Abstract
Ischemic stroke is one of the leading causes of death in the world, and thus is a major public health concern. Atherosclerosis, also known as atherogenesis, is a crucial risk factor for cerebral ischemia, yet how it develops remains largely unknown. It has been found, however, that angiopoietin-like protein 4 (ANGPTL4), a protein expressed in vascular endothelial cells, plays a role in the pathophysiology of atherosclerosis and may therefore be involved in ischemic stroke. ANGPTL4 activity is associated with endothelial cell integrity, inflammation, oxidative stress, and lipid metabolism. ANGPTL4 also serves as a potent inhibitor of the lipoprotein lipase, and may inhibit atherogenesis via regulating inflammatory signaling and lipid metabolism. In addition, ANGPTL4 plays a role in the regulation of oxidative stress. However, there currently exists a controversy on the role of ANGPTL4 in endothelial cells. Some studies indicate that ANGPTL4 can protect the integrity of endothelial cells, while others have shown that it can be destructive to the endothelium, thereby leading to the initiation of atherosclerosis. Thus, the effects of ANGPTL4 on development of atherosclerosis and thereby ischemic stroke, are undefined. Further research is needed to better understand ANGPTL4-mediated signaling pathways in endothelial function and to determine its potentials as therapeutic target for atherosclerosis and ischemic stroke.
Keywords: Ischemic stroke, Angiopoietin-like 4, Atherosclerosis, Endothelial cell, Inflammation, Oxidative stress, Lipid metabolism
1. Introduction
Ischemic stroke, the most common stroke subtype, which occurs when an artery to the brain is blocked, is the second leading cause of death and disability in the world, and as such, has a great impact on public health (Fang et al., 1999; Towfighi et al., 2010). A crucial risk factor for ischemic stroke is atherosclerosis, which is the formation of an arterial plaque that leads to stenosis and occlusion of the artery by its expansion, or acute obstruction of the vessel by the rupture-induced thrombus. Atherosclerosis also involves the hardening of the arterial wall due to the accumulation of cells, cholesterol, and an over-expressed extracellular matrix. Consequently, it is an important risk factor for cardio- and cerebrovascular diseases. Additionally, the pathophysiology of atherosclerosis includes abnormal lipid metabolism and glycometabolism (Rasmussen-Torvik et al., 2011; Stout, 1981; van Diepen et al., 2013), inflammation (van Diepen et al., 2013), and endothelial dysfunction (Mudau et al., 2012).
Recently, it was found that angiopoietin-like protein 4 (ANGPTL4), a secreted protein involved in the regulation of vascular permeability, angiogenesis and inflammatory responses, may play a significant role in the pathophysiology of atherosclerosis (Georgiadi et al., 2013; Katano and Yamada, 2014). The present review therefore seeks to clarify the relationships among ANGPTL4, atherosclerosis, and ischemic stroke.
2. Atherosclerosis
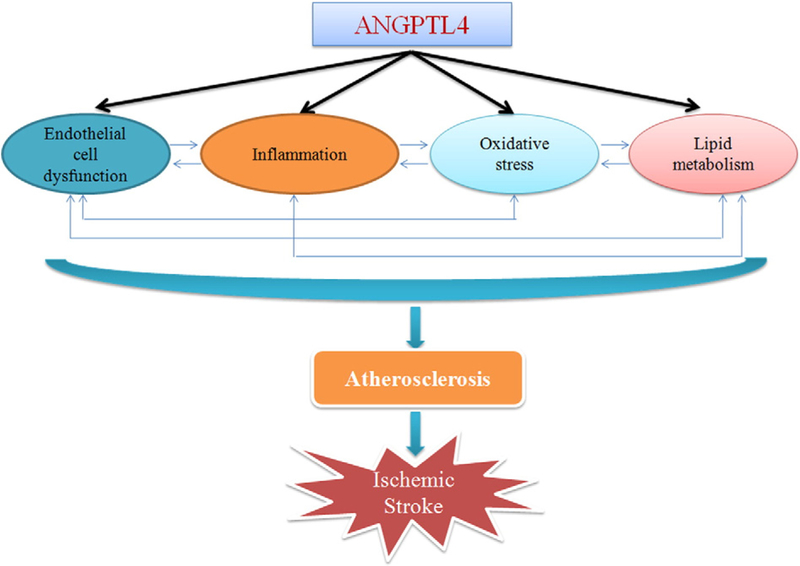
In western societies, atherosclerosis is the underlying cause of approximately 50% of all deaths (Lusis, 2000). Though it is not completely clear how atherosclerosis occurs, endothelial cell dysfunction, inflammation, oxidative stress, and abnormal lipid metabolism are known to contribute to the pathology (Fig. 1). (Hartman and Frishman, 2014; Hodis, 1999; Wong et al., 2012).
Fig. 1.
Potential links between ANGPTL4 and ischemic stroke.
The earliest event in atherogenesis is endothelial cell dysfunction, which contributes to the formation and progression of an atherosclerotic plaque (Sitia et al., 2010). Under normal physiological conditions, endothelial cells maintain a relaxed vascular tone, a low level of oxidative stress, and a balance between pro- and anti-inflammatory molecules. However, this is disrupted in the presence of certain stimuli, such as oxidative stress. This results in a decrease in the production of nitric oxide, along with the induction of angiotensin II, plasminogen activator inhibitor 1, endothelin-1, cellular adhesion molecules, pro-inflammatory cytokines (interleukins 1, 2, and 6), and tumor necrosis factor-α (Dessi et al., 2013; Sitia et al., 2010). These all promote the recruitment of monocytes/macrophages into the sub-endothelial space where they form foam cells by phagocytizing oxidized lipids, creating the hallmark of a fatty streak lesion (Mehrabian and Allayee, 2004; Siegel et al., 2013). Though fatty streaks are not clinically significant, they are the precursors of advanced lesions, characterized by the accumulation of lipid-rich necrotic debris and smooth muscle cells (Lusis, 2000). Smooth muscle cells, activated by angiotensin II and other growth factors, will then migrate into the intimae, proliferate, and form an intermediate lesion (Siegel et al., 2013). Smooth muscle cells can produce cytokines, such as platelet derived growth factor, transforming growth factor β, interferon gamma, macrophage inhibitory factor and monocyte chemoattractant protein, contributing to the initiation and propagation of the inflammatory response (Doran et al., 2008; Gomez and Owens, 2012). If inflammation continues unabated, the accumulation of mononuclear cells, the migration and proliferation of smooth muscle cells, and the formation of fibrous tissue, will lead to the further enlargement and restructuring of the lesion. Eventually it will become a complex core of lipid and necrotic tissue covered by a fibrous cap, called a complicated lesion (Ross, 1999). These lesions can either continue expanding until they totally block blood flow, or they can result in an acute occlusion of the vessel by the rupture-induced thrombus (Lusis, 2000). This eventually leads to ischemic stroke (Madden, 2012). Some studies have also indicated that free fatty acids can trigger the inflammatory response that leads to the initiation of atherosclerosis (Dasu and Jialal, 2011; Schwartz and Reaven, 2012; Soto-Vaca et al., 2013; Tripathy et al., 2003).
