Abstract
Cancer cells constantly face a fluctuating nutrient supply and interference with adaptive responses might be an effective therapeutic approach. It has been discovered that in the absence of glucose, cancer cells can synthesize crucial metabolites by expressing phosphoenolpyruvate carboxykinase (PEPCK, PCK1 or PCK2) using abbreviated forms of gluconeogenesis. Gluconeogenesis, which in essence is the reverse pathway of glycolysis, uses lactate or amino acids to feed biosynthetic pathways branching from glycolysis. PCK1 and PCK2 have been shown to be critical for the growth of certain cancers. In contrast, fructose-1,6-bisphosphatase 1 (FBP1), a downstream gluconeogenesis enzyme, inhibits glycolysis and tumor growth, partly by non-enzymatic mechanisms. This review sheds light on the current knowledge of cancer cell gluconeogenesis and its role in metabolic reprogramming, cancer cell plasticity, and tumor growth.
Keywords: Gluconeogenesis, tumor, metabolic plasticity, starvation, adaptation
1. Nutrient deprivation in cancer
Metabolic pathways in cancer cells are rewired to support proliferation and tumor growth. Already in the 1920s, Nobel laureate Otto Heinrich Warburg described that tumor slices avidly consumed glucose and produced lactate, even under non-hypoxic conditions (aerobic glycolysis) [1]. The “Warburg effect” is found in many aggressive cancers, but also in normal, highly proliferative cells. As outlined in excellent reviews on the topic [2,3], high rates of glycolysis ensure that glycolytic intermediates are available to be shunted to important biosynthetic pathways including: 1) oxidative and non-oxidative branches of the pentose phosphate pathway (PPP), providing ribose-5-phosphate and NADPH; 2) synthesis of glycerol-3-phosphate for lipid biosynthesis; 3) serine and glycine synthesis; and 4) the hexosamine pathway [2,3]. Still, the tumor’s high demand for nutrients is frequently not met by an adequate supply. Although angiogenesis is activated early in cancer growth, the newly formed vascular network is aberrant, with leaky vessels and irregular, fluctuating blood flow (reviewed in [4]). Furthermore, cancers outgrow their supply by continuous proliferation and consumption of nutrients like glucose. At the invasive front of solid tumors, cancer cells coopt existing microvessels. In this context as well, perfusion is frequently inadequate, since the proliferating tumor cells compress the pre-existing vessels [5]. Local steep gradients for oxygen (O2), glucose and other nutrients emerge and tumor-cell derived metabolites, like lactate, accumulate [6] (Fig. 1). In fact, a considerable heterogeneity in tumor perfusion and glucose uptake has been shown in rapidly growing tumors, even on a macroscopic level, which greatly impacts tumor cell metabolism [7]. Overall, the microenvironment in solid tumors is considered to be nutrient-poor. Especially glucose deprivation appears to be common, due to its high rate of consumption by the neoplastic tumor cells. In numerous studies, glucose concentrations have been found to be significantly lower in human tumors than in corresponding normal tissues [8–14]. The mechanisms underlying cancer cell adaptation to such a fluctuating nutrient supply are under intense investigation, since they might reveal specific vulnerabilities of cancer cells.
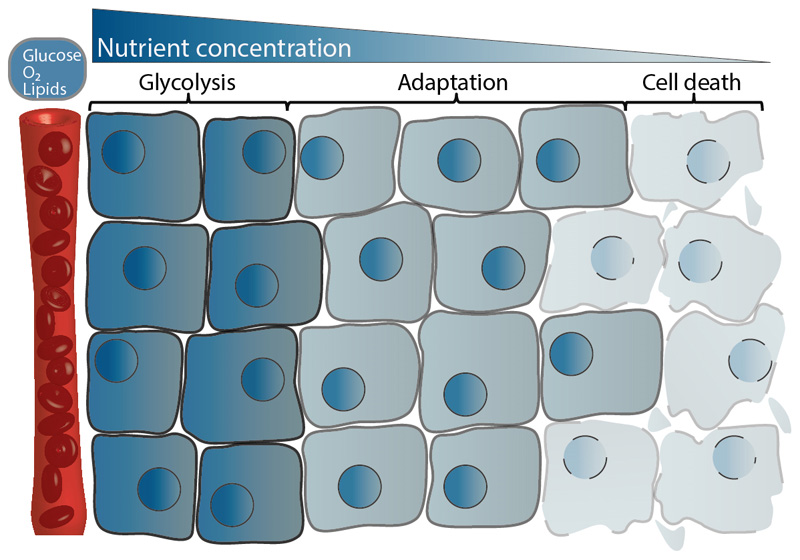
Fig. 1. Effects of nutrient availability on tumor cells.
The concentrations of nutrients like glucose or lipids as well as oxygen (O2) decline with increasing distance from tumor capillaries. Cancer cells adapt to these conditions, partly by utilizing other nutrients than glucose, e.g. lactate. Very low nutrient concentrations and/or oxygen (O2) deprivation induce cell death.
2. Metabolic heterogeneity and use of alternative fuels in cancer cells
Cancer cells are increasingly acknowledged to utilize nutrients other than glucose in a context-dependent manner. An unexpected preference for oxidation of exogenous lactate over glucose has been discovered in certain oxidative tumor cells, sparing glucose for hypoxic, glucose-dependent cancer cells [15]. In human head and neck cancers, lactate metabolism was found to be variable, with some tumors showing net lactate release and some net lactate consumption [16]. Recently, a high contribution of circulating lactate and/or glutamine to TCA cycle intermediates has been shown in genetically engineered lung and pancreatic cancer tumors in mice [17]. Intravenous infusion of 13C-labeled glucose in non-small cell lung cancer (NSCLC) patients revealed high intra- and intertumoral variability in glucose metabolism [7]. Tumor areas with high blood perfusion rather resembled normal lung and showed a considerable contribution of non-glucose precursors to different intermediates, while the use of glucose was higher in poorly perfused tumor areas [7]. A conversion of intravenously administered 13C-lactate to TCA cycle intermediates was shown in human NSCLC in a further study by the same group [18]. Besides lactate, glutamine [19,20], acetate [21–23] and fatty acids [24] also emerged as important non-carbohydrate nutrients in different cancers. Their contribution to cancer metabolism has been reviewed extensively elsewhere [3].
The TCA cycle functions as a central metabolic hub, linking glycolysis, respiration and anabolic biosynthetic pathways (Fig. 2). TCA cycle intermediates are diverted into multiple pathways e.g. nucleic acid, amino acid or fatty acid synthesis (Fig. 2, blue boxes). If glucose is missing, other nutrients entering the TCA cycle can feed into these biosynthetic pathways. However, de novo synthesis of several important cellular building blocks in proliferating cells, including nucleic acids and (glycero-) lipids, requires glycolytic intermediates [25]. Non-carbohydrate precursors can be shuttled via the TCA cycle to glycolytic intermediates, but this pathway requires the action of the gluconeogenesis enzymes phosphoenolpyruvate carboxykinase (PEPCK) and to some extent fructose-1,6-bisphosphatase (FBPase) [25] (Fig. 2, red boxes). Until recently, it was unclear whether glycolytic intermediates are generated in cancer cells via the PEPCK pathway. The emerging role of gluconeogenesis in adaptation of cancer cells to nutrient deprivation on the one hand, and the regulatory properties of gluconeogenesis enzymes on cancer cell metabolism, on the other hand, are discussed here. Highlighting the differential roles of gluconeogenesis enzymes in cancer cells under nutrient-poor as opposed to nutrient-rich conditions and on the metabolic pathways fed by (abbreviated) gluconeogenesis, this review emphasizes other aspects than a recent review on the topic by Wang et al. [26].
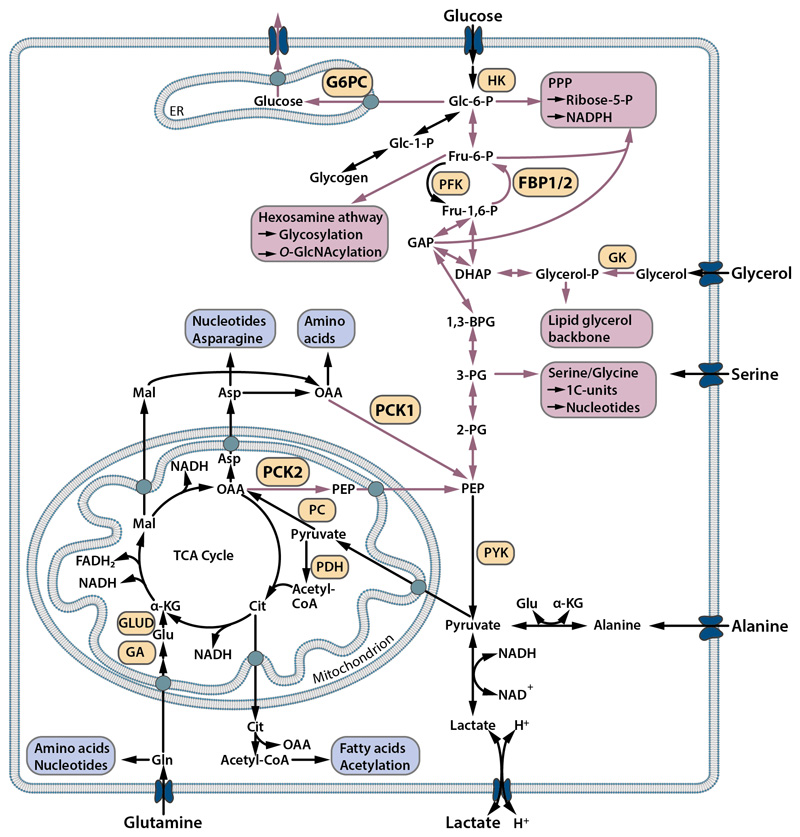
Fig. 2. Scheme of glycolysis, gluconeogenesis and branching biosynthetic pathways.
Gluconeogenesis, the formation of glucose from non-carbohydrate precursors such as amino acids or lactate, is in part the reversal of glycolysis. Phosphoenolpyruvate carboxykinase, which exists as a mitochondrial (PCK2) and a cytosolic isoform (PCK1), mediates the initial step of gluconeogenesis and links the TCA cycle (citric acid cycle, Krebs cycle) and glycolysis/gluconeogenesis. Further steps in gluconeogenesis (red arrows) are mediated by bi-directional glycolytic/gluconeogenesis enzymes and the downstream enzymes fructose-1,6-bisphosphatase (FBPase) and glucose-6-phosphatase (G6PC). Abbreviated gluconeogenesis allows the synthesis of cellular building blocks from non-carbohydrate precursors (red boxes) and requires the action of PCK1/2 and to some extent also FBP1/2. Biosynthetic pathways independent of gluconeogenesis are depicted in blue.
3. Physiological functions of gluconeogenesis enzymes
Gluconeogenesis is the synthesis of glucose from non-carbohydrate precursors. It primarily occurs in the liver, kidney, intestine and skeletal muscle [25,27]. Though many steps in gluconeogenesis are glycolytic reactions run in the reverse direction, three reactions are catalyzed exclusively by gluconeogenesis enzymes. The initial enzyme of gluconeogenesis, PEPCK, forms a bottle neck in cell metabolism linking the TCA cycle and glycolysis/gluconeogenesis (Fig. 2). Two isoforms of PEPCK exist, a cytosolic isoform, PCK1 (also known as PEPCK-C, PEPCK1), and a mitochondrial isoform, PCK2 (PEPCK-M, PEPCK2). PCK1 and PCK2 both catalyze the conversion of oxaloacetate (OAA) and GTP to phosphoenolpyruvate (PEP), CO2 and GDP [25,27]. OAA is generated either in the TCA cycle or by pyruvate carboxylase (PC), which mediates the conversion of pyruvate to OAA. OAA is exported from the mitochondria to the cytosol in the form of malate or aspartate [25,28] (Fig. 2). This is a pre-requisite for further conversion via PCK1, but not via PCK2. Further downstream, gluconeogenesis requires FBPase, which hydrolyzes fructose-1,6-bisphosphate into fructose-6-phosphate and Pi. The two isoforms of FBPase are known, liver FBPase (FBP1) and muscle FBPase (FBP2). The final step of gluconeogenesis is mediated by glucose-6-phosphatase (encoded by G6PC), which hydrolyzes glucose-6-phosphate to glucose and Pi [25] (Fig. 2). Although the role of PCK1 in gluconeogenesis is well known, it has only recently been demonstrated that PCK2 also contributes to gluconeogenesis. Adenoviral overexpression of PCK2 in liver-specific PCK1 knockout mice showed that PCK2 supports hepatic gluconeogenesis, but is less efficient than PCK1 [28]. Silencing of PCK2 with antisense oligonucleotides in rats reduced blood glucose levels, particularly in the fed state, and diminished gluconeogenesis from lactate or amino acids in isolated hepatocytes [29]. PCK1 and PCK2 mediate not only gluconeogenesis but also glyceroneogenesis, an abbreviated metabolic pathway for the generation of glycerol-3-phosphate from non-carbohydrate precursors. Glyceroneogenesis plays an important role in re-esterification of fatty acids in adipose tissue and in the liver [30].
