Abstract
Peter B Bach and colleagues call for an end to contradictory regulatory standards in the US that allow drug manufacturers to boost profits by producing single dose vials containing quantities that increase leftover drug
Even though reducing waste in healthcare is a top priority, analysts have missed the waste that can be created when expensive infused drugs are packaged containing quantities larger than the amount needed.1 2 This is particularly true for drugs for which dosage is based on a patient’s weight or body size and that come in single dose packages. These drugs must be either administered or discarded once open, and because patients’ body sizes are unlikely to match the amount of drug included in the vial, there is nearly always some left over. The leftover drug still has to be paid for, even when discarded, making it possible for drug companies to artificially increase the amount of drug they sell per treated patient by increasing the amount in each single dose vial relative to the typically required dose.
Increasing the amount of drug sold per treated patient also increases profits to doctors and hospitals in the United States. Under a system nicknamed “buy and bill,” doctors and hospitals buy single dose vials of drugs and then bill insurers or patients when they are used. The bill includes a percentage based mark-up which can vary widely, but even low percentages can equate to large amounts of money given that many of the drugs cost thousands of dollars per vial.
Although doctors and hospitals sometimes use leftover drug to treat a subsequent patient, thus reducing the amount of leftover drug for which they bill, this practice is very limited. Safety standards from the US Pharmacopeial Convention permit sharing only if leftover drug is used within six hours, and only in specialised pharmacies.3 4 5
We analysed spending on cancer drugs that are packaged in single dose vials and dosed based on body size in the United States to estimate the extent of the problem. We focused on the US because, unlike in most other Western countries, the government plays no role in how drugs are priced and doctors and hospitals can profit from leftover drugs. Although similar problems exist with other drugs, cancer drugs are expensive and they constitute the largest single category of specialty drug spending.6 Moreover, cancer drugs often have narrow therapeutic and toxicity windows, meaning that dosing is commonly based on a patient’s body size.
How big is the problem?
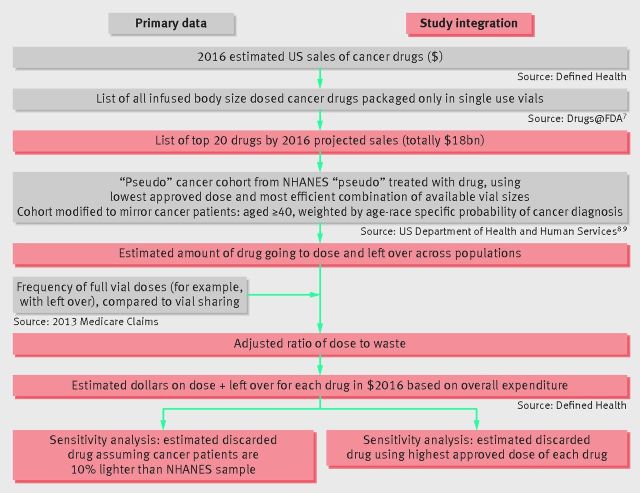
We examined the top 20 cancer drugs that are dosed by body size and packaged in single dose vials (based on 2016 projected sales), which collectively account for 93% of all sales of such drugs. We calculated the total amount of leftover drug and resulting 2016 US revenues for each drug using the method shown in fig 1. In brief, we estimated how often vial sharing occurred by examining how often claims filed with the Medicare program included amounts of drug that did not total the full contents of the vial. We then calculated the most efficient way to combine available vial sizes to achieve the lowest US Food and Drug Administration approved dose in a representative sample of the US population derived from the National Health and Nutrition Examination Survey.7 After correcting for vial sharing percentage, and adjusting the population to mirror a cancer patient population, we apportioned projected 2016 US revenues to administered or leftover drug.8 9When calculating the effect of vial sharing we assumed that doses that were not multiples of available vial sizes had no leftover drug, an assumption that made our estimates of leftover drug conservative.
Fig 1.
