Abstract
Heart failure with preserved ejection fraction (HFpEF) is frequently accompanied by comorbidities and a systemic proinflammatory state, resulting in coronary microvascular dysfunction (CMD), as well as myocardial fibrosis. Recent studies have shown a high prevalence of CMD in HFpEF. The purpose of this study is to examine the relationship between myocardial perfusion reserve (MPR) and diffuse myocardial fibrosis in patients with HFpEF using CMR. A single center study was performed in 19 patients with clinical HFpEF and 15 healthy control subjects who underwent quantitative first-pass perfusion imaging to calculate global MPR. T1 mapping was used to assess fibrosis and to calculate extracellular volume (ECV). Spiral cine displacement encoded stimulated echo (DENSE) was used to calculate myocardial strain. Comprehensive 2D echocardiograms with speckle tracking, cardiopulmonary exercise testing, and BNP levels were also obtained. In patients with HFpEF, mean left ventricular (LV) EF was 61 ± 9% and LV mass index 45 ± 12 g/m2. Compared to controls, HFpEF patients had reduced global MPR (2.29 ± 0.64 vs 3.38 ± 0.76, p = 0.002) and VO2 max (16.5 ± 6.8 vs 30.9 ± 7.7 ml/kg*min, p < 0.001) while ECV (0.29 ± 0.04 vs 0.25 ± 0.04, p = 0.02), pulmonary artery systolic pressure (35.4 ± 13.7 vs 22.3 ± 5.4 mmHg, p = 0.004), and average E/e’ (15.0 ± 7.6 vs 8.6 ± 2.0, p = 0.005) were increased. DENSE peak systolic circumferential strain (p = 0.60) as well as echocardiographic derived global longitudinal strain (p = 0.07) were similar between both groups. The prevalence of CMD, defined as global MPR < 2.5, among the HFpEF group was 69%. In conclusion, HFpEF patients have a high prevalence of CMD and diffuse fibrosis. These parameters may be useful clinical endpoints for future therapeutic trials.
Keywords: Cardiovascular magnetic resonance, coronary microvascular dysfunction, extracellular volume, myocardial perfusion reserve
Background
Heart failure (HF) with preserved ejection (HFpEF) accounts for >50% of HF cases with a mortality similar to that of HF with reduced EF (HFrEF). HFpEF is frequently accompanied by systemic comorbidities such as diabetes, hypertension, and metabolic syndrome.1, 2 A systemic proinflammatory state may be involved in the pathophysiology of HFpEF.2 Inflammation affects the coronary microvascular endothelium which results in vasoconstriction of the coronary microvascular bed in HFpEF patients.2 Coronary microvascular dysfunction (CMD), assessed invasively,3, 4 non-invasively,5, 6 and on autopsy7 may be involved in the pathogenesis of HFpEF. A recent prospective, multi-center study of coronary flow reserve (CFR) in HFpEF found the prevalence of CMD was high (75%).1
CMD assessment by quantitative stress perfusion cardiovascular magnetic resonance (CMR) has not been previously studied in HFpEF. Myocardial perfusion reserve (MPR) by CMR has been validated against invasive9 and noninvasive modalities (PET).10 It has also been shown to provide prognostic utility and can improve diagnostic performance over qualitative assessment of ischemia in CAD.11, 12
CMR has the advantage over other modalities in that it can also quantify diffuse myocardial fibrosis which is a common endpoint of many cellular processes in HFpEF patients7, 14. T1 mapping post-gadolinium and quantification of extracellular volume fraction (ECV) by CMR has been shown to be markers of diffuse myocardial fibrosis.15-19 Thus, myocardial fibrosis and microvascular dysfunction measured by CMR may provide promising therapeutic targets in HFpEF.
The aim of our study was to quantify MPR and diffuse myocardial fibrosis by using CMR in patients with a clinical diagnosis of HFpEF. We hypothesized that these patients will have reduced MPR and increased myocardial fibrosis compared to age-matched controls. Furthermore, we hypothesize that MPR and fibrosis indices would be associated with echocardiographic evidence of diastolic dysfunction, exercise capacity, and peak VO2.
