Abstract
Background
Glioblastoma represents the most common primary brain tumor with a worst prognosis despite developments in neurosurgery and chemoradiotherapy. Detachment of the cells from the primary tumor tissue is a prerequisite for their dispersion and spreading. Initial and incessant dispersal of tumor cells from the primary tumor tissue renders GBM refractory to comprehensive surgical removal and increases the chance of recurrence and poorer prognosis.
Purposes
The current study was designed to investigate the effect of inhibition of MEK-ERK1/2 signaling by PD98059 and U0126 on the growth and migration of glioma cells as well as their adhesion to extracellular matrix.
Methods
MEK-ERK1/2 signaling in U87-MG cells was inhibited by PD98059 and U0126. Migration, proliferation and adhesion were analyzed by scratch-wound assay, MTT assay, cell adhesion assay respectively.
Results
PD98059 and U0126 significantly not only reduced the proliferation of glioma cells and attenuated their migration but also increased their adhesion to gelatin of extracellular matrix.
Conclusion
This study provides the evidence that inhibition of MEK-ERK1/2 signaling enhances the adhesion of glioma cells to gelatin/collagen component of ECM, and decreases the proliferation and migration of the glioma cells. We propose the possible rationale of association between ERK signaling and cell-cell adhesion molecules in glioma microenvironment which regulates the glioma initiation, growth and progression.
Keywords: Glioblastoma, Dispersal, ECM, Gelatinase, MAPK
Introduction
Glioblastoma (GBM) represents the most common primary brain tumor in humans with an average incidence rate of 3.21 per 1,00,000 population [1]. Recent studies have reported the incidence of glioma in India is 1–4 per 1,00,000 [2–4]. The prognosis of GBM remains poor despite developments in neurosurgery and chemoradiotherapy. GBM remains malignant owing to its highly proliferative, migratory and invasive potential [5]. Detachment of cells from primary tumor tissue is a prerequisite for their dispersion and spreading. Initial and incessant dispersal of tumor cells from the primary tumor tissue renders GBM refractory to comprehensive surgical removal and increases the chances of recurrence. Suppression of tumor growth and progression could significantly increase the possibility of total surgical resection and improve the prognosis [6].
Active migration of glioma cells is a prerequisite for invasion, and is a complex and dynamic process involving interaction of tumor cell with its microenvironment [7, 8]. Various researchers, including our study, have reported the factors regulating proliferation, migration and invasion and the extent of growth of glioma cells [8–12]. Tumor cell adhesion to extracellular matrix (ECM) or basement membrane plays a key role in tumor progression [13]. Over the years, several studies have aimed at understanding the mechanisms governing the aggressive behavior of GBM, but it still remains elusive [14–17].
One of the most common signaling cascades dysregulated in cancer is MEK-ERK pathway, which is involved in cancer cell survival [18]. Similarly, in malignant gliomas the RAS/RAF/MEK/ERK pathway is aberrantly activated [19]. An earlier study has shown that the ablation of the MEK1/2 kinases and/or ERK1/2 kinases in mice model of non-small cell carcinoma effectively prevented K-RAS-driven tumor development [20]. There are conflicting reports on the role of ERK1/2 pathway on glioma cells. One study demonstrated that transient activation of ERK1/2 by human chorionic gonadotropin β resulted in migration and invasion [21], while the other study reported that sustained activation of ERK1/2 by Sulforaphane inhibits migration and invasion of glioma cells [17]. Nonetheless, these findings suggest the importance of ERK signaling in growth of glioma necessitating the need for further studies to decipher ERK pathway in glioma biology.
With this viewpoint the current study was designed to investigate the effect of inhibition of MEK-ERK1/2 signaling by PD98059 and U0126 on the growth and migration of glioma cells as well as their adhesion to ECM.
Methods
ERK1/2 inhibitors, U0126 and PD98059 (InvivoGen, USA) were dissolved in dimethyl sulfoxide (DMSO, cat # D2650, Sigma Chemicals, St Louis, MO). U0126–5mg (Cat. #: tlrl-u0126, Working concentration: 10–100 µM, Solubility: DMSO, 10 mg/ml). PD98059–10mg (Cat. # tlrl-pd98, Working concentration: 10–100 μM, Solubility: DMSO 6.5 mg/ml). Concentration of DMSO (cat # D2650 Sigma Chemicals, St Louis, MO) used in the assays ranged between 0.01%-0.1%.
