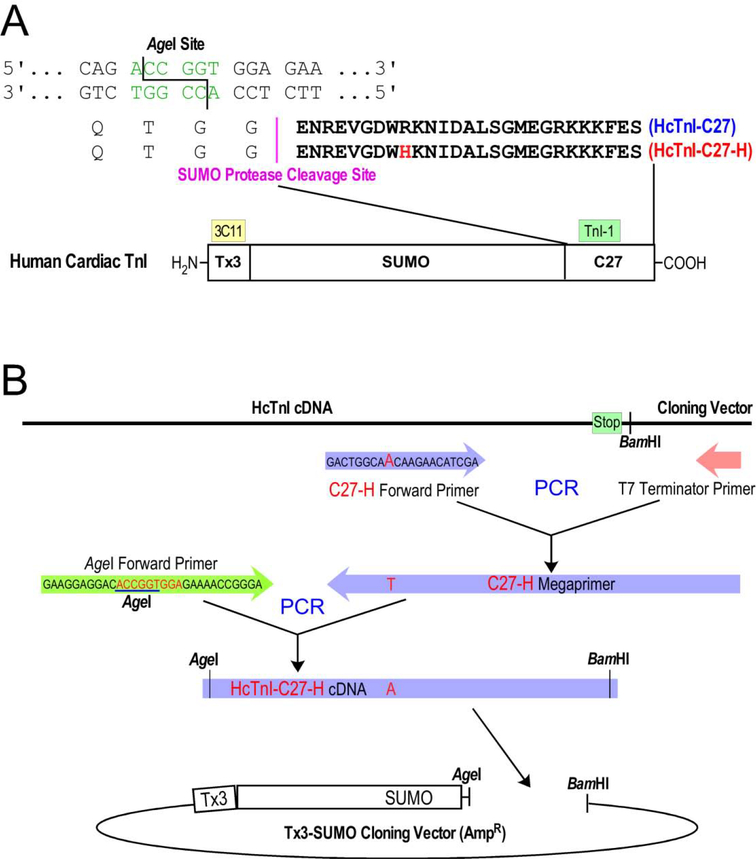
Biologically synthesized cTnI-C27 peptide is desirable for the studies of native structure and physiological function, especially in an initial investigation. Due to difficulties in using bacterial expression system to produce short peptides, we employed a fusion protein approach. cDNA encoding the C-terminal 27 amino acids of human cardiac TnI (HcTnI-C27) was amplified from a full length cDNA using polymerase chain reaction (PCR). The forward PCR primer contained a restriction enzyme AgeI site followed by a Gly codon (GGA) required for the cleavage by small ubiquitin-like modifier (SUMO) protease, which leaves zero residue behind [26–28] (Figure 2A). The reverse PCR primer contained a BamHI restriction site previously used to clone the intact cDNA. The PCR product was double-digested with AgeI and BamHI and purified using agarose gel electrophoresis for insertion into a T7 RNA polymerase-based expression plasmid constructed with a transition metal-binding tag (Tx3) and a SUMO substrate domain [28] followed by an in-frame AgeI site and downstream multi-cloning sites. The DNA ligation product was used for transformation of JM109 competent E. coli cells and antibiotic resistant colonies were screened using PCR. Recombinant expression plasmids were miniprepped and sequence confirmed.
Figure 2: Construction of expression plasmids.
HcTnI-C27 and HcTnI-C27-H peptides were expressed in Tx3-SUMO-fusion proteins. (A) The amino acid sequences of HcTnI-C27 and HcTnI-C27-H peptides, the Tx3-SUMO fusion protein structure and the strategy of using AgeI cloning site at the fusion joint for the recovery of free peptide with zero fusion residue are illustrated. The N-terminal Tx3 tag in the fusion protein for metal affinity purification is recognized by an mAb 3C11 for immunological identification. (B) The two-step PCR procedure to construct an HcTnI-C27-H mutant cDNA into Tx3-SUMO vector is outlined.
A recombinant plasmid expressing SUMO-fused HcTnI-C27 containing the RCM mutation R192H (HcTnI-C27-H) was constructed using PCR. A megaprimer was first made by PCR from wild type human cardiac TnI cDNA template using a forward primer containing the mutant site paired with the same reverse primer used above. This PCR-produced megaprimer was purified for use as the reverse primer to pair with the forward primer containing the AgeI restriction site in a second PCR on wild type full length cDNA template (Figure 2B). The final PCR product was double-digested using AgeI and BamHI, purified on agarose gel and cloned into Tx3-SUMO plasmid as above.
2.2. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
SDS-PAGE was performed as described previously [29]. Briefly, 14% Laemmli SDS-PAGE with an acrylamide:bisacrylamide ratio of 29:1 was used to monitor protein expression. Protein samples were prepared using sample buffer containing 2% SDS and 3% β-mercaptoethanol and run on 0.75 mm gels using a Bio-Rad mini-gel system. Resolved gels were stained with Coomassie Blue R-250 and de-stained using 10% acetic acid.
Tris-Tricine small pore SDS-gels were utilized to resolve small peptides as described previously [29]. Briefly, 15% SDS-PAGE with acrylamide:bisacrylamide ratio of 20:1 was used to monitor the recovery of peptides. The gel was run using different anode and cathode buffers as described previously [30] and processed as above.
2.3. Western Blotting
The protein bands resolved in SDS-PAGE gels were transferred to polyvinylidene difluoride (PVDF) membranes using a Bio-Rad semidry electrotransfer apparatus as described previously [29]. The membranes were stained with Amido Black to verify the effective blotting of the small peptides. The membranes were then blocked with 1% bovine serum albumin (BSA) in Tris-buffered saline (TBS) at room temperature for 30 minutes, and incubated with mAb TnI-1 against an epitope in the C-terminal end segment of TnI [8] or mAb 3C11 against the Tx3 tag [31] at 4°C overnight. Washed with 0.05% Triton X-100 and 0.1% SDS in TBS, the membranes were further incubated with alkaline phosphatase-conjugated goat anti-mouse IgG secondary antibody, washed again, and developed in BCIP-NTB substrate solution as described previously [29].
2.4. Expression and Purification of HcTnI-C27 and HcTnI-C27-H Fusion Proteins and Recovery of Free Peptides
Sequence-confirmed Tx3-SUMO-HcTnI-C27 and Tx3-SUMO-HcTnI-C27-H expression plasmids were used for transformation of BL21(DE3)pLysS E. coli competent cells. The fusion proteins were expressed in LB media cultures upon isopropyl β-D-1-thiogalactopyranoside (IPTG) induction at mid-log phase of growth for 3 hours. Bacterial cells were then harvested by centrifugation, resuspended with lysis buffer containing 6 M urea, 1 M KCl, 20 mM phosphate buffer, pH 7.4, supplemented with 5 mM PMSF, and lysed using a French press. Lysate was centrifuged and the supernatant containing the fusion protein was loaded on a Zn(II) affinity column equilibrated with the same buffer. The column was washed with five bed volumes of lysis buffer and the Tx3-fusion protein was eluted with a step gradient of imidazole. The column fractions were examined with SDS-PAGE and the fusion protein peak was pooled, dialyzed against de-ionized water, and concentrated by lyophilization.
The lyophilized fusion protein was resuspended in a minimal volume of SUMO cleavage buffer (250 mM NaCl, 250 mM sucrose, 2 mM DTT, 40 mM Tris-HCl, pH 7.5). A 1:200 molar ratio of SUMO protease-to-fusion protein was used to cleave HcTnI-C27 and HcTnI-C27-H peptides at 4°C overnight. The cleaved product was loaded onto a re-equilibrated Zn(II) affinity column and the flow-through containing free HcTnI-C27 or HcTnI-C27-H peptide was collected, analyzed with small pore SDS-PAGE, dialyzed against de-ionized water, and then concentrated by lyophilization. The fusion proteins and isolated free peptides were used in the following structural and functional studies.
