ABSTRACT
BACKGROUND:
Treatment of glioblastoma (GB), the most common malignant primary brain tumor in adults, can include alkylating chemo-therapeutic agents. Two molecular biomarkers of treatment response are MGMT (O6-methylguanine-DNA methyltransferase) promoter methylation and IDH (isocitrate dehydrogenase) mutations, which prevent repair of tumor cell DNA damage caused by alkylating chemotherapy. The status of MGMT promoter methylation and IDH mutation are associated with longer survival and a better response to chemotherapy.
OBJECTIVE:
Assess the prognostic value of MGMT methylation status and IDH mutation in adult Saudi glioblastoma patients.
DESIGN:
Retrospective, comparative survival analysis.
SETTING:
Tertiary care center.
PATIENTS AND METHODS:
The status of the MGMT promoter methylation and IDH mutation was assessed in adult patients diagnosed with GB between 2006 and 2019. A PCR-based assay was used to analyze for methylation of the MGMT promoter. A qualitative assay combining PCR clamping and amplification refractory mutation system technology was used to search for any of the 12 most common mutations in IDH1 and IDH2. Differences in survival were compared between those with and without MGMT promoter methylation and IDH mutation and between other subgroups.
MAIN OUTCOME MEASURES:
Survival of GB patients relative to MGMT promoter methylation and IDH mutation status.
SAMPLE SIZE:
146 patients (80 males and 66 females).
RESULTS:
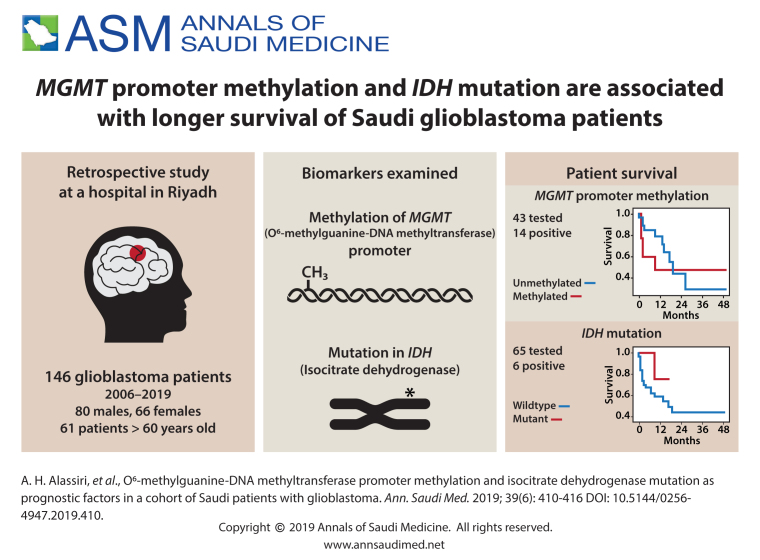
Of 43 (29.5%) cases tested for MGMT promoter methylation, 14 (32.5%) were positive. Of 65 (44.5%) cases screened for IDH mutation, 6 cases (9.2%) tested positive. The 36-month survival rate was 47% for the MGMT methylated cohort compared to 27% for their unmethylated counterparts. The 18-month survival rate for the IDH-mutant was 75% compared to 48% for their IDH-wildtype counterparts.
CONCLUSION:
The findings confirm the positive impact of both MGMT promoter methylation and IDH mutation on the overall survival of Saudi GB patients.
LIMITATIONS:
Single institute study with relatively few tested cases.
CONFLICT OF INTEREST:
None.