Cell culture
Human glioblastoma cell line U87-MG was purchased from National Centre for Cell Science (NCCS), Pune, India. The cells were maintained in Dulbecco’s Modified Eagle’s Medium-High glucose (DMEM) (D5648–1L, Sigma-Aldrich, USA) supplemented with 10% fetal bovine serum (FBS) (Gibco), PenStrep (Gibco) [penicillin (100 U/mL) and streptomycin (100 mg/mL)] at 37°C in a humidified atmosphere containing 5% CO2. Culture medium was exchanged twice a week. Before use, the cells were detached from the flask with 0.05 % trypsin–EDTA (Gibco), centrifuged and the resulting pellet was resuspended in fresh culture medium [12].
Proliferation assay
Proliferation of glioma cells was investigated by 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) assay as described earlier with modification [12]. Briefly, cells were trypsinized and counted in a Neubauer chamber slide using trypan blue dye exclusion method. Viable cells were plated at a density of 1X104 cells per well in 96-well plates in a final volume of 0.1 mL media containing 10 % FBS. After 24 h, cells were washed with Dulbecco’s phosphate buffered saline (DPBS) and treated with various concentrations of ERK1/2 inhibitors (25 µM, 50 µM and 100 µM) followed by 24 h growth in serum-free media. The experiments were conducted in triplicate. The plates were incubated at 37°C in a humidified atmosphere containing 5 % CO2 and 95 % air. After 24 h incubation, 100 µL of 2 mg/mL MTT was added to each well and incubated for 4 h at 37°C. Then, 200 µL of dimethylsulfoxide (DMSO) was added after removing the used media to each well and mixed. Absorbance was measured at 570 nm using a TECAN Infinite M200 multi-well plate reader. Data shown are representative of three independent experiments.
Wound healing migration assay
Migration of U87MG glioma cells was investigated by wound healing migration assay as previously described with minor modification [14]. Cells were counted in a Neubauer chamber slide using trypan blue exclusion method. Viable cells were plated at 4x105 cells per well in 6-well culture plates using growth media containing 10% FBS and allowed to grow for 24 h. The cells were washed with DPBS and pretreated with MEK-ERK1/2 inhibitor in serum-free media for the mentioned time intervals. To the control wells, only DMSO (vehicle control) was added. In vitro scratch wounds were then created by scraping the cell monolayers with a 200 µL sterile pipette tip to create a uniform, cell-free wound area. After washing away suspended cells, fresh serum-free media was added and photomicrograph was taken immediately (time 0 h) with an inverted microscope equipped with a digital camera, and the wounded cultures were allowed to grow for 24 h at 37°C. Another photomicrograph was taken at the same position after 24 h of incubation. Migration was quantified by count-ing cell numbers migrating from the wound edge. Within each assay the experiments were performed in triplicates. Data shown are representative of minimum three independent experiments.
Cell Adhesion Assay
Cell adhesion assay was performed as described earlier with minor modification [22]. Briefly, U87-MG cells (1×105 cells per well) were washed with Dulbecco’s phosphate buffer saline (DPBS) and harvested by trypsin. The cells were seeded in 24 well plate coated with gelatin (Sigma-Aldrich). After 1 h of incubation at 37°C, the unattached cells were removed by washing thrice with PBS. The remaining cells were fixed with methanol and stained with 1% crystal violet and counted under inverted microscope. The number of cells in six random fields was counted for each group. Data shown are representative of minimum three independent experiments.
Statistical Analysis
Results were expressed as mean ± SD. Statistical probability was calculated using GraphPad Prism software version 6.0. Student’s t-test was used to determine the level of significance between groups. p value of <0.05 was considered significant.
Results
Effect of ERK1/2 inhibitors on the proliferation of U87-MG cells
To evaluate the role of MEK-ERK1/2 signaling in glioma cell proliferation, U87-MG cells were incubated with increasing concentrations (25 µM, 50 µM and 100 µM) of MEK-ERK1/2 pathway inhibitors, U0126 and PD98059. Cell proliferation was demonstrated by MTT assay. U0126 and PD98059 reduced proliferation significantly at all the concentrations used in a dose-dependent manner (Fig. 1).