Study flowchart
Table 1 shows the leftover drug from the packaging approaches for the 20 drugs. We estimate total US revenue from these drugs to be $18bn (£12.5bn; €16bn) in 2016, with 10% or $1.8bn from discarded drug. The extent and cost of leftover drug varies according to market size and available vial sizes. For example, in 2016, 7% of $3.9bn in rituximab sales will be on discarded drug, totaling $254m, while 33% of $697m in carfilzomib sales will be discarded, totaling nearly as much, $231m. Sensitivity analyses suggested our results were robust. If every person received the highest dose approved by the FDA, revenue from discarded drugs falls to $1.4bn; if every cancer patients weighed 10% less than the survey participants, the estimate rises to $2bn.
Table 1.
Top 20 infused cancer drugs based on projected 2016 sales sold in single dose vials and dosed based on patient body size
| Drug (brand name), year of FDA approval | Dose of first approved indication (highest approved dose at any time) | Amount of drug in available single dose vials (discontinued vial sizes)* | Vial sharing | 2016 expected sales ($m) | 2016 expected revenue from leftover drug ($m) | ||
|---|---|---|---|---|---|---|---|
| % of leftover drug using only full vials | % doses with vial sharing | % of leftover drug adjusted for frequency of vial sharing† | |||||
| Paclitaxel protein bound (Abraxane), 2005 | Breast 260 mg/m2 | 100 | 9 | 16 | 8 | 960.77 | 76.72 |
| Brentuximab vedotin (Adcetris), 2011 | Lymphoma 1.8 mg/kg | 50 | 15 | 36 | 10 | 292.18 | 29.15 |
| Pemetrexed (Alimta), 2004 | Mesothelioma/lung 500 mg/ m2 | 100, 500 | 5 | 16 | 4 | 1269.04 | 54.64 |
| Bevacizumab (Avastin), 2004 | Colorectal 5 (15) mg/kg | 100, 400 | 11 | 19 | 9 | 3159.32 | 284.49 |
| Ramucirumab (Cryamza), 2014 | Gastric 8 (10) mg/kg | 100, 500 | 7 | 16‡ | 6 | 471.55 | 28.78 |
| Cetuximab (Erbitux), 2004 | Head/neck 250 (400) mg/m2 | 100, 200 | 6 | 19 | 5 | 570.22 | 29.18 |
| Asparaginase Erwinia chrysanthemi (Erwinaze), 2011 | All 25000 IU/ m2 | 10000 | 10 | 16‡ | 8 | 170.40 | 14.13 |
| Eribulin (Halaven), 2010 | Breast 1.4 mg/ m2 | 1 | 15 | 18 | 13 | 167.71 | 21.85 |
| Cabazitaxel (Jevtana), 2010 | Prostate 25 mg/m2 | 60 | 23 | 12 | 21 | 127.96 | 26.89 |
| Ado-trastuzumab emtansine (Kadcyla), 2013 | Breast 3.6 mg/kg | 100, 160 | 7 | 16‡ | 6 | 413.96 | 23.66 |
| Pembrolizumab (Keytruda), 2014 | Melanoma 2 mg/kg | (50), 100 | 24 | 16‡ | 21 | 943.07 | 197.94 |
| Carfilzomib (Kyprolis), 2012 | Myeloma 20 (27) mg/ m2 | 60 | 37 | 16‡ | 33 | 697.65 | 231.45 |
| Filgrastim (Neupogen), 1991 | Neutropenia 5 (10) µg/kg | 300, 480 | 17 | 0§ | 17 | 623.85 | 106.01 |
| Irinotecan liposome (Onivyde), 2015 | Pancreatic 70 mg/m2 | 43 | 7 | 16 | 6 | 118.09 | 7.13 |
| Nivolumab (Opdivo), 2014 | Melanoma 3 mg/kg | 40, 100 | 4 | 16‡ | 3 | 2078.63 | 68.93 |
| Rituximab (Rituxan), 1997 | Non-Hodgkin’s lymphoma 375 (500) mg/m2 | 100, 500 | 7 | 0§ | 7 | 3852.75 | 253.85 |
| Bendamustine (Treanda), 2008 | Chronic lymphocytic leukemia 100 (120) mg/ m2 | 25, 45, 100, 180 | 1 | 6 | 1 | 563.44 | 7.38 |
| Panitumumab (Vectibix), 2006 | Colorectal 6 mg/kg | 100, 200, 400 | 10 | 17 | 8 | 237.41 | 18.72 |
| Bortezomib (Velcade), 2003 | Myeloma:1.3 mg/ m2 | 3.5 | 30 | 16 | 27 | 1160.64 | 308.74 |
| Ipilimumab (Yervoy), 2011 | Melanoma 3 mg/kg | 50, 200 | 10 | 22 | 7 | 620.22 | 46.47 |
| Total | — | — | — | — | — | 18 498.86 | 1836.11 |
*All amounts in mg except for filgrastim (µg) and asparaginase (IU). Filgrastim also sold in single dose prefilled syringes.
