Abstract
Extensive experimental evidence shows that platelets support tumor metastasis. The activation of platelets and the coagulation system play a critical role in the progression of cancer. Within the circulatory system, platelets guard tumor cells from immune elimination and promote their arrest at the endothelium, supporting the establishment of secondaruy lesions. These contributions of platelets to tumor cell survival and spread recognize platelets as a new avenue for therapy.
Cancer patients frequently present with signs of thrombosis, and these are most severe if the disease has progressed to a metastatic stage 1–3. Severe forms of thrombosis include disseminated intravascular coagulation [G], migratory thrombophlebitis [G] and pulmonary embolism [G] as signs of aberrant platelet activation and aggregation 4. However, even if thrombotic events are not detected, coagulation parameters are often elevated in cancer patients, and platelet turnover is generally enhanced. These clinical observations indicate a potential relationship between the blood coagulation system, platelet functions, and cancer spreading via the blood stream. To investigate if a functional relationship may exist between platelet activities and tumor progression, experimental models have been developed to understand how specific platelet functions might contribute to this process. Analytical animal models in which specific platelet functions were altered through drug treatment or controlled genetic ablation have yielded evidence that platelets can guard tumor cells from immune elimination within the circulatory system, promote tumor cell arrest within the vasculature and affect tumor cell survival, thereby supporting the establishment of secondary lesions.
In this Opinion article, we highlight evidence indicating that the activation of platelets and the coagulation system play a critical role in the metastatic progression of cancer. Insight from studies pointing to contributions of platelets to tumor cell survival and spreading support the notion that platelets are important, if not essential, in the development of metastases from the blood stream. The existing knowledge and further mechanistic studies could recognize platelets and their functions as a new avenue for anti-metastatic therapy.
The metastatic process, platelets, and hemostasis
Tumor metastasis to distant organs depends on interactions between tumor cells and the host microenvironment within the circulation, lymphatic vessels and target tissues. This involves blood cells, components of the coagulation system, stromal cells, and the extracellular matrix. Cells within the blood stream that contribute to metastasis are endothelial cells, platelets, lymphocytes, macrophages, mast cells, fibroblasts, and bone marrow-derived progenitor cells 5. The link between platelets and cancer was first documented in the mid 19th century by Trousseau who diagnosed himself and his patients with excessive blood clotting, caused by occult carcinoma that led to the inflammation of blood vessels 6. He observed an association of thrombosis and cancer, and proposed that changes in the hemostatic system support the progression of the malignancy 7. These initial findings suggest that tumors which are capable of activating platelets and forming clots are likely to spread and have a poor outcome. It is now well recognized that in cancer patients, tumor growth is accompanied by the development of an increased tendency toward blood clotting (hypercoagulable state), platelet abnormalities, and an increased risk of thromboembolic disease [G] 1, 4. Moreover, thromboembolism is an early diagnostic feature 4 and a major cause of death 8. Platelet activation is amplified in many cancers and found within the tumor vasculature 9–12. Platelet counts commonly vary during cancer progression and may predict the clinical outcome. Thrombocytosis (high platelet count) associates with poor prognosis in colon, breast, lung, gastric, renal, cervical, pancreatic, endometrial, brain and ovarian cancers 9, 13–19. Thrombocytopenia (low platelet count) also occurs, but is mostly associated with chemotherapy that suppresses platelet production in the bone marrow 20.
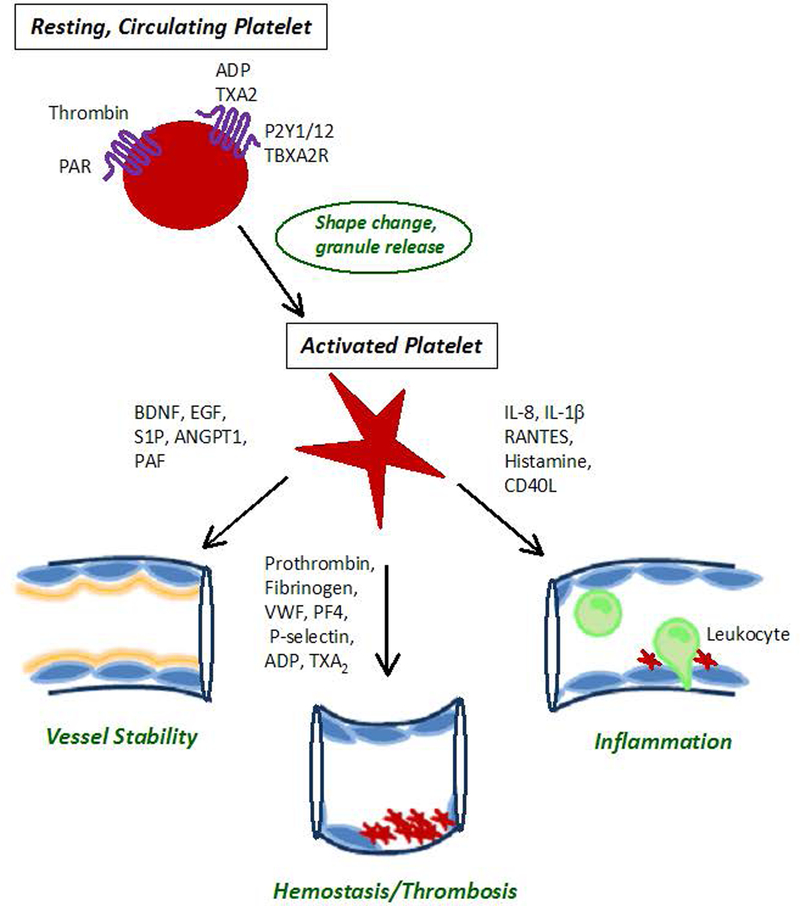
The basic biological functions of platelets in coagulation and hemostasis (Figure 1) are enacted during tumor metastasis. Platelets, or thrombocytes, are small, specialized blood cells released as anuclear cytoplasmic bodies from megakaryocytes in the bone marrow. One liter of blood contains about four hundred billion circulating platelets that are turned over every week. The main function of platelets is to halt hemorrhage after tissue trauma and vascular injury 21, 22. This is initiated through platelet activation, which results in adhesion and release of a multitude of bioactive factors from platelet granules. The process leads to firm attachment of platelets to the injured vessel wall and platelet aggregation induced thrombus formation to seal the wound. Interaction between platelets and damaged endothelium or the subendothelial matrix initiates transient platelet adhesion. Binding of platelet receptors such as glycoprotein VI (GPVI) and glycoprotein Ibα (GPIbα) to exposed collagen and von Willebrand factor (VWF), respectively, leads to activation of platelet integrin αIIbβ3, also known as glycoprotein IIb/IIIa (GPIIb/IIIa), which enables firm adhesion and platelet aggregation. The serine protease thrombin, generated from its latent form in plasma, triggers a strong procoagulant response by fully activating platelets and converting fibrinogen into crosslinked fibrin. This maximizes local thrombus formation and release of platelet granules that contain active proteases, growth factors, matrix proteins and chemokines.
Figure 1. Platelet biology.
During thrombus formation, thrombin activates G protein-linked protease-activated receptors (PARs) at the platelet surface, thereby triggering a flux in intracellular calcium and a decrease in cyclic adenosine monophosphate (cAMP). This allows for rapid reorganization of the platelet cytoskeleton and shape change, maximizing platelet adhesion during thrombus formation. Other platelet agonists include collagen of the subendothelial matrix exposed during vascular injury, adenosine diphosphate (ADP), and thromboxane A2 (TXA2). The latter bind G protein-coupled receptors P2Y and TXA2R respectively, to stimulate platelet degranulation and release of their contents as part of an amplification loop of platelet activation and aggregation. For instance, thrombosis is perpetuated by prothrombin, fibrinogen, von Willebrand factor (VWF), platelet factor 4 (PF4), P-selectin, TXA2, and adenosine diphosphate (ADP) 21, 22. Platelets are now also recognized as modulators of endothelial integrity and immune cell trafficking between the blood stream and sites of inflammation through their released factors 45, 152. Platelets sustain vascular stability through maintenance of endothelial cell junctions via the release of brain-derived neutrophic factor (BDNF), epidermal growth factor (EGF), sphingosine 1 -phosphate (S1P), angiopoietin −1 (ANGPT1), and platelet-activating factor acetylhydrolase (PAF) 152. Activated platelets dispense interleukin-8 (IL-8), IL-1β, C-C motif chemokine 5 (CCL5, also known as RANTES), CD40 ligand (CD40L) and histamine that stimulate endothelial cells to upregulate adhesion receptors and chemokine release to promote leukocyte recruitment and tissue infiltration during the inflammatory response 22, 153. The potential role of platelets in cancer progression and target organ colonization by circulating tumor cells is summarized in this review.
Components of the extrinsic hemostatic pathway, in particular coagulation factor VII (FVII) and tissue factor, are dramatically activated in cancer patients 23. Tissue factor expressed by tumor cells interacts with coagulation factors VII (FVII) and coagulation factor X (FX), and generates thrombin to enhance tumor progression 24, 25. Thrombin not only leads to fibrin formation and activation of platelet receptors that promote thrombosis, but also supports tumor cell malignancy 26–28. Thus, tissue factor and thrombin play critical roles in tumor growth, angiogenesis, and metastasis. These processes are reported in an extensive body of literature and were covered by other reviews 29–31.
A resurgence of inquiry in the field refined the original hypothesis of platelet involvement in tumor metastasis based on the use of transgenic animal models, and thereby tying in a broader knowledge and renewed appreciation of platelets as an important part of the host microenvironment 5, 32–36 (Table 1). Investigation led to a model of platelet-supported tumor metastasis where tumor cells enter the blood stream (intravasation), bind and activate platelets (cohesion) as well as leukocytes. Evidence is emerging that these host cells then assist tumor cell arrest at the vessel wall (adhesion), survival within the vasculature (immune evasion), enabling exit from the circulation (extravasation), and tumor cell survival and proliferation within target tissues of metastasis 8, 37–40. In making this argument, we recognize that there are still many opportunities for research into the role of platelets and that, in many cases, discrete evidence is still needed. However based on reported results, we suggest that the hemostatic properties of platelets critically contribute to metastasis and should be considered in cancer treatment and targeted therapy.
Table 1.
Key platelet molecules involved in tumor metastasis, and models used for their identification
| Platelet or progenitor molecule |
Mouse model Genotype Phenotype / background |
Conclusion and implicated role in tumor metastasis |
|---|---|---|
| NF-E2 |
NF-E2−/− Complete platelet deficiency Immune deficient host |
Platelets support experimental metastasis 60 |
| Gαq |
Gαq−/− Defective platelet activation Immune competent host |
Platelet activation protects tumor cells from natural killer cells; platelets support spontaneous metastasis 51 |
| PAR4 |
Par4−/− Impaired platelet activation Immune competent host |
Thrombin-activated platelets enhance metastasis 60 |
| P-selectin |
P-selectin−/− Immune competent host |
Platelets cohere with colon cancer cells and leukocytes to enhance metastasis 49, 81 |
| GPIbα |
GpIbα−/− Immune competent host |
GPIbα supports experimental metastasis of melanoma cells 106 |
| GPVI |
GpVI−/− Immune competent host |
GPVI enhances experimental metastasis of melanoma and lewis lung carcinoma 53 |
| LPA |
Platelet depleted mice LPA receptor overexpressing tumor cells |
Platelet-derived LPA enhances bone metastasis and growth of breast cancer cells 48 |
| β3 integrin |
Integrin β3 −/− Immune competent host |
αIIbβ3 on platelets supports bone metastasis 92 |
Gαq (Guanine-nucleotide-binding protein Gq), GPIbα (Glycoprotein Ibα), GPVI (Glycoprotein VI) LPA (Lysophosphatidic acid), NFE2 (Nuclear factor (erythroid-derived 2)), Par4 (Protease-activated receptor 4)
Platelet role in angiogenesis and tumor vascular remodeling
Vascularization within a tumor is a portal through which tumor cells can enter the blood stream to disseminate. Platelets may stabilize vessel growth during tumor development and thus contribute to this process.
