ABSTRACT
BACKGROUND:
Familial Mediterranean fever (FMF), an autosomal recessive, autoinflammatory disease that is common in Arabs, Jews, Armenians and Turks, is caused by mutations in the MEFV gene, which encodes a protein called pyrin. The disease is characterised by recurrent fever, peritonitis, pleuritis, abdominal pain and arthralgia.
OBJECTIVE:
Determine the distributions of MEFV mutations and their relationship with clinical manifestations.
DESIGN:
Retrospective, descriptive.
SETTING:
Turkish community.
SUBJECTS AND METHODS:
The study included patients with complaints related to FMF who were admitted to the research hospital of Cumhuriyet University between 2005 and 2017. FMF was diagnosed by physical examination using the Tel-Hashomer criteria. MEFV mutations were detected by reverse hybridization strip assay and pyrosequencing.
MAIN OUTCOME MEASURE:
The prevalence of specific MEFV gene mutations in a large cohort of Middle Anatolia.
SAMPLE SIZE:
10 033 patients admitted, 1223 with confirmed mutations.
RESULTS:
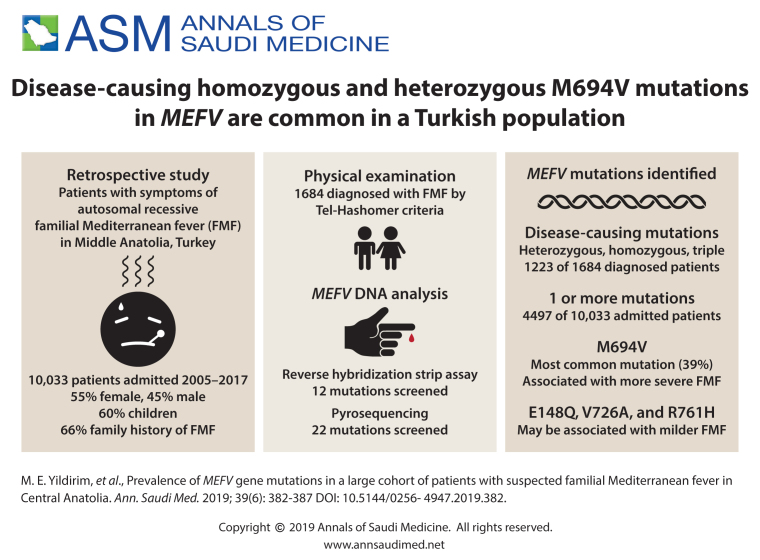
Of 1684 patients diagnosed by Tel-Hashomer criteria, mutation screening confirmed that 1223 patients (72.6%) had FMF. Male/female ratio of the FMF patients was 1.3:1. One or more FMF mutations were found in 4497 patients (44.8%). 3262 had heterozygous or carrier mutations, 821 had compound heterozygous mutation, 381 had homozygous mutations, and 21 had triple mutations. Sixty-six percent had a family history of the disease and 13.7% of the patients had parental consanguinity. Main symptoms found in the patients were abdominal pain (85.2%), fever (84%), chest pain (30.2%), arthralgia (28.6%), rash or erysipelas-like erythema (8.2%). The most common mutation in this population was M694V (39%) of 5753 alleles.
CONCLUSION:
M694V was the most frequent mutation in our population (Middle Anatolia, Turkey) and cause severe forms of the disease. Patients with E148Q, V726A and R761H mutations may have milder FMF symptoms. There was a high rate of carriers in our study group.
LIMITATIONS:
Amyloidosis, an important complication of the disease, needs to be analyzed.
CONFLICT OF INTEREST:
None.