2.5. Synthetic HcTnI-C27 and HcTnI-C27-H Peptides
Free HcTnI-C27 and HcTnI-C27-H peptides were also chemically synthesized at a purity of >98% using a commercial service (Peptide 2.0 Inc., Chantilly, VA). After verification of their mAb TnI-1 epitope configuration and tropomyosin binding as described below, the chemically synthesized peptides were used in skinned muscle contractility studies.
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
The ELISA procedure used in the present study for epitope structure and protein binding studies was performed as described previously [32]. Briefly, 96-well microtiter plates were coated with SUMO-HcTnI-C27 fusion protein, SUMO-HcTnI-C27-H fusion protein, purified bovine cardiac TnI, or R192H mutant mouse cardiac TnI (2 μg/mL in Buffer A containing 0.1 M KCl, 3 mM MgCl2, 10 mM PIPES, pH 7.0, 100 μL/well) at 4°C overnight. The plates were washed with Buffer T (0.05% Tween-20 in Buffer A) for 10 minutes and tapped dry. The wells were blocked using Buffer B (1% BSA in Buffer T) at room temperature for one hour. After washing again with Buffer T, serial dilutions of primary mAb against the coated protein in Buffer D (dilution buffer, 0.1% BSA in Buffer T) were added to the plate to incubate at room temperature for two hours. After Buffer T washes, horse radish peroxidase (HRP)-conjugated anti-mouse immunoglobulin secondary antibody was added to incubate at room temperature for one hour. After final washes, H2O2-2,2′-azinobis-(3-ethylbenzthiazolinesulfonic acid) substrate solution was added for colorimetric development at room temperature and the plate was read using an automated microplate reader at 420 nm at 5 min intervals for 30 minutes.
The ELISA experiments were done in triplicate wells. Optical density data from the time point just before the end of the linear course of color development were used to plot the titration curves. Each experiment was repeated one or more times to confirm the results.
2.7. Competitive ELISA
Competitive tropomyosin-binding experiments were performed using a derivative protocol of the microtiter plate ELISA as described previously [32]. Purified bovine cardiac α-tropomyosin was coated on 96-well plate at 5 μg/mL in Buffer A, 100 μL/well, at 4°C overnight. Washed and blocked as above, bovine cardiac TnI at a predetermined concentration that produces sub-maximal binding for the immobilized tropomyosin was added together with serial dilutions of HcTnI-C27 or HcTnI-C27-H peptides for incubation at room temperature for 2 hours. The competition between the cardiac TnI C-terminal peptides and intact cardiac TnI for tropomyosin binding was measured via mAb 4H6 that recognizes an epitope in intact TnI but not in the C-terminal end peptide [22] and HRP-anti-mouse IgG secondary antibody using standard ELISA procedure as described above.
To compare the epitope configuration of chemically synthesized HcTnI-C27 and HcTnIC27-H peptides with that of the biologically synthesized counterparts, competitive ELISA affinity titrations against mAb TnI-1 were performed. 96-well microtiter plates were coated with purified bovine cardiac TnI in Buffer A and incubated at 4°C overnight. mAb TnI-1 at a predetermined concentration that produces sub-maximal binding for the immobilized bovine cardiac TnI was added together with serial dilutions of biologically or chemically synthesized HcTnI-C27 and HcTnI-C27-H peptides. The effects of the peptides on competing with intact cardiac TnI for mAb TnI-1 were measured via HRP-anti-mouse IgG secondary antibody using standard ELISA procedure as described above
The competitive ELISA experiments were done in triplicate wells and each assay was repeated one or more times.
2.8. Contractility Measurements Using Membrane Permeabilized Muscle Preparations
Permeabilized rat and mouse left ventricular papillary muscles were prepared using a skinned cryosection method. Immediately after euthanasia, papillary muscles were dissected with a portion of ventricular wall in one end and the valve tendon in the other end. The isolated papillary muscle was pinned down on a cork at the two ends using 30 gauge needles. A small drop of optimal cutting temperature (O.C.T.) compound was used to fill the space between the muscle tissue and the surface of the cork before flash freezing by submerging in liquid nitrogen. The frozen papillary muscle was sectioned longitudinally at the thickness of 35 μm using a cryostat and collected on a glass slide. Four stacked razor blades were used to cut the muscle sections longitudinally into 140–150 μm wide strips. The muscle strips were washed with a relaxing buffer (BES 40 mM, EGTA 10 mM, MgCl2 6.86 mM, ATP 5.96 mM, DTT 1 mM, creatine phosphate 33 mM, creatine kinase 200U/mL, K-propionate 3.28 mM, pH 7.0, plus protease inhibitor cocktail) and stored in a 35 mm dish at −20°C in relaxing buffer containing 50% glycerol until the use in force-pCa studies.
Extensor digitorum longus (EDL) muscles were obtained from adult C57B/L6 mice immediately after euthanasia to prepare chemically permeabilized muscle preparations as described previously [33, 34]. Briefly, whole EDL muscles were excised from mice and dissected along the fibers in the relaxing buffer. The strips dissected were washed with relaxing buffer and stored in 50% glycerol in relaxing buffer in a 35 mm dish at −20°C until used in force-pCa studies.
For contractility studies, the storage dish was placed on a thermal-controlled metal stage at 0°C under a dissection scope. Cryosectioned cardiac muscle strips selected with cardiomyocytes clearly organized along the long axis and EDL muscle fibers were mounted between two aluminum T-clips and transferred to an 8-chamber thermo-controlled stage (802D, Aurora Scientific) on an inverted microscope in relaxation buffer at 6–8°C. Seen through a 20X lens, the muscle preparation was connected to a force transducer (403A, Aurora Scientific) and a length controller (322C, Aurora Scientific). The buffer was then switched to a skinning solution (relaxation buffer containing 1% Triton X-100) for 20 min to further permeabilize the muscle strips. After a wash with relaxation buffer, the permeabilized muscle strip was placed in pCa 9.0 buffer made by mixing the relaxing buffer (pCa 10.0) with an activation buffer (BES 40 mM, EGTA 10 mM, MgCl2 6.64 mM, ATP 6.23 mM, DTT 1 mM, CaCl2 10 mM, creatine phosphate 33 mM, creatine kinase 200U/mL, K-propionate 2.09 mM, pH 7.0, plus protease inhibitor cocktail, pCa 4.0) [34] and the sarcomere length was measured through a digital camera attached to the microscope and adjusted to 2.0 μm and 2.3 μm for the cardiac muscle preparations or 2.7 μm for the EDL muscle fibers. Calcium activated force was examined at pCa 6.5, 6.3, 6.0, 5.8, 5.5, 5.0, and 4.5 at 15°C as described previously [34]. The series of pCa buffers were made by mixing the relaxation buffer and activation buffer with the free [Ca2+] calculated using Fabiato’s program [35]. HcTnI-C27 peptide was then added at 20 μM and the force-pCa measurements were repeated. The force-pCa curves were plotted and fitted using Hill exponential equation for data analysis [34].
2.9. Data Analysis
Statistical analysis was performed using Student’s t-test to compare paired data points.
3. Results
3.1. Biological Synthesis of C-terminal Peptide of Human Cardiac TnI
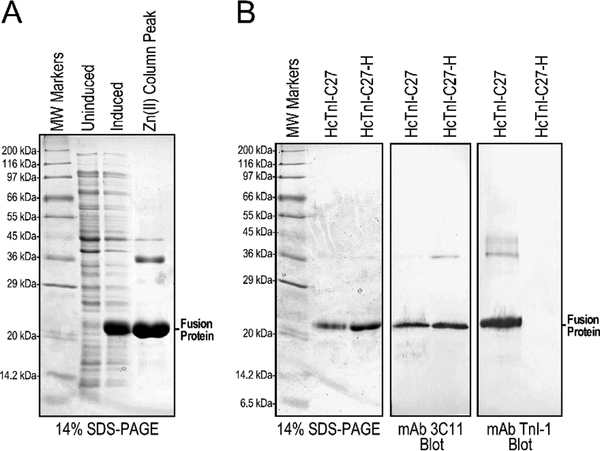
The Tx3-SUMO-HcTnI-C27 fusion protein was readily expressed in E. coli and purified using Zn(II) affinity column (Figure 3A). Similarly high level expression (data not shown) and effective one step purification was obtained for the Tx3-SUMO-HcTnI-C27-H fusion protein (Figure 3B).
