Abstract
The limited scope of DNA-compatible chemistry restricts the types of chemical features that can be incorporated into DNA-encoded libraries (DELs). Here, a method to synthesize DNA-conjugated polycyclic isoxazolidines via a [3+2] nitrone–olefin cycloaddition is described. The reaction is compatible with many olefin-containing substrates and diverse N-alkylhydroxylamines. The ability to perform subsequent DNA ligation and PCR amplification was also confirmed. This methodology facilitates the synthesis of DELs containing topographically complex compounds with underexplored chemical features.
Graphical Abstract
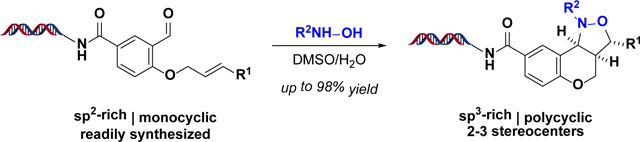
DNA-encoded libraries (DELs) of small molecules can facilitate the identification of protein “binders”1,2 in affinity-based biochemical assays.3–6 DELs combine synthetic organic chemistry and DNA barcoding to generate libraries of compounds that are each covalently linked to a unique, amplifiable tag that records the synthetic reactions from which individual compounds derive.7 DELs are most often used to identify molecules that engage an immobilized protein target.8 This strategy has thus far yielded binders of therapeutically relevant proteins such as kinases,9 phosphatases,10 G-protein coupled receptors (GPCRs),11 and others.12
It is possible that the full potential of DEL-mediated target-based screening has not been realized because, despite recent advances in DNA-compatible chemistry,13–16 the types of structural features found in DELs remain limited.17 To generate libraries, DNA-encoded compounds are often synthesized in parallel with their barcodes using split–pool synthesis. The presence of DNA and water during synthesis, however, restricts the chemistry that may be used to build DELs. Many robust and commonly used transformations (e.g. cross-couplings, amidations, and SNAr reactions)18 tend to produce compounds rich in sp2-hybridized carbons. Therefore, significant gaps exist in the types of molecular architectures found in DELs.19 In an effort to address these shortcomings, a novel, open-source collaboration involving a small group of scientists at the Novartis Institutes for BioMedical Research and the Broad Institute was recently established. The work reported here describes an early example of this collaboration.
We identified the intramolecular [3+2] nitrone–olefin cycloaddition as a transformation that can fill some of the structural gaps in current DELs. Earlier reports have shown this reaction to be both water- and DNA-compatible,20,21 though it has never been used for DEL synthesis, and previous DNA-compatible examples were performed using DNA-templated synthesis with activated olefins and pre-formed nitrones.20
From a structural standpoint, this reaction converts sp2-rich starting materials to sp3-rich isoxazolidines containing at least two stereogenic elements. The isoxazolidine motif is rarely found in DELs, but it resides in several alkaloid natural products and other bioactive compounds.22,23 Furthermore, unlike most other ring-forming reactions (e.g. ring-closing metathesis), it can introduce appendage diversity via condensation of N-alkylhydroxylamines with aldehydes or ketones to form nitrones in situ. The ability to perform ring closure and appendage diversification simultaneously is appealing because split–pool synthesis often makes post-reaction purification challenging. Therefore, DEL pathways should be limited to a handful of steps to mitigate buildup of DNA damage and unwanted by-products, among other factors.24
We first synthesized on-DNA salicylaldehyde derivative 1 and treated it with methylhydroxylamine; we used the double-stranded DNA “headpiece” commonly used in split–pool DEL synthesis.6 Our initial experiments suggested that conversion to the desired isoxazolidine 2 could be increased by raising the reaction temperature (Table 1; entries 1–2). Performing the reaction at 100 °C, however, resulted in decomposition of the DNA barcode via depurination that was detectable by UPLC-MS analysis (Table 1; entry 3). The use of organic base (Table 1; entry 4) or additional equivalents of hydroxylamine (Table 1; entry 5) resulted in low conversion and substantial by-product formation. While high conversions could be achieved in 4 h at 90 °C in basic phosphate buffer (Table 1; entry 6), even higher conversions were observed in acidic phosphate buffer (Table 1; entry 7). However, the substrate was more prone to depurination in acidic conditions (Table 1; entry 8), so milder buffering systems were explored (Table 1; entries 9–12). Ultimately, eight volumes of 0.1 M NaOAc buffer yielded the highest conversion (Table 1; entry 12). The cis ring fusion of 2 was confirmed via NMR-based characterization of an off-DNA analog (see Supporting Information for details).