INTRODUCTION
Familial Mediterranean Fever (FMF) is an inflammatory disease inherited in an autosomal recessive pattern. Signs and symptoms include recurrent attacks of fever, peritonitis, pleuritis, abdominal pain, arthralgia and skin rash defined as erysipelas-like erythema.1 The disease is particularly common in people from the Mediterranean region, including Arabs, Jews, Armenians and Turks.2 In general, FMF is diagnosed on the basis of clinical findings, but the disease is known to be caused by mutations in the MEFV gene located on the short arm of chromo-some 16.3 The MEFV gene consists of 10 exons and to date, more than 280 MEFV sequence variants have been isolated.4 MEFV encodes pyrin (also known as marenostrin), a protein involved in innate immunity.5 MEFV expression is increased in response to inflammatory activators such as interferon-[.alpha] (IFN-[.alpha]) and tumour necrosis factor-[.alpha] (TNF-[.alpha]), indicating that the main function of pyrin is regulation of the innate immune response.6
FMF and the types of mutations involved vary geographically.7 Therefore, the diagnosis requires significant clinical suspicion and certain molecular evidence, especially in societies where the prevalence is low.8 Acute phase reactants such as the erythrocyte sedimentation rate (ESR), C reactive protein (CRP), fibrinogen, serum amyloid A (SAA) rise during FMF attacks.9 Although the phenotype-genotype correlation of FMF has not yet been clarified, several studies have suggested that more severe disease and an increased susceptibility to amyloidosis are associated with specific MEFV mutations such as M694V.10,11 The main complication of FMF is the development of amyloidosis, especially renal amyloidosis, which causes kidney failure.12 We retrospectively analyzed the molecular screenings of 10033 patients with complaints of recurrent fever, abdominal and chest pain, arthralgia, rashes and similar problems. Patients had nonspecific symptoms as well as typical findings of FMF disease.
SUBJECTS AND METHODS
Patients with abdominal and chest pain, joint pain, fever and other similar complaints who presented to between 2005 and 2017 to Cumhuriyet University Research Hospital in Sivas, a Middle Anatolian city in Turkey, were included in this retrospective study. All of the patients were from Middle Anatolian cities in Turkey. Informed consent had been taken from the patients at the time of enrollment. Our study was approved by the Cumhuriyet University ethics committee (2017-05/08). Clinical and demographic data such as age, sex, consanguinity and main symptoms such as fever, abdominal pain, arthralgia, chest pain, erysipelas-like erythema had been previously recorded.
The patients were diagnosed using the Tel-Hashomer criteria.13 The findings were recorded on the basis of major criteria (recurrent febrile episodes with serositis, amyloidosis of the AA type, favorable response to colchicine) and minor criteria (recurrent febrile episodes, erysipelas-like erythema, FMF in a first-degree relative). MEFV gene screening of the patients was carried out in the second, third, fifth and tenth exon by two different methods: Reverse hybridization strip assay (Vienna Lab, FMF StripAssay, GMBH, Austria) and pyrosequencing (PyroMark, Qiagen, Germany). For the strip assay, total genomic DNA was extracted from peripheral blood samples with a DNA isolation kit (Invitek Invisorb Spin Blood Kit, Germany). Multiplex PCR amplification was performed using biotinylated primers. PCR products were incubated to nitrocellulose strips for reverse hybridization. The process was completed with color development and the detection of signals in Auto-LIPA (Auto-LIPA Innogenetics). For pyrosequencing, genomic DNA was isolated using the EZ1 blood mini kit and PCR was performed using the PyroMark PCR Kit (Qiagen, Germany) with 15 minutes of denaturation at 95°C. The DNA was amplified for 45 cycles under the following conditions: 30 sec 94°C, 30 seconds at 60°C and 30 seconds at 72°C. PCR products (10 µL) were mixed with streptavidin-conjugated sepharose material in binding buffer (70 µL). They were collected using a vacuum preparation workstation. The sequencing primer was added with the annealing buffer and they were kept at 80°C for 2 minutes. Pyrosequencing reaction was completed by processing with PyroMark Q24 instrument (Qiagen, Germany).
