Abstract
Background
Treatment of spinal cord injury (SCI) induced neuropathic pain (NP) proves to be extremely clinically challenging as the mechanism behind SCINP is poorly understood. Matrix metalloproteinase (MMP) is largely responsible for the early disruption of the blood spinal cord barrier. This system initiates macrophage infiltration and degradation of myelin, which plays a pivotal role in how NP occurs. In a recent study, we demonstrated that folic acid (FA) treatment to cSCI rats reduced NP and improved functional recovery by repressing MMP-2 expression. We hypothesize that MMP-2 expression is suppressed because FA actively methylates the DNA sequence that encodes for the MMP-2 protein. However, modulation of MMP-2 expression for alleviation of NP is only pertinent to the mid- to late-phase of injury. Therefore, we need to explore alternate therapeutic methods to target the early- to mid-phase of injury to wholly alleviate NP.
Purpose
Furthering our previous findings on inhibiting MMP-2 expression by FA in mid- and late- phase following cSCI in rats, we hypothesized that FA will methylate and suppress MMP-9 expression during the early- phase, day 1, 3, 7 post cSCI and mid- phase (day 18 post cSCI), in comparison with MMP-2 expression during mid- and the late-phase of cSCI.
Methods
Adult male Sprague Dawley rats (250–270g) underwent cSCI, using a NYU impactor, with 12.5 gm/cm injury. The spinal cord-injured animals were treated intraperitoneally (i.p.) with a standardized dose of FA (80 μg/kg body weight) on day 1, 2, 3, prior to cSCI, followed by daily injection up to 14 or 17 days post-cSCI in different experiments. Animals were euthanized on day 1, 3, 7 post cSCI (early- phase), day 18 post cSCI (mid- phase), and day 42 post cSCI (late-phase) and the epicenter region of injured spinal cord were harvested for MMP-9 and MMP-2 expression analysis by Western blots technique.
Results
i) During early-phase on day 1, 3, and 7, the quantitation displayed no statistical significance in MMP-9 expression, between water- and FA- injected rats. ii) On day 18 post-cSCI, FA significantly modulates the expression of MMP-9 (p = 0.043) iii) Comparing results with MMP-2 expression and inhibition, FA significantly modulates the expression of MMP-2 on day 18 post cSCI (FA- and water-injected rats (p = 0.003). iv) In addition, FA significantly modulates the expression of MMP-2 on day 42 post-cSCI comparing FA- and water- injected rat groups (p = 0.034).
Conclusion
We report that FA administration results in alleviating cSCI-induced NP by inhibiting MMP-9 in the proposed mid- phase of cSCI. However, FA administration resulted in MMP-2 decline during both mid- through late- phase following cSCI. Our study elucidates a new phase of cSCI, the mid-phase. We conclude that further investigation on discovering and quantifying the nature of the mid- phase of SCI injury is needed.
Keywords: Matrix metalloproteinses (MMPs), MMP-9, MMP-2, Folic acid (FA), Demethylation
Introduction
In modern medicine, there is a lack of therapeutic options for spinal cord injury (SCI). Early secondary pathogenesis following SCI is believed to be mediated by inflammatory responses and matrix metalloproteinases (MMPs) [1]. MMPs are endopeptidases that contribute to growth, development, wound healing, and pathologies such as arthritis and cancer; participation in these processes is done through the degradation of extracellular matrix (ECM) molecules [2]. Furthermore, MMP activity is much more directed and causes the release of cryptic information from the ECM. By precisely cleaving large insoluble ECM components and ECM-associated molecules, MMPs liberate bioactive fragments and growth factors and change ECM architecture, all of which influence cellular behavior. Thus, MMPs have become a focal point for understanding matrix biology [3].