As tumors and metastases expand, they must recruit new blood vessels to provide nutrients and oxygen for further lesion growth. Blood vessel formation within tumors is called angiogenesis. Angiogenesis was originally hypothesized by Judah Folkman 41 as a necessary mechanism for tumor progression to prevent hypoxia-induced growth stagnation and necrosis. The finding that platelets release vascular endothelial growth factor (VEGF) upon activation was the first evidence that platelets promote angiogenesis 42. Platelets were then formally hypothesized by Folkman and colleagues 43 to contribute to tumor angiogenesis. Even though platelets are poised to alter the tumor vasculature 9, initial evidence in mouse models remains equivocal. Platelets contain a multitude of factors that are released during platelet activation and which regulate the angiogenic process. These factors are either anti-angiogenic (angiopoietin −1 (ANGPT1, also known ANG-1), sphingosine 1 -phosphate (S1P), serotonin, thrombospondin −1 (THBS1)), or pro-angiogenic (VEGFA, epidermal growth factor (EGF), basic fibroblast growth factor (bFGF, also known as FGF2)), or function as cytokines (interleukin −1β (IL-1β), interleukin −8 (IL-8)) and proteases (matrix metalloproteinase −2 (MMP2), matrix metalloproteinase −9, (MMP9)) (Table 2) 44, 45. Platelet activation can occur locally within the tumor vascular environment, as well as systemically as seen in patients with prostrate, breast, lung, colon or gastric cancer 8. The leaky tumor vasculature may expose matrix proteins such as collagen, a potent activator of platelets. Tumor cells could act indirectly through binding of VWF to initiate platelet tethering, given that VEGF stimulates VWF secretion by endothelial cells 46. Furthermore, systemic deployment of C-X-C motif ligand 12 (CXCL12, also known as SDF-1) from platelets regulates neoangiogenesis through the recruitment of hematopoietic progenitors 47.
Table 2.
Platelet surface molecules and released factors with known role in cancer
| Name | Function | Name | Function |
|---|---|---|---|
| Tumor Promoting | Tumor Promoting | ||
| Cell Surface | α Granules | ||
| αIIbβ3 | Firm platelet adhesion and aggregation; tumor cell metastasis 63 | CCL2 | Macrophage chemokine; tumor cell invasion and tumor macrophage infiltration 158 |
| GPIbα | Platelet adhesion and recovery from vascular injury especially under high shear blood flow; tumor cell metastasis 112 | CCL3 | Proinflammatory cytokine 153 |
| GPVI | Platelet adhesion, activation and aggregation; tumor cell metatasis 55 | CCL5 | Chemokine that recruits leukocytes during inflammation; tumor progression 88 |
| P-selectin | Stabilize platelet aggregation and leukocyte arrest on endothelium; tumor growth and metastasis 51 | CCL7 | Proinflammatory, macrophage chemokine 159 |
| PAR4 | Thrombin receptor that activates platelets; enhances metastasis 62 | Gro-α | Neutrophil chemokine and monocyte arrest 160 |
| Gαq | α subunit of G protein mediates signaling during platelet a ctivation; enhances metastasis 53 |
CXCL5 | Proinflammatory cytokine 160 |
| α Granules | IL-8 | Proinflammatory, leukocyte chemoattractant, angiogenesis 160 | |
| Prothrombin | Activated by tissue factor to convert fibrinogen to fibrin, procoagulant, tumor progression 25 |
CXCL12 | Chemokine; tumor progression and metastasis 5 |
| SERPINE1 | Inhibits plasminogen activation and fibrinolysis; tumor invasion and angiogenesis 161 | BDNF | Neuronal development t and function; receptor Trkb supports tumorigenesis and metastasis162 |
| Plasminogen | Protease that breaks down fibrin in blood clots 161 | TP | Tumor progression and angiogenesis; chemotherapy activation 163 |
| uPA | Serine proteinase that converts plasminogen to plasmin 161 | Dense Granules | |
| EGF | Enhances cell growth, proliferation, differentiation and migration 9 | Serotonin | Platelet aggregation, vascular tone, and cardiac functions; tumor angiogenesis and metastasis 124 |
| TGF-β | Regulates inflammation; both enhancing and inhibitory roles in tumorigenesis and progression 164 | Histamine | Proinflammatory, increases vascular permeability 165 |
| PDGF | Enhances stromal cell survival, proliferation and migration, angiogenesis 9 | ATP | Triggers calcium influx and amplifies platelet aggregation 22 |
| bFGF | Mitogenic, tumor progression and angiogenesis 9 | ADP | Potent activator of platelets, adhesion and aggregation 22 |
| HGF | Tumor progression, metastasis, and angiogenesis 9 | IL-1β | Proinflammatory cytokine, induces angiogenesis 153 |
| IGF1 | Mitogenic, tumor progression and angiogenesis 9 | VEGF | Regulates vascular permeability and growth, stimulates angiogenesis 43 |
| VEGF | Regulates vascular permeability and growth, stimulates angiogenesis 43 | Tumor Inhibiting | |
| ANGPT1 | Maintains vascular integrity; tumor progression and angiogenesis 166 | α Granules | |
| MMP-1 | Tissue remodeling and matrix degradation 106 | PF4 | Promotes blood coagulation; inhibits tumor growth and angiogenesis 133 |
| MMP-2 | Tissue remodeling, matrix degradation and tumor cell invasion 106 | TSP-1 | Enhances platelet aggregation and inhibits angiogenesis 52 |
| MMP-9 | Matrix degradation and tumor cell invasion, metastasis 104 | VWF | Matrix protein, forms multimers to aggregate platelets, binds GPIb-V-IX; inhibits tumor cell metastasis 115 |
| Fibrinogen | Coagulation, precursor of fibrin clots, matrix protein, platelet adhesion and aggregation; protects and adheres tumor cells in circulation 53 | PGK | Glycolytic enzyme; anti-angiogenic factor 167 |
| Fibronectin | Extracellular matrix protein, binds integrins, fibrin, and collagen; tumor cell invasion and metastasis 73 |
TIMP | Inhibits matrix metalloproteinases, antiangiogenic 106 |
| PAF acetylhydrolase | Inflammation and hemostasis, lipid messenger 168 | Collagen α1(XVIII) chain isoform 1 precursor (Endostatin) | Angiogenesis inhibitor, derived from collagen type XVIII 43 |
| S1P | Vascular permeability, cell invasion and survival 169 | Dense Granules | |
| LPA | Cell proliferation, survival and migration 170 | TFPI | Serine protease inhibitor, inactivates TF-FXII coagulation 171 |
| P-selectin | Cell adhesion molecule, proinflammatory, marker of platelet activation; tumor progression 51 | PF4 | Promotes blood coagulation; inhibits tumor growth and angiogenesis 133 |
ADP (Adenosine diphosphate), ATP (Adenosine triphosphate), ANGPT1 (Angoipoietin-1), bFGF (Basic fibroblast growth factor), BDNF (Brain-derived neutrophic factor), CCL (C-C motif chemokine), CXCL5 (C-X-C motif chemokine), EGF (Epidermal growth factor), GPIbα (Glycoprotein Ibα), GPVI (Glycoprotein VI), Gro-α (Growth-regulated protein homolog), HGF (Hepatocyte growth factor), IGF1 (Insulin-like growth factor 1), IL(Interleukin), LPA (Lysophosphatidic acid), MMP-2 (Matrix metalloproteinase-2), PGK (Phosphoglycerate kinase), SERPINE1 (serpin peptidase inhibitor, also known as plasminogen activator inhibitor-1, PAI 1), PF4 (Platelet factor 4), PAF (Platelet-activating factor acetylhydrolase), PDGF (Platelet-derived growth factor), PGK (Phosphoglycerate kinase), S1P (Sphingosine 1 -phosphate ), TFPI (Tissue factor pathway inhibitor), TIMP (Tissue inhibitor of metalloproteinases), TSP-1 (Thrombospondin-1), TP (Thymidine phosphorylase), TGF-β (Transforming growth factor-β), uPA (Urokinase plasminogen activator), VEGF (Vascular endothelial growth factor), VWF (von Willebrand factor). See 45 for additional information.
Due to the regulatory impact of platelets on vascular remodeling, they were surmised to help shape the tortuous vasculature within tumors. Platelets have been proposed to alter blood vessels through localized release of platelet factors 43. Interestingly, platelets have compartmentalized pro- and anti-angiogenic α granules, which may be differentially released depending on the stimuli. For example, granules laden with VEGF are selectively released after ligation of protease-activated receptor PAR1, while granules containing endostatin are released upon PAR4 activation 48, 49. These findings not only explain a pivotal role of platelets in regulating hemostasis, but also suggest that tumor cells could use platelets to balance an angiogenic milieu. In a similar way as platelet-derived LPA enhances bone metastatic growth and progression 50, platelet-released factors may play a role in tumor progression, and therefore warrant further investigation. The attenuated tumor growth observed in P-selectin-deficient mice 51 could affect metastasis based on the impaired recruitment of leukocytes, although the contributions of endothelial and platelet P-selectin are indiscriminate. In contrast, an initial study demonstrates that the transplantation of wild-type bone marrow into thrombospondin-1-deficient mice resulted in diminished tumor growth 52. This implies that platelet delivery of an angiogenesis inhibitor may suppress tumor progression. Nonetheless, there is no change in tumor growth in mice deficient in fibrin, platelet activation or GPVI expression, and there are no overt vascular differences from the wild-type 53–55. Fundamental questions of how platelets affect tumor angiogenesis, and in which way their pro- or anti-angiogenic capacities contribute to tumor progression remain to be investigated further.
A recent study provides evidence that platelets prevent hemorrhaging within a tumor 56, indicating that the hemostatic functions of platelets are likely critical for maintaining vascular integrity in tumors. Antibody-induced thrombocytopenia in mice was immediately followed by erupted hemorrhage within tumors, which resulted in reduced tumor cell proliferation and increased apoptosis 56. The severe bleeding could be rescued by reconstitution with resting platelets, but not with platelets that were experimentally exhausted of their granule content after ex vivo thrombin activation. This indicates that secretion of platelet granules is crucial for platelet regulation of tumor vascular homeostasis. The resulting impact on tumor growth would be interesting to be studied further. It was proposed that platelets stabilize the tumor vasculature through anti-angiogenic factors such as ANGPT1, and serotonin, which presumably counteract VEGFA released from the tumor cells 56. Another possibility is that platelets dampen inflammation and local tissue damage through modulation of leukocyte recruitment or cytokine release 57. Therefore, tumors may develop a dependence on platelets for preventing hemorrhage during tumor growth and angiogenesis.
