Abstract
Background:
Fragile X syndrome (FXS), the most common inherited cause for intellectual disability, is associated with alterations in cholinergic among other neurotransmitter systems. The present study investigated the effects of donepezil hydrochloride, a cholinesterase inhibitor that has potential to correct aberrant cholinergic signaling.
Method:
Forty-two individuals with FXS (mean age=19.61) were randomized to receive 2.5mg-10.0 mg of donepezil (N=20, 7 females) or placebo (N=22, 8 females) per day. One individual in the active group withdrew at week 7. Outcomes included the contingency naming test, the aberrant behavior checklist, and behavior and brain activation patterns during a functional MRI (fMRI) gaze discrimination task.
Results:
There were no significant differences between active and placebo groups on cognitive (contingency naming task) or behavioral (total score or subscales of the aberrant behavior checklist) outcomes. At baseline, the active and placebo groups did not differ in fMRI activation patterns during the gaze task. After 12 weeks of treatment the active group displayed reduced activation in response to the averted vs. direct gaze contrast, relative to the placebo group, in the left superior frontal gyrus.
Conclusions:
Reduced functional brain activation for the active group may represent less arousal in response to direct eye gaze, relative to the placebo group. Change in fMRI activation patterns may serve as a more sensitive metric and predictor of response to treatment when compared to cognitive and behavioral assessments. Our results suggest that donepezil may have an impact on brain functioning, but longer term follow up and concomitant behavioral intervention may be required to demonstrate improvement in cognition and behavior.
Introduction
Advances in neurobiological research have led to the discovery of disease-specific mechanisms that underlie particular neuropsychiatric disorders. This growing body of empirical evidence spans several interdisciplinary fields and includes molecular information from animal models (Chang et al., 2008) and neuroimaging research describing aberrant neurocircuitry in humans with specific disorders (Bruno et al., 2014). These investigations seek to inform disease-specific, targeted interventions that could lead to substantial improvement over current symptom-focused treatments for such disorders (Insel, 2014). Fragile X syndrome (FXS), the most common inherited cause for intellectual disability and the most common single gene cause for autism symptoms (Hagerman et al., 2010), is one disorder that has received a great deal of attention (Berry-Kravis, 2014) but for which no effective targeted therapy yet exists.
The genetic etiology of FXS is a repeat expansion in the fragile X mental retardation-1 gene (FMR1) on the X chromosome. This mutation is associated with absent or greatly reduced production of the fragile X mental retardation protein (FMRP), a protein that is critical for neurodevelopmental processes including synaptic plasticity and dendritic pruning (Swanger and Bassell, 2011). Recent research in the mouse model of FXS (Fmr1 knockout (KO)) indicates that the downstream effects of reduced FMRP include alterations in multiple neurotransmitter systems (Davidovic et al., 2011). Silencing FMRP in the Fmr1 KO mouse results in amplified signaling through specific G protein coupled receptors – group I metabotropic glutamate receptors (mGluR1 and mGluR5) (Bear et al., 2004), and muscarinic acetylcholine receptors (mAChRs) (Veeraragavan et al., 2011). Targeted therapies aimed at correcting aberrant receptor signaling have demonstrated promise in rescuing phenotypes in animal models. For example, an mGluR5 antagonist rescued the abnormal spine length in the Fmr1 KO mouse (Pop et al., 2014). Additionally, decreased GABA-ergic inhibition has been reported in the Fmr1 KO mouse (D’Hulst and Kooy, 2009). Treatment with GABA-ergic compounds rescues phenotypes including aberrant courtship behavior and excessive protein translation in the dfmr mutant fly (Olmos-Serrano et al., 2010). Results of human treatment trials aimed at correcting aberrant glutamatergic (Berry-Kravis et al., 2016; Jacquemont et al., 2011) or GABAergic signaling (Berry-kravis et al., 2017) have thus far been unsuccessful at ameliorating behavioral symptoms.
Cholinergic signaling has received relatively less attention as a pharmacological target in FXS, although multiple lines of research indicate the potential effectiveness of targeting this system. Overactive mAChR signaling has been demonstrated in the drosophila model (Chang et al., 2008) and the Fmr1 KO mouse (Veeraragavan et al., 2011). Genetic reduction of mAChR signaling in the Fmr1 KO mouse resulted in rescue of phenotypes including anxiety, aberrant sensory motor gating and perseverative behaviors (Veeraragavan et al., 2012). High FMR1 gene expression is present in cholinergic neurons in the basil forebrain (nucleus basalis) during human fetal development (Abitbol et al., 1993) and this region demonstrates reduced brain activation in individuals with FXS (Greicius et al., 2004). Correspondingly, magnetic resonance spectroscopy (MRS) in humans with FXS revealed reduced levels of choline in the caudate nucleus (Bruno et al., 2013) and dorsolateral prefrontal cortex (Kesler et al., 2009). While levels of choline as revealed by MRS may not directly represent the effects of altered cholinergic signaling, they do represent an indirect link between animal model and human brain system alterations. Cognitive weaknesses in individuals with FXS include difficulties with executive function (Hooper et al., 2018; Martin et al., 2016) and attention (Cornish et al., 2007). Other prominent phenotypic features include hyperarousal (Hessl et al., 2006) and aberrant sensory motor gating (Hessl et al., 2009). These symptoms may be linked to cholinergic disfunction, given the cholinergic system’s role in attention and executive function (Hasselmo and Sarter, 2011), cortical arousal and plasticity (Lin et al., 2015), and sensory-motor learning (Deng et al., 2007).
The present study sought to test the efficacy of donepezil, an acetylcholinesterase-inhibitor that slows the degradation of synaptic acetylcholine thereby increasing its availability. This agent is thought to increase available acetylcholine in the brain (Liang and Tang, 2004). Donepezil is FDA approved for the treatment of mild to moderate Alzheimer’s disease and has been shown to improve cognition in typically developing individuals. (Reches et al., 2014) In children and adolescents with autism spectrum disorder, donepezil treatment was associated with reductions in hyperactivity and irritability (Hardan and Handen, 2002). Donepezil treatment was also associated with reduction in tics in individuals with tics and attention deficit hyperactivity disorder (Cubo et al., 2008). A case study in a male fragile X premutation carrier indicated improvements in cognition following donepezil treatment (Bourgeois et al., 2006). It is worth noting that the fragile X premutation is associated with a unique set of cognitive and behavioral outcomes and is considered a condition separate from full mutation FXS (Bailey et al., 2008). One small (N= 20 total, 10 in active group) double blind study in males with FXS reported no change in cognition following donepezil administration (Sahu et al., 2013). However, a preliminary, open label study by our group demonstrated positive effects on cognition and behavior following treatment with donepezil for individuals with FXS (Kesler et al., 2009). We hypothesized that donepezil would improve cognitive functioning and behavior as demonstrated in the open label study.