MMPs are responsible for early disruption of the blood spinal cord barrier, which initiates macrophage infiltration and degradation of myelin [4]. The expression of MMP-1, -2, -9 and -12 have been confirmed during first week of post-traumatic human SCIs [5] and they are involved in the destructive inflammatory events of protein breakdown, phagocytosis by infiltrating neutrophils and macrophages and enhancing permeability of the blood spinal cord barrier. This leads to hyper excitability of afferents with action potentials (APs) outlasting the stimulus, creating central sensitization, a common mechanism of neuropathic pain (NP) [6]. In addition, MMPs are an integral contributor to inhibitory glial scar formation [6] and both MMP-2 and MMP-9 have been found to participate in this process [6]. The key findings of the study [6] highlight the mechanisms that differentiate between early and late phases of NP pathophysiology. In a nerve injury model, following injury, MMP-9 induces and MMP-2 maintains NP through interleukin-1β cleavage and microglia activation at early phase. Inhibition of MMP may provide a novel therapeutic approach for the treatment of NP at different phases [6]. Through this mechanism, MMPs limit functional recovery after SCI by the modulation of early vascular events [1]. However, the significant induction of these MMPs was not supplemented by the expression of their inhibitors, evident in animal studies, which allows these proteins to exert their effects in the spinal cord lesion.
Observed in an olfactory nerve injury model, MMP-9 expression levels rapidly increase immediately following an injury, which is consistent with the early phase of NP and corresponds to neuronal degeneration and increased glial activity [6]. On the other hand, MMP-2 displays a delayed response and peaks significantly later than MMP-9 [6]. Furthermore, using MMP-9 KO mice, MMP-2 expression has been found to be independent of MMP-9 expression, suggesting that MMP-2 and MMP-9 may play different roles in the injury and repair processes [7]. MMP-2 and MMP-9 have been hypothesized, in an acute SCI model, to contribute to the disruption of the blood-spinal cord barrier and the influx of leukocytes into a SCI, as well as apoptosis [8]. In addition, MMP-9 and MMP-2 have been found to regulate inflammation and NP after peripheral nerve injury and may contribute to SCI-induced pain [8]. As an example, early pharmacologic inhibition of MMPs, or the gelatinases (MMP-2 and MMP-9), have resulted in long-term neurological recovery and is associated with reduced glial scarring and NP [8].
The folate pathway may play a crucial role in the regeneration and repair of the adult CNS after SCI injury, evident in the rodent model. This repair occurs, in part, via DNA methylation, which is a major epigenetic mechanism [9]. The effect of folate on the regeneration of afferent spinal neurons has been found to be biphasic and dose dependent, which closely relates to its dose range with the expression of de novo DNA methyltransferases as well as global and gene-specific DNA methylation [10]. Although unprecedented, the FDA approved dietary folic acid supplement has been found to function as a key methyl donor through the folate pathway, thus leading to higher levels of methylation in CNS associated gene, two of which encode for MMP-2 and -9 expression [9]. Therefore, increased DNA methylation levels in the promoters of MMPs and DNMT inhibitors, as found by previous studies, may result in reduction of NP and increased functional recovery. This highlights the underlying epigenetic mechanisms that occur in neuro-repair [11]. Thus, manipulation of the methylation environment may offer new and safer therapeutic avenues to alleviate NP and promote regeneration and functional recovery after SCI.
Furthermore, while exploring these mechanisms, we have reported chronic NP is associated with changes in the expression of MMP-2, β-catenin, and ERK. Our data suggests that the transient up-regulation of phosphorylated ERK (phospho-ERK) is involved in the initial up-regulation of both β-catenin and MMP-2 following cSCI induced NP states [12]. In addition, we have tested the role of folic acid (FA) in modulating MMP-2 expression. FA implementation in SCI injuries has demonstrated alleviation of NP and functional recovery improvement in SCI rodents [13]. To explore the benefits of FA in SCI recovery and NP alleviation, we have targeted MMP-9 regulation during the early stage and MMP-2 in late stage, as these are the times in which MMP-9 and MMP-2 are at peak expression, respectively. Using natural methylaters, such as folic acid, we are employing a safer way to alleviate NP and enhance axonal regeneration and functional recovery. This new pharmacological approach, with a non-toxic FDA approved dietary supplement, can be a highly rewarding way to treat NP. We hypothesize that FA will methylate and suppress MMP-9 expression during the early phase and MMP-2 expression during the late phase of SCI to promote perpetual alleviation of NP.
Methods
Animals
Adult male Sprague Dawley rats, weighing 250 to 280 g weight, were used for this study. This protocol was in accordance with NIH guidelines and approved by the University of Wisconsin Institutional Animal Care and Use Committee (IACUC).