3. Angiopoietin-like 4 (ANGPTL4)
The human ANGPTL4 gene is well conserved across different species and shares a very similar amino acid sequence with mice and chimpanzees, approximately 77% and 99%, respectively (Zhu et al., 2012). The gene is located on chromosome 19p13.3, and encodes a 45–65 kDa glycoprotein (Zhu et al., 2012). ANGPTL4 belongs to the family of angiopoietins and angiopoietin-like proteins, which are characterized by the presence of an N-terminal coiled-coil domain and a C-terminal fibrinogen-like domain (Kim et al., 2000). ANGPTL4, discovered in 2000, was originally classified as an adipokine, as it is associated with lipid metabolism (Zhu et al., 2012). Recently, however, studies have found that ANGPTL4 is also involved in the regulation of vascular permeability, angiogenesis, and inflammatory responses (Georgiadi et al., 2013; Guo et al., 2014; Katano and Yamada, 2014). ANGPTL4 can be induced by glucocorticoid and nuclear hormone receptors, such as the peroxisome proliferator-activated receptor β/δ, which is able to induce the expression of ANGPTL4 via a peroxisome proliferator-activated receptor response element in both human and mouse ANGPTL4 genes (Guo et al., 2014). ANGPTL4 may also be induced by hypoxia and fasting, and can thus be downregulated by hyperoxia treatment (Drager et al., 2013; Goodin et al., 2013; Lichtenstein and Kersten, 2010; Quintero et al., 2012). It has been suggested that ANGPTL4 is involved in atherogenesis (Georgiadi et al., 2013; Lichtenstein et al., 2010). Lichtenstein et al. found that ANGPTL4 can prevent macrophage activation and foam cell formation (Lichtenstein et al., 2010). Moreover, Georgiadi et al. showed that over-expression of ANGPTL4 protects against atherosclerosis development (Georgiadi et al., 2013). The mechanisms by which ANGPTL4 regulates atherogenesis are, however, still not entirely known.
3.1. ANGPTL4 in endothelial cell integrity
3.1.1. Endothelial cell function
Endothelial cells are the major component of the endothelial barrier, which regulates the transportation of solutes, large molecules, and cells, across the vessel wall. Disruption of the endothelial barrier leads to changes in vascular permeability. There are three types of junctions that help to maintain the integrity of the endothelium: adherence junctions, tight junctions, and gap junctions. The adherence and tight junction proteins are directly linked to the actin cytoskeleton, and are connected to adjacent endothelial cells (Komarova and Malik, 2010). Loss of endothelial cell integrity is an early event following oxidant-mediated injury, which contributes to the initiation of atherosclerosis (Simoneau et al., 2012). There are several agents and signaling pathways that regulate vascular permeability, including thrombin, histamine, vascular endothelial growth factor (VEGF), and angiopoietin.
3.1.2. The two-sided role of ANGPTL4 in endothelial cell integrity
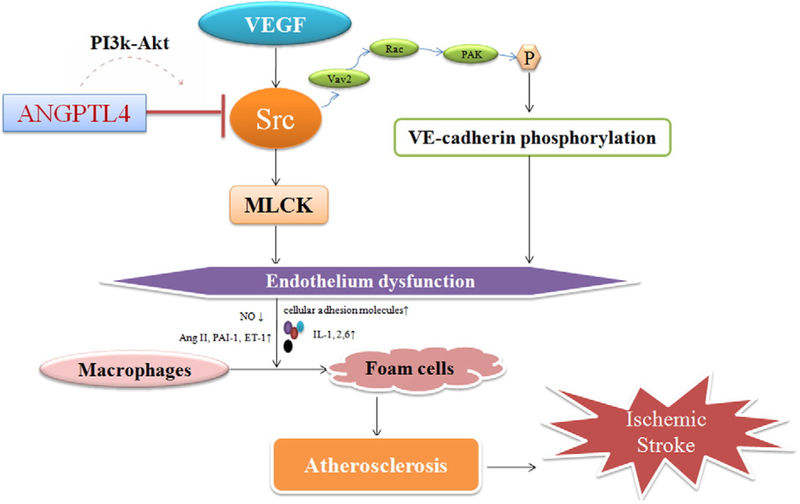
Though ANGPTL4 is widely expressed in vascular endothelial cells, its role in endothelial cells is controversial (Galaup et al., 2006; Huang et al., 2011; Kim et al., 2000). Kim et al. reported that ANGPTL4 protects endothelial cells from apoptosis through endocrine activity (Kim et al., 2000). Galaup et al. also reported that ANGPTL4 inhibited the increase in histamine-induced vascular permeability (Galaup et al., 2006). They further showed that the expression of the VEGF Receptor-2 (R-2) and vascular endothelial (VE)-cadherin were decreased, while Src kinase phosphorylation increased. Following ischemia–reperfusion in ANGPTL4-deficient mice, Src kinase signals downstream of the VEGFR-2. This leads to altered VEGF-R2/VE-cadherin complexes, and disruption of adherence junctions in the endothelial cells, resulting in an increase in vascular permeability (Galaup et al., 2012). However, treatment with recombinant ANGPTL4 was able to reverse the dissociation of the VEGF-R2/VE-cadherin complex, and reduce myocardial infarct size (Galaup et al., 2012). Bouleti et al.’s finding was in line with previous results, as they showed that ANGPTL4 could reduce the loss of vascular integrity in ischemic stroke by attenuating Src kinase signaling. Accordingly, the brain water content of ANGPTL4-treated mice was decreased, compared to vehicle-treated animals (Bouleti et al., 2013). Ito et al. also found that both VEGF-induced angiogenesis and vascular leakiness in vivo were inhibited by administration of ANGPTL4 (Ito et al., 2003). Additionally, Goodin et al. found that the activation of ANGPTL4 may protect against cerebral edema by inhibiting VEGF signaling (Goodin et al., 2013).