Methods
Between May 2015 and November 2018, patients with a confirmed clinical diagnosis of HFpEF were prospectively enrolled in this single center study. This study was performed under a locally approved IRB protocol (HSR# 17997), and all patients gave written informed consent before study enrollment. The following strict criteria were required for study inclusion: age 18–85, New York Heart Association (NYHA) functional class ≥ II or BNP ≥ 150 pg/mL, EF> 45%, and at least grade 1 diastolic dysfunction by echo or elevated pulmonary capillary wedge pressure on right heart catheterization. Exclusion criteria were history of known myocardial infarction, severe valvular heart disease, secondary causes of hypertension, infiltrative (i.e. amyloidosis, sarcoidosis) or hypertrophic cardiomyopathy, pericardial disease, primary pulmonary arterial hypertension, or previously reduced left ventricular (LV) ejection fraction (EF) with recovery of function. Subjects with contraindications to MRI such as metallic implants, severe claustrophobia, pacemakers/defibrillators, and estimated GFR < 45 mL/min/1.73 m2 were also excluded. History of persistent atrial fibrillation with HR > 100 bpm were excluded due to concern regarding poor image quality. Patients with a contraindication to adenosine or who refused adenosine were excluded from stress myocardial perfusion imaging but were eligible for the remainder of the study, including native and contrast-enhanced T1 mapping. One patient screened for the study was excluded due to presence of previously unknown constrictive pericarditis.
Patients were compared to 15 age matched normal controls. Age-matched healthy controls, free of known cardiovascular disease were recruited. All subjects underwent a history and physical examination, electrocardiogram (ECG), cardiopulmonary exercise testing, comprehensive transthoracic echocardiogram (including strain), blood tests, and CMR.
CMR studies were performed on a 1.5 Tesla scanner (Avanto or Aera, Siemens Healthineers, Erlangen, Germany). Protocol included scout imaging followed by balanced steady-state free precession cine sequences to determine LV volumes, mass, and function. A short-axis stack of 8 mm thick short axis images with 2 mm gap covered the entire LV. Three long-axis images were obtained (2, 3, and 4-chamber views). A spiral cine displacement encoding with stimulated echoes (DENSE) pulse sequence was used to quantify peak systolic circumferential strain and early diastolic strain rate. 20, 24, 25 Base- and mid-ventricular short-axis modified Look-Locker inversion recovery (MOLLI) T1 mapping images, were then obtained prior to contrast administration, at 5- and 10 minutes after rest perfusion, and at 5-, 10-, and 15 minutes after stress perfusion imaging.
For stress perfusion assessment, 3 short axis slice locations were imaged per heart beat over a 60 heart beat acquisition during an IV bolus of 0.075 mmol/kg of gadolinium contrast (Magnevist, Bayer Healthcare, Whippany, New Jersey, USA) injected via power injector at 4 mL/s. Adenosine was infused at 140 μg/kg per minute through a peripheral IV line for 3–4 minutes during the stress imaging. Rest perfusion imaging was performed 15 minutes following stress imaging. Quantitative first-pass perfusion imaging was performed using a dual-sequence approach using a vendor-supplied works in progress (WIP) package. The saturation-recovery gradient echo pulse sequence acquires a low-resolution arterial input function (AIF) image, and 3 myocardial tissue function (TF) images every RR interval. Two proton density weighted images were acquired for the AIF and TF for correcting surface-coil related intensity inhomogeneity and Bloch simulation modeling was used to convert the signal intensity into gadolinium concentration units as previously described.26, 27
Late gadolinium enhancement (LGE) using phase-sensitive inversion recovery pulse sequences were also obtained 5 minutes after resting perfusion using standard SCMR guideline protocols.28
Cine CMR images were analyzed by an experienced investigator using QMASS (Medis Medical Imaging Systems, Leiden, the Netherlands). Short axis cine images were contoured for each short-axis slice, with total LV mass, end-diastolic volume, end-systolic volume, stroke volume and EF measured.
Perfusion quantification was performed on a pixel-wise basis using the constrained Fermi function deconvolution method implemented in MATLAB (Mathworks, Natick, Massachusetts, USA).13 MPR was calculated as a ratio of the stress perfusion divided by rest perfusion for each subject. Figure 1 illustrates the perfusion maps for a patient with reduced global MPR.
Figure 1.

Pixel based perfusion maps and bulls-eye plots at rest (top) and stress (bottom) for 3 short axis slices. Global MPR for this patient was: 2.19 ± 0.31.
