Abstract
Objectives:
Quaternary ammonium (QA) methacrylate monomers have been extensively investigated and demonstrate excellent antibacterial properties. However, the presence of ester bonds makes them prone to degradation in the oral cavity. In this study, ester-free QA monomers based on meth-acrylamides were synthesized and screened for polymerization kinetics, mechanical properties and antibacterial effects.
Materials and Methods:
Tertiary quaternary ammonium acrylamides (AM) and methacrylamides (MAM) with alkyl side chain lengths of 9 and 14 carbons (C9 and C14) were synthesized and incorporated at 10 wt% into experimental composites based on BisGMA:TEGDMA (1:1), camphorquinone/ethyl-4-dimethylaminobenzoate (0.2/0.8 wt%) and 70 wt% barium glass fillers. Analogous methacrylate versions (MA) were used as controls. Degree of conversion (DC) and rate of polymerization (RP) during photoactivation (800 mW/cm2) were followed in real-time with near-IR. Flexural Strength (FS) and Modulus (E) were measured on 2×2×25 mm bars in 3-point bending after 24h dry storage and 7-day storage in water at 37°C. Antimicrobial properties and biofilm adhesion (fouling) were evaluated by bioluminescence (Luciferase Assay) and biofilm removal by water spray microjet impingement test, respectively. Cytotoxicity was assessed by MTT assay on dental pulp stem cells (DPSC). Data were analyzed with one-way ANOVA/Tukey’s test (α=0.05).
Results:
DC was similar for all groups tested (~70%). Both MAMs and C14-AM presented significantly lower RP. Under dry conditions, FS (110–120 MPa) and E (8–9 GPa) were similar for all groups. After water storage, all materials presented FS/E similar to the control, except for C14-AM (for FS) and C14-MAM (for E), which were lower. All C14 versions were strongly antibacterial, decreasing the titer counts of biofilm by more than two orders of magnitude in comparison to the control. C9 monomers did not present significant antibacterial nor antifouling properties. And biofilms had approximately equivalent adhesion on the C9 composites as on the control. Cytotoxicity did not show significant differences between the MA and AM versions and the control group.
Conclusions:
C14-QA monomers based on methacrylates and meth-acrylamides present strong antibacterial properties, and in general, similar conversion/mechanical properties compared to the methacrylate control.
Keywords: antimicrobial materials, polymerization, dental adhesives, S. mutans, Biofilm
Graphical Abstract
1. INTRODUCTION
The lifetime of resin composite dental restorations remains much shorter than desirable, and more than fifty percent of the restorations require replacement in less than 10 years [1]. Biofilm colonization of restorative surfaces is thought to be one of the main issues leading to secondary caries, which in turn is one of the leading causes for replacement [2]. The addition of quaternary ammonium methacrylates (QAM’s) into the resin composite and dental adhesive compositions has been proposed as an anti-bacterial strategy [3]. The main advantage with this system is that it is possible to co-polymerize the antimicrobial monomer with the main organic matrix in the dental composite, which in theory precludes the release of the compounds [4]. This is advantageous because the presence of leachates presents cytotoxicity concerns, decreased mechanical properties over time, and only short-term effectiveness (assuming re-charging is not effective) [4].
As a cationic antimicrobial agent, the QAM’s biocidal effect is related to a high binding affinity with bacterial cell walls [5, 6]. In general, these compounds are composed of quaternary nitrogens associated with a long-chain alkylic substituent that is responsible for piercing and disrupting the cell envelope [4]. The length of the side alkyl chain has been demonstrated to greatly influence the antimicrobial effect, especially with gram-positive species [3, 6]. Longer carbon side chain lengths (up to 16–18 carbons) have been associated with stronger antimicrobial properties due to increased hydrophobicity and charged area, which enhances the likelihood of interaction and penetration of the side chain into the hydrophobic bacterial membrane [5, 6] leading to its physical disruption [7]. Side chains longer than the critical threshold, conversely, have been associated with less pronounced antibacterial effect, and this is due to the likelihood of curling, which in turn may sterically hinder the positively charged quaternary ammonium groups and block electrostatic interactions [6].
To date, quaternary ammonium monomers have been made polymerizable by the addition of ester-containing methacrylate functionalities, which are highly susceptible to enzymatic and hydrolytic degradation [8]. In an attempt to produce more degradation resistant antimicrobial polymers, the substitution of the ester groups with other functionalities, such as methacrylamides, seems to be an opportune approach [8]. Acrylamides have been used for quite some time in many other applications, such as papermaking, as stabilizers for emulsion polymerization in cosmetics, and as flocculent and biocides in wastewater treatment [9]. By combining the antimicrobial potential of quaternary ammonium monomers with the hydrolytic stability of methacrylamides, it is possible to envision monomers for dental applications that would be able to attract and kill the bacteria [6, 7]. Combined with the appropriate length of alkylic side chain, the monomers would also have cell-bursting capability, especially needed for antibacterial effect against gram-positive species [10]. Finally, the combination of a degradation-resistant polymerizable functionality would increase polymer longevity.
Therefore, the aim of this study was to synthesize quaternary ammonium methacrylate, acrylamide and methacrylamide compounds with side alkyl chain lengths of 9 or 14 carbons and characterize them for their potential use as additives for resin composites and adhesives by studying their kinetics of polymerization, mechanical properties, antimicrobial and antifouling properties. The tested hypothesis is that the antimicrobial effect will be potentiated in acrylamides and methacrylamides with longer chains without significantly compromising the mechanical properties and the polymerization reaction.
2. MATERIALS & METHODS
2.1. Compound synthesis
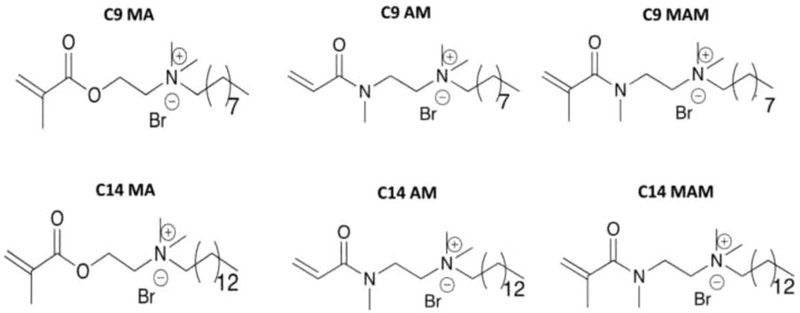
Unless otherwise stated, all reagents and solvents were purchased from commercial suppliers and used without further purification. Quaternary ammonium monomers containing 9 or 14-carbon chain lengths functionalized with methacrylate (MA), acrylamide (AM), and methacrylamide (MAM) (Figure 1) were synthesized using the procedure detailed in the supplemental materials. Solvents were of analytical grade. All reactions were conducted in standard, dry glassware and under an inert atmosphere of nitrogen unless otherwise noted. 13C-NMR and 1H-NMR spectra were recorded on a Bruker AMX-400 MHz spectrometer using CDCl3 and D2O at 25 °C. 1H-NMR chemical shifts are reported in ppm relative to CHCl3 (7.26) in CDCl3, H2O (4.79) in D2O and Me4Si (TMS) was used as internal standard. Chemical shifts are reported as δ values in parts per million (ppm) and coupling constants (J) are reported in Hertz. Fourier transform-Infrared (FT-IR) spectra were recorded on a Thermo Scientific Nicolet spectrophotometer. Solid samples were obtained using KBr pellets and liquid samples were obtained using NaCl crystals. Melting points were recorded on Electrothermal MET-TEMP capillary melting point apparatus and are uncorrected. Spectra were recorded over the 400–4000 cm−1 range without base line corrections. High Resolution Mass Spectrometer Electrospray Ionization HRMS (ESI) was recorded on a high-resolution (30000) LTQ-Orbitrap mass spectrometer. All mass spectrometer samples were dissolved in a MeOH/H2O (1:9) mixture.