Spinal cord injury
Adult male Sprague Dawley rats (250–270g) underwent cSCI, using a NYU impactor, with 12.5 gm/cm injury as per protocol used in earlier studies and well established in our lab [12–23]. A total of 40 male rats were used to assure an adequate number of animals exhibiting NP behavior. Briefly, following the induction of adequate inhalational anesthesia (Isoflurane, induction 5%, maintenance 2.5%, in a 50: 50 mixtures of oxygen and nitrous oxide), a T9 laminectomy was performed following an impaction, which was done by dropping a 10-g weight from a height of 12.5 mm. Sham control rats underwent laminectomy, but no contusion. Rats underwent manual bladder expression for a week after injury or until bladder control was re-established.
Drug administration
Briefly, the spinal cord-injured animals were treated intraperitoneally (IP) with a standardized dose of FA (80 μg/kg body weight) [13]. For all phases, rats were treated for 3 days prior to the cSCIs. For the early/acute phases, in addition to prior 3-day treatment animals were also treated with FA post cSCI injury until one day before sacrifice day which are 1, 3, and 7. Similarly, for the mid phase of injury, in addition to prior treatment, rat cohorts were treated on post cSCI day 0 to 17 and sacrificed on day 18. For the late phase of injury, post cSCI, rat cohorts were treated on days 0 to day 14.
Spinal cord harvest
Animals were euthanized on day 1, 3, 7 post cSCI (early phase), day 18 post cSCI (mid phase), and day 42 post cSCI (late phase). The epicenter region of injuries harvested for MMP-9 and MMP-2 expression analysis.
Western Blot analysis
Tissue samples from the epicenter of the injury were lysed using a lysis buffer (Sigma Chemical Co., St. Louis, MO, USA). The protein content was determined through the Lowry’s method. Pre-stained molecular mass markers (Bio-Rad, Hercules, CA, USA) and the tissues samples (30μg/lane) denatured in SDS reducing buffer solution (1:2 by volume, Bio-Rad, Hercules, CA, USA). Electrophoresis was performed in 12.5% tris-HCL gels (Bio-Rad). The resolved proteins were then transferred to a polyvinylidene difluoride membrane (PVDF, 0.2 µm; Bio-Rad, Hercules, CA, USA) and incubated in 5% non-fat dry milk in tris-buffered saline (TBS) for 30 min. For MMP9 detection the blot was incubated overnight at 4°C with polyclonal antibody against MMP9 (Anti-MMP9 metalloproteinase, mouse monoclonal IgG2a; obtained from UC Davis/NIH NeuroMab Facility, supported by NIH grant and maintained by the Department of Neurobiology) followed by rinsing the blot in TBS and incubated with standard anti-mouse IgG (1:2,000) for 1.5 h at room temperature. For MMP2 detection the blot was incubated overnight at 4°C with polyclonal antibody against MMP2 (H-76, 1:500; Santa Cruz Biotechnology, Inc.) followed by rinsing the blot in TBS and incubated with corresponding horseradish peroxidase-conjugated secondary IgG (1:2,000; Santa Cruz Biotechnology, CA, USA) for 1.5 h at room temperature. Bound antibody visualized using the enhanced chemiluminescent solution (Pierce, Thermo Fisher Scientific, and Rockford, IL, USA) as per the manufacturer’s instructions. The chemiluminescent signal captured on autoradiographs (Eastman Kodak, Rochester, NY, USA) and scanned. The signal’s intensity (including a blank region) quantitated using the NIH Image J software. The immunoblot was treated in stripping buffer (Pierce, Thermo Fisher Scientific, Rockford, IL, USA) for 30 min and then re-probed with a mouse monoclonal antibody against β-actin (1:4,000; Sigma, St. Louis, MO, USA) to quantitate the expression of β-actin. Control and treatment values corrected for blank values and normalized to their respective β-actin band intensity. β-actin band intensity analyzed using ImageJ.
Statistical analysis
Statistical significance was determined using a one-way ANOVA with Bonferroni’s correction for all pairwise comparisons; a significance level, α, of 0.05 was used.
Results
MMP-9 and MMP-2 Expression Analysis Post-cSCI
To determine the efficacy of FA in MMP-2 and MMP-9 altered expression, we tested MMP-2 and MMP-9 expression in the spinal cord epicenter tissue from SCI rodents treated with either FA or water IP. Western blots were created using the epicenter tissues, from the SCI injured rats, harvested on days 1, 3, 7, 18, and 42.