†Based on (discarded percentage assuming full vials×proportion of full vials)/((discarded percentage assuming full vials×proportion of full vials)+average dose).
‡Based on median of drugs for which there were available data.
§Billed in full vial or full prefilled syringe units.
The proportion of drug left over varies from 1% to 33%. Between these extremes are drugs such as bevacizumab, which comes in both 100 mg and 500 mg vials, and ipilimumab, which comes in both 40 mg and 100 mg. About 9% and 7% of these drugs, respectively, is left over. Yet small percentages can still lead to large dollar amounts. The October 2015 Medicare Average Sales Price files show that a dose of ipilumumab might cost $29 000,10 meaning that the 7% left over would generate an additional $2000 in revenue for the company for each vial sold.
How drug quantity affects profits and waste
The effect of different approaches to packaging for single dose vials is illustrated by the two drugs bendamustine and bortezomib. Bendamustine, a drug for leukemia, is sold in a broad array of single dose vials (25, 45, 100, and 180 mg) that can be combined to reach its dose of 100 mg/m2 nearly precisely (fig 2). Vial combinations cover every 5 mg interval across the typical adult dose range of 110 mg to 310 mg, with the exception of 130 mg and 155 mg. We calculate that only 1% of bendamustine is wasted. Bortezomib on the other hand, a drug to treat multiple myeloma, is available in the US in only a 3.5 mg vial, much larger than the average required dose, which we calculate to be 2.5 mg based on the drug’s dose of 1.3 mg/m2 and the average weight of a cancer patient. Our estimate is that 27% to 30% of bortezomib sales in the US are related to leftover drug equating to $309m. The large vial size of bortezomib seems to be unique to the US market. The drug is sold in 1 mg vials in the UK.11
Fig 2.
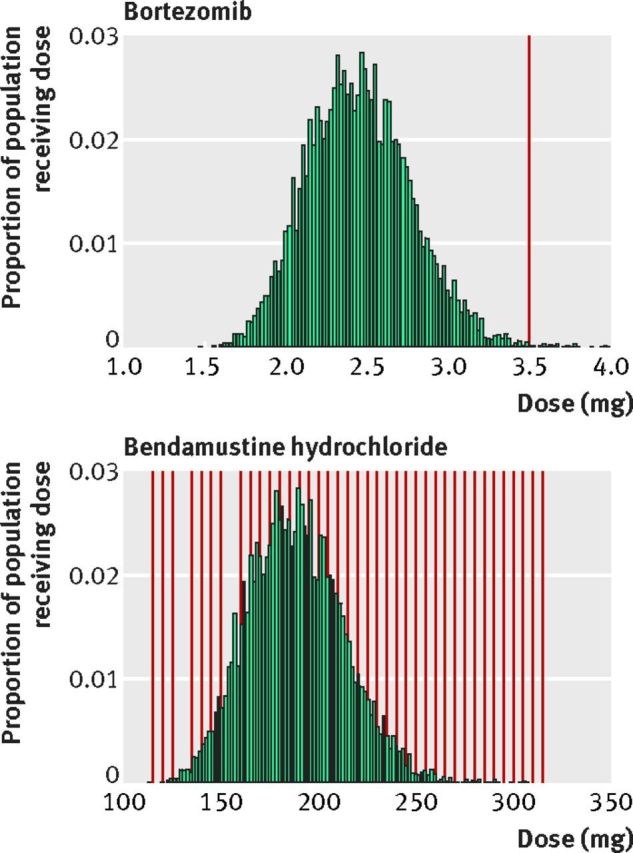
Distribution of FDA approved dose (green histogram) in the US population of cancer patients, and available combinations of full vial contents (red lines) to achieve that dose for bortezomib (top) and bendamustine (bottom)
Pembrolizumab provides another example of how vial sizes can influence revenues. When it was initially approved in the US in September 2014, the drug was sold in 50 mg vials (as a powder that needs to be reconstituted into a liquid). But in February 2015 the manufacturer introduced a larger 100 mg vial (as a liquid) and stopped distributing the 50 mg vials to the US market. Five months later, in July 2015, pembrolizumab was approved in Europe, where it is sold in the smaller 50 mg vials as a powder.
