Abstract
Objective:
We report the immune response during a case of acute HEV response in a patient with an acute hepatitis E virus (HEV) genotype 3 infection in the Netherlands.
Methods:
Cytokine evaluation was performed via multiplex cytokine array for 65 immune markers in plasma during the different phases of hepatitis.
Results:
The patient initially presented with features typical of acute viral hepatitis, with detectable HEV RNA in blood. This evolved into a cholestatic disease following peripheral clearance of the virus, leading to the demise of the patient. Real time PCR revealed the presence of HEV in liver tissue, suggestive of active intrahepatic infection despite clearance in blood. During the phase of detectable HEV RNA in serum, there was a surge in T-cell-related immune mediators, as well as interferon alpha and interferon gamma-induced protein 10 (IP-10), characteristic of a viral infection. After clearance of the virus in the blood and development of cholestatic hepatitis, several inflammatory markers subsided, followed by an increase in immune factors related to anti-inflammatory activity, as well as monocyte/macrophage-related markers, likely due to the intrahepatic presence of the virus.
Conclusions:
This report describes the dissociation of intra- and extra-hepatic immune responses during acute HEV infection. As shedding of the virus became solely intrahepatic, an immune profile reflective of the activity of hepatic resident cells was observed.
Keywords: HEV, Immune-regulation, Cholestatic hepatitis
Introduction
Hepatitis E virus (HEV), specifically genotype 3, has been recognized as a cause of chronic hepatitis in immunosuppressed individuals, particularly in solid organ transplant recipients in high-resource countries (Debes et al., 2016; Kamar et al., 2008). In recent years, some limited insight into the immune modulation triggered by chronic HEV infection has been obtained. However, acute symptomatic HEV genotype 3 infection is rare in resource-rich countries (Brost et al., 2010; Saint-Jacques et al., 2016). Consequently, there is a paucity of knowledge about the immune processes involved during acute HEV infection with genotype 3. This case report describes a patient with fatal autochthonous cholestatic HEV in the Netherlands, with an analysis of the patient’s peripheral immune responses.
Case report
A73-year-old male with a history of diabetes mellitus, presented with a 2-week history of fatigue and dark urine. His vital signs were within normal limits and his physical examination was remarkable for jaundice.
Laboratory investigations showed an aspartate aminotransferase (AST) level of 4700 IU/ml, alanine aminotransferase (ALT) of 5600 IU/ml, total bilirubin of 15 mg/dl, alkaline phosphatase of 149 IU/ml, international normalized ratio (INR) of 1.2, and ferritin level of 19 000 ng/ml. A complete blood count and chemistry panels were normal. The patient denied taking any new medications, over-the-counter products, or ingesting mushrooms. He denied the use of alcohol, cigarettes, or illicit drugs. He had no history of recent travel and no close contacts reported any recent illnesses.
An infectious work-up revealed negative serology for common viral hepatitis including hepatitis A, B, and C viruses, Epstein–Barr virus (EBV), herpes zoster virus (HSV), and cytomegalovirus (CMV). Serologies for HEV IgG and IgM were positive, and PCR for HEV was positive at 8.3 × 104 IU/ml. Further HEV amplification determined the presence of HEV genotype 3. Computed tomography of the abdomen revealed a possible nodular liver contour, but no evidence of portal hypertension. The patient was diagnosed with acute autochthonous HEV infection. Due to the possible nodular contour, there was concern for liver cirrhosis in this patient, but a fibroscan study did not indicate advanced fibrosis.
Following admission, the patient’s liver enzyme levels showed a downward trend. He showed signs of clinical recovery and was discharged home with no treatment and a diagnosis of acute hepatitis E in a recovering phase.
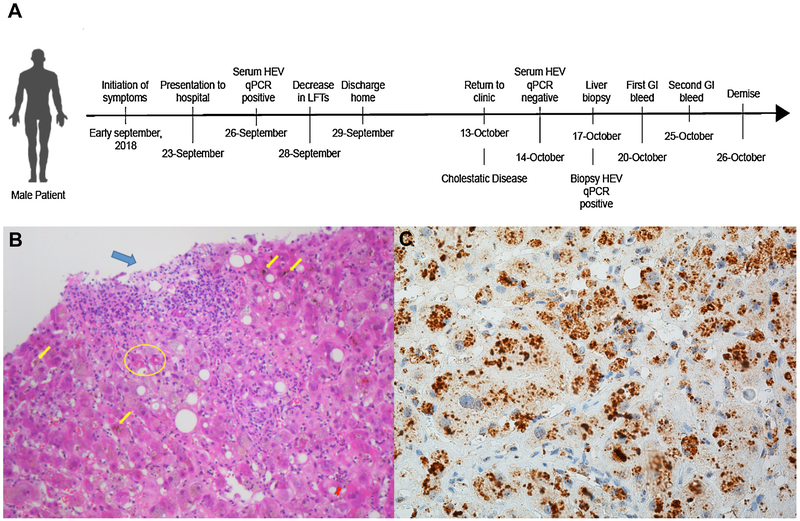
At the patient’s follow-up visit 2 weeks later in the outpatient clinic, the patient continued to express fatigue. His ALT and AST levels were 372 IU/ml and 470 IU/ml, respectively, but his total bilirubin level had increased to 33 mg/dl. The patient was readmitted to hospital (for a chronology of events, see Figure 1A). Repeat PCR for HEV RNA in blood was negative, indicating no peripheral viral activity (at the time no feces were sent for HEV assessment). It was decided to obtain a liver biopsy to aid in the diagnosis, which revealed mild lobular and portal inflammatory infiltrates with obvious bilirubinostasis and slight periportal fibrosis, with no evidence of cirrhosis (Figure 1B). The biopsy showed no evidence of medication- or toxin-induced liver disease. A diagnosis of cholestatic hepatitis was made. Over the following week, the patient developed an upper gastrointestinal bleed secondary to duodenal ulcers, suffered further hepatic as well as renal decompensation, and passed away.