Table 1.
Initial optimization of on-DNA [3+2] nitrone–olefin cycloaddition
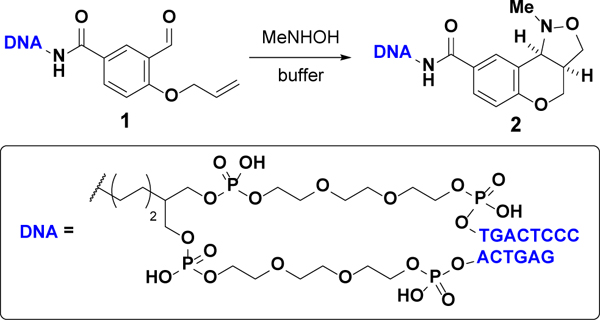 | ||||||
|---|---|---|---|---|---|---|
| entry | time (h) |
temp (°C) |
MeNHOH (equiv) |
buffer | buffer volsg |
2 (%)h |
| 1 | 12 | 70 | 25 | Na2HPO4a | 2 | 70 |
| 2 | 12 | 90 | 25 | Na2HPO4a | 2 | 88 |
| 3 | 12 | 100 | 25 | Na2HPO4a | 2 | 84f |
| 4 | 12 | 70 | 25 | pyrrolidineb | 0.1 | 54 |
| 5 | 12 | 90 | 100 | Na2HPO4a | 2 | 64 |
| 6 | 4 | 90 | 25 | Na2HPO4a | 0.5 | 86 |
| 7 | 4 | 90 | 25 | NaH2PO4c | 0.1 | 93 |
| 8 | 12 | 90 | 25 | NaH2PO4c | 0.5 | 81f |
| 9 | 4 | 90 | 25 | NaH2PO4d | 2 | 90 |
| 10 | 4 | 90 | 25 | NaH2PO4d | 8 | 71 |
| 11 | 4 | 90 | 25 | NaOAce | 2 | 91 |
| 12 | 4 | 90 | 25 | NaOAce | 8 | >98 |
1.0 M aq buffer (pH 9.2).
0.2 M DMA solution.
1.0 M aq buffer (pH 4.2).
0.1 M aq buffer (pH 5.9).
0.1 M aq buffer (pH 5.0).
Significant DNA damage observed.
Compared to amount of 1 stock solution (0.8 mM) added.
Determined by UPLC-MS.
We then studied the compatibility of this transformation with additional hydroxylamines; the reaction conditions were modified slightly to account for the increased complexity and steric bulk of the nitrone intermediates (Table S1). While methyl-, ethyl-, and n-hexylhydroxylamine afforded clean conversions to the desired isoxazolidines (Table 2; entries 1–3), by-products were observed when 1 was treated with iPrNHOH (Table 2; entry 4). We identified one species as the obligatory nitrone intermediate; it both had the same mass as the desired isoxazolidine product and appeared to convert to it over time (Figure S1). The other species, which was independent of the hydroxylamine in the reaction, was an oxime-containing product likely derived from interception of the nitrone intermediate by an exogenous nucleophile. Both products are consistent with the increased steric bulk of the nitrone slowing the rate of the intramolecular cyclization.
Table 2.