Figure 1.
Quaternary ammonium monomers containing 9 or 14-carbon chain length functionalized with methacrylate (MA), acrylamide (AM), and methacrylamide (MAM) synthesized and tested in this study.
2.2. Composites
To prepare the composites, QA monomers were mixed at 10 wt% with Bisphenol A Glycidyl Methacrylate (BisGMA) and triethylene glycol dimethacrylate (TEGDMA) (50:50 wt%), both from Esstech Inc. (Esstech Inc., Essington, PA, USA). Filler was added at 70 wt% (methacrylate silanized barium silicate glass particles, 0.7 μm, Esstech Inc.). Materials were made photopolymerizable by the addition of camphoroquinone andethyl-4-dimethylaminobenzoate (0.2 and 0.8 wt%, respectively), purchased from Sigma Aldrich (Milwaukee, WI, USA). A composition without any QA monomer was added as control. All photocuring procedures were carried out using a Bluephase G2 (Ivoclar Vivadent, Schaan, Liechtenstein) with 10 mm diameter tip and exitant irradiance of 700 mW/cm2.
2.3. Kinetics of polymerization and Degree of Conversion
Discs (8 mm in diameter and 0.8 mm in thickness), sandwiched between two glass slides, were photoactivated for 60 s (n=3) with the light guide placed 2 cm away from the glass slide surface, delivering 350 mW/cm2 to the surface of the specimen. Spectra were collected in real-time during 180 s, with 2 scans per spectrum at 4 cm−1 resolution. The degree of conversion was calculated based on the area of the methacrylate vinyl overtone in near-IR at 6165 cm−1, and the rate of polymerization was calculated as the first derivative of the conversion vs. time curve [11].
2.4. Degradation at low pH
Aqueous solutions with pH values 1, 2 and 4 were prepared using deuterium oxide (D2O, pH 9.8) and adjusted by adding hydrochloric acid (20 w/w % in D2O). 1.2 mL, 60 mM solutions of each of the tested uncured monomers were prepared at 4 different pH values (n = 3). The aqueous solutions were stored at 37°C, except when the NMR experiments were carried out (room temperature, 20–25°C). A 45-pulse was used for NMR observation, with accumulation and repetition times of 1000 and 3.8 s, respectively. The percentage of degradation was calculated based on the appearance of methacrylic acid (MAA), the main degradation product. The integrals of peaks corresponding to the absorption of the vinyl protons in MAA (protons appearing at 6.10 – 6.09 and 5.70 – 5.71 ppm) were measured before and after monomer incubation.
2.5. Mechanical Properties
The flexural strength (FS) and elastic modulus (E) were measured in three-point bending, according to ISO 4049. Twelve rectangular bars (2.0 × 2.0 × 25.0 mm) per group were obtained from metal molds placed between two glass slides. Bars were photoactivated for 20 s in three consecutive and overlapping exposures, directly over one side of the specimen at 800 mW/cm2 (Demi Plus, Dentsply-Sirona, Milford, DE, USA) as assessed with a radiometer (Optilux, SDS-Kerr, Orange, CA, USA). The excess flash material was carefully removed with a scalpel blade and sandpaper. Half of the specimens were subjected to the test after 24 hours dry storage, and the other half after 7 days water storage at room temperature. Specimens were tested at 0.5 mm/min of cross-head speed on a 20 mm span (Criterion, MTS, Eden Prairie, WI, USA). Elastic modulus (GPa) was calculated according to equation 1:
| Equation (1) |
where L is the maximum load (N), D the span between the supports (mm), w the specimen width (mm) and h the specimen height (mm).
Flexural Strength (MPa) was calculated according to equation 2 (4):
| Equation (2) |
2.6. Antimicrobial activity evaluation – Luciferase reporter assay
Sample preparation and bioluminescence assay
Resin composite discs (6 mm in diameter and 2 mm in thickness) were prepared in silicone molds sandwiched between two glass slides and photoactivated for 60 s on each side as described above (n=6). After 24 hours, the top surface was ground with 600 grit SiC paper and the surface roughness was evaluated (Surftest Mitutoyo, Japan – Cut-off length = 0.25mm, tracing speed = 1.0 mm/s, roughness parameter = Ra, and measuring range = Ra. 0.05–10 μm), in order to standardize surface roughness between 2 and 2.5 μm. For the purpose of disinfection and sterilization, the discs were immersed in isopropyl alcohol for 20 minutes. The discs were stored dry for 24 h before the luciferase assay was conducted.
Bioluminescent S. mutans strain IdhRenGSm, a derivative of wild type UA159, was used in this study. This manipulation of recombinant strains was described in a previous study [12]. The strain was started from a frozen stock and, then, the bacteria were streaked out onto an agar plate grown out overnight in a 37°C incubator. Planktonic cultures of IdhRenGSm [13] were grown in TH culture medium supplemented with 0.3% yeast extract (THY) for 16 hours under anaerobic conditions at 37°C. A 1:500 dilution of the inoculum was added to TH biofilm growth medium supplemented with 1.0% (w/v) sucrose. Aliquots (1.0mL) were dispensed into the wells of sterile 48-well plates (Falcon, Corning, USA) containing the sterile discs. Discs in media without inoculum served as the sterility control. The plates were incubated for 24 hours on an orbital shaker agitated at 40 rpm in a CO2 incubator at 37°C. Discs were carefully moved to 24-well black plates (Black Visiplate TC, Wallac, Finland) containing 0.5 ml of fresh media per well and incubated at 37°C for 1 hour. 5 μl of Coelenterazineh ethanol solution was added to each well and the bioluminescence activity measured immediately in a GloMax® Discover Multimode Microplate Reader GM3000 (Promega Corporation). Data are presented in relative light units (RLU).
Biofilm adhesion strength - Impingement test
Immediately after Luciferase Assay, biofilm adhesion strength was tested by the microjet impingement method (MJI) [14]. A 0.20-mm ID nozzle was oriented perpendicular to the disk-shaped specimens at a height of 0.4-mm and dispensed a stream of water for 5 seconds at 0.31 MPa. Biofilms were stained with 2% crystal violet aqueous solution for 2 min and imaged by light microscopy. NIH ImageJ software was used to determine the area of removed material from each biofilm, indicative of loss of adhesion in percentage.