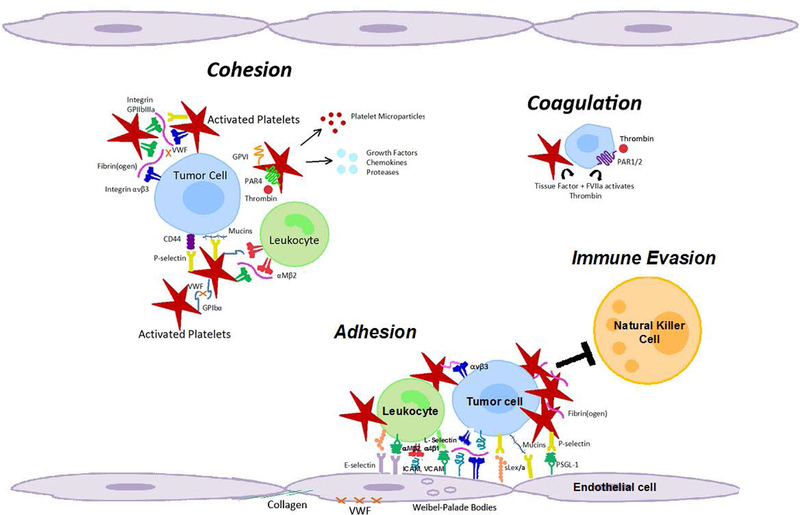
While platelet factors help to regulate tumor blood vessel integrity, adhesive platelet functions and thrombus formation, surprisingly, are apparently not required 58. Mice deficient in either platelet GPIbα, VWF, P-selectin or integrin αIIbβ3 did not show tumor hemorrhaging. However, angiogenesis analyzed in the cornea of the eye clearly involved platelet adhesion to prevent hemorrhage, as seen in mice rendered thrombocytopenic or mice lacking platelet receptor GPIbα 59. Supporting this premise, previous in vivo imaging of platelets in tumors showed platelet rolling along the endothelium, but neither firm adhesion nor thrombus formation within the tumor microcirculation 60. The slowed blood flow within tumors may allow for localized platelet granule release 43, 58 and not require involvement of receptors and ligands that mediate platelet adhesion under higher shear conditions. While platelets and their hemostatic functions impact vascular integrity and remodeling within tumors, there is no direct evidence that platelets also affect tumor cell intravasation and access to the circulation. As the molecular mechanisms though which platelets contribute to primary tumor growth and angiogenesis still need to be explored further, convincing evidence already indicates a critical role of platelets in promoting tumor cell metastasis from the blood stream (Figure 2).
Figure 2. Molecular coordination between platelets and tumor cells supports metastasis from the blood stream.
As platelets become activated, they undergo a shape change, increase their adhesiveness, release granules and microparticles, and perpetuate cohesion of heteroaggregates containing tumor cells, platelets and leukocytes. Platelet granules contain growth factors, chemokines, and proteases. Cohesion: Circulating tumor cells can interact with activated platelets and leukocytes, and form heteroaggregates that support attachment to the endothelium and thereby contribute to metastasis. Coagulation: Platelet-initiated coagulation steps can activate receptors on tumor cells (e.g. PAR1/2) and promote release of tissue factor that further enhances procoagulant activity. Adhesion: Initial, transient adhesion involving selectins, mucins, CD44, and other sialyl Lewis X/A containing glycoconjugates is followed by firm attachment mediated by integrins and intercellular cell adhesion molecules. Immune evasion: Multivalent plasma proteins form intercellular bridges, and activated platelets and fibrin(ogen) protect tumor cells from natural killer cell lysis during hematogenous metastasis. Coincidently, tissue factor and FVIIa activate thrombin to cleave protease-activated receptors on platelets and tumor cells to promote invasion and metastasis. GP (Glycoprotein), PAR (Protease-activated receptor), ICAM (Intercellular adhesion molecule, ICAM-1), VCAM (Vascular cell adhesion molecule, VCAM-1), PSGL1 (P-selectin-glycoprotein ligand 1), sLex/a (Sialyl Lewis X/A antigens), VWF (Von Willebrand factor).
Survival of tumor cells and immune evasion
The microenvironment within the blood stream is hostile for circulating tumor cells and their survival before extravasation is critical for metastasis. Antibody-induced 61 or genetic 62 depletion of platelets inhibits metastasis, whereas platelet reconstitution restores metastatic activity 63 as seen in several independent mouse models. A definitive study explored a model that lacks circulating platelets based on nuclear factor (erythroid-derived 2) (NFE2) knockout which interrupts platelet production through interfering with megakaryocyte maturation 62 (Table 1). Experimental metastasis is nearly completely inhibited in these mice. These results, together with reduced metastasis in PAR4- and fibrinogen-knockout mice, indicate that platelets and their activation play an essential role in hematogenous tumor cell spreading and involve thrombin-dependent and -independent mechanisms 62. Platelets of Par4−/− mice are unable to aggregate in response to thrombin, but their thromboxane and GPVI receptors are not disturbed 61,64. Treatment with thrombin inhibitor hiruidin further reduces metastasis in PAR4 null mice, implying a mechanism that involves other thrombin ligands such as fibrinogen 65, or PARs expressed by the tumor cells. In fact, host deficiencies in PAR1 and PAR2, where endothelial responses to coagulation proteases - but not those of the experimentally introduced tumor cells - are attenuated, did not provide protection against metastasis 62. Thrombin activates PAR1 and PAR2 receptors expressed by host and tumor cells, and generally supports tumor growth, angiogenesis and metastasis 66, 67.
Mechanisms by which platelets assist hematogenous metastasis depend on the ability of platelets to act in concert with coagulation factors in protecting tumor cells within the blood stream. When proteases of the hierarchical coagulation cascade activate thrombin, this protein cleaves fibrinogen and induces fibrin formation. During this process, platelets crosslink through adhesion molecules, such as integrin αIIbβ3, which once activated binds soluble fibrinogen and then supports thrombus formation and platelet incorporation into fibrin clots. Newly activated platelets perpetuate thrombosis with discharge of additional fibrinogen, VWF, and fibronectin. Early observers showed that entanglements of platelets and fibrin surround tumor cells that are arrested within the vasculature 68–70. Leukocytes and natural killer cells (NK cells) are found in close proximity of these metastasizing cells 71. NK cells can disable blood borne cancer cells by direct contact and lysis. However, adhesion of platelets to tumor cells and their incorporation into platelet heteroaggregates may shield the tumor cells from NK cell activity. This could explain why clearance of circulating tumor cells by NK cells is incomplete and allows cancer spreading from the blood stream 72. Furthermore, incorporation of plasma fibronectin into fibrin clots enhances metastasis to the lungs, but less so to other sites, as seen in mice that were postnatally depleted of the plasma fibronectin gene in the liver 73.
The involvement of NK cells and hemostatic factors in metastasis was elegantly explored through development of novel mouse and cell models 74. Platelets and tissue factor, and thus thrombin generation, were clearly identified as nodes in the impact that NK cells exert on the metastatic process. Fibrin deposition, induced by tumor cell-associated tissue factor, impedes tumor cell recognition by NK cells and thereby enhances metastasis through cloaking tumor cells within the circulation 53, 75. This concept is based on studies of experimental and spontaneous metastasis in mice that lack Gαq, fibrinogen, or NK cells 51 (Table 1). Mice lacking the α subunit of the heterotrimeric guanine-nucleotide-binding protein Gq (Gαq), a protein critical for platelet activation, were mostly unable to support metastasis after tumor cell injection into the venous circulation. Spontaneous metastasis from primary subcutaneous tumors was also attenuated, while tumor growth itself was not affected. Similar results were seen in fibrinogen-deficient mice and in another study using mice treated with an inhibitor of platelet aggregation 53, 76. In the presence of NK cells, platelet activation is required for tumor cell survival. Notably, the blocking of metastasis by Gαq or fibrinogen knockdown was abrogated when NK cells were immunologically or genetically depleted. This clearly indicates that the supportive effects of platelet activation and fibrinogen involve tumor cell shielding from NK cell function 51 (Figure 2).
The same group later recognized NK cell-independent mechanisms that determine early tumor cell survival in the target organ microvasculature. However, it was clearly documented that even NK-independent survival requires thrombin generation 53, 75. In an elegant approach, tumor cells were generated from murine fibroblasts of tissue factor-deficient embryos by transformation with Ha-Ras. Tumor growth and metastasis of these cells was then studied in immuno competentmice lacking either tissue factor, Gαq, fibrinogen, NK cell function, or which were severely depleted of prothrombin 75. Results from this model indicated that tumor associated tissue factor and distal components of the hemostatic system are important, but not critical, for initial tumor cell localization in the lung microvasculature. However, genetic reduction of prothrombin profoundly reduced the survival of the lodged tumor cells, and thereby determined early success of metastasis development 53, 75, 77. In the presence of NK cells, platelet activation is required for tumor cell survival. Together, these findings suggest that the platelet-fibrin axis plays an important part in immune evasion. Platelets also contribute to NK cell-independent intravascular tumor cell survival. The exact mechanisms of how platelet activation and fibrin clotting protect from NK cells still need to be defined. Current hypotheses include that platelets provide a physical barrier to NK cell contact, or that platelets exert paracrine suppression of NK-mediated cytolytic activity 78. A recent study illuminated one such mechanism by demonstrating that transforming growth factor-β (TGF-β) from platelets diminishes NK granule mobilization, cytotoxicity, and interferon-γ (IFN-γ) secretion 79. Several other soluble mediators, including prostaglandin E2 (PGE2) that were suggested to participate, need to be studied for this capacity 78. Overall, the ability of platelets to induce formation of heteroaggregates between tumor cells, platelets and leukocytes may play an important part in determining tumor cell survival within the microvasculature of target organs of metastasis.
Tumor cell interaction with vascular cells
Transient interactions
The process of tumor cell arrest within the microvasculature of target organs is difficult to study because these adhesive events are rare in a living host. Therefore, tumor cell arrest in vivo cannot be analyzed as reliably and quantitatively as the attachment of circulating leukocytes during inflammation. Furthermore, in vitro models can mimic the complexity of the intravascular situation only in certain aspects. Nevertheless, studies focused on tumor cell interaction with vascular cells at early time points after introducing malignant cells into the circulation of drug treated or genetically altered animals have shed light on tumor cell arrest in target organ vessels 51, 80. These approaches have also yielded information on adhesion receptors and ligands that mediate tumor cell anchorage at the endothelium and the following early events of metastatic colonization as summarized below.
Evidence suggests that rolling and tethering of circulating tumor cells to the vessel wall, and formation of heteroaggregates between tumor cells, platelets and leukocytes can be mediated by selectins, a family of transmembrane cell adhesion molecules. Selectins enable white blood cell trafficking by promoting their transient adhesion within the bloodstream during inflammation. Selectins are expressed by platelets, endothelial cells, and leukocytes, and metastasis studies indicate that platelets can use selectins to support initial transient tumor cell interaction with the endothelium in a manner similar to that observed during recruitment of leukocytes in inflammation 81 (Figure 2).