MMP-9 Expression Days 1, 3 and 7: Early Phase of SCI
On days 1, 3 and 7 post SCI (early phase of SCI), rats injected with standardized doses of FA or water were sacrificed for MMP-9 expression analysis. The quantitation displayed no statistical significance difference, for days 1, 3, and 7, in MMP-9 expression between water- (n = 2) and FA- injected rats (n = 2) (p>0.05) (p-values for days 1, 3, and 7 are 0.175, 0.877, and 0.384, respectively) (Figures 1).
Fig. 1:
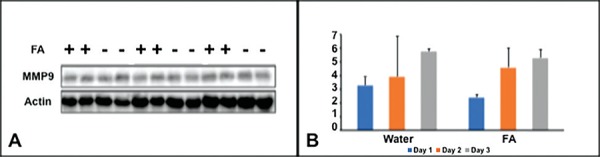
Expression of MMP-9 in early phase of SCI. A: Western blots of MMP-9 on Day 1, Day 3 and Day 7 in Folic acid treated and non-treated animals. B: The normalized intensity values expressed as mean ± SE. The quantitation displayed no statistical significance, for days 1, 3, and 7, in MMP-9 expression between water- and FA- injected rats (p>0.05).
MMP-9 Expression Day 18: Mid Phase of SCI
For mid phase analysis, FA or water was administered IP for 3 days pre-cSCI and 17 days post-cSCI followed by western blot analysis on day 18th. The quantitation of data displayed statistical significant difference between FA- (n = 4) and water- injected rat groups (n = 4) (p<0.05) when looking at MMP-9 expression (Figure 1). Treatment with Folic acid (3 days prior to injury and 17 days post injury) significantly (p-value = 0.043) modulates the expression of MMP-9 on day 18, upon the harvest of epicenter tissues (Figures 2).
Fig. 2:
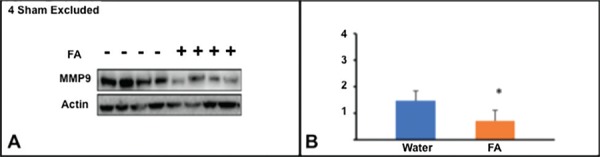
Expression of MMP-9 in mid phase of SCI. A: Western blots of MMP-9 on Day 18 in Folic acid treated and non-treated animals. B: The normalized intensity values expressed as mean ± SE. The quantitation of data displayed statistical difference between FA- and water- injected rat groups (*p<0.05)
MMP-2 Expression Mid Phase (Day 18) and Late Phase (Day 42) of SCI
On day 18 post-cSCI, FA (n = 9) and water (n = 7) injected animals displayed statistically significant difference (p<0.05). FA significantly modulates the expression of MMP-2 on day 18, upon the harvest of epicenter tissues, given injections for 3 days pre- and 17 days post-cSCI (Figures 3).
Fig. 3:
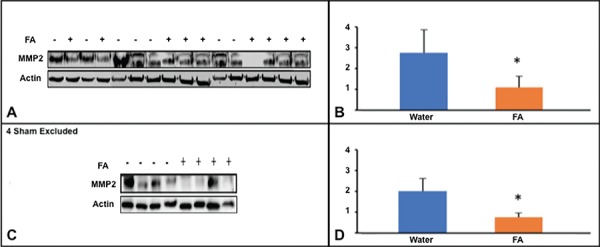
Expression of MMP-2 in mid phase and late phase of SCI A: Western blots of MMP-2 on day 18 from FA treated and non-treated animals. B: The normalized intensity values of blots represented in A, expressed as mean ± SE C: Western blots of MMP-2 on day 42 from FA treated and non-treated animals. D: The normalized intensity values of blots represented in C, expressed as mean ± SE.
The quantitation of data displayed statistical difference between FA- and water- injected rat groups (*p<0.05)
Similarly on day 42 post-cSCI the quantitation of western blots revealed statistical difference between the FA- (n = 4) and water- (n = 4) injected rat groups (p-values for day 18 and day 42 are 0.003 and 0.034, respectively) suggesting FA can modulates the expression of MMP-2 in late phase as well (Figures 3).