Twelve MEFV mutations (E148Q, P369S, F479L, M680I (G/C), M680I (G/A), 1692del, M694V, M694I, A744S, R761H, K695R, V726A) were screened by the reverse hybridization strip assay method and 22 mutations by pyrosequencing. Statistical analysis was performed with IBM SPSS (Armonk, NY: IBM Corp) version 22 to evaluate the demographic characteristics and the distribution of FMF mutations.
RESULTS
The 10 033 patients were composed of 5498 females (54.8%) and 4535 males (45.2%). About 60 percent (n=6032) were children (younger than 18 years of age). The main clinical symptoms were abdominal pain (85.2%, n=8548), fever (84%, n=8427), chest pain (30.2%, 3029), arthralgia (28.6%, n=2869) and rash or erysipelas-like erythema (8.2%, n=820). One-third of patients had nonspecific symptoms such as headache, diarrhea, visual defects, hearing loss, vertigo. There was a high degree of parental consanguinity in the families of the patients (13.7%). Sixty-six percent had a family history of the disease. One or more FMF mutations were found in 4497 patients screened by both methods. Of the 4497 cases, 1223 had compound heterozygous, homozygous or triple mutations. Although pyrosequencing is more comprehensive, there was no difference in the mutations found by the two methods of genetic screening. Mutation screening with both methods revealed heterozygous mutation in 3262 (72.5%) patients, who were carriers. The most common mutation in the heterozygous cases was M694V (35.8%) followed by E148Q (29.3%) and M680I (G/C) (12.8%) mutations (Table 1).
Table 1.
Heterozygous mutations (n=3262).
| M694V | 1167 (35.8) |
| E148Q | 955 (29.3) |
| M680I (G/C) | 416 (12.8) |
| V726A | 330 (10.1) |
| A744S | 132 (4) |
| P369S | 116 (3.5) |
| R761H | 74 (2.3) |
| K695R | 33 (1) |
| F479L | 28 (0.9) |
| M694I | 8 (0.2) |
| M680I (G/A) | 3 (0.1) |
Data are number of patients (%).
FMF was diagnosed in 1684 patients by physical examination on the basis of the Tel-Hashomer criteria, but mutation screenings confirmed only 1223 FMF patients (689 males and 534 females). These confirmed FMF cases constituted 12.2% of all patients admitted to the hospital with similar complaints. The mean age of these patients was 22.2 (14.1) (range: 3-82 years). Most (67.1%, n=821) had compound heterozygous mutations (Table 2) and 381 patients had homozygous mutations (Table 3). The most common mutation in homozygous cases was also M694V (67.4%). Twenty-one of the patients (1.7%) had triple mutations (complex alleles) (Table 4). Family analyses detected double mutations on a single allele in 12 patients. Although 268 people (15.9% of 1684) had only one mutation, treatment was initiated in different departments. No mutation was detected in the remaining 193 subjects (11.5%).
Table 2.
Compound heterozygous mutations (n=821) in the 1223 with confirmed FMF.
| E148Q+M694V | 167 (20.3) |
| M680I (G/C)+M694V | 166 (20.2) |
| V726A+M694V | 143 (17.4) |
| M680I (G/C)+V726A | 72 (8.8) |
| E148Q+P369S | 63 (7.7) |
| R761H+M694V | 37 (4.5) |
| E148Q+V726A | 31 (3.8) |
| E148Q+M680I (G/C) | 30 (3.7) |
| M680I (G/C)+R761H | 23 (2.8) |
| A744S+M694V | 14 (1.7) |
| A744S+E148Q | 14 (1.7) |
| P369S+M694V | 11 (1.4) |
| V726A+R761H | 8 (1) |
| F479L+V726A | 6 (0.7) |
| E148Q+F479L | 5 (0.6) |
| M680I (G/C)+A744S | 4 (0.5) |
| F479L+M694V | 4 (0.5) |
| E148Q+R761H | 4 (0.5) |
| V726A+A744S | 3 (0.4) |
| F479L+M680I (G/C) | 2 (0.3) |
| E148Q+M694I | 2 (0.3) |
| K695R+M694V | 1 (0.1) |
| K695R+R761H | 1 (0.1) |
| E148Q+M680I (G/A) | 1 (0.1) |
| K695R+M680I (G/C) | 1 (0.1) |
| K695R+E148Q | 1 (0.1) |
| P369S+A744S | 1 (0.1) |
| P369S+M680I (G/C) | 1 (0.1) |
| M680I (G/A)+M694V | 1 (0.1) |
| M694I+M680I (G/C) | 1 (0.1) |
| K695R+A744S | 1 (0.1) |
| A744S+R761H | 1 (0.1) |
| P369S+V726A | 1 (0.1) |
Data are number of patients (%).