Selectins contain a conserved lectin domain that recognizes and binds glycoconjugates [G] on other cells 81. All selectins recognize ligands that contain tetrasaccharide sialyl Lewis X/A antigens 82, and can require the clustering of selectin molecules for high affinity binding 81. Some of these glycoconjugate ligands include P-selectin-glycoprotein ligand-1, mucins and CD44 variants, many of which are expressed by metastatic tumor cells 82. There are at least three selectin types. L-selectin is constitutively expressed on leukocytes. E- and P-selectin are present on endothelial cells and platelets, respectively, but only when these are activated. Upon activation, P-selectin translocates to the cell surface from Weibel-Palade bodies [G] in endothelial cells, and from α granules in platelets.
Results from P-selectin knockout mice, established on an immunodeficient background, indicated that tumor growth and metastasis of colon carcinoma cells involves P-selectin function and platelets. This was clearly evident at early steps of tumor cell interaction with the pulmonary microvasculature during metastasis 51, 83. P-selectin on endothelial cells was shown to contribute to experimental metastasis as well, as seen in irradiated wild-type mice after reconstitution with P-selectin-deficient bone marrow 84. In this elegant study, intravascular tumor cell rolling and metastasis were attenuated, but not as strongly as in P-selectin null mice (Table 1). Thus, this model allowed to dissect contributions of P-selectin on endothelial cells and platelets 84. Metastasis can also be attenuated by the anticoagulant heparin. In addition to inhibiting thrombin, heparin binding can mimic negatively charged mucins at the tumor cell surface 71. Thereby, heparin impedes tumor cell binding to P-selectin on platelets 29, 71, 85, 86. P-selectin-dependent tumor cell tethering is mediated in part through binding to tumor cell mucins, which also induce platelet aggregation 51, 83. These interactions were demonstrated using co-cultures of platelets, colon carcinoma mucins, and activated endothelium. The results may have relevance for human colon carcinomas that express mucin type P-selectin ligands. Non-mucin ligands of L- and P-selectins act synergistically to enhance metastasis through tumor cell binding to leukocytes and platelets 87. In static co-cultures of tumor cells, platelets and leukocytes, selectin-dependent interaction between these cell types induces activation of endothelial cells to release C-C motif chemokine 5 (CCL5, also known as RANTES). In vivo, CCL5 likely expressed by activated endothelium recruits monocytes to further increase metastasis 88. Therefore, these findings suggest that platelet-dependent activation of endothelial cells is required for monocytes to home to and promote development of the micrometastatic niche. Under shear stress [G] generated by blood flow, CD44 is a dominant ligand on colon cancer cells that mediates their binding to platelet P-selectin. This engagement can be competitively inhibited by fibrin, but not by fibrinogen 89. Evidence supports that CD44-fibrin interaction helps to establish firm adhesion between platelets and tumor cells. Thus, a model emerges in which tumor cells co-opt platelets and leukocytes to form heteroaggregates, which may travel within the circulation and then tether and roll along activated areas of the endothelium in a P-selectin-dependent manner. The tethering process precedes higher affinity adhesion and tumor cell arrest 90 as a prerequisite for extravasation from the bloodstream.
Adhesion and selection of a metastatic phenotype
Adhesion receptors on platelets can crosslink with tumor cells and leukocytes to help establish firm tumor cell arrest within the vasculature. Platelets assist the attachment of tumor cells to endothelial cells and avidly bind exposed subendothelial matrix proteins. In this process, platelets promote selection of tumor cells with a metastatic phenotype (Figure 2).
The transition from selectin-dependent tumor cell rolling along the endothelium 90 to firm arrest is mediated by integrins. The main platelet integrin involved in this process is integrin αIIbβ3. In hemostasis, this receptor becomes activated in response to platelet agonist stimulation or platelet interaction with collagen at exposed sites of the subendothelial matrix. Activated integrin αIIbβ3 can bind soluble fibrinogen, which then forms inter-platelet bridges and leads to thrombus formation 91 (Figure 1). Fibrinogen also crosslinks tumor cells and platelets. Platelet integrin αIIbβ3 and tumor cell receptors, such as integrin αvβ3, mediate tumor cell-platelet cohesion that helps to overcome shear forces generated by blood flow, in lodging circulating tumor cells to the vessel wall 92. Inhibitors of αIIbβ3 reduce tumor cell colonization e.g. of the lungs 63, 93. This indicates that αIIbβ3 coordinates tumor cell-platelet cohesion and adhesion as important steps in metastasis. Tumor cell integrin αvβ3 then perpetuates adhesion, migration, and invasiveness in cooperation with platelets 92, 94. For instance, knockout mice lacking the β3 integrin subunit gene have reduced bone metastases and bone destruction. This is because platelet integrin αIIbβ3 is required for platelet aggregation and critically contributes to homing of metastatic cells to the bone microenvironment, where osteoclast integrin αvβ3 mediates bone destruction in growing metastatic lesions 95. Direct evidence for platelet involvement in tumor cell lodging within target organ microvessels was obtained from lung preparations, isolated at early time points after tumor cell injection into the venous circulation and infusion with fluorescently labeled antibodies to identify the tumor cells, platelets and fibrin clots 80. Tumor cells were found spread within pulmonary microvessels 2–6 hours post-injection, and they were associated with platelets and fibrin clots. Inhibition of clot formation with hirudin reduced tumor cell retention in the lung 80. Platelets further support bone metastatic growth and cytokine-induced bone destruction by producing lysophosphatidic acid (LPA) in response to stimulation by tumor cells 50, 96. Inhibition of platelet aggregation with Integrilin, an antagonist of platelet integrin αIIbβ3, blocks bone metastasis and thereby identifies platelets as a critical source of LPA in the tumor microenvironment 50, 96.
Generally, cell attachment to the vessel wall critically depends on the affinity state of integrin adhesion receptors 97. This is well established for platelets and leukocytes 91. The specialized functions of these cells in repair of vascular injury and inflammation require that the cells rapidly respond to local stimuli and engage in adhesive interactions that support their arrest at the vessel wall. Agonist stimulation leads to conformational changes of critical integrins, such as αIIbβ3 on platelets, and αMβ2 and α4β1 on leukocytes, and transition of the receptors into a high affinity state 98. Once activated, the receptors can bind ligands from solution, leading to cell-cell cohesion and high affinity interactions with ligands or counter receptors on endothelial cells and proteins of the subendothelial matrix 99–102. During metastasis, activation of platelets, leukocytes and potentially tumor cells can support their cohesion and firm arrest at the vascular endothelium, involving multiple receptors and ligands on each cell type. These include platelet integrin αIIbβ3, leukocyte β2 integrins and α4β1, tumor cell and endothelial cell β1 integrins and αvβ3, intercellular adhesion molecules ICAM, VCAM, as well as P-, L-, and E-selectins 82 (Figure 2).
Analyzing the adhesive behavior of blood borne tumor cells in a blood perfusion model [G] revealed that tumor cells can also express key integrins, such as αvβ3, in distinct states of activation 103. Only the high affinity, activated form of αvβ3 on tumor cells mediates their interaction with platelets and supports tumor cell arrest during blood flow 103. In this way, platelet-supported anchorage selects for a subpopulation of tumor cells that constitutively express the high affinity form of αvβ3 104. In subsequent steps of the metastatic cascade, this high affinity receptor further promotes tumor cell invasion by enhancing protease activation 104, 105, 106. Furthermore, the high affinity form of tumor cell integrin αvβ3 stimulates tumor cell survival and proliferation in target organ tissues, as recently shown for breast cancer brain metastases. Activated αvβ3 elicits brain lesion growth through continuous, hypoxia-independent expression of tumor cell VEGF by inhibiting 4EBP1, a translational repressor known as eukaryotic translation initiation factor 4E-binding protein 1107. Thus, platelet-mediated selection of tumor cells expressing a high affinity receptor can identify a subset of tumor cells with an aggressive metastatic potential. Interestingly, some cancer patients apparently produce inhibitory antibodies against high affinity αvβ3 which inhibit tumor cell platelet interaction and target organ colonization from the blood stream 108. These antibodies can also repress the growth of established, late stage metastases 109. In the absence of β3 integrin expression, alternate mechanisms can drive tumor growth and progression. For example, mammary carcinoma development and spontaneous lung metastasis were essentially unaffected in mice deficient for the β3 and β5 subunit genes or a combination of both, when the disease was driven by the epidermal growth factor receptor 2 (ERBB2, HER2/neu) oncogene110.
Molecules mediating tumor cell migration and adhesion may also bind directly to counterreceptors on platelets. For example, at the leading edge of invasive cells, poliovirus receptor (also known as nectin-like molecule 5, NECL5) colocalizes with integrin αvβ3 111. The NECL5 receptor on colon cancer cells enhances experimental metastasis in a platelet-dependent manner. The process involves adhesion to a counter-receptor on platelets and thereby indirectly enables tumor cell attachment to the vascular endothelium. While intriguing, this mechanism awaits further investigation 111.
Involvement of the subendothelial matrix
Another important platelet adhesion receptor that potentially contributes to tumor progression is the GPIb-IX-V receptor complex. In hemostasis, this complex is central to thrombus formation primarily in the arterial circulation. The interaction between platelet GPIbα and VWF allows platelets to tether to the vascular endothelium, especially in the presence of high shear forces generated by arterial blood flow. This adhesion process enables platelets to recognize exposed sites of extracellular matrix, leading to platelet binding, activation, and thrombus formation 21. The importance of the extracellular arrangement of GPIbα in cancer progression is highlighted in several studies 112, 113. It was demonstrated that experimental metastasis of melanoma cells is reduced in immune competent mice lacking GPIbα 112. In contrast, wild-type mice treated with monovalent antibody fragments directed against GPIbα showed enhanced metastasis of the same melanoma cell type. However, no underlying mechanism was identified 114. Platelet GPIbα might be involved in tumor progression in a number of different ways. The receptor can bind thrombin and VWF. Which ligand is important, and which step of the metastatic cascade may involve GPIbα binding of either of these ligands still needs to be explored. For example, it was shown that VWF can actually inhibit experimental metastasis from the blood stream 115, 116 while thrombin clearly supports this process.
An important trigger of platelet interaction with the vessel wall is the exposure to subendothelial matrix proteins. When platelets encounter denuded sites of subendothelial matrix, they attach to these sites and become activated. During the metastatic process, microlesions in the endothelium could exist in the metastatic niche or be generated through leukocyte- or tumor cell-induced endothelial retraction [G]. Retraction exposes collagen, which is avidly recognized by platelet integrins, such as α2β1, as well as by the platelet glycoprotein receptor for collagen VI (GPVI) 21. GPVI ligation causes platelet activation, aggregation and further amplifies platelet binding to collagen. Whether this mechanism contributes to tumor metastasis is still unclear, but tumor cell colonization of the lungs from the blood stream was found reduced in GPVI-deficient mice 55.
Extravasation
Platelets can modulate the permeability of the vasculature. Platelet-mediated enhancement of vascular permeability, for example, helps immune cells to colonize sites of inflammation. It is possible that platelets might similarly assist tumor cells to exit the blood stream and enter target organs of metastasis. It is likely that such a process involves release of platelet granules and mircoparticles (Box 1).
Box 1. Platelet microparticles.