INTRODUCTION
Glioblastoma (GB) is the most common primary malignant brain tumor in adults. The estimated annual incidence is 3.19 cases per 100 000 population.1,2 According to the 2016 WHO classification of central nervous system (CNS) tumors, GB can be classified into two main molecular subtypes: IDH-wildtype and IDH-mutant. The IDH-wildtype GB accounts for about 90% of cases, arises de novo, and usually affects adults over 55 years of age. On the contrary, the IDH-mutant GB accounts for about 10% of cases, arises secondarily to lower grade diffuse astrocytomas, and tends to affect younger individuals.3
The majority of GBs (>90%) present with a short history of neurological deterioration in the absence of precursor lesions, i.e., de novo.4 The incidence of GB increases with age, being more common in the age group 75-84 years. There is a male predominance, with a male-to-female ratio of 1.6:1.5
Standard therapy for patients with GB comprises maximal safe resection followed by concomitant radiotherapy and temozolomide (TMZ). Despite multi-modal therapy, the mean overall survival is estimated to be 14-21 months and the 5-year survival rate is less than 3%.6 Factors influencing survival include age, extent of resection, tumor location/size, post-operative Karnofsky performance status scale, and the MGMT (O6-methylguanine-DNA methyltransferase) promoter methylation status.1
MGMT promoter methylation and IDH (isocitrate dehydrogenase) mutations serve as molecular biomarkers of treatment response. MGMT is a DNA repair protein that rescues tumor cells by removing alkylation from the O6 position of guanine caused by the use of the alkylating agent, temozolomide.7 The latter is considered the first-line chemotherapeutic agent for glioblastoma. Data concerning the status of MGMT promoter methylation and IDH mutation in Saudi GB patients is scanty. Comparable findings in the international literature cannot be assumed to apply to Saudi patients. The main aim of the study was to assess the prognostic value of MGMT promoter methylation and IDH mutation in a cohort of patients with glioblastoma diagnosed and treated at a tertiary care medical center. We also analyzed overall survival.
PATIENTS AND METHODS
This retrospective cohort study was conducted to assess the status of MGMT promoter methylation and IDH mutation and their impact on survival among Saudi patients with GB diagnosed at a reference laboratory between June 2006 and March 2019. All included patients underwent either a stereotactic biopsy or debulking procedure with or without adjuvant chemoradiotherapy. Only biopsy-proven GB cases meeting the inclusion criteria were retrospectively evaluated. The overall survival was calculated from the date of diagnosis to the date of death or the last date of follow-up visit for patients who were still alive. The diagnoses were confirmed by two Canadian board-certified neuropathologists. Pediatric cases, defined at our institution as equal to or less than 18 years of age, were excluded. Ethical approval was obtained from the Institutional review board (RC15/074/R).
The pathology reports of the GB cases were retrieved from the archives of the anatomic pathology division. Patient demographics, glioblastoma characteristics, date of diagnosis, surgery type, adjuvant therapy, date of death or last follow-up visit were collected either from the electronic medical records or manually from medical records department. The specifics of radiotherapy were obtained directly from the radiation oncology department. The MGMT promoter methylation analyses were performed at using a methylationspecific PCR-based assay.
The IDH mutation status was initially assessed by immunohistochemistry using the antibody IDH1 clone H09. This antibody detects the most common mutation (R132H). Negative cases were further analyzed by RT-qPCR if the patient was <55 years old. Mapping of the viable tumor area and calculation of the tumor percentage was performed by certified neuropathologists (first and fourth authors) using hematoxylin and eosin-stained representative slides for enrichment by macrodissection. A qualitative assay that combines PCR clamping and amplification refractory mutation system technology was used to detect the presence of seven mutations in IDH1 (Arg132His, Arg132Cys, Arg132Ser, Arg132Gly, Arg132Leu, Arg132Val, and Arg100Gln) and five mutations in IDH2 (Arg172Lys, Arg172Met, Arg172Trp, Arg172Ser, and Arg172Gly). This assay utilizes Qiagen therascreen IDH1/2 RGQ PCR Kit CE using real-time PCR on the Rotor-Gene Q 5plex HRM Instrument. Mutation nomenclature is based on Ensembl accession number ENST00000345146 and GRCh37 genome reference.