Discussion
MMPs are integral contributors to inhibitory glial scar formation [24, 25] and are responsible for early disruption of the blood spinal cord barrier [4], which initiates macrophage infiltration and degradation of myelin basic proteins. This leads to hyper excitability of afferents with action potentials (APs), outlasting the stimulus creating central sensitization, which is a common mechanism of NP [6, 26, 27]. Both MMP-2 and MMP-9 are known to participate in this process [6, 28]. Data have shown that inhibiting MMP-9 and MMP-2 significantly decreases NP in animals that have undergone spinal nerve ligation (SNL) [6]. In addition, MMP-9 and MMP-2 limit functional recovery after SCI by modulation of early vascular events [1]. The ability to effectively treat post-cSCI/NP with specific MMP-2 and MMP-9 inhibitors would represent a significant step forward in determining the underlying mechanisms behind the development of NP. In line with current and preliminary research, we predicted that high levels of MMP-9 expression would occur in the early phase and high levels of MMP-2 would occur in the late phase of injury.
Studies have shown that promoter methylation suppresses the transcription of MMP-9; intrathecal administration of DNA methyltransferase (DNMT) inhibitor, following chronic constriction injury (CCI), results in the suppression of DNA methylation and alleviation of NP in rodents [29]. Comparable to rodents, it has been shown that FA, an FDA approved dietary supplement and key methyl donor in the CNS, increases axonal regeneration and repair of an injured CNS via methylation [9,10, 30, 31, 32].
Our data shows that FA treatment, after cSCI in rodents, alters the expression of MMP-9 and MMP-2, alleviates NP, and improves functional recovery after SCI. Although MMP-9 is purported to be modulated by FA during the early phase of a SCI injury, the results demonstrate that during the early phase of SCI, FA is unable to have significant modulation of MMP-9 expression. In a comparable model of spinal nerve ligation, the primary difference was found in MMP-9 expression, in which it exhibited rapid and transient upregulation in injured dorsal root ganglion (DRG) primary sensory neurons during the early phase of NP [6]. MMP-9 produced in the injured DRG neurons serves as one of the triggers for spinal microglia activation and NP development and that MMP-9-induced pathophysiology involves IL-1β cleavage and microglia p38 activation [6]. The differences reported in MMP-9 expression level in our study and this study can be attributed to a variety of reasons: (i) difference in tissue analysis (i.e. epicenter of injured tissue, targeting spinal astrocytes, vs DRG); (ii) cell type response; (iii) intrathecal administration vs intraperitoneal injection.
Nonetheless, our results demonstrate two key features of FA (i) It exhibits a significant effect on MMP-9 and MMP-2 expression; (ii) Modulation of MMP-9 and MMP-2 expression is time and location dependent. As exemplified by the data, and novel research, MMP-9 modulation, by FA, occurs when MMP-9 is expected to have the greatest amount of expression (i.e. early phase of injury). On the other hand, MMP-2 modulation, by FA, expresses the greatest amount of modulation at the late phase of SCI, which is due to the latency of MMP-2 expression from a SCI injury. In addition, MMP-9 induces NP through interleukin-1β cleavage and microglia activation at early times, whereas MMP-2 maintains NP through same mechanism at later times. Common folate deficiencies can be attributed to diet, intestinal degenerative diseases (i.e. Crohn’s Disease), or defects in homocysteine methyltransferase, which results in methyl traps that mimic folate deficiency [33]. As we report in this study, FA administration results in alleviating cSCI-induced NP by suppression MMP expression. Considering both the novelty and efficacy of folic acid in SCI induced NP treatment, those that experience folate deficiencies may be more susceptible to SCI injuries [33].
The time line for observations in our cSCI model is 42 days as compared with other NP models demonstrating results in 14 days, which also provide a needed longitudinal study of expression of MMP-9. Because of the implications of a new phase of injury that our study shows, it is important that researchers focus their studies on discovering and quantifying the nature of both early- and mid- phase/transition phase of SCI injury.
Acknowledgement
Funding support for this project is from the University of Wisconsin Hospital and Clinics Department of Neurosurgery. The authors are thankful to Dr. Roberts J Dempsey, Chair, Department of Neurological surgery for support and constant encouragement to undertake this research.
Authorship contribution
Manuscript preparation (GSM, JN, GRB, and JK), data analysis (GSM, CKS, and JN), experimental techniques (GSM, CKS and JN), animal handling (NAY, SB, and GSM), figure construction (JN), presentation (SB and SES), manuscript editing (SK). Experimental design (GSM and CKS), equal first authorship (GSM and JM) project oversight (DKR).