Antimicrobial activity evaluation after extraction of potential leachates
Additional discs of selected resin composite formulations were prepared as described above. The groups showing the best antimicrobial activity (determined to be C14-MA and C14-MAM) were selected and compared with the control (same formulation without antimicrobial monomer). The discs (n=6) were stored in one of the following conditions: (1) dry storage, (2) 24 hours in MiliQ water, and (3) 48 hours in MiliQ water (n=6). The initial mass (M1), the mass after water incubation (M2), and the final mass (M3) were recorded for each sample. Water sorption (WS) and solubility (SL) in μg/mm3 were calculated according to the following equations:
Where V is the volume of the cylindrical specimen (in mm3).
The leachates were analyzed by NMR after water evaporation and resuspension in deuterated chloroform. After mass stabilization, the antimicrobial activity was assessed by Luciferase assay, as described above. This was done to assess the antimicrobial activity in specimens free of potentially unbound quaternary ammonium monomers leaching out of the composite.
2.8. Biocompatibility Assay
Disc-shaped samples 8 mm in diameter and 2 mm in thickness were prepared from silicone molds laminated between two glass slides and photocured for 60 s as described above, from both the bottom and top surfaces (n=5). Dental pulp stem cells (DPSC – Lonza, Basel, Switzerland) were grown in α MEM (Gibco, ThermoFisher Scientific, Waltham, USA) supplemented with L-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin (Sigma-Aldrich), and 10% embryonic stem cell fetal bovine serum (eFBS, ThermoFisher). Culture media was changed every 3 days. Cells were maintained in a humidified incubator (5% CO2, 37°C). After reaching 80% of confluency, cells were treated with 0.05% trypsin and passaged to subsequent T75 culture plates. Only cells from passages 3 to 10 were used. Next, DPSCs were cultured in 24-well plates, seeded at 2.5 × 104 DPSCs/well. After 24 hours, confluency was confirmed, medium changed, and the discs were placed on top of the cell monolayer and incubated for 48 hours. At the end of the incubation time, 50 μl of tetrazolium-based colorimetric assay (MTT) was added in each well and, after 3 hours 500 μl of dimethyl sulfoxide (DMSO). Wells containing cells without discs and only medium were added as positive and medium controls, respectively. The optical density (OD) readings were taken at 570 nm and 630 nm wavelengths and, after subtracting OD630 from OD570, the cell viability (CV - %) was calculated according to the following equation:
| Equation (3) |
where: CV (%) is percentage of cell viability ; ODpositive control is the optical density of the positive control; ODmedium is the optical density of the medium, and ODcells exper is the optical density of the experimental samples.
2.9. Microtensile bond strength of selected groups
The two best materials based on the microbiological, mechanical and degradation assays were selected to be used as fully formulated adhesives and compared to a control. Formulations were tested for kinetics of polymerization (as described above) and microtensile bond strength (μTBS). The tested groups were composed of: 60 wt% BisGMA + 40 wt% HEMA (control), 60% wt% BisGMA + 30 wt% HEMA + 10 wt% C14 MA, and 60% wt BisGMA + 30 wt% HEMA + 10 wt% C14 MAM. As photoinitiators/inhibitors, camphorquinone (0.2 wt%) with ethyl-4-dimethylaminobenzoate (0.8 wt%) and butylated hydroxytoluene (0.2 wt%) were added to the mixtures. For μTBS, 40 vol% of ethanol was included into the adhesive compositions, similar to commercial adhesive formulations.
Eighteen caries-free third human molars were used (n=6). Briefly, after coronal enamel removal, an artificial smear layer was created by abrading the occlusal surface for 30s with a wet #600 SiC paper. The surface was etched with 37% phosphoric acid for 15 s, rinsed and carefully dried in order to keep it moistened. A first layer of the adhesive was actively applied on the dentin surface for 20 s and the solvent evaporated by an air dry jet for 10 s. A second adhesive layer was applied and photocured for 20 s (Bluephase, Ivoclar Vivadent, Schaan, Liechtenstein) delivering 800 mW/cm2 directly above the adhesive layer (~1 mm). Irradiance was measured daily with a radiometer (Optilux, SDS-Kerr, Orange, CA, USA). Resin composite restoration was built up in two 2 mm increments each photocured for 30s. After 24 hours, teeth were cut in a slow speed diamond saw (Accutom, Struers, Cleveland, OH, USA) to obtain sticks of 1 mm2 in cross-sectional area. Half of the sticks were tested for μTBS in the universal testing machine after 24 hours storage on a 500N load cell at 0.5mm/min.