The increased revenue from the change is substantial. Consider a 70 kg patient who requires a dose of 140 mg (the drug is dosed at 2 mg/kg). When the drug was sold in 50 mg vials, reaching the desired dose would require three 50 mg vials and leave 10 mg unused. But with only 100 mg vials available, 60 mg is left over. According to the Medicare Not Otherwise Classified October 2015 file, which lists Medicare’s reimbursement rates for these drugs, each milligram of pembrolizumab costs around $50. In this example the change in vial size alone increases the revenues for the company from leftover drug by sixfold, from $500 to $3000, for a single dose. We estimate that the additional revenue to the company from the packaging change over the next five years will be $1.2bn, which comes on top of the $1.2bn they would have gained from leftover drug with the 50 mg package (table 2). Similarly, by only selling bortezomib in the US in the larger 3.5 mg vials rather than the 1 mg vials sizes available in Europe, the manufacturer, Millennium, will increase its 2016 US revenues by $130m (data not shown).11
Table 2.
Projected revenue from sales of pembrolizumab comparing scenarios with revenue only from administered drug, revenue based on 50 mg vial sizes with reimbursement for leftover drug, and revenue based on 100 mg vial sizes with reimbursement for leftover drug. Data based on pooled analyst estimates compiled by Defined Health.
| Year of sales | Revenue from dose only ($m) | Revenue from dose and leftover using 50 mg vials ($m) | Revenue from dose and leftover using 100 mg vials ($m) |
|---|---|---|---|
| 2016 | 762 | 862 | 964 |
| 2017 | 1335 | 1510 | 1690 |
| 2018 | 1991 | 2253 | 2520 |
| 2019 | 2346 | 2654 | 2969 |
| 2020 | 2687 | 3040 | 3401 |
| Total | 9121 | 10 320 | 11 544 |
Effect on hospitals and patients
We have focused on how much money companies earn in terms of revenues from leftover drug, not how much payers and patients are spending on them, which is a larger number due to the fact that distributing intermediaries and treating doctors and hospitals mark-up drugs when they bill for them. The mark-up varies considerably. In public insurance programs such as the Federal Medicare program the mark-up set by Congress is 6% and is currently 4%. For commercial insurance, which is the more common coverage in the United States, payers have reported that they pay mark-ups to doctors and hospitals in the order of 22% and 142%, respectively.12 In hospitals that use the distribution channel 340B, mark-ups in the Medicare program have been estimated to be 58%.13 14 15 The mark-up for commercially insured patients at these types of hospitals is even greater. So although it is hard to precisely estimate the additional profit that will come to doctors and hospitals from billing for leftover cancer drugs, our estimate is that it will almost certainly exceed $1bn in 2016.
The additional costs to patients, who are charged for leftover drug just as they are for drug they have received is also likely to be substantial. Medicare Part B, covering roughly half of cancer patients, includes 20% coinsurance with no upper limit, and 14% of beneficiaries have no additional coverage for their coinsurance.16 Private insurance generally has out of pocket maximums that many patients with cancer reach regardless.