The primary outcome was executive functioning assessed by the contingency naming task (CNT, (Anderson et al., 2000)). Aberrant behaviors represent particularly maladaptive symptoms for families and individuals with FXS (Hustyi et al., 2014). Thus, our secondary outcome was behavior as quantified by the Aberrant behavior checklist (ABC, (Aman et al., 1995)). The CNT and ABC were chosen based on the results of our open label study (Kesler et al., 2009) and because they have been used in several other studies of individuals with FXS (Bennetto et al., 2001; Berry-kravis et al., 2017; Hooper et al., 2018; Lightbody et al., 2006). We also used functional MRI (fMRI) as an exploratory neuroimaging outcome to assess potential circuitry changes that may underlie behavioral response to donepezil. With fMRI we examined neural processing of face and gaze stimuli, given that eye contact avoidance is a particularly prominent symptom in individuals with FXS (Hall et al., 2006). Using a face-gaze paradigm, we have previously demonstrated FXS-specific patterns of sensitization (increasing activation over time) in the amygdala (Watson et al., 2008) and in widespread frontal, temporal and occipital cortical regions (Bruno et al., 2014).
Materials and methods
Participants
Stanford University’s Institutional Review Board approved all protocols (protocol ID 13773). This clinical trial has been registered with ClinicalTrials.gov (Identifier: ).
Participants and/or parents gave written informed consent and assent to participate. Participants were required to meet the following criteria: a) a confirmed genetic diagnosis of fragile X syndrome with FMR1 full mutation, b) age >=12, <=29 years, c) Verbal IQ >= 50 or <=75 based on the Wechsler Abbreviated Scale for Intelligence (Wechsler, 1999), d) Tanner pubertal stage >= 3 (based on parent rating of schematic drawings representing the five standard Tanner stages of pubertal development (Marshall and Tanner, 1969, 1970)). Potential participants were excluded if they presented with evidence of any of the following: a) current or lifetime DSM-IV diagnosis of bipolar disorder, schizophrenia, schizoaffective disorder, or psychotic disorder, NOS based upon reported history, b) poorly controlled seizure disorder or taking more than one anticonvulsant (subjects who were taking carbamazepine, phenytoin, or phenobarbital were excluded due to potential interaction effects with donepezil), c) concomitant or anticipated use of other medications having prominent effects on the cholinergic system (e.g., bethanechol, benztropine, atropine, succinylcholine), d) concomitant or anticipated use of medications or nutritional supplements that have the potential to significantly alter donepezil levels, clinical effects or adverse reactions (antifungal agents, corticosteroids, erythromycin, beta-blockers, calcium channel blockers, NSAIDs, gingko biloba, St. John’s wort), e) medical illnesses where donepezil could worsen the condition such as asthma, cardiac conduction abnormalities, urinary obstruction or gastrointestinal disease with gastric bleeding, f) Pregnancy or sexually active females not using a reliable method of contraception. All participant medications and dosage were stable during the trial with the exception of donepezil or placebo administration. See Table 2 for included participant medications.
Table 2.
Participant medications
| Active | Placebo | |
|---|---|---|
| N (N female) | 19 (7) | 22 (8) |
| N Any medications | 7 | 9 |
| N Stimulant | 3 | 7 |
| N SSRI/SNRI | 7 | 8 |
| N Minocycline | 0 | 3 |
| N Other | 2 | 4 |
The Any medications category includes counts of participants who were taking one or more medication across any of the classes, including Other. Other includes antipsychotics, anticonvulsants medication and alpha 2 receptor agonists. Groups did not differ in the overall number of individuals taking medications or within each class (all p’s >0.10).
Study design
The study was conducted at Stanford University. Recruitment and follow up were completed between September, 2009 and October, 2013. Participants were recruited across the United States and Canada through advertisements, referrals, word of mouth and nation-wide groups. Pharmacy-controlled randomization was used to assign individuals to receive donepezil (N=20, 7 females) or a placebo (N=22, 8 females) and pills were identical. Randomization was stratified by sex and age (12–20 and 21–29 years). Studies using PET in older adults with Alzheimer’s disease indicate that a 5mg dose was sufficient to achieve up to 60% AChE inhibition in the brain (Bencherif et al., 2002; Okamura et al., 2008). In the present study, dose was titrated from 2.5mg to 5mg during the first week and from 5mg to 10mg during the second week as tolerated in each arm of the study. The Dosage Record and Treatment Emergent Symptom Scale (DOTES. (Garvey et al., 1991)) was used to monitor side effects and adverse events. If the study physician or parents detected any potential adverse events, the subject’s dose was decreased to 5mg or 2.5mg/day. The participants, their parents, all researchers and study physicians were blind to treatment group. The sample size was chosen based on a-priori power calculations and our preliminary, open label trial (Kesler et al., 2009). The study was completed upon collection of follow up data for all enrolled participants.
The primary and secondary outcomes were compared between the active and placebo groups at study endpoint using ANOVA with covariates included in the model to account for baseline performance level.
Primary and secondary outcomes
The primary outcome was executive functioning as measured by the CNT (Anderson et al., 2000). The CNT assesses inhibition and set switching and is modeled after the Stroop Color Word Test. Participants were presented with a series of shapes in three different colors each containing a same or different shape within the larger outside shape. Each person completed three trials of this task 1) naming the color of shapes, 2) naming the shape of the outside figure while ignoring the shape of the inside figure, and 3) naming either the color or the shape of the outside figure according to a predetermined rule: “If the inside shape matches the outside shape, name the color, otherwise, name the outside shape.” For each trial the number of correct responses per minute was calculated and trials 2 and 3 were used as outcome measures. The first rule (naming) did not require any inhibition or switching and was thus too simple to be informative as an outcome measure. The fourth rule, switching, was not used in the present study as it was too difficult for most of our participants with FXS. The secondary outcome was behavior as assessed by the ABC, a parent rating scale measuring problem behaviors (Aman et al., 1995). We used the total score as our outcome measure based on our open label study (Kesler et al., 2009). We examined the FXS-specific coding which includes subscales for irritability, hyperactivity, lethargy, and social avoidance (Sansone et al., 2012) in an exploratory analysis.
Neuroimaging methodology
fMRI scanning was performed on a 3 Tesla General Electric Signa scanner (Milwaukee, WI) using a standard 8-channel headcoil. Participants were free from MRI contraindications and met screening criteria for ability to tolerate fMRI procedures (e.g. ability to hold still, minimal sensitivity to loud noises). Prior to scanning, participants practiced lying motionless in an MRI simulator and experienced the sounds and sights of an MRI scanner (Epstein et al., 2007). Practice continued until the participant could hold motionless (movement<1mm measured by potentiometer) for 10 minutes. Participants also practiced the imaging task described below with images not used in the actual fMRI task.