Fig. 1:
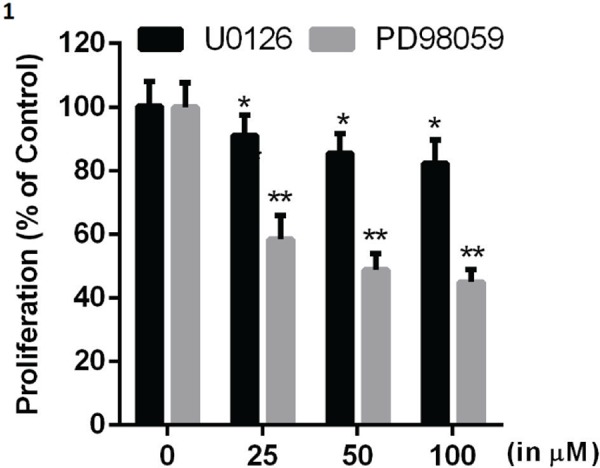
Effect of ERK1/2 inhibitors on glioma cell proliferation: U87-MG cells were treated with U0126/PD98059 at the indicated concentrations for 24 h and proliferation of glioma cells was evaluated. Data are presented as the mean ± SD. Statistics were performed using Student’s t-test. *p<0.05 and **p<0.01 in comparison to respective control. Data shown are representative of three independent experiments carried out in triplicate.
Effect of ERK1/2 inhibitors on migration of U87-MG glioma cells
The migration of U87-MG cells treated with U0126 or PD98059 and untreated controls was analyzed using wound healing migration assay. Quantification of migrated cells confirmed that the migration of U87-MG cells treated with U0126 or PD98059 was significantly reduced as compared to untreated (untreated control for U0126 contained 0.025% DMSO, and for PD98059 contained 0.05% of DMSO) cells (Fig. 2 a, c).
Fig. 2:
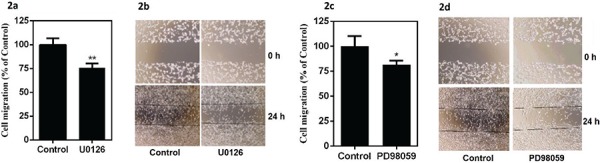
Effect of ERK1/2 inhibitors on glioma cell migration: U87-MG cells were treated with U0126 (25 µM) or PD98059 (50 μM) for 24 h. (a, c) Glioma cell migration. (b, d) Representative pictures of U87-MG wound healing migration assay. Data are presented as the mean ± SD. Statistics were performed using Student’s t-test. *p<0.05 and **p<0.01 in comparison to respective control. Data shown are representative of three independent experiments carried out in triplicate.
Effect of ERK1/2 inhibitors on adhesion of glioma cells to gelatin
Tumor cell adhesion to the extracellular matrix is implicated in tumor cell motility. We found that U87-MG cell attachment to gelatin-coated surface significantly increased on inhibition of ERK signaling by U0126/PD98059 as compared to untreated (untreated control for U0126 contained 0.025% DMSO, and for PD98059 contained 0.05% of DMSO) cells (Fig. 3).
Fig. 3:
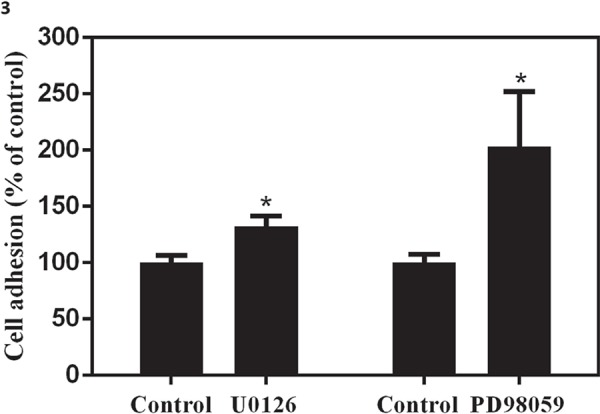
Effect of ERK1/2 inhibitors on glioma cell adhesion: U87-MG cells were treated with U0126 (25 µM) or PD98059 (50 μM) for 24 h and adhesiveness was evaluated. Data are presented as the mean ± SD. Statistics were performed using Student’s t-test. *p<0.05 in comparison to control. Data shown are representative of three independent experiments carried out in duplicate.