Figure 3: Expression and purification of Tx3-SUMO-HcTnI-C27 and Tx3-SUMO-HcTnI-C27-H fusion proteins.
(A) The SDS-gel shows an example of induced expression of Tx3- SUMO-HcTnI-C27 fusion protein in E. coli and effective one-step Zn(II) column purification. (B) The Western blots of purified fusion proteins showed that while mAb 3C11 bound to both Tx3-SUMO-HcTnI-C27 and Tx3-SUMO-HcTnI-C27-H via the metal binding tag in the fusion carrier, mAb TnI-1 has a strong binding to Tx3-SUMO-HcTnI-C27 indicating preserved epitope structure, which is abolished in the Tx3-SUMO-HcTnI-C27-H mutant. MW, molecular weight.
While the purified Tx3-SUMO-HcTnI-C27 and Tx3-SUMO-HcTnI-C27-H fusion proteins are both recognized by the anti-Tx3 tag mAb 3C11 in Western blot, only Tx3-SUMOHcTnI-C27 was reactive to mAb TnI-1 (Figure 3B). The result is consistent with previous Western blot studies in intact cardiac TnI where the myopathic single amino acid R192H substitution completely abolished the epitope recognized by mAb TnI-1 [25].
The results that the HcTnI-C27 peptide retains the mAb TnI-1 epitope structure when fused with an unrelated carrier protein indicate its retention of a native conformation. This epitope structure is preserved or intrinsically resumed after the denaturing process of SDS-PAGE and Western blotting (Figure 3B).
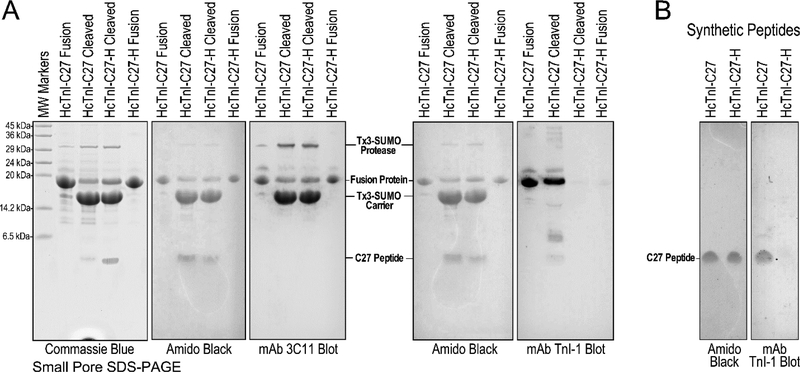
SUMO protease cleavage of the fusion proteins released the HcTnI-C27 and HcTnI-C27-H peptides (Figure 4A). The released peptides were separated from the carrier protein, any un-cleaved fusion protein and the protease, which all have the metal binding tag were absorbed by the post-cleavage Zn(II) column.
Figure 4: Cleavage of C27 peptides from fusion proteins and preservation of mAb TnI epitope in HcTnI-C27 peptide.
(A) The 15% small pore SDS-PAGE gels show the cleavage of Tx3-SUMO-HcTnI-C27 and Tx3-SUMO-HcTnI-C27-H fusion proteins. Western blot using mAb 3C11 against the metal-binding tag in carrier protein detected the intact fusion proteins and the cleaved carrier, as well as the recombinant SUMO protease that has the metal-binding tag for rapid purification. Western blot using mAb TnI-1 detected the HcTnI-C27 fusion protein and free HcTnI-C27 peptide released by SUMO protease digestion but not the carrier protein. The binding of mAb TnI-1 is lost for the HcTnI-C27-H mutant peptide. (B) The preservation of mAb TnI-1 epitope in wild type but not mutant C27 peptide was more clearly demonstrated by Western blot using chemically synthesized peptides. Amido Black stains of the PVDF membranes prior to blocking and mAb incubation are shown to verify the effective blotting of the small peptides. MW, molecular weight.
The identities of the cleavage products were verified by Western blotting using mAb 3C11 against the Tx3 tag and mAb TnI-1 against the HcTnI-C27 epitope (Figure 4A). After cleavage from the fusion protein, the isolated HcTnI-C27 peptide remains reactive to mAb TnI-1 in Western blot (Figure 4A), further demonstrating that this short peptide structure is able to configure the native epitope conformation independently and after the denaturing process of SDS-PAGE and Western blotting whereas the HcTnI-C27-H mutant peptide cleaved from the fusion protein remains non-reactive to mAb TnI-1 (Figure 4A). The results suggest that the C-terminal end segment of TnI is a structural domain that forms the native conformation when isolated from the TnI backbone. This observation was confirmed by mAb TnI-1 Western blot using chemically synthesized HcTnI-C27 and HcTnI-C27-H peptides (Figure 4B).
3.2. HcTnI-C27 Peptide Retains Native Configuration in Non-denaturing Conditions
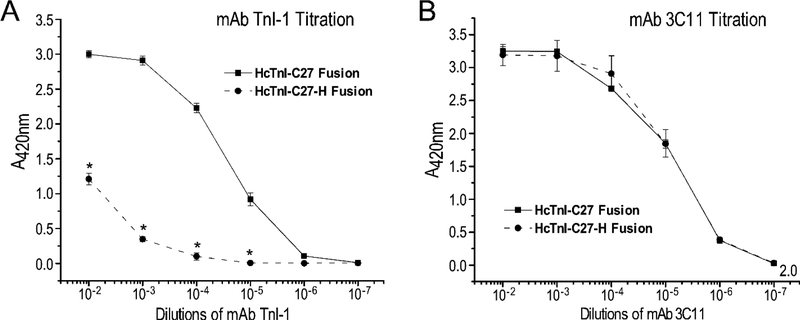
To confirm the observations in Western blotting studies, ELISA titrations further showed that Tx3-SUMO-HcTnI-C27 fusion protein reacts with mAb TnI-1 in a non-denaturing condition. The titration curve in Figure 5A shows a saturable binding of mAb TnI-1 to Tx3-SUMO-HcTnI-C27 fusion protein immobilized to microtiter plate, which is nearly identical to that of the coating control using anti-Tx3 tag mAb 3C11 (Figure 5B).
Figure 5: ELISA titration of mAbs TnI-1 and 3C11 against Tx3-SUMO-HcTnI-C27 and Tx3-SUMO-HcTnI-C27-H fusion proteins.
The fusion proteins were coated on microtiter plate to incubate with serial dilutions of the mAbs for ELISA titration as described in the methods. (A) mAb TnI-1 showed high affinity binding to Tx3-SUMO-HcTnI-C27, which was significantly decreased but still clearly detectable for Tx3-SUMO-HcTnI-C27-H. (B) mAb 3C11 titration curves against the metal tag in fusion proteins confirmed comparable amounts of Tx3-SUMO HcTnI-C27 and Tx3-SUMO-HcTnI-C27-H coated on the microtiter plate. *P < 0.0001 in paired Student’s t-test.
While Tx3-SUMO-HcTnI-C27-H fusion protein completely lost binding to mAb TnI-1 under the post-denaturing Western blotting condition (Figure 4B), it showed a clearly detectable but significantly decreased binding to mAb TnI-1 in ELISA titration under non-denaturing conditions (Figure 5A). This result demonstrates that while the myopathic R192H mutation [16] significantly alters the conformation and function of the C-terminal end segment of cardiac TnI, the mAb TnI-1 epitope is partially preserved in the Tx3-SUMO-HcTnI-C27-H fusion protein.