Functionally linked to their downstream anabolic pathways, PCK1 and PCK2 also control the removal of TCA cycle anions (cataplerosis). If anaplerosis (entry of anions into the TCA cycle) is increased e.g. after conversion of glutamine to glutamate and finally to α-ketoglutarate (α-KG), the influx must be balanced by cataplerosis. Accordingly, PCK1 was shown to promote TCA cycle flux in the liver [31,32] and in the small intestine [33]. PCK2 was found to be involved in regulating TCA cycle flux in insulin-producing β-cells [34]. Immunohistochemical analysis of different human tissues suggests that PCK1 and FBP1 are quite broadly expressed [35], but the function of PCK1/2 or FBP1 in non-gluconeogenic or non-glyceroneogenic tissues remains still elusive. Interestingly, PCK1 overexpression in Caenorhabditis elegans extends lifespan [36], however the mechanism is not entirely clear.
It is well known that under glucose starvation, many cells can generate energy (ATP) from alternative fuels in the presence of functioning mitochondria, e.g. from ketone bodies or fatty acids. However, glycolytic intermediates are needed in highly proliferative cells that require ribose-5-phosphate to synthesize nucleotides or glycerol-3-phosphate to synthesize membrane glycerophospholipids [2,3]. Given the fact that gluconeogenic and glycolytic pathways share common intermediates, gluconeogenesis might potentially be an alternative source of biosynthetic precursors under glucose deprivation. It has long been recognized that gluconeogenesis is indispensable for growth of bacteria or yeast on non-fermentable (hexose sugar free) carbon sources [37,38]. Recently, our group and others have identified such an adaptive mechanism in certain tumor cells.
4. Role of PCK1/2 in cancer
Although gluconeogenesis enzymes were previously assumed to be absent from cancers not arising in gluconeogenic organs, several studies have demonstrated their functional expression in diverse cancers as mediators of abbreviated forms of gluconeogenesis. As will be outlined in detail, gluconeogenesis enzymes allow the synthesis of crucial intermediates and biomass in cancer cells under glucose deprivation, while they also regulate glycolysis and the TCA cycle.
4.1. PCK1 and PCK2 enhance metabolic flexibility in cancer cells
Elevated expression of the upstream gluconeogenesis enzyme PCK2 has been noted in the context of mutant KRAS in colon cancer cells and PCK2 was upregulated in colon carcinoma samples compared to normal colon tissue [39]. In a proteomics analysis PCK2 has been found to be highly elevated in brain metastatic cells derived from breast cancer compared to the parental breast cancer cells or bone metastatic cells [40]. The functional significance of PCK2 has not been analyzed in these studies. In 2014 our group reported that the gluconeogenic pathway is active in cancer cells not arising from a gluconeogenic organ [41]. We found that PCK2 mRNA expression and activity were increased in human lung cancer (NSCLC) samples compared to normal lung tissue, although PCK2 was also detectable in bronchial epithelial cells in normal lung, but not in alveolar cells [41]. Low glucose conditions led to upregulation of PCK2 expression and activity in lung cancer cell lines [41]. PCK1 was expressed only at very low levels. While a net production of lactate under high glucose medium was found in different NSCLC cell lines, there was a net consumption of lactate under low glucose conditions [41]. In fact, stable isotopic labeling showed that lactate was converted to PEP in glucose-starved cancer cells, confirming PCK2 activity in the direction of gluconeogenesis [41]. Silencing of PCK2 significantly compromised cancer cell survival under glucose deprivation in two of the three NSCLC cell lines. PCK1/2 inhibition further enhanced apoptosis in NSCLC cells growing as 3-dimensional spheroids, which are known to exhibit gradients for glucose and O2 [41].
Mendez-Lucas et al. [42] reported functional PCK2 expression in cancer cells and its regulation by stress pathways shortly thereafter. PCK2 was abundant in different cancer cell lines, while PCK1 expression was low [42]. KRAS transformed NIH-3T3 fibroblasts showed enhanced PCK2 mRNA compared to the non-tumorigenic parental cell line. In breast cancer cells, silencing of PCK2 slightly reduced glucose consumption, lactate production and proliferation under normal conditions. Apoptosis induction by glutamine deprivation or by endoplasmic reticulum (ER) stress was significantly increased [42]. Thus, PCK2 was identified as a component of the amino acid response and unfolded protein response in cancer cells.
In an unbiased metabolomics and gene expression analysis of lung cancer cells, PCK2-mediated gluconeogenesis and serine synthesis were found to be upregulated in glucosefree medium [43]. PCK2 silencing reduced proliferation of different NSCLC cell lines under these conditions [43]. Importantly, in two different NSCLC cell lines, PCK2 silencing clearly reduced growth of subcutaneous xenografts in mice [43]. PCK2 silencing prevented lung cancer xenografts from growing beyond microscopic size in vivo, as recently published [44]. In vitro analyses showed reduced colony forming ability of PCK2 silenced lung cancer cells under glucose- and serum starvation [44].
PCK1 was found to be expressed in the majority of colon cancers and moderate to high PCK1 immunohistochemistry scores were more frequent in colon carcinoma than in normal colon mucosa [45]. Silencing of PCK1 decreased proliferation and clonogenic growth of colon cancer cells in glucose-containing medium and clearly reduced colon cancer xenograft growth [45]. PCK1 was found to operate in both, the gluconeogenic or the reverse, anaplerotic direction, depending on the glucose concentration in the medium [45]. These properties of PCK1 may further enhance the metabolic flexibility of cancer cells. Full conversion of gluconeogenic precursors to glucose was not observed [45], which is not surprising since the production and release of glucose would be of no apparent benefit for the cancer cells. Interestingly, PCK1 silencing has been shown to reduce cancer growth in a drosophila brain tumor model that was rescued by increasing NAD+ or oxidizing cytosolic NADH [46].
4.2. Metabolic downstream pathways of PCK1 and PCK2 in cancer cells
Under glucose starvation, cancer cells appear to redistribute glycolytic/gluconeogenic intermediates to downstream pathways that are crucial for survival and/or proliferation. As expected, only a minor proportion of PEP generated by PCK2 was converted (back) to pyruvate and to the TCA cycle in glucose-deprived lung cancer cells [43]. Instead, serine and glycine were synthesized de novo [43]. Abbreviated gluconeogenesis via PCK2 generated 40% of cellular serine under glucose depletion in lung cancer cells [43]. Thus, a large proportion of serine may derive from gluconeogenesis in cancer cells. Amplification of the serine synthesis enzyme phosphoglycerate dehydrogenase and a dependency of cancer cells on de novo serine synthesis have been described in different cancers [47,48]. Serine is a precursor in numerous biosynthetic pathways, including the synthesis of glycine, cysteine and sphingosine [47–49]. Together with its downstream product glycine, serine fuels one-carbon metabolism, which is important for nucleotide synthesis and restoration of the NADPH/NADP+ ratio [49]. In fact, glutamine-derived carbons were detected in the purine nucleotide ATP in cells grown under glucose-free conditions, suggesting that PCK2 contributes to nucleotide synthesis via serine de novo formation and one-carbon metabolism [43].
Recently, our group has shown that PCK2 mediates glyceroneogenesis and thereby contributes to biomass synthesis in cancer cells under glucose deprivation [44]. Carbons derived from glutamine and lactate were transferred onto the glycerol backbone of glycerophospholipids (GPL) via PCK2 under low but not under high glucose conditions [44]. Importantly, PCK2 silencing led to a 30-50% reduction of absolute levels of the GPL phosphatidylethanolamine under glucose- and serum deprivation [44]. Colony formation was inhibited by different constructs of PCK2 shRNA under glucose starvation, and the effect was partly reversed by exogenous phosphatidylethanolamine [44]. These results show that glyceroneogenesis via PCK2 is an important pathway necessary for de novo GPL synthesis and GPL homeostasis in glucose-deprived cancer cells.
The PPP generates ribose-5-phosphate for nucleotide synthesis and/or NADPH, required for cellular antioxidant functions. A series of stable isotopic tracer experiments demonstrated that in PCK1 positive colon cancer cells, there is carbon flow from glutamine via the TCA cycle to (RNA) ribose, which was enhanced by low glucose [45]. Similarly, ribose-5-phosphate generation from 13C-glutamine was found in glucose-deprived metastatic breast cancer cells, mediated by FBP2 [50]. The hexosamine pathway, which branches from fructose-6-phosphate, generates N-acetylglucosamine (GlcNAc), an intermediate required for a posttranslational modification (O-GlcNAcylation) as well as protein glycosylation [51]. O-GlcNAcylation, which is increased in cancer cells, has been linked to nutrient sensing and is required for signaling and transcription [51]. Whether gluconeogenesis contributes to maintaining the hexosamine pathway in cancer cells remains unknown. Together these studies show that metabolic flexibility of cancer cells is highly increased by PCK1/2 and suggest that PCK1 or PCK2 are required in different cancers to maintain the levels of precursors for biomass production under glucose deprivation (Fig. 3).
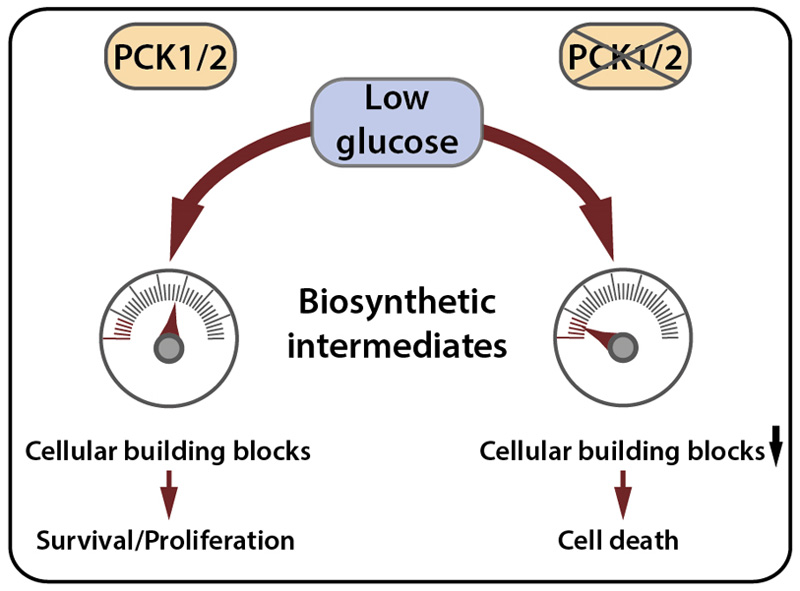
Fig. 3. PCK1/2 confer metabolic plasticity under glucose starvation.
PCK1/2 allow numerous biosynthetic pathways (the generation of serine/glycine, glycerol-3-phosphate or ribose-5-phosphate) to be fed from alternative nutrients under glucose starvation. If PCK1 or PCK2 are absent or silenced, gluconeogenesis is inhibited, resulting in a lack of cellular building blocks and decreased cell survival or proliferation, as has been shown in several studies on non-hepatic cancer cells.