Several pathways may be involved in the regulation of endothelial cell integrity and atherosclerosis by ANGPTL4: (1) ANGPTL4 can counteract VEGF signaling, thereby diminishing Src-signaling downstream (Bouleti et al., 2013). The myosin light chain kinase (MLCK) activity is then also inhibited, leading to reduced MLCK-dependent endothelial hyperpermeability (Dudek et al., 2002; Rigor et al., 2013). (2) Activation of Src family kinases, and the subsequent VE-cadherin phosphorylation, plays a key role in the induction of permeability by growth factors and inflammatory cytokines (Gavard et al., 2008; Lambeng et al., 2005). Orsenigo et al. found that the Src-mediated VE-cadherin phosphorylation occurs mostly in veins. Inhibition of Src by ANGPTL4 blocks VE-cadherin phosphorylation and the bradykinin-induced increase in vessel permeability (Orsenigo et al., 2012). (3) ANGPTL4 can inhibit the recruitment of phosphorylated Src to the VEGFR-2 after VEGF stimulation by activating phosphatidylinositol 3-kinase (PI3K)/Akt signaling, thus protecting the VEGFR-2/VE-cadherin complexes from disruption, and preserving vascular integrity (Dudek et al., 2002; Liu et al., 2006; Rigor et al., 2013). These all show a role for ANGPTL4 in endothelial cell integrity/disruption. It is well established that endothelial cell dysfunction is the earliest event in atherogenesis, which is an important risk factor for ischemic stroke. As such, it is believed that ischemic stroke may be prevented by the protection of endothelial cell integrity (Fig. 2). On this basis, ANGPTL4 may be a viable therapeutic target for ischemic stroke.
Fig. 2.
Possible pathways linking ANGPTL4, endothelial integrity, atherosclerosis, and ischemic stroke. VEGF: vascular endothelial growth factor; MLCK: myosin light chain kinase; PI3K/ Akt: phosphatidylinositol 3-kinase (PI3K)/Akt; NO: nitric oxide; Ang II: angiotensin II; ET-1: endothelin 1; IL-1,2,6: interleukins 1, 2, and 6.
Despite the positive outcomes attained from ANGPTL4 treatment, as described above, other groups have presented findings that contradict these. For example, Huang et al. reported that the c-terminal fibrinogen-like domain of ANGPTL4 could disrupt the integrity of the vascular endothelium by directly interacting with integrin α5β1, VE-cadherin and claudin-5 in a sequential manner. ANGPTL4 can bind to integrin α5β1 and activate integrin α5β1-mediated Rac1/PAK signaling to weaken cell–cell contacts (Huang et al., 2011). Furthermore, it was also found that ANGPTL4 could lead to the degradation of VE-cadherin and claudin-5, resulting in endothelial cell disruption (Huang et al., 2011). Padua et al. reported that tumor cell-derived ANGPTL4 disrupted vascular endothelial cell–cell junctions, increased the permeability of lung capillaries, and facilitated the trans-endothelial transport of tumor cells (Padua et al., 2008). We postulate, based on these findings, that the different effects of ANGPTL4 on vascular integrity may be dependent on the specific tissue type, the physiology and pathophysiology micro-environment. Further studies are needed to clearly delineate the functions and effects of ANGPTL4 on the endothelium.
3.2. ANGPTL4 in inflammation
3.2.1. Inflammation in atherosclerosis
Inflammatory processes are a part of the initiation, progression and rupture of lipid-rich atherosclerotic plaques (Montero-Vega, 2012; Ross, 1999). At every stage of the disease, the inflammatory response in atherogenesis is mediated by monocyte-derived macrophages and specific subsets of T lymphocytes (Jonasson et al., 1986; Ross, 1999). The role of macrophages in foam cell formation in the vascular wall has been well established (Van Eck et al., 2000).
Several factors and agents can induce inflammation, including lipoproteins, homocysteine, hypertension, diabetes, etc (Ross, 1999; Siegel et al., 2013). Circulating free fatty acids are also known as risk factors for cardiovascular inflammation. Soto-Vaca et al. found that free fatty acids may induce low-grade inflammation in human coronary arterial cells (Soto-Vaca et al., 2013). Furthermore, Dasu and Jialal found that free fatty acids exacerbated high glucose-induced toll-like receptor expression and activity in monocytes with excess superoxide release, enhanced nuclear factor-κB (NF-κB) activity, and increased the release of pro-inflammatory factors (Dasu and Jialal, 2011). Furthermore, Tripathy et al. reported that increased FFA concentration induced oxidative stress, thereby promoting inflammation (Tripathy et al., 2003).
