Abstract
Background.
Acute stroke due to supratentorial intracerebral haemorrhage is associated with high morbidity and mortality. Open craniotomy haematoma evacuation has not been found to have any benefit in large randomised trials. We assessed whether minimally invasive catheter evacuation followed by thrombolysis (MISTIE), with the aim of decreasing clot size to 15 mL or less, would improve functional outcome in patients with intracerebral haemorrhage.
Methods.
MISTIE III was an open-label, blinded endpoint, phase 3 trial done at 78 hospitals in the USA, Canada, Europe, Australia, and Asia. We enrolled patients aged 18 years or older with spontaneous, non-traumatic, supratentorial intracerebral haemorrhage of 30 mL or more. We used a computer-generated number sequence with a block size of four or six to centrally randomise patients to image-guided MISTIE treatment (1·0 mg alteplase every 8 h for up to nine doses) or standard medical care. Primary outcome was good functional outcome, defined as the proportion of patients who achieved a modified Rankin Scale (mRS) score of 0–3 at 365 days, adjusted for group differences in prespecified baseline covariates (stability intracerebral haemorrhage size, age, Glasgow Coma Scale, stability intraventricular haemorrhage size, and clot location). Analysis of the primary efficacy outcome was done in the modified intention-to-treat (mITT) population, which included all eligible, randomly assigned patients who were exposed to treatment. All randomly assigned patients were included in the safety analysis. This study is registered with ClinicalTrials.gov, number .
Findings.
Between Dec 30, 2013, and Aug 15, 2017, 506 patients were randomly allocated: 255 (50%) to the MISTIE group and 251 (50%) to standard medical care. 499 patients (n=250 in the MISTIE group; n=249 in the standard medical care group) received treatment and were included in the mITT analysis set. The mITT primary adjusted efficacy analysis estimated that 45% of patients in the MISTIE group and 41% patients in the standard medical care group had achieved an mRS score of 0–3 at 365 days (adjusted risk difference 4% [95% CI –4 to 12]; p=0·33). Sensitivity analyses of 365-day mRS using generalised ordered logistic regression models adjusted for baseline variables showed that the estimated odds ratios comparing MISTIE with standard medical care for mRS scores higher than 5 versus 5 or less, higher than 4 versus 4 or less, higher than 3 versus 3 or less, and higher than 2 versus 2 or less were 0·60 (p=0·03), 0·84 (p=0·42), 0·87 (p=0·49), and 0·82 (p=0·44), respectively. At 7 days, two (1%) of 255 patients in the MISTIE group and ten (4%) of 251 patients in the standard medical care group had died (p=0·02) and at 30 days, 24 (9%) patients in the MISTIE group and 37 (15%) patients in the standard medical care group had died (p=0·07). The number of patients with symptomatic bleeding and brain bacterial infections was similar between the MISTIE and standard medical care groups (six [2%] of 255 patients vs three [1%] of 251 patients; p=0·33 for symptomatic bleeding; two [1%] of 255 patients vs 0 [0%] of 251 patients; p=0·16 for brain bacterial infections). At 30 days, 76 (30%) of 255 patients in the MISTIE group and 84 (33%) of 251 patients in the standard medical care group had one or more serious adverse event, and the difference in number of serious adverse events between the groups was statistically significant (p=0·012).
Interpretation.
For moderate to large intracerebral haemorrhage, MISTIE did not improve the proportion of patients who achieved a good response 365 days after intracerebral haemorrhage. The procedure was safely adopted by our sample of surgeons.
Introduction
Worldwide, more than 5 million brain haemorrhages occur annually.1 Intracerebral haemorrhage has greater morbidity and mortality than ischaemic stroke;1–3 however, no evidence-based primary treatment exists.2,4 Large, pragmatic trials of craniotomy,5,6 a therapy used in routine practice, have not improved functional outcome or mortality. Findings from non-surgical trials of aggressive, early haemostasis4 and early blood pressure reduction7,8 showed volume stabilisation of haematoma growth (1–5 mL), but no change in functional outcome or mortality. We designed the Minimally Invasive Surgery Plus Alteplase for Intracerebral Hemorrhage Evacuation (MISTIE) III trial to assess whether image-guided, minimally invasive surgery followed by gentle thrombolytic irrigation of the catheterised intracerebral haemorrhage clot alters functional outcome.9–12 The aim of our trial was to determine whether the MISTIE procedure could achieve the benefits of clot evacuation and improve functional outcome at 365 days after stroke without procedure-related safety events or additional brain injury beyond the risks associated with standard care in an intensive care unit.
Methods
Study design and patients
MISTIE III was a randomised, controlled, open-label, blinded endpoint, phase 3 explanatory trial13,14 of image-guided,11 catheter-based10 removal of intracerebral haemorrhage of 30 mL or more, measured with the ABC/2 method,15 done at 78 hospitals in the USA, Canada, Europe, Australia, and Asia (appendix).
Each hospital obtained local institutional review board or ethics committee approval. Eligible patients were aged 18 years or older with a spontaneous, non-traumatic, supratentorial intracerebral haemorrhage of 30 mL or more due to cerebral small-vessel disease, with a Glasgow Coma Scale (GCS) score of 14 or less or National Institutes of Health Stroke Scale (NIHSS) score of 6 or higher, an mRS score of 0 or 1 before the bleed, and an intracerebral haemorrhage that remained the same size (growth <5 mL) for at least 6 h after diagnostic CT. We did not enrol patients with expressed care limitations or those deemed to have life-threatening mass effect requiring surgery. The full list of inclusion and exclusion criteria is in the appendix.
Local principal investigators determined eligibility and stability of haematoma growth, and written informed consent was then obtained from all patients or their legal representatives. The study protocol is available at https://clinicaltrials.gov/ct2/show/NCT01827046.
Randomisation and masking
Patients were randomly allocated by site personnel using a central web-based enrolment system and presentation data. The trial statisticians (RET, GY) used a computer-generated number sequence to randomly allocate patients to the MISTIE or standard medical care groups. Block randomisation was used (block sizes of four or six) to ensure masking of randomisation status. After the site block was fulfilled, covariate-adaptive randomisation was used with the aim of minimising differences in three baseline severity factors: age, presentation GCS, and intracerebral haemorrhage size. This method, which used only baseline covariate information from previous participants, has been described elsewhere.16 Study personnel were masked to outcome, which was determined by an adjudication committee masked to treatment allocation.16,17
Procedures
We managed the risks of initial haematoma growth or instability16,18 by use of a stability protocol that combined normalisation of coagulation variables, guideline-based blood pressure management, and repeat CT assessment of clot size, measured with the ABC/2 method. 6 h or more after diagnostic CT, patients had to have an international normalised ratio of 1·3 or less, a normal activated partial thromboplastin time, and blood pressure stability.19 The stability CT ensured the intracerebral haemorrhage clot had not expanded by 5 mL or more, defined as the absence of active bleeding before treatment,12 and provided image demonstration of a safe starting point for clot reduction therapy. The CT could be repeated every 6 h until the clot stabilised or just before the 72 h eligibility window closed, whichever occurred first. CT angiography was done to exclude underlying pathology as the source of bleeding; a formal angiogram was encouraged in patients with equivocal findings on vascular pathology screening.10,20 MRI and magnetic resonance angiogram were used when clinically indicated.21,22 The trial sites shared planned catheter insertion trajectories describing the skull entry site and planned linear path to the haematoma target with the trial’s surgical centre for joint review.
Surgeons followed a 10-step protocol that defined the surgical task and its endpoint (decrease in clot size to <15 mL, nine doses, or safety endpoint; appendix). Under general anaesthesia, after burr hole placement, we used image guidance to place a rigid cannula within the middle two-thirds of the overall haematoma short axis to a targeted point 75% or greater along the long axis of the clot, through a burr hole or twist drill opening. Clot aspiration was done with a 10 mL handheld syringe until first resistance. A soft catheter was then placed with image guidance into the residual haematoma, tunnelled subcutaneously, and connected to a three-way stopcock and closed drainage system. Postoperative CT was done to confirm positioning of the soft catheter and stability of the residual haematoma and catheter tract. If the catheter did not engage the clot, it was removed and repositioned. 6 h or more after catheter placement, we administered alteplase directly into the clot through the catheter, at 1·0 mg in 1 mL followed by 3 mL flush every 8 h, for up to nine doses. Administration of alteplase was stopped when the trial-defined surgical aim (residual haematoma ≤15 mL) was reached, nine doses of alteplase were given, or on occurrence of a clinically symptomatic rebleeding event, defined as a sustained decrease of more than 2 points on the GCS motor score, with CT-demonstrated enlargement of the intracerebral haemorrhage. All doses were followed by a 3 mL flush of preservative-free normal saline, and the system was closed for 1 h to allow drug–clot interaction and then reopened to allow for gravitational drainage. Subsequent CT scans were done for any safety concern or every 24 h. Ongoing surgeon training and mentoring and monitoring of the surgical task was overseen by the trial’s surgical committee and dedicated surgical centre.23
We used the American Heart Association and European Stroke Organisation recommendations for treatment of non-traumatic spontaneous intracerebral haemorrhage,19,21 which enabled a standard approach to monitoring patients’ airways, ventilation, intracranial pressure, sedation, and pharmacological treatment of intracranial mass effect (appendix). Patients allocated to the standard medical care group had follow-up CT scans and other monitoring assessments on the same schedule as those in the intervention group.