Figure 1.
(A) Graphic describing the chronological order of presentation, laboratory values, and clinical course. (B) Liver biopsy (hematoxylin–eosin ×200): mild portal (blue arrow) and mild lobular (green arrow) inflammation; bilirubinostasis (yellow arrows) and Mallory hyalin in the cytoplasm of hepatocytes (yellow circle). (C) Immunohistochemistry of liver biopsy (J2 × 40): anti-ds-RNA antibody staining (brown) in cytoplasmic and perinuclear areas.
Results
To obtain greater insight into the immune processes active during the acute HEV infection in this patient, a microsphere-based multiplex protein assay was performed. It was observed that during the acute phase of the HEV infection, expressed by positive HEV RNA in serum and high levels of transaminases, dramatically increased levels of all immune analytes were present in the patient as compared to two healthy controls of similar age and sex (Figure 2, left panel). These included elevated T-cell-related cytokines such as interleukin (IL)-2 and interferon gamma (IFN-γ), and monocyte-derived cytokines such as tumor necrosis factor (TNF), IL-12p70, IL-18, and macrophage colony-stimulating factor (M-CSF), as well as markers characteristic of viral infections such as interferon alpha (IFN-α) and IP-10. These mediators have all been shown to increase during acute viral hepatitis from different viruses, suggesting no major differences in initial immune response for HEV compared to other viral infections (Stacey et al., 2009).
Figure 2.
Differential levels of immune markers in plasma following changes in HEV RNA levels in blood. Arrows pointing up describe an increase and arrows pointing down describe a decrease. The left panel describes the fold change compared to controls; the panel on the right describes the fold change after negativization of HEV RNA in blood.
Following negativization of serum HEV RNA and the decrease in liver transaminases, several markers, including soluble IL-2 receptor (sIL-2R), IP-10, and eotaxin 3 normalized, as would be expected with resolution of the viral hepatitis (Figure 2, right panel). However, after peripheral HEV clearance, a further increase in the T-cell-derived cytokines IL-2, IFN-γ, and IL-10 was observed, likely reflecting strong induction of proinflammatory Th1 responses that are counteracted by the activity of the anti-inflammatory cytokine IL-10. Also, the levels of IFN-α as well as important proinflammatory mediators, such as IL-15, TNF, and IL-1β produced by Kupffer cells, monocytes, and hepatocytes, remained elevated or were even further augmented. Despite the absence of detectable HEV RNA in serum, significantly elevated levels of bilirubin were observed. Surprisingly, examination of the liver biopsy by HEV RNA PCR demonstrated a positive result (5.7 × 105 arbitrary units (AU)). For comparison, we added a negative biopsy from a chronic HCV patient (<200 copies/g) and a positive sample from a patient with acute HEV (6.1 × 108 arbitrary units (AU)). Staining of the biopsy with the J2 antibody for double-stranded RNA (ds-RNA) was positive, whereas uninfected control liver was negative (Figure 1C). These findings indicate that although HEV RNA was cleared from blood, the virus was still present and active in the liver.
Discussion
This case emphasizes the possibility of acute cholestatic viral hepatitis E due to the presence of intrahepatic HEV RNA with negative peripheral RNA, which has not been reported before. Interestingly, during the cholestatic phase and peripheral clearance of HEV in blood, there was an inflammatory cascade related to monocyte/Kupffer cell activation. This clinical manifestation possibly suggests a response of the intrahepatic component of the immune system that could have led to the cholestatic pattern observed. Therefore, one could speculate that while the peripheral inflammatory pattern normalized, an augmented intrahepatic immune response was observed due to the presence of the virus in this environment, arguing for a dissociation of intra- and extra-hepatic immune responses.