Table 3.
Homozygous mutations in 1223 patients with confirmed FMF mutations (n=381).
| Homozygous mutations | Patients (n) | % |
|---|---|---|
| M694V | 257 | 67.4 |
| M680I (G/C) | 60 | 15.7 |
| E148Q | 32 | 8.4 |
| V726A | 22 | 5.8 |
| R761H | 5 | 1.3 |
| P369S | 2 | 0.5 |
| A744S | 1 | 0.3 |
| M694I | 1 | 0.3 |
| M680I (G/A) | 1 | 0.3 |
Table 4.
Triple mutations (complex alleles) (n=21) in 1223 patients with confirmed FMF mutations.
| E148Q+P369S+M694V | 4 (19) |
| E148Q+P369S+M680I (G/C) | 3 (14.2) |
| E148Q+E148Q+P369S | 3 (14.2) |
| E148Q+M694V+M680I (G/C) | 2 (9.5) |
| E148Q+F479L+M680I (G/C) | 1 (4.8) |
| E148Q+M694V+K695R | 1 (4.8) |
| M680I (G/C)+M694V+V726A | 1 (4.8) |
| E148Q+M694V+V726A | 1 (4.8) |
| E148Q+M680I (G/C)+V726A | 1 (4.8) |
| P369S+M680I (G/C)+M694V | 1 (4.8) |
| E148Q+P369S+F479L | 1 (4.8) |
| E148Q+P369S+P369S | 1 (4.8) |
| M694V+M694V+E148Q | 1 (4.8) |
Data are number of patients (%).
M694V was the most frequent mutation (39%) involved in 2245 alleles of the 5753 alleles. Other common mutations were E148Q (23.8%), M680I(G/C) (14.8%) and V726A (11.2%) in a Middle Anatolian population. M680I (G/A) was the rarest mutation (0.1%), followed by M694I (0.2%), K695R (0.7%) and F479L (0.8%) respectively (Table 5). Our study population had a high frequency of carriers. The male/female ratio of the FMF patients was 1.3:1. Of the confirmed FMF patients (n=1223), 325 had prominent findings (more severe forms of the disease in terms of attack frequency, its duration and extent) and 64% of the 325 (208 individuals) had either homozygous M694V mutation or compound heterozygous mutation containing M694V.
Table 5.
Allelic frequency (n=5753 alleles).