Discussion
Extracellular matrix constitutes an important component of tumor microenvironment [23]. Two crucial steps involved in invasion of glioma cells are adhesion of glioma cell to the ECM and then degradation of ECM. Collagen forms one of the important constituents of ECM of which collagen type IV and type XVI are upregulated in glioma [24]. The current study demonstrates that inhibition of MEK-ERK1/2 signaling by PD98059 and U0126, increases adhesion of glioma cells to gelatin/collagen which might reduce cell movement.
Since cell-matrix adhesion play a role in migration of glioma cells through ECM components, and in proliferation of glioma cells through integrins and proteoglycans, we further evaluated the effect of inhibition of MEK-ERK1/2 signaling on migration and proliferation. Also, proliferative and migratory processes play an essential role in glial tumor development and progression. Findings of current study illustrate that MEK-ERK1/2 signaling inhibitors PD98059 and U0126 significantly reduce proliferation and migration of glioma cells. Matrix-interaction domain of collagen has binding sites for proteoglycans and a study has reported chondroitin sulphate proteoglycans modulate the ecto-5’-NT/CD73 activity of ECM and thereby cell adhesion [25]. It is important to note that glioma microenvironment derived chondroitin sulphate proteoglycans promote proliferation of glioma cells; while constituents of ECM including collagen, laminin and fibronectin play a major role in migration of glioma cells [24]. These observations indicate interaction between glioma cell adhesion, tumor cell-ECM proliferation and migration. In the light of these evidences, it can be surmised that antiproliferative and antimigratory effect of ERK inhibitors are probably due to increased adhesion of glioma cells to ECM.
The level of phosphorylated-ERK is increased in high-grade gliomas with aggressive growth and invasive behavior (WHO grade IV) compared to low-grade gliomas (WHO grade II) [9]. Additionally, our study indicated that inhibition of ERK signaling inhibited the migration and proliferation. Interestingly, cell-cell adhesion molecules like intracellular adhesion molecule-1 have been implicated in tumor transformation and metastasis via tumor cell adhesion to the vascular endothelium [10]. Also, signaling proteins like focal adhesion kinase integrate signals from integrins to the actin filaments during cell migration [26]. Off late, a study reported the delay in dispersal of glioma cells by the inhibitor of ERK1/2 phosphorylation, PD0325901 and the decrease in motility of GBM mass [6], which could be due to increased adhesion.
Majority of glioma adhere to aberrant RTK/RAS/RAF/MEK signaling cascade for their growth and progression. Moreover, multiple oncogenic signaling pathways converge in the genesis of glioblastoma, with variations in the signaling network due to multifaceted crosstalk, hence making them less amenable to significant inhibition by monotherapies or polytherapy [27]. It is crucial to understand the mechanisms involved in glioblastoma to tailor in depth and to find appropriate rationale for the formulation of effective combination as well as personalized treatments. Therapies targeting containment of cell dispersal or enhancing adhesion might prove successful in improving the survival of glioma patients. Hence, identifying novel drugs targeting signaling pathways implicated in adhesion of glioma cells to ECM could provide valuable insight into the complex clinicopathological mechanisms involved in glioma and help in better management of glioblastoma (6).
To summarize, this study provides the evidence that inhibition of MEK-ERK1/2 signaling increases adhesion of glioma cells to gelatin/collagen component of ECM, and decreases the proliferation and migration of the glioma cells. We propose possible rationale of association between ERK signaling and cell-cell adhesion molecules in glioma microenvironment which regulates the glioma initiation, growth and progression. This demands further in-depth studies to establish the interaction which could provide newer insights into molecular signaling and potential therapeutic targets for glioma.
Acknowledgment
Authors gratefully acknowledge the English language editing by Prof. Chandrajit Prasad. Palaniswamy R acknowledges the senior research fellowship from the Council of Scientific and Industrial Research (CSIR), India.
Authorship contribution
PR: Performed the experiments and Analyzed the data.
NDN: Conceived and designed the experiments. Analyzed the data and written the manuscript.
MB: Analyzed the data and written the manuscript.
Abbreviation
- DMEM:
Dulbecco’s Modified Eagle’s Medium
- DMSO:
Dimethyl sulfoxide
- ERK:
Extracellular signaling-regulated kinase
- ECM:
Extracellular matrix
- GBM:
Glioblastoma
- MAPK:
Mitogen-activated protein kinase
- MEK:
MAPK extracellular signaling-regulated kinase kinase
- MMPs:
Matrix metalloproteinases
- MTT:
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
Footnotes
Source of funding
This work was supported by research grant from Science and Engineering Research Board (SERB), New Delhi, India [grant number: EMR/2014/000937], and a research grant from Department of Biotechnology (DBT), New Delhi, India [grant number: BT/PR3431/MED/30/648/2011] to Nandakumar Dalavaikodihalli Nanjaiah.