Statistical Analysis
Data for each evaluation was tested for normality (Anderson-Darling) and homoscedasticity (Bartlett/Levene), and then analyzed with one-way ANOVA and Tukey’s test for multiple comparisons for all experimental tests, except for Antimicrobial activity evaluation after leachates extraction and WS/SL data, which were assessed by two-way ANOVA. The significance level was set at α = 0.05.
3. RESULTS
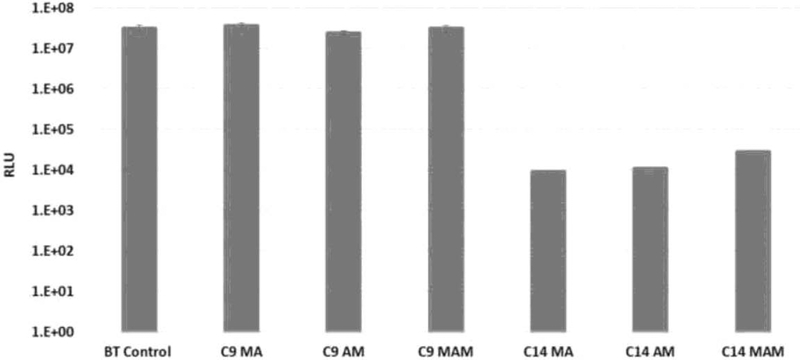
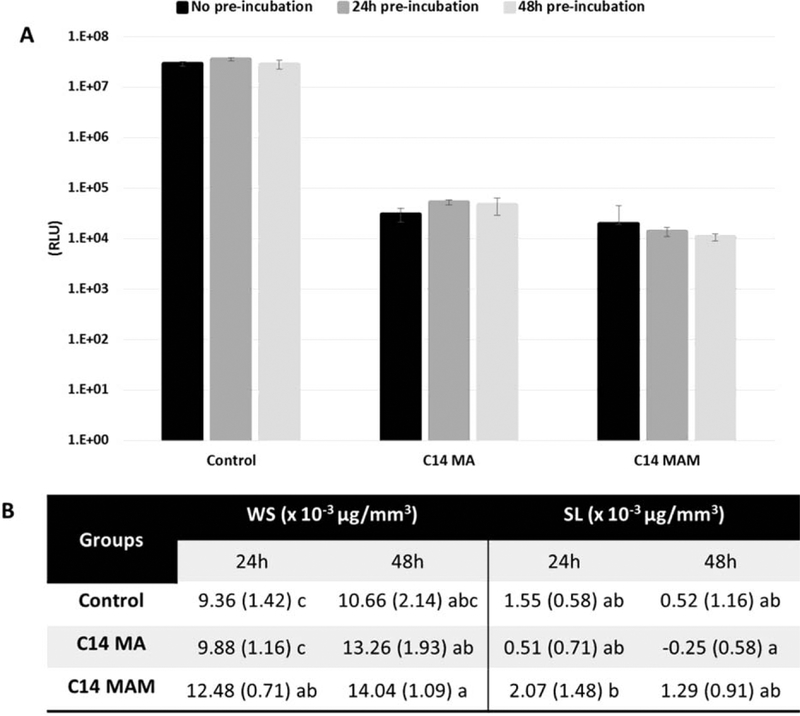
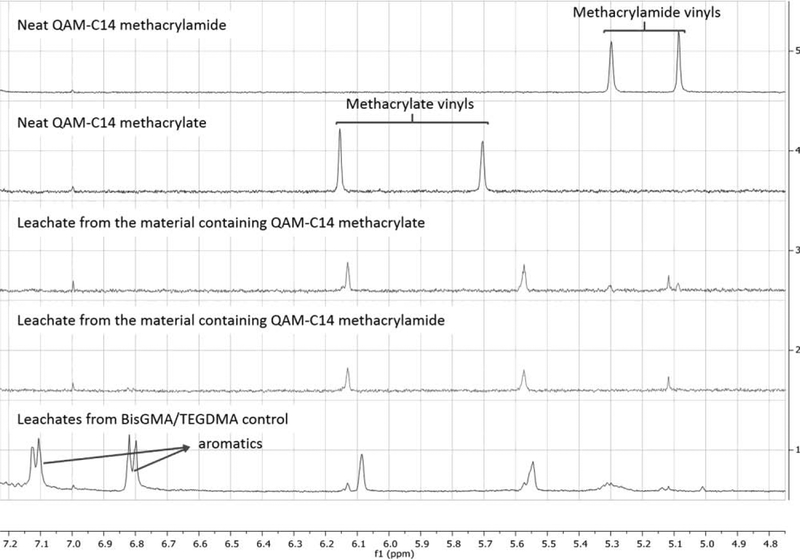
Luciferase Assay results showed statistically greater (p<0.001) antimicrobial properties for all quaternary ammonium monomers with 14 carbons on the side alkyl chain, regardless of the functionality. Among C9 variations, acrylamide and methacrylamide presented significantly lower luciferase activity than the methacrylate, but both were similar to the control (Figure 2). For the compositions analyzed after the extraction of potential leachates from discs incubated in water for 24 and 48h, both tested monomers (C14 MA and MAM) showed significant (p=0.002) reduction in biofilm formation in relation to the control (99.86% and 99.95% respectively) (Figure 3A). The overall mass of leachates extracted was very low, with calculated WS/SL values being statistically similar to the control (p=0.0033 for WS and p=0.0052 for SL). The WS and SL values ranged from 13.5 to 7.8 and from 0.25 to −2.07 μg/mm3, respectively (Figure 3B). The analysis of the extracted compounds by 1H-NMR (method detailed in the supplemental materials) showed the presence of methacrylate peaks only (Figure 4).
Figure 2.
Plots of bacterial bioluminescence activity measured by Luciferase Assay after 24 hours of biofilm growth. Relative light unit (RLU) is the result of the luciferase reaction and proportional to the expression of the luciferase gene and indicates metabolic activity in the biofilms adherent on the composite disks. The results represent the average of 5 disks, with the error bar indicating the standard deviation. Bars with the same letter indicate statistically similar values (p≥0.05).
Figure 3.
(A) Plots of antimicrobial activity assessed by Luciferase Assay after 24 hours of biofilm growth on discs pre-incubated under different conditions. Bars with the same letter indicate statistically similar values. (B) Water Sorption (WS) and Solubility (SL) for control, C14 MA and C14 MAM discs preincubated for 24h or 48h before biofilm growth incubation. Values followed by the same letter within the same test indicate statistically similar values (α=5%).
Figure 4.
Stacked 1H-NMR spectra for (top to bottom): neat QAM-C14 methacrylamide and neat QAMC14 methacrylate (top two spectra), and leachates obtained after immersion of polymerized discs made from materials containing BisGMA/TEGDMA and either QAM-C14 methacrylate, QAM-C14 methacrylamide or no QAM monomer serving as a control (bottom three spectra). The protons corresponding to the vinyls in each of the neat monomers are identified. The leachate spectra demonstrate an absence of methacrylamide vinyl protons.
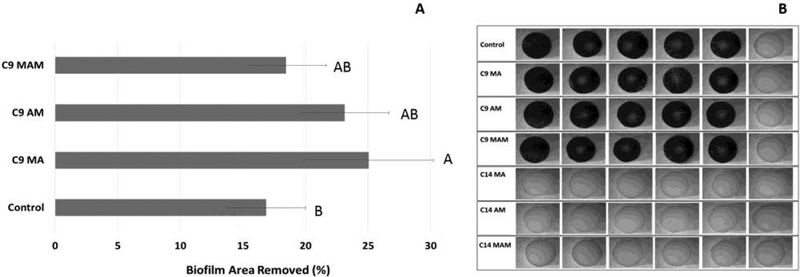
No biofilm adhesion was detected for any of the monomers with 14 carbons on the alkyl side chain. For the C9 versions, the addition of QA methacrylate resulted in statistically lower biofilm adhesion on disk surfaces as compared with the control (25% versus 17%, respectively; p=0.017). Acrylamide and methacrylamide showed intermediate results (Figure 5A). Photographs of the stained specimens for all groups are shown in Figure 5B.
Figure 5.
(A) Biofilm area removed (%) from the disk surfaces after microjet impingement test. Quaternary ammonium salts with longer chain length did not have biofilm adherent on the disks, which made it unnecessary to subject them the impingement test. The results represent the mean (± s.d.) of 4 disks. Disc 1 was not subjected to impingement test and was used as negative control to compare the effectiveness of the method. Bars with the same letter indicate statistically similar values. (B) Biofilm adherent on disks surface after crystal violet staining.
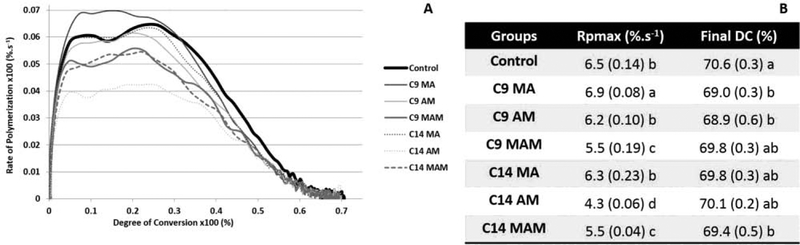
Kinetics of polymerization for the tested materials are shown in Figure 6A. In terms of maximum rate of polymerization (Rpmax), within the same chain length, acrylamide (C9 AM and C14 AM) and methacrylamide (C9 MAM and C14 MAM) versions showed statistically lower values than methacrylates (C9 MA and C14 MA; p<0.001). Resin composites based on methacrylates presented similar or higher Rpmax compared to the control group. The degree of conversion (DC) ranged between 68.9 to 70.1%, and were statistically similar for all groups (Figure 6B).