MGMT promoter methylation analysis was performed abroad at Mayo Laboratories, Rochester, Minnesota, USA, using methylation-specific polymerase chain reaction (PCR) analysis. At least 40% viable tumor present in the unstained section prepared from the formalin-fixed, paraffin-embedded tissue block is required. In general, a 6 mm × 3 mm area of tumor tissue cut at 5-micron thickness is the minimum amount of tumor tissue needed. So for each case to be tested, a set of one hematoxylin-and-eosin stained slide accompanied by 5 unstained slides, all prepared from the same representative paraffin block, were sent for the analysis. A modification of the real-time, methylationspecific PCR assay described by Kitange et al, is used to test tumor DNA for the presence of methylation of the promoter of the MGMT gene.8
Data analysis was performed using IBM SPSS version 23. Descriptive statistics were used to summarize the generated data which contained the following variables: MGMT promoter methylation status, IDH mutation status, tumor location, surgery type, and type of adjuvant therapy. These are described using frequency tables and measures of central tendency and range. Inferential statistics were used to calculate standard deviation, confidence interval, and the P value. The significance level for the P value is .05. Kaplan-Meier survival analysis was performed to assess the overall survival. The Mantel-Cox log-rank test was performed to compare the survival curves between the MGMT promoter methylated group vs. the unmethylated group, and the IDH-wildtype vs. IDH-mutant group. A multivariate analysis was performed using a Cox proportional hazards model to identify the hazard ratios for other factors that might influence overall survival, including age, gender, tumor laterality, tumor size, lobe involved, extent of resection, and adjuvant therapy on survival. All tests with a P value of <.05 were considered statistically significant.
RESULTS
Of 195 GB cases initially retrieved from the archives of the anatomic pathology division, 49 cases did not meet the inclusion criteria. For the 146 cases in the analysis, the age range was between 18 and 91 years and the mean (SD) age at diagnosis was 55.7 (15.2) years (Table 1). Most patients were diagnosed between the age of 46-60 years. Males were slightly predominant (n=80; 54.7%). Of the 43 cases tested for MGMT promoter methylation, only 14 cases (32.5%) were methylated versus 29 unmethylated (67.4%). Of 65 cases tested for IDH mutation, most cases (n=59; 90.7%) were of the IDH-wildtype and only 6 cases (9.2%) tested positive for the most common mutation IDH1 (R132H). Interestingly, only three of the tested cases harbored both the MGMT promoter methylation and the IDH mutation and the overall survival for these three patients was 9, 14, and 17, months. The 36-month survival rate was 47% for the methylated cohort compared to 27% for their unmethylated counterparts. The 18-month survival rate for the IDH-mutant was 75% compared to 48% for their IDH-wildtype counterparts.
Table 1.
Patient clinical and demographic characteristics at baseline.
| Age group | |
| 18-30 | 10 (6.8) |
| 31-45 | 28 (19.7) |
| 46-60 | 51 (34.9) |
| 61-75 | 46 (31.5) |
| 76-91 | 11 (7.5) |
| Gender | |
| Males | 80 (54.7) |
| Females | 66 (45.2) |
| MGMT | |
| Methylated | 14 (32.5) |
| Unmethylated | 29 (67.4) |
| IDH | |
| Wildtype | 59 (90.7) |
| Mutant | 6 (9.2) |
| Resection extent | |
| Subtotal resection | 99 (67.8) |
| Biopsy | 47 (32.1) |
| Adjuvant therapy | |
| None | 66 (45.2) |
| Radiotherapy | 15 (10.2) |
| (Radiotherapy + Temozolomide) | 65 (44.5) |
| Methylated | |
| (Radiotherapy + Temozolomide) | 8 (57.1) |
| Radiotherapy | 1 (7.1) |
| Subtotal resection | 3 (21.4) |
| Biopsy | 2 (14.2) |
| Unmethylated | |
| (Radiotherapy + Temozolomide) | 15 (51.7) |
| Radiotherapy | 3 (10.3) |
| Subtotal resection | 8 (27.5) |
| Biopsy | 3 (10.3) |
Data are number (%). MGMT: O6-methylguanine-DNA methyltransferase; IDH: isocitrate dehydrogenase.