Whole brain T2*-weighted gradient echo spiral images were acquired with high-order shimming (echo time (TE)=30ms, repetition time (TR)= 2000ms, flip angle=80°, FOV=22cm, acquisition matrix = 64×64, approximate voxel size = 4.0×3.4×3.4mm, 30 axial-oblique slices: 4.0mm thick, 1.0mm skip). Participants viewed color photographs of college-aged models with neutral expressions. Fifty facial stimuli (25 females) were presented across two runs (3 minutes 58 seconds each). Twenty-five faces were oriented with gaze directly toward the participant and 25 with gaze averted away. Each face was presented for 3 seconds, followed by a blank screen for 500 msec and a jittered (1–6 sec) inter stimulus interval during which a fixation cross was displayed. Participants were instructed to press button 1 if the person in the picture was looking at them, or button 2 if looking away. Responses and reaction times were collected via button box which was placed in a single hand.
High-resolution T1-weighted anatomical images, collected during the same imaging session, facilitated normalization to standard space (FSPGR, TE=3.4ms, TR=8.5ms, flip angle=15°, FOV=220×165mm, slice thickness=1.7mm, 124 coronal slices; matrix = 256×192; acquired resolution = 0.86×0.86×1.7mm).
Research staff blind to treatment group performed all fMRI image analyses, including quality assessment, using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/). Preprocessing included realignment and correction for motion and global signal intensity artifacts using the ArtRepair toolbox (http://cibsr.stanford.edu/tools/methods/artrepair-software.html). Volumes with movement>0.5mm per TR or global signal intensity fluctuations>1.5% were de-weighted and repaired using interpolation between the nearest non-outlier scans. Participants whose fMRI series required repair for>25% of volumes were removed from analyses. Images were co-registered to the participant’s anatomical image, normalized to the MNI template, and spatially smoothed using a 6mm full-width at half maximum Gaussian kernel.
After preprocessing the images, statistical analysis was first performed at the individual level using a general linear model. The design was modeled as a boxcar, convolved with SPM canonical hemodynamic response function. First level statistics included fixed effects modeling to identify activation to direct and averted gaze stimuli separately (direct, averted) and overall (direct+averted). First level, within-subjects contrasts included modeling averted>direct conditions. We chose to examine averted>direct gaze as our primary contrast given previous results demonstrating a FXS specific pattern of brain activation in response to this contrast (Watson et al., 2008). Due to significantly reduced sample size completing neuroimaging at both baseline and endpoint (N=18 vs N=27 completing fMRI at time 2) a two-sample T-test was employed to compare activation maps between the active and placebo groups at each timepoint. We also used ANOVA to assess the interaction of gaze (direct or averted) by group by time but, due to the reduced sample size for this comparison we consider the T-tests the primary neuroimaging outcome. Responses to the fMRI task were analyzed using ANOVA including only individuals who had complete, usable fMRI data at both baseline and endpoint whereas the follow-up T-tests for accuracy and reaction time included individuals who were missing fMRI data at one time point.
Results
Demographic and background characteristics
Forty-five individuals with FXS (age 12–29 years) were assessed for eligibility and 42 were enrolled in this 12-week placebo-controlled trial (Figure 1). The medication was well tolerated and there were no serious adverse events. One male in the active group withdrew at week 7 due to complaints of nausea. This participant had been reduced to dosage of 2.5 mg at the time of withdrawal. Nineteen individuals in the active group and 22 individuals in the placebo group completed the 12-week trial and were included in the analysis of primary and secondary outcomes. These individuals were titrated to the 10mg dose as planned. Active and placebo groups did not differ on age, sex and estimated full scale IQ measured by the Wechsler Abbreviated Scale for Intelligence (all p’s>0.10, Table 1).
Figure 1.
Participant flow diagram.
Table 1.
Group descriptive statists
| Active | Placebo | Statistical comparison | ||||
|---|---|---|---|---|---|---|
| N | 19 (7 females) | 22 (8 females) | ||||
| Mean | Standard Deviation | Mean | Standard Deviation | |||
| Age at enrollment | 18.95 | 4.49 | 20.18 | 5.05 | t(39)=−0.821, p>0.01 | |
| Full Scale IQ | 63.11 | 11.08 | 62.14 | 11.74 | t(39)=−0.270, p>0.01 | |
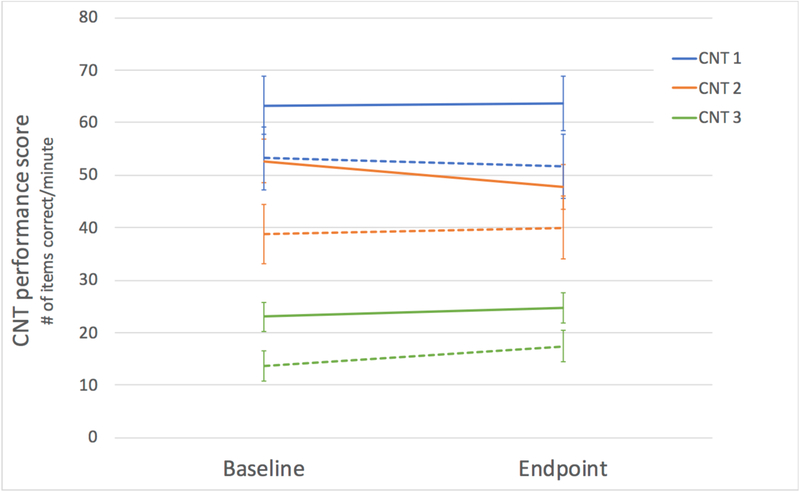
| Contingency Naming 1 | Baseline | 62.56 | 25.39 | 53.25 | 28.26 | F(1,38)=0.002, p>0.10, |
| Endpoint | 63.69 | 22.76 | 51.74 | 28.32 | ||
| Contingency Naming 2 | Baseline | 52.36 | 19.01 | 38.77 | 26.49 | F(1,38)=0.06, p>0.10, |
| Endpoint | 47.83 | 18.69 | 39.98 | 28.05 | ||
| Contingency Naming 3 | Baseline | 23.00 | 12.90 | 13.77 | 13.44 | F(1,38)=0.05 p>0.10, |
| Endpoint | 24.79 | 12.57 | 17.47 | 14.48 | ||
| ABC total score | Baseline | 19.74 | 15.76 | 30.77 | 25.55 | F(1,36)=0.02, p>0.10 |
| Endpoint | 17.56 | 13.65 | 24.43 | 23.27 | ||
| ABC Irritability | Baseline | 4.00 | 6.29 | 8.64 | 10.43 | F(1,36)=0.05, p>0.10 |
| Endpoint | 2.78 | 3.35 | 6.10 | 7.69 | ||
| ABC Hyperactivity | Baseline | 2.53 | 3.27 | 5.45 | 6.36 | F(1,36)=0.60, p>0.10 |
| Endpoint | 2.33 | 2.89 | 4.19 | 5.77 | ||
| ABC Lethargy | Baseline | 4.16 | 3.45 | 4.86 | 4.38 | F(1,36)=0.11, p>0.10 |
| Endpoint | 3.89 | 4.57 | 4.67 | 5.76 | ||
| ABC Social avoidance | Baseline | 3.84 | 2.73 | 3.23 | 3.01 | F(1,36)=0.002, p>0.10 |
| Endpoint | 3.33 | 2.77 | 2.81 | 3.60 | ||
All values are mean (standard deviation) with the exception of N. The contingency naming scores represent the number of correct items per minute. Full scale IQ = Wechsler abbreviated scale of intelligence. Contingency Naming = contingency naming task, number of correct items per minute are reported for trials 1, 2 and 3. Trial 1 is reported for completeness but not used in analysis. Endpoint is 12 weeks following baseline. ABC = the aberrant behavior checklist. Total score reflects the original coding and the subscales represent the FXS specific coding. Statistical comparisons for CNT and ABC are ANOVAs comparing active and placebo groups at study endpoint with covariates to account for baseline performance level.