When platelets are activated, they relinquish surface membrane particles (30–100 nm) and exosomes (100 nm-1 μm) derived from α granules fused with the plasma membrane. Both particle types contain parent platelet membrane proteins and lipids 137. Platelet microparticles pack a repertoire of surface receptors including integrin αIIbβ3, the GPIbα-IX-V receptor complex, P-selectin, CXCR4, bioactive lipids such as LPA and S1P, cytoplasmic and secreted proteins. Furthermore, platelet microparticles provide a procoagulant membrane surface for the activation of thrombin 137. Blood borne microparticles are also formed from endothelial cells, leukocytes and tumor cells, and contribute to many pathological disease states, especially cancer 138, 139. The association of platelet microparticles with individual circulating tumor cells or with tumor cells in heteroaggregates could potentially add to their expression of adhesion molecules, stimulate the release of cytokines, and activate intracellular signaling pathways. As a consequence, downstream effects might alter the reactivity of blood borne tumor cells with the vasculature, stimulate adhesion and proliferation of cancer cells and impact metastasis. Preliminary studies demonstrate an increased invasiveness, migration, proliferation and experimental metastasis of tumor cells when incubated with platelet microparticles 140–144. A mechanistic involvement of platelet microparticles in tumor progression has not been investigated in detail. However, high levels of these particles correlate with aggressive tumors and a poor clinical outcome 145–151.
Platelets and blood vessel permeability
While the profuse release of adhesive molecules, cytokines and mitogens from platelet granules apparently contributes to modulation of immune surveillance, it may also facilitate tumor cell extravasation, survival and growth, although this is mostly speculation for now. The integrity of the vascular endothelium within the microenvironment of attached tumor cell-platelet heteroaggregates may be directly, or indirectly, affected by growth factors released from platelet α granules, including platelet-derived growth factor (PDGF), TGF-β, EGF, insulin-like growth factor −1 (IGF-1), and VEGFA 44, 45. Platelets also release the endothelial agonist S1P, a potent inhibitor of vascular leakage 117, 118. In addition to S1P, platelets are major stores for LPA. Both are signaling molecules that affect cytoskeletal rearrangement, actin changes, cell-cell communication, and influence endothelial permeability. S1P decreases the permeability of endothelial cells, while LPA stabilizes the integrity of certain endothelial types but not in others 119, 120. For instance, LPA notably induces permeability in brain microvascular endothelial cells 121. Thus, platelets may affect blood vessel integrity and could impact tumor cell extravasation. Another potent bioactive factor released from platelets is serotonin. Platelets take up serotonin from plasma, store it in dense granules, and release it upon activation. This leads to modulation of vascular tone, inducing either vasoconstriction or vasodilation 122. Introduction of tumor cells into the circulation of mice can increase plasma levels of serotonin significantly 123. Blockade of serotonin receptors or calcium channels hinders experimental tumor metastasis, as seen in the liver 123, 124. This exemplifies another means by which interference with platelet activation can augment metastasis. An additional potent vasoactive and inflammatory factor released by platelets is histamine. Histamine increases vascular permeability and thus augments leukocyte extravasation. Moreover, platelets release CCL5 which recruits monocytes and eosinophils and triggers further histamine release 125. Together, the integration of circulating tumor cells into platelet-leukocyte heteroaggregates, arrested at the vascular endothelium, generates a rich microenvironment for the incorporated tumor cells that might enhance their extravasation and invasion of target tissues. These hypotheses have yet to be examined.
Therapeutic Implications
Physicians currently administer anticoagulants to prevent and treat venous thromboembolism in cancer patients 126. Going beyond the use of anticoagulants for prophylaxis in paraneoplastic disease [G], these compounds need to be further investigated for the treatment of tumor progression. Low molecular weight heparin has potential to do both 29, 85, 127. Antithrombotic therapies such as Aspirin, Warfarin and Cox inhibitors improve cancer patient survival, but not always. These agents have many targets, apart from tumor cells (platelets predominantly express Prostaglandin G/H synthase 1, also known as Cyclooxygenase-1 COX1) 85, 128, 129, and bleeding side effects may occur. Antiplatelet drugs reduce experimental metastasis in some animal models, but not in others 8. Furthermore, the use of mouse models to assess platelet function in human cancer is limited due to species-specific differences. In particular, human platelets are primarily activated through the PAR1 thrombin receptor and coactivated by PAR4, whereas mouse platelets do not express PAR1 and are prominently activated via PAR4.
Platelets should be considered in the management and detection of human cancers 130–132. The prometastatic properties of platelets raise questions of whether chemotherapy-induced thrombocytopenia and platelet restoration in these patients affect disease progression and outcome. Intriguingly, platelets specifically sequester regulators of angiogenesis (VEGF, platelet factor 4 (PF4), THBS1) released from tumors, as seen in mouse models 52, 133, 134. Increased levels of these factors are detectable in platelets, but not plasma or serum, when dormant tumors are microscopic (<1 mm) 134. Thus, tumor associated alterations of platelet granule contents could serve as valuable biomarkers for cancer progression.
Antiplatelet drugs used for the intervention of cardiovascular disease, such as integrin αIIbβ3 antagonists might be studied for their effects on tumor progression, metastasis and overall survival in clinical trials. Therapies targeting P-selectin 135, GPIb-IX-V, and GPVI could also be explored. Adverse effects due to normal platelet physiology may be overcome by targeting of pathological tumor cell-platelet interactions while maintaining normal platelet functions. So far, no specific platelet function targeted therapy has been included in standard cancer treatment. Furthermore, studies investigating possible links between heritable platelet dysfunctions in the human population and the incidence of metastatic disease have not been undertaken. The overall evidence from experimental models on contributions of platelets to cancer progression, however, predicts that a better understanding of the molecular mechanisms of platelet involvement will help identify promising new clinical approaches.
Perspectives
This Opinion article highlights evidence for a role of platelets in tumor metastasis. Metastasis is a complex process and platelets may contribute to its success at distinct stages of progression. Before the onset of tumor cell dissemination, platelets may affect the development and architecture of the tumor vasculature. The most profound influence of platelets on metastasis likely occurs when tumor cells have entered the blood stream. In that dynamic environment, platelet functions can affect the progression of hematogenous metastasis by complementing tumor cell adhesive properties, providing protection from immune elimination and, through their intricate interactions, select tumor cells with a metastatic phenotype. Future studies might show that the multitude of growth factors and cytokines released from platelet granules also directly impact tumor cell survival and proliferation, and the recruitment of inflammatory cells into the microenvironment of nascent metastatic lesions. Thus, investigation of platelets, their surface receptors, released products, and their critical influence on coagulation pathways, will help elucidate mechanisms of tumor metastasis, as well as vascular development and stability that support metastatic growth.
The changing requirements by disseminating tumor cells during metastasis provide challenging questions to address and understand the complexity of platelet involvement in different stages of cancer progression. Transgenic mouse models with defined aberrations in platelet and other host cell type functions will help to further elucidate critical molecular mechanisms. For example, one such model that has not been explored in the context metastasis is a model of Glanzmann’s thrombasthenia 136. This model recapitulates a human disease where platelets lack or have aberrant expression of integrin αIIbβ3. Models like this offer the opportunity to explore contributions of platelet receptors and functions that are key to platelet adhesion, aggregation and activation.
Important questions to be addressed are: Which stages of tumor progression are affected by platelet hemostatic functions? When are platelet adhesive functions critical to support or influence tumor progression? When and how is platelet involvement in inflammatory functions crucial? New concepts include the identification and properties of premetastatic niches, and how platelets might contribute to their development in distant organs. Platelet functions during the recruitment of bone marrow progentior cells may be involved 34, 47. The development of megakaryocyte lineage-specific mouse models will help delineate platelet contributions, among those of other hematopoietic cells, during the progression of cancer and establishment of metastatic disease.
Supplementary Material
Glossary
- Disseminated intravascular coagulation:
pathological activation of coagulation (blood clotting) mechanisms that lead to formation of small blood clots in blood vessels throughout the body4.
- Migratory thrombophlebitis:
malignancy-associated hypercoagulable state leading to spontaneous platelet-rich clots in veins anywhere in the body that dynamically form, resolve and reappear7.
- Pulmonary embolism:
Blockage of the main artery of the lung or one of its branches by a platelet-rich clot that originated in the venous circulation and dislodged from where it initially formed2.
- Thromboembolic disease:
condition caused by traveling blood clots or emboli that occlude blood vessels; commonly manifesting as deep vein thrombosis or pulmonary embolism in cancer patients2, 3.
- Glycoconjugates:
carbohydrate-linked lipids and proteins, which include receptors and ligands that function as cell adhesion molecules82.
- Weibel-Palade bodies:
secretory granules of endothelial cells that particularly store and secrete multimerized von Willebrand Factor, and which translocate P-selectin to the membrane surface upon cell activation154.
- Shear stress:
the velocity of flowing blood is highest in the center of vessels and decreases toward the vessel wall, resulting in differential flow that generates a shearing force21.
- Blood perfusion model:
parallel plate flow chamber system in which adhesive interactions between blood borne tumor cells, blood cells, platelets and endothelial cells are monitored and measured by video microscopy92.
- Endothelial retraction:
disruption of adherence junctions of endothelial cells creates gaps in the endothelial monolayer and increases vascular permeability while exposing matrix proteins of the basement membrane155.
- Paraneoplastic disease:
disease induced as a result of tumor burden; generally caused by the release of tumor-derived hormones, peptides, or cytokines or by the misguided destruction of normal tissue by immune cells targeted against malignant cells156, 157.