3.2.2. ANGPTL4 in inflammation and atherosclerosis
Lipoprotein lipase, produced by macrophages, has pro-atherogenic effects via the bridging of lipoproteins to cells and the extracellular matrix, thus promoting the retention of lipoproteins in the artery wall and the formation of foam cells (Makoveichuk et al., 2012). Lipoprotein lipase is also able to hydrolyze triglycerides, releasing fatty acids in plasma lipoproteins for metabolic use in muscles and adipose tissue. ANGPTL4 serves as a potent inhibitor of lipoprotein lipase. Hence, it can inhibit atherogenesis via regulating inflammatory signaling. Lichtenstein et al. found that ANGPTL4, induced by chyle, protected against the severe pro-inflammatory effects of saturated fats by inhibiting the low density lipoprotein-dependent uptake of fatty acids by macrophages in the mesenteric lymph node. They also found that ANGPTL4 reduced macrophage foam cell formation, inflammatory gene expression, and the chyle-induced activation of the endoplasmic reticulum (Lichtenstein et al., 2010). Their results indicate that ANGPTL4 is involved in both inflammation and atherogenesis. Moreover, Georgiadi et al. demonstrated that ANGPTL4 reduced foam cell formation and decreased atherosclerosis in atherosclerosis-prone apolipoprotein E*3-Leiden mice. In their study, the authors found that ANGPTL4Tg.E*3-Leiden mice exhibited less pro-inflammatory markers, with decreased accumulation of monocytes/macrophages in the atherosclerotic plaque, suggesting an anti-inflammatory role of ANGPTL4 in atherosclerosis development. It should be pointed out that the levels of plasma triglycerides and cholesterol were similar between ANGPTL4Tg.E*3-Leiden mice and control E*3-Leiden mice, which indicated that the anti-inflammatory effect of ANGPTL4 did not rely on its mediation of lipid metabolism (Georgiadi et al., 2013).
3.2.3. From ANGPTL4 to ischemic stroke: possible pathways in inflammation
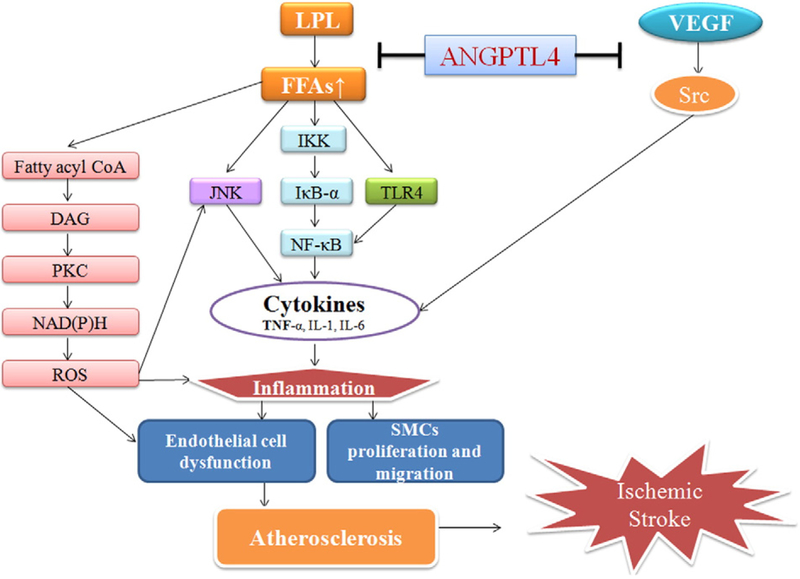
There are several pathways involved in ANGPTL4’s regulation of inflammation: (1) Acute elevation of plasma free fatty acids can activate the pro-inflammatory IκB kinase/IκB-α/NF-κB and c-Jun N-terminal kinase pathways, resulting in the increased hepatic expression of several pro-inflammatory cytokines, including tumor necrosis factor-α, interleukin-1β and interleukin-6 (Boden, 1998; Boden et al., 2005; Itani et al., 2002; Ozcan et al., 2004). (2) The elevation of fatty acids may activate toll-like receptor-4 signaling in adipocytes and macrophages, leading to activation of the NF-κB pathway, which then activates the transcription of certain pro-inflammatory genes that encode molecules such as cytokines, chemokines and other effectors of the innate immune response (Dasu and Jialal, 2011). (3) Increased concentration of plasma free fatty acids induces the accumulation of fatty acid coenzyme A and diacylglycerol, and activates protein kinase C, which has been shown to activate nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, elevating reactive oxygen species, and decreasing nitric oxide in vascular endothelial cells (Boden, 1998). ANGPTL4 can inhibit the activation of lipoprotein lipase, and thus decrease the production of fatty acids. (4) Sarang et al. found that Src family kinases were involved in the regulation of pro-inflammatory cytokine production. ANGPTL4 can inhibit Src kinase signaling, reducing the expression of several pro-inflammatory cytokines as a result (Sarang et al., 2011). Since inflammation plays a key role in every stage of atherogenesis, and atherogenesis is a crucial risk factor for ischemic stroke, we postulate that the inhibition of inflammation by ANGPTL4 can retard the process of atherosclerosis-related ischemic stroke (Fig. 3).
Fig. 3.
Possible pathways linking ANGPTL4, inflammation, atherosclerosis and ischemic stroke. LPL: lipoprotein lipase; FFAs: free fatty acids; CoA: coenzyme A; DAG: diacylglycerol; PKC: protein kinase C; ROS: reactive oxygen species; IKK: IkB kinase: JNK: c-Jun N-terminal kinases; TLR4: toll-like receptor 4; TNF-α: tumor necrosis factor-α; SMCs: smooth muscle cells.
3.3. ANGPTL4, oxidative stress, and atherosclerosis
3.3.1. Oxidative stress in atherosclerosis
Increasing evidence has highlighted the role of oxidative stress in the promotion of atherosclerotic cardiovascular disease. Firstly, oxidative stress can induce endothelial cell dysfunction, the initial step of atherogenesis. Secondly, oxidative stress causes oxidation of low density lipoproteins, which are phagocytized by macrophages, promoting the formation of foam cells. Thirdly, the increase in reactive oxygen species reduces the production and consequent bioavailability of nitric oxide, resulting in vasoconstriction, platelet aggregation, and adhesion of neutrophils to the endothelium. In addition, oxidative stress may affect atherogenesis by increasing transcription factors such as activator protein 1 and NF-κB, which can further increase the expression of adhesion molecules (Vogiatzi et al., 2009). Factors that lead to atherosclerosis, such as free fatty acids, also facilitate the production of reactive oxygen species, increasing oxidative stress (Wang et al., 2009; Zhou et al., 2009, 2013).