T1 quantification was performed using manual segmentation of the myocardium in MATLAB. The partition coefficient of gadolinium, λ, was calculated from the slope of the linear fit of the plot of 1/T1 myocardium versus 1/T1 blood. ECV was calculated as λ*(1-hematrocrit).29, 30 Hematocrit was measured on the day of the CMR study. Peak systolic circumferential strain and early diastolic strain rates were computed offline from DENSE images with a custom MATLAB script using previously described methods.20, 31, 32
All study participants underwent comprehensive 2-D with Doppler, tissue Doppler imaging, and speckle tracking using commercially available ultra sound systems (GE Healthcare, General Electric Corp., Waukesha, WI, USA, Philips Medical Systems). LV function, diastolic function, and global longitudinal strain (GLS) were quantified as recommended by the American Society of Echocardiography/European Association of Cardiovascular Imaging.33, 34 GLS was analyzed using vendor-independent speckle-tracking echocardiographic software (Tomtec 2D Cardiac Performance Analysis, Tom Tec Imaging Systems, Munich, Germany) on optimized (maximized frame rate, minimizing foreshortening) two-, three-, and four-chamber apical view images. If regional tracking was suboptimal in more than two myocardial segments in a single view then GLS calculation was not performed.
All exercise tests were performed at our center’s cardiopulmonary exercise laboratory. Metabolic measures were determined during a continuous progressive treadmill protocol (Cardiac Science treadmill, Waukesha, Wisconsin). VO2 max was chosen as the highest one minute value obtained at volitional exhaustion. Respiratory exchange ratio (RER) ≥ 1.1, Borg Rating of Perceived Exertion (RPE) > 18, and age predicted maximal HR were also used as indicators of attaining VO2 max.
Analysis was performed using SAS, version 9.4 (SAS Institute, Inc. Cary, North Carolina, USA). Continuous normally distributed data are presented as mean ± SD, and were compared using two-sided t-tests. Categorical data are presented as N and percentages and compared using chi-square analysis and the Fisher’s exact test where appropriate. For all statistics a p <0.05 was considered statistically significant. Pearson test was used to determine the correlation of different covariates with ECV and MPR.
Results
A total of 34 subjects were included in the study, 19 patients with HFpEF (63 ± 11 years, 42% female) and 15 healthy age-matched controls (59 ± 9 years, 60% female). A comparison of demographic and clinical characteristics between HFpEF and control group is summarized in Table 1. There was no significant difference in age, gender, systolic blood pressure, or history of tobacco use. Average heart rate and body mass index were significantly higher in the HFpEF group. There are notable differences in cardiovascular risk factors between the two groups. The HFpEF group had a significantly higher prevalence of hypertension (84%), hyperlipidemia (74%), and diabetes (58%). In addition, there were more African Americans in the HFpEF group (26%). As expected, there were more patients in the HFpEF group taking cardiovascular medications including diuretics, beta blockers, ACE-inhibitor or ARB, and statins. 95% of the HFpEF patients were on a loop-diuretic at the time of enrollment. BNP was elevated in HFpEF patients compared to normal controls.
Table 1.
Baseline Characteristic of HFpEF Patients and Controls
| HFpEF (n = 19) | Controls (n =15) | p-value | |
|---|---|---|---|
| Age (years) | 63 ± 11 | 59 ± 9 | p = 0.22 |
| Body Mass Index (kg/m2) | 35 ± 7 | 27 ± 5 | p < 0.001 |
| Female (n,%) | 8 (42) | 9 (60) | p = 0.49 |
| Heart Rate (bpm) | 74 ± 14 | 64 ± 12 | p = 0.03 |
| SBP (mmHg) | 128 ± 22 | 135 ± 11 | p = 0.33 |
| DBP (mmHg) | 74 ± 14 | 71 ± 10 | p = 0.41 |
| African American | 5 (26) | 0 (0) | p = 0.05 |
| Smoking (n,%) | 2 (11) | 0 (0) | p = 0.50 |
| Hypertension (n,%) | 16 (84) | 3 (20) | p < 0.001 |
| Hyperlipidemia (n,%) | 14 (74) | 1 (7) | p < 0.001 |
| Diabetes (n,%) | 11 (58) | 0 (0) | p < 0.001 |
| Loop diuretic (n,%) | 18 (95) | 1 (7) | p < 0.001 |
| Beta Blocker (n,%) | 14 (74) | 0 (0) | p < 0.001 |
| ACE-I or ARB (n,%) | 14 (74) | 3 (20) | p = 0.005 |
| Statin (n,%) | 14 (74) | 1 (7) | p < 0.001 |
| BNP (pg/ml) | 135 ± 153 | 26 ± 37 | p = 0.01 |
| Hematocrit | 39 ± 5 | 44 ± 4 | p = 0.007 |
Values are presented as mean ± SD or n (%). ACEI = angiotensin converting enzyme inhibitors, ARB = angiotensin II receptor blocker, BNP = brain natriuretic peptide, DBP = diastolic blood pressure, and SBP = systolic blood pressure.