Scope of hydroxylamine with substrate 1
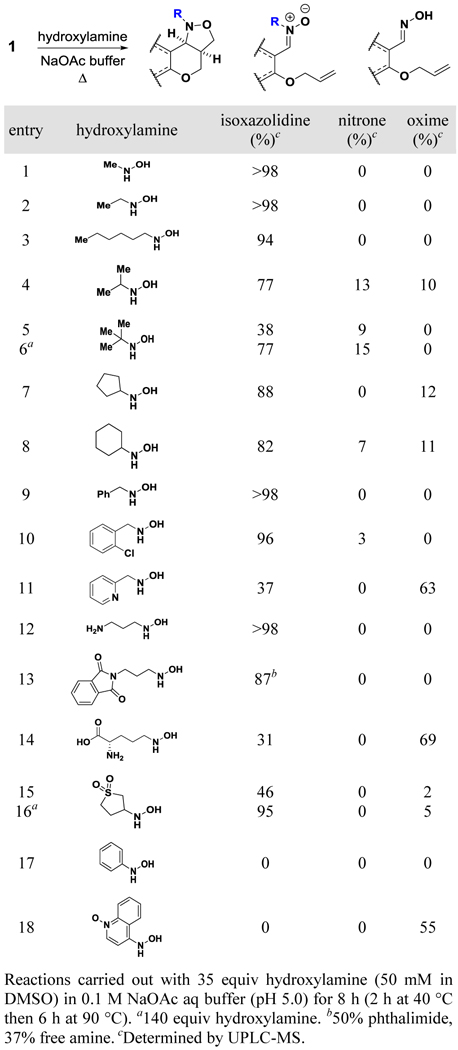 |
The steric bulk of tBuNHOH (Table 2; entry 5) hindered both the [3+2] cycloaddition and condensation to the nitrone; however, increasing the amount of hydroxylamine 4-fold (Table 2; entry 6) yielded substantially higher isoxazolidine formation. Cyclopentyl- and cyclohexylhydroxylamine achieved 88% and 82% conversion, respectively (Table 2; entries 7–8), both of which were higher than iPrNHOH (77%). These differences in reactivity underscore the outsized impact of steric effects upon the success of the reaction.
We then tested hydroxylamines containing functional groups other than saturated hydrocarbons. Benzyl-containing hydroxylamines were very successful (Table 2; entries 9–10), but an electrophilic 2-pyridine derivative yielded high amounts of oxime (Table 2; entry 11). Both free and protected amines are compatible with the reaction, though we observed some phthalimide deprotection (Table 2; entries 12–13). N-hydroxy-L-ornithine generated high levels of oxime (Table 2; entry 14), potentially via an intramolecular 5-exo-tet cyclization to generate the on-DNA oxime and the presumed ornithine-derived proline. A bulky sulfone-containing reagent initially afforded low consumption of 1, but additional hydroxylamine furnished excellent results (Table 2; entries 15–16). Finally, treatment with aromatic hydroxylamines resulted in no isoxazolidine formation (Table 2; entries 17–18).
Next, we studied the effect of olefin substitution. Ten additional on-DNA substrates (3–12) were synthesized, and each was initially tested with three hydroxylamines (Figure 1). Unsurprisingly, due to the sensitivity of the reaction to steric effects (see Table 2; entries 1–8), all experiments with prenylated substrate 3 resulted in poor isoxazolidine formation.
Figure 1.
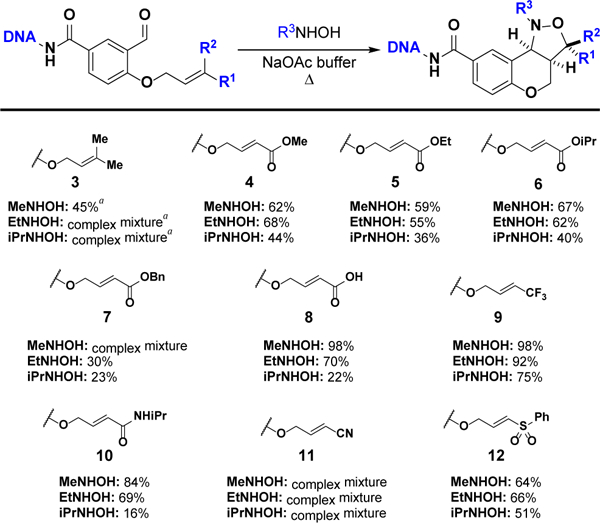
Conversions to isoxazolidines for on-DNA substrates 3–12. Reactions carried out with 40 equiv hydroxylamine (50 mM in DMSO) in 1.0 M NaOAc aq buffer (pH 5.0) for 8 h at 40 °C. a35 equiv hydroxylamine for 2 h at 40 °C then 6 h at 90 °C.