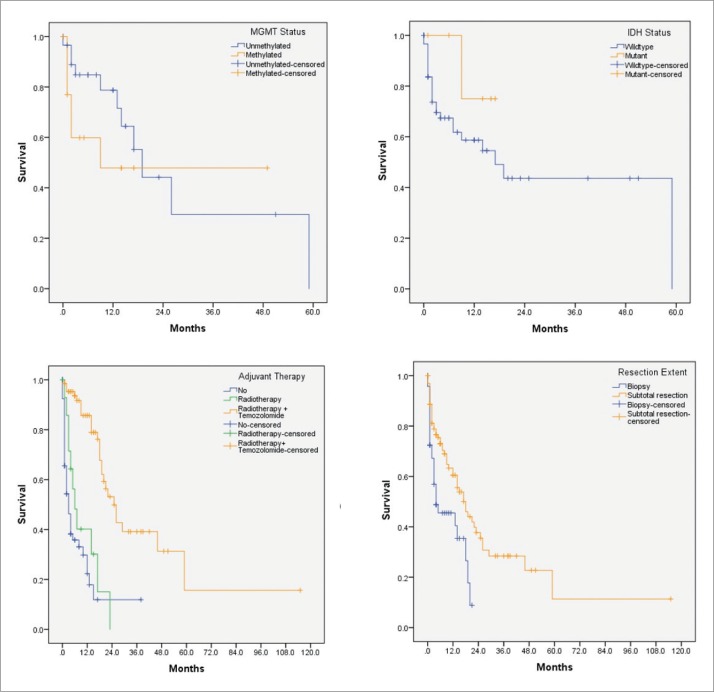
Two thirds of the patients (N=99; 67.8%) underwent subtotal resection of the tumor. Sixty-five patients (44.5%) received combined adjuvant chemoradiotherapy. Patients who received only radiotherapy had a hazard ratio or risk of death of 3.79 (P<.001). Patients who did not receive adjuvant chemoradiotherapy had a hazard ratio of 5.34 (P<.001) (Table 2) Median and percent survival at different time points are shown in Table 3. Figure 1 shows Kaplan-Meier survival curves by subgroups.
Table 2.
Multivariate analysis using the Cox proportional hazard model.
| Hazard ratio | 95% CI | P value | |
|---|---|---|---|
| Age | |||
| <60 years | Reference | - | - |
| ≥60 years | 1.77 | 1.08-2.9 | .022 |
| Tumor size | 1.04 | 0.92-1.17 | .491 |
| Gender | |||
| Males | Reference | - | - |
| Females | 0.99 | 0.61-1.61 | .970 |
| Laterality | |||
| Right | Reference | - | - |
| Left | 1.03 | 0.61-1.73 | .905 |
| Bilateral | 2.62 | 1.27-5.39 | .009 |
| Lobe | |||
| Singular | Reference | - | - |
| Multiple | 1.5 | 0.90-2.5 | .112 |
| Resection extent | |||
| Subtotal resection | Reference | - | - |
| Biopsy | 1.43 | 0.85-2.43 | .175 |
| Adjuvant therapy | |||
| Radiotherapy + Temozolomide | Reference | - | - |
| Radiotherapy | 3.79 | 1.723-8.34 | .001 |
| None | 5.34 | 2.90-9.83 | <.001 |
Figure 1.
Survival curves by MGMT promoter methylation status, IDH mutation status, adjuvant therapy, and extent of resection.
Table 3.