| M694V | 2245 (39) |
| E148Q | 1367 (23.8) |
| M680I (G/C) | 849 (14.8) |
| V726A | 643 (11.2) |
| P369S | 213 (3.7) |
| A744S | 172 (3) |
| R761H | 158 (2.7) |
| F479L | 47 (0.8) |
| K695R | 39 (0.7) |
| M694I | 13 (0.2) |
| M680I (G/A) | 7 (0.1) |
Data are number (%)
DISCUSSION
FMF is considered the prototype of the autoinflammatory diseases, characterised by recurrent febrile attacks without specific autoantibodies.14 Early age at the beginning of the symptoms is one of the main features of the disease. About 90% of patients experience their attacks before the age of 20 years.15 Researchers have detected a high frequency of MEFV mutation carriers in distinct regions of Iran, Turkey, and Armenia.2,16
The rate of patients with MEFV gene mutations in our study was 44.8% and the most frequent mutation of the group was M694V (39%). We detected high parental consanguinity (13.7%) in the study group. A high rate of carriers was also determined in our population with 3262 cases (32.5% of the patients admitted to the hospital). We found M694V mutation in the majority of FMF patients who had prominent findings (64%). The presence of the M694V mutation in most of the severe FMF cases suggests that this mutation causes more serious clinical findings. Ozen et al also reported that M694V mutation in exon 10 is usually associated with the most severe phenotype in FMF.17 On the basis of physical examination, blood tests and anamnesis findings in our study, we thought that patients with homo-zygous E148Q (32 cases), V726A (22 cases) and R761H (5 cases) mutations had a milder form of the disease in terms of attack frequency, duration of attack, severity of pain and fever and the levels of acute phase reactants such as CRP and fibrinogen. In a study by el-Garf et al, it was stated that the people who have a low frequency of the M694V and higher frequency of V726A mutation tend to have milder versions of FMF with a low incidence of amyloidosis.18 Cekin et al reported that E148Q and V726A mutations produce a milder phenotype of FMF.19 Some researchers described the E148Q variant as a disease-causing mutation with low penetrance.20
The types of common mutations vary from region to region. For example, although the M694V mutation was one of the most frequent mutations in Mediterranean region, Tsuchiya-Suzuki and colleagues found no M694V mutation in a study with a group of 80 patients in Japan.21 In a study by Kishida et al, while the most frequent mutation in Japanese patients was E148Q, common mutations of Mediterranean populations such as M694V, V726A, and M680I were not detected and the M694I mutation was associated with a more severe clinical course.22 Some investigators have pointed out that the M694I mutation is found predominantly in Arabs.23
While 268 patients had heterozygous mutations in our study group, they were accepted as FMF cases in various clinical departments and treatment was started. These patients were clinically diagnosed. Some rare mutations might have been skipped in the screening. In addition, the possibility of dominant inheritance may be considered in relation to mutations in these patients. We ignored the possibility of misdiagnosis.
Although FMF is accepted as an autosomal recessive inherited disease, there are arguments that dominant inheritance may also occur and the presence of only a single mutation can cause FMF symptoms.20 Booty et al had sequenced the MEFV gene by standard capillary electrophoresis in patients who were clinically diagnosed with FMF and they declared the existence of a significant subset of FMF patients who were carriers of only one MEFV mutation.24 Rowczenio et al claimed that the p.M694del variant is associated with autosomal dominantly inherited FMF in Northern European Caucasians.25 Ben-Chetrit et al asserted that about one-third of FMF patients carry a single mutation on one allele and in this context, this disease might be transferred as an autosomal dominant trait with partial penetration.26 There are a lot of FMF patients who present with typical FMF manifestations and respond to colchicine and carry only a single heterozygous MEFV mutation. Unlike classical Mendelian inheritance, a complex and multifactorial form of FMF is possible and symptoms may be milder or less typical in heterozygotes.27
In conclusion, we report the prevalence of various MEFV mutations, the frequency of clinical findings of the patients and a high rate of carriers in a large cohort. In such a large population, it is also necessary to analyze the possible complications (especially amyloidosis) and their relationship with mutations. A limitation of this study is that we could not follow the patients who developed amyloidosis. This analysis is important for the development of amyloidosis and the association of this complication with mutations.
Funding Statement
None.