4.3. Role of PCK1 and PCK2 beyond anabolism
Increased expression of PCK2 in neuroendocrine tumors of the pancreas compared to non-neoplastic islet cells and a proliferation promoting effect of PCK2 were found in pancreatic neuroendocrine cancer cells associated with increased expression of tRNA processing genes [52]. In prostate cancer, a higher expression level of PCK2 has been reported in metastases compared to primary tumors or normal prostate [53]. High PCK2 expression in prostate cancer tissue was associated with worse overall survival [53]. In this study prostate cancer tumor initiating cells (TIC) showed greatly elevated PCK2 expression compared to their non-stem cell like counterparts. PCK2 silencing reduced the proportion of TIC, diminished their sphere-forming ability and inhibited growth of prostate cancer nodules in vivo [53]. Glycolysis was decreased by PCK2 silencing, suggesting a role of PCK2 in promoting the glycolytic switch [53]. Interestingly, PCK2 silencing slightly enhanced survival of TIC in medium without glucose [53].
Similarly, an upregulation of PCK1 has been observed in tumor-repopulating (stem-cell like) cells from murine hepatocarcinoma (liver cancer), melanoma and lymphoma [54]. PCK1 expression promoted glycolysis and glucose consumption in melanoma tumor-repopulating cells [54]. Silencing of PCK1 in these cells reduced the capacity to form colonies in 3D fibrin gels in vitro, slowed nodule growth in the skin and impaired metastasis formation in the lungs [54]. However, a subsequent study by the same research group showed that PCK2 was rather reduced in melanoma tumor-repopulating cells in contrast to PCK1 [55]. Overexpression of PCK2 decreased glucose uptake, reduced the levels of fumarate, citrate and lipids in high glucose medium and reduced tumorigenesis in vivo and colony formation in vitro [55]. Thus, in some cancer cells PCK1/2 might play a role in regulating TCA cycle flux, both, under glucose starvation or normal glucose conditions.
4.5. Crosstalk of PCK1/2 with signaling and epigenetics
Metabolites are continuously sensed in cells and their abundance can greatly impact signaling and cell fate. Mechanistic target of rapamycin complex 1 (mTORC1) is one of the most important nutrient-regulated signaling nodes, which activates protein synthesis and cell growth if amino acids and glucose are available [56]. On the other hand, adenosine monophosphate-activated protein kinase (AMPK) is activated under low energy stress by an increase in AMP (adenosine monophosphate) and downregulates energy consuming processes, while activating autophagy [56]. Both, PCK1 and PCK2, have been shown to modulate cell signaling, which was at least partially attributable to alterations in metabolite levels. PCK1 overexpression in colon cancer cells led to an enhancement, while PCK1 silencing inhibited glucose uptake and lactate production [45]. The effect was due to enhanced mTORC1 activation by PCK1 following increased glutamine uptake [45]. In tumor-repopulating melanoma cells, overexpression of PCK2 decreased levels of fumarate under nutrient-replete conditions [55]. As a consequence, the stability of hypoxia-inducible factor 1α (HIF-1α) under hypoxia was decreased, since fumarate regulates prolyl hydroxylase activity [55]. Additionally, the reduction of fumarate by PCK2 overexpression altered DNA methylation due to modified activity of Ten-eleven translocation (TET) enzymes [55]. In gastric cancer cells, PCK1 enhanced extracellular signal-regulated kinase 1/2 activation and expression of matrix metalloproteinase 9 [57]. This effect was associated with an increased uptake of glucose and glutamine and with increased invasion and migration in normal glucose containing medium [57]. PCK2 has been found to have an interesting role in regulating protein acetylation in prostate TIC [53]. PCK2 silencing elevated acetyl-CoA levels due to enhanced production from citrate via ATP citrate lyase (ACLY), increasing histone and total protein lysine acetylation [53]. Importantly, reducing protein acetylation by inhibiting ACLY reversed the reduction in TIC numbers by PCK2 silencing, showing that PCK2 elevates TIC numbers in prostate cancer by suppressing protein acetylation [53].
4.6. PCK1/2 downregulation in liver and kidney cancer
In contrast to the predominant tumor-promoting role of PCK2 or PCK1 observed in non-hepatic cancers, PCK1 was downregulated in hepatocellular carcinoma (HCC) (Table 1). PCK1 overexpression induced apoptosis or inhibited proliferation and suppressed growth of HCC in vivo in different studies (Table 1). In one study, the ability to survive under low glucose conditions was decreased under forced PCK1 and partially under PCK2 overexpression, due to a considerable decrease in ATP levels, while effects under high glucose were quite variable [68]. These results are contradictory to the data obtained in different non-hepatic cancer cells and might be related to the reported pivotal role of PCK1 in regulating TCA cycle flux in the liver [31,32]. In clear cell renal cell carcinoma (ccRCC), the most common type of kidney cancer, PCK1 was decreased in several studies (Table 1). Thus, downregulation of gluconeogenesis enzymes appears to be advantageous for cancer development in gluconeogenic tissues and constitutes part of the metabolic rewiring necessary to support growth and proliferation; in contrast, abbreviated gluconeogenesis facilitates tumor cell proliferation and survival in cancers originating in non-gluconeogenic tissues.
Table 1. Tumor promoting and tumor suppressor activity of PCK1/2.
| I. Tumors originating in non-gluconeogenic tissues | ||
|---|---|---|
| Alteration in tumor tissue | Biological effect | |
| PCK1 | Primary tumors |
In
vitro
|
| PCK2 |
Primary tumors
|
In
vitro
|
| II. Liver and kidney cancer | ||
| Alteration in tumor tissue | Biological effect | |
| PCK1 | In vitro | |
| PCK2 |
|
|
5. Role of FBP1/2 and G6PC in cancer
The expression of the distal gluconeogenesis enzymes FBP1, FBP2 and G6PC is highly variable in tumors. FBP1 or FBP2 are frequently downregulated compared to normal tissue; however, in some contexts FBPase activity is protumorigenic. Here we summarize the present body of literature on the function of FBP1, FBP2 and G6PC in cancer, focusing on the distinction between enzymatic and non-enzymatic effects under different nutritional conditions.
5.1. FBP2 is required for glucose-independent growth in metastatic breast cancer cells
FBP2, the muscle isoform, was shown to be upregulated in human breast cancer brain metastases compared to paired samples of primary breast cancer [50]. It was enhanced in brain metastatic cancer cells, in contrast to the parental breast cancer cells and mediated de novo ribose-5-phosphate and purine synthesis from 13C-labeled glutamine under glucose limitation [50]. FBP2 silencing greatly compromised viability and proliferation of the metastatic cancer cells under glucose deprivation and delayed the growth of orthotopically implanted brain metastases in mice [50].
5.2. Tumor-suppressive function of FBP1
In contrast, numerous studies suggest a tumor-suppressive role of FBP1, and partly also FBP2 (summarized in Table 2). FBP1 expression was shown to be downregulated in different cancers and low FBP1 expression was frequently associated with poor overall survival (Table 2). FBP1 overexpression suppressed proliferation in HCC cells and other tumor types and significantly reduced tumor growth in vivo in numerous studies (Table 2). When the role of FBP1 was studied in vitro under nutrient-replete conditions, it was observed to inhibit glycolysis in different cancer cells, including breast cancer [71,72], pancreatic cancer [73,74], lung cancer [75,76] and HCC cells [77–79]. The reduction of proliferation by FBP1 appears to be related to the decrease in glucose uptake. Likewise, overexpression of FBP1 in ccRCC diminished proliferation and migration and led to a reduction of glycolysis [69]. Importantly, the metabolic and proliferation-promoting effects were partially independent of its enzymatic activity but mediated by the interaction of FBP1 with HIFs [69] (Fig. 4). A non-enzymatic function of FBP1 regulating extracellular signal-regulated kinase (ERK) signaling has been shown to be responsible for the suppression of growth of pancreatic cancer xenografts in vivo [74]. Besides inhibiting proliferation, FBP1 was shown to affect epithelial to mesenchymal transition (EMT), an important step in tumor metastasis. Restoration of FBP1 expression suppressed the EMT phenotype, migration and tumor growth in Snail overexpressing HCC cancer cells [80] and reduced EMT in gastric cancer cells [81]. Only limited knowledge exists on the role of FBP2 in cancer. In contrast to the tumor promoting role of FBP2 in metastatic breast cancer [50], FBP2 inhibited glycolysis and growth of gastric cancer cells [82].
Table 2. Tumor promoting and tumor suppressor activity of FBP1/2.
| I. Tumors originating in non-gluconeogenic tissues | ||
|---|---|---|
| Alteration in tumor tissue | Biological effect | |
| FBP1 |
Primary tumors
|
In
vitro
|
| FBP2 |
Primary tumors
|
In
vitro
|
| II. Liver and kidney cancer | ||
| Alteration in tumor tissue | Biological effect | |
| FBP1 |
In
vitro
|
|
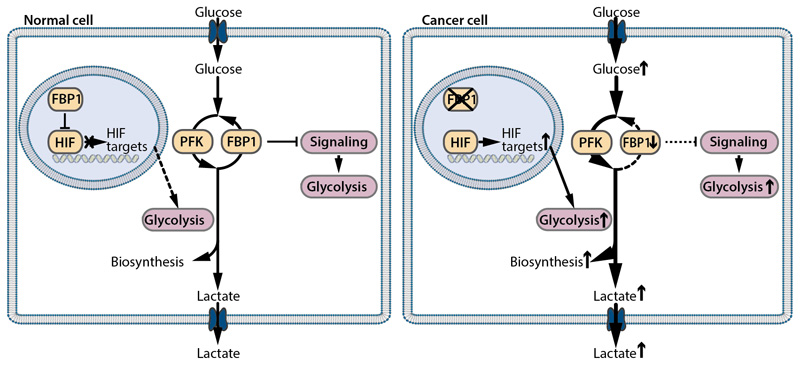
Fig. 4. Effects of FBP1 on glycolysis by canonical and non-canonical activities.
Besides its gluconeogenic function that opposes glycolysis (canonical function), FBP1 inhibits glycolytic gene expression by a direct action on different signaling pathways (non-canonical function). In kidney cancer cells FBP1 binds to HIF-1α or HIF-2α in the nucleus and reduces HIF-induced expression of glycolytic genes. In cancer cells, FBP1 expression is frequently decreased to promote glycolysis and the Warburg effect.
Together, these studies suggest that FBP1 and possibly also FBP2 act to inhibit glycolysis and may play a tumor-suppressive role in tumor cells, both via enzymatic and non-enzymatic mechanisms (Fig. 3). This is in contrast to the finding that FBP2 may be required to confer metabolic flexibility under glucose deprivation. Interestingly, FBP1 expression inhibited tumor growth also in cancer types that displayed growth enhancement by PCK2, e.g. in breast and lung cancer (Table 2). In the non-oxidative PPP, de novo synthesis of ribose-5-phosphate starts from both, fructose-6-phosphate and glyceraldehyde-3-phosphate (GAP) (Fig. 2). Thus, even in the absence of FBPase, abbreviated gluconeogenesis can contribute carbons to ribose-5-phosphate. Alternatively, nucleotide degradation via salvage pathways might ensure sufficient ribose-5-phosphate for biosynthetic purposes.
5.3. G6PC activity is linked to glycogen turnover in cancer cells
G6PC hydrolyzes glucose-6-phosphate and thus mediates the final step in gluconeogenesis but also in glycogen degradation e.g. in the liver. In HCC, G6PC expression [61–63] and activity [61] were reported to be downregulated compared with adjacent tumor-free tissues, and similar results were obtained in kidney cancer [63,69]. G6PC overexpression reduced HCC growth in mice in vivo [62]. These results are reminiscent of the findings on PCK1 in tumors arising in gluconeogenic tissues. In contrast, G6PC expression was enhanced in glioblastoma, a highly malignant brain cancer, compared to noncancerous human cortex [92]. TIC isolated from glioblastoma showed an upregulation of G6PC by the glycolysis inhibitor 2-desoxyglucose (2DG) [92]. G6PC silencing reduced proliferation and migration in glioblastoma cells and invasion in vivo, which was especially pronounced after 2DG treatment and recovery. Mechanistically, G6PC silencing led to glycogen accumulation and a reduced activation of AKT (protein kinase B) [92]. High G6PC expression was found to be associated with poor overall and disease-free survival in ovarian cancer [93]. Silencing or inhibition of G6PC in ovarian cancer cells led to reduced proliferation and invasion and an accumulation of glycogen in vitro [93]. In both studies a tumor-promoting role of G6PC has been observed in the presence of abundant glucose, suggesting a role of G6PC in glucose cycling (glycogen degradation) rather than in gluconeogenesis.