Patients returned to the clinic on days 30, 180, and 365, and were contacted by telephone on days 90 and 270. A certified examiner assessed patients using the modified Rankin Scale (mRS), Barthel Index, Stroke Impact Scale, Glasgow Outcome Scale, extended Glasgow Outcome Scale (eGOS), and NIHSS (clinic visits only). mRS interviews on days 30, 180, and 365 were recorded and transferred to an independent jury masked to treatment allocation at the University of Glasgow (Glasgow, UK) for scoring16 (appendix).
To optimise accuracy and minimise investigator bias, a core imaging laboratory used semi-automated segmentation and Hounsfield thresholds to measure clot volumes. Measurement of clot volumes was done with the image processing software OsiriX MD (version 9.0.1) on Digital Imaging and Communications in Medicine images of each patient’s stability and treatment scans. This approach has been validated for accuracy and inter-rater reliability.24 We used fully monitored core laboratory measurements in all analyses. The core laboratory defined location as either lobar or deep (putamen or thalamus) and final location was adjudicated by a neuroradiologist (DG).
Outcomes
The primary efficacy outcome was good functional outcome, defined as the proportion of patients who achieved an mRS score of 0–3 at 365 days, adjusted for group differences in prespecified baseline covariates (stability intracerebral haemorrhage size, age, GCS, stability intraventricular haemorrhage size, and clot location). Missing day 365 mRS scores were estimated from earlier timepoints using the targeted maximum likelihood estimator to adjust for censoring for the primary efficacy outcome only.25 Secondary outcomes were eGOS score dichotomised as good (4–8) vs poor (1–3) at 365 days after stroke, all-cause mortality 365 days after stroke, association between functional outcome and clot removal quantified as weighted clot volume by area under the curve (AUC) and clot volume at end of treatment, patient disposition, efficacy 180 days after stroke, type and intensity of intensive care unit management; and health-related quality of life (electronic visual analogue scale and EQ-5D scores). Our protocol identified a plan to assess the possible influence of variation in procedural performance on functional outcome as determined via as-treated analysis. Exploratory analyses of the primary outcome at 365 days post intracerebral haemorrhage were logistic regression on dichotomised, adjudicated, cross-sectional mRS score (0–3 vs 4–6); ordinal mRS score (0–6); longitudinal mRS score (0–3 vs 4–6); severity subgroup analyses; cross-sectional mRS score (0–3 vs 4–6) for demographic subgroups, including for race and sex; and analysis of cross-sectional mRS score (0–3 vs 4–6) that models random effects for hospital sites. Safety outcomes were all-cause mortality at 30 days, procedure-related mortality at 7 days, bacterial brain infection at 30 days, and symptomatic bleeding within 72 h after last dose. All adverse and serious adverse events were centrally assessed during acute treatment, and all serious adverse events were assessed by a safety committee with an independent medical monitor (CSK) until the end of follow-up. An independent Data Safety and Monitoring Board (DSMB) appointed by the study sponsor managed the trial.
Statistical analysis
On the basis of the assumption that 25% of patients would have an mRS score of 0–3 in the standard medical care group versus 38% of patients in the MISTIE group, we estimated that 500 patients (approximately 250 patients in each treatment group) would provide 88% power at α level 0·05 to detect an average effect size of 13%. Furthermore, this sample size would have 77% power to detect a more conservative effect size of 11%. The sample size was calculated on the basis of MISTIE II,12 which provided evidence of increasing frequency of mRS scores in the 0–2 categories between 180 and 365 days with an unadjusted proportion of mRS 0–3 increasing to 14%. We updated the statistical analysis plan in light of the results of MISTIE II and based the primary outcome on 365-day outcomes because no plateau was observed for good functional outcome (mRS score 0–3) at 180 days.12
Analysis of the primary efficacy outcome was done in the modified intention-to-treat (mITT) population, which included all eligible, randomly assigned patients who were exposed to treatment. All randomly assigned patients were included in the safety analyses. To estimate the mean treatment effect in the primary analysis, we used the longitudinal targeted maximum likelihood estimator of Van der Laan and Gruber.25 This statistical method adjusts for chance imbalances in prespecified baseline variables between treatment groups, and also accounts for missing outcome data by a combination of regression modelling and inverse probability of censoring weighting. For the primary outcome analysis, the proportions of patients with an mRS score of 0–3 were compared between the MISTIE and control group, and adjusted for baseline (prerandomisation) variables used in the covariate adaptive randomisation (ie, clot volume, age, and severity of impairment as measured by GCS) and for clinically established severity variables (intraventricular haemorrhage size and intracerebral haemorrhage clot location [lobar vs deep]). Efficacy analyses of the ordinal shift in mRS score were done for the full range of adjudicated mRS scores (0–6) using the Cochran-Mantel-Haenszel shift test followed by proportional odds logistic regression subject to the validity of shift analysis model assumptions.
The seven secondary analyses were ordered according to perceived importance (as listed in the statistical analysis plan): eGOS; mortality; mediation of benefit by clot removal, as defined by time averaged clot removal and by clot remaining at end of treatment (an as-treated analysis of participants reaching the surgical goal of <15 mL clot size); patient disposition; 180 day results; intensive care unit care; and quality of life. Analysis of secondary outcomes was done in the mITT population. Variable by treatment interactions terms were included in multivariable models to estimate treatment effects for population subgroups. Wald tests were used to test the null hypothesis that all interaction terms were equal to zero for variables with more than two categories. The DSMB monitored defined thresholds for mortality, rebleeding, and infection, and safety analyses included between-group comparisons of serious adverse events by patient, Standardised Medical Dictionary for Regulatory Activities (MedDRA) code, and organ system. Overall, the statistical analysis plan included 54 pre-planned analyses (including primary, secondary, exploratory, and safety analyses). A p value of less than 0·05 was considered to indicate a statistically significant difference, however, any single analyses at this level of significance should be interpreted with caution because the study-wide type I error rate was not controlled at this level due to multiplicity of analyses. This study is registered with ClinicalTrials.gov, number NCT01827046.