3.3.2. ANGPTL4 in oxidative stress
Recently, Georgiadi et al. found that the induction of ANGPTL4 by dietary fatty acids was mediated by peroxisome proliferator-activated receptor β/δ. This was part of a feedback mechanism to protect cardiomyocytes from lipid overload and the resulting fatty acid-induced oxidative stress (Georgiadi et al., 2010). However, Zhu et al., from their study in tumors, arrived at a different conclusion. They demonstrated that ANGPTL4 elevated superoxide (O2−•) levels and maintained a high superoxide/hydrogen peroxide (O2−•/H2O2) ratio in tumor cells. ANGPTL4 deficiency resulted in diminished O2−• production and a reduced O2−•/H2O2 ratio, creating a cellular environment conducive to apoptosis (Zhu et al., 2011). Further research is needed to verify the relationship between ANGPTL4 and oxidative stress.
3.4. ANGPTL4, triglycerides and atherosclerosis
Several clinical studies have suggested that high triglyceride content is an independent risk factor for the development of atherosclerosis (Ikeda et al., 2014; Labreuche et al., 2009; Patsch et al., 1992; Schwartz and Reaven, 2012). Though there are many studies on the relevance of triglycerides in atherosclerosis, the direct effect of triglycerides on the initiation of atherosclerosis is not yet known, as triglycerides themselves cannot be detected in the atherosclerotic region (Matsumoto et al., 2014). It has been reported that hypertriglyceridemia can cause decreased serum high density lipoprotein cholesterol levels, increased remnant lipoproteins, small low density lipoproteins and thrombogenic conditions. In addition, triglyceride-rich lipoproteins were reported to be associated with endothelial inflammation and dysfunction, which promotes atherosclerosis formation (Mano et al., 1996; Miller et al., 2011; Nakaya, 2002; Wang et al., 2011).
Notably, it was found that over-expression of ANGPTL4 in mice leads to extremely high levels of triglycerides in blood, while ANGPTL4 knock-out mice had much lower triglyceride levels in the blood (Yoshida et al., 2002). Against this background, ANGPTL4 may promote atherogenesis by increasing triglycerides. Conversely, ANGPTL4 can also reduce the risk of atherogenesis by inhibiting lipoprotein lipase and decreasing the level of fatty acids.
4. ANGPTL4 and ischemic stroke
Several studies have shown that ANGPTL4 may reduce the risk of atherosclerosis in animal models. Clinical studies are required to verify whether the same would be true in humans. Katano et al. studied the role of ANGPTL4 in carotid plaques. They found that, in highly calcified plaques, ANGPTL4 was up-regulated for anti-angiogenic modulation, in line with the down-regulation of fibroblast growth factor receptor 2, which contributed to the stability of the plaques (Katano and Yamada, 2014). It was highlighted that plaques, particularly unstable ones, play a vital role in atherosclerotic diseases such as large-artery atherosclerosis, coronary heart disease, and stroke. Understanding the relationship between ANGPTL4 and the stability of atherosclerotic plaques would, therefore, enhance our knowledge of and ability to intervene in these diseases.
5. Conclusion
ANGPTL4 is involved in the pathophysiology of atherosclerosis and may therefore be associated with ischemic stroke, as atherosclerosis is a major risk factor for ischemic stroke. ANGPTL4 has effects on endothelial cell integrity, inflammation, oxidative stress, and lipid metabolism. However, both positive and negative effects have been reported. The overall effect of ANGPTL4 on atherosclerosis and ischemic stroke is therefore undefined. Further research is needed to better understand ANGPTL4-mediated signaling pathways in endothelial function and to determine its potentials as therapeutic target for atherosclerosis and ischemic stroke.
List of abbreviations
- ANGPTL4
angiopoietin-like protein 4
- NF-κB
nuclear factor-κB
- VEGF
vascular endothelial growth factor
- VE-cadherin
vascular endothelial cadherin
Footnotes
Declaration of interests
The authors have no competing interests to declare.
References
- Boden G, 1998. Free fatty acids (FFA), a link between obesity and insulin resistance.Front. Biosci 3, d169–d175. [DOI] [PubMed] [Google Scholar]
- Boden G, She P, Mozzoli M, Cheung P, Gumireddy K, Reddy P, Xiang X, Luo Z, Ruderman N, 2005. Free fatty acids produce insulin resistance and activate the proinflammatory nuclear factor-kappaB pathway in rat liver. Diabetes 54, 3458–3465. [DOI] [PubMed] [Google Scholar]
- Bouleti C, Mathivet T, Coqueran B, Serfaty JM, Lesage M, Berland E, Ardidie-Robouant C, Kauffenstein G, Henrion D, Lapergue B, Mazighi M, Duyckaerts C, Thurston G, Valenzuela DM, Murphy AJ, Yancopoulos GD, Monnot C, Margaill I, Germain S, 2013. Protective effects of angiopoietin-like 4 on cerebrovascular and functional damages in ischaemic stroke. Eur. Heart J 34, 3657–3668. [DOI] [PubMed] [Google Scholar]