6. Regulation of gluconeogenesis enzyme expression in normal and cancer cells
The regulation of PCK1 expression in gluconeogenic tissues upon fasting, which has been studied for decades, has been reviewed in detail elsewhere [94,95]. In summary, glucagon and glucocorticoids are the best characterized activators of PCK1 transcription in the liver, while insulin inhibits PCK1 expression. Glucagon activates cAMP regulatory element-binding protein (CREB), which acts in concert with multiple co-activators to induce PCK1 [94,95]. Acting antagonistically, insulin leads to the recruitment of phosphoinositide 3-kinase (PI3K) to the plasma membrane upon binding to its receptor, which generates phosphatidylinositol-3,4,5-triphosphate (PIP3). PIP3 in turn inhibits different transcription factors via AKT, including forkhead box protein O1 (FoxO1) [94,95]. In tumor cells, PCK1 and PCK2 are rather regulated in a cell-autonomous manner than by insulin or glucagon. In HepG2 liver cancer cells, insulin had no effect on PCK1 expression [96]. Accordingly, levels of PCK1 or G6PC were unaffected by fasting in liver cancer tissue in mice, although an increase by exogenous glucocorticoids was observed, both in vivo and in vitro [62]. PCK1 levels and activity are also regulated by post-translational modification. The turnover of PCK1 protein has been found to be controlled by acetylation [97]. High glucose levels destabilize PCK1 by stimulating its acetylation which promotes its ubiquitinylation and subsequent degradation, while deacetylation e.g. by sirtuin 2 stabilizes PCK1 [97]. The different signaling pathways regulating expression of gluconeogenesis enzymes in cancer cells have been reviewed elsewhere in detail [26] and are briefly summarized here.
The tumor suppressor p53 activated the expression of PCK2 and G6PC in HepG2 liver cancer cells [98]. Contrary to these results, ectopic expression of p53 efficiently downregulated the expression of PCK1 and G6PC, by sirtuin 6-mediated nuclear exclusion of transcription factor FoxO1, in different cancer cell lines, and in mouse liver [99]. The effect of p53 on gluconeogenesis gene expression thus appears to be context-dependent. A posttranslational mechanism of PCK1 regulation has been described in HCC cells involving increased degradation via sumoylation [65]. PCK1 sumoylation was enhanced in HCC cells due to repressed Nur77 [65]. Notably, PCK1 and G6PC were downregulated in liver and kidney cancer cells, but not in a panel of tumor cell lines from other tissues, by mTORC2, one of the complex forming members of mTOR [63]. Silencing of the mitotic kinase polo-like kinase 1 (PLK1) dramatically reduced levels of PCK1 mRNA and protein in melanoma cells, while FBP1 expression was increased, suggesting that the two enzymes are not regulated in the same direction in certain cancer cell types [100].
In contrast to the large body of literature on the regulation of PCK1 expression, especially in liver cells and adipocytes, little is known about the regulation of PCK2. PCK2 was upregulated under low glucose conditions in different cancer cell lines [41–44]. Moreover, ER stress and glutamine deprivation increased PCK2 expression [42]. The upregulation of PCK2 by HIFs in lung cancer cells [43] points to a crosstalk of hypoxia-induced pathways and gluconeogenesis in cancer cells. More research is warranted to characterize the factors regulating the expression of PCK1 or PCK2 in cancer cells in vivo.
In an interesting study using tumor tissue derived from rapid autopsies, PCK1 and PC were found to be among the most highly upregulated genes in liver metastases from human pancreatic cancer compared to a standard reference tissue [58]. In contrast, PCK1 or PC mRNA were not elevated in primary pancreatic cancer [58]. PCK2 was also upregulated in liver metastases, but downregulated in skeletal muscle metastases [58]. These site-specific alterations of gluconeogenic gene expression in metastases, that have also been observed in breast cancer metastases [40,50] and prostate cancer metastases [53] (Tables 1 and 2), are of major interest. These results suggest that the specific environment at the metastatic site might contribute to the upregulation of gluconeogenic genes.
Physiologically, FBPase activity is tightly regulated by metabolic intermediates. FBP1 and FBP2 are inactivated by fructose-2,6-bisphosphate and AMP [101]. In different cancers, including gastric cancer [84], HCC [77,86], colon carcinoma [86], NSCLC [102] and breast cancer [103] hypermethylation of the FBP1 promoter decreased expression of FBP1. Similar findings have been obtained for FBP2 promoter hypermethylation in breast cancer [103]. In ccRCC [104] and HCC [77] there is also gene copy number loss. NSCLC cell lines showed reduced FBP1 expression, which was reversed by silencing of Zinc finger E-box-binding homeobox 1 (ZEB1) [75]. FBP1 protein may also be post-translationally regulated in cancer cells by ubiquitination and proteasomal degradation [90]. Interestingly, FBP1 was significantly downregulated following HRAS-induced transformation in NIH3T3 mouse fibroblasts [84]. The regulation of FBPase activity in the direction of gluconeogenesis and the modulation of FBP1 or FBP2 expression by the glucose availability in cancer cells is still unclear and should be addressed in future studies.
7. Present concepts
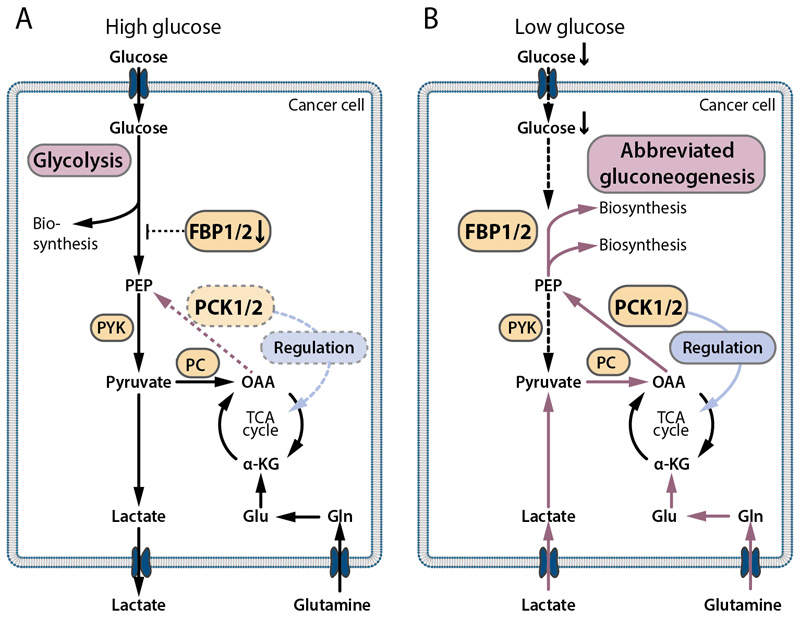
The studies discussed here show that gluconeogenesis enzymes exert context-dependent and highly important functions in cancer cells (Fig. 5). When glucose is abundant, most cancer cells are glycolytic and the suppression of FBP1 mediated inhibition of glycolysis is an advantage (Fig. 5A). Under these conditions PCK1/2 may play a role by regulating the flux through the TCA cycle in certain cancers (Fig. 5A). This cataplerotic function of PCK1/2 may be either tumor-promoting or tumor-suppressive, depending on the cancer type and context.
Fig. 5. Present concepts of gluconeogenesis in cancer cells under different microenvironmental conditions.
(A) Under high glucose supply, most cancer cells perform glycolysis for biosynthesis, lactate is released. In many cancers, this aerobic glycolysis is facilitated by a downregulation of FBP1, since FBP1 inhibits glycolysis and glycolytic gene expression via enzymatic and non-enzymatic mechanisms. In some tumors, PCK1 or PCK2 act under high or moderate glucose concentrations to regulate TCA cycle flux. (B) Under glucose deprivation, many cancer cells exhibit abbreviated forms of gluconeogenesis via PCK1 or PCK2 and to some extent FBPase, which allows feeding of biosynthetic pathways from lactate or glutamine, if glucose is missing. This promotes cancer cell survival in the hostile, glucose-deprived microenvironment of solid cancers. Additionally, PCK1/2 have a regulatory function on the TCA cycle flux. PCK1/2 and/or FBP1/2 expression occurs in a context-dependent manner and is highly heterogenous among different cancers.
In contrast, under low glucose conditions, PCK1 and PCK2 confer metabolic flexibility in different cancer types by allowing the synthesis of ribose-5-phosphate, glycerol-3-phosphate and serine and hence nucleotides and lipids from non-carbohydrate precursors (Fig. 5B). The preference for PCK1 or PCK2 in glucose-deprived cancer cells appears to be largely determined by the tissue of origin. An upregulation of PCK1/2 or FBP2 was found in metastases (Tables 1 and 2), which might be related to the nutrient-poor metabolic microenvironment in progressed cancers or to altered nutrient availability at the metastatic site. However, in some tumors, PCK1 or PCK2 are absent or expressed at very low levels. Alternative pathways or nutrient scavenging strategies might be activated in these cells, as will be discussed below. The metabolic pathways mediated by gluconeogenesis enzymes in cancer cells are reminiscent of the physiological abbreviated gluconeogenesis in adipocytes and liver cells (glyceroneogenesis). In cancer cells, however, abbreviated gluconeogenesis is not hormonally regulated but activated in a cell-autonomous manner, especially under low glucose conditions. In rapidly growing cancers like lung cancer, expression of PCK1 or PCK2 might be important for cancer growth, because of the high anabolic demand and frequently insufficient supply. Many different gluconeogenic precursors, like lactate, glutamine, and several other amino acids can serve as precursors for (abbreviated) gluconeogenesis in glucose-starved cancer cells in vivo, which leads to enormous versatility of PCK1/2 mediated anabolic pathways.
As already outlined in the review by Wang et al. [26], contradictory findings on PCK1/2 have been reported in cancers originating from gluconeogenic organs as opposed to non-gluconeogenic organs. Mostly anti-tumorigenic effects of PCK1 or PCK2 have been described in gluconeogenic organs, liver and kidney, while in cancers arising from non-gluconeogenic organs mostly tumor promoting effects have been found (Table 1). These controversial results may be related to the fact that the physiological function of PCK1 and PCK2 as gluconeogenesis enzymes is lost during carcinogenesis. HCC tissue and kidney cancer tissue do not contribute to maintaining blood glucose levels. Lower PEPCK activities have been measured in the context of abbreviated gluconeogenesis in tumor cells, than in starved mouse liver [41]. This indicates that physiological gluconeogenesis requires higher gluconeogenesis enzyme activities than abbreviated gluconeogenesis in tumor cells. Kidney cancers, especially ccRCC, are typically well perfused tumors due to constitutive HIF stabilization and angiogenesis [105]. Therefore, differences in tumor perfusion and the metabolic tumor microenvironment might also play a role. Moreover, metabolic requirements for growth might differ across different cancer types. For example, a downregulation of the serine synthesis pathway enzymes and an upregulation of pyruvate dehydrogenase, the enzyme diverting pyruvate away from the glycolytic pathway to form acetyl-CoA, have been noted in HCC [70], which is in contrast to other tumor types.