Role of the funding source
The National Institute of Neurological Disorders and Stroke provided input on study design during the grant review process, and the DSMB provided input during active recruitment. The funder had no role in data collection, analysis, or interpretation or writing of the report. The National Institute of Neurological Disorders and Stroke and Genentech approved the decision to submit for publication. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
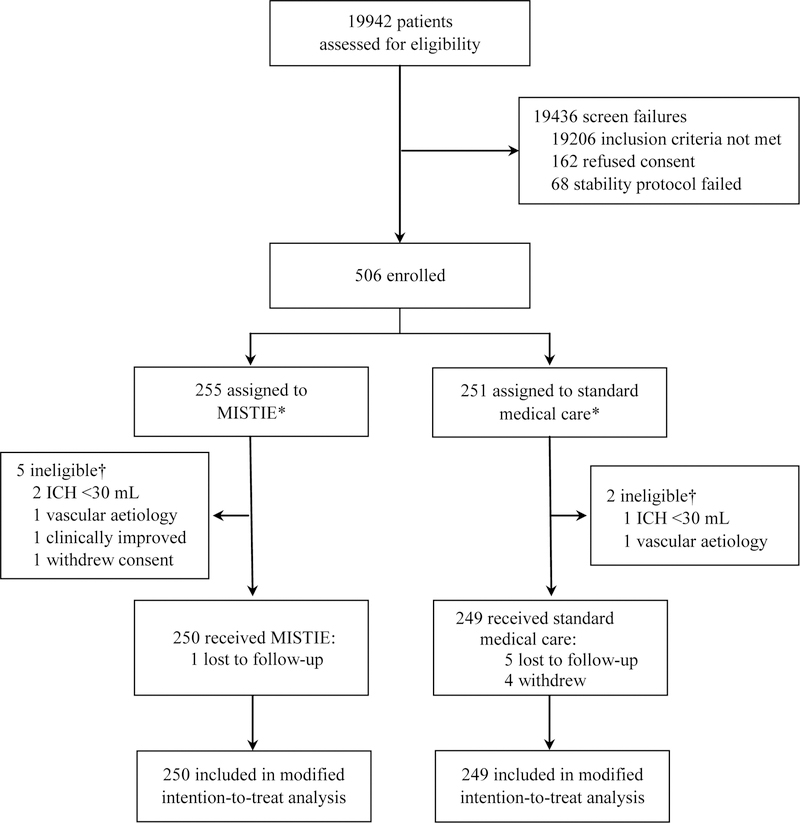
Between Dec 30, 2013, and Aug 15, 2017, 19 942 patients were assessed for eligibility, of whom 506 patients were randomly assigned to the two study groups: 255 patients to receive MISTIE and 251 to receive standard medical care. 499 patients (n=250 in the MISTIE group; n=249 in the standard medical care group) received treatment and were included in the mITT analysis set (figure 1). 305 (61%) of 499 patients were men and 194 (39%) were women (median age 62 years [IQR 52–71]). 307 (62%) of 499 clots were located in the basal ganglia and 192 (38%) in the lobar region. At presentation, median clot volume was 41·8 mL (IQR 30·8–54·5) for intracerebral haemorrhage and 0 mL (0–1·9) for intraventricular haemorrhage; median GCS score was 10 (IQR 8–13) and median NIHSS score was 19 (IQR 15–23). Age, GCS score, clot size, intraventricular haemorrhage volume, and withdrawal of care was similar between groups at presentation and after stability had been achieved (table 1). Medical conditions were similar between the groups, but a higher number of patients were on anticoagulants at the time of bleed in the MISTIE group than the standard medical care group. Reduction in clot volume between randomisation and the end of treatment (mean 3·6 days [95% CI 3·4–3·7]) is shown in the appendix. The mean reduction in haematoma size was 69% (SD 20) in the MISTIE group versus 3% (12) in the standard medical care group. The mean end-of-treatment volume was 16 mL (SD 13) for patients in the MISTIE group versus 47 mL (18) for patients in the standard medical care group (mean difference 32 mL, 95% CI 30–34; p<0·0001). 146 (58%) of 250 patients in the MISTIE group achieved the surgical aim (residual haematoma ≤15 mL) compared with two (<1%) of 249 patients in the standard medical care group (table 2). Clot removal was more consistent among surgeons who had done ten or more procedures than those who had done less than ten procedures (appendix). Clinical deterioration led to craniotomy or craniectomy in four patients in the MISTIE group and 17 patients in the standard medical care group. Two additional patients in the MISTIE group and two patients in the standard medical care group had craniotomies after 30 days for new bleeding events unrelated to the trial. The final mRS score was assessed on Sept 17, 2018.
Figure 1: Trial profile.
*All randomly assigned patients (n=506) were included in safety analyses and sensitivity analyses. Loss to follow-up occurred when the patient could not be contacted. Withdrawal occurred when the patient or family refused to complete the planned follow-up. †Patients were randomly assigned in error and thus were excluded.
Table 1:
Demographic and baseline characteristics of patients
| MISTIE (n=250) |
Medical (n=249) |
|
|---|---|---|
| Demographic variables | ||
| Age (years) | 62 (52–70) | 62 (53–71) |
| Men | 159 (63.6) | 146 (58.6) |
| Race | ||
| Black | 46 (18.4) | 41 (16·5) |
| White | 190 (76.0) | 184 (73·9) |
| Other | 13 (5.2) | 24 (9·6) |
| Unknown | 1 (0·4) | 0 (0·0) |
| Ethnicity: Hispanic/Latino | 34 (13·6) | 34 (13·7) |
| Baseline variables | ||
| Tobacco use | 50 (20·0) | 39 (15·7) |
| Cocaine use | 11 (4·4) | 9 (3·6) |
| Anticoagulated | 24 (9·6) | 10 (4·0) |
| Hormone replacement therapy | 1 (0·4) | 3 (1·2) |
| Hyperlipidaemia medication compliant | 96 (38·4) | 93 (37·4) |
| On antiplatelets | 67 (26·8) | 77 (30·9) |
| Diabetes | 72 (28·8) | 67 (26·9) |
| Hypertension | 241 (96·4) | 240 (96·4) |
| Other cardiovascular disease | 38 (15·2) | 34 (13·7) |
| GCS score at randomisation* | ||
| 3–8 | 64 (25·6) | 63 (25·3) |
| 9–12 | 111 (44·4) | 108 (43·4) |
| 13–15 | 75 (30·0) | 78 (31·3) |
| NIHSS score at randomisation | 19 (15–23) | 19 (15–23) |
| Diagnostic CT (at presentation) | ||
| ICH volume (mL) | 42·7 (30·4–54·5) | 41·5 (30·9–55·3) |
| IVH volume (mL) | 0 (0–1·7) | 0 (0–1·9) |
| Stability CT (prior to randomisation) | ||
| ICH volume (mL) | 45·8 (35·4–59·6) | 45·3 (35·4–57·2) |
| IVH volume (mL) | 0·3 (0–3·1) | 0·4 (0–3·2) |
| Ventilated at randomisation | 107 (42·8) | 102 (41.0) |
| Blood pressure at presentation | ||
| Systolic BP (mm Hg) | 177 (155–208) | 176 (158–200) |
| Diastolic BP (mm Hg) | 99 (85–113) | 98 (84–114) |
| Blood pressure at randomisation | ||
| Systolic BP (mm Hg) | 138 (130–148) | 138 (131–148) |
| Diastolic BP (mm Hg) | 70 (63–78) | 69 (60–77) |
| Ictus to diagnostic CT time (hours) | 2·2 (1·1–6·0) | 1·9 (1·2–4·8) |
| Ictus to stability CT time (hours) | 36·4 (23·4–52·6) | 36·3 (23·6–48·6) |
| Clot location: deep | 163 (65·2) | 144 (57·8) |
| mRS score before stroke | ||
| 0 | 230 (92·0) | 233 (93·6) |
| 1 | 20 (8·0) | 16 (6·4) |
Data are median (IQR) or n (%). Five patients in the MISTIE group and two patients in the control group were randomly assigned in error and thus were excluded. GCS=Glasgow Coma Scale. NIHSS=National Institutes of Health Stroke Scale. ICH=intracerebral haemorrhage. IVH=intraventricular haemorrhage. BP=blood pressure. mRS=modified Rankin Scale.
GCS scores range from 15 (fully conscious) to 3 (deep coma).
Table 2:
Treatment variables
| MISTIE (n=250) |
Medical (n=249) |
p value | |
|---|---|---|---|
| Ictus to randomisation time (hours) | 47 (33–60) | 46 (36–58) | 0·817 |
| Ictus to end of treatment (EOT) time (hours)* | 123 (104–150) | 115 (103–127) | <0·0001 |
| MISTIE procedure duration (hours) | 1 (1–1) | NA | NA |
| Number of alteplase doses | 4 (2–6) | NA | NA |
| EOT CT | |||
| ICH volume (mL) | 12·5 (7·6–21·0)† | 43·7 (33·6–56·3)† | <0·0001 |
| IVH volume (mL) | 0·2 (0–1·5) | 0·3 (0–1·9) | 0·137 |
| EOT ICH remaining ≤15 mL | 148 (59·7%) | 2 (0·8%) | <0·0001 |
| Withdrawal of care | 26 (10·4%) | 35 (14·1%) | 0·213 |
| Days in ICU | 10 (7–17) | 10 (5–16) | 0·460 |
| Days to return home | 55 (34–105) | 62 (35–100) | 0·846 |
| ICP events monitored | |||
| % subjects with any ICP ≥20 mm Hg | 34 (13·6%) | 38 (15·3%) | 0·598 |
| % subjects with any CPP <70 mm Hg | 9/34 (26·5%) | 22/38 (57·9%) | 0·007 |
| % ICP readings ≥20 mm Hg† | 23/690 (3·3%) | 67/711 (9·4%) | 0·01 |
| % CPP readings <70 mm Hg† | 64/690 (9·3%) | 159/711 (22·4%) | 0·04 |
| One or more ICP therapies | 25/34 (73·5%) | 26/38 (68·4%) | 0·634 |
Data are median (IQR), mean (SD), or n/N (%). NA=not applicable. ICH=intracerebral haemorrhage. IVH=intraventricular haemorrhage. ICU=intensive care unit. ICP=intracranial pressure. CPP=cerebral perfusion pressure.
End of treatment time calculated as 24 h after last dose for patients in the MISTIE group who received alteplase and as the sum of randomisation time plus median surgical end of treatment time for patients in the standard medical care group. This achieved a virtual dosing endpoint without alteplase dosing for each individual patient in the standard medical care groups.
Adjusted for number of readings per patient.
Data were available for 240 patients in the MISTIE group and 238 patients in the standard medical care group. Additional outcome variable data are included in the appendix.