REFERENCES
- 1.Coskun S, Ustyol L, Bayram Y, Bektas MS, Gulsen S, Çim A, et al. The spectrum of MEFV gene mutations and genotypes in Van province, the eastern region of Turkey, and report of a novel mutation (R361T). Gene 2015; 562 (1): 128–131 [DOI] [PubMed] [Google Scholar]
- 2.Beheshtian M, Izadi N, Kriegshauser G, Kahrizi K, Mehr EP, Rostami M, et al. Prevalence of common MEFV mutations and carrier frequencies in a large cohort of Iranian populations. J Genet. 2016; 95 (3): 667-674. [DOI] [PubMed] [Google Scholar]
- 3.Haghighat M, Moghtaderi M, Farjadian S.. Genetic Analysis of Southwestern Iranian Patients with Familial Mediterranean Fever. Rep Biochem Mol Biol. 2017; 5 (2): 117-120. [PMC free article] [PubMed] [Google Scholar]
- 4.Gangemi S, Manti S, Procopio V, Casciaro M, Di Salvo E, Cutrupi M, et al. Lack of clear and univocal genotype-phenotype correlation in familial Mediterranean fever patients: A systematic review. Clin Genet. 2018; 94 (1): 81-94. [DOI] [PubMed] [Google Scholar]
- 5.Battal F, Silan F, Topaloglu N, Aylanç H, Yıldırım S, Binnetoglu FK, et al. The MEFV gene pathogenic variants and phenotypegenotype correlation in children with familial Mediterranean fever in the Çanakkale population. Balkan J Med Genet. 2017; 19 (2): 23-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mansfield E, Chae JJ, Komarow HD, Brotz TM, Frucht DM, Aksentijevich I, et al. The familial Mediterranean fever protein, pyrin, associates with microtubules and colocalizes with actin filaments. Blood 2001; 98 (3): 851-859. [DOI] [PubMed] [Google Scholar]
- 7.Ece A, Cakmak E, Uluca U, Kelekci S, Yolbas I, Günes A, et al. The MEFV mutations and their clinical correlations in children with familial Mediterranean fever in southeast Turkey. Rheumatol Int. 2014; 34 (2): 207–212. [DOI] [PubMed] [Google Scholar]
- 8.Sandhya P, Vellarikkal SK, Nair A, Ravi R, Mathew J, Jayarajan R, et al. Egyptian tale from India: application of whole-exome sequencing in diagnosis of atypical familial Mediterranean fever. Int J Rheum Dis. 2017; 20 (11): 1770-1775. [DOI] [PubMed] [Google Scholar]
- 9.Korkmaz C, Ozdogan H, Kasapcopur O, Yazici H.. Acute phase response in familial Mediterranean fever. Ann Rheum Dis 2002; 61 (1): 79-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dusunsel R, Dursun I, Gunduz Z, Poyr-azoglu MH, Gurgoze MK, Dundar M.. Geno-type – phenotype correlation in children with familial Mediterranean fever in a Turkish population. Pediatr Int. 2008; 50 (2): 208–212 [DOI] [PubMed] [Google Scholar]
- 11.Bilge SY, Solmaz D, Senel S, Emmungil H, Kılıç L, Öner SY, et al. Exon 2: Is it the good police in familial mediterranean fever? Eur J Rheumatol. 2019; 6 (1): 34-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nursal AF, Turkmen E, Uzun Kaya S, Tekcan A, Sezer O, Celik SD, et al. Angiotensin Converting Enzyme Gene Insertion/Deletion Variant and Familial Mediterranean Fever-related Amyloidosis. Iran J Kidney Dis. 2018; 12 (3): 150-155. [PubMed] [Google Scholar]
- 13.Sag E, Demirel D, Demir S, Atalay E, Akca U, Bilginer Y, et al. Performance of the new ‘Eurofever/PRINTO classification criteria’ in FMF patients. Semin Arthritis Rheum. 2019; 7 pii: 10.1016/j.semarthrit.2019.08.004 [DOI] [PubMed] [Google Scholar]
- 14.Maconi G, Obici L, Carmagnola S, Guzzetti S.. Autoinflammatory diseases as a cause of acute abdominal pain in the emergency department. Clin Exp Rheumatol. 2018; 110 (1): 39-43. [PubMed] [Google Scholar]