- Dasu MR, Jialal I, 2011. Free fatty acids in the presence of high glucose amplify monocyte inflammation via Toll-like receptors. Am. J. Physiol. Endocrinol. Metab 300, E145–E154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessi M, Noce A, Bertucci P, Manca di Villahermosa S, Zenobi R, Castagnola V, Addessi E, Di Daniele N, 2013. Atherosclerosis, dyslipidemia, and inflammation: the significant role of polyunsaturated fatty acids. ISRN Inflamm 2013, 191823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran AC, Meller N, McNamara CA, 2008. Role of smooth muscle cells in the initiation and early progression of atherosclerosis. Arterioscler. Thromb. Vasc. Biol 28, 812–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drager LF, Yao Q, Hernandez KL, Shin MK, Bevans-Fonti S, Gay J, Sussan TE, Jun JC, Myers AC, Olivecrona G, Schwartz AR, Halberg N, Scherer PE, Semenza GL, Powell DR, Polotsky VY, 2013. Chronic intermittent hypoxia induces atherosclerosis via activation of adipose angiopoietin-like 4. Am. J. Respir. Crit. Care Med 188, 240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek SM, Birukov KG, Zhan X, Garcia JG, 2002. Novel interaction of cortactin with endothelial cell myosin light chain kinase. Biochem. Biophys. Res. Commun 298, 511–519. [DOI] [PubMed] [Google Scholar]
- Fang XH, Kronmal RA, Li SC, Longstreth WT Jr., Cheng XM, Wang WZ, Wu S, Du XL, Siscovick D, 1999. Prevention of stroke in urban China: a community-based intervention trial. Stroke 30, 495–501. [DOI] [PubMed] [Google Scholar]
- Galaup A, Cazes A, Le Jan S, Philippe J, Connault E, Le Coz E, Mekid H, Mir LM, Opolon P, Corvol P, Monnot C, Germain S, 2006. Angiopoietin-like 4 prevents metastasis through inhibition of vascular permeability and tumor cell motility and invasiveness. Proc. Natl. Acad. Sci. U. S. A 103, 18721–18726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galaup A, Gomez E, Souktani R, Durand M, Cazes A, Monnot C, Teillon J, Le Jan S, Bouleti C, Briois G, Philippe J, Pons S, Martin V, Assaly R, Bonnin P, Ratajczak P, Janin A, Thurston G, Valenzuela DM, Murphy AJ, Yancopoulos GD, Tissier R, Berdeaux A, Ghaleh B, Germain S, 2012. Protection against myocardial infarction and no-reflow through preservation of vascular integrity by angiopoietin-like 4. Circulation 125, 140–U557. [DOI] [PubMed] [Google Scholar]
- Gavard J, Patel V, Gutkind JS, 2008. Angiopoietin-1 prevents VEGF-induced endothelial permeability by sequestering Src through mDia. Dev. Cell 14, 25–36. [DOI] [PubMed] [Google Scholar]
- Georgiadi A, Lichtenstein L, Degenhardt T, Boekschoten MV, van Bilsen M, Desvergne B, Muller M, Kersten S, 2010. Induction of cardiac Angptl4 by dietary fatty acids is mediated by peroxisome proliferator-activated receptor beta/delta and protects against fatty acid-induced oxidative stress. Circ. Res 106, 1712–1721. [DOI] [PubMed] [Google Scholar]
- Georgiadi A, Wang Y, Stienstra R, Tjeerdema N, Janssen A, Stalenhoef A, van der Vliet JA, de Roos A, Tamsma JT, Smit JW, Tan NS, Muller M, Rensen PC, Kersten S, 2013. Overexpression of angiopoietin-like protein 4 protects against atherosclerosis development. Arterioscler. Thromb. Vasc. Biol 33, 1529–1537. [DOI] [PubMed] [Google Scholar]
- Gomez D, Owens GK, 2012. Smooth muscle cell phenotypic switching in atherosclerosis. Cardiovasc. Res 95, 156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodin JL, Pizarro-Matos JM, Prasad BM, Seiter TJ, Weaver CR, Muza SR, Beidleman BA, Wood JC, 2013. Evaluating the molecular basis for acute mountain sickness: hypoxia response gene expression patterns in warfighters and murine populations. Mil. Med 178, 1256–1263. [DOI] [PubMed] [Google Scholar]
- Guo L, Li SY, Ji FY, Zhao YF, Zhong Y, Lv XJ, Wu XL, Qian GS, 2014. Role of Angptl4 in vascular permeability and inflammation. Inflamm. Res 63, 13–22. [DOI] [PubMed] [Google Scholar]
- Hartman J, Frishman WH, 2014. Inflammation and atherosclerosis: a review of the role of interleukin-6 in the development of atherosclerosis and the potential for targeted drug therapy. Cardiol. Rev 22, 147–151. [DOI] [PubMed] [Google Scholar]
- Hodis HN, 1999. Triglyceride-rich lipoprotein remnant particles and risk of atherosclerosis. Circulation 99, 2852–2854. [DOI] [PubMed] [Google Scholar]
- Huang RL, Teo Z, Chong HC, Zhu P, Tan MJ, Tan CK, Lam CR, Sng MK, Leong DT, Tan SM, Kersten S, Ding JL, Li HY, Tan NS, 2011. ANGPTL4 modulates vascular junction integrity by integrin signaling and disruption of intercellular VE-cadherin and claudin-5 clusters. Blood 118, 3990–4002. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Hirano K, Fukushima N, Sawa Y, 2014. A novel type of human spontaneous coronary atherosclerosis with triglyceride deposition. Eur. Heart J 35, 875. [DOI] [PubMed] [Google Scholar]
- Itani SI, Ruderman NB, Schmieder F, Boden G, 2002. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes 51, 2005–2011. [DOI] [PubMed] [Google Scholar]
- Ito Y, Oike Y, Yasunaga K, Hamada K, Miyata K, Matsumoto S, Sugano S, Tanihara H, Masuho Y, Suda T, 2003. Inhibition of angiogenesis and vascular leakiness by angiopoietin-related protein 4. Cancer Res 63, 6651–6657. [PubMed] [Google Scholar]
- Jonasson L, Holm J, Skalli O, Bondjers G, Hansson GK, 1986. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis 6, 131–138. [DOI] [PubMed] [Google Scholar]
- Katano H, Yamada K, 2014. Upregulation of ANGPTL4 messenger RNA and protein in severely calcified carotid plaques. J. Stroke Cerebrovasc. Dis 23, 933–947. [DOI] [PubMed] [Google Scholar]