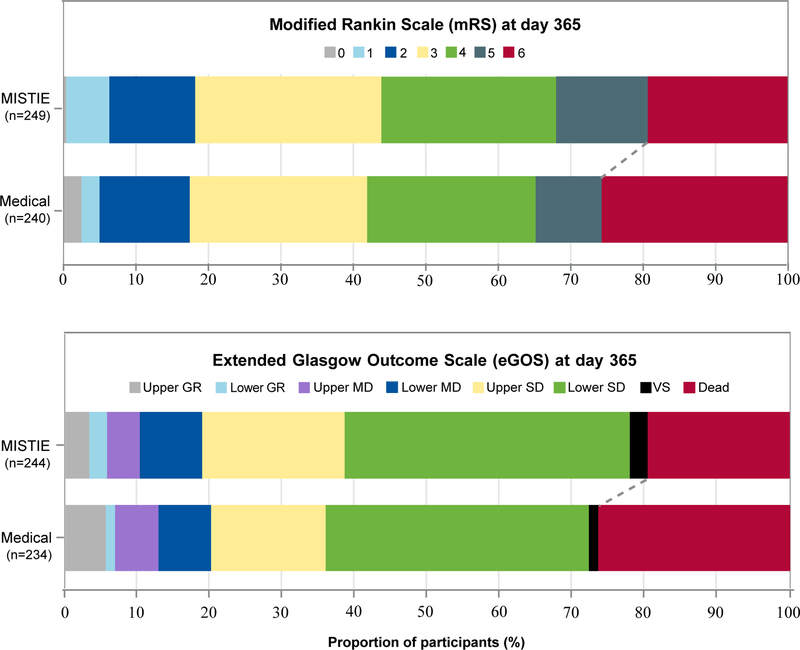
Of the 489 patients with available mRS scores at 365 days, 110 (44%) of 249 patients in the MISTIE group and 100 (42%) of 240 patients in the standard medical care group had achieved an mRS score 0–3 at 365 days (figure 2). For the mITT analysis set, adjusting for baseline variables, primary adjusted efficacy analysis estimated that 45% of patients in the MISTIE group and 41% patients in the standard medical care group had achieved an mRS score of 0–3 (absolute risk difference 4% [95% CI –4 to 12]; p=0·33). 94 (39%) of 244 patients in the MISTIE group and 84 (36%) of 234 patients in the standard medical care group achieved an eGOS score of 4–8 (adjusted risk difference 4·2% (95% CI –3·3 to 11·8%; p=0·28) 365 days after stroke. Inclusion of all randomly assigned patients (n=506) in the sensitivity analysis did not change the primary result.
Figure 2: Independently adjudicated functional outcomes at 365 days post stroke in the modified intention-to-treat analysis set.
mRS scores range from 0 (no disability) to 6 (death); eGOS scores range from 8 (upper good recovery) to 1 (death). GR=good recovery. MD=moderate disability. SD=severe disability. VS=vegetative state.
The proportion of 365-day mRS 0–3 was 110 (44.2%) in the MISTIE group vs 100 (41.7%) in the medical group. mRS 4–6 was 139 (55.8%) in the MISTIE group and 140 (58.3%) in the medical group. mRS scores were missing for 10 out of 499 patients; of the 10, 6 subjects were lost to follow-up, and 4 refused further participation in the study.
The proportion of 365-day eGOS 4–8 (upper severe disability through upper good recovery) was 94 (38.5%) in the MISTIE group vs 84 (35.9%) in the medical group. eGOS scores 1–3 (lower severe disability through death) were 150 (61.5%) in the MISTIE group and 150 (64.1%) in the medical group. eGOS scores were missing for 21 out of 499 patients, out of which 11 completed the study with no eGOS reported, 6 were lost to follow-up, and 4 refused further participation. See appendix for detailed ordinal measures for mRS and eGOS.
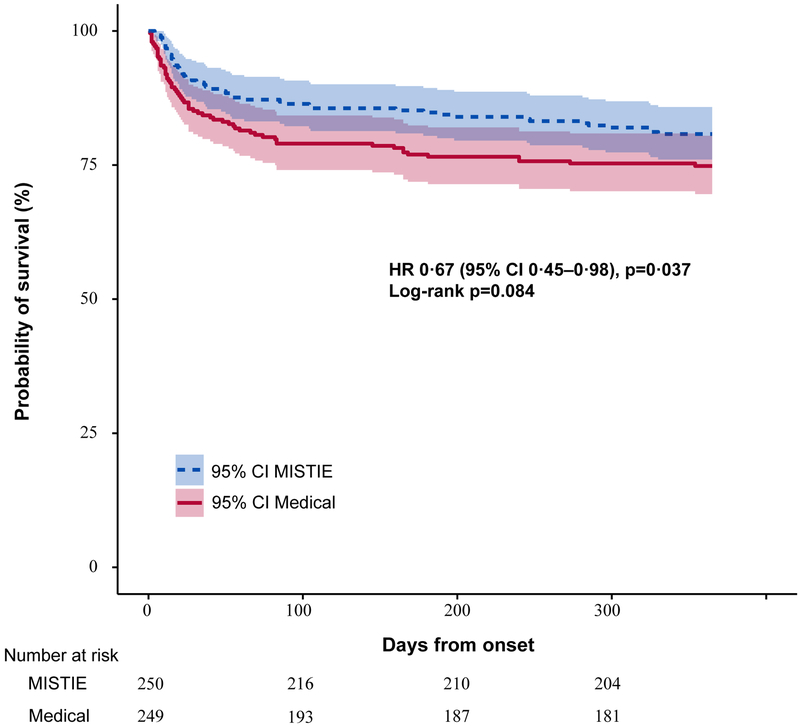
Estimated all-cause mortality (figure 3) differed by 6–8% throughout the 365-day period (log-rank p=0·08), and was significantly lower in the MISTIE group than the standard medical care group at 365 days (severity-adjusted Cox proportional hazard ratio 0·67, 95% CI 0·45–0·98; p=0·037). Analysis of the association between clot removal and functional outcome, adjusted for the same baseline variables as the primary efficacy analysis, showed extent of removal was correlated with mRS scores of 0–3 (odds ratio 0·68 [95% CI 0·59–0·78; p<0·0001; appendix). Similarly, the as-treated analyses comparing the proportion of patients who achieved the surgical aim (clot size ≤15 mL) in the MISTIE group with the standard medical care group showed a 10·5% increase in risk difference (95% CI 1·0–20·0; p=0·03) in mRS scores of 0–3 at 365 days after controlling for variation in initial severity factors and severity matching (appendix). These secondary analyses are not adjusted for multiplicity and should be interpreted as exploratory.
Figure 3: Kaplan-Meier survival estimates from day of randomisation to observed day of death with truncation at day 365.
Estimated survival probabilities were higher throughout 365 days of follow-up with MISTIE compared to medical treatment (p=0.084). Shading shows 95% CI.
Intracranial pressure elevation and cerebral perfusion pressure depression were less common in the MISTIE group than the standard medical care group (table 2). Patient disposition, 180-day function, other intensive care unit care, and quality of life were similar between the groups (appendix). Additional subgroup analyses, the as-treated analyses, site random effects analyses, and a detailed assessment of serious adverse events by patient, MedDRA code, and organ system were consistent with the primary safety analyses (appendix) and suggest that the frequency of serious adverse events was similar between the groups. At 30 days, 56 (13%) of 443 patients had returned home, and by 365 days, 314 (83%) of 379 patients were living at home or in acute rehabilitation (appendix).
Specified safety event rates did not reach a-priori determined DSMB review thresholds throughout the trial. However, the number of deaths in the MISTIE group was lower than in the standard medical care group at 7 days (two [1%] vs ten [4%]; p=0·018), 30 days (24 [9%] vs 37 [14%]; p=0·066; table 3), and 180 days (39 [15%] vs 57 [23%]; p=0·033). At 30 days, 76 (30%) of 255 patients in the MISTIE group and 84 (33%) of 251 patients in the standard medical care group had one or more serious adverse event (table 4), and the difference in number of serious adverse events between the groups was statistically significant (p=0·012; table 3). The proportion of patients with asymptomatic bleeding was significantly higher in the MISTIE group than the standard medical care group (81 [32%] of 255 patients vs 21 [8%] of 251 patients; p<0·0001). Other safety measures were similar between groups (table 3).
Table 3:
Safety outcomes
| Study stop threshold |
MISTIE (n=255) |
Medical (n=251) |
p value | |
|---|---|---|---|---|
| Died within 7 days | 10% | 2 (0·8%) | 10 (4·0%) | 0·018 |
| Died within 30 days | 60% | 24 (9·4%) | 37 (14·7%) | 0·066 |
| Died within 180 days | NA | 39 (15·3%) | 57 (22·7%) | 0·033 |
| Bacterial brain infection within 30 days | 15% | 2 (0·8%) | 0 | 0·160 |
| Symptomatic brain bleeds within 72 h after last dose* | 25% | 6 (2·4%) | 3 (1·2%) | 0·325 |
| Asymptomatic brain bleeds within 72 h after last dose* | NA | 81 (31·8%) | 21 (8·4%) | <0·0001 |
| Number of serious adverse events within 30 days | NA | 126 | 142 | 0·012 |
Data are n, or n (%). NA=not applicable.