Figure 6.
(A) Polymerization rate (%.s−1) as a function of DC (%) for experimental resin composites mixed with 10 wt% QA salts. Vinyl conversion was followed in real time for 180 seconds during the photoactivation carried out with 800 mW/cm2. (B) Rpmax (%.s−1) and final DC (%) for all experimental groups. Values followed by the same letter within the same column are statistically similar.
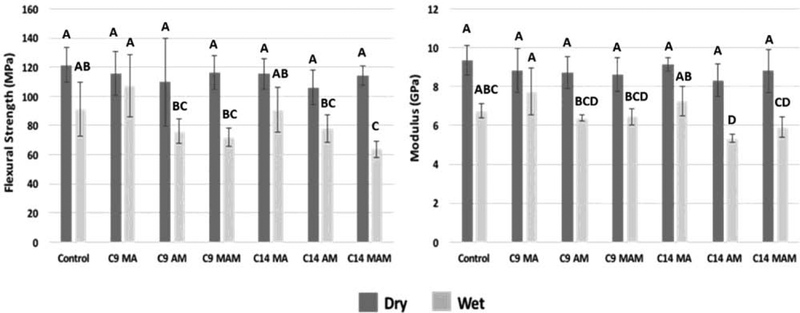
There was no significant difference between groups for flexural strength and elastic modulus after 24 hours dry storage (p=0.653 and p=0.517, respectively). After 7 days water storage, within the same chain length, methacrylates had greater flexural strength and modulus than the methacrylamide and acrylamide versions (p<0.001 and p<0.001, respectively). However, with a few exceptions (FS of C14 MAM and E of C14 AM), all materials showed flexural strength and modulus statistically similar to the control (Figure 7).
Figure 7.
Mean (± s.d.) of six bars for (A) Flexural Strength (MPa) and (B) Modulus of Elasticity after 24 hours dry and 7 days water storage.. Bars with the same letter within the same storage condition are statistically similar.
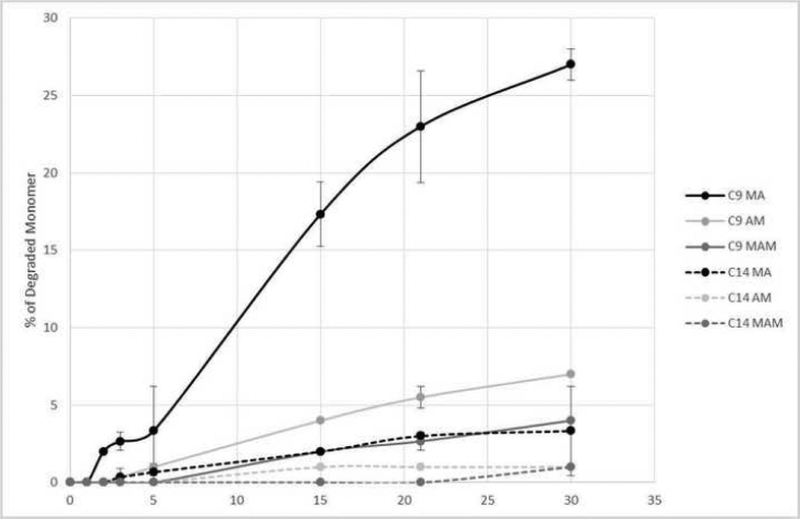
The 1H-NMR analysis of the uncured monomers showed higher degradation for C9 and C14 methacrylate compounds (28.4±0.78% and 3.47±0.63%, respectively) at pH 1 compared with the analogous acrylamide and methacrylamide versions (p<0.05), Figure 8). The degradation of C9 acrylamide and methacrylamide were 8.25 and 3.85 %, respectively. Overall, C14 versions were more stable, with virtually no degradation even after 30 days in extremely acidic conditions, for both amide versions (Figure 8). At pH 2 and 4 all tested monomers showed less than 2% degradation.
Figure 8.
Percentage of degraded quaternized ammonium methacrylate, acrylamide and methacrylamide monomers with 9 and 14 carbon chain lengths at pH 1 during 30 days.
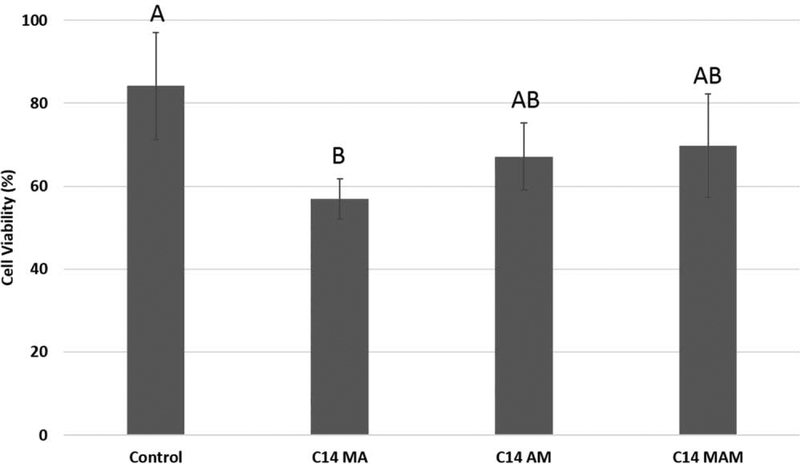
The results of the cytotoxicity assay indicated cell viability of 84%, 56%, 67% and 69% for control, C14 MA, AM, and MAM versions, respectively (Figure 9). The statistical analysis showed significant difference between the groups (p = 0.005), with only C14 MA presenting results significantly lower than the control.
Figure 9.
Effect of cured resin composites containing C14 quaternized monomers and a control formulation without antimicrobial monomer on the proliferation of DPSC cells after 48h-incubation as assessed by the MTT assay. The results are normalized to wells containing cells without discs and bars with the same letter indicate statistically similar values.
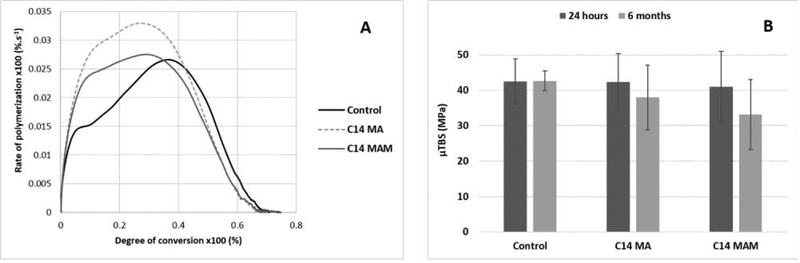
Results for polymerization kinetics and microtensile bond strength of the fully formulated adhesive materials are shown in Figures 10A and B, respectively. No statistical difference was detected among the materials in terms of the final DC. The two QA-containing materials showed lower maximum rate of polymerization in comparison with the control. There were no statistical differences in terms of μTBS at either evaluation time among the materials.
Figure 10.