Primary and secondary outcomes
Analyses included all participants completing the trial (active N=19, placebo N=22). Results are presented in Table 1 and Figures 2 and 3. The active group demonstrated significantly higher baseline performance scores on CNT trial 3 (t(40)=2.23, p=0.031), and a trend for higher scores on CNT trial 2 (t(40)=1.86, p=0.071). Additional scores for the CNT are presented in Supplementary Table 1. There were no group differences in baseline scores on the ABC total (t(39)=−1.63, p>0.10). At week 12 (endpoint), there were no significant group differences in CNT trial 3 or trial 2 after controlling for baseline performance (rule 3: F(1,38)=0.05 p>0.10, ; rule 2: F(1,38)=0.06, p>0.10, ). One individual in the active group and one in the placebo group were missing the ABC at follow-up assessment. There were no significant group differences on ABC total score after controlling for baseline performance (F(1,36)=0.02, p>0.10, ). There were also no significant differences for any of the original subscales, or the FXS specific coding of subscales (all p’s>0.10). The pattern of significance remained the same for all CNT and ABC outcomes when controlling for age at enrollment in addition to baseline performance (all p’s>0.10).
Figure 2.
Contingency naming test scores pre and post treatment. CNT = contingency naming test, scores represent the number of correct items per minute. Solid lines indicate active group scores and dashed lines indicate placebo group scores. Error bars represent standard error.
Figure 3.
Aberrant behavior checklist scores pre and post treatment. ABC = aberrant behavior checklist. Solid lines indicate active group scores and dashed lines indicate placebo group scores. Error bars represent standard error.
Neuroimaging results
At baseline, fMRI data from 12 participants in the active group and 11 in the placebo group were usable based on image quality. At week 12, fMRI data from 16 participants in the active group and 11 in the placebo group was usable. Please see Table 3 for the number of participants completing fMRI scanning in each group at each time.
Table 3.
Number of individuals in each group who were included in fMRI study
| Active | Placebo | ||
|---|---|---|---|
| Baseline | Completed fMRI scan | 13 | 14 |
| Excluded for motion* | 1 | 3 | |
| Included in fMRI analyses | 12 | 11 | |
| Endpoint | Completed fMRI scan | 17 | 13 |
| Excluded for motion* | 1 | 2 | |
| Included in fMRI analyses | 16 | 11 |
Includes number of individuals excluded based on quality of fMRI data (repair for>25% of volumes, see Methods for details).
Behavioral performance during the fMRI task at baseline and at endpoint indicated no significant group difference in accuracy or reaction time for direct or averted gaze stimuli (all p’s >0.10, Figure 4). However, a significant group by time interaction was found in reaction time for the averted gaze stimuli (p = 0.024). Follow up T-tests for the averted condition indicate a trend for faster reaction times in the active group post treatment (t(19) = −1.97, p = 0.06). The groups did not differ on reaction time pre-treatment (p>0.10). Judging eye gaze is a known deficit among individuals with FXS, thus, as in our previous study, (Bruno et al., 2014) primary fMRI analysis included all individuals (who passed fMRI quality control as described above) and parallel analyses were conducted excluding participants who 1) did not respond on more than 40% of trials and 2) responded by pressing either button 1 or button 2 for all trials. Four participants (3 in the active group and 1 in the placebo group) were excluded based on these criteria.
Figure 4.
fMRI results Top panel: Group level performance for judging eye gaze during fMRI task. Squares represent mean values and error bars indicate standard error. RT = reaction time. Bottom panel: Group difference in brain activation after treatment with donepezil. Note that comparison of group activation differences pre-treatment revealed no significant group difference (p<0.05, FWE). Brain activation patterns at follow up shown in A) sagittal, B) coronal and C) axial views. In B and C left side of images = left side of brain. Yellow color indicates regions for which individuals in the placebo group demonstrated greater activation relative to the active group (T values for direct>averted gaze stimuli, height threshold = 0.001, corrected for multiple comparison, FWE < 0.05). Cross hair corresponds to peak at x=−18, y=36, z=40. Sub cluster peaks were found at x=−22, y=24, z=34 and x=−10, y=34, z=36.
At baseline, there were no significant group differences in brain activation (active vs. placebo) when responses to direct and averted gaze stimuli were contrasted (p>0.10, corrected for family-wise error rate, (FWE)). This result remained consistent when individuals were excluded due to performance as indicated above.
At endpoint, the active group demonstrated significantly less activation than the placebo group when responses to direct and averted gaze stimuli were contrasted (direct>averted, height threshold=0.001, corrected for multiple comparison, FWE<0.05). The activation cluster corresponding to this group difference was located in the left superior frontal gyrus (peak x=−18, y=36, z=40, Figure 4.) This cluster remained significant when individuals were excluded for performance as indicated above. There was no significant gaze by group by time interaction (FWE corrected p>0.10) using a whole brain approach. A post hoc region of interest analysis indicated a significant group by time interaction (F(1,16) = 8.63, p = 0.01) within the cluster demonstrating a group difference at study endpoint (Supplementary Figure 1). Follow up t-tests revealed a trend for decreasing activation for the active group (t(10) = 1.91, p = 0.085) and increasing in activation for the placebo group (t(6) = −2.81, p = 0.031). As with analysis of the behavioral responses, the ANOVA and follow-up t-tests included only individuals with usable fMRI data at baseline and endpoint. Finally, we explored within-group correlations between brain activation for this significant cluster and change in CNT and ABC scores which revealed no significant relationships (all p’s >0.10).
There were no group differences that survived FWE correction (p<0.05) at baseline or endpoint when contrasting responses to all gaze stimuli with fixation periods (averted + direct>fixation) or when contrasting responses to each gaze stimuli with fixation (direct>fixation and averted>fixation). However, with a relaxed threshold (p<0.001 uncorrected), the active group demonstrated less activation than the placebo group for the direct gaze>fixation contrast in the left superior frontal gyrus at endpoint. No other differences relative to fixation were revealed with a similar relaxed threshold. This result remained consistent when individuals were excluded for performance as indicated above. We also performed an exploratory post-hoc region of interest analysis using 5 ROIs reported previously (Bruno, et al., 2014) as demonstrating differences between individuals with FXS and an age- and symptom-matched comparison group. We did not observe treatment-specific differences in brain activity for any of the examined ROIs (anterior cingulate, right angular gyrus, left middle/lateral occipital gyrus, left fusiform and left frontal cortex, Brodmann Area 6).