- Kim I, Kim HG, Kim H, Kim HH, Park SK, Uhm CS, Lee ZH, Koh GY, 2000. Hepatic expression, synthesis and secretion of a novel fibrinogen/angiopoietin-related protein that prevents endothelial-cell apoptosis. Biochem. J 346 (Pt 3), 603–610. [PMC free article] [PubMed] [Google Scholar]
- Komarova Y, Malik AB, 2010. Regulation of endothelial permeability via paracellular and transcellular transport pathways. Annu. Rev. Physiol 72, 463–493. [DOI] [PubMed] [Google Scholar]
- Labreuche J, Touboul PJ, Amarenco P, 2009. Plasma triglyceride levels and risk of stroke and carotid atherosclerosis: a systematic review of the epidemiological studies. Atherosclerosis 203, 331–345. [DOI] [PubMed] [Google Scholar]
- Lambeng N, Wallez Y, Rampon C, Cand F, Christe G, Gulino-Debrac D, Vilgrain I, Huber P, 2005. Vascular endothelial-cadherin tyrosine phosphorylation in angiogenic and quiescent adult tissues. Circ. Res 96, 384–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein L, Kersten S, 2010. Modulation of plasma TG lipolysis by angiopoietin-like proteins and GPIHBP1. Biochim. Biophys. Acta 1801, 415–420. [DOI] [PubMed] [Google Scholar]
- Lichtenstein L, Mattijssen F, de Wit NJ, Georgiadi A, Hooiveld GJ, van der Meer R, He Y, Qi L, Koster A, Tamsma JT, Tan NS, Muller M, Kersten S, 2010. Angptl4 protects against severe proinflammatory effects of saturated fat by inhibiting fatty acid uptake into mesenteric lymph node macrophages. Cell Metab 12, 580–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZJ, Xiao M, Balint K, Soma A, Pinnix CC, Capobianco AJ, Velazquez OC, Herlyn M, 2006. Inhibition of endothelial cell proliferation by Notch1 signaling is mediated by repressing MAPK and PI3K/Akt pathways and requires MAML1. FASEB J 20, 1009–1011. [DOI] [PubMed] [Google Scholar]
- Lusis AJ, 2000. Atherosclerosis. Nature 407, 233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden JA, 2012. Role of the vascular endothelium and plaque in acute ischemic stroke.Neurology 79, S58–S62. [DOI] [PubMed] [Google Scholar]
- Makoveichuk E, Sukonina V, Kroupa O, Thulin P, Ehrenborg E, Olivecrona T, Olivecrona G, 2012. Inactivation of lipoprotein lipase occurs on the surface of THP-1 macrophages where oligomers of angiopoietin-like protein 4 are formed. Biochem. Biophys. Res. Commun 425, 138–143. [DOI] [PubMed] [Google Scholar]
- Mano T, Masuyama T, Yamamoto K, Naito J, Kondo H, Nagano R, Tanouchi J, Hori M, Inoue M, Kamada T, 1996. Endothelial dysfunction in the early stage of atherosclerosis precedes appearance of intimal lesions assessable with intravascular ultra-sound. Am. Heart J 131, 231–238. [DOI] [PubMed] [Google Scholar]
- Matsumoto S, Gotoh N, Hishinuma S, Abe Y, Shimizu Y, Katano Y, Ishihata A, 2014. The role of hypertriglyceridemia in the development of atherosclerosis and endothelial dysfunction. Nutrients 6, 1236–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrabian M, Allayee H, 2004. Vascular cross-talk: a conversation. Arterioscler. Thromb. Vasc. Biol 24, 1748–1749. [DOI] [PubMed] [Google Scholar]
- Miller M, Stone NJ, Ballantyne C, Bittner V, Criqui MH, Ginsberg HN, Goldberg AC, Howard WJ, Jacobson MS, Kris-Etherton PM, Lennie TA, Levi M, Mazzone T, Pennathur S, 2011. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation 123, 2292–2333. [DOI] [PubMed] [Google Scholar]
- Montero-Vega MT, 2012. The inflammatory process underlying atherosclerosis. Crit. Rev. Immunol 32, 373–462. [DOI] [PubMed] [Google Scholar]
- Mudau M, Genis A, Lochner A, Strijdom H, 2012. Endothelial dysfunction: the early predictor of atherosclerosis. Cardiovasc. J. Afr 23, 222–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaya N, 2002. Hypertriglyceridemia as a cause of atherosclerosis. Nihon Rinsho 60,860–867. [PubMed] [Google Scholar]
- Orsenigo F, Giampietro C, Ferrari A, Corada M, Galaup A, Sigismund S, Ristagno G, Maddaluno L, Koh GY, Franco D, Kurtcuoglu V, Poulikakos D, Baluk P, McDonald D, Lampugnani MG, Dejana E, 2012. Phosphorylation of VE-cadherin is modulated by haemodynamic forces and contributes to the regulation of vascular permeability in vivo. Nat. Commun 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS, 2004. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306, 457–461. [DOI] [PubMed] [Google Scholar]
- Padua D, Zhang XHF, Wang QQ, Nadal C, Gerald WL, Gomis RR, Massague J, 2008. TGF beta primes breast tumors for lung metastasis seeding through angiopoietin-like4. Cell 133, 66–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsch JR, Miesenbock G, Hopferwieser T, Muhlberger V, Knapp E, Dunn JK, Gotto AM Jr., Patsch W, 1992. Relation of triglyceride metabolism and coronary artery disease. Studies in the postprandial state. Arterioscler. Thromb 12, 1336–1345. [DOI] [PubMed] [Google Scholar]
- Quintero P, Gonzalez-Muniesa P, Garcia-Diaz DF, Martinez JA, 2012. Effects of hyperoxia exposure on metabolic markers and gene expression in 3T3-L1 adipocytes. J. Physiol. Biochem 68, 663–669. [DOI] [PubMed] [Google Scholar]