Table 2 summarizes the CMR, echocardiographic, and CPET test results for the HFpEF and control groups. There was no between group difference in LVEF, mass index, or end-diastolic volume index. 16 of 19 HFpEF patients and all 15 controls underwent stress perfusion CMR. 3 patients did not undergo stress, 1 opted not to undergo stress, and 2 had technical difficulties with perfusion. Global myocardial perfusion was lower in the HFpEF group (2.29 ± 0.64 vs 3.38 ± 0.76, p = 0.002). Furthermore, 69% of the HFpEF group had CMD, defined as a global MPR < 2.5. ECV was higher in the HFpEF group (0.29 ± 0.04 vs 0.25 ± 0.04, p = 0.02). However, native T1 was similar between groups 6/19 (32%) HFpEF patients had LGE, 3 patients had subendocardial LGE in a single vessel territory, and 3 had LGE in non-ischemic patterns (mid-wall and RV insertion site). DENSE peak systolic circumferential strain and early diastolic circumferential strain rate were similar between groups. Global longitudinal strain (GLS) by echo trended towards a decrease in GLS in the HFpEF group. All 15 patients in the control group had sufficient echo quality to perform strain analysis. Only 12 (63%) of patients in the HFpEF group met appropriate image quality for performing strain analysis. The HFpEF group had a significantly higher pulmonary artery systolic (PASP) (35.4 ± 13.7 vs 22.3 ± 5.4 mmHg, p = 0.004) and average E/e’ (15.0 ± 7.6 vs 8.6 ± 2.0, p = 0.005) but similar left atrial volume index. HFpEF group had a significantly lower VO2max (16.5 ± 6.8 vs 30.9 ± 7.7 ml/kg*min, p < 0.001) and METS (4.7 ± 1.9 vs 8.8 ± 2.2 kcal/kg*h, p < 0.001) compared to the control group.
Table 2.
CMR, Echocardiogram, and CPET Parameters comparing HFpEF patients and Controls
| HFpEF (n = 19) | Controls (n =15) | p-value | |
|---|---|---|---|
| LVEF (%) | 61 ± 9 | 65 ± 5 | p = 0.13 |
| LVMI (g/m2) | 45 ± 12 | 46 ± 11 | p = 0.76 |
| LVEDVI (ml/m2) | 70 ± 14 | 70 ± 14 | p = 0.96 |
| Peak average circumferential strain | −0.15 ± 0.03 | −0.16 ± 0.03 | p = 0.60 |
| Average Circumferential e'SR (s−1) | 1.37 ± 0.65 | 1.26 ± 0.48 | p = 0.59 |
| Native T1 (ms) | 1040 ± 74 | 1020 ± 43 | p = 0.36 |
| λ | 0.48 ± 0.05 | 0.45 ± 0.05 | p = 0.14 |
| ECV | 0.29 ± 0.04 | 0.25 ± 0.04 | p = 0.02 |
| Global Myocardial Perfusion Reserve* | 2.29 ± 0.64 | 3.38 ± 0.76 | p = 0.002 |
| Stress Perfusion* | 1.62 ± 0.52 | 1.78 ± 0.57 | p = 0.43 |
| Rest Perfusion* | 0.73 ± 0.23 | 0.54 ± 0.19 | p = 0.02 |
| LA Volume Index (ml/m2) | 34.5 ± 15.4 | 26.5 ± 7.2 | p = 0.09 |
| PASP (mmHg) | 35.4 ± 13.7 | 22.3 ± 5.4 | p = 0.004 |
| Average E/e' | 15.0 ± 7.6 | 8.6 ± 2.0 | p = 0.005 |
| GLS (%) | −17.9 ± 3.5 | −20.5 ± 3.6 | p = 0.07 |
| VO2max (ml/kg*min) | 16.5 ± 6.8 | 30.9 ± 7.7 | p < 0.001 |
| METS (kcal/kg*h) | 4.7 ± 1.9 | 8.8 ± 2.2 | p < 0.001 |
16/19 HFpEF patients and all control subjects underwent stress perfusion CMR.
Values are presented as mean ± SD. ECV = extracelluar volume, E/e' = early mitral inflow velocity/ mitral annular early diastolic velocity ratio, e'SR = early diastolic strain rate, GLS = global longitudinal strain, LVEDVI = left ventricular end diastolic volume index, LVEF = left ventricular ejection fraction, LVMI = left ventricular mass index, METS = metabolic equivalents, PASP = pulmonary artery systolic pressure, VO2max = maximum oxygen consumption, and λ = partition coefficient.