For patients in the MISTIE group who achieved the endpoint before alteplase dosing and for patients in the standard medical care group, this was calculated as the corresponding median time from randomisation. Safety analyses were done for the full randomised cohort (n=506), including the seven patients randomised in error.
Table 4:
Serious adverse events
| Stroke to day 30 | Stroke to end of follow-up | |||
|---|---|---|---|---|
| Body system classification | MISTIE (n=255) |
Medical (n=251) |
MISTIE (n=255) |
Medical (n=251) |
| Cardiac disorders | 4 (1.6%) | 1 (0.4%) | 8 (3.1%) | 6 (2.4%) |
| Gastrointestinal disorders | 3 (1.2%) | 4 (1.6%) | 4 (1.6%) | 6 (2.4%) |
| General disorders and administration site conditions | 13 (5.1%) | 26 (10.4%) | 25 (10.0%) | 37 (14.7%) |
| Hepatobiliary disorders | 0 | 0 | 1 (0.4%) | 1 (0.4%) |
| Infections, non-neurologic | 2 (0.8%) | 4 (1.6%) | 6 (2.4%) | 8 (3.2%) |
| Injury, poisoning, and procedural complications | 3 (1.2%) | 0 | 6 (2.4%) | 3 (1.2%) |
| Metabolism and nutrition disorders | 1 (0.4%) | 0 | 1 (0.4%) | 0 |
| Musculoskeletal and connective tissue disorders | 0 | 0 | 0 | 1 (0.4%) |
| Neoplasms (benign, malignant, and unspecified) | 1 (0.4%) | 0 | 2 (0.8%) | 1 (0.4%) |
| Nervous system disorders | 17 (6.7%) | 33 (13.1%) | 36 (14.5%) | 49 (19.5%) |
| Psychiatric disorders | 0 | 1 (0.4%) | 2 (0.8%) | 2 (0.8%) |
| Renal and urinary disorders | 0 | 1 (0.4%) | 0 | 1 (0.4%) |
| Respiratory, thoracic, and mediastinal disorders | 26 (10.2%) | 14 (5.6%) | 33 (13.3%) | 19 (7.6%) |
| Vascular disorders | 6 (2.4%) | 0 (0.0%) | 7 (2.8%) | 0 |
Data are n (%).
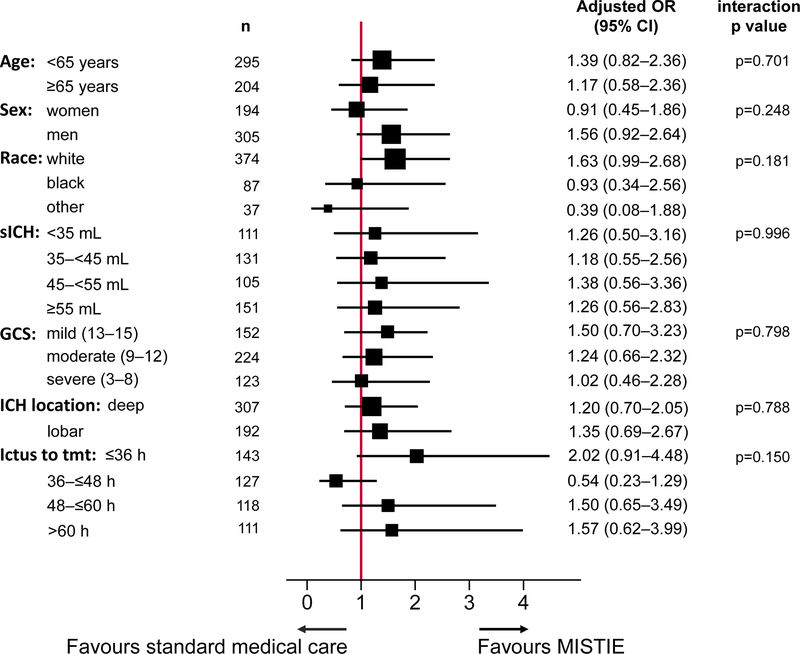
Additional analyses of the primary efficacy outcome by ordinal methods, longitudinal methods, demographic characteristics, and site characteristics yielded the same results as the adjusted primary efficacy analyses (appendix). Subgroup analyses did not identify substantial differences in outcome with regard to age, intracerebral haemorrhage volumes, or time to randomisation (figure 4). The test for homogeneity of treatment effect (based on testing for interaction) was not rejected at α level 0·05. Routine ordinal analysis did not meet proportionality assumptions. Therefore, generalised ordered logistic regression analyses, adjusting for the same baseline variables as the primary efficacy analysis, were used to compare patients in the MISTIE group with those in the standard medical group with different 365-day mRS scores; scores 0–2 were combined into a single category due to small numbers. The estimated odds ratios for mRS scores higher than 5 versus 5 or less, higher than 4 versus 4 or less, higher than 3 versus 3 or less, and higher than 2 versus 2 or less were 0·60 (p=0·03), 0·84 (p=0·42), 0·87 (p=0·49), and 0·82 (p=0·44), respectively (appendix). There was no evidence of an increase in the proportion of patients with an mRS score of 4 or 5 in the MISTIE group versus the standard medical care group, although it is not possible to determine the mRS disability distribution for patients who would have died with standard of care but would have survived with MISTIE.
Figure 4: Subgroup analyses.
Forest plot of interaction terms, adjusted for age, sex, race, intracerebral haemorrhage location, intracerebral haemorrhage stability, time from stroke to treatment initiation, and GCS score (mild, moderate, severe) at presentation. The test for homogeneity of treatment effect (based on testing for interaction) was not rejected at α level 0·05. The size of points indicates the relative sizes of the subgroups. GCS scores range from 15 (fully conscious) to 3 (deep coma). sICH=stability intracerebral haemorrhage. GCS=Glasgow Coma Scale.
Discussion
In the MISTIE III trial, aspiration and thrombolytic irrigation of the intracerebral haematoma with alteplase via an image-guided catheter did not improve functional outcomes compared with standard medical care in patients with large intracerebral haemorrhage. Our secondary analyses were not adjusted for multiplicity and should be interpreted as exploratory. Mortality at 365 days seemed to be lower in the MISTIE group than the standard medical care group, without a net increase in the proportion of patients with severe disability. This increased survival is, at best, a modest effect based on a secondary analysis. Exploratory analyses of clot removal showed an association between extent of removal and lower mRS scores (0–3). This association could be due to a benefit of the procedure or due to unmeasured confounding (eg, individuals with haemorrhage shape or location more amenable to MISTIE reduction of clot volume might have better prognoses unrelated to treatment).
The MISTIE procedure was safe with regard to serious bleeding and infection, and procedural risk was similar between groups. These findings suggest MISTIE has few negative consequences. Time spent in intensive care units, withdrawal of care, use of external ventricular drains, and intracranial pressure therapies did not seem to account for observed differences in the secondary outcomes between treatment groups. MISTIE cannot be recommended as an intervention to improve functional outcome for all patients with intracerebral haemorrhage until the desired reduction in haematoma size is uniformly achieved.
Study limitations include the open-label design, the size of the mITT analysis set (n=499), and use of 78 sites and 114 surgeons from resource-rich tertiary referral centres and universities. The use of a surgical core laboratory and the additional oversight provided in phase 3 trials are factors that influence generalisability. However, these same limitations allowed for detailed monitoring of data accuracy, enabled planning and performance of the surgical task, and avoided caregiver bias. Therefore, the neutral result in MISTIE III indicates that the MISTIE procedure should not be adopted pragmatically. Assessment of the surgical data indicates that when MISTIE achieves the protocol-defined surgical aim, a benefit in mortality and functional improvement seems possible. The inclusion criterion of clinical stability excluded patients with a poor prognosis. Imprecision exists in the mRS and eGOS. However, the use of an adjudication committee with multiple reviews of mRS limits this problem. Both scales provide similar results, thus providing external validation of each other. A limitation of the clot removal analyses is potential bias due to unmeasured confounding, since clot volume removal was not randomised. The absence of blinding did not protect against undertreatment or overtreatment bias associated with assignment to a particular intervention. Our mortality analysis was secondary and was not adjusted for multiple secondary analyses and thus should be interpreted as exploratory, rather than confirmatory.