Discussion
The present study sought to assess the cognitive, behavioral and neuro-functional changes associated with a targeted intervention in individuals with the FMR1 full mutation. In this double-blind, placebo-controlled treatment study the pharmacological agent donepezil, an acetylchoenesterase inhibitor, was used to enhance cholinergic signaling. Findings did not reveal significant improvements in cognition or behavior following twelve weeks of treatment with donepezil. However, our neuroimaging results provide evidence for change in neural circuitry as measured by functional brain activation patterns post treatment. Brain activation patterns may represent a sensitive, objective and early predictive measure of treatment response that is independent of change in cognition/behavior. In particular, brain imaging outcomes may help us understand the initial response to pharmacological treatment and, as such, could be useful in planning and carrying out future treatment studies.
The primary and secondary outcomes of the present study indicated that executive function measured by CNT and problem behaviors measured by the ABC did not show improvement in response to treatment with donepezil. Specifically, we did not demonstrate a significant group difference between active and placebo groups post treatment, when controlling for pretreatment differences. These results are in contrast to our open label treatment study where improvements in both CNT performance and reduction in problem behaviors measured by ABC were observed (Kesler et al., 2009). However, the open label study’s conclusions regarding the effect of Donepezil should be interpreted with caution due to the lack of a placebo group and potential practice effects. The baseline performance differences (significantly higher CNT performance and a trend for lower ABC scores in the active group) may have obscured potential positive treatment effects. Future research with larger sample sizes would help to avoid pretreatment cognitive/behavioral group differences after randomization. A recent double blind study in a smaller group of individuals with FXS also failed to find significant effects of treatment with donepezil when measuring cognitive outcomes including performance on the Stanford – Binet test of intelligence (Sahu et al., 2013). Possible reasons for negative results in our trial and others are discussed in detail below but here we discuss the importance of considering that cognitive/behavioral symptoms associated with FXS may not be readily altered based on pharmacological treatment alone.
Cognitive and behavioral symptoms in affected individuals are very complicated as they develop progressively in association with education, training and life experiences (Klaiman et al., 2014; Quintin et al., 2015). Accordingly, specific cognitive-behavioral training/intervention may be required (in addition to an “effective” pharmacologic agent) to result in measurable improvement in these complex cognitive and behavioral processes. This is particularly true for adolescents and adults for whom behaviors and habits have been shaped over many years of development. Behavioral treatments have been successful in some individuals with idiopathic autism spectrum disorder (ASD), specifically in terms of improving intellectual and adaptive functioning (Klintwall et al., 2015) as well as reducing problem behaviors (Heyvaert et al., 2014). These behavioral interventions have yet to be thoroughly investigated in individuals with FXS who have a similar yet distinct set of cognitive/behavioral symptoms when compared to individuals with idiopathic ASD. Behavioral interventions may provide the means that permit an affected individual to alter his or her cognition/behavior and thus, will be important in the field of FXS research going forward.
When compared to cognitive or behavioral assessment, neuroimaging represents a more direct measure of change in nervous system functioning. Precedence for using neuroimaging as a treatment outcome has been established in the field of Alzheimer’s disease (Beckett et al., 2011). Within the context of FXS, previous neuroimaging evidence suggests that aberrant brain functioning (as measured by fMRI) may represent an intermediate endophenotype between the downstream effects of reduced/absent FMRP and cognitive/behavioral symptoms (Bruno et al., 2014; Menon et al., 2004). Thus, measurement of brain functioning may be a particularly useful intermediate outcome and may demonstrate response to treatment that precedes cognitive or behavioral changes. In the present study we revealed differences in neural circuitry between the active and placebo groups following treatment and no differences in brain activation at baseline. These results establish preliminary evidence for the effects of donepezil treatment on neural circuitry.
The group differences following treatment indicated significantly less activation in the active group relative to the placebo group within the left superior frontal gyrus for the direct relative to the averted gaze contrast. This region overlaps with a subset of regions for which individuals with FXS demonstrated sensitization to repeated presentations of face/gaze stimuli (Bruno et al., 2014). Reduced activation (for direct vs. averted gaze) in the frontal lobe in the present study, in the active relative to the placebo group, may represent overall less sensitization (less arousal) in response to direct eye gaze in the group receiving donepezil. In support of this interpretation, we did observe less activation for the active relative to the placebo group in the direct gaze vs. fixation contrast (p<0.001, uncorrected). Additionally, our post hoc analysis of activation (direct vs. averted gaze) in the left superior frontal gyrus cluster indicates decreasing activation for the active group and increasing activation for the placebo group. These results suggest that donepezil may be effective at normalizing the aberrant neurophenotype previously associated with FXS (Bruno et al., 2014). This interpretation is also consistent with recent findings that application of a cholinergic agonist enhanced PFC connectivity in the Fmr1 KO mouse (Luongo et al., 2016). An alternative interpretation is that the increased activation in the active group reflects increasing regulation of negative stimuli (direct eye gaze) as the left superior frontal gyrus is involved in regulation of negative affect (Mak et al., 2009).
Our study was limited in that we did not measure eye gaze directly while participants were in the scanner. Furthermore, we did not observe a significant gaze by group by time interaction due to significantly reduced sample size of individuals completing longitudinal MRI scanning. We also did not reveal any relationship between brain activation and change in ABC or CNT scores although this is likely due to lack of significant change in cognition or behavior. Follow-up work will be important to fully understand donepezil’s effects on cortical circuitry, cognition and behavior in individuals with FXS. Studies have demonstrated that donepezil treatment is associated with changes in fMRI activation patterns for individuals with Alzheimer’s (Goveas et al., 2011; Kircher et al., 2005) and mild cognitive impairment (Petrella et al., 2009). Given that these studies used much different task paradigms and patent populations than those used in the present study, comparison of the specific activation pattern changes is not appropriate. However, these studies do show changes in activation that suggest a normalization of a potential aberrant neural phenotype, which is in line with our current findings. Further investigation of the validity and reliability of fMRI as a treatment outcome is warranted.
As argued previously (Budimirovic et al., 2017), choosing an appropriate treatment outcome measure is a critical decision in clinical trials. While biomarkers may represent sensitive treatment endpoints, demonstration of change in brain functioning is not sufficient to demonstrate the clinical utility of a treatment. Ultimately, we seek to demonstrate that a target is effective in ameliorating the symptoms associated with the specific disorder. The complicated cognitive/behavioral symptoms associated with FXS may require concurrent pharmacological and cognitive-behavioral intervention. Combining a pharmacological treatment with a cognitive-behavioral intervention may represent the most promising treatment strategy; for example, and this strategy has proven effective in other disorders where monotherapies were not successful (Taylor et al., 2017). Our results indicating a treatment-specific change in brain functioning provide evidence for utilizing donepezil as a pharmacological agent in a combined therapy.