Subpopulation analysis of estimated median survival and survival percentages.
| Variable | N (%) | Median survival (95% CI) in months | Overall survival (%) | P value | |||
|---|---|---|---|---|---|---|---|
| 12 months | 18 months | 36 months | 60 months | ||||
| MGMT | .339 | ||||||
| Methylated | 14 (32.5) | 9 (-) | 47 | 47 | 47 | NR | |
| Unmethylated | 29 (67.4) | 19 (13.8-24.1) | 77 | 55 | 28 | NR | |
| IDH | .254 | ||||||
| Wildtype | 59 (90.7) | 17 (5.5-28.4) | 58 | 48 | 43 | NR | |
| Mutant | 6 (9.2) | NR | 75 | 75 | NR | NR | |
| Age | <.001 | ||||||
| ≥60 years | 61 (41.7) | 6 (3.1-8.8) | 40 | 25 | 12 | NR | |
| <60 years | 85 (58.2) | 20 (14-25.9) | 77 | 57 | 43 | 14 | |
| Gender | .431 | ||||||
| Males | 80 (54.7) | 17 (13.6-20.3) | 58 | 38 | 21 | NR | |
| Females | 66 (45.2) | 10 (1.7-18.2) | 47 | 37 | 24 | 9 | |
| Resection extent | .003 | ||||||
| Biopsy | 47 (32.1) | 4 (1-13.4) | 40 | 9 | NR | NR | |
| Subtotal resection | 99 (67.8) | 18 (12.8-23.1) | 55 | 45 | 28 | 12 | |
| Adjuvant therapy | <.001 | ||||||
| None | 66 (45.2) | 3 (1.5-4.4) | 17 | 12 | 12 | NR | |
| Radiotherapy | 15 (10.2) | 6 (2.6-9.3) | 40 | 15 | NR | NR | |
| Radiotherapy + Temozolomide | 65 (44.5) | 25 (19-30.9) | 85 | 78 | 38 | 15 | |
NR: Not Reached; MGMT: O6-methylguanine-DNA methyltransferase; IDH: isocitrate dehydrogenase. Statistical analysis by the log-rank test.
DISCUSSION
Glioblastoma arises either de novo (primary) or as a progression from low-grade diffuse astrocytomas (secondary). Isocitrate dehydrogenase (IDH1, IDH2) gene mutations are common in secondary glioblastomas and considered an earlier event in the neoplastic progression. The vast majority of glioblastomas are primary.4 MGMT gene encodes for a DNA repair enzyme that is crucial for glioblastoma cells in repairing their DNA damage secondary to chemotherapy. The latter includes temozolomide that crosslinks DNA by alkylating at the O6 of guanine. Methylation of the promoter for MGMT gene shuts down its expression.9
In the present study, we investigated the association between MGMT promoter methylation and IDH mutation and outcome in a cohort of Saudi GB patients diagnosed and treated at a tertiary care center. As expected, the IDH mutant and MGMT promoter methylated subgroups seemed to fair better than their wild counterparts at 18 and 36 months. The P values in these subgroups were statistically insignificant due to the relatively low number of tested cases. MGMT promoter methylation predicts a better response to the alkylating chemotherapeutic agent temozolamide.10 It also serves as a good prognostic factor, being most frequently observed among long-term GB survivors.11-16 IDH mutation is associated with a better prognosis and is common in secondary glioblastomas.17 The latter is known to constitute only a minority of GB cases as also found in our study.
The median survival for patients who underwent only biopsy and subtotal resection was 4 and 18 months, respectively. The difference between the two survival curves was statistically significant (P=.003). According to Chaichana et al, patients who undergo more resections will have a significantly prolonged survival.18 However, regardless of the extent of resection, glioblastomas tend to recur.
In the current study, tumor topography did not seem to have an influence on survival. However, subtotal resection of the tumor and the concurrent administration of chemoradiotherapy predicted survival. Patients who did not receive adjuvant chemoradiotherapy, had a hazard ratio of 5.34 (P<.001). In contrast, patients who received only radiotherapy, had a hazard ratio of 3.79 (P<.001).