- 15.Yasar Bilge NS, Sari I, Solmaz D, Senel S, Emmungil H, Kilic L, et al. Comparison of early versus late onset familial Mediterranean fever. Int J Rheum Dis. 2018; 21 (4): 880-884. [DOI] [PubMed] [Google Scholar]
- 16.Ozdemir O, Sezgin I, Kurtulgan HK, Candan F, Koksal B, Sumer H, et al. Prevalence of known mutations in the MEFV gene in a population screening with high rate of carriers. Mol Biol Rep. 2011; 38 (5): 3195–3200. [DOI] [PubMed] [Google Scholar]
- 17.Ozen S, Demirkaya E, Amaryan G, Koné-Paut I, Polat A, Woo P, et al. Results from a multicentre international registry of familial Mediterranean fever: impact of environment on the expression of a monogenic disease in children. Ann Rheum Dis. 2014; 73 (4): 662-667. [DOI] [PubMed] [Google Scholar]
- 18.el-Garf A, Salah S, Iskander I, Salah H, Amin SN.. MEFV mutations in Egyptian patients suffering from familial Mediterranean fever: analysis of 12 gene mutations. Rheumatol Int. 2010; 30 (10): 1293-1298. [DOI] [PubMed] [Google Scholar]
- 19.Cekin N, Akyurek ME, Pinarbasi E, Ozen F.. MEFV mutations and their relation to major clinical symptoms of Familial Mediterranean Fever. Gene 2017; 626: 9–13 [DOI] [PubMed] [Google Scholar]
- 20.Migita K, Izumi Y, Jiuchi Y, Iwanaga N, Kawahara C, Agematsu K, et al. Familial Mediterranean fever is no longer a rare disease in Japan. Arthritis Res. Ther. 2016; 18: 175 10.1186/s13075-016-1071-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsuchiya-Suzuki A, Yazaki M, Nakamura A, Yamazaki K, Agematsu K, Matsuda M, et al. Clinical and genetic features of familial Mediterranean fever in Japan. J Rheumatol. 2009; 36: (8) 1671-1676. [DOI] [PubMed] [Google Scholar]
- 22.Kishida D, Nakamura A, Yazaki M, Tsuchiya-Suzuki A, Matsuda M, Ikeda S.. Genotypephenotype correlation in Japanese patients with familial Mediterranean fever: differences in genotype and clinical features between Japanese and Mediterranean populations. Arthritis Res Ther. 2014; 16 (5): 439 10.1186/s13075-014-0439-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Majeed HA, El-Khateeb M, El-Shanti H, Rabaiha ZA, Tayeh M, Najib D.. The spectrum of familial Mediterranean fever gene mutations in Arabs: report of a large series. Semin Arthritis Rheum. 2005; 34 (6): 813-818. [DOI] [PubMed] [Google Scholar]
- 24.Booty MG, Chae JJ, Masters SL, Remmers EF, Barham B, Le JM, et al. Familial Mediterranean fever with a single MEFV mutation: Where is the second hit? Arthritis Rheum. 2009; 60 (6): 1851-1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rowczenio DM, Iancu DS, Trojer H, Gilbertson JA, Gillmore JD, Wechalekar AD, et al. Autosomal dominant familial Mediterranean fever in Northern European Caucasians associated with deletion of p.M694 residue—a case series and genetic exploration. Rheumatology (Oxford). 2017; 56 (2): 209-213. [DOI] [PubMed] [Google Scholar]
- 26.Ben-Chetrit E, Peleg H, Aamar S, Heyman SN.. The spectrum of MEFV clinical presentations–is it familial Mediterranean fever only? Rheumatology (Oxford). 2009; 48 (11): 1455–1459. [DOI] [PubMed] [Google Scholar]
- 27.Jéru I, Hentgen V, Cochet E, Duquesnoy P, Le Borgne G, Grimprel E, et al. The risk of familial Mediterranean fever in MEFV heterozygotes: a statistical approach. PLoS One. 2013; 8 (7): e68431 10.1371/journal.pone.0068431. [DOI] [PMC free article] [PubMed] [Google Scholar]