Conflict of interest: The authors have no conflicts of interest to declare.
References
- 1.Ostrom QT, Gittleman H, Truitt G, Boscia A, Kruchko C, Barnholtz-Sloan JS. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2011–2015. Neuro-oncology. 2018;20(suppl_4):iv1–iv86. doi: 10.1093/neuonc/noy131. Epub 2018/11/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mukherjee R, Das T, Roy K, Mukherjee J. Current Understanding of Epidemiology, Genetic Etiology and Treatment of Gliomas from Indian Population. Chemo Open Access. 2016;5(203):2. [Google Scholar]
- 3.Kumawat R, Gowda SH, Debnath E, Rashid S, Niwas R, Gupta Y et al. Association of Single Nucleotide Polymorphisms (SNPs) in Genes Encoding for Folate Metabolising Enzymes with Glioma and Meningioma in Indian Population. Asian Pacific journal of cancer prevention: APJCP. 2018;19(12):3415–25. doi: 10.31557/apjcp.2018.19.12.3415. Epub 2018/12/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jaiswal J, Shastry AH, Ramesh A, Chickabasaviah YT, Arimappamagan A, Santosh V. Spectrum of primary intracranial tumors at a tertiary care neurological institute: A hospital-based brain tumor registry. Neurology India. 2016;64(3):494–501. doi: 10.4103/0028-3886.181535. Epub 2016/05/06. [DOI] [PubMed] [Google Scholar]
- 5.Zhang X, Zhang W, Cao WD, Cheng G, Zhang YQ. Glioblastoma multiforme: Molecular characterization and current treatment strategy (Review). Exp Ther Med. 2012;3(1):9–14. doi: 10.3892/etm.2011.367. Epub 2012/09/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shannon S, Jia D, Entersz I, Beelen P, Yu M, Carcione C et al. Inhibition of glioblastoma dispersal by the MEK inhibitor PD0325901. BMC Cancer. 2017;17(1):121. doi: 10.1186/s12885-017-3107-x. Epub 2017/02/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maestro RD, Shivers R, McDonald W, Maestro AD. Dynamics of C6 astrocytoma invasion into three-dimensional collagen gels. J Neurooncol. 2001;53(2):87–98. doi: 10.1023/a:1012236830230. Epub 2001/11/22. [DOI] [PubMed] [Google Scholar]
- 8.Fathima Hurmath K, Ramaswamy P, Nandakumar DN. IL-1beta microenvironment promotes proliferation, migration, and invasion of human glioma cells. Cell Biol Int. 2014;38(12):1415–22. doi: 10.1002/cbin.10353. Epub 2014/07/24. [DOI] [PubMed] [Google Scholar]
- 9.Kim JY, Kim YJ, Lee S, Park JH. The critical role of ERK in death resistance and invasiveness of hypoxia-selected glioblastoma cells. BMC Cancer. 2009;9:27. doi: 10.1186/1471-2407-9-27. Epub 2009/01/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobayashi H, Boelte KC, Lin PC. Endothelial cell adhesion molecules and cancer progression. Curr Med Chem. 2007;14(4):377–86. doi: 10.2174/092986707779941032. Epub 2007/02/20. [DOI] [PubMed] [Google Scholar]
- 11.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–7. doi: 10.1038/nature01322. Epub 2002/12/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramaswamy P, Aditi Devi N, Hurmath Fathima K, Dalavaikodihalli Nanjaiah N. Activation of NMDA receptor of glutamate influences MMP-2 activity and proliferation of glioma cells. Neurol Sci. 2014;35(6):823–9. doi: 10.1007/s10072-013-1604-5. Epub 2014/01/01. [DOI] [PubMed] [Google Scholar]
- 13.Maziveyi M, Alahari SK. Cell matrix adhesions in cancer: The proteins that form the glue. Oncotarget. 2017;8(29):48471–87. doi: 10.18632/oncotarget.17265. Epub 2017/05/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hurmath FK, Mittal M, Ramaswamy P, Umamaheswara Rao GS. Dalavaikodihalli Nanjaiah N. Sevoflurane and thiopental preconditioning attenuates the migration and activity of MMP-2 in U87MG glioma cells. Neurochemistry international. 2016;94:32–8. doi: 10.1016/j.neuint.2016.02.003. Epub 2016/02/16. [DOI] [PubMed] [Google Scholar]