Chen et al conducted a comprehensive systematic review and a meta-analysis investigating the association between the MGMT promoter methylations and prognosis for patients diagnosed with glioblastoma. The meta-analysis included 22 studies on the relationship between MGMT promoter methylation and the overall survival of GBM patients, and 12 studies investigating the association between MGMT and progression-free survival (PFS). The conclusion was that there is a significant OS and PFS advantage in those who were MGMT promoter methylated vs. unmethylated MGMT.19
In the present study, 41.7% of the patients (n=61) were older than 60 years of age. Older patients diagnosed with glioblastoma are less tolerable to treatment than younger patients, making treatment for glioblastoma in the elderly less aggressive.20-24 According to Iwamoto et al, age is considered the most significant factor for patients offered resection, radiotherapy, or chemotherapy.25
Finally, one limitation that needs to be acknowledged in our study is the fact that the total number of cases analyzed for the MGMT promoter methylation and IDH mutation is relatively low. Despite this and to our best knowledge, this is the first study to correlate these genetic factors with survival in GB in Saudi Arabia, reflecting a single institution experience over a span of thirteen years. The results are in keeping with the conclusion from similar international studies. Our study confirms the positive impact of both MGMT promoter methylation and IDH mutation on the overall survival of Saudi GB patients in keeping with what is largely known in this regard. Routine analysis of these two molecular biomarkers enables healthcare providers to prognosticate and predict response to adjuvant therapy. It will be interesting to assess whether the relationship between MGMT and IDH is inclusive or exclusive and perform the analyses in a bigger sample size.
Funding Statement
None.
REFERENCES
- 1.Roh TH, Park HH, Kang S-G, Moon JH, Kim EH, Hong C-K, et al. Long-term outcomes of concomitant chemoradiotherapy with temozolomide for newly diagnosed glioblastoma patients. Medicine (Baltimore). 2017. July;96(27):e7422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ostrom QT, Gittleman H, Liao P, Rouse C, Chen Y, Dowling J, et al. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2007-2011. Neuro Oncol. 2014. October 1;16(suppl 4):iv1–63. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25304271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016. June 9;131(6):803-20. [DOI] [PubMed] [Google Scholar]
- 4.Ohgaki H, Kleihues P.. Genetic Pathways to Primary and Secondary Glioblastoma. Am J Pathol. 2007. May;170(5):1445-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ostrom QT, Gittleman H, Farah P, Ondracek A, Chen Y, Wolinsky Y, et al. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2006-2010. Neuro Oncol. 2013. November 1;15(suppl 2):ii1-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stewart BW, Wild C, International Agency for Research on Cancer, World Health Organization . World cancer report 2014. p 630. [Google Scholar]
- 7.Karsy M, Neil JA, Guan J, Mahan MA, Colman H, Jensen RL.. A practical review of prognostic correlations of molecular bio-markers in glioblastoma. Neurosurg Focus. 2015. March;38(3):E4. [DOI] [PubMed] [Google Scholar]
- 8.Kitange GJ, Carlson BL, Mladek AC, Decker PA, Schroeder MA, Wu W, Grogan PT, et al. : Evaluation of MGMT promoter methylation status and correlation with temozolomide response in orthotopic glioblastoma xenograft model. J Neurooncol 2009. March;92(1):23-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005. March 10; 352(10):997-1003. [DOI] [PubMed] [Google Scholar]
- 10.Weller M, Stupp R, Reifenberger G, Brandes AA, van den Bent MJ, Wick W, et al. MGMT promoter methylation in malignant gliomas: ready for personalized medicine? Nat Rev Neurol. 2010. January 8;6(1):39-51. [DOI] [PubMed] [Google Scholar]