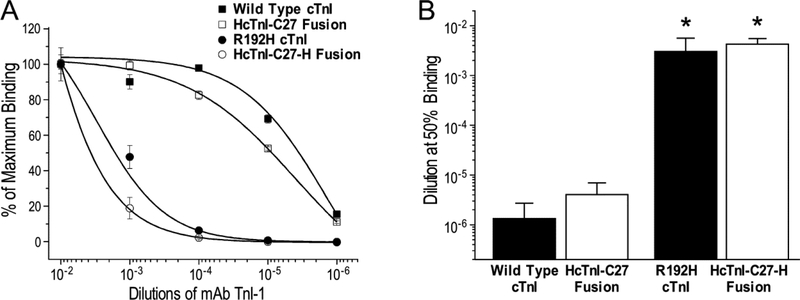
The retained native conformation of HcTnI-C27 peptide in the fusion protein and the alteration due to the R192H mutation were further compared to that of the C-terminal end segment residing in situ in intact cardiac TnI. The results of mAb TnI-1 titration in Figure 6 showed that the binding affinity of Tx3-SUMO-HcTnI-C27 fusion protein for mAb TnI-1 is very similar to that of intact wild type bovine cardiac TnI whereas the R192H mutation produced similar decreases in the affinity for mAb TnI-1 in Tx3-SUMO-HcTnI-C27-H fusion protein and in intact mouse cardiac TnI engineered with the RCM mutation. The similar epitope conformation of HcTnI-C27 peptide in fusion with an unrelated carrier protein and in situ in cardiac TnI further demonstrates its nature as an independent structural domain in troponin complex.
Figure 6: Similar affinities of mAb TnI-1 for HcTnI-C27 residing in SUMO fusion protein and in cardiac TnI.
The ELISA titration curves normalized to maximum binding (A) showed that mAb TnI-1 binds its epitope in Tx3-SUMO-HcTnI-C27 fusion protein and in wild type cardiac TnI (cTnI) with similar affinities as reflected by the mAb TnI-1 dilutions for 50% maximum binding (B), which were similarly decreased by the R to H single amino acid substitution in Tx3-SUMO-HcTnI-C27-H fusion protein and in situ in R192H RCM mutant cardiac TnI (*P < 0.005 compared with the wild type control in Student’s t test).
3.3. HcTnI-C27 Peptide Binds Tropomyosin
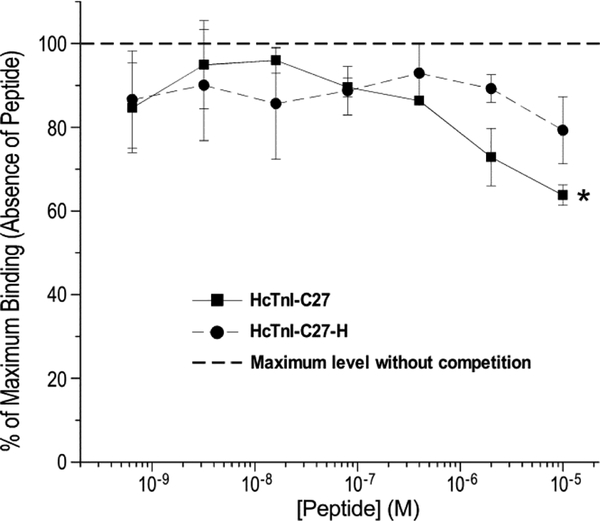
Implicating a potential role in TnI’s inhibitory regulation of muscle contractility, we previously found that when residing in the troponin complex, the C-terminal end segment of TnI possesses a Ca2+-regulated, relatively low-affinity but saturable binding to tropomyosin [18]. To demonstrate that this biochemical activity is retained with the isolated HcTnI-C27 peptide, the results of competitive tropomyosin binding study in Figure 7 showed that the presence of HcTnI-C27 peptide in a physiological solution produced a dose-dependent competition with intact bovine cardiac TnI for the binding to tropomyosin immobilized on microtiter plate. This function was diminished with the HcTnI-C27-H mutant peptide. The results demonstrate retained biochemical activity of HcTnI-C27 peptide in isolation from the TnI backbone.
Figure 7: Isolated HcTnI-C27 peptide retains the binding affinity for tropomyosin.
The competitive ELISA titration curves normalized to the maximum binding of intact cardiac TnI for tropomyosin without competition showed a dose-dependent competitive effect of HcTnI-C27 peptide (*P < 0.05 in paired Student’s t test), which was diminished for the HcTnI-C27-H RCM mutant peptide.
3.4. Chemically synthesized HcTnI-C27 and HcTnI-C27-H peptides exhibit epitope conformations similar to their biological-made counterparts in physiologic solution
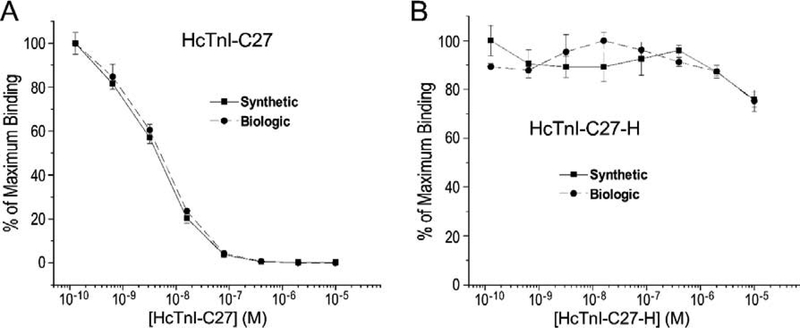
Upon demonstrating the preserved native epitope structure and biochemical activity of HcTnI-C27 and HcTnI-C27-H peptides made from bacterial expression via the rather complex procedure, we evaluated chemically synthesized peptides in order to use them in further structural and functional studies. The competitive ELISA titration results in Figure 8A demonstrated that synthetic and biologically expressed HcTnI-C27 peptides had nearly identical effectiveness on competing in a physiologic solution with intact cardiac TnI coated on microtiter plate for the binding of mAb TnI-1. Consistently, chemically synthesized and biologically made HcTnI-C27-H mutant peptides both drastically lost the binding affinity for mAb TnI-1 (Figure 8B).
Figure 8: Synthetic and biologically made HcTnI-C27 peptides have similar epitope conformation in physiologic buffer.
Normalized to the maximum binding of a pre-determined concentration of mAb TnI-1 to intact cardiac TnI immobilized on microtiter plate, the competition curves of serial dilutions of chemically synthesized and biologically made HcTnI-C27 peptides were nearly identical, reflecting their comparable epitope conformation in a physiologic buffer (A). Synthetic and biologically made HcTnI-C27-H mutant peptides both showed drastically diminished ability in competing for mAb TnI-1, reflecting similarly altered conformation of the epitope (B).