(A) Rate of polymerization (%.s−1) as a function of degree of conversion (%) for fully formulated adhesive compositions. Control and C14 MAM showed similar maximum rate of polymerization and final degree of conversion (2.66 and 2.74%.s−1 and 74.7 and 74.1%, respectively). C14 MA showed the highest values of maximum rate of polymerization (3.3%.s−1), which did not translate to higher final degree of conversion (71.8%). In terms of degree of conversion at maximum rate of polymerization, the control group showed the highest values (36.7%). (B) Dentin μTBS results after 24 hours and 6 months for the tested adhesives. There was no significant difference between the groups at 24 hours (p = 0.942) or at 6 months (p = 0.149). Additionally, all groups showed statistical similarity between 24 hours and 6 months (p = 0.467, 0.199 and 0.102, for Control, C14 MA and C14 MAM, respectively).
4. DISCUSSION
Resin-based materials are highly susceptible to plaque accumulation, which likely contributes to secondary caries and decreased longevity of the restoration [4, 15]. In an attempt to overcome this issue, studies have focused on the development of antibacterial agents that can be chemically bound within the polymeric carrier via functional polymerizable groups [4]. Quaternary ammonium (QA) compounds have been commonly incorporated in the polymer network to impart antibacterial activity [16]. QA compounds are considered to be broad-spectrum bactericides, with a typical contact-kill antimicrobial mechanism, so there is interest in making such charges permanently available on the surface of dental materials. In this context, QA have been synthesized with methacrylate or acrylate groups incorporated in the structure [7]. As long as the material does not degrade, having the charge immobilized at the surface via the formation of a polymer network decreases the likelihood that the QA compound will leach out, potentially increasing the longevity of the antibacterial effect, and decreasing cytotoxicity concerns [7]. Thus far, ester-containing methacrylates have been used as the polymerizable moiety, which makes them susceptible to hydrolytic and enzymatic degradation [8], compromising the long-term antimicrobial effect. Therefore, the use of more hydrolytically-stable (meth)acrylamides is a logical approach to improve the stability of the antibacterial surface.
Previous studies in the pharmaceutical field showed that a homopolymer of primary amine-containing methacrylamide monomer provided significantly higher antimicrobial activity than that of the poly(methacrylate) version, and this effect was attributed to the increased hydration on the polymer backbone [17, 18]. Surprisingly, in spite of the somewhat common practice in pharmaceutical applications [19–22], the incorporation of methacrylamide functionalities as antimicrobial agents has not been extrapolated to dental materials. In this work, our goals was to combine the stability of (meth)acrylamide compounds to the potent antimicrobial activity of QA compounds. More specifically against gram-positive bacteria such as S. mutans, the compounds evaluated here also contained membrane-disrupting side alkyl chains. The length of this chain is strongly related to the antimicrobial activity as it modulates electrostatic interactions of the cell membrane with the cationic nitrogen of the ammonium group [23]. In addition, the side chain acts as a “lancet” that allows for cell wall disruption via physical penetration of this hydrophobic group. At the optimal length, this enables the alkyl-ammonium group to physically interrupt all key bacteria-cell functions [7].
The Luciferase assay and crystal violet staining results showed no biofilm formation for all C14 monomers, regardless of the polymerizable functionality. The C9 versions did not affect biofilm formation, irrespective of the polymerizable group. Among C9 materials, all QA versions showed similar performance with biofilm removal ranging between 18 and 25%. The similarity between the methacrylate, acrylamide and methacrylamide in terms of antimicrobial activity and biofilm attachment was somewhat unexpected since the replacement of ester groups with more hydrophilic amide linkages increases the polarity near the polymer backbone, which would be translated into greater attraction of water and, ultimately, maximized biocidal effect. It is likely that the increase in polarity was too modest to demonstrate this effect. However, this strongly suggests that the role of the alkyl chain length is predominant in determining the antimicrobial activity. It has been demonstrated that, in static culture, the adhesion of late stage colonizers is facilitated by the early stage colonizers, even if the latter are killed [24]. In a static culture incubated for a relatively long period of time (24 h), it is possible that the less potent C9 version allowed for the adhesion of at least some early colonizers, which then provided anchoring for subsequent layers of bacteria. Conversely, the C14 versions do not allow bacteria adhesion at all. Combined, the results from the luciferase and impingement test suggest that the biofilm inhibition shown here is driven by a mechanism related to the inhibition of bacterial adhesion, perhaps in combination with cell-disrupting contact-kill, as has been postulated previously [25]. The examination of early biofilm formation might have provided greater insight into the behavior of monomers with different polymerizable groups.
Longer cationic polymer chains show a more effective interaction with the bacterial cytoplasmic membranes, which increases the antimicrobial effect [3, 25]. Using compounds with structures similar to the ones evaluated here, recent studies reported on the antimicrobial effect of QA-containing monomers with systematically varied side chain lengths containing 3–18 carbons [25, 26]. The results indicated that, at least for S. mutans (gram positive), the optimal side chain length was 16 carbons. This allowed for bacterial cell wall disruption while still allowing for the quaternary ammonium site on the composite to interact with the bacteria [25, 26]. In the current, slightly shorter side chains with 9 or 14 carbons were selected to ensure that the quaternary ammonium moiety was exposed.
In general, all C14 monomers were more resistant to hydrolysis than their C9 counterparts, which can be explained by the increase in hydrophobicity imparted by the additional carbon atoms. The hydrolytic degradation is considerably faster and progressed to a greater extent in the C9 monomers due to their enhanced hydrophilic character. This is expected to increase the rate of water diffusion into the material and to the final overall water content, all as a result of the higher molecular mobility, greater density of hydrophilic terminal carboxyl and hydroxyl groups, and greater susceptibility to the formation of water soluble oligomers. This susceptibility was very evident at pH 1, but not surprisingly absent with lower acidity at pH 2 or 4. In addition, D2O is known to cause hydrolysis at a much slower rate than water [27]. The use of D2O was justified based on its technical convenience for use with 1H-NMR, but likely underestimated the amount of degradation, especially during the first few days of evaluation. Therefore, the final incubation period of 30 days was used to overcome the reduced initial rate of degradation, and allow for fair comparisons among materials. Indeed, the greater susceptibility of methacrylates to hydrolysis was highlighted in this test, especially for the monomer with the shorter side chain, likely due to the hydrolysis of the ester groups to produce methacrylic acid [28].
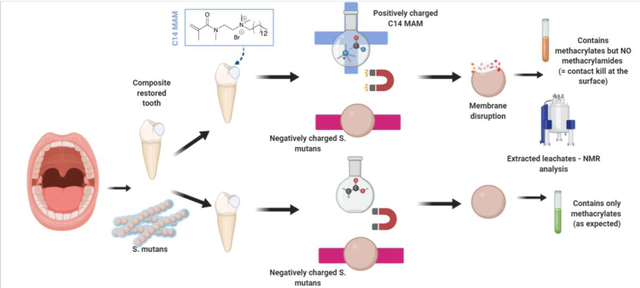
One concern that arises is the possibility that quaternary ammonium monomers might exert their antimicrobial effect via the lixiviation of unreacted species, which would limit the antimicrobial effects to the availability of free monomer and also raise concerns about the mechanical integrity and biocompatibility of the material. For that reason, in this study the antimicrobial activity was also evaluated for selected compositions using specimens that had been pre-incubated in water for 24 h and 48 h, based on a previous study showing that in 48 h polymer discs are saturated by water and most of the unreacted monomers are extracted [29]. The leachates were analyzed by 1H-NMR and confirmed that (meth)acrylamides peaks were not detected in the leachates obtained at 24 or 48 h, and most of what was extracted were unreacted methacrylate monomers. In addition, and importantly, the C14 monomers tested demonstrated the same potent antibacterial effect regardless of the pre-incubation period. This verifies that the quaternary ammonium moieties available at the surface are responsible for the observed antimicrobial effect.