8. Open questions and future challenges
Inhibition of gluconeogenesis enzymes could potentially be exploited to prevent tumor cell adaptation to the nutrient-starved microenvironment. This model is supported by the observed decline in biosynthetic intermediates as well as end products, such as specific classes of phospholipids [44], in glucose-deprived cancer cells. The efficacy of gluconeogenesis inhibition, however, may likely depend on the extent of nutrient deprivation in the specific tumor and on the availability of non-carbohydrate precursors (lactate, amino acids). The existing literature suggests that a possible effect of gluconeogenesis enzyme inhibition might be context-related and highly dependent on the target. Especially the canonical and non-canonical functions of FBP1 as inhibitor of glycolysis and cell signaling must be considered. Conversely, any treatment options aiming at FBP1 re-expression in FBP1-low cancer might promote cancer cell metabolic plasticity and a potential growth advantage under starvation.
Robust anticancer effects upon silencing of PCK1 or PCK2 have been observed in different in vivo models of primary or metastatic non-hepatic cancers (Table 1), however data on the effect of inducible silencing or inhibition of these enzymes in already established tumors are missing. Moreover, the contribution of PCK1 or PCK2 to biosynthetic activities in cancer cells in their 3D context and tumor microenvironment is still poorly understood. In a study on glucose metabolism in patient-derived lung cancer xenografts, there was a considerable scrambling of the tracer (the appearance of intermediates with only partial labeling) at the level of the glycolytic intermediate fructose-6-phosphate [106]. This was suggested by the authors to implicate active gluconeogenesis in the tumor tissue [106]. However, the authors pointed out that the PPP could contribute to the 13C scrambling in sugar phosphates [106]. Lactate has been found to contribute to TCA cycle intermediates in different cancers [7,17,18]; however, a possible further conversion along the gluconeogenesis pathway is still elusive. When NSCLC patients or tumor-bearing mice were infused with 13C-lactate, 13C-lactate was in fact taken up by the tumors and converted to TCA cycle metabolites, but only a minor pool of 3-phosphoglycerate (3-PG) or PEP was labeled, likely from systemic gluconeogenesis [18]. According to the authors, the 3-PG and PEP label might have derived from labeled plasma glucose due to systemic gluconeogenesis [18]. While this study does not support a major contribution of gluconeogenesis from lactate in NSCLC, the utilization of glutamine, as suggested by several in vitro studies, has not been addressed. Future studies using different stable isotope labeled gluconeogenic precursors are warranted.
Alternative pathways feeding the pools of glycolytic/gluconeogenic intermediates besides gluconeogenesis might potentially play a role in glucose-deprived cancer cells. Fructose and galactose are less abundant in blood than glucose, however both may eventually enter glycolysis. Still, the availability of fructose may be high in some settings. A high fructose diet has recently been shown to enhance intestinal tumor growth in mice [107]. Fructose enhanced glucose uptake by the tumor cells, but it was also used as a fuel. When tumors where incubated in 13C-fructose in the absence of glucose ex vivo, a considerable conversion of fructose to glycolytic intermediates was found [107]. Recently, a novel metabolic pathway has been described in cancer cells, that leads to the generation of glycolytic intermediates from thymidine catabolism, similar to a known degradation pathway in prokaryotes [108]. These pathways may act in addition to gluconeogenesis to enhance metabolic plasticity in glucose deprived cancer cells. Nutrient scavenging via macropinocytoysis followed by digestion of the scavenged macromolecules is an important, oncogene-activated mechanism [109,110]. Many different components of the extracellular space can be scavenged, including albumin, as well as necrotic cell debris [111]. Macropinocytosis has been shown to enhance proliferation and survival of cancer cells under starvation and to be important for cancer growth in vivo [109,110]. It is unclear today, whether PCK1/2 activation and macropinocytosis represent different strategies of cancer cell adaptation to starvation or whether they act in concert. A recent study suggests that macropinocytosis-derived amino acids are precursors for gluconeogenesis in cancer cells [112].
Microvascular density [113] and high fluorodeoxyglucose positron emission tomography (FDG-PET) signals [114,115] have been shown to be correlated with a poor prognosis in different cancers. Although these findings do not support nutrient starvation as major driver of cancer progression, still the local or temporal adaptation to nutrient-poor conditions might be a prerequisite for metabolically highly active tumors to thrive. Abbreviated gluconeogenesis may be a survival promoting metabolic pathway in a subset of cancer cells that continue anabolic activity despite poor perfusion. Interestingly, stem-cell like tumor subpopulations and metastases showed high PCK1/2 expression across different tumor entities and gluconeogenesis promoted metastasis and colony forming capacities. To clarify, which intrinsic (cell-autonomous) or extrinsic (microenvironment-driven) signals regulate gluconeogenesis in different cancer types is of major interest and should be addressed in future studies. Moreover, it is unclear, how the gluconeogenic phenotype is linked to the glycolytic switch, and which intratumoral subpopulations, e.g. invasive versus proliferative cells preferentially utilize this pathway.
In the hostile tumor microenvironment, tumor cells and tumor-infiltrating immune cells compete for nutrients [116] and anti-tumor effector functions might potentially be influenced by the activities of gluconeogenesis enzymes in the immune cells as well. Little is known about the abundance and function of the different gluconeogenesis enzymes in tumor-infiltrating immune cells. Overexpression of FBP1 in natural killer cells inhibited their tumoricidal activity [117]. In contrast, abrogation of PCK1 dependent PPP decreased levels of glutathione, increased levels of reactive oxygen species and depleted CD8+ memory T-cells [118]. Lactate released from colon carcinoma cells induced PCK1 in monocytes [119]. It will be important to determine the role of gluconeogenesis enzymes not only in the tumor cells, but also in the immune cells.
In summary, a growing body of literature on gluconeogenesis in cancer shows that tumor cells exploit or regulate gluconeogenesis enzymes that also have distinct functions in normal cells, allowing growth and survival in their specific metabolic microenvironment. Gluconeogenesis enzymes, which mediate important anabolic biosynthetic pathways and regulate multiple cellular functions, thus might represent interesting therapeutic targets for cancer therapy.
Acknowledgements
We would like to thank Eugenia Lamont, Medical University of Graz, for carefully proof-reading the manuscript.
Funding
This work was supported by the Austrian Science Fund (FWF) [grant number P 28692-B31].
Abbreviations
- 1,3-BPG
1,3-bisphosphoglycerate
- 2-PG
2-phosphoglycerate
- 3-PG
3-phosphoglycerate
- α-KG
α-ketoglutarate
- AKT
protein kinase B
- AMP
adenosine monophosphate
- ATP
adenosine triphosphate
- Asp
aspartate
- cAMP
cyclic adenosine monophosphate
- ccRCC
clear cell renal cell carcinoma
- Cit
citrate
- DHAP
dihydroxyacetone phosphate
- EMT
epithelial to mesenchymal transition
- ER
endoplasmic reticulum
- FADH2
reduced flavin adenine dinucleotide
- FBPase
fructose-1,6-bisphosphatase
- FBP1
liver FBPase
- FBP2
muscle FBPase
- FOXO1
forkhead box protein O1
- Fru-1,6-P
fructose-1,6-bisphosphate
- Fru-6-P
fructose-6-phosphate
- G6PC
glucose-6-phosphatase
- GA
glutaminase
- GAP
glyceraldehyde-3-phosphate
- GK
glycerol kinase
- Glc-1-P
glucose-1-phosphate
- Glc-6-P
glucose-6-phosphate
- Gln
glutamine
- Glu
glutamate
- GLUD
glutamate dehydrogenase
- GPL
glycerophospholipid
- HCC
hepatocellular carcinoma
- HIF
hypoxia-inducible factor
- HK
hexokinase
- Mal
malate
- mTORC
mechanistic target of rapamycin complex
- NADH
reduced nicotinamide adenine dinucleotide
- NADPH
reduced nicotinamide adenine dinucleotide phosphate
- NSCLC
non-small cell lung cancer
- OAA
oxaloacetate
- O-GlcNAc
O-linked N-acetylglucosamine
- PC
pyruvate carboxylase
- PCK1
cytosolic isoform of PEPCK
- PCK2
mitochondrial isoform of PEPCK
- PDH
pyruvate dehydrogenase
- PEP
phosphoenolpyruvate
- PEPCK
phosphoenolpyruvate carboxykinase
- PFK
phosphofructokinase
- PYK
pyruvate kinase
- PPP
pentose phosphate pathway
- TCA cycle
tricarboxylic acid cycle
Footnotes
Declarations of interest
None.
References
- [1].Warburg O, Posener K, Negelein E. On the metabolism of carcinoma cells. Biochem Zeitschrift. 1924;152:309–344. [Google Scholar]
- [2].Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Pavlova NN, Thompson CB. The emerging hallmarks of cancer metabolism. Cell Metab. 2016;23:27–47. doi: 10.1016/j.cmet.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Martin JD, Fukumura D, Duda DG, Boucher Y, Jain RK. Reengineering the tumor microenvironment to alleviate hypoxia and overcome cancer heterogeneity. Cold Spring Harb Perspect Med. 2016;6 doi: 10.1101/cshperspect.a027094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Voutouri C, Kirkpatrick ND, Chung E, Mpekris F, Baish JW, Munn LL, Fukumura D, Stylianopoulos T, Jain RK. Experimental and computational analyses reveal dynamics of tumor vessel cooption and optimal treatment strategies. Proc Natl Acad Sci U S A. 2019;116:2662–2671. doi: 10.1073/pnas.1818322116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Vaupel P. Tumor microenvironmental physiology and its implications for radiation oncology. Semin Radiat Oncol. 2004;14:198–206. doi: 10.1016/j.semradonc.2004.04.008. [DOI] [PubMed] [Google Scholar]
- [7].Hensley CT, Faubert B, Yuan Q, Lev-Cohain N, Jin E, Kim J, Jiang L, Ko B, Skelton R, Loudat L, Wodzak M, et al. Metabolic heterogeneity in human lung tumors. Cell. 2016;164:681–694. doi: 10.1016/j.cell.2015.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hirayama A, Kami K, Sugimoto M, Sugawara M, Toki N, Onozuka H, Kinoshita T, Saito N, Ochiai A, Tomita M, Esumi H, et al. Quantitative metabolome profiling of colon and stomach cancer microenvironment by capillary electrophoresis time-of-flight mass spectrometry. Cancer Res. 2009;69:4918–4925. doi: 10.1158/0008-5472.CAN-08-4806. [DOI] [PubMed] [Google Scholar]