References
- 1.Khorana AA & Connolly GC Assessing risk of venous thromboembolism in the patient with cancer. J. Clin. Oncol 27, 4839–4847 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lyman GH & Khorana AA Cancer, clots and consensus: new understanding of an old problem. J. Clin. Oncol 27, 4821–4826 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khorana AA & Fine RL Pancreatic cancer and thromboembolic disease. The Lancet Oncology 5, 655–663 (2004). [DOI] [PubMed] [Google Scholar]
- 4.Lugassy G, Falanga A, Kakkar A & Rickles F Thrombosis and Cancer (ed. Lugassy G, Falanga A, Kakkar A, Rickles F) (Informa Health Care, London, New York, 2004). [Google Scholar]
- 5.Joyce JA & Pollard JW Microenvironmental regulation of metastasis. Nat. Rev. Cancer 9, 239–252 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trousseau A Phlegmasia alba dolens. (ed. JB B) (1865). [Google Scholar]
- 7.Khorana AA Malignancy, thrombosis and Trousseau: the case for an eponym. J. of Thromb. and Haemost 1, 2463–2465 (2003). [DOI] [PubMed] [Google Scholar]
- 8.Sierko E & Wojtukiewicz MZ Inhibition of platelet function: does it offer a chance of better cancer progression control? Semin. Thromb. Hemost 33, 712–721 (2007). [DOI] [PubMed] [Google Scholar]
- 9.Sierko E & Wojtukiewicz MZ Platelets and angiogenesis in malignancy. Semin. Thromb. Hemost 30, 95–108 (2004). [DOI] [PubMed] [Google Scholar]
- 10.Prisco D et al. Platelet activation and platelet lipid composition in pulmonary cancer Prostaglandins Leukot. Essent. Fatty Acids 53, 65–68 (1995). [DOI] [PubMed] [Google Scholar]
- 11.Blann AD et al. Increased soluble P-selectin in patients with haematological and breast cancer: a comparison with fibrinogen, plasminogen activator inhibitor and von Willebrand factor. Blood Coagul. Fibrinolysis 12, 43–50 (2001). [DOI] [PubMed] [Google Scholar]
- 12.Verheul HMW et al. Platelet and coagulation activation with vascular endothelial growth factor generation in soft tissue sarcomas. Clin. Cancer Res 6, 166–171 (2000). [PubMed] [Google Scholar]
- 13.Erdemir F et al. Clinical significance of platelet count in patients with renal cell carcinoma. Urol. Int 79, 111–116 (2007). [DOI] [PubMed] [Google Scholar]
- 14.Costantini V, Zacharski LR, Moritz TE & Edwards RL The platelet count in carcinoma of the lung and colon Thromb. Haemost 64, 501–505 (1990). [PubMed] [Google Scholar]
- 15.Ayhan A et al. The value of preoperative platelet count in the prediction of cervical involvement and poor prognostic variables in patients with endometrial carcinoma. Gynecol. Oncol 103, 902–905 (2006). [DOI] [PubMed] [Google Scholar]
- 16.Taucher S et al. Impact of pretreatment thrombocytosis on survival in primary breast cancer. Thromb. Haemost 89, 1098–1106 (2003). [PubMed] [Google Scholar]
- 17.Brown KM, Domin C, Aranha GV, Yong S & Shoup M Increased preoperative platelet count is associated with decreased survival after resection for adenocarcinoma of the pancreas. Am. J. Surg 189, 278–282 (2005). [DOI] [PubMed] [Google Scholar]
- 18.Bensalah K et al. Prognostic value of thrombocytosis in renal cell carcinoma. J. Urol 175, 859–863 (2006). [DOI] [PubMed] [Google Scholar]
- 19.Brockmann MA et al. Preoperative thrombocytosis predicts poor survival in patients with glioblastoma. Neuro-Oncology 9, 335–342 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaushansky K Historical review: megakaryopoiesis and thrombopoiesis. Blood 111, 981–986 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruggeri ZM & Mendolicchio GL Adhesion mechanisms in platelet function. Circ. Res 100, 1673–1685 (2007). [DOI] [PubMed] [Google Scholar]
- 22.Davi G & Patrono C Platelet activation and atherothrombosis. N. Engl. J. Med 357, 2482–2494 (2007). [DOI] [PubMed] [Google Scholar]
- 23.Kakkar AK, Deruvo N, Chinswangwatanakul V, Tebbutt S & Williamson RCN Extrinsic-pathway activation in cancer with high factor VIIA and tissue factor. Lancet 346, 1004–1005 (1995). [DOI] [PubMed] [Google Scholar]
- 24.Mueller BM, Reisfeld RA, Edgington TS & Ruf W Expression of tissue factor by melanoma cells promotes efficient hematogenous metastasis. Proc. Natl. Acad. Sci. U. S. A 89, 11832–11836 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruf W & Mueller BM Thrombin generation and the pathogenesis of cancer. Semin. Thromb. Hemost 32, 061,068 (2006). [DOI] [PubMed] [Google Scholar]
- 26.Esumi N, Fan D & Fidler IJ Inhibition of murine melanoma experimental metastasis by recombinant desulfatohirudin, a highly specific thrombin inhibitor. Cancer Res 51, 4549–4556 (1991). [PubMed] [Google Scholar]
- 27.Nierodzik ML, Plotkin A, Kajumo F & Karpatkin S Thrombin stimulates tumor-platelet adhesion in vitro and metastasis in vivo. The Journal of Clinical Investigation 87, 229–236 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu L, Lee M, Campbell W, Perez-Soler R & Karpatkin S Role of endogenous thrombin in tumor implantation, seeding, and spontaneous metastasis. Blood 104, 2746–2751 (2004). [DOI] [PubMed] [Google Scholar]
- 29.Nierodzik ML & Karpatkin S Thrombin induces tumor growth, metastasis, and angiogenesis: evidence for a thrombin-regulated dormant tumor phenotype. Cancer Cell 10, 355–362 (2006). [DOI] [PubMed] [Google Scholar]
- 30.Ruf W Tissue factor and PAR signaling in tumor progression. Thromb. Res 120, S7–S12 (2007). [DOI] [PubMed] [Google Scholar]
- 31.Ruf W, Yokota N & Schaffner F Tissue factor in cancer progression and angiogenesis. Thromb. Res 125, S36–S38 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gupta GP & Massague J Cancer metastasis: Building a framework. Cell 127, 679–695 (2006). [DOI] [PubMed] [Google Scholar]
- 33.Nguyen DX, Bos PD & Massague J Metastasis: from dissemination to organ-specific colonization. Nat. Rev. Cancer 9, 274–U65 (2009). [DOI] [PubMed] [Google Scholar]
- 34.Psaila B & Lyden D The metastatic niche: adapting the foreign soil. Nat. Rev. Cancer 9, 285–93 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steeg PS Tumor metastasis: mechanistic insights and clinical challenges. Nat. Med 12, 895–904 (2006). [DOI] [PubMed] [Google Scholar]
- 36.Chambers AF, Groom AC & MacDonald IC Dissemination and growth of cancer cells in metastatic sites. Nat. Rev. Cancer 2, 563–572 (2002). [DOI] [PubMed] [Google Scholar]
- 37.Honn KV, Tang DG & Chen YQ Platelets and cancer metastasis-more than an epiphenomenon. Semin. Thromb. Hemost 18, 392–415 (1992). [DOI] [PubMed] [Google Scholar]
- 38.Mehta P Potential role of platelets in the pathogenesis of tumor-metastasis. Blood 63, 55–63 (1984). [PubMed] [Google Scholar]
- 39.Jurasz P, Alonso-Escolano D & Radomski MW Platelet-cancer interactions: mechanisms and pharmacology of tumour cell-induced platelet aggregation. Br. J. Pharmacol 143, 819–826 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Erpenbeck L & Schon MP Deadly allies: the fatal interplay between platelets and metastasizing cancer cells. Blood 115, 3427–3436 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Folkman J et al. Tumor angiogenesis-therapeutic implications. N. Engl. J. Med 285, 1182–1186 (1971). [DOI] [PubMed] [Google Scholar]
- 42.Mohle R, Green D, Moore M, Nachman R & Rafii S Constitutive production and thrombininduced release of vascular endothelial growth factor by human megakaryocytes and platelets. Proc. Natl. Acad. Sci. U. S. A 94, 663–668 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pinedo HM, Verheul HMW, D’Amato RJ & Folkman J Involvement of platelets in tumour angiogenesis? The Lancet 352, 1775–1777 (1998). [DOI] [PubMed] [Google Scholar]
- 44.Coppinger JA et al. Characterization of the proteins released from activated platelets leads to localization of novel platelet proteins in human atherosclerotic lesions. Blood 103, 2096–2104 (2004). [DOI] [PubMed] [Google Scholar]
- 45.Smyth SS et al. Platelet functions beyond hemostasis. J. of Thromb. and Haemost 7, 1759–1766 (2009). [DOI] [PubMed] [Google Scholar]
- 46.Brock TA, Dvorak HF & Senger DR Tumor-secreted vascular permeability factor increases cytosolic Ca2+ and von Willebrand factor release in human endothelial cells. Am. J. Pathol 138, 213–221 (1991). [PMC free article] [PubMed] [Google Scholar]
- 47.Jin DK et al. Cytokine-mediated deployment of SDF-1 induces revascularization through recruitment of CXCR4+ hemangiocytes. Nat. Med 12, 557–567 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma L et al. Proteinase-activated receptors 1 and 4 counter-regulate endostatin and VEGF release from human platelets. Proc. Natl. Acad. Sci. U. S. A 102, 216–220 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Italiano JE, Jr et al. Angiogenesis is regulated by a novel mechanism: pro- and antiangiogenic proteins are organized into separate platelet α granules and differentially released. Blood 111, 1227–1233 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boucharaba A et al. Platelet-derived lysophosphatidic acid supports the progression of osteolytic bone metastases in breast cancer. J. Clin. Invest 114, 1714–1725 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim YJ, Borsig L, Varki NM & Varki A P-selectin deficiency attenuates tumor growth and metastasis. Proc. Natl. Acad. Sci. U. S. A 95, 9325–9330 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zaslavsky A et al. Platelet-derived thrombospondin-1 is a critical negative regulator and potential biomarker of angiogenesis. Blood 115, 4605–4613 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Palumbo JS et al. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood 105, 178–185 (2005). [DOI] [PubMed] [Google Scholar]
- 54.Palumbo JS et al. Spontaneous hematogenous and lymphatic metastasis, but not primary tumor growth or angiogenesis, is diminished in fibrinogen-deficient mice. Cancer Res 62, 6966–6972 (2002). [PubMed] [Google Scholar]
- 55.Jain S, Russell S & Ware J Platelet glycoprotein VI facilitates experimental lung metastasis in syngenic mouse models. J. of Thromb. and Haemost 7, 1713–1717 (2009). [DOI] [PubMed] [Google Scholar]
- 56.Ho-Tin-Noe B, Goerge T, Cifuni SM, Duerschmied D & Wagner DD Platelet granule secretion continuously prevents intratumor hemorrhage. Cancer Res 68, 6851–6858 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ho-Tin-Noe B et al. Innate immune cells induce hemorrhage in tumors during thrombocytopenia. Am. J. Pathol 175, 1699–1708 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ho-Tin-Noe B, Goerge T & Wagner DD Platelets: guardians of tumor vasculature. Cancer Res 69, 5623–5626 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kisucka J et al. Platelets and platelet adhesion support angiogenesis while preventing excessive hemorrhage. Proc. Natl. Acad. Sci. U. S. A 103, 855–860 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Manegold PC, Hutter J, Pahernik SA, Messmer K & Dellian M Platelet-endothelial interaction in tumor angiogenesis and microcirculation. Blood 101, 1970–1976 (2003). [DOI] [PubMed] [Google Scholar]