Ethical statement
Approval was obtained from Institutional Animal Care and Use Committee (IACUC) of University of Wisconsin-Madison via protocol id: M005530-A01 (PI: Gurwattan Miranpuri).
Footnotes
Conflict of interest
The authors report no conflict of interest.
References
- 1.Noble LJ, Donovan F, Igarashi T et al. Matrix metalloproteinases limit functional recovery after spinal cord injury by modulation of early vascular events. J Neuroscience. 2002;22(17):7526–35. doi: 10.1523/JNEUROSCI.22-17-07526.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mott JD, Werb Z. Regulation of matrix biology by matrix metalloproteinases. Curr Opin Cell Biol. 2004;16(5):558–64. doi: 10.1016/j.ceb.2004.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costanzo RM and, Perrino LA. Peak in matrix metalloproteinase-2 levels observed during recovery from olfactory nerve injury. Neuroreport. 2008;19(3):327–31. doi: 10.1097/WNR.0b013e3282f50c7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whetstone WD, Hsu JY, Eisenberg M et al. Blood-spinal cord barrier after spinal cord injury: relation to revascularization and wound healing. J Neurosci Res. 2003;74(2):227–39. doi: 10.1002/jnr.10759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buss A, Pech K, Kakulas BA et al. Matrix metalloproteinases and their inhibitors in human traumatic spinal cord injury. BMC Neurol. 2007;7:17. doi: 10.1186/1471-2377-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawasaki Y, Xu ZZ, Wang X et al. Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nat Med. 2008;14(3):331–6. doi: 10.1038/nm1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang H, Chang M, Hansen CN et al. Role of matrix metalloproteinases and therapeutic benefits of their inhibition in spinal cord injury. Neurotherapeutics. 2011;8(2):206–20. doi: 10.1007/s13311-011-0038-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lakhan SE and, Avramut M. Matrix Metalloproteinases in Neuropathic Pain and Migraine: Friends, Enemies, and Therapeutic Targets. Pain Res Treat. doi: 10.1155/2012/952906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iskandar BJ, Rizk E, Meier B et al. Folate regulation of axonal regeneration in the rodent central nervous system through DNA methylation. J Clin Invest. 2010;120(5):1603–16. doi: 10.1172/JCI40000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iskandar BJ, Nelson A, Resnick D et al. Folic acid supplementation enhances repair of the adult central nervous system. Ann Neurol. 2004;56(2):221–7. doi: 10.1002/ana.20174. [DOI] [PubMed] [Google Scholar]
- 11.Kronenberg G, Endres M. Neuronal injury: folate to the rescue? J Clin Invest. 2010;120(5):1383–6. doi: 10.1172/JCI40764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miranpuri GS, Schomberg DT, Alrfaei B et al. Role of matrix metalloproteinases 2 in spinal cord injury-induced neuropathic pain. Ann Neurosci. 2016;23(1):25–32. doi: 10.1159/000443553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miranpuri GS, Meethal SV, Sampene E et al. Folic acid modulates MMP2 expression, alleviates neuropathic pain and improves functional recovery in spinal cord injured rats. Ann Neurosci. 2017;24:74–81. doi: 10.1159/000475896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmed MM, Lee H, Clark Z et al. Pathogenesis of spinal cord injury induced edema and neuropathic pain: expression of multiple isoforms of wnk1. Ann Neurosci. 2014;21(3):97–103. doi: 10.5214/ans.0972.7531.210305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim HT, Kim T, Novotny B et al. Thermal hyperalgesia assessment for rats after spinal cord injury: developing a valid and useful pain index. Spine J. 2014;14(6):984–89. doi: 10.1016/j.spinee.2013.09.051. [DOI] [PubMed] [Google Scholar]
- 16.Lee HK, Ahmed MM, King KC et al. Persistent phosphorylation of NKCC1 and WNK1 in the epicenter of the spinal cord following contusion injury. Spine J. 2014;14(5):777–81. doi: 10.1016/j.spinee.2013.06.100. [DOI] [PubMed] [Google Scholar]