3.5. HcTnI-C27 Peptide Reduces Ca2+ Sensitivity of Skinned Muscle Preparations
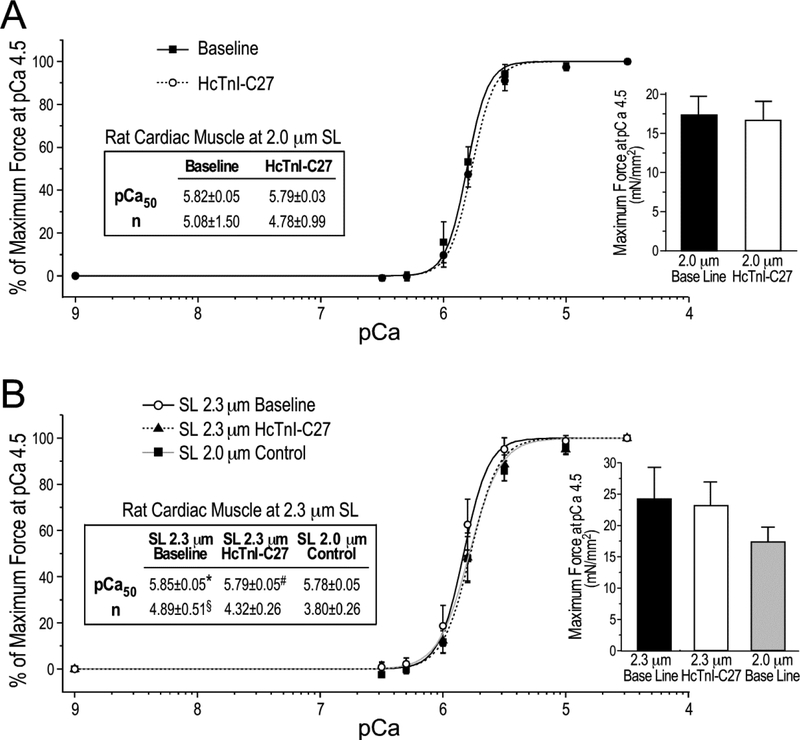
To investigate the function of the C-terminal end segment of TnI in regulating muscle contraction, the effect of wild type HcTnI-C27 peptide on the force-pCa relationship of cardiac muscle was first studied using skinned rat ventricular papillary muscle sections at sarcomere lengths of 2.0 μm and 2.3 μm. The contractility results in Figure 9A showed that HcTnI-C27 peptide had a small effect on lowering pCa50 and cooperativity at sarcomere lengths of 2.0 μm. However, it produced a statistically significant right-shift of pCa50 at sarcomere lengths of 2.3 μm with a trend of decreasing cooperativity (Figure 9B). The maximum force production was not affected by the addition of HcTnI-C27 peptide (the bar graphs in Figure 9).
Figure 9. HcTnI-C27 peptide reduces Ca2+ sensitivity in skinned rat cardiac muscle strips without decreasing maximum force production.
Ca2+-activated isometric force of skinned rat papillary muscle at sarcomere lengths (SL) of 2.0 μm and 2.3 μm in the absence or presence of 20 μM HcTnI-C27 peptide was plotted as Hill fitted force-pCa curves normalized to the maximum force at pCa 4.5. At sarcomere length of 2.0 μm, HcTnI-C27 peptide resulted in a small right-shift of the force-pCa curve (P = 0.055 in one-tail Student t test) (A). At sarcomere length of 2.3 μm, however, the addition of HcTnI-C27 peptide significantly decreased Ca2+ sensitivity and cooperativity (n), completely diminishing the Ca2+-sensitization effect of increasing sarcomere length from 2.0 μm to 2.3 μm. Values are presented as mean ± SE. N = 4 for SL 2.0 μm group and n = 3 for SL 2.3 μm group. The bar graphs show that the maximum force production was not affected by the addition of HcTnI-C27 peptide. Statistical analysis was done using paired Student’s t test. pCa50, Ca2+ concentration for 50% maximum force. *P < 0.05 vs the SL 2.0 μm control; #P < 0.05 vs the SL 2.3 μm baseline in the absence of HcTnI-C27 peptide; §P < 0.05 vs SL 2.0 μm control in one-tail Student t test.
It is interesting that the effect of HcTnI-C27 on Ca2+-desensitization and the effect of increasing sarcomere length on increasing Ca2+-sensitivity are both more notable at higher [Ca2+] in the isometric contractility assay, corresponding to the activated state of myofilaments (Figure 9B). As an outcome, the effect of HcTnI-C27 peptide diminished the Ca2+-sensitization effect of increasing sarcomere length from 2.0 μm to 2.3 μm (Figure 9B). The results demonstrate that the adding of free HcTnI-C27 peptide to skinned muscle preparation produces a myofilament activation- as well as sarcomere length-dependent Ca2+-desensitization.
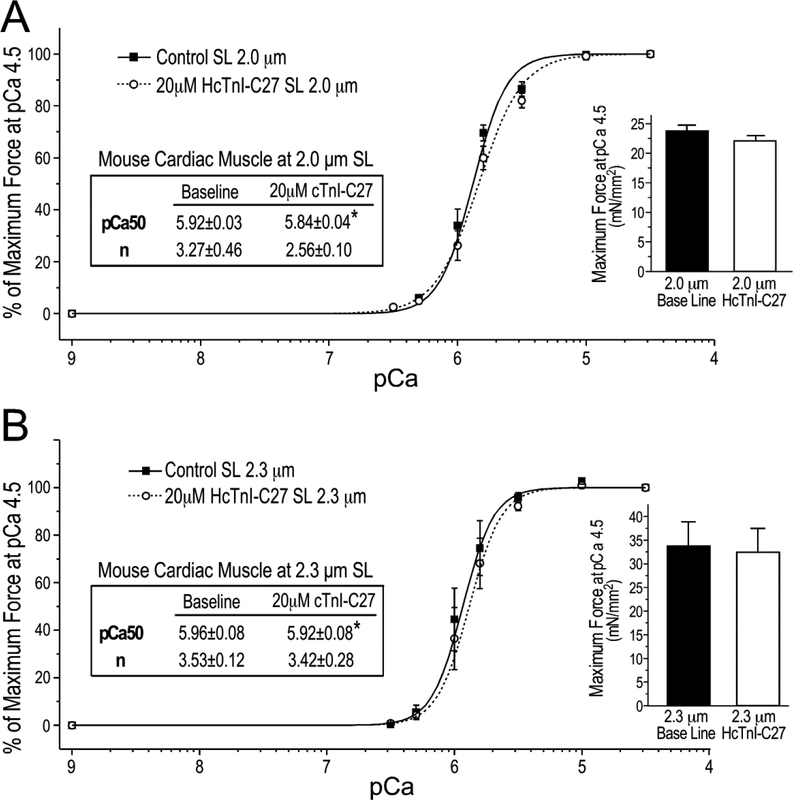
The effect of HcTnI-C27 peptide on myofilament Ca2+-sensitivity and force development was confirmed using skinned mouse ventricular papillary muscle preparations. The results in Figure 10 showed that the addition of HcTnI-C27 peptide reproduced the Ca2+-desensitization effect with right-shifts of the force-pCa curves in skinned mouse cardiac muscle strips. Different from that seen in rat cardiac muscle (Figure 9), the effect was more obvious at sarcomere length of 2.0 μm in the activated state with significantly lowered cooperativity in comparison to that at sarcomere length of 2.3 μm (Figure 10). One possible explanation for the different sarcomere length dependence of HcTnI-C27’s Ca2+ desensitization effect on mouse and rat cardiac muscles could be a species-specific difference in cardiomyocyte compliance, which is known to affect myofilament force-pCa relationships [36]. Supporting this notion, we detected a trend of more increase in the passive tension of skinned mouse cardiac muscle at pCa 9 when increasing the sarcomere length from 2.0 μm to 2.3 μm than that in the rat cardiac muscle preparations (data not shown). The higher passive tension reflects a lower compliance of mouse cardiac muscle than that of rat, which may confer difference myofilament responses to the changes of sarcomere length. This hypothesis and its implication on the desensitization effect of HcTnI-C27 peptide is worth further investigating.
Figure 10. HcTnI-C27 reduces Ca2+ sensitivity of skinned mouse cardiac muscle.
The Force-pCa curves show that HcTnI-C27 treatment decreased Ca2+-sensitivity of skinned mouse ventricular papillary muscle at sarcomere lengths (SL) of 2.0 μm (A) and 2.3 μm (B) with decreased cooperativity (n) at SL of 2.0 μm. The maximum force production was not significantly affected by the addition of HcTnI-C27 peptide (the bar graphs). Ca50, Ca2+ concentration for 50% maximum force. Values are presented as mean ± SE. N = 3 for 2.0 μm and n = 4 for 2.3 μm groups. Statistical analysis was done using paired Student’s t test. *P < 0.05 vs the HcTnI-C27 peptide-absent baseline.