Although we focused on cancer, the problem of mismatched single dose vials and doses is not unique to the disease. The asthma drug omalizumab has approved doses in 75 mg intervals, but the company only sells 150 mg vials in the United States, even though it has an approved 75 mg vial size. The drug infliximab, one of the largest selling drugs in the United States with expected 2015 revenues of $4.3bn, is available in only 100 mg single dose vials. It is also dosed based on body size and using the same methods we applied to the cancer drugs, this packaging generates around $500m in additional revenues from leftover drug.
How can we stop the waste?
Regularly and systematically discarding expensive drugs is antithetical to efforts to reduce spending on healthcare services that provide no value. Policy makers should therefore explore approaches that would reduce or eliminate paying for leftover drug. Current regulatory standards could be viewed as contradictory, or at least as ambiguous (box). The FDA calls on companies to balance vial contents so that leftover drug is minimized yet they should also provide enough drug that more than one vial is rarely needed for a single dose.17 Guidance on vial sharing is also inconsistent. The Centers for Medicare and Medicaid Services essentially encourages it; the Centers for Disease Control and Prevention states that it is unsafe (box).18 19
Federal agency guidelines and advisories regarding proper drug quantity and use of drugs contained in single dose vials (SDVs)
FDA guideline 20—“Significantly more drug than is required for a single dose may result in the misuse of the leftover drug product. Similarly, the need to combine several single-dose vials for a single patient dose may lead to medication errors and microbial contamination”
Centers for Medicare and Medicaid Services advisory 21—“It is permissible for healthcare personnel to administer repackaged doses derived from SDVs to multiple patients, provided that each repackaged dose is used for a single patient in accordance with applicable storage and handling requirements”
Centers for Disease Control and Prevention guideline 22—“Vials labeled by the manufacturer as ‘single dose’ or ‘single use’ should only be used for a single patient. These medications typically lack antimicrobial preservatives and can become contaminated and serve as a source of infection when they are used inappropriately”
Several policy options merit exploration. Regulators could require manufacturers to provide drugs in a reasonable set of size options to ensure the amount of wasted drug is low, say 3%. This is achievable, as table 3 shows. If all of our suggestions were adopted, it would lower revenue from leftover drug from $1.8bn to $400m and, including the reductions to doctor and hospital mark-ups on leftover drug, would save around $2bn in total. An alternative would be to leave manufacturers free to select their vial sizes but also require them to refund the cost of leftover drug. This could be achieved through certified disposal and a virtual return.
Table 3.
Proposed additional single dose vial sizes to reduce the amount of waste on leftover drug for 18 out of 20 top selling cancer drugs in our analysis for which we propose one additional size and estimation of effect on waste in 2016
| Generic name | Currently available vial sizes (mg) | Proposed additional vial size | Estimated waste in 2016 ($m) | Value of drug in additional vial ($)* | |
|---|---|---|---|---|---|
| With existing vials | With additional vial | ||||
| Paclitaxel protein bound | 100 | 30 | 77 | 8 | 293 |
| Brentuximab vedotin | 50 | 10 | 29 | 6 | 1193 |
| Pemetrexed | 500, 100 | 60 | 55 | 11 | 367 |
| Bevacizumab | 400, 100 | 20 | 284 | 60 | 139 |
| Ramucirumab | 500, 100 | 40 | 29 | 6 | 432 |
| Cetuximab | 200, 100 | 50 | 29 | 15 | 267 |
| Asparaginase Erwinia chrysanthemi | 10000† | 3000† | 14 | 2 | 1129 |
| Eribulin | 1 | 0.25 | 22 | 6 | 256 |
| Cabazitaxel | 60 | 2.5 | 27 | 3 | 372 |
| Ado-trastuzumab emtansine | 160, 100 | 20 | 24 | 12 | 584 |
| Pembrolizumab | 100, (50)‡ | 10 | 198 | 24 | 457 |
| Carfilzomib | 60 | 2.5 | 231 | 19 | 78 |
| Irinotecan liposome | 43 | 10 | 14 | 1 | 389 |
| Nivolumab | 100, 40 | 10 | 69 | 35 | 254 |
| Rituximab | 500, 100 | 40 | 254 | 53 | 300 |
| Panitumumab | 400, 200, 100 | 30 | 19 | 2 | 303 |
| Bortezomib | 3.5 | 0.25 | 309 | 48 | 117 |
| Ipilimumab | 200, 50 | 10 | 46 | 10 | 1388 |
| Total | — | — | 1843.11 | 434.25 | — |
*Based on October 2015 ASP files.10
†International Units.