However, since the monomers were still present in the leachates, albeit in a concentration similar to the control, the biocompatibility of the formulations with marked biocidal effect was evaluated using DPSCs. DPSCs were chosen for this study because they are well characterized stem cells that can provide a reliable indication of the compounds’ biocompatibility [30]. The specimens containing the C14 methacrylamide and acrylamide monomers resulted in cell viability similar to the control (around 80%), with only the C14-methacrylate version resulting in statistically lower cell viability (around 60%). Cytotoxic potential has been reported for specific acrylamide and methacrylamide monomers, though most studies report on free monomers in culture [31]. In this study, the MTT assay was conducted with polymerized discs without any pre-incubation, so any potential leachates were still present in the disc placed into the culture medium. The methacrylamides and acrylamides showed high degree of conversion (70% on average), which in turn reduced the solubility of these compositions, explaining their improved biocompatibility in relation to the C14 methacrylate. This shows the potential for (meth)acrylamides to be used as safe quaternary-ammonium-containing monomers for dental applications.
In terms of kinetics of polymerization, in all cases, the use of QA MAM and AM decreased the Rpmax in comparison to the methacrylate version, but no difference was observed in terms of the final DC. This was somewhat expected based on previous studies demonstrating lower reactivity of tertiary acrylamides and methacrylamides [32, 33]. As mentioned above, the analysis of the leachates from the polymerized discs after storage did not indicate the presence of tertiary methacrylamide monomers, which would have pointed to their lower reactivity. Together with the high conversion, this suggests that the monomers were successfully co-polymerized with the methacrylate and became part of the polymeric matrix. In addition, the monomers are relatively high in molecular weight and bear hydrophobic side chains, which pose a challenge to their extraction by an aqueous medium. The high values of DC may be explained based on the modified spatial arrangement of the monomers The presence of quaternary ammonium cations close to the oxygen in the amide functionality is hypothesized to induce an overall shift in the electron density of the amide, decreasing the intrinsic double bond component in the Ccarbonyl-N bond and therefore increasing the bond length to a dimension similar to that of a single bond. This could be sufficient for the steric strain to decrease to such a level that would favor the polymerization of this type of monomer.
In terms of mechanical properties, in dry conditions no difference was observed between the groups for both flexural strength and elastic modulus. After wet storage, all materials experienced some reduction in mechanical properties. For methacrylates, the drop resulted in FS and E that were statistically similar to the control. This was generally the case for the other materials as well, with two exceptions where the value was statistically lower than the control: for FS, with the material containing C14 MAM; for E, with the material containing C14 AM. These results were expected since (meth)acrylamides are known to swell and retain a significant fraction of water within their structure [34]. This implies that the use of these monomers for applications with high mechanical demands, such as composites, may not be indicated. However, because the decrease in mechanical properties after storage was not significantly greater than with methacrylates, it is possible to speculate that in situations where the mechanical requirements are less stringent, as is the case with the adhesive layer, that all the C14 materials will have adequate properties and may be used to make successful materials. In fact, having a potent antimicrobial at the bonded interface, which is one of the most susceptible regions of the restoration, might prove to be clinically important.
In general, incorporation of QAs in the dental adhesives did not affect the kinetics of polymerization nor the μTBS. It is especially important that the bond strengths with the materials containing these compounds were stable even after 6 months water storage. In conjunction with their significant antimicrobial effect, these monomers are promising in terms of their use as dental adhesives which could potentially inhibit bacterial colonization on surfaces as well as in interfacial gaps, therefore preventing secondary caries and, ultimately, dental restoration failure [35]. Additionally, the results show that amide-based adhesives are more degradation resistant than the methacrylate-based adhesives and might be able to maintain higher bond strength even after storage times longer than the 6 months evaluated here. Therefore, by combining greater and more stable dentin bond strength with demonstrated antimicrobial properties, a new avenue is opened for the incorporation of antimicrobial monomers into the dental adhesive system, which can represent a significant advance in relation to the currently available materials.
5. CONCLUSION
The synthesis of quaternary ammonium with polymerizable amide functionalities resulted in no significant effects on the cytotoxicity, polymerization kinetics, dry/wet mechanical properties (with a few exceptions) or medium-term bond strengths. In terms of antimicrobial properties, the alkyl chain length played a more crucial role than the polymerizable functionality, with C14 monomers completely preventing biofilm formation. The use of amide-based dental adhesives with antimicrobial properties might prevent bacterial re-colonization at the tooth-restoration interface, especially when gaps are present, and this has the potential to significantly improve restoration longevity.
Supplementary Material
Statement of significance:
This work demonstrates the viability of methacrylamides and acrylamides as potential components in dental restorative materials with antimicrobial properties. The use of ester-free polymerizable functionalities has the potential of improving the degradation resistance of these materials long-term. The use of (meth)acrylamides did not interfere with the antimicrobial potential of quaternary ammonium-based materials.