- 61.Gasic GJ, Gasic TB & Stewart CC Antimetastatic effects associated with platelet reduction. Proc. Natl. Acad. Sci. U. S. A 61, 46–52 (1968). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Camerer E et al. Platelets, protease-activated receptors, and fibrinogen in hematogenous metastasis. Blood 104, 397–401 (2004). [DOI] [PubMed] [Google Scholar]
- 63.Karpatkin S, Pearlstein E, Ambrogio C & Coller BS Role of adhesive proteins in platelet tumor interaction in vitro and metastasis in vivo. J. Clin. Invest 81, 1012–1019 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sambrano GR, Weiss EJ, Zheng Y-W, Huang W & Coughlin SR Role of thrombin signalling in platelets in haemostasis and thrombosis. Nature 413, 74–78 (2001). [DOI] [PubMed] [Google Scholar]
- 65.Palumbo JS et al. Fibrinogen is an important determinant of the metastatic potential of circulating tumor cells. Blood 96, 3302–3309 (2000). [PubMed] [Google Scholar]
- 66.Versteeg HH et al. Protease-activated receptor (PAR) 2, but not PAR1, signaling promotes the development of mammary adenocarcinoma in polyoma middle T mice. Cancer Res 68, 7219–7227 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hu L, Roth JM, Brooks P, Ibrahim S & Karpatkin S Twist is required for thrombininduced tumor angiogenesis and growth. Cancer Res 68, 4296–4302 (2008). [DOI] [PubMed] [Google Scholar]
- 68.Jones DS, Wallace AC & Fraser EE Sequence of events in experimental metastases of walker 256 tumor-light, immunofluorescent, and electron microscopic observations. J. Natl. Cancer Inst 46, 493-& (1971). [PubMed] [Google Scholar]
- 69.Crissman JD, Hatfield J, Schaldenbrand M, Sloane BF & Honn KV Arrest and extravasation of B16 amelanotic melanoma in murine lungs. A light and electron microscopic study. Lab. Invest 53, 470–478 (1985). [PubMed] [Google Scholar]
- 70.Sindelar WF, Tralka TS & Ketcham AS Electron microscopic observations on formation of pulmonary metastases. J. Surg. Res 18, 137–161 (1975). [DOI] [PubMed] [Google Scholar]
- 71.Borsig L et al. Heparin and cancer revisited: mechanistic connections involving platelets, P-selectin, carcinoma mucins, and tumor metastasis. Proc. Natl. Acad. Sci. U. S. A 98, 3352–3357 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nieswandt B, Hafner M, Echtenacher B & Mannel DN Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res 59, 1295–1300 (1999). [PubMed] [Google Scholar]
- 73.Malik G et al. Plasma fibronectin promotes lung metastasis by contributions to fibrin clots and tumor cell invasion. Cancer Res 70, 4327–4334 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Palumbo JS Mechanisms linking tumor cell-associated procoagulant function to tumor dissemination. Semin. Thromb. Hemost 34, 154–160 (2008). [DOI] [PubMed] [Google Scholar]
- 75.Palumbo JS et al. Tumor cell-associated tissue factor and circulating hemostatic factors cooperate to increase metastatic potential through natural killer cell-dependent and-independent mechanisms. Blood 110, 133–141 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wenzel J, Zeisig R & Fichtner I Inhibition of metastasis in a murine 4T1 breast cancer model by liposomes preventing tumor cell-platelet interactions. Clin. Exp. Metastasis 27, 25–34 (2010). [DOI] [PubMed] [Google Scholar]
- 77.Offermanns S, Toombs CF, Hu Y-H & Simon MI Defective platelet activation in G-α-qdeficient mice. Nature 389, 183–186 (1997). [DOI] [PubMed] [Google Scholar]
- 78.Palumbo JS & Degen JL Mechanisms linking tumor cell-associated procoagulant function to tumor metastasis. Thromb. Res 120, S22–S28 (2007). [DOI] [PubMed] [Google Scholar]
- 79.Kopp HG, Placke T & Salih HR Platelet-derived transforming growth factor-β downregulates NKG2D thereby inhibiting natural killer cell antitumor reactivity. Cancer Res 69, 7775–7783 (2009). [DOI] [PubMed] [Google Scholar]
- 80.Im JH et al. Coagulation facilitates tumor cell spreading in the pulmonary vasculature during early metastatic colony formation. Cancer Res 64, 8613–8619 (2004). [DOI] [PubMed] [Google Scholar]
- 81.Läubli H & Borsig L Selectins promote tumor metastasis. Semin. Cancer Biol (2010). [DOI] [PubMed] [Google Scholar]
- 82.Konstantopoulos K & Thomas SN Cancer cells in transit: the vascular interactions of tumor cells. Annu. Rev. Biomed. Eng 11, 177–202 (2009). [DOI] [PubMed] [Google Scholar]
- 83.Kim YJ, Borsig L, Han HL, Varki NM & Varki A Distinct selectin ligands on colon carcinoma mucins can mediate pathological interactions among platelets, leukocytes, and endothelium. Am. J. Pathol 155, 461–472 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ludwig RJ et al. Endothelial P-selectin as a target of heparin action in experimental melanoma lung metastasis. Cancer Res 64, 2743–2750 (2004). [DOI] [PubMed] [Google Scholar]
- 85.Mousa SA & Petersen LJ Anti-cancer properties of low-molecular-weight heparin: preclinical evidence. Thromb. Haemost 102, 258–267 (2009). [DOI] [PubMed] [Google Scholar]
- 86.Varki AP, Varki NM & Borsig L Selectins, heparins, and cancer: rationale for clinical trials. Blood 112, 1348–1348 (2008). [Google Scholar]
- 87.Borsig L, Wong R, Hynes RO, Varki NM & Varki A Synergistic effects of L- and P-selectin in facilitating tumor metastasis can involve non-mucin ligands and implicate leukocytes as enhancers of metastasis. Proc. Natl. Acad. Sci. U. S. A 99, 2193–2198 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Laubli H, Spanaus KS & Borsig L Selectin-mediated activation of endothelial cells induces expression of CCL5 and promotes metastasis through recruitment of monocytes. Blood 114, 4583–4591 (2009). [DOI] [PubMed] [Google Scholar]
- 89.Alves CS, Burdick MM, Thomas SN, Pawar P & Konstantopoulos K The dual role of CD44 as a functional P-selectin ligand and fibrin receptor in colon carcinoma cell adhesion. Am. J. of Physiol. and Cell Physiol 294, C907–C916 (2008). [DOI] [PubMed] [Google Scholar]
- 90.McCarty OJT, Mousa SA, Bray PF & Konstantopoulos K Immobilized platelets support human colon carcinoma cell tethering, rolling, and firm adhesion under dynamic flow conditions. Blood 96, 1789–1797 (2000). [PubMed] [Google Scholar]
- 91.Shattil SJ, Kim C & Ginsberg MH The final steps of integrin activation: the end game. Nat. Rev. Mol. Cell Biol 11, 288–300 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Felding-Habermann B, Habermann R, Saldivar E & Ruggeri ZM Role of β3 integrins in melanoma cell adhesion to activated platelets under flow. J. Biol. Chem 271, 5892–5900 (1996). [DOI] [PubMed] [Google Scholar]
- 93.Amirkhosravi A et al. Inhibition of tumor cell-induced platelet aggregation and lung metastasis by the oral GpIIb/IIIa antagonist XV454. Thromb. Haemost 90, 549–554 (2003). [DOI] [PubMed] [Google Scholar]
- 94.Desgrosellier JS & Cheresh DA Integrins in cancer: biological implications and therapeutic opportunities. Nat. Rev. Cancer 10, 9–22 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bakewell SJ et al. Platelet and osteoclast β3 integrins are critical for bone metastasis. Proc. Natl. Acad. Sci. U. S. A 100, 14205–14210 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gupta GP & Massague J Platelets and metastasis revisited: a novel fatty link. J. Clin. Invest 114, 1691–1693 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Arnaout MA, Mahalingam B & Xiong J-P Integrin structure, allostery, and bidirectional signaling. Annu. Rev. Cell Dev. Biol 21, 381–410 (2005). [DOI] [PubMed] [Google Scholar]
- 98.Rose DM, Alon R & Ginsberg MH Integrin modulation and signaling in leukocyte adhesion and migration. Immunol. Rev 218, 126–134 (2007). [DOI] [PubMed] [Google Scholar]
- 99.Alcaide P, Auerbach S & Luscinskas FW Neutrophil recruitment under shear flow: it’s all about endothelial cell rings and gaps. Microcirculation 16, 43–57 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zarbock A & Ley K Mechanisms and consequences of neutrophil interaction with the endothelium. Am. J. Pathol 172, 1–7 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Barthel SR, Johansson MW, McNamee DM & Mosher DF Roles of integrin activation in eosinophil function and the eosinophilic inflammation of asthma. J. Leukoc. Biol 83, 1–12 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hyduk SJ & Cybulsky MI Role of α4β1 integrins in chemokine-induced monocyte arrest under conditions of shear stress. Microcirculation 16, 17–30 (2009). [DOI] [PubMed] [Google Scholar]
- 103.Felding-Habermann B et al. Integrin activation controls metastasis in human breast cancer. Proc. Natl. Acad. Sci. U. S. A 98, 1853–1858 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rolli M, Fransvea E, Pilch J, Saven A & Felding-Habermann B Activated integrin αvβ3 cooperates with metalloproteinase MMP-9 in regulating migration of metastatic breast cancer cells. Proc. Natl. Acad. Sci. U. S. A 100, 9482–9487 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Felding-Habermann B Targeting tumor cell-platelet interaction in breast cancer metastasis. Pathophysiol. of Haemost. and Thromb 33, 56–58 (2003). [DOI] [PubMed] [Google Scholar]
- 106.Deryugina E & Quigley J Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 25, 9–34 (2006). [DOI] [PubMed] [Google Scholar]
- 107.Lorger M, Krueger JS, O’Neal M, Staflin K & Felding-Habermann B Activation of tumor cell integrin αvβ3 controls angiogenesis and metastatic growth in the brain. Proc. Natl. Acad. Sci. U. S. A 106, 10666–10671 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Felding-Habermann B et al. Combinatorial antibody libraries from cancer patients yield ligandmimetic Arg-Gly-Asp-containing immunoglobulins that inhibit breast cancer metastasis. Proc. Natl. Acad. Sci. U. S. A 101, 17210–17215 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Staflin K et al. Targeting activated integrin αvβ3 with patient-derived antibodies impacts latestage multiorgan metastasis. Clin. Exp. Metastasis 27, 217–231 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Taverna D, Crowley D, Connolly M, Bronson RT & Hynes RO A direct test of potential roles for β3 and β5 integrins in growth and metastasis of murine mammary carcinomas. Cancer Res. 65, 10324–10329 (2005). [DOI] [PubMed] [Google Scholar]
- 111.Morimoto K et al. Interaction of cancer cells with platelets mediated by Necl-5/poliovirus receptor enhances cancer cell metastasis to the lungs. Oncogene 27, 264–273 (2007). [DOI] [PubMed] [Google Scholar]
- 112.Jain S et al. Platelet glycoprotein Ibα supports experimental lung metastasis. Proc. Natl. Acad. Sci. U. S. A 104, 9024–9028 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kitagawa H et al. Involvement of platelet membrane glycoprotein Ib and glycoprotein IIb/IIIa complex in thrombin-dependent and -independent platelet aggregations induced by tumor cells. Cancer Res. 49, 537–541 (1989). [PubMed] [Google Scholar]
- 114.Erpenbeck L, Nieswandt B, Schon M, Pozgajova M & Schon MP Inhibition of platelet GPIbα and promotion of melanoma metastasis. J. Invest. Dermatol 130, 576–586 (2009). [DOI] [PubMed] [Google Scholar]