Several other factors may have contributed to our lack of significant treatment effects on cognition and behavior. First, we targeted only one of several neurotransmitter systems known to be affected in FXS (Davidovic et al., 2011). A treatment that targets multiple neurotransmitter systems (or a combination of several pharmacological treatments) may be required. Second, correcting cholinergic dysfunction at a relatively late phase in brain development (adolescence/young adulthood) may not be sufficient to result in changes in cognition or behavior. It is highly likely that cholinergic dysfunction contributes to shaping FXS-specific brain development and correction of these deficits at a very early stage would be required to induce cognitive/behavioral changes. The 12-week duration of the present study may not have been sufficiently long to allow for improvements in cognition and behavior. Finally, including individuals taking other medications was a necessary limitation of the present study (Table 2). We excluded individuals who were taking medications known to have an effect on the cholinergic system and the active and placebo groups did not differ in the number of individuals taking medications overall or in each class (all p’s >0.10). Therefore, is unlikely that medication use drove group differences in the fMRI outcome or obscured actual differences in the cognitive/behavioral outcomes.
The present study represents the first pharmacological treatment trial in individuals with FXS to include a functional neuroimaging outcome. We report negative cognitive and behavioral outcomes following treatment with donepezil, yet we demonstrate a treatment-specific pattern of brain activation at study endpoint. Importantly, the brain activation patterns following treatment with donepezil may indicate normalization of an aberrant neuroimaging phenotype (i.e. sensitization of brain responses) previously associated with FXS. Additional studies will be required to understand the potential therapeutic effect of donepezil for individuals with FXS. Improving cognitive and behavioral symptoms associated with the disorder remains the primary end goal of targeted therapies. However, brain-imaging metrics that capture intermediate phenotypes of the disorder, such as the functional imaging outcome presented here, represent a promising strategy for measuring initial response. Brain response to pharmacological treatments could also help to inform future studies aimed at combining pharmacological with behavioral therapies.
Data availability
Anonymized data will be shared following reasonable request from a qualified investigator. Requests can be made via email to the corresponding author.
Supplementary Material
Acknowledgements:
We gratefully acknowledge the support of The Lucas Service Center at Stanford, and the families who participated in this research.
Funding: Grant support was provided by the NIH (R34-MH085899, ALR) and Autism Speaks (ALR). SMH’s effort was supported in part by Stanford Spectrum Child Health, Pilot Early Career Award, Brain & Behavior Foundation, NARSAD Young Investigator Award and NIH Career Development Award (K25-AG050759).
Appendix
| Name | Location | Role | Contribution |
|---|---|---|---|
| Jennifer Bruno, PhD | Stanford University | Author | Analyzed the data; drafted the manuscript for intellectual content; contributed to data acquisition |
| S.M. Hadi Hosseini | Stanford University | Author | Analyzed the data; drafted the manuscript for intellectual content |
| Amy A. Lightbody, Ph.D. | Stanford University | Author | Involved in the study concept and design; drafted the manuscript for intellectual content |
| Mai K. Manchanda, Psy.D. | Stanford University | Author | Major role in the acquisition of data |
| Allan L. Reiss, M.D. | Stanford University | Author | Design and conceptualized study; analyzed the data; drafted the manuscript for intellectual content |
Footnotes
Supplemental file: Consort checklist
ClinicalTrials.gov Identifier:
Declaration of Interest: Dr. Bruno reports consulting for Balance Pharmaceuticals regarding clinical trial data for research in Down syndrome. The remaining authors report no conflicts of interest.
References:
- Abitbol M, Menini C, Delezoide AL, et al. (1993) Nucleus basalis magnocellularis and hippocampus are the major sites of FMR-1 expression in the human fetal brain. Nature genetics 4(2): 147–53. DOI: 10.1038/ng0693-147. [DOI] [PubMed] [Google Scholar]
- Aman MG, Burrow WH and Wolford PL (1995) The Aberrant Behavior Checklist-Community - Factor Validity and Effect of Subject Variables for Adults in Group Homes. American Journal on Mental Retardation 100(3): 283–292. [PubMed] [Google Scholar]
- Anderson P, Anderson V, Northam E, et al. (2000) Standardization of the Contingency Naming Test (CNT) for school-aged children: A measure of reactive flexibility. Clinical Neuropsychological Assessment 1: 247–273. [Google Scholar]
- Bailey DB, Raspa M, Olmsted M, et al. (2008) Co-occurring conditions associated with FMR1 gene variations: Findings from a national parent survey. American Journal of Medical Genetics, Part A 146(16): 2060–2069. DOI: 10.1002/ajmg.a.32439. [DOI] [PubMed] [Google Scholar]
- Bear MF, Huber KM and Warren ST (2004) The mGluR theory of fragile X mental retardation. Trends in Neurosciences 27(7): 370–377. DOI: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Beckett LA, Ph D, Harvey DJ, et al. (2011) The Alzheimer’s Disease Neuroimaging Initiative: Annual Change in Biomarkers and Clinical Outcomes. Alzheimer’s & Dementia 6(3): 257–264. DOI: 10.1016/j.jalz.2010.03.002.The. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bencherif B, Endres CJ, Musachio JL, et al. (2002) PET imaging of brain acetylcholinesterase using [11C]CP-126,998, a brain selective enzyme inhibitor. Synapse 45(1): 1–9. DOI: 10.1002/syn.10072. [DOI] [PubMed] [Google Scholar]
- Bennetto L, Taylor AK, Pennington BF, et al. (2001) Profile of cognitive functioning in women with the fragile X mutation Neuropsychology 15(2). American Psychological Association (APA): 290–299. DOI: 10.1037/0894-4105.15.2.290. [DOI] [PubMed] [Google Scholar]
- Berry-kravis E, Hagerman R, Visootsak J, et al. (2017) Arbaclofen in fragile X syndrome : results of phase 3 trials. Journal of Neurodevelopmental Disorders: 1–18. DOI: 10.1186/s11689-016-9181-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry-Kravis E (2014) Mechanism-based treatments in neurodevelopmental disorders: Fragile X syndrome Pediatric Neurology 50(4). Elsevier Inc: 297–302. DOI: 10.1016/j.pediatrneurol.2013.12.001. [DOI] [PubMed] [Google Scholar]
- Berry-Kravis E, Des Portes V, Hagerman R, et al. (2016) Mavoglurant in fragile X syndrome: Results of two randomized, double-blind, placebo-controlled trials. Science translational medicine 8(321): 321ra5 DOI: 10.1126/scitranslmed.aab4109. [DOI] [PubMed] [Google Scholar]