To determine if favorable electronics could outweigh the penalty of increased steric bulk, we synthesized DNA-linked substrates featuring a variety of electron-withdrawing groups on the reactive olefin (4–12; Figure 1). For each substrate, significant product formation was observed after 8 h at just 40 °C. Even under these mild conditions, however, saponification was observed with esters 4–7. Of these four substrates, isopropyl ester 6 exhibited the lowest susceptibility to hydrolysis. Substrates 8–10 often yielded high conversions and simple reaction profiles. Though treatment of 8 and 10 with iPrNHOH resulted in low isoxazolidine formation, in each case the only other major product was the nitrone intermediate. Nitrile 11, on the other hand, always generated a complex mixture of products that could not be easily identified by UPLC-MS analysis alone. Finally, sulfone 12 afforded moderate isoxazolidine formation with all three hydroxylamines.
Having seen that activated olefins accelerate on-DNA cyclizations dramatically, we studied the hydroxylamine scope further with substrates 6 and 8–10 (Table 3). We tailored the reaction parameters for each substrate to yield maximal isoxazolidine formation under the mildest possible conditions; 9 achieved very similar results with the harsher conditions associated with 8 and 10 (Table S2), which suggests 8–10 (or derivatives) could be pooled for DEL synthesis.
Table 3.
Scope of hydroxylamine with 6 and 8–10
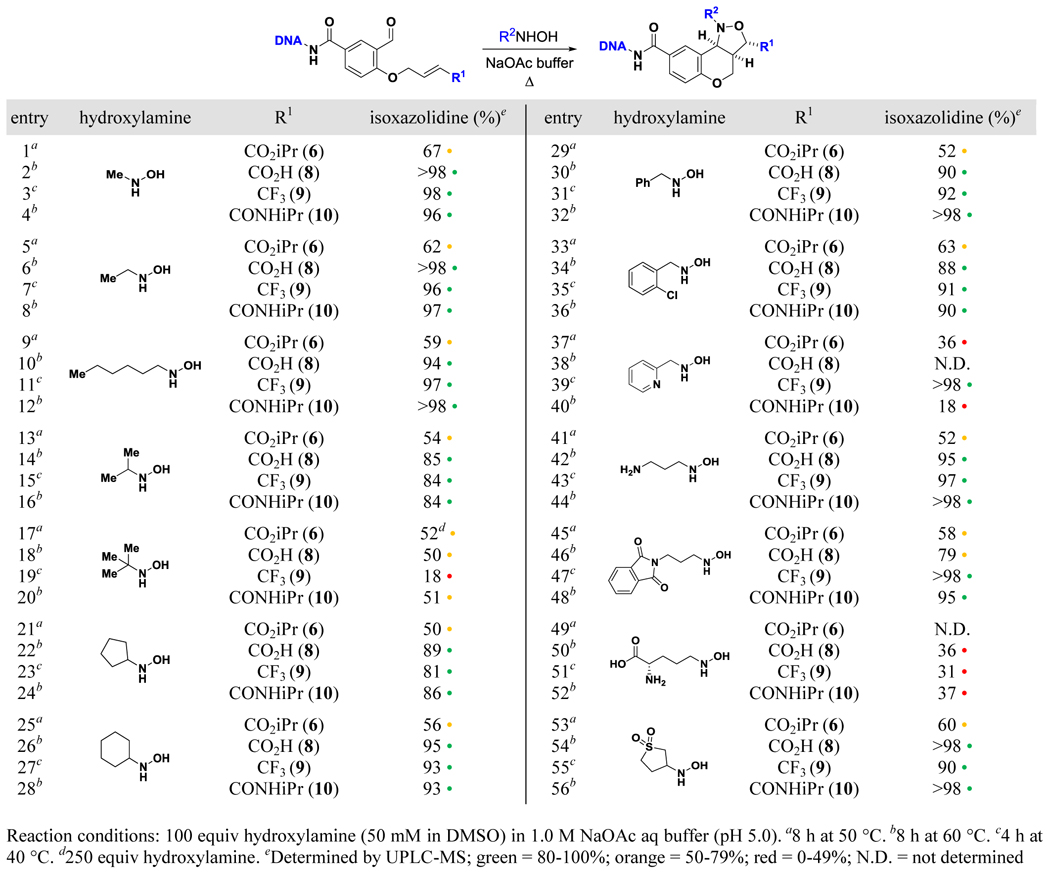 |
Using these optimized conditions, treatment with methyl-and n-alkylhydroxylamines largely resulted in excellent isoxazolidine formation (Table 3; entries 1–12). Experiments with ester 6 were less successful due to incomplete consumption of starting material and the formation of saponification by-products; this trend continued throughout the study. All substrates yielded slightly lower conversions with iPrNHOH (Table 3; entries 13–16). Lower conversions still were seen with tBuNHOH (Table 3; entries 17–20) due to sluggish nitrone formation and cyclization. Cyclopentylhydroxylamine behaved similarly to iPrNHOH (Table 3; entries 21–24), but reactions with the bulkier cyclohexyl reagent were superior due to decreased oxime formation (Table 3; entries 25–28). Next, reactions with benzyl-containing hydroxylamines proceeded smoothly (Table 3; entries 29–36). Most experiments with the pyridine-containing reagent, however, suffered from high rates of oxime formation, though 9 was sufficiently reactive to undergo cyclization before this side-reaction could occur (Table 3; entries 37–40). Both free and phthalimide-protected