- 17.Ahmed MM, Rajpal S, Sweeney C et al. Cannabinoid subtype-2 receptors modulate the antihyperalgesic effect of WIN 55,212–2 in rats with neuropathic spinal cord injury pain. Spine J. 2010;10(12):1049–54. doi: 10.1016/j.spinee.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 18.Cramer SW, Baggott C, Cain J et al. The role of cation-dependent chloride transporters in neuropathic pain following spinal cord injury. Mol Pain. 2008;4:36. doi: 10.1186/1744-8069-4-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rajpal S, Gerovac TA, Turner NA et al. Antihyperalgesic effects of vanilloid-1 and bradykinin-1 receptor antagonists following spinal cord injury in rats. J Neurosurg Spine. doi: 10.3171/spi.2007.6.5.420. [DOI] [PubMed] [Google Scholar]
- 20.Park SW, Yi JH, Miranpuri G et al. Thiazolidinedione class of peroxisome proliferator-activated receptor gamma agonists prevents neuronal damage, motor dysfunction, myelin loss, neuropathic pain, and inflammation after spinal cord injury in adult rats. J Pharmacol Exp Ther. 2007;320(3):1002–12. doi: 10.1124/jpet.106.113472. [DOI] [PubMed] [Google Scholar]
- 21.DomBourian MG, Turner NA, Gerovac TA et al. B1 and TRPV-1 receptor genes and their relationship to hyperalgesia following spinal cord injury. Spine. 2006;31(24):2778–82. doi: 10.1097/01.brs.0000245865.97424.b4. [DOI] [PubMed] [Google Scholar]
- 22.Resnick DK, Schmitt C, Miranpuri GS et al. Molecular evidence of repair and plasticity following spinal cord injury. Neuroreport. 2004;15(5):837–39. doi: 10.1097/00001756-200404090-00020. [DOI] [PubMed] [Google Scholar]
- 23.Moran PM, Sampene E, Oakes SG et al. Immediate administration of COX inhibitors in spinal cord contusion injury and a current review of COX Therapy. Med Res Innov. doi: 10.15761/MRI.1000122. [DOI] [Google Scholar]
- 24.Hsu JY, Bourguignon LY, Adams CM et al. Matrix metalloproteinase-9 facilitates glial scar formation in the injured spinal cord. J Neurosci. 2008;28(50):13467–77. doi: 10.1523/JNEUROSCI.2287-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Filous AR, Miller JH, Coulson-Thomas YM et al. Immature astrocytes promote CNS axonal regeneration when combined with chondroitinase ABC. Dev. Neurobiol. 2010;70(12):826–41. doi: 10.1002/dneu.20820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kobayashi K, Yamanaka H, Fukuoka T et al. P2Y12 Receptor Upregulation in Activated Microglia Is a Gateway of p38 Signaling and Neuropathic Pain. J. Neurosci. 2008;28(11):2892–02. doi: 10.1523/JNEUROSCI.5589-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Devor M. Sodium channels and mechanisms of neuropathic pain. J Pain. 2006;7(1 Suppl 1):S3–S12. doi: 10.1016/j.jpain.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 28.Chandler S, Coates R, Gearing A et al. Matrix metalloproteinases degrade myelin basic protein. Neurosci Lett. 1995;201(3):223–26. doi: 10.1016/0304-3940(95)12173-0. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Liu C, Guo QL et al. Intrathecal 5-azacytidine inhibits global DNA methylation and methyl- CpG-binding protein 2 expression and alleviates neuropathic pain in rats following chronic constriction injury. Brain Res. 2011;1418:64–9. doi: 10.1016/j.brainres.2011.08.040. [DOI] [PubMed] [Google Scholar]
- 30.Van den Veyver IB. Genetic effects of methylation diets. Annu Rev Nutr. 2002;22:255–82. doi: 10.1146/annurev.nutr.22.010402.102932. [DOI] [PubMed] [Google Scholar]
- 31.Bailey LB. Folate. In: Bowman and B, Russell R, editors. Present Knowledge in Nutrition. Washington, DC: International Life Sciences Institute; 2001. pp. 278–01. [Google Scholar]
- 32.Carmel R. Folic Acid. In: Shils M, Shike M, Ross A, Caballero and B, Cousins R, editors. Modern Nutrition in Health and Disease. Baltimore, MD: Lippincott Williams & Wilkins; 2005. pp. 470–81. [Google Scholar]
- 33.Shane B, Stokstad EL. Vitamin B12-folate interrelationships. Annu Rev Nutr. 1985;5:115–41. doi: 10.1146/annurev.nu.05.070185.000555. [DOI] [PubMed] [Google Scholar]