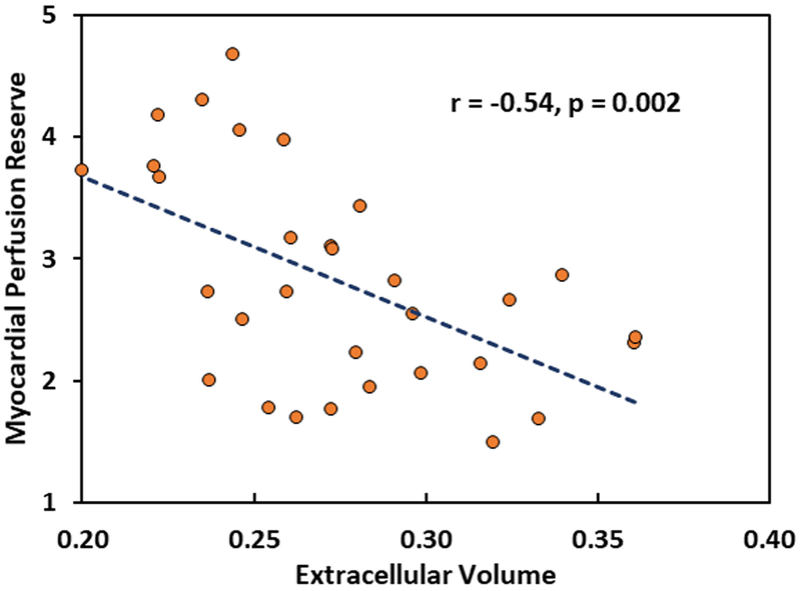
Correlation between MPR or ECV and different covariates are summarized in Table 3. VO2 max was positively correlated with MPR while average E/e’ and PASP were negative correlated. PASP had the strongest negative correlation. VO2 max had negative correlation with ECV while PASP had a positive correlation. Average E/e’ had no correlation with ECV. In addition, a significant negative correlation between ECV and MPR can be seen in Figure 2.
Table 3.
Pearson Correlation Coefficients
| Pearson r | p | |
|---|---|---|
| ECV | ||
| VO2max | −0.52 | 0.002 |
| Average E/e' | 0.34 | 0.057 |
| PASP | 0.51 | 0.006 |
| MPR | ||
| VO2max | 0.40 | 0.028 |
| Average E/e' | −0.47 | 0.008 |
| PASP | −0.60 | 0.001 |
ECV = extracelluar volume, E/e' = early mitral inflow velocity/ mitral annular early diastolic velocity ratio, METS = metabolic equivalents, PASP = pulmonary artery systolic pressure, and VO2max = maximum oxygen consumption.
Figure 2.
Scatter plot of correlation between ECV and MPR.
Discussion
The present study demonstrates that patients with a clinical diagnosis of HFpEF have reduced MPR and increased myocardial fibrosis as compared to age matched controls. In addition, global MPR and ECV were inversely correlated. Patients in the HFpEF cohort had significantly reduced VO2 max, increased E/e’ and PASP on echo, and elevated BNP, thus confirming their clinical HFpEF. VO2 max, average E/e’, and PASP also correlated with MPR with PASP having the highest negative correlation. The absence of LVH and known obstructive CAD underscores that the reduction in global MPR is not due to these factors. This suggests that CMD and diffuse fibrosis may play an important role in the pathophysiology of HFpEF and potentially be key targets for therapeutic interventions.
In this cohort, the prevalence of CMD, defined as global MPR < 2.5,1, 35 was high (69%). This is consistent with the high (75%) prevalence of CMD reported in a recent large multi-center study of coronary flow reserve (CFR) assessment using adenosine stress transthoracic Doppler echocardiography in patients with HFpEF.1 This adds to the emerging literature suggesting that HFpEF is a systemic disorder associated with endothelial dysfunction.1-7 This is the first study to assess CMD in HFpEF by CMR first pass perfusion imaging. Fully quantitative CMR has recently identified reduced MPR in patients with typical angina and risk factors for CMD who had no obstructive coronary artery disease.13
CMR has the advantage over other modalities in that in can also quantify diffuse myocardial fibrosis. ECV has been shown to be elevated in patients with LVH.20 ECV has also recently been studied in HFpEF patients and found to help differentiate them from hypertensive heart disease.21 Despite our cohort demonstrating no hypertrophy, ECV was still elevated compared to the control group. Interestingly our study showed that measurements of myocardial strain, both by CMR and echocardiography, were not reduced in the HFpEF group, although there was a trend towards decreased GLS (p = 0.07). A recent study among patients with HFpEF, hypertensive patients, and healthy controls showed that although both GLS and ECV could discriminate between hypertensive heart disease and HFpEF, ECV was a better discriminatory marker of HFpEF (AUC 0.88) than GLS (AUC 0.78)21. Their cohort had significantly less diabetics compared to the present study (9.7% vs 58%) and higher LV mass index (70.8 ± 20.2 vs 45 ± 12 g/m2) suggesting that in a HFpEF cohort predominated by diabetic patients without LVH, increase in ECV occurs before impairment in strain. Furthermore, this study illustrates the challenge of acquiring high quality images for speckle tracking assessment of GLS in HF patients which was only achievable in <2/3 of the HFpEF cohort.