amines were again tolerated (Table 3; entries 41–48), and minimal deprotection was observed under these milder conditions. N-hydroxy-L-ornithine generally furnished high amounts of oxime by-product and complex reaction profiles (Table 3; entries 49–52), but the sulfone-containing hydroxylamine yielded excellent results (Table 3; entries 53–56). We did not observe products of conjugate addition—which is a known DNA-compatible reaction25—in any experiment.
The compatibility of this transformation with several commonly used functional groups in DNA-compatible chemistry—free and protected amines, carboxylic acids, and aryl halides—highlights its utility for DEL design. In strategically designed on-DNA chemical pathways, these chemical handles (e.g. the carboxylic acid of 8) would be functionalized either before or after cyclization to enable to the construction of large, combinatorial DELs containing rare structural features.
We have shown that this methodology can achieve high yields with a variety of substrates. But to be suitable for DEL synthesis, it must also leave the DNA barcode capable of undergoing enzymatic DNA ligation and PCR amplification.26 To assess post-reaction DNA integrity (Figure 2), we performed cycloadditions with substrates 1, 9, and 10 using each scaffold’s optimized reaction conditions. Then, we ligated an oligonucleotide to the reaction products to generate a barcode comparable in size to those found in a 3-cycle DEL. Finally, we successfully amplified all three barcodes by PCR and detected no loss of informational integrity (Figures S2–S5).
Figure 2.
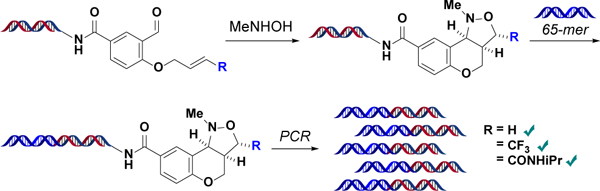
Schematic for three-step assessment of DNA integrity. DNA attached to cycloaddition substrates 1, 9, and 10 successfully underwent DNA ligation and PCR amplification after [3+2] nitrone–olefin cycloaddition.
In conclusion, we have developed a DNA-compatible [3+2] nitrone–olefin cycloaddition capable of generating polycyclic isoxazolidines. This transformation routinely eclipses 80% product formation with a variety of N-alkylhydroxylamines and olefin-containing substrates, which makes it appealing for DEL construction. We also confirmed that three sets of our optimized reaction conditions did not damage the DNA barcode or prevent subsequent ligation and amplification steps required for DEL synthesis.
DELs that incorporate this transformation may begin to fill some of the structural—and perhaps functional—gaps left by current on- and off-DNA small-molecule libraries. Efforts to leverage this reaction most effectively for DEL synthesis are ongoing, and we aim to describe these at a later date.