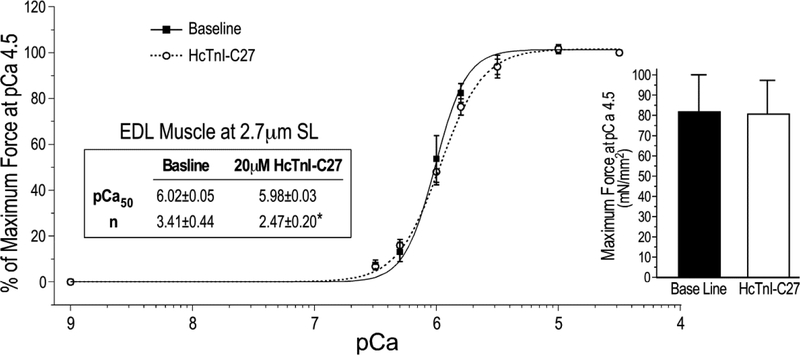
Since the C-terminal end segment of TnI is conserved in cardiac and skeletal muscle isoforms of TnI (Figure 1), we also tested HcTnI-C27 peptide for its effect on the contractility of skinned mouse EDL muscle fibers. The results in Figure 11 showed that at physiologic sarcomere length of 2.7 μm, the presence of 20 μM HcTnI-C27 peptide produced a myofilament Ca2+-desensitization effect predominantly in the activated state with significantly decreased cooperativity. The maximum force production was not affected by the addition of HcTnI-C27 peptide. Reproduction of the physiological effect of HcTnI-C27 peptide in a skeletal muscle preparation adds an evidence for the conserved function of the C-terminal end segment of TnI.
Figure 11. Ca2+-desensitization effect of HcTnI-C27 peptide in skinned mouse EDL muscle.
Force-pCa curves of skinned EDL muscle fibers at sarcomere length (SL) of 2.7 μm in the absence or presence of 20 μM HcTnI-C27 peptide showed a decrease in cooperativity (n) upon HcTnI-C27 treatment due to the decreases in myofilament Ca2+ sensitivity in the activated state at higher Ca2+ concentrations without change of maximum force production. Values are presented as mean ± SE. N = 4 in each group. *P < 0.05 vs the baseline in one-tail Student’s t test.
4. Discussion
We previously reported that the C-terminal end segment of TnI is a Ca2+-modulated allosteric structure exposed in troponin complex [8]. Based on the fact that this structure is also the most conserved segment of TnI among the three muscle type TnI isoforms and across vertebrate species [8], we subsequently demonstrated that the molecular conformation of this structure in troponin is regulated by Ca2+, corresponding to a saturable binding to tropomyosin at pCa 9 but not at pCa 4 [18]. NMR and molecular modeling also indicated that the C-terminal end segment of TnI is a Ca2+-modulated mobile domain in reconstituted troponin complex [19]. Our present study further characterized the C-terminal end peptide of TnI in isolation from the TnI backbone. By demonstrating the preservation of native epitope structure in HcTnI-C27 peptide with retained biochemical activity to bind tropomyosin, the destructive effect of myopathic mutation R192H, and the effect of HcTnI-C27 peptide on myofilament Ca2+-desensitization, the results present the following novel findings.
4.1. The Highly Conserved C-terminal End Segment of TnI Is Able to Function as A Standing Alone Regulatory Structure
We previously studied intact TnI and C-terminal truncated TnI to locate the mAb TnI-1 epitope to the C-terminal end segment [8], and demonstrated its destruction by the single amino acid substitution mutation R192H [16]. By engineering and characterization of the C27 peptides, results of the present study demonstrate that HcTnI-C27 fully retains the native conformation of mAb TnI-1 epitope with biochemical and physiologic activities when fused with the unrelated protein SUMO or as a free peptide while the significant structural alteration by the R192H mutation is also independent of the TnI backbone. The fact that the native epitope structure of HcTnI-C27 was retained after undergoing the denaturing condition during SDS-PAGE and Western blotting further indicates its intrinsic property of maintaining and/or restoring the native conformation. These data not only provide evidence for the structural independence of the C-terminal end domain but also supported the subsequent studies on its intrinsic biological activity.
The finding that free HcTnI-C27 peptide retains the biochemical function of binding tropomyosin with an affinity similar to that of intact TnI solidifies the previously reported Ca2+-regulated interaction of the C-terminus of TnI with tropomyosin [18]. Numerous studies have investigated the structural basis of TnI’s inhibitory function during Ca2+-regulated contraction and relaxation of striated muscles [9, 15]. The highly conserved structure of the C-terminal end segment of TnI, its binding to tropomyosin at low [Ca2+] [18], its NMR structure as a mobile domain of troponin [19], and the diminished inhibitory function of myopathic cardiac TnI mutation R192H strongly support our observation that the C-terminal end segment of TnI is an inhibitory regulatory structure.
The effect of free HcTnI-C27 peptide on Ca2+-desensitization further support the notion that TnI C-terminal end segment may play regulatory function as a stand-alone peptide to modulate muscle contractility, providing a novel tool to study the structure-function relationship of troponin in myofilament Ca2+-regulation.
4.2. Insights into the Myopathic Mutation R192H of Cardiac TnI’s Loss of Inhibitory Function
The altered molecular conformation of the C-terminal end segment of cardiac TnI due to the R192H mutation corresponds to a significant decrease in the binding affinity for tropomyosin (Figure 7), indicting this loss of function as a pathogenic mechanism to cause RCM. We previously showed that the C-terminal end segment of TnI binds tropomyosin at low Ca2+ state [18]. The diminished binding affinity for tropomyosin due to R192H mutation may impair the inhibitory function of troponin during the relaxation of cardiac muscle to cause diastolic dysfunction [16, 25].
While wild type HcTnI-C27 peptide is able to strongly compete with intact TnI for the binding of mAb TnI-1 at ~0.1 μM, HcTnI-C27-H had only minimum effect (Figure 8). This change in epitope structure demonstrates that the single amino acid R192H RCM mutation produces a significant conformational alteration in the C-terminal end segment of cardiac TnI. In contrast to the completely abolished recognition of mAb TnI-1 in Western blot (Figures 3 and 4), this partially preserved epitope structure nonetheless indicates that the R192H mutation does not completely destroy the intrinsic folding and overall conformation of the C-terminal end domain of TnI under native conditions, but may have decreased the structural stability to reduce the resistance to denaturing conditions or impaired the ability of restoring native conformation. To promote its native folding and/or conformational stability may be explored for the treatment of cTnI R192H RCM. Further studies are needed to compare HcTnI-C27-H mutant with wild type peptides for their difference in structural stability relating to the effect on Ca2+ regulation of myofibril contractility.
4.3. The C-terminal End Domain of TnI As A Modulator of Muscle Contractility
When added to skinned cardiac (Figures 9 and 10) and skeletal (Figure 11) muscle preparations, free HcTnI-C27 peptide produces a Ca2+-desensitization effect without significant effect on maximum force production. Since the C-terminal end peptide recapitulates its structure and regulatory function in intact TnI, this effect is consistent with the noted inhibitory function of the C-terminal end domain of TnI in the Ca2+-regulation of muscle contraction.