Our study also had strengths. We used a standard task description and a high level of safety was achieved in a large group of surgeons who had not previously done the procedure. The challenges associated with performing the standardised MISTIE task seem remediable. Since MISTIE is a new procedure, it is not surprising that technical excellence was more difficult to achieve than safe performance. Results from our as-treated analysis suggest reduction in clot size to 15 mL or less as an aim for the MISTIE procedure. Additional analyses of factors associated with less optimal haematoma evacuation are planned. More work is needed to enhance effective targeting and dosing. Some surgical situations might require specific strategies,23 such as lobar haematomas since the round shape and large size might require more than one catheter. Posterior haematomas require additional entry points and targeting precision. Securing catheters can be improved further. Although surgeons were able to remove large amounts of intracerebral haemorrhage clots despite little previous experience with the MISTIE, protocol adherence to the task of decreasing the haematoma to 15 mL or less improved with site and surgeon experience (appendix). Established methods can be used to address the issue of improved surgical task performance26 and interventional team performance.27 An additional strength of this study was the use of widely accepted evidence-based guidelines, which seem to have resulted in uniform medical management.
Similarities and differences exist between our results and those reported for other surgical trials of craniotomy. The low mortality (20%) observed and the proportion of patients who achieved good functional outcomes (>40% of all patients) at 365 days were not expected. In the MISTIE II trial,12 at 365 days, 38% of patients had died and 20% had achieved good functional outcomes in the control group. A similar proportion of patients (around 30%) achieved good outcomes in the STICH I and II trials;5,6 however, mortality was higher in STICH I (37%) and STICH II (21%).6 The proportion of patients with lobar haematomas was higher in the standard care group than the MISTIE group, a location previously associated with improved prognosis.6 Other factors affecting severity, such as age, GCS, intracerebral haemorrhage size, and intraventricular haemorrhage size, were similar in both MISTIE trials.12 Although inclusion criteria (eg, stability) could account for the baseline decrease in mortality in both trial groups compared with previous trials, the improved mortality with the MISTIE procedure is associated with the intervention, not the selection criteria. This finding provides biological plausibility that reduction of mass effect and tissue injury might be lessened using the MISTIE procedure. Although baseline mortality was higher in other reports, these findings mimic the specific effect sizes suggested in previous meta-analyses of mortality benefit.28–30 The longitudinal data show functional independence at 30 days had increased by four times by 365 days. At 365 days post stroke, 80% of all survivors were living at home or in active rehabilitation in both treatment groups. An important generalisable conclusion is that the prognosis for large intracerebral haemorrhages might not be as poor as suggested by common prognostic scales.31,32 Additionally, our data demonstrate that withdrawal of care is strongly associated with mortality in this set of patients who received intensive care unit treatment.33 Each of these findings suggests a policy of aggressive care for patients who are healthy without advance directives is a wise choice, if such care optimises patient preference.
Important technical differences exist between craniotomy, the MISTIE procedure, and other evacuation techniques, however, evaluations have been limited by lack of data, study size, scope of variables collected, scope of monitoring, and the use of central adjudication for procedural perfomance;34–38 most of these techniques have much higher indices of mechanical tissue trauma. These techniques might have advantages over the MISTIE procedure; other surgical approaches should be investigated with the same rigour used to develop the evidence for MISTIE. Without trials that balance known factors that independently influence functional outcome and mortality, there is a risk of repeated overinterpretation of therapeutic benefit when shifts in baseline severity between the control and intervention groups account for the putative benefit.4,39 A similar problem exists when the generalisability of surgical task performance is not assessed. This problem has been well documented in the development of successful ischaemic stroke therapies16,40 and should also be applied to intracerebral haemorrhage. MISTIE III provides additional evidence that the occurrence of intracerebral haemorrhage should not uniformly trigger a nihilistic clinical response.19 The predicted poor prognosis of large intracerebral haemorrhage is potentially altered by aggressive care. The components of care investigated in the MISTIE trial include early blood pressure control, stabilisation of bleeding, and tissue-sparing, large reduction of haematoma size to 15 mL or less. To better assess the effect of MISTIE on functional survival, a trial in which training and clinical experience can direct consistent removal of haematoma volumes to 15 mL or less is needed.
Research in context
Evidence before this study
Recent meta-analyses have shown that stereotaxis plus thrombolysis and other non-craniotomy surgical techniques could decrease mortality and poor functional outcome after intracerebral haemorrhage with approximate effect sizes of up to 0·50. However, high-quality evidence from well conducted stroke trials is limited, since only three of 14 studies identified in a recent meta-analysis met all Cochrane Review criteria. Available studies were mostly small, unblinded, did not use standardised surgical definitions or monitoring of the surgical task, and none assessed the generalisability of surgical performance or the association between performance and clinical outcome. Thus, we did a meta-analysis of multisite trials of catheter-based minimally invasive surgery plus thrombolysis in which outcomes were assessed using the modified Rankin scale (mRS) or extended Glasgow Outcome Scale at 180 days (appendix). The results of our meta-analysis found no significant benefit of minimally invasive surgery (odds ratio [OR] 0·61, 95% CI 0·29–1·26), which is consistent with current guidelines regarding the MISTIE intervention. The European Stroke Organisation and American Heart Association advise additional evidence is needed to recommend use of minimally invasive surgery plus thrombolysis in routine care. Biologically plausible mechanisms for surgical removal exist. Clinical injury from intracerebral haemorrhage is directly associated with clot size, with about 10% improvement in good outcomes for each 10 mL decrease in 10–50 mL clots (OR 1·4 per 10 mL). However, benefit from mechanical reduction of clot size has been difficult to demonstrate in humans. The International Surgical Trial in Intracerebral Haemorrhage (STICH) I and II studies provided strong indications that pragmatic use of open craniotomy does not yield an effect size of 10–15% in mortality or functional benefit. The MISTIE surgical task was designed to eliminate craniotomy-associated injury including cortical incision, electrocautery, toxic exposure to thrombin, and additional mechanical manipulations of brain tissue. This surgical procedure was rigorously standardised in a multisite study overseen by a core surgical laboratory. MISTIE II results provided evidence of safety of this non-craniotomy procedure, identified the best dose of thrombolytic drug to be used for clot volume reduction, and demonstrated a treatment effect for mRS 0–3 that was substantial (>10% absolute benefit) and was sustained for 1 year. The conclusions of MISTIE II required testing in a large population to assure generalisability, including the presence or absence of beneficial functional or mortality change, safety of this surgical-drug combination, and assessment of the range of performance of a standardised surgical technique and the impact of procedural performance on outcome.
Added value of this study
Despite the absence of benefit for our primary outcome in MISTIE III, data demonstrate that approximately 43% of all patients achieved a good functional outcome, even those with large intracerebral haemorrhage. These changes in functional outcome are consistent with a low frequency of withdrawal of care, guideline-driven intensive care unit care, and achievement of stability of haematoma growth. However, the fact that the proportion of all patients who achieved a good outcome was higher than expected is not solely because the patients were healthy; average patient severity matched or exceeded that of the patients included in the STICH trials. Accounting for all differences in functional outcome between randomised trials remains difficult. The secondary endpoints indicate acceptable safety and a slight decrease in mortality attributable to the MISTIE technique. The estimated mortality difference between the MISTIE and standard care treatment groups was modest, with a number needed to treat of 17 to preserve one life; however, mortality was not the primary outcome and as a result of multiple testing this estimate is exploratory. Our findings show the MISTIE technique can be safely adopted by a broad group of newly trained neurosurgeons. The exploratory secondary results suggest that clot size reduction to 15 mL or less in the MISTIE group was associated with better mRS scores at 365 days in patients who were stabilised. This finding identifies a threshold of procedural outcome, which would need to be evaluated in a future trial.
Implications of all the available evidence
The pragmatic use of MISTIE cannot be recommended. The overall functional results strongly support an active approach to care for patients with intracerebral haemorrhage who fit the enrolment criteria. For the entire trial cohort, around 43% of patients had good functional outcomes, and 80% of patients were living at home or in active rehabilitation 365 days after stroke with an acceptable quality of life. The data provide a sound basis to avoid limitation of care in patients with intracerebral haemorrhage. The MISTIE approach did not increase dependency and mortality might be decreased.
The safety profile of the MISTIE procedure shows clot size reduction can be achieved with similar safety to standard medical care. Exploratory analyses also suggest that reduction in clot size to 15 mL or less is associated with functional improvement. These findings require further study of the MISTIE technique with a greater emphasis on consistently achieving clot reduction to 15 mL or less. A detailed analysis of elements of surgical performance in MISTIE III is ongoing.