Supplementary Material
ACKNOWLEDGMENT
We thank Liam Hudson, Jeremy Mason, and Matthias Westphal (each Novartis Postdoctoral Scholars), Shawn Nelson Jr., Wenyu Wang, and Bruce Hua (each at the Broad Institute) for helpful discussions. We also thank Patrick O’Hearn (Broad), Jennifer Poirier (Novartis), Philip Michaels (Novartis), and John Capece (Novartis) for analytical support. Bennett Meier (Broad) provided critical feedback on the manuscript. This work was supported by NIH R01GM038627, NIH R35GM127045 (both to S.L.S.), and the Novartis Institutes for BioMedical Research. C.J.G. is supported by a National Science Foundation Graduate Research Fellowship (DGE1144152). S.L.S. is a member of the Novartis Faculty of Scholars.
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website.
Detailed methods for all chemical and biological procedures, characterization data (including NMR spectra) for off-DNA synthetic intermediates, UPLC-MS data for optimized on-DNA chemical reactions, Supporting Figures and Tables, and additional details regarding reagents and instruments used (PDF)
S.L.S. is a member of the Board of Directors of the Genomics Institute of the Novartis Research Foundation (“GNF”); a shareholder and member of the Board of Directors of Jnana Therapeutics; a shareholder of Forma Therapeutics; a shareholder of and adviser to Decibel Therapeutics and Eikonizo Therapeutics; an adviser to Eisai, Inc., the Ono Pharma Foundation, and F-Prime Capital Partners; and a Novartis Faculty Scholar.
REFERENCES
- (1).Schreiber S A Chemical Biology View of Bioactive Small Molecules and a Binder-Based Approach to Connect Biology to Precision Medicines. bioRxiv 2018. DOI: 10.1101/386904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Schreiber SL A Chemical Biology View of Bioactive Small Molecules and a Binder-Based Approach to Connect Biology to Precision Medicines. Isr. J. Chem. 2019. DOI: 10.1002/ijch.201800113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Goodnow RA; Dumelin CE; Keefe AD DNA-Encoded Chemistry: Enabling the Deeper Sampling of Chemical Space. Nat. Rev. Drug Discov. 2017, 16 (2), 131–147. [DOI] [PubMed] [Google Scholar]
- (4).Brenner S; Lerner RA Encoded Combinatorial Chemistry. Proc. Natl. Acad. Sci. 1992, 89 (12), 5381–5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Arico-Muendel CC From Haystack to Needle: Finding Value with DNA Encoded Library Technology at GSK. Med. Chem. Commun. 2016. [Google Scholar]
- (6).Clark MA; Acharya RA; Arico-Muendel CC; Belyanskaya SL; Benjamin DR; Carlson NR; Centrella PA; Chiu CH; Creaser SP; Cuozzo JW; et al. Design, Synthesis and Selection of DNA-Encoded Small-Molecule Libraries. Nat. Chem. Biol. 2009, 5 (9), 647–654. [DOI] [PubMed] [Google Scholar]
- (7).Neri D; Lerner RA DNA-Encoded Chemical Libraries: A Selection System Based on Endowing Organic Compounds with Amplifiable Information. Annu. Rev. Biochem. 2018, 87 (1), 479–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Decurtins W; Wichert M; Franzini RM; Buller F; Stravs MA; Zhang Y; Neri D; Scheuermann J Automated Screening for Small Organic Ligands Using DNA-Encoded Chemical Libraries. Nat. Protoc. 2016, 11 (4), 764–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Yang H; Medeiros PF; Raha K; Elkins P; Lind KE; Lehr R; Adams ND; Burgess JL; Schmidt SJ; Knight SD; et al. Discovery of a Potent Class of PI3Kα Inhibitors with Unique Binding Mode via Encoded Library Technology (ELT). ACS Med. Chem. Lett. 2015, 6 (5), 531–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Gilmartin AG; Faitg TH; Richter M; Groy A; Seefeld MA; Darcy MG; Peng X; Federowicz K; Yang J; Zhang S-Y; et al. Allosteric Wip1 Phosphatase Inhibition through Flap-Subdomain Interaction. Nat. Chem. Biol. 2014, 10, 181. [DOI] [PubMed] [Google Scholar]