- Bourgeois JA, Farzin F and Brunberg JA (2006) Dementia with mood symptoms in a fragile X premutation carrier with the fragile X-associated tremor/ataxia syndrome: clinical intervention with donepezil and …. Journal of …: 171–177. [DOI] [PubMed] [Google Scholar]
- Bruno J, Shelly E, Quintin E-M, et al. (2013) Aberrant basal ganglia metabolism in fragile X syndrome: a magnetic resonance spectroscopy study Journal of Neurodevelopmental Disorders 5(1). Springer Nature: 20 DOI: 10.1186/1866-1955-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno JL, Garrett AS, Quintin EM, et al. (2014) Aberrant face and gaze habituation in fragile X syndrome. American Journal of Psychiatry 171(10): 1099–1106. DOI: 10.1176/appi.ajp.2014.13111464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budimirovic DB, Berry-Kravis E, Erickson CA, et al. (2017) Updated report on tools to measure outcomes of clinical trials in fragile X syndrome. Journal of Neurodevelopmental Disorders 9(1). Journal of Neurodevelopmental Disorders: 14 DOI: 10.1186/s11689-017-9193-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S, Bray SM, Li Z, et al. (2008) Identification of small molecules rescuing fragile X syndrome phenotypes in Drosophila. Nature chemical biology 4(4): 256–63. DOI: 10.1038/nchembio.78. [DOI] [PubMed] [Google Scholar]
- Cornish K, Scerif G and Karmiloff-Smith A (2007) Tracing syndrome specifit trajectories of attention across the life span. Cortex 43: 672–685. [DOI] [PubMed] [Google Scholar]
- Cubo E, Fernndez Jaén A, Moreno C, et al. (2008) Donepezil use in children and adolescents with tics and attention-deficit/hyperactivity disorder: An 18-week, single-center, dose-escalating, prospective, open-label study. Clinical Therapeutics 30(1): 182–189. DOI: 10.1016/j.clinthera.2008.01.010. [DOI] [PubMed] [Google Scholar]
- D’Hulst C and Kooy RF (2009) Fragile X syndrome: from molecular genetics to therapy. Journal of Medical Genetics 46(9). BMJ: 577–584. DOI: 10.1136/jmg.2008.064667. [DOI] [PubMed] [Google Scholar]
- Davidovic L, Navratil V, Bonaccorso CM, et al. (2011) A metabolomic and systems biology perspective on the brain of the Fragile X syndrome mouse model. Genome Research 21: 2190–2202. DOI: 10.1101/gr.116764.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng P, Zhang Y and Xu ZC (2007) Involvement of Ih in Dopamine Modulation of Tonic Firing in Striatal Cholinergic Interneurons. Journal of Neuroscience 27(12): 3148–3156. DOI: 10.1523/JNEUROSCI.5535-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein JN, Casey BJ, Tonev ST, et al. (2007) Assessment and prevention of head motion during imaging of patients with attention deficit hyperactivity disorder Psychiatry Research: Neuroimaging 155(1). Elsevier BV: 75–82. DOI: 10.1016/j.pscychresns.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey CA, Gross D and Freeman L (1991) Assessing Psychotropic Medication Side Effects Among Children A Reliability Study. Journal of Child and Adolescent Psychiatric Nursing. DOI: 10.1111/j.1744-6171.1991.tb00509.x. [DOI] [PubMed] [Google Scholar]
- Goveas JS, Xie C, Ward BD, et al. (2011) Recovery of hippocampal network connectivity correlates with cognitive improvement in mild Alzheimer’s disease patients treated with donepezil assessed by resting-state fMRI. Journal of Magnetic Resonance Imaging 34(4): 764–773. DOI: 10.1002/jmri.22662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Boyett-Anderson JM, Menon V, et al. (2004) Reduced basal forebrain and hippocampal activation during memory encoding in girls with fragile X syndrome NeuroReport 15(10). Ovid Technologies (Wolters Kluwer Health): 1579–1583. DOI: 10.1097/01.wnr.0000134472.44362.be. [DOI] [PubMed] [Google Scholar]
- Hagerman R, Hoem G and Hagerman P (2010) Fragile X and autism: Intertwined at the molecular level leading to targeted treatments. Mol Autism 2010/09/23(1): 12 DOI: 2040-2392-1-12 [pii] 10.1186/2040-2392-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall S, DeBernardis M and Reiss A (2006) Social Escape Behaviors in Children with Fragile X Syndrome. Journal of Autism and Developmental Disorders 36(7): 935–947. DOI: 10.1007/s10803-006-0132-z. [DOI] [PubMed] [Google Scholar]
- Hardan AY and Handen BL (2002) A Retrospective Open Trial of Adjunctive Donepezil in Children and Adolescents with Autistic Disorder. Journal of Child and Adolescent Psychopharmacology 12(3): 237–241. DOI: 10.1089/104454602760386923. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME and Sarter M (2011) Modes and models of forebrain cholinergic neuromodulation of cognition Neuropsychopharmacology 36(1). Nature Publishing Group: 52–73. DOI: 10.1038/npp.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessl D, Glaser B, Dyer-Friedman J, et al. (2006) Social behavior and cortisol reactivity in children with fragile X syndrome. Journal of Child Psychology and Psychiatry 47(6): 602–610. DOI: 10.1111/j.1469-7610.2005.01556.x. [DOI] [PubMed] [Google Scholar]
- Hessl D, Berry-Kravis E, Cordeiro L, et al. (2009) Prepulse inhibition in fragile X syndrome: Feasibility, reliability, and implications for treatment. American Journal of Medical Genetics, Part B: Neuropsychiatric Genetics 150(4): 545–553. DOI: 10.1002/ajmg.b.30858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyvaert M, Saenen L, Campbell JM, et al. (2014) Efficacy of behavioral interventions for reducing problem behavior in persons with autism: An updated quantitative synthesis of single-subject research. Research in Developmental Disabilities. DOI: 10.1016/j.ridd.2014.06.017. [DOI] [PubMed] [Google Scholar]
- Hooper SR, Hatton D, Sideris J, et al. (2018) Developmental trajectories of executive functions in young males with fragile X syndrome Research in Developmental Disabilities 81(May). Elsevier: 73–88. DOI: 10.1016/j.ridd.2018.05.014. [DOI] [PubMed] [Google Scholar]
- Hustyi KM, Hall SS, Jo B, et al. (2014) Research in Developmental Disabilities Longitudinal trajectories of aberrant behavior in fragile X syndrome Research in Developmental Disabilities 35(11). Elsevier Ltd.: 2691–2701. DOI: 10.1016/j.ridd.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR (2014) The NIMH Research Domain Criteria (RDoC) Project: Precision Medicine for Psychiatry. American Journal of Psychiatry 171(4): 395–397. DOI: 10.1007/s00335-013-9476-9. [DOI] [PubMed] [Google Scholar]
- Jacquemont S, Curie A, des Portes V, et al. (2011) Epigenetic Modification of the FMR1 Gene in Fragile X Syndrome Is Associated with Differential Response to the mGluR5 Antagonist AFQ056 Science Translational Medicine 3(64). American Association for the Advancement of Science (AAAS): 64ra1–64ra1. DOI: 10.1126/scitranslmed.3001708. [DOI] [PubMed] [Google Scholar]
- Kesler SR, Lightbody A a and Reiss AL (2009) Cholinergic dysfunction in fragile X syndrome and potential intervention: A preliminary 1 H MRS study. American Journal of Medical Genetics Part A 149A(3): 403–407. DOI: 10.1002/ajmg.a.32697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircher TJ, Erb M, Grodd W, et al. (2005) Cortical Activation During Cholinesterase-Inhibitor Treatment in Alzheimer Disease Preliminary Findings From a Pharmaco-fMRI Study American Journal of Geriatric Psychiatry 13(11). American Association for Geriatric Psychiatry: 1006–1013. DOI: 10.1097/00019442-200511000-00012. [DOI] [PubMed] [Google Scholar]