Supplementary Material
Acknowledgments
We thank the patients involved in MISTIE III, their families, and the investigators and coordinators who cared for them. We thank Prelude Dynamics for developing the data management and randomisation systems and Newcastle University, Newcastle Upon Tyne Hospitals Foundation Trust, Alan Cohen and Cohen Consultants (Liège, Belgium), Mawdsley-Brooks & Co (Salford, UK), and the Coordination Centre for Clinical Trials (Heidelberg, Germany) for supporting the trial in the European Union. We thank the National Institutes of Health-appointed Data Safety and Monitoring Board, Colin P Derdeyn (chair), Kyra J Becker, James C Torner, and Brian L Hoh, for guidance and oversight of the safety of the trial participants. MISTIE III was supported by a grant from the National Institutes of Health National Institute of Neurological Disorders and Stroke (1U01NS080824) and materials grants from Genentech.
Funding.
National Institute of Neurological Disorders and Stroke and Genentech. Genentech provided alteplase at no cost to all sites in North America. HA, RA, IAA, AJB-H, YH, KL, NM, PLM, WAM, AS, EAS, and WZ report grants from the National Institute of Neurological Disorders and Stroke (NINDS), during the conduct of the study. CSA reports grants from the National Health and Medical Research Council of Australia, grants and personal fees from Takeda, and personal fees from Amgen and Boehringer Ingelheim, outside the submitted work. DB reports non-financial support from Evgen Pharma and personal fees from ReNeuron, outside the submitted work. MRC reports grants from IMRIS, the Head for the Cure Foundation, and the Mrs Carol Rossfeld and the Alex and Alice Aboussie Family Charitable Foundation, outside the submitted work. JD has received honoraria from Bayer, Pfizer, BMS, Daiichi Sankyo, and Boehringer Ingelheim; is an investigator in a Servier and MicroTransponder trial; and reports fees for medico-legal work and grants from the Chief Scientist’s Office, Stroke Association, British Heart Foundation, National Institute for Health Research (NIHR), Chest Heart Stroke Scotland, and the Neurosciences Foundation, outside the submitted work. DG reports grants from National Institutes of Health (NIH), Focused Ultrasound Foundation, Microvention, and Insightec. BG reports grants from Johns Hopkins University, during the conduct of the study. DFH reports grants from NINDS, non-financial support from Genentech, during the conduct of the study; and personal fees from BrainScope, Neurotrope, Op2Lysis, Portola Pharmaceuticals, and Medtronic, outside the submitted work. MRH reports grants from NIH, during the conduct of the study. JH reports Longeviti stock ownership and personal fees from medico-legal, outside the submitted work. KRL reports personal fees from Boehringer Ingelheim, outside the submitted work. SWM reports grants from NINDS, during the conduct of the study, and personal fees from Johns Hopkins University, outside the submitted work. ADM reports grants from NIH, during the conduct of the study; non-financial support from the Newcastle Neurosurgery Foundation; and personal fees from Stryker and Draeger, outside the submitted work. HP reports grants from NIHR Efficacy and Mechanism Evaluation and Health Foundation and personal fees from medico-legal work. RET reports grants from NIH/NINDS, during the conduct of the study and personal fees from Neurotrope Bioscience, outside the submitted work. PV is an adviser with stock equity for InTouch Health and an adviser for M Dialysis and Moberg Medical. GY reports grants from NINDS, during the conduct of the study and personal fees from the RAND Corporation, Health Advisory Services, outside the submitted work. All other authors declare no competing interests.
Footnotes
Data sharing
The MISTIE III trial data, including de-identified participant data, will be made available indefinitely at the National Institute of Neurological Disorders and Stroke data archive (https://www.ninds.nih.gov/) and the Virtual International Stroke Trials Archive (http://www.virtualtrialsarchives.org/vista/) by Dec 31, 2019. To gain access, requesters will need to sign a data-access agreement. The study protocol, statistical analysis plan, and informed consent form will be included, but are also available at ClinicalTrials.gov.
Contributor Information
Daniel F Hanley, Division of Brain Injury Outcomes, Johns Hopkins University, Baltimore, MD, USA.
Richard E Thompson, Johns Hopkins University Bloomberg School of Public Health, Department of Biostatistics, Baltimore, MD, USA.
Michael Rosenblum, Johns Hopkins University Bloomberg School of Public Health, Department of Biostatistics, Baltimore, MD, USA.
Gayane Yenokyan, Johns Hopkins University Bloomberg School of Public Health, Department of Biostatistics, Baltimore, MD, USA.
Karen Lane, Division of Brain Injury Outcomes, Johns Hopkins University, Baltimore, MD, USA.
Nichol McBee, Division of Brain Injury Outcomes, Johns Hopkins University, Baltimore, MD, USA.
Steven W Mayo, Emissary International LLC, Austin, TX, USA.
Amanda J Bistran-Hall, Division of Brain Injury Outcomes, Johns Hopkins University, Baltimore, MD, USA.
Dheeraj Gandhi, University of Maryland, Baltimore, MD, USA.
W Andrew Mould, Division of Brain Injury Outcomes, Johns Hopkins University, Baltimore, MD, USA.
Natalie Ullman, The Children’s Hospital, Philadelphia, PA, USA.
Hasan Ali, Division of Brain Injury Outcomes, Johns Hopkins University, Baltimore, MD, USA.
J Ricardo Carhuapoma, Johns Hopkins University, School of Medicine, Baltimore, MD, USA.
Carlos S Kase, Emory University, Atlanta, GA, USA.
Kennedy R Lees, University of Glasgow, Glasgow, UK.
Jesse Dawson, University of Glasgow, Institute of Cardiovascular and Medical Sciences, College of Medical, Veterinary and Life Sciences, Glasgow, UK.
Alastair Wilson, University of Glasgow, Glasgow, UK.
Joshua F Betz, Johns Hopkins University Bloomberg School of Public Health, Department of Biostatistics, Baltimore, MD, USA.
Elizabeth A Sugar, Johns Hopkins University Bloomberg School of Public Health, Department of Biostatistics, Baltimore, MD, USA.
Yi Hao, Division of Brain Injury Outcomes, Johns Hopkins University, Baltimore, MD, USA.
Radhika Avadhani, Division of Brain Injury Outcomes, Johns Hopkins University, Baltimore, MD, USA.
Jean-Louis Caron, University of Texas Health, San Antonio, TX, USA.
Mark R Harrigan, University of Alabama, Birmingham, AL, USA.
Andrew P Carlson, University of New Mexico, Albuquerque, NM, USA.
Diederik Bulters, University Hospital Southampton NHS Foundation Trust, Southampton, UK.
David LeDoux, Hofstra Northwell School of Medicine, Hempstead, NY, USA.
Judy Huang, Johns Hopkins University, School of Medicine, Baltimore, MD, USA.
Cully Cobb, Mercy Neurological Institute Stroke Center, Sacramento, California, USA.
Gaurav Gupta, Rutgers-Robert Wood Johnson Medical School, New Brunswick, NJ, USA.
Ryan Kitagawa, University of Texas, McGovern Medical Center, Houston, TX, USA.
Michael R Chicoine, Washington University School of Medicine, St. Louis, MO, USA.
Hiren Patel, Salford Royal Hospital, Salford, UK.
Robert Dodd, Stanford University School of Medicine, Stanford, California, USA.
Paul J Camarata, University of Kansas, Kansas City, KS, USA.
Stacey Wolfe, Wake Forest School of Medicine, Winston-Salem, North Carolina, USA.
Agnieszka Stadnik, University of Chicago, Chicago, IL, USA.
P Lynn Money, University of Chicago, Chicago, IL, USA.
Patrick Mitchell, Newcastle Royal Infirmary, UK.
Rosario Sarabia, Hospital Universitario Rio Hortega, Valladolid, Spain.
Sagi Harnof, Rabin Medical Center, Israel.
Pal Barzo, University of Szeged, Szeged, Hungary.
Andreas Unterberg, University of Heidelberg, Heidelberg, Germany.
Jeanne S Teitelbaum, Montreal Neurological Institute and Hospital at McGill University, Montreal, Quebec, Canada.
Weimin Wang, Guangzhou Neuroscience Institute, Guangzhou Liuhua Qiao Hospital, Guangzhou, Guangdong, China.
Craig S Anderson, The George Institute for Global Health China at Peking University Health Science Center, Beijing, China; The George Institute for Global Health, Faculty of Medicine, University of New South Wales, Sydney, Australia.
A David Mendelow, Newcastle University, Newcastle upon Tyne, UK.
Barbara Gregson, Newcastle University, Newcastle upon Tyne, UK.
Scott Janis, National Institutes of Health, National Institute of Neurological Disorders and Stroke, Bethesda, MD, USA.
Paul Vespa, University of California, Los Angeles, CA, USA.
Wendy Ziai, Division of Brain Injury Outcomes, Johns Hopkins University, Baltimore, MD, USA.
Mario Zuccarello, University of Cincinnati, Cincinnati, OH, USA.
Issam A Awad, University of Chicago, Chicago, IL, USA.