The effect of additional free HcTnI-C27 peptide may reflect an augmentation of the in situ inhibitory function of the C-terminal end domain of TnI in facilitating myocardial relaxation. Its effect on decreasing the cooperativity of force-pCa curves reflects a selective decrease of myofilament Ca2+-sensitivity at the higher Ca2+ concentrations corresponding to the activated state. This functional feature of the C-terminal end segment of TnI indicates a novel myofilament mechanism to modulate the kinetics of muscle contractility. The post-activation Ca2+-desensitization of myofibrils lays a basis for slowing down late-systolic velocity to prolong ventricular ejection time without reducing maximum force development. Over two-thirds of the left ventricular stroke volume of human heart is produced in the rapid ejection phase [37]. We have demonstrated that a small prolongation of the rapid ejection time by moderate reduction of myocardial contractile velocity significantly increases left ventricular stroke volume without increasing the ventricular peak systolic pressure [38]. Therefore, enhancing the function of the C-terminal end domain of TnI presents a plausible mechanism to treat heart failure.
Diastolic heart failure, i.e., heart failure with preserved ejection fraction, HFpEF [39], is a challenging clinical condition. It is characterized by inefficient filling of the heart chambers during diastole resulting in reduced stroke volume by the Frank-Starling mechanism that involves the function of cardiac TnI [40, 41]. The post-activation Ca2+-desensitization function of the C-terminal end domain of TnI merits further investigation for the significance in facilitating myocardial relaxation and the treatment of HFpEF.
4.4. HcTnI-C27 Free Peptide as A Myofilament Ca2+-Desensitizing Reagent
Heart failure is the most common end stage condition of cardiovascular diseases. After decades of intensive research and numerous clinical trials, specific and effective treatments remain to be developed [42, 43]. Beta blockers have been commonly utilized in the treatment of heart failure by enhancing ventricular filling and lowering vascular resistance; however, beta blockers are notably negative inotropes and thereby have the potential to weaken force production [44, 45]. On the other hand, positively inotropic drug such as digitalis and other Ca2+ enhancers drastically increase myocardial energetic expenditure with very limited long-term benefit [46]. There is no specific treatment for HFpEF [39]. Pharmacological therapy for diastolic cardiac dysfunction is currently multifactorial and involves addressing diuresis, heart rate control, reducing myocardial hypertrophy and ventricular relaxation [42, 43, 47].
Therefore, a new generation of heart failure treatment, which selectively targets specific steps of the cardiac muscle contraction and relaxation cycle, needs to be developed in order to effectively treat systolic and diastolic heart failures while minimizing side effects. The predominantly post-activation myofilament Ca2+-desensitizing effect of HcTnI-C27 peptide demonstrates a promising approach to selectively modulate contractile kinetics downstream of Ca2+-activation without reducing maximum force production. The data laid a foundation for applying this endogenous small peptide in the treatment of heart failure, especially the treatment of diastolic heart failure. Further studies on high resolution structure of the peptide will lead the development of HcTnI-C27 into a myofilament Ca2+-desensitizer drug.
Highlights.
The C-terminal end segment of troponin I is a highly conserved structure
Deletion or point mutation in this segment impairs cardiac muscle relaxation
TnI C-terminal terminal peptide retains native conformation and binds tropomyosin
The C27 peptide reduces myofibril Ca2+-sensitivity without decreasing maximum force
The results demonstrate a potential mechanism for the treatment of heart failure
Acknowledgements
We thank Ms. Hui Wang for technical support.
Sources of Funding
This work is supported in part by grants from the National Institutes of Health (HL-127691 and 138007 to JPJ).
Abbreviations
- cTnI
cardiac troponin subunit I
- EDL
Extensor digitorum longus
- HcTnI-C27
C-terminal 27 amino acid peptide of human cardiac TnI
- HcTnI-C27-H
R192H mutant of the C-terminal 27 amino acid peptide of human cardiac TnI
- pCa50
Ca2+ concentration for 50% maximum force
- SUMO
small ubiquitin-like modifier
- Tx3
a transition metal-binding tag
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None
References
- [1].Krans JL, The Sliding Filament Theory of Muscle Contraction, Nature Education 3(9) (2010) 66. [Google Scholar]
- [2].Yanagida T, Arata T, Oosawa F, Sliding distance of actin filament induced by a myosin crossbridge during one ATP hydrolysis cycle., Nature 316(6026) (1985) 366. [DOI] [PubMed] [Google Scholar]
- [3].Spudich JA, Huxley HE, Finch JT, Regulation of skeletal muscle contraction: II. Structural studies of the interaction of the tropomyosin-troponin complex with actin, Journal of Molecular Biology 72(3) (1972) 619–620. [DOI] [PubMed] [Google Scholar]
- [4].Greaser ML, Gergely J, Reconstitution of troponin activity from three protein components., Journal of Biological Chemistry 246(13) (1971) 4226–4233. [PubMed] [Google Scholar]
- [5].Lymn RW, Taylor EW, Mechanism of adenosine triphosphate hydrolysis by actomyosin., Biochemistry 10(25) (1971) 4617–4624. [DOI] [PubMed] [Google Scholar]
- [6].Narita A, Yasunaga T, Ishikawa T, Mayanagi K, Wakabayashi T, Ca2+-induced switching of troponin and tropomyosin on actin filaments as revealed by electron cryo-microscopy, Journal of Molecular Biology 308(2) (2001) 241–261. [DOI] [PubMed] [Google Scholar]
- [7].Gomes AV, Potter JD, Szczesna-Cordary D, The Role of Troponins in Muscle Contraction, IUBMB life 54(6) (2002) 323–333. [DOI] [PubMed] [Google Scholar]
- [8].Jin J-P, Yang F-W, Yu Z-B, Ruse CI, Bond M, Chen A, The Highly Conserved COOH Terminus of Troponin I Forms a Ca2+-Modulated Allosteric Domain in the Troponin Complex, Biochemistry 40(8) (2001) 2623–2631. [DOI] [PubMed] [Google Scholar]
- [9].Sheng J-J, Jin J-P, TNNI1, TNNI2 and TNNI3: Evolution, Regulation, and Protein Structure-Function Relationships Gene 576(1) (2016) 385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gambarin FI, Tagliani M, Arbustini E, Pure restrictive cardiomyopathy associated with cardiac troponin I gene mutation: Mismatch between the lack of hypertrophy and the presence of disarray, Heart 94(10) (2008) 1257. [DOI] [PubMed] [Google Scholar]
- [11].Doolan A, Tebo M, Ingles J, Nguyen L, Tsoutsman T, Lam L, Chiu C, Chung J, Weintraub RG, Semsarian C, Cardiac troponin I mutations in Australian families with hypertrophic cardiomyopathy: clinical, genetic and functional consequences, Journal of Molecular and Cellular Cardiology 38(2) (2005) 387–393. [DOI] [PubMed] [Google Scholar]
- [12].Lu Q-W, Wu X-Y, Morimoto S, Inherited cardiomyopathies caused by troponin mutations, Journal of geriatric cardiology 10(1) (2013) 91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Parvatiyar MS, Pinto JR, Dweck D, Potter JD, Cardiac Troponin Mutations and Restrictive Cardiomyopathy, Journal of Biomedicine and Biotechnology 2010 (2010) 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Willott RH, Gomes AV, Chang AN, Parvatiyar MS, Pinto JR, Potter JD, Mutations in Troponin that cause HCM, DCM AND RCM: What can we learn about thin filament function?, Journal of Molecular and Cellular Cardiology 48(5) (2010) 882–892. [DOI] [PubMed] [Google Scholar]