- Klaiman C, Quintin E-M, Jo B, et al. (2014) Longitudinal profiles of adaptive behavior in fragile X syndrome. Pediatrics 134(2): 315–24. DOI: 10.1542/peds.2013-3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klintwall L, Eldevik S and Eikeseth S (2015) Narrowing the gap: Effects of intervention on developmental trajectories in autism. Autism 19(1): 53–63. DOI: 10.1177/1362361313510067. [DOI] [PubMed] [Google Scholar]
- Liang YQ and Tang XC (2004) Comparative effects of huperzine A, donepezil and rivastigmine on cortical acetylcholine level and acetylcholinesterase activity in rats Neuroscience Letters 361(1–3): 56–59. DOI: 10.1016/j.neulet.2003.12.071. [DOI] [PubMed] [Google Scholar]
- Lightbody AA, Hall SS and Reiss AL (2006) Chronological age, but not FMRP levels, predicts neuropsychological performance in girls with fragile X syndrome Am. J. Med. Genet 141B(5). Wiley-Blackwell: 468–472. DOI: 10.1002/ajmg.b.30307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S-C, Brown RE, Hussain Shuler MG, et al. (2015) Optogenetic Dissection of the Basal Forebrain Neuromodulatory Control of Cortical Activation, Plasticity, and Cognition. Journal of Neuroscience 35(41): 13896–13903. DOI: 10.1523/JNEUROSCI.2590-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luongo FJ, Horn ME and Sohal VS (2016) Putative microcircuit-level substrates for attention are disrupted in mouse models of autism Biological Psychiatry 79(8). Elsevier: 667–675. DOI: 10.1016/j.biopsych.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak AKY, Hu Z guo, Zhang JX, et al. (2009) Neural correlates of regulation of positive and negative emotions: An fMRI study. Neuroscience Letters 457(2): 101–106. DOI: 10.1016/j.neulet.2009.03.094. [DOI] [PubMed] [Google Scholar]
- Marshall WA and Tanner JM (1969) Variations in pattern of pubertal changes in girls. Archives of Disease in Childhood 44(235): 291–303. DOI: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WA and Tanner JM (1970) Variations in pattern of pubertal changes in boys. Obstetrical & Gynecological Survey 45(13): 13–23. DOI: 10.1097/00006254-197007000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, Quintin E-M, Hall SS, et al. (2016) The Role of Executive Function in Independent Living Skills in Female Adolescents and Young Adults With Fragile X Syndrome. American Journal on Intellectual and Developmental Disabilities 121(5): 448–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Leroux J, White CD, et al. (2004) Frontostriatal deficits in fragile X syndrome: relation to FMR1 gene expression. Proceedings of the National Academy of Sciences of the United States of America 101(10): 3615–3620. DOI: 10.1073/pnas.0304544101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura N, Funaki Y, Tashiro M, et al. (2008) In vivo visualization of donepezil binding in the brain of patients with Alzheimer’s disease. British Journal of Clinical Pharmacology 65(4): 472–479. DOI: 10.1111/j.1365-2125.2007.03063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmos-Serrano JL, Paluszkiewicz SM, Martin BS, et al. (2010) Defective GABAergic Neurotransmission and Pharmacological Rescue of Neuronal Hyperexcitability in the Amygdala in a Mouse Model of Fragile X Syndrome Journal of Neuroscience 30(29). Society for Neuroscience: 9929–9938. DOI: 10.1523/jneurosci.1714-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrella JR, Prince SE, Krishnan S, et al. (2009) Effects of donepezil on cortical activation in mild cognitive impairment: A pilot double-blind placebo-controlled trial using functional MR imaging. American Journal of Neuroradiology 30(2): 411–416. DOI: 10.3174/ajnr.A1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pop AS, Levenga J, De Esch CEF, et al. (2014) Rescue of dendritic spine phenotype in Fmr1 KO mice with the mGluR5 antagonist AFQ056/Mavoglurant. Psychopharmacology 231(6): 1227–1235. DOI: 10.1007/s00213-012-2947-y. [DOI] [PubMed] [Google Scholar]
- Quintin E-M, Jo B, Hall SS, et al. (2015) The cognitive developmental profile associated with fragile X syndrome: A longitudinal investigation of cognitive strengths and weaknesses through childhood and adolescence. Development and Psychopathology 8: 1–13. DOI: 10.1017/S0954579415001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reches A, Laufer I, Ziv K, et al. (2014) Network dynamics predict improvement in working memory performance following donepezil administration in healthy young adults. NeuroImage 88 Elsevier Inc.: 228–241. DOI: 10.1016/j.neuroimage.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu JK, Gulati S, Sapra S, et al. (2013) Effectiveness and Safety of Donepezil in Boys With Fragile X Syndrome: A Double-Blind, Randomized, Controlled Pilot Study. Journal of Child Neurology 28(5): 570–575. DOI: 10.1177/0883073812449381. [DOI] [PubMed] [Google Scholar]
- Sansone SM, Widaman KF, Hall SS, et al. (2012) Psychometric study of the aberrant behavior checklist in fragile X syndrome and implications for targeted treatment. Journal of Autism and Developmental Disorders 42(7): 1377–1392. DOI: 10.1007/s10803-011-1370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanger S a and Bassell GJ (2011) Making and breaking synapses through local mRNA regulation Current Opinion in Genetics & Development 21(4). Elsevier Ltd: 414–421. DOI: 10.1016/j.gde.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JH, Lebowitz ER, Jakubovski E, et al. (2017) Monotherapy Insufficient in Severe Anxiety? Predictors and Moderators in the Child/Adolescent Anxiety Multimodal Study Journal of Clinical Child and Adolescent Psychology 00(00). Routledge: 1–16. DOI: 10.1080/15374416.2017.1371028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeraragavan S, Bui N, Perkins JR, et al. (2011) The modulation of fragile X behaviors by the muscarinic M4 antagonist, tropicamide. Behavioral neuroscience 125(5): 783–90. DOI: 10.1037/a0025202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeraragavan S, Graham D, Bui N, et al. (2012) Genetic reduction of muscarinic M4 receptor modulates analgesic response and acoustic startle response in a mouse model of fragile X syndrome (FXS) Behavioural brain research 228(1). Elsevier B.V.: 1–8. DOI: 10.1016/j.bbr.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson C, Hoeft F, Garrett AS, et al. (2008) Aberrant brain activation during gaze processing in boys with fragile X syndrome. Archives of general psychiatry 65(11): 1315–1323. DOI: 10.1001/archpsyc.65.11.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (1999) Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corperation. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized data will be shared following reasonable request from a qualified investigator. Requests can be made via email to the corresponding author.