References
- 1.Krishnamurthi RV, Feigin VL, Forouzanfar MH, et al. Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet Glob Health 2013; 1: e259–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hachinski V, Donnan GA, Gorelick PB, et al. Stroke: working toward a prioritized world agenda. Int J Stroke 2010; 5: 238–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cadilhac DA, Dewey HM, Vos T, Carter R, Thrift AG. The health loss from ischemic stroke and intracerebral hemorrhage: evidence from the North East Melbourne Stroke Incidence Study (NEMESIS). Health Qual Life Outcomes 2010; 8: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mayer SA, Brun NC, Begtrup K, et al. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med 2008; 358: 2127–37. [DOI] [PubMed] [Google Scholar]
- 5.Mendelow AD, Gregson BA, Fernandes HM, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet 2005; 365: 387–97. [DOI] [PubMed] [Google Scholar]
- 6.Mendelow AD, Gregson BA, Rowan EN, Murray GD, Gholkar A, Mitchell PM. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): a randomised trial. Lancet 2013; 382: 397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson CS, Heeley E, Huang Y, et al. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med 2013; 368: 2355–65. [DOI] [PubMed] [Google Scholar]
- 8.Qureshi AI, Palesch YY. Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH) II: design, methods, and rationale. Neurocrit Care 2011; 15: 559–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wagner KR, Xi G, Hua Y, et al. Ultra-early clot aspiration after lysis with tissue plasminogen activator in a porcine model of intracerebral hemorrhage: edema reduction and blood-brain barrier protection. J Neurosurg 1999; 90: 491–98. [DOI] [PubMed] [Google Scholar]
- 10.Montes JM, Wong JH, Fayad PB, Awad IA. Stereotactic computed tomographic-guided aspiration and thrombolysis of intracerebral hematoma protocol and preliminary experience. Stroke 2000; 31: 834–40. [DOI] [PubMed] [Google Scholar]
- 11.Rohde V, Rohde M, Reinges L, Mayfrank L, Gilsbach JM. Frameless stereotactically guided catheter placement and fibrinolytic therapy for spontaneous intracerebral hematomas: technical aspects and initial clinical results. Minim Invasiv Neurosurg 2000; 43: 9–17. [DOI] [PubMed] [Google Scholar]
- 12.Hanley DF, Thompson RE, Muschelli J, et al. Safety and efficacy of minimally invasive surgery plus alteplase in intracerebral haemorrhage evacuation (MISTIE): a randomised, controlled, open-label, phase 2 trial. Lancet Neurol 2016; 15: 1228–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sedgwick P. Explanatory trials versus pragmatic trials. BMJ 2014; 349: g6694. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz D, Lellouch J. Explanatory and pragmatic attitudes in therapeutical trials. J Clin Epidemiol 1967; 20: 637–48. [DOI] [PubMed] [Google Scholar]
- 15.Kothari RU, Brott T, Broderick JP. The ABCs of measuring intracerebral hematoma volumes. Stroke 1996; 27: 1304–05. [DOI] [PubMed] [Google Scholar]
- 16.Hanley DF, Lane K, McBee N, et al. Thrombolytic removal of intraventricular haemorrhage in treatment of severe stroke: results of the randomised, multicentre, multiregion, placebo-controlled CLEAR III trial. Lancet 2017; 389: 603–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McArthur KS, Johnson PCD, Quinn TJ, et al. Improving the efficiency of stroke trials. Stroke 2013; 44: 3422–48. [DOI] [PubMed] [Google Scholar]
- 18.Anderson CS, Selim MH, Molina CA, Qureshi AI. Intensive blood pressure lowering in intracerebral hemorrhage. Stroke 2017; 48: 2034–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morgenstern L, Hemphill JC 3rd, Anderson C, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2010; 41: 2108–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Josephson CB, White PM, Krishan A, Al-Shahi Salman R. Computed tomography angiography or magnetic resonance angiography for detection of intracranial vascular malformations in patients with intracerebral haemorrhage. Cochrane Database Syst Rev 2014; 9: CD009372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steiner T, Al-Shahi Salman R, Beer R, et al. European Stroke Organisation (ESO) guidelines for the management of spontaneous intracerebral hemorrhage. Int J Stroke 2014; 9: 840–55. [DOI] [PubMed] [Google Scholar]
- 22.Cordonnier C, Salman RA-S, Bhattacharya JJ, et al. Differences between intracranial vascular malformation types in the characteristics of their presenting haemorrhages: prospective, population-based study. J Neurol Neurosurg Psychiatry 2008; 79: 47. [DOI] [PubMed] [Google Scholar]
- 23.Fam MD, Hanley D, Stadnik A, et al. Surgical performance in minimally invasive surgery plus recombinant tissue plasminogen activator for intracerebral hemorrhage evacuation phase III clinical trial. Neurosurgery 2017; 81: 860–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mould WA, Carhuapoma JR, Muschelli J, et al. Minimally invasive surgery plus recombinant tissue-type plasminogen activator for intracerebral hemorrhage evacuation decreases perihematomal edema. Stroke 2013; 44: 627–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Laan MJ, Gruber S. Targeted minimum loss based estimation of causal effects of multiple time point interventions. Int J Biostat 2012; 8: 1–39. [DOI] [PubMed] [Google Scholar]
- 26.Willaert WI, Van Herzeele I. Carotid artery stenting–strategies to improve procedural performance and reduce the learning curve. Interv Cardiol 2013; 8: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Menon BK, Almekhlafi MA, Pereira VM, et al. Optimal workflow and process-based performance measures for endovascular therapy in acute ischemic stroke: analysis of the Solitaire FR thrombectomy for acute revascularization study. Stroke 2014; 45: 2024–29. [DOI] [PubMed] [Google Scholar]
- 28.Scaggiante J, Zhang X, Mocco J, Kellner CP. Minimally invasive surgery for intracerebral hemorrhage: an updated meta-analysis of randomized controlled trials. Stroke 2018; 49: 2612–20. [DOI] [PubMed] [Google Scholar]
- 29.Wang J-W, Li J- P, Song Y- L, et al. Stereotactic aspiration versus craniotomy for primary intracerebral hemorrhage: a meta-analysis of randomized controlled trials. PLoS One 2014; 9: e107614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou X, Chen J, Li Q, et al. Minimally invasive surgery for spontaneous supratentorial intracerebral hemorrhage: a meta-analysis of randomized controlled trials. Stroke 2012; 43: 2923–30. [DOI] [PubMed] [Google Scholar]
- 31.Hemphill JC 3rd, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke 2001; 32: 891–97. [DOI] [PubMed] [Google Scholar]
- 32.Morgenstern LB, Zahuranec DB, Sánchez BN, et al. Full medical support for intracerebral hemorrhage. Neurology 2015; 84: 1739–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Becker KJ, Baxter AB, Cohen WA, et al. Withdrawal of support in intracerebral hemorrhage may lead to self-fulfilling prophecies. Neurology 2001; 56: 766–72. [DOI] [PubMed] [Google Scholar]
- 34.Labib MA, Shah M, Kassam AB, et al. The safety and feasibility of image-guided brainpath-mediated transsulcul hematoma evacuation: a multicenter study. Neurosurgery 2017; 80: 515–24. [DOI] [PubMed] [Google Scholar]
- 35.Fiorella D, Arthur AS, Mocco J. 305 The INVEST trial: a randomized, controlled trial to investigate the safety and efficacy of image-guided minimally invasive endoscopic surgery with apollo vs best medical management for supratentorial intracerebral hemorrhage. Neurosurgery 2016; 63: 187. [Google Scholar]
- 36.Vespa P, Hanley D, Betz J, et al. ICES (Intraoperative Stereotactic Computed Tomography-Guided Endoscopic Surgery) for brain hemorrhage: a multicenter randomized controlled trial. Stroke 2016; 47: 2749–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang W-Z, Jiang B, Liu G-M, et al. Minimally invasive craniopuncture therapy vs. conservative treatment for spontaneous intracerebral hemorrhage: results from a randomized clinical trial in China. Int J Stroke 2009; 4: 11–16. [DOI] [PubMed] [Google Scholar]
- 38.Sun H, Liu H, Li D, Liu L, Yang J, Wang W. An effective treatment for cerebral hemorrhage: minimally invasive craniopuncture combined with urokinase infusion therapy. Neurol Res 2010; 32: 371–77. [DOI] [PubMed] [Google Scholar]
- 39.Mayer SA, Brun NC, Begtrup K, et al. Recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med 2005; 352: 777–85. [DOI] [PubMed] [Google Scholar]
- 40.Sajobi TT, Singh G, Lowerison MW, et al. Minimal sufficient balance randomization for sequential randomized controlled trial designs: results from the ESCAPE trial. Trials 2017; 18: 516. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.