- 11.Esteller M, Garcia-Foncillas J, Andion E, Goodman SN, Hidalgo OF, Vanaclocha V, et al. Inactivation of the DNA-Repair Gene MGMT and the Clinical Response of Gliomas to Alkylating Agents. N Engl J Med. 2000. November 9;343(19):1350-4. [DOI] [PubMed] [Google Scholar]
- 12.Hegi ME, Diserens A-C, Godard S, Dietrich P-Y, Regli L, Ostermann S, et al. Clinical trial substantiates the predictive value of O-6-methylguanine-DNA methyltransferase promoter methylation in glioblastoma patients treated with temozolomide. Clin Cancer Res. 2004. March 15;10(6):1871-4. [DOI] [PubMed] [Google Scholar]
- 13.Hegi ME, Diserens A-C, Gorlia T, Hamou M-F, de Tribolet N, Weller M, et al. MGMT Gene Silencing and Benefit from Temozolomide in Glioblastoma. N Engl J Med. 2005. March 10;352(10):997-1003. [DOI] [PubMed] [Google Scholar]
- 14.Herrlinger U, Rieger J, Koch D, Loeser S, Blaschke B, Kortmann R-D, et al. Phase II Trial of Lomustine Plus Temozolomide Chemotherapy in Addition to Radiotherapy in Newly Diagnosed Glioblastoma: UKT-03. J Clin Oncol. 2006. Sep. 20;24(27):4412-7. [DOI] [PubMed] [Google Scholar]
- 15.Weller M, Felsberg J, Hartmann C, Berger H, Steinbach JP, Schramm J, et al. Molecular Predictors of Progression-Free and Overall Survival in Patients With Newly Diagnosed Glioblastoma: A Prospective Translational Study of the German Glioma Network. J Clin Oncol. 2009. Dec. 1;27(34):5743-50. [DOI] [PubMed] [Google Scholar]
- 16.Gorlia T, van den Bent MJ, Hegi ME, Mirimanoff RO, Weller M, Cairncross JG, et al. Nomograms for predicting survival of patients with newly diagnosed glioblastoma: prognostic factor analysis of EORTC and NCIC trial 26981-22981/CE.3. Lancet Oncol. 2008. January;9(1):29-38. [DOI] [PubMed] [Google Scholar]
- 17.Ichimura K, Pearson DM, Kocialkowski S, Backlund LM, Chan R, Jones DT, et al. IDH1 mutations are present in the majority of common adult gliomas but rare in primary glioblastomas. Neuro Oncol. 2009;11:341-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaichana KL, Zadnik P, Weingart JD, Olivi A, Gallia GL, Blakeley J, et al. Multiple resections for patients with glioblastoma: prolonging survival. J Neurosurg. 2013. April;118(4):812-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y, Hu F, Zhou Y, Chen W, Shao H, Zhang Y.. MGMT Promoter Methylation and Glioblastoma Prognosis: A Systematic Review and Meta-analysis. Arch Med Res. 2013. May;44(4):281-90. [DOI] [PubMed] [Google Scholar]
- 20.Brandes AA, Franceschi E, Tosoni A, Benevento F, Scopece L, Mazzocchi V, et al. Temozolomide concomitant and adjuvant to radiotherapy in elderly patients with glioblastoma. Cancer. 2009. Aug. 1;115(15):3512-8. [DOI] [PubMed] [Google Scholar]
- 21.Kimple RJ, Grabowski S, Papez M, Collichio F, Ewend MG, Morris DE.. Concurrent Temozolomide and Radiation, a Reasonable Option for Elderly Patients With Glioblastoma Multiforme? Am J Clin Oncol. 2009. October;33(3):1. [DOI] [PubMed] [Google Scholar]
- 22.Lawrence YR, Wang M, Dicker AP, Andrews D, Curran WJ, Michalski JM, et al. Early toxicity predicts long-term survival in high-grade glioma. Br J Cancer. 2011. April 12;104(9):1365-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sijben AE, McIntyre JB, Roldán GB, Easaw JC, Yan E, Forsyth PA, et al. Toxicity from chemoradiotherapy in older patients with glioblastoma multiforme. J Neurooncol. 2008. August 9;89(1):97-103. [DOI] [PubMed] [Google Scholar]
- 24.Minniti G, De Sanctis V, Muni R, Filippone F, Bozzao A, Valeriani M, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma in elderly patients. J Neurooncol. 2008. May 5;88(1):97-103. [DOI] [PubMed] [Google Scholar]
- 25.Iwamoto FM, Reiner AS, Panageas KS, Elkin EB, Abrey LE.. Patterns of care in elderly glioblastoma patients. Ann Neurol. 2008. December;64(6):628-34. [DOI] [PubMed] [Google Scholar]