- 15.Gao YF, Zhu T, Chen J, Liu L, Ouyang R. Knockdown of collagen alpha-1(III) inhibits glioma cell proliferation and migration and is regulated by miR128–3p. Oncology letters. 2018;16(2):1917–23. doi: 10.3892/ol.2018.8830. Epub 2018/07/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kohsaka S, Hinohara K, Wang L, Nishimura T, Urushido M, Yachi K et al. Epiregulin enhances tumorigenicity by activating the ERK/MAPK pathway in glioblastoma. Neuro-oncology. 2014;16(7):960–70. doi: 10.1093/neuonc/not315. Epub 2014/01/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li C, Zhou Y, Peng X, Du L, Tian H, Yang G et al. Sulforaphane inhibits invasion via activating ERK1/2 signaling in human glioblastoma U87MG and U373MG cells. PloS one. 2014;9(2):e90520. doi: 10.1371/journal.pone.0090520. Epub 2014/03/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Redman EK, Brookes PS, Karcz MK. Role of p90(RSK) in regulating the Crabtree effect: implications for cancer. Biochemical Society transactions. 2013;41(1):124–6. doi: 10.1042/bst20120277. Epub 2013/01/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brennan CW, Verhaak RG, McKenna A, Campos B, Noushmehr H, Salama SR et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462–77. doi: 10.1016/j.cell.2013.09.034. Epub 2013/10/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanclemente M, Francoz S, Esteban-Burgos L, Bousquet-Mur E, Djurec M, Lopez-Casas PP et al. c-RAF Ablation Induces Regression of Advanced Kras/Trp53 Mutant Lung Adenocarcinomas by a Mechanism Independent of MAPK Signaling. Cancer Cell. 2018;33(2):217–28.e4. doi: 10.1016/j.ccell.2017.12.014. Epub 2018/02/06. [DOI] [PubMed] [Google Scholar]
- 21.Li Z, Du L, Li C, Wu W. Human chorionic gonadotropin beta induces cell motility via ERK1/2 and MMP-2 activation in human glioblastoma U87MG cells. J Neurooncol. 2013;111(3):237–44. doi: 10.1007/s11060-012-1017-y. Epub 2012/12/13. [DOI] [PubMed] [Google Scholar]
- 22.Liu P, Cai J, Dong D, Chen Y, Liu X, Wang Y et al. Effects of SOX2 on Proliferation, Migration and Adhesion of Human Dental Pulp Stem Cells. PloS one. 2015;10(10):e0141346. doi: 10.1371/journal.pone.0141346. Epub 2015/10/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. doi: 10.1016/j.cell.2011.02.013. Epub 2011/03/08. [DOI] [PubMed] [Google Scholar]
- 24.Shimizu T, Kurozumi K, Ishida J, Ichikawa T, Date I. Adhesion molecules and the extracellular matrix as drug targets for glioma. Brain tumor pathology. 2016;33(2):97–106. doi: 10.1007/s10014-016-0261-9. Epub 2016/03/20. [DOI] [PubMed] [Google Scholar]
- 25.Cappellari AR, Vasques GJ, Bavaresco L, Braganhol E, Battastini AM. Involvement of ecto-5’-nucleotidase/CD73 in U138MG glioma cell adhesion. Molecular and cellular biochemistry. 2012;359(1–2):315–22. doi: 10.1007/s11010-011-1025-9. Epub 2011/08/23. [DOI] [PubMed] [Google Scholar]
- 26.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6(1):56–68. doi: 10.1038/nrm1549. Epub 2005/02/03. [DOI] [PubMed] [Google Scholar]
- 27.Shingu T, Holmes L, Henry V, Wang Q, Latha K, Gururaj AE et al. Suppression of RAF/MEK or PI3K synergizes cytotoxicity of receptor tyrosine kinase inhibitors in glioma tumor-initiating cells. J Transl Med. 2016;14:46. doi: 10.1186/s12967-016-0803-2. Epub 2016/02/11. [DOI] [PMC free article] [PubMed] [Google Scholar]