- [9].Rocha CM, Barros AS, Gil AM, Goodfellow BJ, Humpfer E, Spraul M, Carreira IM, Melo JB, Bernardo J, Gomes A, Sousa V, et al. Metabolic profiling of human lung cancer tissue by 1H high resolution magic angle spinning (HRMAS) NMR spectroscopy. J Proteome Res. 2010;9:319–332. doi: 10.1021/pr9006574. [DOI] [PubMed] [Google Scholar]
- [10].Duarte IF, Rocha CM, Barros AS, Gil AM, Goodfellow BJ, Carreira IM, Bernardo J, Gomes A, Sousa V, Carvalho L. Can nuclear magnetic resonance (NMR) spectroscopy reveal different metabolic signatures for lung tumours? Virchows Arch. 2010;457:715–725. doi: 10.1007/s00428-010-0993-6. [DOI] [PubMed] [Google Scholar]
- [11].Ziebart T, Walenta S, Kunkel M, Reichert TE, Wagner W, Mueller-Klieser W. Metabolic and proteomic differentials in head and neck squamous cell carcinomas and normal gingival tissue. J Cancer Res Clin Oncol. 2011;137:193–199. doi: 10.1007/s00432-010-0875-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Urasaki Y, Heath L, Xu CW. Coupling of glucose deprivation with impaired histone H2B monoubiquitination in tumors. PLoS One. 2012;7:e36775. doi: 10.1371/journal.pone.0036775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wang L, Chen J, Chen L, Deng P, Bu Q, Xiang P, Li M, Lu W, Xu Y, Lin H, Wu T, et al. 1H-NMR based metabonomic profiling of human esophageal cancer tissue. Mol Cancer. 2013;12 doi: 10.1186/1476-4598-12-25. 25-4598-12-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Rocha CM, Barros AS, Goodfellow BJ, Carreira IM, Gomes A, Sousa V, Bernardo J, Carvalho L, Gil AM, Duarte IF. NMR metabolomics of human lung tumours reveals distinct metabolic signatures for adenocarcinoma and squamous cell carcinoma. Carcinogenesis. 2015;36:68–75. doi: 10.1093/carcin/bgu226. [DOI] [PubMed] [Google Scholar]
- [15].Sonveaux P, Vegran F, Schroeder T, Wergin MC, Verrax J, Rabbani ZN, De Saedeleer CJ, Kennedy KM, Diepart C, Jordan BF, Kelley MJ, et al. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest. 2008;118:3930–3942. doi: 10.1172/JCI36843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Richtsmeier WJ, Dauchy R, Sauer LA. In vivo nutrient uptake by head and neck cancers. Cancer Res. 1987;47:5230–5233. [PubMed] [Google Scholar]
- [17].Hui S, Ghergurovich JM, Morscher RJ, Jang C, Teng X, Lu W, Esparza LA, Reya T, Le Z, Yanxiang Guo J, White E, et al. Glucose feeds the TCA cycle via circulating lactate. Nature. 2017;551:115–118. doi: 10.1038/nature24057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Faubert B, Li KY, Cai L, Hensley CT, Kim J, Zacharias LG, Yang C, Do QN, Doucette S, Burguete D, Li H, et al. Lactate metabolism in human lung tumors. Cell. 2017;171:358–371.e9. doi: 10.1016/j.cell.2017.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, Thompson CB. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci U S A. 2007;104:19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wise DR, DeBerardinis RJ, Mancuso A, Sayed N, Zhang XY, Pfeiffer HK, Nissim I, Daikhin E, Yudkoff M, McMahon SB, Thompson CB. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci U S A. 2008;105:18782–18787. doi: 10.1073/pnas.0810199105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Comerford SA, Huang Z, Du X, Wang Y, Cai L, Witkiewicz AK, Walters H, Tantawy MN, Fu A, Manning HC, Horton JD, et al. Acetate dependence of tumors. Cell. 2014;159:1591–1602. doi: 10.1016/j.cell.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Mashimo T, Pichumani K, Vemireddy V, Hatanpaa KJ, Singh DK, Sirasanagandla S, Nannepaga S, Piccirillo SG, Kovacs Z, Foong C, Huang Z, et al. Acetate is a bioenergetic substrate for human glioblastoma and brain metastases. Cell. 2014;159:1603–1614. doi: 10.1016/j.cell.2014.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Schug ZT, Peck B, Jones DT, Zhang Q, Grosskurth S, Alam IS, Goodwin LM, Smethurst E, Mason S, Blyth K, McGarry L, et al. Acetyl-CoA synthetase 2 promotes acetate utilization and maintains cancer cell growth under metabolic stress. Cancer Cell. 2015;27:57–71. doi: 10.1016/j.ccell.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Nieman KM, Kenny HA, Penicka CV, Ladanyi A, Buell-Gutbrod R, Zillhardt MR, Romero IL, Carey MS, Mills GB, Hotamisligil GS, Yamada SD, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17:1498–1503. doi: 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Berg JM, Tymoczko JL, Stryer L. Glycolysis and gluconeogenesis. In: Freeman WH, editor. Biochemistry. W.H. Freeman and Company; New York: 2012. pp. 469–514. [Google Scholar]
- [26].Wang Z, Dong C. Gluconeogenesis in cancer: function and regulation of PEPCK, FBPase, and G6Pase. Trends Cancer. 2019;5:30–45. doi: 10.1016/j.trecan.2018.11.003. [DOI] [PubMed] [Google Scholar]
- [27].Beale EG, Harvey BJ, Forest C. PCK1 and PCK2 as candidate diabetes and obesity genes. Cell Biochem Biophys. 2007;48:89–95. doi: 10.1007/s12013-007-0025-6. [DOI] [PubMed] [Google Scholar]
- [28].Mendez-Lucas A, Duarte JA, Sunny NE, Satapati S, He T, Fu X, Bermudez J, Burgess SC, Perales JC. PEPCK-M expression in mouse liver potentiates, not replaces, PEPCK-C mediated gluconeogenesis. J Hepatol. 2013;59:105–113. doi: 10.1016/j.jhep.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Stark R, Guebre-Egziabher F, Zhao X, Feriod C, Dong J, Alves TC, Ioja S, Pongratz RL, Bhanot S, Roden M, Cline GW, et al. A role for mitochondrial phosphoenolpyruvate carboxykinase (PEPCK-M) in the regulation of hepatic gluconeogenesis. J Biol Chem. 2014;289:7257–7263. doi: 10.1074/jbc.C113.544759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hanson RW, Reshef L. Glyceroneogenesis revisited. Biochimie. 2003;85:1199–1205. doi: 10.1016/j.biochi.2003.10.022. [DOI] [PubMed] [Google Scholar]
- [31].Hakimi P, Johnson MT, Yang J, Lepage DF, Conlon RA, Kalhan SC, Reshef L, Tilghman SM, Hanson RW. Phosphoenolpyruvate carboxykinase and the critical role of cataplerosis in the control of hepatic metabolism. Nutr Metab (Lond) 2005;2 doi: 10.1186/1743-7075-2-33. 33-7075-2-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Burgess SC, He T, Yan Z, Lindner J, Sherry AD, Malloy CR, Browning JD, Magnuson MA. Cytosolic phosphoenolpyruvate carboxykinase does not solely control the rate of hepatic gluconeogenesis in the intact mouse liver. Cell Metab. 2007;5:313–320. doi: 10.1016/j.cmet.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Potts A, Uchida A, Deja S, Berglund ED, Kucejova B, Duarte JAG, Fu X, Browning JD, Magnuson MA, Burgess SC. Cytosolic phosphoenolpyruvate carboxykinase as a cataplerotic pathway in the small intestine. Am J Physiol Gastrointest Liver Physiol. 2018;315:G249–G258. doi: 10.1152/ajpgi.00039.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Stark R, Pasquel F, Turcu A, Pongratz RL, Roden M, Cline GW, Shulman GI, Kibbey RG. Phosphoenolpyruvate cycling via mitochondrial phosphoenolpyruvate carboxykinase links anaplerosis and mitochondrial GTP with insulin secretion. J Biol Chem. 2009;284:26578–26590. doi: 10.1074/jbc.M109.011775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Yanez AJ, Nualart F, Droppelmann C, Bertinat R, Brito M, Concha II, Slebe JC. Broad expression of fructose-1,6-bisphosphatase and phosphoenolpyruvate carboxykinase provide evidence for gluconeogenesis in human tissues other than liver and kidney. J Cell Physiol. 2003;197:189–197. doi: 10.1002/jcp.10337. [DOI] [PubMed] [Google Scholar]
- [36].Yuan Y, Hakimi P, Kao C, Kao A, Liu R, Janocha A, Boyd-Tressler A, Hang X, Alhoraibi H, Slater E, Xia K, et al. Reciprocal changes in phosphoenolpyruvate carboxykinase and pyruvate kinase with age are a determinant of aging in Caenorhabditis elegans. J Biol Chem. 2016;291:1307–1319. doi: 10.1074/jbc.M115.691766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Fraenkel DG, Horecker BL. Fructose-1, 6-diphosphatase and acid hexose phosphatase of Escherichia coli. J Bacteriol. 1965;90:837–842. doi: 10.1128/jb.90.4.837-842.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Valdes-Hevia MD, de la Guerra R, Gancedo C. Isolation and characterization of the gene encoding phosphoenolpyruvate carboxykinase from Saccharomyces cerevisiae. FEBS Lett. 1989;258:313–316. doi: 10.1016/0014-5793(89)81682-5. [DOI] [PubMed] [Google Scholar]
- [39].Chun SY, Johnson C, Washburn JG, Cruz-Correa MR, Dang DT, Dang LH. Oncogenic KRAS modulates mitochondrial metabolism in human colon cancer cells by inducing HIF-1alpha and HIF-2alpha target genes. Mol Cancer. 2010;9 doi: 10.1186/1476-4598-9-293. 293-4598-9-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Chen EI, Hewel J, Krueger JS, Tiraby C, Weber MR, Kralli A, Becker K, Yates JR, 3rd, Felding-Habermann B. Adaptation of energy metabolism in breast cancer brain metastases. Cancer Res. 2007;67:1472–1486. doi: 10.1158/0008-5472.CAN-06-3137. [DOI] [PubMed] [Google Scholar]
- [41].Leithner K, Hrzenjak A, Trotzmuller M, Moustafa T, Kofeler HC, Wohlkoenig C, Stacher E, Lindenmann J, Harris AL, Olschewski A, Olschewski H. PCK2 activation mediates an adaptive response to glucose depletion in lung cancer. Oncogene. 2015;34:1044–1050. doi: 10.1038/onc.2014.47. [DOI] [PubMed] [Google Scholar]
- [42].Mendez-Lucas A, Hyrossova P, Novellasdemunt L, Vinals F, Perales JC. Mitochondrial phosphoenolpyruvate carboxykinase (PEPCK-M) is a pro-survival, endoplasmic reticulum (ER) stress response gene involved in tumor cell adaptation to nutrient availability. J Biol Chem. 2014;289:22090–22102. doi: 10.1074/jbc.M114.566927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Vincent EE, Sergushichev A, Griss T, Gingras MC, Samborska B, Ntimbane T, Coelho PP, Blagih J, Raissi TC, Choiniere L, Bridon G, et al. Mitochondrial phosphoenolpyruvate carboxykinase regulates metabolic adaptation and enables glucose-independent tumor growth. Mol Cell. 2015;60:195–207. doi: 10.1016/j.molcel.2015.08.013. [DOI] [PubMed] [Google Scholar]