- 115.Terraube V et al. Increased metastatic potential of tumor cells in von Willebrand factor-deficient mice. J. of Thromb. and Haemost 4, 519–526 (2006). [DOI] [PubMed] [Google Scholar]
- 116.Terraube V, Marx I & Denis CV Role of von Willebrand factor in tumor metastasis. Thromb. Res 120, S64–S70 (2007). [DOI] [PubMed] [Google Scholar]
- 117.Marsolais D & Rosen H Chemical modulators of sphingosine 1-phosphate receptors as barrieroriented therapeutic molecules. Nat. Rev. Drug Discov 8, 297–307 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yatomi Y et al. Sphingosine 1 -phosphate as a major bioactive lysophospholipid that is released from platelets and interacts with endothelial cells. Blood 96, 3431–3438 (2000). [PubMed] [Google Scholar]
- 119.Schaphorst KL et al. Role of sphingosine 1 -phosphate in the enhancement of endothelial barrier integrity by platelet-released products. Am. J. Physiol. Lung Cell Mol. Physiol 285, L258–267 (2003). [DOI] [PubMed] [Google Scholar]
- 120.Yin F & Watsky MA LPA and S1P increase corneal epithelial and endothelial cell transcellular resistance. Invest. Ophthalmol. Vis. Sci 46, 1927–1933 (2005). [DOI] [PubMed] [Google Scholar]
- 121.Sarker MH, Hu DE & Fraser PA Regulation of cerebromicrovascular permeability by lysophosphatidic acid. Microcirculation 17, 39–46 (2010). [DOI] [PubMed] [Google Scholar]
- 122.Côté F, Fligny C, Fromes Y, Mallet J & Vodjdani G Recent advances in understanding serotonin regulation of cardiovascular function. Trends in Molecular Medicine 10, 232–238 (2004). [DOI] [PubMed] [Google Scholar]
- 123.Skolnik G, Bagge U, Dhlstrom A & Ahlman H The importance of 5-HT for tumor cell lodgement in the liver. Int. J. Cancer 33, 519–523 (1984). [DOI] [PubMed] [Google Scholar]
- 124.Skolnik G, Bagge U, Blomqvist G, Djarv L & Ahlman H The role of calcium channels and serotonin (5-HT2) receptors for tumour cell lodegment in the liver. Clin. Exp. Metastasis 7, 169–174 (1989). [DOI] [PubMed] [Google Scholar]
- 125.Kuna P et al. RANTES, a monocyte and T lymphocyte chemotactic cytokine releases histamine from human basophils. J. Immunol 149, 636–642 (1992). [PubMed] [Google Scholar]
- 126.Lyman GH et al. American society of clinical oncology guideline: recommendations for venous thromboembolism prophylaxis and treatment in patients with cancer. J. Clin. Oncol 25, 5490–5505 (2007). [DOI] [PubMed] [Google Scholar]
- 127.Varki A Trousseau’s syndrome: multiple definitions and multiple mechanisms. Blood 110, 1723–1729 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Elwood PC, Gallagher AM, Duthie GG, Mur LAJ & Morgan G Aspirin, salicylates, and cancer. The Lancet 373, 1301–1309 (2009). [DOI] [PubMed] [Google Scholar]
- 129.Mackman N Triggers, targets and treatments for thrombosis. Nature 451, 914–918 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Verheul HMW et al. Platelets take up the monoclonal antibody bevacizumab. Clin. Cancer Res 13, 5341–5347 (2007). [DOI] [PubMed] [Google Scholar]
- 131.Kanter J, Khan SY, Kelher M, Gore L & Silliman CC Oncogenic and angiogenic growth factors accumulate during routine storage of apheresis platelet concentrates. Clin. Cancer Res 14, 3942–3947 (2008). [DOI] [PubMed] [Google Scholar]
- 132.Foss B & Bruserud O Platelet functions and clinical effects in acute myelogenous leukemia. Thromb. Haemost 99, 27–37 (2008). [DOI] [PubMed] [Google Scholar]
- 133.Cervi D et al. Platelet-associated PF-4 as a biomarker of early tumor growth. Blood 111, 1201–1207 (2008). [DOI] [PubMed] [Google Scholar]
- 134.Klement GL et al. Platelets actively sequester angiogenesis regulators. Blood 113, 2835–2842 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Borsig L The role of platelet activation in tumor metastasis. Expert Rev. of Anticancer Ther 8, 1247–1255 (2008). [DOI] [PubMed] [Google Scholar]
- 136.Fang J et al. Therapeutic expression of the platelet-specific integrin, αIIbβ3, in a murine model for Glanzmann thrombasthenia. Blood 106, 2671–2679 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Freyssinet J-M & Toti F Formation of procoagulant microparticles and properties. Thromb. Res 125, S46–S48 (2010). [DOI] [PubMed] [Google Scholar]
- 138.Muralidharan-Chari V, Clancy JW, Sedgwick A & D’Souza-Schorey C Microvesicles: mediators of extracellular communication during cancer progression. J. Cell Sci 123, 1603–1611 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Castellana D, Toti F & Freyssinet JM Membrane microvesicles: macromessengers in cancer disease and progression. Thromb. Res 125, S84–S88 (2010). [DOI] [PubMed] [Google Scholar]
- 140.Baj-Krzyworzeka M et al. Platelet-derived microparticles stimulate proliferation, survival, adhesion, and chemotaxis of hematopoietic cells. Exp. Hematol 30, 450–459 (2002). [DOI] [PubMed] [Google Scholar]
- 141.Janowska-Wieczorek A, Marquez-Curtis LA, Wysoczynski M & Ratajczak MZ Enhancing effect of platelet-derived microvesicles on the invasive potential of breast cancer cells. Transfusion (Paris). 46, 1199–1209 (2006). [DOI] [PubMed] [Google Scholar]
- 142.Janowska-Wieczorek A et al. Microvesicles derived from activated platelets induce metastasis and angiogenesis in lung cancer. Int. J. Cancer 113, 752–760 (2005). [DOI] [PubMed] [Google Scholar]
- 143.Dashevsky O, Varon D & Brill A Platelet-derived microparticles promote invasiveness of prostate cancer cells via upregulation of MMP-2 production. Int. J. Cancer 124, 1773–1777 (2009). [DOI] [PubMed] [Google Scholar]
- 144.Kalinkovich A et al. Functional CXCR4-expressing microparticles and SDF-1 correlate with circulating acute myelogenous leukemia cells. Cancer Res 66, 11013–11020 (2006). [DOI] [PubMed] [Google Scholar]
- 145.Kim HK et al. Elevated levels of circulating platelet microparticles, VEGF, IL-6 and RANTES in patients with gastric cancer: possible role of a metastasis predictor. Eur. J. Cancer 39, 184–191 (2003). [DOI] [PubMed] [Google Scholar]
- 146.Helley D et al. Platelet microparticles: a potential predictive factor of survival in hormonerefractory prostate cancer patients treated with docetaxel-based chemotherapy. Eur. Urol 56, 479–484 (2009). [DOI] [PubMed] [Google Scholar]
- 147.Hron G et al. Tissue factor-positive microparticles: cellular origin and association with coagulation activation in patients with colorectal cancer. Thromb. Haemost 97, 119–123 (2007). [PubMed] [Google Scholar]
- 148.Tesselaar ME et al. Microparticle-associated tissue factor activity: a link between cancer and thrombosis? J. of Thromb. and Haemost 5, 520–527 (2007). [DOI] [PubMed] [Google Scholar]
- 149.Toth B et al. Platelet-derived microparticles and coagulation activation in breast cancer patients. Thromb. Haemost 100, 663–669 (2008). [PubMed] [Google Scholar]
- 150.Thomas GM et al. Cancer cell-derived microparticles bearing P-selectin glycoprotein ligand 1 accelerate thrombus formation in vivo. J. Exp. Med 206, 1913–1927 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tilley RE, Holscher T, Belani R, Nieva J & Mackman N Tissue factor activity is increased in a combined platelet and microparticle sample from cancer patients. Thromb. Res 122, 604–609 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Nachman RL & Rafii S Platelets, petechiae, and preservation of the vascular wall. N. Engl. J. Med 359, 1261–1270 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Von Hundelshausen P & Weber C Platelets as immune cells: bridging inflammation and cardiovascular disease. Circ. Res 100, 27–40 (2007). [DOI] [PubMed] [Google Scholar]
- 154.Vischer U & Wagner D CD63 is a component of Weibel-Palade bodies of human endothelial cells. Blood 82, 1184–1191 (1993). [PubMed] [Google Scholar]
- 155.Honn KV et al. Enhanced endothelial cell retraction mediated by 12(S)-HETE: a proposed mechanism for the role of platelets in tumor cell metastasis. Exp. Cell Res 210, 1–9 (1994). [DOI] [PubMed] [Google Scholar]
- 156.Pelosof LC & Gerber DE Paraneoplastic syndromes: an approach to diagnosis and treatment. Mayo Clin. Proc 85, 838–854 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Franchini M, Montagnana M, Favaloro EJ & Lippi G The bidirectional relationship of cancer and hemostasis and the potential role of anticoagulant therapy in moderating thrombosis and cancer spread. Semin. Thromb. Hemost 35, 644–653 (2009). [DOI] [PubMed] [Google Scholar]
- 158.Popivanova BK et al. Blockade of a chemokine, CCL2, reduces chronic colitis-associated carcinogenesis in mice. Cancer Res 69, 7884–7892 (2009). [DOI] [PubMed] [Google Scholar]
- 159.Jung D-W et al. Tumor-stromal crosstalk in invasion of oral squamous cell carcinoma: a pivotal role of CCL7. Int. J. Cancer 127, 332–344 (2010). [DOI] [PubMed] [Google Scholar]
- 160.Keeley EC, Mehrad B, Strieter RM, George FVW & George K in Adv. Cancer Res 91–111 (Academic Press, 2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bajou K et al. Absence of host plasminogen activator inhibitor 1 prevents cancer invasion and vascularization. Nat. Med 4, 923–928 (1998). [DOI] [PubMed] [Google Scholar]
- 162.Geiger TR & Peeper DS Critical role for TrkB kinase function in anoikis suppression, tumorigenesis, and metastasis. Cancer Res 67, 6221–6229 (2007). [DOI] [PubMed] [Google Scholar]
- 163.Bronckaers A, Gago F, Balzarini J & Liekens S The dual role of thymidine phosphorylase in cancer development and chemotherapy. Med. Res. Rev 29, 903–953 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ikushima H & Miyazono K TGFβ signalling: a complex web in cancer progression. Nat. Rev. Cancer 10, 415–424 (2010). [DOI] [PubMed] [Google Scholar]
- 165.Medina VA & Rivera ES Histamine receptors and cancer pharmacology. Br. J. Pharmacol 161, 755–767 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Falcon BL et al. Contrasting actions of selective inhibitors of angiopoietin-1 and angiopoietin-2 on the normalization of tumor blood vessels. Am. J. Pathol 175, 2159–2170 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ho M-Y et al. Nucleotide-binding domain of phosphoglycerate kinase 1 reduces tumor growth by suppressing COX-2 expression. Cancer Science 101, 2411–2416 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Melnikova V & Bar-Eli M Inflammation and melanoma growth and metastasis: the role of platelet-activating factor (PAF) and its receptor. Cancer Metastasis Rev 26, 359–371 (2007). [DOI] [PubMed] [Google Scholar]
- 169.Visentin B et al. Validation of an anti-sphingosine-1-phosphate antibody as a potential therapeutic in reducing growth, invasion, and angiogenesis in multiple tumor lineages. Cancer Cell 9, 225–238 (2006). [DOI] [PubMed] [Google Scholar]
- 170.David M et al. Cancer cell expression of autotaxin controls bone metastasis formation in mouse through lysophosphatidic acid-dependent activation of osteoclasts. PLoS ONE 5, e9741 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Amirkhosravi A et al. The role of tissue factor pathway inhibitor in tumor growth and metastasis. Semin. Thromb. Hemost 33, 643,652 (2007). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.