‡No longer marketed.
One pattern sometimes seen in clinical practice is to round up doses to the quantity in the full vial, thus changing dosing from body sized based to “flat” or “fixed” dosing. The approach is problematic not only because it leads some patients to receive too high a dose and others too low when compared to the FDA approved dose, but also because it does not reduce spending on leftover drug. It merely changes clinician behavior from discarding leftover drug to infusing leftover drug into patients.
Policy makers should also revisit the current FDA guidance on the appropriate packaging of infused drugs in single dose vials and encourage the FDA, CDC, Centers for Medicare and Medicaid Services, and US Pharmacopeial Convention to reconcile their views on vial contents and vial sharing. Such steps could lead to savings for our healthcare system without sacrificing health outcomes. Opportunities to eradicate waste of this kind are rare.
Key messages
Many infused cancer drugs are packaged in single dose vials but dosed based on body size, often resulting in leftover drug
All the drug in the vial has to be paid for, making wasted drug a source of unnecessary spending
Drug companies will earn around $1.8bn from leftover cancer drugs in the United States in 2016
Manufacturers should be required to package drugs in quantities that allow better matching with required doses or enable virtual return of leftover drug
We thank Coral Atoria for help with the analysis of Medicare claims data and Raina H Jain for research and editorial assistance.
Contributors and Sources: PBB is guarantor, and is the director of the Center for Health Policy and Outcomes at Memorial Sloan Kettering Cancer Center (MSKCC). Rena M. Conti is a health economist at the University of Chicago. Raymond J. Muller is the Associate Director of Pharmacy Services at MKSCC, and Geoffrey C. Schnorr was a project coordinator in the Center for Health Policy and Outcomes at MSKCC. Leonard B. Saltz is the Chair, Pharmacy and Therapeutics Committee at MSKCC. All authors analyzed and interpreted the data. PBB drafted the manuscript. PBB, RMC, RJM, GCS, and LBS revised the manuscript and all authors approved the final version. All authors had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding: This study was funded by internal Memorial Sloan Kettering Cancer Center funds and by Memorial Sloan Kettering Cancer Center Support Grant/Core Grant P30 CA 008748.
Competing Interests: We have read and understood BMJ policy on declaration of interests and declare the following interests: PBB reports personal fees from Association of Community Cancer Centers, America's Health Insurance Plans, AIM Specialty Health, American College of Chest Physicians, American Society of Clinical Oncology, Barclays, Defined Health, Express Scripts, Genentech, Goldman Sachs, McKinsey and Company, MPM Capital, National Comprehensive Cancer Network, Novartis, Biotechnology Industry Organization, American Journal of Managed Care, Boston Consulting Group, Foundation Medicine; LBS reports grants from Taiho Pharmaceuticals; RJM reports personal fees from Amgen, Hospira, Seattle Genetics, Sunesis, Amneal Biosciences, Magellan Medication Management System, and is an uncompensated member of the national clinical advisory committee for the Institute of Safe Medication Practices.
References
- 1.Berwick DM, Hackbarth AD. Eliminating waste in US health care. JAMA 2012;307:1513-6. 10.1001/jama.2012.362. 22419800. [DOI] [PubMed] [Google Scholar]
- 2.Lien K, Cheung MC, Chan KK. Adjusting for drug wastage in economic evaluations of new therapies for hematologic malignancies: a systematic review. J Oncol Pract 2015 . Sep 1 JOP.2015.005876. [DOI] [PubMed]
- 3.Smith RS. A 2-year retrospective review of vial sharing options for the compounding of cytotoxics. Eur J Hosp Pharm 2015;22:161-4 10.1136/ejhpharm-2014-000547. . [DOI] [Google Scholar]
- 4. United States Pharmacopeia, ed. USP< 797> Guidebook to pharmaceutical compounding-sterile preparations.United States Pharmacopeial Convention, 2008.