- [44].Leithner K, Triebl A, Trotzmuller M, Hinteregger B, Leko P, Wieser BI, Grasmann G, Bertsch AL, Zullig T, Stacher E, Valli A, et al. The glycerol backbone of phospholipids derives from noncarbohydrate precursors in starved lung cancer cells. Proc Natl Acad Sci USA. 2018;115:6225–6230. doi: 10.1073/pnas.1719871115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Montal ED, Dewi R, Bhalla K, Ou L, Hwang BJ, Ropell AE, Gordon C, Liu WJ, DeBerardinis RJ, Sudderth J, Twaddel W, et al. PEPCK coordinates the regulation of central carbon metabolism to promote cancer cell growth. Mol Cell. 2015;60:571–583. doi: 10.1016/j.molcel.2015.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hussain R, Shaukat Z, Khan M, Saint R, Gregory SL. Phosphoenolpyruvate carboxykinase maintains glycolysis-driven growth in Drosophila tumors. Sci Rep. 2017;7 doi: 10.1038/s41598-017-11613-2. 11531-017-11613-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Possemato R, Marks KM, Shaul YD, Pacold ME, Kim D, Birsoy K, Sethumadhavan S, Woo HK, Jang HG, Jha AK, Chen WW, et al. Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature. 2011;476:346–350. doi: 10.1038/nature10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Locasale JW, Grassian AR, Melman T, Lyssiotis CA, Mattaini KR, Bass AJ, Heffron G, Metallo CM, Muranen T, Sharfi H, Sasaki AT, et al. Phosphoglycerate dehydrogenase diverts glycolytic flux and contributes to oncogenesis. Nat Genet. 2011;43:869–874. doi: 10.1038/ng.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Locasale JW. Serine, glycine and one-carbon units: cancer metabolism in full circle. Nat Rev Cancer. 2013;13:572–583. doi: 10.1038/nrc3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Chen J, Lee HJ, Wu X, Huo L, Kim SJ, Xu L, Wang Y, He J, Bollu LR, Gao G, Su F, et al. Gain of glucose-independent growth upon metastasis of breast cancer cells to the brain. Cancer Res. 2015;75:554–565. doi: 10.1158/0008-5472.CAN-14-2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Ma Z, Vosseller K. Cancer metabolism and elevated O-GlcNAc in oncogenic signaling. J Biol Chem. 2014;289:34457–34465. doi: 10.1074/jbc.R114.577718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Chu PY, Jiang SS, Shan YS, Hung WC, Chen MH, Lin HY, Chen YL, Tsai HJ, Chen LT. Mitochondrial phosphoenolpyruvate carboxykinase (PEPCK-M) regulates the cell metabolism of pancreatic neuroendocrine tumors (pNET) and de-sensitizes pNET to mTOR inhibitors. Oncotarget. 2017;8:103613–103625. doi: 10.18632/oncotarget.21665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Zhao J, Li J, Fan TWM, Hou SX. Glycolytic reprogramming through PCK2 regulates tumor initiation of prostate cancer cells. Oncotarget. 2017;8:83602–83618. doi: 10.18632/oncotarget.18787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Li Y, Luo S, Ma R, Liu J, Xu P, Zhang H, Tang K, Ma J, Zhang Y, Liang X, Sun Y, et al. Upregulation of cytosolic phosphoenolpyruvate carboxykinase is a critical metabolic event in melanoma cells that repopulate tumors. Cancer Res. 2015;75:1191–1196. doi: 10.1158/0008-5472.CAN-14-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Luo S, Li Y, Ma R, Liu J, Xu P, Zhang H, Tang K, Ma J, Liu N, Zhang Y, Sun Y, et al. Downregulation of PCK2 remodels tricarboxylic acid cycle in tumor-repopulating cells of melanoma. Oncogene. 2017;36:3609–3617. doi: 10.1038/onc.2016.520. [DOI] [PubMed] [Google Scholar]
- [56].Efeyan A, Comb WC, Sabatini DM. Nutrient-sensing mechanisms and pathways. Nature. 2015;517:302–310. doi: 10.1038/nature14190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Li Y, Zhang M, Dorfman RG, Pan Y, Tang D, Xu L, Zhao Z, Zhou Q, Zhou L, Wang Y, Yin Y, et al. SIRT2 Promotes the migration and invasion of gastric cancer through RAS/ERK/JNK/MMP-9 pathway by increasing PEPCK1-related metabolism. Neoplasia. 2018;20:745–756. doi: 10.1016/j.neo.2018.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Chaika NV, Yu F, Purohit V, Mehla K, Lazenby AJ, DiMaio D, Anderson JM, Yeh JJ, Johnson KR, Hollingsworth MA, Singh PK. Differential expression of metabolic genes in tumor and stromal components of primary and metastatic loci in pancreatic adenocarcinoma. PLoS One. 2012;7:e32996. doi: 10.1371/journal.pone.0032996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Fernandez-Coto DL, Gil J, Hernandez A, Herrera-Goepfert R, Castro-Romero I, Hernandez-Marquez E, Arenas-Linares AS, Calderon-Sosa VT, Sanchez-Aleman MA, Mendez-Tenorio A, Encarnacion-Guevara S, et al. Quantitative proteomics reveals proteins involved in the progression from non-cancerous lesions to gastric cancer. J Proteomics. 2018;186:15–27. doi: 10.1016/j.jprot.2018.07.013. [DOI] [PubMed] [Google Scholar]
- [60].Park JW, Kim SC, Kim WK, Hong JP, Kim KH, Yeo HY, Lee JY, Kim MS, Kim JH, Yang SY, Kim DY, et al. Expression of phosphoenolpyruvate carboxykinase linked to chemoradiation susceptibility of human colon cancer cells. BMC Cancer. 2014;14 doi: 10.1186/1471-2407-14-160. 160-2407-14-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Wang B, Hsu SH, Frankel W, Ghoshal K, Jacob ST. Stat3-mediated activation of microRNA-23a suppresses gluconeogenesis in hepatocellular carcinoma by downregulating glucose-6-phosphatase and peroxisome proliferator-activated receptor gamma, coactivator 1 alpha. Hepatology. 2012;56:186–197. doi: 10.1002/hep.25632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Ma R, Zhang W, Tang K, Zhang H, Zhang Y, Li D, Li Y, Xu P, Luo S, Cai W, Ji T, et al. Switch of glycolysis to gluconeogenesis by dexamethasone for treatment of hepatocarcinoma. Nat Commun. 2013;4 doi: 10.1038/ncomms3508. 2508. [DOI] [PubMed] [Google Scholar]
- [63].Khan MW, Biswas D, Ghosh M, Mandloi S, Chakrabarti S, Chakrabarti P. mTORC2 controls cancer cell survival by modulating gluconeogenesis. Cell Death Discov. 2015;1:15016. doi: 10.1038/cddiscovery.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Shi H, Fang R, Li Y, Li L, Zhang W, Wang H, Chen F, Zhang S, Zhang X, Ye L. The oncoprotein HBXIP suppresses gluconeogenesis through modulating PCK1 to enhance the growth of hepatoma cells. Cancer Lett. 2016;382:147–156. doi: 10.1016/j.canlet.2016.08.025. [DOI] [PubMed] [Google Scholar]
- [65].Bian XL, Chen HZ, Yang PB, Li YP, Zhang FN, Zhang JY, Wang WJ, Zhao WX, Zhang S, Chen QT, Zheng Y, et al. Nur77 suppresses hepatocellular carcinoma via switching glucose metabolism toward gluconeogenesis through attenuating phosphoenolpyruvate carboxykinase sumoylation. Nat Commun. 2017;8 doi: 10.1038/ncomms14420. 14420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Tuo L, Xiang J, Pan X, Gao Q, Zhang G, Yang Y, Liang L, Xia J, Wang K, Tang N. PCK1 Downregulation Promotes TXNRD1 Expression and Hepatoma Cell Growth via the Nrf2/Keap1 Pathway. Front Oncol. 2018;8:611. doi: 10.3389/fonc.2018.00611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Tang Y, Zhang Y, Wang C, Sun Z, Li L, Cheng S, Zhou W. Overexpression of PCK1 gene antagonizes hepatocellular carcinoma through the activation of gluconeogenesis and suppression of glycolysis pathways. Cell Physiol Biochem. 2018;47:344–355. doi: 10.1159/000489811. [DOI] [PubMed] [Google Scholar]
- [68].Liu MX, Jin L, Sun SJ, Liu P, Feng X, Cheng ZL, Liu WR, Guan KL, Shi YH, Yuan HX, Xiong Y. Metabolic reprogramming by PCK1 promotes TCA cataplerosis, oxidative stress and apoptosis in liver cancer cells and suppresses hepatocellular carcinoma. Oncogene. 2018;37:1637–1653. doi: 10.1038/s41388-017-0070-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Li B, Qiu B, Lee DS, Walton ZE, Ochocki JD, Mathew LK, Mancuso A, Gade TP, Keith B, Nissim I, Simon MC. Fructose-1,6-bisphosphatase opposes renal carcinoma progression. Nature. 2014;513:251–255. doi: 10.1038/nature13557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Gao Y, Wang X, Sang Z, Li Z, Liu F, Mao J, Yan D, Zhao Y, Wang H, Li P, Ying X, et al. Quantitative proteomics by SWATH-MS reveals sophisticated metabolic reprogramming in hepatocellular carcinoma tissues. Sci Rep. 2017;7 doi: 10.1038/srep45913. 45913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Dong C, Yuan T, Wu Y, Wang Y, Fan TW, Miriyala S, Lin Y, Yao J, Shi J, Kang T, Lorkiewicz P, et al. Loss of FBP1 by Snail-mediated repression provides metabolic advantages in basal-like breast cancer. Cancer Cell. 2013;23:316–331. doi: 10.1016/j.ccr.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Li K, Ying M, Feng D, Du J, Chen S, Dan B, Wang C, Wang Y. Fructose-1,6-bisphosphatase is a novel regulator of Wnt/beta-Catenin pathway in breast cancer. Biomed Pharmacother. 2016;84:1144–1149. doi: 10.1016/j.biopha.2016.10.050. [DOI] [PubMed] [Google Scholar]
- [73].Zhu Y, Shi M, Chen H, Gu J, Zhang J, Shen B, Deng X, Xie J, Zhan X, Peng C. NPM1 activates metabolic changes by inhibiting FBP1 while promoting the tumorigenicity of pancreatic cancer cells. Oncotarget. 2015;6:21443–21451. doi: 10.18632/oncotarget.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Jin X, Pan Y, Wang L, Ma T, Zhang L, Tang AH, Billadeau DD, Wu H, Huang H. Fructose-1,6-bisphosphatase inhibits ERK activation and bypasses gemcitabine resistance in pancreatic cancer by blocking IQGAP1-MAPK interaction. Cancer Res. 2017;77:4328–4341. doi: 10.1158/0008-5472.CAN-16-3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Zhang J, Wang J, Xing H, Li Q, Zhao Q, Li J. Down-regulation of FBP1 by ZEB1-mediated repression confers to growth and invasion in lung cancer cells. Mol Cell Biochem. 2016;411:331–340. doi: 10.1007/s11010-015-2595-8. [DOI] [PubMed] [Google Scholar]
- [76].Dai J, Ji Y, Wang W, Kim D, Fai LY, Wang L, Luo J, Zhang Z. Loss of fructose-1,6-bisphosphatase induces glycolysis and promotes apoptosis resistance of cancer stem-like cells: an important role in hexavalent chromium-induced carcinogenesis. Toxicol Appl Pharmacol. 2017;331:164–173. doi: 10.1016/j.taap.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Hirata H, Sugimachi K, Komatsu H, Ueda M, Masuda T, Uchi R, Sakimura S, Nambara S, Saito T, Shinden Y, Iguchi T, et al. Decreased expression of fructose-1,6-bisphosphatase associates with glucose metabolism and tumor progression in hepatocellular carcinoma. Cancer Res. 2016;76:3265–3276. doi: 10.1158/0008-5472.CAN-15-2601. [DOI] [PubMed] [Google Scholar]
- [78].Yang J, Wang C, Zhao F, Luo X, Qin M, Arunachalam E, Ge Z, Wang N, Deng X, Jin G, Cong W, et al. Loss of FBP1 facilitates aggressive features of hepatocellular carcinoma cells through the Warburg effect. Carcinogenesis. 2017;38:134–143. doi: 10.1093/carcin/bgw109. [DOI] [PubMed] [Google Scholar]
- [79].Yang J, Jin X, Yan Y, Shao Y, Pan Y, Roberts LR, Zhang J, Huang H, Jiang J. Inhibiting histone deacetylases suppresses glucose metabolism and hepatocellular carcinoma growth by restoring FBP1 expression. Sci Rep. 2017;7 doi: 10.1038/srep43864. 43864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Liu GM, Li Q, Zhang PF, Shen SL, Xie WX, Chen B, Wu J, Hu WJ, Huang XY, Peng BG. Restoration of FBP1 suppressed Snail-induced epithelial to mesenchymal transition in hepatocellular carcinoma. Cell Death Dis. 2018;9 doi: 10.1038/s41419-018-1165-x. 1132-018-1165-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Li J, Wang Y, Li QG, Xue JJ, Wang Z, Yuan X, Tong JD, Xu LC. Downregulation of FBP1 promotes tumor metastasis and indicates poor prognosis in gastric cancer via regulating epithelial-mesenchymal transition. PLoS One. 2016;11:e0167857. doi: 10.1371/journal.pone.0167857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Li H, Wang J, Xu H, Xing R, Pan Y, Li W, Cui J, Zhang H, Lu Y. Decreased fructose-1,6-bisphosphatase-2 expression promotes glycolysis and growth in gastric cancer cells. Mol Cancer. 2013;12 doi: 10.1186/1476-4598-12-110. 110-4598-12-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Zhao W, Yang S, Chen J, Zhao J, Dong J. Forced overexpression of FBP1 inhibits proliferation and metastasis in cholangiocarcinoma cells via Wnt/beta-catenin pathway. Life Sci. 2018;210:224–234. doi: 10.1016/j.lfs.2018.09.009. [DOI] [PubMed] [Google Scholar]