Multiple techniques have been used to assess microvascular function.35 The advent of noninvasive techniques increase the feasibility of diagnosing CMD without the associated risk of catheter-based techniques. Doppler echocardiography techniques rely on the quality of acoustic windows and are not often used in the clinical setting. PET perfusion imaging has become a gold standard; however CMR has the added advantage of including fibrosis and strain imaging. The global MPR measured in our cohort (2.29 ± 0.64) was very similar to measurements by PET (2.16 ± 0.69) in a cohort of HFpEF patients without known obstructive epicardial CAD.5 Our study is consistent with recent data that less than one third of patients with HFpEF don’t have evidence of CMD.1 Future studies are needed to assess if MPR can be used to assess the stage of disease progression in HFpEF. Clinical trials targeting fibrosis and perfusion in the HFPEF population are needed.
This study is limited by a relatively small sample size; however, this is the only study to date evaluating quantitative CMR first pass perfusion imaging in HFpEF. Furthermore the study was adequately powered to highlight important differences in CMR, echocardiographic, and CPET parameters in a cohort with pure HFpEF (no LVH, no infiltrative cardiomyopathies and normal EF).
In conclusion, we have demonstrated by quantitative CMR perfusion imaging that HFpEF patients without LVH and with normal myocardial strain, have diffuse fibrosis and a high prevalence of CMD. Comprehensive evaluation of perfusion, fibrosis, and strain by CMR may become important endpoints for therapeutic efficacy in HFpEF.
Acknowledgments
The authors would like to acknowledge the help of our study coordinators and nurses Jayne Missel RN, and Caroline Flournoy PhD, our research CMR technologists Joseph Hylton, RT(R)MR and Jose Reyes RT(R)MR, and our research echo sonographers Kimberly Chadwell RDCS, Jason Higginson RDCS, and Dale E. Fowlder MD, RDCS.
Funding: Drs. Löffler, Balfour, and Shaw were supported by NIH 5T32EB003841. Dr. Salerno was funded by NIH R01 HL131919. This work was funded by the UVA-AstraZeneca Alliance.
Footnotes
Disclosures: Dr. Kramer is a consult for Bayer. Drs. Salerno and Epstein receive research support from Siemens Healthineers. Dr. Salerno receives grant support from Astra Zeneca. Dr. Gan is an employee of Astra Zeneca.
References
- 1.Shah SJ, Lam CSP, Svedlund S, Saraste A, Hage C, Tan RS, Beussink-Nelson L, Fermer ML, Broberg MA, Gan LM, Lund LH. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF, Eur Heart J 2018;39:3439–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation, J Am Coll Cardiol 2013;62:263–271. [DOI] [PubMed] [Google Scholar]
- 3.Sucato V, Evola S, Novo G, Sansone A, Quagliana A, Andolina G, Assennato P, Novo S. Angiographic Evaluation of Coronary Microvascular Dysfunction in Patients with Heart Failure and Preserved Ejection Fraction, Microcirculation 2015;22:528–533. [DOI] [PubMed] [Google Scholar]
- 4.Dryer K, Gajjar M, Narang N, Lee M, Paul J, Shah AP, Nathan S, Butler J, Davidson CJ, Fearon WF, Shah SJ, Blair JEA. Coronary microvascular dysfunction in patients with heart failure with preserved ejection fraction, Am J Physiol Heart Circ Physiol 2018;314:H1033–H1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Srivaratharajah K, Coutinho T, deKemp R, Liu P, Haddad H, Stadnick E, Davies RA, Chih S, Dwivedi G, Guo A, Wells GA, Bernick J, Beanlands R, Mielniczuk LM. Reduced Myocardial Flow in Heart Failure Patients With Preserved Ejection Fraction, Circ Heart Fail 2016;9:e002562. [DOI] [PubMed] [Google Scholar]