This case also highlights potential explanations for the lack of correlation between HEV RNA detection and HEV IgM levels when diagnosing acute HEV. The authors of several studies have reported difficulties in diagnosing acute HEV due to individuals presenting positivity in either one or the other test, but not both. This has generally been attributed to the lack of standardized PCR techniques or poor sensitivities of IgM ELISA tests (Debes et al., 2016; Cattoir et al., 2017). However, as in our patient, a lack of peripheral blood HEV RNA concomitant with intrahepatic replication could explain this dissociation and should be kept in mind in the appropriate clinical setting. Moreover, as HEV can replicate in the liver without replication in blood (such as in our patient), in situations where liver biopsy is not possible, consideration should be given to stool HEV PCR assessment (which represents shedding from bile) or repetition of HEV IgM. These tests, if properly applied, could increase the odds of diagnosis in cases of high suspicion.
Methods
Assessment of levels of cytokines/chemokines
Plasma was collected and multi-analyte profiling of cytokines, chemokines, growth factors, and other proteins was performed using the Procarta Plex Human Immune Monitoring Panel (Affymetrix; eBioscience, Vienna, Austria). The panel measured 65 proteins simultaneously (Spaan et al., 2016). The assay was conducted according to the manufacturer’s instructions, similarly to our previous studies (Spaan et al., 2016). All samples were analyzed in one run. Data were analyzed using Procarta Plex Analyst 1.0.
Liver tissue HEV PCR
RNA was isolated from formalin-fixed paraffin-embedded (FFPE) core needle liver biopsies using the RNA isolation RNAeasy FFPE Kit (Qiagen, Hilden, Germany). Positive (acute HEV) and negative (chronic HCV) biopsies were included as controls. All samples were screened for the presence of HEV RNA using an ISO15189:2012-validated, internally controlled quantitative real-time RT-PCR, as described previously (Pas et al., 2012).
Immunohistochemical staining for double-stranded RNA
Immunohistochemistry was performed with an automated, validated, and accredited staining system (Ventana Benchmark ULTRA; Ventana Medical Systems, Tucson, AZ, USA) using the Optiview Universal DAB Detection Kit (#760–700). In brief, following deparaffinization and heat-induced antigen retrieval, the tissue samples were incubated with mouse monoclonal antibody anti-ds-RNA (J2, English and Scientific Consulting Kft.) for 30min. Incubation was followed by hematoxylin II counter-staining for 12 min and then blue coloring reagent for 8 min, according to the manufacturer’s instructions.
Funding
This work was supported by the VIRGO consortium, the Robert Wood Johnson Foundation (AMFP), American College of Gastroenterology, and NIH-NCI grant number R21 CA215883-01A1.
Role of the funding source
None of the funding sources stated had any influence on the study design, interpretation of data, or writing of the manuscript.
Footnotes
Ethical approval
This study was approved by the Ethics Committee of Erasmus MC, Rotterdam, the Netherlands.
Conflict of interest
No conflict of interest declared by any of the authors.
References
- Brost S, Wenzel JJ, Ganten TM, Filser M, Flechtenmacher C, Boehm S, et al. Sporadic cases of acute autochthonous hepatitis E virus infection in Southwest Germany. J Clin Virol 2010;47:89–92. [DOI] [PubMed] [Google Scholar]
- Cattoir L, Van Hoecke F, Van Maerken T, Nys E, Ryckaert I, De Boulle M, et al. Hepatitis E virus serology and PCR: does the methodology matter?. Arch Virol 2017;38:1062–9. [DOI] [PubMed] [Google Scholar]
- Debes JD, Pisano MB, Lotto M, Re V. Hepatitis E virus infection in the HIV-positive patient. J Clin Virol 2016;80:102–6. [DOI] [PubMed] [Google Scholar]
- Kamar N, Selves J, Mansuy JM, Ouezzani L, Péron JM, Guitard J, et al. Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N Engl J Med 2008;358:811–7. [DOI] [PubMed] [Google Scholar]
- Pas SD, de Man RA, Mulders C, Balk AH, van Hal PT, Weimar W, et al. Hepatitis E virus infection among solid organ transplant recipients, the Netherlands. Emerg Infect Dis 2012;18:869–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Jacques P, Tissot-Dupont H, Colson P. Autochthonous infection with hepatitis E virus related to subtype 3a, France: a case report. Ann Hepatol 2016;15:438–41. [DOI] [PubMed] [Google Scholar]
- Spaan M, van Oord G, Kreefft K, Hou J, Hansen BE, Janssen HL, et al. Immunological analysis during interferon-free therapy for chronic hepatitis C virus infection reveals modulation of the natural killer cell compartment. J Infect Dis 2016;213:216–23. [DOI] [PubMed] [Google Scholar]
- Stacey AR, Norris PJ, Qin L, Haygreen EA, Taylor E, Heitman J, et al. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J Virol 2009;83:3719–33. [DOI] [PMC free article] [PubMed] [Google Scholar]