- [15].Sheng J-J, Jin J-P, Gene regulation, alternative splicing, and posttranslational modification of troponin subunits in cardiac development and adaptation: a focused review, Frontiers in Physiology 5 (2014) 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Du J, Zhang C, Liu J, Sidky C, Huang XP, A point mutation (R192H) in the C-terminus of human cardiac troponin I causes diastolic dysfunction in transgenic mice, Archives of Biochcemistry and Biophysics 456(2) (2006) 143–150. [DOI] [PubMed] [Google Scholar]
- [17].Murphy AM, Kögler H, Georgakopoulos D, McDonough JL, Kass DA, Eyk JEV, Marbán E, Transgenic Mouse Model of Stunned Myocardium, Science 287(5452) (2000) 488–491. [DOI] [PubMed] [Google Scholar]
- [18].Zhang Z, Akhter S, Mottl S, Jin J-P, Calcium-Regulated Conformational Change in the COOH-Terminal End Segment of Troponin I and Its Binding to Tropomyosin, The FEBS Journal 278(18) (2011) 3348–3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Murakami K, Yumoto F, Ohko S.-y., Yasunaga T, Tanokura M, Wakabayashi T, Structural Basis for Ca2C-regulated Muscle Relaxation at Interaction Sites of Troponin with Actin and Tropomyosin, Journal of Molecular Biology 352(1) (2005) 178–201. [DOI] [PubMed] [Google Scholar]
- [20].Takeda S, Yamashita A, Maeda K, Maéda Y, Structure of the core domain of human cardiac troponin in the Ca2+-saturated form, Nature 424(6944) (2003) 35. [DOI] [PubMed] [Google Scholar]
- [21].Vinogradova MV, Stone DB, Malanina GG, Karatzaferi C, Cooke R, Mendelson RA, Fletterick RJ, Ca(2+)-regulated structural changes in troponin., Proceedings of the National Academy of Sciences 102(14) (2005) 5038–5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Akhter S, Jin J-P, Distinct conformational and functional effects of two adjacent pathogenic mutations in cardiac troponin I at the interface with troponin T, FEBS Open Bio 5 (2015) 64–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zhang Z, Biesiadecki BJ, Jin J-P, Selective deletion of the NH2-terminal variable region of cardiac troponin T in ischemia-reperfusion by myofibril-associated μ-calpain cleavage, Biochemistry 45(38) (2007) 11681–11694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Yu Z-B, Zhang L-F, Jin J-P, A proteolytic NH2-terminal truncation of cardiac troponin I that is up-regulated in simulated microgravity, Journal of Biological Chemistry 276(19) (2001) 15753–15760. [DOI] [PubMed] [Google Scholar]
- [25].Li Y, Zhang L, Jean-Charles P-Y, Nan C, Chen G, Tian J, Jin J-P, Gelb IJ, Huang X, Dose-dependent diastolic dysfunction and early death in a mouse model with cardiac troponin mutations, Journal of Molecular and Cellular Cardiology 62 (2013) 227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Malakhov MP, Mattern MR, Malakhova OA, Drinker M, Weeks SD, Butt TR, SUMO fusions and SUMO-specific protease for efficient expression and purification of proteins, Journal of Structural and Functional Genomics 5(1–2) (2004) 75–86. [DOI] [PubMed] [Google Scholar]
- [27].Catanzariti A-M, Soboleva TA, Jans DA, Board PG, Baker RT, An efficient system for high-level expression and easy purification of authentic recombinant proteins, Protein Science 13(5) (2004) 1331–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Amarasinghe C, Jin J-P, The Use of Affinity Tags to Overcome Obstacles in Recombinant Protein Expression and Purification, Protein and Peptide Letters 22(10) (2015) 885–892. [DOI] [PubMed] [Google Scholar]
- [29].Jin J-P, Cloned Rat Cardiac Titin Class I and Class II Motifs EXPRESSION, PURIFICATION, CHARACTERIZATION, AND INTERACTION WITH F-ACTIN, Journal of Biological Chemistry 270(12) (1995) 6908–6916. [PubMed] [Google Scholar]
- [30].Schägger H, Jagow GV, Tricine-Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis for the Separation of Proteins in the Range from 1 to 100 kDa Analytical biochemistry 166(2) (1987) 368–379. [DOI] [PubMed] [Google Scholar]
- [31].Liu R, Calponin And Cytoskeleton Dynamics In Macrophage Functions And The Pathogenesis Of Atherosclerosis, Wayne State University, Wayne State University; Dissertations, 2016. [Google Scholar]
- [32].Biesiadecki BJ, Jin J-P, A high-throughput solid-phase microplate protein-binding assay to investigate interactions between myofilament proteins., Journal of Biomedicine and Biotechnology 2011 (2011) 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ochala J, Lehtokari V-L, Iwamoto H, Li M, Feng H-Z, Jin J-P, Yagi N, Wallgren-Pettersson C, Penisson-Besnier I, Larsson L, Disrupted myosin cross-bridge cycling kinetics triggersmuscle weakness in nebulin-related myopathy, The FASEB Journal 25(6) (2011) 1903–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Roche SM, Gumucio JP, Brooks SV, Mendias CL, Claflin DR, Measurement of Maximum Isometric Force Generated by Permeabilized Skeletal Muscle Fibers, Journal of Visualized Experiments 100 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Fabiato A, Fabiato F, Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells., Journal of Physiology (Paris) 75(5) (1979) 463–505. [PubMed] [Google Scholar]
- [36].Patrick SM, Hoskins AC, Kentish JC, White E, Shiels HA, Cazorla O, Enhanced length-dependent Ca2+ activation in fish cardiomyocytes permits a large operating range of sarcomere lengths, Journal of Molecular and Cellular Cardiology 48(5) (2010) 917–924. [DOI] [PubMed] [Google Scholar]
- [37].Pelech AN, The physiology of cardiac auscultation, Pediatric Clinics 51(6) (2004) 1515–1535. [DOI] [PubMed] [Google Scholar]
- [38].Feng H-Z, Biesiadecki BJ, Yu Z-B, Hossain MM, Jin J-P, Restricted N-terminal truncation of cardiac troponin T: a novel mechanism for functional adaptation to energetic crisis., Journal of Physiology 586(14) (2008) 3537–3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Melenovsky V, Borlaug BA, Rosen B, Hay I, Ferruci L, Morell CH, Lakatta EG, Najjar SS, Kass DA, Cardiovascular features of heart failure with preserved ejection fraction versus nonfailing hypertensive left ventricular hypertrophy in the urban Baltimore community: the role of atrial remodeling/dysfunction., Journal of the American College of Cardiology 49(2) (2007) 198–207. [DOI] [PubMed] [Google Scholar]
- [40].Shiels HA, White E, The Frank–Starling mechanism in vertebrate cardiac myocytes., Journal of Experimental Biology 211(13) (2008) 2005–2013. [DOI] [PubMed] [Google Scholar]
- [41].Allen DG, Kentish JC, The cellular basis of the length-tension relation in cardiac muscle, Journal of Molecular and Cellular Cardiology 17(9) (1985) 821–840. [DOI] [PubMed] [Google Scholar]
- [42].Ha J-W, Oh JK, Therapeutic Strategies for Diastolic Dysfunction: A Clinical Perspective, Journal of Cardiovascular Ultrasound 17(3) (2009) 86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Satpathy C, Mishra TK, Satpathy R, Satpathy HK, Barone E, Diagnosis and Management of Diastolic Dysfunction and Heart Failure, American Family Physician 73(5) (2006) 841–846. [PubMed] [Google Scholar]
- [44].Arlock P, Wohlfart B, Sjöberg T, Steen S, The negative inotropic effect of esmolol on isolated cardiac muscle, Scandinavian Cardiovascular Journal 39(4) (2005) 250–254. [DOI] [PubMed] [Google Scholar]
- [45].Bristow MR, Shakar SF, Linseman JV, Lowes BD, Inotropes and beta-blockers: is there a need for new guidelines?, Journal of Cardiac Failure 7(2) (2001) 8–12. [DOI] [PubMed] [Google Scholar]
- [46].Sasayama S, Inotropic agents in the treatment of heart failure: despair or hope?, Cardiovascular drugs and therapy 10(6) (1997) 703–709. [DOI] [PubMed] [Google Scholar]
- [47].Aziz F, TK L-A, Enweluzo C, Dutta S, Zaeem M, Diastolic Heart Failure: A Concise Review, Journal of Clinical Medicine Research 5(5) (2013) 327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]