- 6.Kato S, Saito N, Kirigaya H, Gyotoku D, Iinuma N, Kusakawa Y, Iguchi K, Nakachi T, Fukui K, Futaki M, Iwasawa T, Kimura K, Umemura S. Impairment of Coronary Flow Reserve Evaluated by Phase Contrast Cine-Magnetic Resonance Imaging in Patients With Heart Failure With Preserved Ejection Fraction, J Am Heart Assoc 2016;5:e002649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mohammed SF, Hussain S, Mirzoyev SA, Edwards WD, Maleszewski JJ, Redfield MM. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction, Circulation 2015;131:550–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taqueti VR, Solomon SD, Shah AM, Desai AS, Groarke JD, Osborne MT, Hainer J, Bibbo CF, Dorbala S, Blankstein R, Di Carli MF. Coronary microvascular dysfunction and future risk of heart failure with preserved ejection fraction, Eur Heart J 2018;39:840–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lockie T, Ishida M, Perera D, Chiribiri A, De Silva K, Kozerke S, Marber M, Nagel E, Rezavi R, Redwood S, Plein S. High-resolution magnetic resonance myocardial perfusion imaging at 3.0-Tesla to detect hemodynamically significant coronary stenoses as determined by fractional flow reserve, J Am Coll Cardiol 2011;57:70–75. [DOI] [PubMed] [Google Scholar]
- 10.Morton G, Chiribiri A, Ishida M, Hussain ST, Schuster A, Indermuehle A, Perera D, Knuuti J, Baker S, Hedstrom E, Schleyer P, O’Doherty M, Barrington S, Nagel E. Quantification of absolute myocardial perfusion in patients with coronary artery disease: comparison between cardiovascular magnetic resonance and positron emission tomography, J Am Coll Cardiol 2012;60:1546–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sammut EC, Villa ADM, Di Giovine G, Dancy L, Bosio F, Gibbs T, Jeyabraba S, Schwenke S, Williams SE, Marber M, Alfakih K, Ismail TF, Razavi R, Chiribiri A. Prognostic Value of Quantitative Stress Perfusion Cardiac Magnetic Resonance, JACC Cardiovasc Imaging 2018;11:686–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel AR, Antkowiak PF, Nandalur KR, West AM, Salerno M, Arora V, Christopher J, Epstein FH, Kramer CM. Assessment of advanced coronary artery disease: advantages of quantitative cardiac magnetic resonance perfusion analysis, J Am Coll Cardiol 2010;56:561–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zorach B, Shaw PW, Bourque J, Kuruvilla S, Balfour PC,Jr, Yang Y, Mathew R, Pan J, Gonzalez JA, Taylor AM, Meyer CH, Epstein FH, Kramer CM, Salerno M. Quantitative cardiovascular magnetic resonance perfusion imaging identifies reduced flow reserve in microvascular coronary artery disease, J Cardiovasc Magn Reson 2018;20:14–018-0435–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure: Part I: diagnosis, prognosis, and measurements of diastolic function, Circulation 2002;105:1387–1393. [DOI] [PubMed] [Google Scholar]
- 15.Iles L, Pfluger H, Phrommintikul A, Cherayath J, Aksit P, Gupta SN, Kaye DM, Taylor AJ. Evaluation of diffuse myocardial fibrosis in heart failure with cardiac magnetic resonance contrast-enhanced T1 mapping, J Am Coll Cardiol 2008;52:1574–1580. [DOI] [PubMed] [Google Scholar]
- 16.Flett AS, Hayward MP, Ashworth MT, Hansen MS, Taylor AM, Elliott PM, McGregor C, Moon JC. Equilibrium contrast cardiovascular magnetic resonance for the measurement of diffuse myocardial fibrosis: preliminary validation in humans, Circulation 2010;122:138–144. [DOI] [PubMed] [Google Scholar]
- 17.Duca F, Kammerlander AA, Zotter-Tufaro C, Aschauer S, Schwaiger ML, Marzluf BA, Bonderman D, Mascherbauer J. Interstitial Fibrosis, Functional Status, and Outcomes in Heart Failure With Preserved Ejection Fraction: Insights From a Prospective Cardiac Magnetic Resonance Imaging Study, Circ Cardiovasc Imaging 2016;9:e005277. [DOI] [PubMed] [Google Scholar]
- 18.Salerno M, Kramer CM. Myocardial Extracellular Volume: Unifying Form and Function in Heart Failure With Preserved Ejection Fraction∗, Journal of the American College of Cardiology 2016;67:1826–1828. [DOI] [PubMed] [Google Scholar]
- 19.Salerno M Seeing the unseen fibrosis in heart failure with preserved ejection fraction, JACC Cardiovasc Imaging 2014;7:998–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuruvilla S, Janardhanan R, Antkowiak P, Keeley EC, Adenaw N, Brooks J, Epstein FH, Kramer CM, Salerno M. Increased extracellular volume and altered mechanics are associated with LVH in hypertensive heart disease, not hypertension alone, JACC Cardiovasc Imaging 2015;8:172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mordi IR, Singh S, Rudd A, Srinivasan J, Frenneaux M, Tzemos N, Dawson DK. Comprehensive Echocardiographic and Cardiac Magnetic Resonance Evaluation Differentiates Among Heart Failure With Preserved Ejection Fraction Patients, Hypertensive Patients, and Healthy Control Subjects, JACC Cardiovasc Imaging 2018;11:577–585. [DOI] [PubMed] [Google Scholar]