- Rasmussen-Torvik LJ, Li M, Kao WH, Couper D, Boerwinkle E, Bielinski SJ, Folsom AR, Pankow JS, 2011. Association of a fasting glucose genetic risk score with sub-clinical atherosclerosis: the Atherosclerosis Risk in Communities (ARIC) study. Diabetes 60, 331–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigor RR, Shen Q, Pivetti CD, Wu MH, Yuan SY, 2013. Myosin light chain kinase signaling in endothelial barrier dysfunction. Med. Res. Rev 33, 911–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R, 1999. Atherosclerosis—an inflammatory disease. N. Engl. J. Med 340, 115–126. [DOI] [PubMed] [Google Scholar]
- Sarang Z, Koroskenyi K, Pallai A, Duro E, Melino G, Griffin M, Fesus L, Szondy Z, 2011. Transglutaminase 2 null macrophages respond to lipopolysaccharide stimulation by elevated proinflammatory cytokine production due to an enhanced alphavbeta3 integrin-induced Src tyrosine kinase signaling. Immunol. Lett 138, 71–78. [DOI] [PubMed] [Google Scholar]
- Schwartz EA, Reaven PD, 2012. Lipolysis of triglyceride-rich lipoproteins, vascular inflammation, and atherosclerosis. Biochim. Biophys. Acta 1821, 858–866. [DOI] [PubMed] [Google Scholar]
- Siegel D, Devaraj S, Mitra A, Raychaudhuri SP, Raychaudhuri SK, Jialal I, 2013. Inflammation, atherosclerosis, and psoriasis. Clin. Rev. Allergy Immunol 44, 194–204. [DOI] [PubMed] [Google Scholar]
- Simoneau B, Houle F, Huot J, 2012. Regulation of endothelial permeability and transendothelial migration of cancer cells by tropomyosin-1 phosphorylation. Vasc. Cell 4, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitia S, Tomasoni L, Atzeni F, Ambrosio G, Cordiano C, Catapano A, Tramontana S, Perticone F, Naccarato P, Camici P, Picano E, Cortigiani L, Bevilacqua M, Milazzo L, Cusi D, Barlassina C, Sarzi-Puttini P, Turiel M, 2010. From endothelial dysfunction to atherosclerosis. Autoimmun. Rev 9, 830–834. [DOI] [PubMed] [Google Scholar]
- Soto-Vaca A, Losso JN, McDonough K, Finley JW, 2013. Differential effect of 14 free fatty acids in the expression of inflammation markers on human arterial coronary cells. J. Agric. Food Chem 61, 10074–10079. [DOI] [PubMed] [Google Scholar]
- Stout RW, 1981. Blood glucose and atherosclerosis. Arteriosclerosis 1, 227–234. [DOI] [PubMed] [Google Scholar]
- Towfighi A, Ovbiagele B, Saver JL, 2010. Therapeutic milestone: stroke declines from the second to the third leading organ- and disease-specific cause of death in the United States. Stroke 41, 499–503. [DOI] [PubMed] [Google Scholar]
- Tripathy D, Mohanty P, Dhindsa S, Syed T, Ghanim H, Aljada A, Dandona P, 2003. Elevation of free fatty acids induces inflammation and impairs vascular reactivity in healthy subjects. Diabetes 52, 2882–2887. [DOI] [PubMed] [Google Scholar]
- van Diepen JA, Berbee JFP, Havekes LM, Rensen PCN, 2013. Interactions between inflammation and lipid metabolism: relevance for efficacy of anti-inflammatory drugs in the treatment of atherosclerosis. Atherosclerosis 228, 306–315. [DOI] [PubMed] [Google Scholar]
- Van Eck M, Zimmermann R, Groot PH, Zechner R, Van Berkel TJ, 2000. Role of macrophage-derived lipoprotein lipase in lipoprotein metabolism and atherosclerosis. Arterioscler. Thromb. Vasc. Biol 20, E53–E62. [DOI] [PubMed] [Google Scholar]
- Vogiatzi G, Tousoulis D, Stefanadis C, 2009. The role of oxidative stress in atherosclerosis. Hell. J. Cardiol 50, 402–409. [PubMed] [Google Scholar]
- Wang H, Li H, Hou Z, Pan L, Shen X, Li G, 2009. Role of oxidative stress in elevated blood pressure induced by high free fatty acids. Hypertens. Res 32, 152–158. [DOI] [PubMed] [Google Scholar]
- Wang YI, Schulze J, Raymond N, Tomita T, Tam K, Simon SI, Passerini AG, 2011. Endothelial inflammation correlates with subject triglycerides and waist size after a high-fat meal. Am. J. Physiol. Heart Circ. Physiol 300, H784–H791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong BW, Meredith A, Lin D, McManus BM, 2012. The biological role of inflammation in atherosclerosis. Can. J. Cardiol 28, 631–641. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Shimizugawa T, Ono M, Furukawa H, 2002. Angiopoietin-like protein 4 is a potent hyperlipidemia-inducing factor in mice and inhibitor of lipoprotein lipase.J. Lipid Res 43, 1770–1772. [DOI] [PubMed] [Google Scholar]
- Zhou H, Liu X, Liu L, Yang Z, Zhang S, Tang M, Tang Y, Dong Q, Hu R, 2009. Oxidative stress and apoptosis of human brain microvascular endothelial cells induced by free fatty acids. J. Int. Med. Res 37, 1897–1903. [DOI] [PubMed] [Google Scholar]
- Zhou HG, Liu L, Zhang Y, Huang YY, Tao YH, Zhang S, Su JJ, Tang YP, Guo ZL, Hu RM, Dong Q, 2013. Glutathione prevents free fatty acids-induced oxidative stress and apoptosis in human brain vascular endothelial cells through Akt pathway. CNS Neurosci. Ther 19, 252–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu P, Tan MJ, Huang RL, Tan CK, Chong HC, Pal M, Lam CR, Boukamp P, Pan JY, Tan SH, Kersten S, Li HY, Ding JL, Tan NS, 2011. Angiopoietin-like 4 protein elevates the prosurvival intracellular O2(−):H2O2 ratio and confers anoikis resistance to tumors. Cancer Cell 19, 401–415. [DOI] [PubMed] [Google Scholar]
- Zhu P, Goh YY, Chin HF, Kersten S, Tan NS, 2012. Angiopoietin-like 4: a decade of research. Biosci. Rep 32, 211–219. [DOI] [PubMed] [Google Scholar]