Acknowledgements:
the authors acknowledge NIH-NIDCR for financial support (U01-DE023756, R01-DE026113 and K02-DE025280 to CP and R35-DE028252 to JM) and 3M-ESPE for the donation of Filtek Supreme, used in the microtensile bond strength experiments. The authors also express gratitude to Dr. Luiz Bertassoni for facilitating the cytotoxicity experiments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES CITED
- [1].Gordan VV, Riley JL 3rd, Rindal DB, Qvist V, Fellows JL, Dilbone DA, Brotman SG, Gilbert GH, National Dental Practice-Based Research Network Collaborative G. Repair or replacement of restorations: A prospective cohort study by dentists in The National Dental Practice-Based Research Network. Journal of the American Dental Association (1939) 2015;146:895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Rasines Alcaraz MG, Veitz-Keenan A, Sahrmann P, Schmidlin PR, Davis D, Iheozor-Ejiofor Z. Direct composite resin fillings versus amalgam fillings for permanent or adult posterior teeth. Cochrane Database of Systematic Reviews 2014. [DOI] [PubMed] [Google Scholar]
- [3].Zhou H, Weir MD, Antonucci JM, Schumacher GE, Zhou XD, Xu HH. Evaluation of three-dimensional biofilms on antibacterial bonding agents containing novel quaternary ammonium methacrylates. Int J Oral Sci 2014;6:77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Beyth N, Yudovin-Farber I, Bahir R, Domb AJ, Weiss EI. Antibacterial activity of dental composites containing quaternary ammonium polyethylenimine nanoparticles against Streptococcus mutans. Biomaterials 2006;27:3995–4002. [DOI] [PubMed] [Google Scholar]
- [5].Tiller JC, Liao C-J, Lewis K, Klibanov AM. Designing surfaces that kill bacteria on contact. Proceedings of the National Academy of Sciences 2001;98:5981–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Li F, Weir MD, Xu HHK. Effects of quaternary ammonium chain length on antibacterial bonding agents. Journal of dental research 2013;92:932–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Simoncic B, Tomsic B. Structures of Novel Antimicrobial Agents for Textiles - A Review. Textile Research Journal 2010;80:1721–37. [Google Scholar]
- [8].Delaviz Y, Finer Y, Santerre JP. Biodegradation of resin composites and adhesives by oral bacteria and saliva: a rationale for new material designs that consider the clinical environment and treatment challenges. Dent Mater 2014;30:16–32. [DOI] [PubMed] [Google Scholar]
- [9].Song BK, Cho MS, Yoon KJ, Lee DC. Dispersion polymerization of acrylamide with quaternary ammonium cationic comonomer in aqueous solution. Journal of Applied Polymer Science 2003;87:1101–8. [Google Scholar]
- [10].Ren X, Liang J. 9 - Smart anti-microbial composite coatings for textiles and plastics In: Montemor MF, editor. Smart Composite Coatings and Membranes: Woodhead Publishing; 2016. p. 235–59. [Google Scholar]
- [11].Stansbury JW, Dickens SH. Determination of double bond conversion in dental resins by near infrared spectroscopy. Dental Materials 2001;17:71–9. [DOI] [PubMed] [Google Scholar]
- [12].Merritt J, Kreth J, Qi F, Sullivan R, Shi W. Non-disruptive, real-time analyses of the metabolic status and viability of Streptococcus mutans cells in response to antimicrobial treatments. Journal of Microbiological Methods 2005;61:161–70. [DOI] [PubMed] [Google Scholar]
- [13].Merritt J, Senpuku H, Kreth J. Let there be bioluminescence: development of a biophotonic imaging platform for in situ analyses of oral biofilms in animal models. Environmental microbiology 2016;18:174–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kreth J, Hagerman E, Tam K, Merritt J, Wong DT, Wu BM, Myung NV, Shi W, Qi F. Quantitative analyses of Streptococcus mutans biofilms with quartz crystal microbalance, microjet impingement and confocal microscopy. Biofilms 2004;1:277–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cazzaniga G, Ottobelli M, Ionescu AC, Paolone G, Gherlone E, Ferracane JL, Brambilla E. In vitro biofilm formation on resin-based composites after different finishing and polishing procedures. J Dent 2017;67:43–52. [DOI] [PubMed] [Google Scholar]
- [16].Resuggan JCL. The antibacterial activity of quaternary ammonium compounds. Proceedings of the Society for Applied Bacteriology 1952;15:166–71. [Google Scholar]
- [17].Palermo EF, Kuroda K. Chemical Structure of Cationic Groups in Amphiphilic Polymethacrylates Modulates the Antimicrobial and Hemolytic Activities. Biomacromolecules 2009;10:1416–28. [DOI] [PubMed] [Google Scholar]
- [18].Palermo EF, Sovadinova I, Kuroda K. Structural Determinants of Antimicrobial Activity and Biocompatibility in Membrane-Disrupting Methacrylamide Random Copolymers. Biomacromolecules 2009;10:3098–107. [DOI] [PubMed] [Google Scholar]
- [19].Exley SE, Paslay LC, Sahukhal GS, Abel BA, Brown TD, McCormick CL, Heinhorst S, Koul V, Choudhary V, Elasri MO, Morgan SE. Antimicrobial Peptide Mimicking Primary Amine and Guanidine Containing Methacrylamide Copolymers Prepared by Raft Polymerization. Biomacromolecules 2015;16:3845–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Paslay LC, Abel BA, Brown TD, Koul V, Choudhary V, McCormick CL, Morgan SE. Antimicrobial Poly(methacrylamide) Derivatives Prepared via Aqueous RAFT Polymerization Exhibit Biocidal Efficiency Dependent upon Cation Structure. Biomacromolecules 2012;13:2472–82. [DOI] [PubMed] [Google Scholar]
- [21].Solovsky MV, Ulbrich K, Kopecek J. Synthesis of N-(2-hydroxypr opyl) methacrylamide copolymers with antimicrobial activity. Biomaterials 1983;4:44–8. [DOI] [PubMed] [Google Scholar]
- [22].Santos RM, Fonseca CA, Mendonça VP, Branco R, Serra CA, Morais VP, Coelho FJ. Recent Developments in Antimicrobial Polymers: A Review. Materials 2016;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Yang Y, Cai Z, Huang Z, Tang X, Zhang X. Antimicrobial cationic polymers: from structural design to functional control. Polymer Journal 2017;50:33. [Google Scholar]
- [24].Garrett TR, Bhakoo M, Zhang Z. Bacterial adhesion and biofilms on surfaces. Progress in Natural Science 2008;18:1049–56. [Google Scholar]
- [25].Zhang K, Cheng L, Weir MD, Bai YX, Xu HH. Effects of quaternary ammonium chain length on the antibacterial and remineralizing effects of a calcium phosphate nanocomposite. Int J Oral Sci 2016;8:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Cheng L, Zhang K, Zhang N, Melo MAS, Weir MD, Zhou XD, Bai YX, Reynolds MA, Xu HHK. Developing a New Generation of Antimicrobial and Bioactive Dental Resins. J Dent Res 2017;96:855–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Brescia F, Mer VKL. The Kinetics of the Hydrolysis of Ethyl Orthoformate in D2O-H2O Mixtures. Journal of the American Chemical Society 1938;60:1962–7. [Google Scholar]
- [28].Munksgaard EC, Freund M. Enzymatic hydrolysis of (di)methacrylates and their polymers. Scandinavian journal of dental research 1990;98:261–7. [DOI] [PubMed] [Google Scholar]
- [29].Ferracane JL, Condon JR. Rate of elution of leachable components from composite. Dental Materials 1990;6:282–7. [DOI] [PubMed] [Google Scholar]
- [30].Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proceedings of the National Academy of Sciences of the United States of America 2000;97:13625–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bakopoulou A, Papadopoulos T, Garefis P. Molecular toxicology of substances released from resin-based dental restorative materials. International journal of molecular sciences 2009;10:3861–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sato T, Hirohata S, Fujimoto T, Seno M. Synthesis and radical polymerization of N,N-diethyl-α-fluoroacrylamide. European Polymer Journal 2001;37:275–80. [Google Scholar]
- [33].Otsu T, Inoue M, Yamada B, Mori T. Structure and reactivity of vinyl monomers: Radical reactivities of N-substituted acrylamides and methacrylamides. Journal of Polymer Science: Polymer Letters Edition 1975;13:505–10. [Google Scholar]
- [34].Ahmed EM. Hydrogel: Preparation, characterization, and applications: A review. Journal of Advanced Research 2015;6:105–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Feuerstein O, Matalon S, Slutzky H, Weiss EI. Antibacterial properties of self-etching dental adhesive systems. The Journal of the American Dental Association 2007;138:349–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.