- 22.Roy C, Slimani A, de Meester C, Amzulescu M, Pasquet A, Vancraeynest D, Beauloye C, Vanoverschelde JL, Gerber BL, Pouleur AC. Associations and prognostic significance of diffuse myocardial fibrosis by cardiovascular magnetic resonance in heart failure with preserved ejection fraction, J Cardiovasc Magn Reson 2018;20:55–018-0477–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schelbert EB, Fridman Y, Wong TC, Abu Daya H, Piehler KM, Kadakkal A, Miller CA, Ugander M, Maanja M, Kellman P, Shah DJ, Abebe KZ, Simon MA, Quarta G, Senni M, Butler J, Diez J, Redfield MM, Gheorghiade M. Temporal Relation Between Myocardial Fibrosis and Heart Failure With Preserved Ejection Fraction: Association With Baseline Disease Severity and Subsequent Outcome, JAMA Cardiol 2017;2:995–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim D, Gilson WD, Kramer CM, Epstein FH. Myocardial tissue tracking with two-dimensional cine displacement-encoded MR imaging: development and initial evaluation, Radiology 2004;230:862–871. [DOI] [PubMed] [Google Scholar]
- 25.Zhong X, Spottiswoode BS, Meyer CH, Kramer CM, Epstein FH. Imaging three-dimensional myocardial mechanics using navigator-gated volumetric spiral cine DENSE MRI, Magn Reson Med 2010;64:1089–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsu LY, Jacobs M, Benovoy M, Ta AD, Conn HM, Winkler S, Greve AM, Chen MY, Shanbhag SM, Bandettini WP, Arai AE. Diagnostic Performance of Fully Automated Pixel-Wise Quantitative Myocardial Perfusion Imaging by Cardiovascular Magnetic Resonance, JACC Cardiovasc Imaging 2018;11:697–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cernicanu A, Axel L. Theory-based signal calibration with single-point T1 measurements for first-pass quantitative perfusion MRI studies, Acad Radiol 2006;13:686–693. [DOI] [PubMed] [Google Scholar]
- 28.Kramer CM, Barkhausen J, Flamm SD, Kim RJ, Nagel E, Society for Cardiovascular Magnetic Resonance Board of Trustees Task Force on Standardized Protocols. Standardized cardiovascular magnetic resonance (CMR) protocols 2013 update, J Cardiovasc Magn Reson 2013;15:91–429X-15–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kellman P, Wilson JR, Xue H, Ugander M, Arai AE. Extracellular volume fraction mapping in the myocardium, part : evaluation of an automated method, J Cardiovasc Magn Reson 2012;14:63–429X-14–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salerno M, Kramer CM. Advances in parametric mapping with CMR imaging, JACC Cardiovasc Imaging 2013;6:806–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spottiswoode BS, Zhong X, Lorenz CH, Mayosi BM, Meintjes EM, Epstein FH. Motion-guided segmentation for cine DENSE MRI, Med Image Anal 2009;13:105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spottiswoode BS, Zhong X, Hess AT, Kramer CM, Meintjes EM, Mayosi BM, Epstein FH. Tracking myocardial motion from cine DENSE images using spatiotemporal phase unwrapping and temporal fitting, IEEE Trans Med Imaging 2007;26:15–30. [DOI] [PubMed] [Google Scholar]
- 33.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging, J Am Soc Echocardiogr 2015;28:1–39.e14. [DOI] [PubMed] [Google Scholar]
- 34.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF,3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu BA, Waggoner AD. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging, J Am Soc Echocardiogr 2016;29:277–314. [DOI] [PubMed] [Google Scholar]
- 35.Marinescu MA, Loffler AI, Ouellette M, Smith L, Kramer CM, Bourque JM. Coronary microvascular dysfunction, microvascular angina, and treatment strategies, JACC Cardiovasc Imaging 2015;8:210–220. [DOI] [PMC free article] [PubMed] [Google Scholar]