- (11).Ahn S; Kahsai AW; Pani B; Wang QT; Zhao S; Wall AL; Strachan RT; Staus DP; Wingler LM; Sun LD; et al. Allosteric “Beta-Blocker” Isolated from a DNA-Encoded Small Molecule Library. Proc Natl Acad Sci U S A 2017, 114 (7), 1708–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Salamon H; Klika Škopić M; Jung K; Bugain O; Brunschweiger A Chemical Biology Probes from Advanced DNA-Encoded Libraries. ACS Chem. Biol. 2016, 11 (2), 296–307. [DOI] [PubMed] [Google Scholar]
- (13).Wang J; Lundberg H; Asai S; Martín-Acosta P; Chen JS; Brown S; Farrell W; Dushin RG; O’Donnell CJ; Ratnayake AS; et al. Kinetically Guided Radical-Based Synthesis of C(Sp 3 )−C(Sp 3 ) Linkages on DNA. Proc. Natl. Acad. Sci. 2018, 115 (28), E6404–E6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Li H; Sun Z; Wu W; Wang X; Zhang M; Lu X; Zhong W; Dai D Inverse-Electron-Demand Diels–Alder Reactions for the Synthesis of Pyridazines on DNA. Org. Lett. 2018, 20 (22), 7186–7191. [DOI] [PubMed] [Google Scholar]
- (15).Wang X; Sun H; Liu J; Dai D; Zhang M; Zhou H; Zhong W; Lu X Ruthenium-Promoted C–H Activation Reactions between DNA-Conjugated Acrylamide and Aromatic Acids. Org. Lett. 2018, 20 (16), 4764–4768. [DOI] [PubMed] [Google Scholar]
- (16).Ruff Y; Berst F Efficient Copper-Catalyzed Amination of DNA-Conjugated Aryl Iodides under Mild Aqueous Conditions. Medchemcomm 2018, 9 (7), 1188–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Franzini RM; Randolph C Chemical Space of DNA-Encoded Libraries. J. Med. Chem. 2016, 59 (14), 6629–6644. [DOI] [PubMed] [Google Scholar]
- (18).Satz AL; Cai J; Chen Y; Goodnow R; Gruber F; Kowalczyk A; Petersen A; Naderi-Oboodi G; Orzechowski L; Strebel Q DNA Compatible Multistep Synthesis and Applications to DNA Encoded Libraries. Bioconjug. Chem. 2015, 26 (8), 1623–1632. [DOI] [PubMed] [Google Scholar]
- (19).Gerry C; Wawer M; Clemons P; Schreiber S DNA Barcoding a Complete Matrix of Stereoisomeric Small Molecules. ChemRxiv 2018. DOI: 10.26434/chemrxiv.7289471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Gartner ZJ; Kanan MW; Liu DR Expanding the Reaction Scope of DNA-Templated Synthesis. Angew. Chemie Int. Ed. 2002, 41 (10), 1796–1800. [DOI] [PubMed] [Google Scholar]
- (21).Gholami MR; Habibi Yangjeh A Kinetics of 1,3-Dipolar Cycloaddition Reaction between C,N-Diphenylnitrone and Dimethyl Fumarate in Various Solvents and Aqueous Solutions. Int. J. Chem. Kinet. 2000, 32 (7), 431–434. [Google Scholar]
- (22).Xie J; Xue Q; Jin H; Li H; Cheng Y; Zhu C A Visible-Light-Promoted Aerobic C-H/C-N Cleavage Cascade to Isoxazolidine Skeletons. Chem. Sci 2013, 4 (3), 1281–1286. [Google Scholar]
- (23).Berthet M; Cheviet T; Dujardin G; Parrot I; Martinez J Isoxazolidine: A Privileged Scaffold for Organic and Medicinal Chemistry. Chem. Rev. 2016, 116 (24), 15235–15283. [DOI] [PubMed] [Google Scholar]
- (24).Franzini RM; Neri D; Scheuermann J DNA-Encoded Chemical Libraries: Advancing beyond Conventional Small-Molecule Libraries. Acc. Chem. Res. 2014, 47 (4), 1247–1255. [DOI] [PubMed] [Google Scholar]
- (25).Gartner ZJ; Liu DR The Generality of DNA-Templated Synthesis as a Basis for Evolving Non-Natural Small Molecules. J. Am. Chem. Soc. 2001, 123 (28), 6961–6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Malone ML; Paegel BM What Is a “DNA-Compatible” Reaction? ACS Comb. Sci. 2016, 18 (4), 182–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