- [84].Liu X, Wang X, Zhang J, Lam EK, Shin VY, Cheng AS, Yu J, Chan FK, Sung JJ, Jin HC. Warburg effect revisited: an epigenetic link between glycolysis and gastric carcinogenesis. Oncogene. 2010;29:442–450. doi: 10.1038/onc.2009.332. [DOI] [PubMed] [Google Scholar]
- [85].Li H, Li M, Pang Y, Liu F, Sheng D, Cheng X. Fructose1,6bisphosphatase1 decrease may promote carcinogenesis and chemoresistance in cervical cancer. Mol Med Rep. 2017;16:8563–8571. doi: 10.3892/mmr.2017.7665. [DOI] [PubMed] [Google Scholar]
- [86].Chen M, Zhang J, Li N, Qian Z, Zhu M, Li Q, Zheng J, Wang X, Shi G. Promoter hypermethylation mediated downregulation of FBP1 in human hepatocellular carcinoma and colon cancer. PLoS One. 2011;6:e25564. doi: 10.1371/journal.pone.0025564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Shi L, He C, Li Z, Wang Z, Zhang Q. FBP1 modulates cell metabolism of breast cancer cells by inhibiting the expression of HIF-1alpha. Neoplasma. 2017;64:535–542. doi: 10.4149/neo_2017_407. [DOI] [PubMed] [Google Scholar]
- [88].Liu Y, Jiang Y, Wang N, Jin Q, Ji F, Zhong C, Zhang Z, Yang J, Ye X, Chen T. Invalidation of mitophagy by FBP1-mediated repression promotes apoptosis in breast cancer. Tumour Biol. 2017;39 doi: 10.1177/1010428317708779. 1010428317708779. [DOI] [PubMed] [Google Scholar]
- [89].Ning XH, Li T, Gong YQ, He Q, Shen QI, Peng SH, Wang JY, Chen JC, Guo YL, Gong K. Association between FBP1 and hypoxia-related gene expression in clear cell renal cell carcinoma. Oncol Lett. 2016;11:4095–4098. doi: 10.3892/ol.2016.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Jin X, Pan Y, Wang L, Zhang L, Ravichandran R, Potts PR, Jiang J, Wu H, Huang H. MAGE-TRIM28 complex promotes the Warburg effect and hepatocellular carcinoma progression by targeting FBP1 for degradation. Oncogenesis. 2017;6:e312. doi: 10.1038/oncsis.2017.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Tuo L, Xiang J, Pan X, Hu J, Tang H, Liang L, Xia J, Hu Y, Zhang W, Huang A, Wang K, et al. PCK1 negatively regulates cell cycle progression and hepatoma cell proliferation via the AMPK/p27(Kip1) axis. J Exp Clin Cancer Res. 2019;38 doi: 10.1186/s13046-019-1029-y. 50-019-1029-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Abbadi S, Rodarte JJ, Abutaleb A, Lavell E, Smith CL, Ruff W, Schiller J, Olivi A, Levchenko A, Guerrero-Cazares H, Quinones-Hinojosa A. Glucose-6-phosphatase is a key metabolic regulator of glioblastoma invasion. Mol Cancer Res. 2014;12:1547–1559. doi: 10.1158/1541-7786.MCR-14-0106-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Guo T, Chen T, Gu C, Li B, Xu C. Genetic and molecular analyses reveal G6PC as a key element connecting glucose metabolism and cell cycle control in ovarian cancer. Tumour Biol. 2015;36:7649–7658. doi: 10.1007/s13277-015-3463-6. [DOI] [PubMed] [Google Scholar]
- [94].Yang J, Reshef L, Cassuto H, Aleman G, Hanson RW. Aspects of the control of phosphoenolpyruvate carboxykinase gene transcription. J Biol Chem. 2009;284:27031–27035. doi: 10.1074/jbc.R109.040535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Chakravarty K, Cassuto H, Reshef L, Hanson RW. Factors that control the tissue-specific transcription of the gene for phosphoenolpyruvate carboxykinase-C, Crit. Rev Biochem Mol Biol. 2005;40:129–154. doi: 10.1080/10409230590935479. [DOI] [PubMed] [Google Scholar]
- [96].Yeagley D, Moll J, Vinson CA, Quinn PG. Characterization of elements mediating regulation of phosphoenolpyruvate carboxykinase gene transcription by protein kinase A and insulin. Identification of a distinct complex formed in cells that mediate insulin inhibition. J Biol Chem. 2000;275:17814–17820. doi: 10.1074/jbc.M909842199. [DOI] [PubMed] [Google Scholar]
- [97].Jiang W, Wang S, Xiao M, Lin Y, Zhou L, Lei Q, Xiong Y, Guan KL, Zhao S. Acetylation regulates gluconeogenesis by promoting PEPCK1 degradation via recruiting the UBR5 ubiquitin ligase. Mol Cell. 2011;43:33–44. doi: 10.1016/j.molcel.2011.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Goldstein I, Yizhak K, Madar S, Goldfinger N, Ruppin E, Rotter V. P53 promotes the expression of gluconeogenesis-related genes and enhances hepatic glucose production. Cancer Metab. 2013;1 doi: 10.1186/2049-3002-1-9. 9-3002-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Zhang P, Tu B, Wang H, Cao Z, Tang M, Zhang C, Gu B, Li Z, Wang L, Yang Y, Zhao Y, et al. Tumor suppressor p53 cooperates with SIRT6 to regulate gluconeogenesis by promoting FoxO1 nuclear exclusion. Proc Natl Acad Sci U S A. 2014;111:10684–10689. doi: 10.1073/pnas.1411026111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Gutteridge RE, Singh CK, Ndiaye MA, Ahmad N. Targeted knockdown of polo-like kinase 1 alters metabolic regulation in melanoma. Cancer Lett. 2017;394:13–21. doi: 10.1016/j.canlet.2017.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Dzugaj A. Localization and regulation of muscle fructose-1,6-bisphosphatase, the key enzyme of glyconeogenesis. Adv Enzym Regul. 2006;46:51–71. doi: 10.1016/j.advenzreg.2006.01.021. [DOI] [PubMed] [Google Scholar]
- [102].Dong Y, Huaying S, Danying W, Chihong Z, Ruibin J, Xiaojiang S, Jianguo F. Significance of methylation of FBP1 gene in non-small cell lung cancer. Biomed Res Int. 2018;2018 doi: 10.1155/2018/3726091. 3726091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Bigl M, Jandrig B, Horn LC, Eschrich K. Aberrant methylation of human L- and M-fructose 1,6-bisphosphatase genes in cancer. Biochem Biophys Res Commun. 2008;377:720–724. doi: 10.1016/j.bbrc.2008.10.045. [DOI] [PubMed] [Google Scholar]
- [104].Dondeti VR, Wubbenhorst B, Lal P, Gordan JD, D'Andrea K, Attiyeh EF, Simon MC, Nathanson KL. Integrative genomic analyses of sporadic clear cell renal cell carcinoma define disease subtypes and potential new therapeutic targets. Cancer Res. 2012;72:112–121. doi: 10.1158/0008-5472.CAN-11-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Turner KJ, Moore JW, Jones A, Taylor CF, Cuthbert-Heavens D, Han C, Leek RD, Gatter KC, Maxwell PH, Ratcliffe PJ, Cranston D, et al. Expression of hypoxia-inducible factors in human renal cancer: relationship to angiogenesis and to the von Hippel-Lindau gene mutation. Cancer Res. 2002;62:2957–2961. [PubMed] [Google Scholar]
- [106].Sun RC, Fan TW, Deng P, Higashi RM, Lane AN, Le AT, Scott TL, Sun Q, Warmoes MO, Yang Y. Noninvasive liquid diet delivery of stable isotopes into mouse models for deep metabolic network tracing. Nat Commun. 2017;8 doi: 10.1038/s41467-017-01518-z. 1646-017-01518-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Goncalves MD, Lu C, Tutnauer J, Hartman TE, Hwang SK, Murphy CJ, Pauli C, Morris R, Taylor S, Bosch K, Yang S, et al. High-fructose corn syrup enhances intestinal tumor growth in mice. Science. 2019;363:1345–1349. doi: 10.1126/science.aat8515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Tabata S, Yamamoto M, Goto H, Hirayama A, Ohishi M, Kuramoto T, Mitsuhashi A, Ikeda R, Haraguchi M, Kawahara K, Shinsato Y, et al. Thymidine catabolism as a metabolic strategy for cancer survival. Cell Rep. 2017;19:1313–1321. doi: 10.1016/j.celrep.2017.04.061. [DOI] [PubMed] [Google Scholar]
- [109].Kamphorst JJ, Nofal M, Commisso C, Hackett SR, Lu W, Grabocka E, Vander Heiden MG, Miller G, Drebin JA, Bar-Sagi D, Thompson CB, et al. Human pancreatic cancer tumors are nutrient poor and tumor cells actively scavenge extracellular protein. Cancer Res. 2015;75:544–553. doi: 10.1158/0008-5472.CAN-14-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Commisso C, Davidson SM, Soydaner-Azeloglu RG, Parker SJ, Kamphorst JJ, Hackett S, Grabocka E, Nofal M, Drebin JA, Thompson CB, Rabinowitz JD, et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature. 2013;497:633–637. doi: 10.1038/nature12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Kim SM, Nguyen TT, Ravi A, Kubiniok P, Finicle BT, Jayashankar V, Malacrida L, Hou J, Robertson J, Gao D, Chernoff J, et al. PTEN deficiency and AMPK activation promote nutrient scavenging and anabolism in prostate cancer cells. Cancer Discov. 2018;8:866–883. doi: 10.1158/2159-8290.CD-17-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Hodakoski C, Hopkins BD, Zhang G, Su T, Cheng Z, Morris R, Rhee KY, Goncalves MD, Cantley LC. Rac-mediated macropinocytosis of extracellular protein promotes glucose independence in non-small cell lung cancer. Cancers (Basel) 2019;11 doi: 10.3390/cancers11010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Hasan J, Byers R, Jayson GC. Intra-tumoural microvessel density in human solid tumours. Br J Cancer. 2002;86:1566–1577. doi: 10.1038/sj.bjc.6600315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Berghmans T, Dusart M, Paesmans M, Hossein-Foucher C, Buvat I, Castaigne C, Scherpereel A, Mascaux C, Moreau M, Roelandts M, Alard S, et al. European Lung Cancer Working Party for the IASLC Lung Cancer Staging Project, Primary tumor standardized uptake value (SUVmax) measured on fluorodeoxyglucose positron emission tomography (FDG-PET) is of prognostic value for survival in non-small cell lung cancer (NSCLC): a systematic review and meta-analysis (MA) by the European Lung Cancer Working Party for the IASLC Lung Cancer Staging Project. J Thorac Oncol. 2008;3:6–12. doi: 10.1097/JTO.0b013e31815e6d6b. [DOI] [PubMed] [Google Scholar]
- [115].Pak K, Cheon GJ, Nam HY, Kim SJ, Kang KW, Chung JK, Kim EE, Lee DS. Prognostic value of metabolic tumor volume and total lesion glycolysis in head and neck cancer: a systematic review and meta-analysis. J Nucl Med. 2014;55:884–890. doi: 10.2967/jnumed.113.133801. [DOI] [PubMed] [Google Scholar]
- [116].Geltink RIK, Kyle RL, Pearce EL. Unraveling the complex interplay between T cell metabolism and function. Annu Rev Immunol. 2018;36:461–488. doi: 10.1146/annurev-immunol-042617-053019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Cong J, Wang X, Zheng X, Wang D, Fu B, Sun R, Tian Z, Wei H. Dysfunction of natural killer cells by FBP1-induced inhibition of glycolysis during lung cancer progression. Cell Metab. 2018;28:243–255.e5. doi: 10.1016/j.cmet.2018.06.021. [DOI] [PubMed] [Google Scholar]
- [118].R Ma, Ji T, Zhang H, Dong W, Chen X, Xu P, Chen D, Liang X, Yin X, Liu Y, Ma J, et al. A Pck1-directed glycogen metabolic program regulates formation and maintenance of memory CD8(+) T cells. Nat Cell Biol. 2018;20:21–27. doi: 10.1038/s41556-017-0002-2. [DOI] [PubMed] [Google Scholar]
- [119].Wei L, Zhou Y, Yao J, Qiao C, Ni T, Guo R, Guo Q, Lu N. Lactate promotes PGE2 synthesis and gluconeogenesis in monocytes to benefit the growth of inflammation associated colorectal tumor. Oncotarget. 2015;6:16198–16214. doi: 10.18632/oncotarget.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]