- 5.Fasola G, Aprile G, Marini L, Follador A, Mansutti M, Miscoria M. Drug waste minimization as an effective strategy of cost-containment in oncology. BMC Health Serv Res 2014;14:57. 10.1186/1472-6963-14-57. 24507545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.IMS Institute for Healthcare Informatics. Medicines use and spending shifts: a review of the use of medicines in the US in 2014. 2015. www.imshealth.com/en/thought-leadership/ims-institute/reports/medicines-use-in-the-us-2014.
- 7.US Food and Drug Administration. Drugs@FDA: FDA approved drug products. 2015. www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm.
- 8. US Department of Health and Human Services. National health and nutrition examination survey. Health professionals home interview Hyattsville.Centers for Disease Control and Prevention, National Centers for Health Statistics, 2004.
- 9.Howlader N, Noone A, Krapcho M, et al. SEER cancer statistics review, 1975-2011.National Cancer Institute, 2014.
- 10.Center for Medicare and Medicaid Services. Medicare Part B drug average sales price: manufacturer reporting of average sales price (ASP) data. 2015. www.cms.gov/Medicare/Medicare-Fee-for-Service-Part-B-Drugs/McrPartBDrugAvgSalesPrice/index.html
- 11.European Medicines Agency. EPAR summary for the public: Velcade 2015. www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/000539/WC500048136.pdf.
- 12.Newcomer LN. Changing physician incentives for cancer care to reward better patient outcomes instead of use of more costly drugs. Health Aff (Millwood) 2012;31:780-5. 10.1377/hlthaff.2012.0002. 22492895. [DOI] [PubMed] [Google Scholar]
- 13.Office of Inspector General. Part B payments for 340B-purchased drugs. 2015. Available from: http://oig.hhs.gov/oei/reports/oei-12-14-00030.pdf.
- 14.Conti RM, Bach PB. Cost consequences of the 340B drug discount program. JAMA 2013;309:1995-6. 10.1001/jama.2013.4156. 23609758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conti RM, Bach PB. The 340B drug discount program: hospitals generate profits by expanding to reach more affluent communities. Health Aff (Millwood) 2014;33:1786-92. 10.1377/hlthaff.2014.0540. 25288423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaiser Family Foundation. Medigap reform: setting the context for understanding recent proposals. 2014 http://kff.org/medicare/issue-brief/medigap-reform-setting-the-context/.
- 17. US Food and Drug Administration. Code of federal regulations—title 21—food and drugs. FDA, 2013.
- 18.Centers for Medicare and Medicaid Services. Safe use of single dose/single use medications to prevent healthcare-associated infections. 2012 www.cms.gov/Medicare/Provider-Enrollment-and-Certification/SurveyCertificationGenInfo/Downloads/Survey-and-Cert-Letter-12-35.pdf.
- 19.Centers for Disease Control and Prevention. Protect patients against preventable harm from improper use of single-dose/single-use vials. 2012 www.cdc.gov/injectionsafety/CDCposition-SingleUseVial.html.
- 20.USDHHS. FDA, CDER, CBER. Allowable excess volume and labeled vial fill size in injectable drug and biological products: guidance for industry. 2015. www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm389069.pdf.
- 21.Centre for Medicare and Medicaid Services. Safe use of single dose/single use medications to prevent healthcare-associated infections. www.cms.gov/Medicare/Provider-Enrollment-and-Certification/SurveyCertificationGenInfo/Downloads/Survey-and-Cert-Letter-12-35.pdf.
- 22.CDC. Protect patients against preventable harm from improper use of single–dose/single–use vials. 2012. www.cdc.gov/injectionsafety/CDCposition-SingleUseVial.html.