Abstract
Typhoid fever caused by Salmonella enterica serovar Typhi (S.Typhi) continues to be a major problem, especially in developing countries. Due to the rapid emergence of multi-drug-resistant (MDR) strains, which limits the efficacy of conventional antibiotics as well as problems associated with the existing vaccines, efforts are being made to develop effective prophylactic agents. CdtB subunit of typhoid toxin was selected for assessing its vaccine potential due to its high conservation throughout the Typhi strains. In-vitro assessment of DNase activity of cloned and purified CdtB protein showed a significant decrease in the band intensity of DNA. The measure of metabolic activity and morphological alterations assessed using different cell lines in the presence of CdtB protein showed no significant signs of toxicity. These observations were further strengthened by cell cycle analysis, assessed by flow cytometry. Keeping these observations in mind, the immunoprotective potential of CdtB was assessed using S.Typhi induced mouse peritonitis model. A significant titer of IgG antibodies (>128000) against CdtB protein was recorded in the immunized mice by enzyme-linked immunosorbent assay (ELISA), which was also validated by immunoblotting. Active immunization with the protein protected 75% mice against a lethal dose of S.Typhi Ty2. The data indicated a significant (up to 5 log) reduction in the bacterial load in the spleen and liver of immunized-infected mice compared to control (unimmunized-infected) mice which might have resulted in the modulation of histoarchitecture of spleen and liver and the levels of cytokines (IL-6, TNF-α and IL-10) production; thereby indicating the effectiveness of the subunit. The observations deduced from the study give the proof of concept of immunogenic potential of protein. However, further studies involving the immunoreactivity of CdtB with the statistically significant number of sera samples obtained from the human patients would be helpful in establishing the relevance of CdtB protein in humans and for making the strategies to develop it as an effective vaccine candidate.
Subject terms: Protein vaccines, Pathogens
Introduction
Typhoid fever caused by Salmonella enterica serovar Typhi is characterized as a severe systemic febrile illness that accounts for approximately 600,000 deaths annually around the world1,2. S.Typhi is a Gram-negative, facultative anaerobic bacilli that belong to family Enterobacteriaceae. It usually spreads through contaminated food and water sources and is endemic in developing countries particularly in the Asian continent where public health measures are not up to the standard3–5. Antibiotics such as amoxicillin, chloramphenicol, and co-trimoxazole have been the mainstay of the treatment against S.Typhi. However, due to increase in the emergence of resistant strains these conventional antibiotics are becoming ineffective6. Recently in 2017, WHO has announced the list of antibiotic-resistant priority pathogens and mentioned S. Typhi as one of them7. Hence, to avoid the emergence of resistant variants of this organism; thus reducing the impact of infectious disease especially in endemic areas novel biotherapeutic or prophylactic approaches are direly needed.
In context to prophylactic measures, different types of vaccines against typhoid fever starting from whole-cell vaccines to subunit and to multivalent conjugated vaccines have been tried and tested to prove their efficacy for providing protection. Earlier inactivated (killed) and live whole-cell vaccines were in use8. However, due to the high incidence of associated adverse (systemic and local) reactions, short-term immunity provided by the killed vaccine; and requirement of multi-dose regimen, unsuitability for immunocompromised persons, and danger of reverting back to a virulent form of live attenuated whole-cell vaccine had rendered them unsuitable for the use9. Later on, with the advancement in the knowledge of important Salmonella antigens playing role in its pathogenesis has shifted focus towards the development of subunit vaccines. The Vi-capsular polysaccharide, one of the virulence factors is being exploited most in the development of subunit10, conjugates11,12 and multivalent vaccines13. Typherix® (Vi-capsular polysaccharide vaccine), Peda-typhTM (conjugate vaccine) and Ty21a are commercially available parenteral vaccines that are also associated with some limitations such as local reactogenicity and multiple-dose regime13. Despite of tremendous efforts, the search for an effective vaccine against Salmonella is going on because of the above stated drawbacks of the existing vaccines8,13,14.
Typhoid toxin, decade ago identified as a potential virulence factor of S. Typhi and reported to play a role in the pathogenicity of S. Typhi15 can prove to be an effective vaccine candidate. In a persistent infection murine model, it has been observed that typhoid toxin is involved in the establishment of S. Typhi persistent infection most likely by altering the immune cell functions to its favor, although the complete underlying mechanism(s) is still not well understood16,17. Typhoid toxin has been documented to be produced intra-cellularly in the infected mammalian cells, which is then exported to the extracellular environment by a unique transport mechanism facilitated by Salmonella-containing vacuole15,18 (SCV). This mechanism helps toxin to traffic out of the infected cell to intoxicate other target cells, expressing the particular type of sialic acid which is abundantly expressed in humans. Typhoid toxin belongs to AB type toxin family with a slight difference as it has two components in A subunit instead of one, that is CdtB (cytolethal distending toxin B) and PltA (pertussis-like toxin A) and PltB (pertussis-like toxin B) as B subunits. CdtB and PltA form the active catalytic part of toxin while PltB helps in binding to the target cell19. CdtB subunit has a deoxyribonuclease like activity that inflicts DNA-damage thus inducing cell cycle arrest (mainly at G2 phase) and cellular distention thereby leading to apoptosis or senesence of the infected cell18,20–22. The potential link of typhoid toxin in its nano-bound form to the life-threatening symptomatology of typhoid fever suggests that it could serve as the basis for the development of sorely needed preventive strategy against the typhoid fever.
To the best of our knowledge, this is the first report indicating the immunoprotective potential of the CdtB subunit of the complexed typhoid toxin using a mouse peritonitis model with a view of intervening Salmonella infection.
Material and Methods
Bacterial strains
Salmonella enterica serovar Typhi strain Ty2 (initially procured from the CRI, Kasauli, India) and the clinical isolates (obtained from Government Medical College and Hospital, Sector- 32, Chandigarh and All India Institute of Medical Science, New Delhi) were used during the study. BL21 (DE3) E.coli competent host cell was used during the study. The bacterial cell suspension was prepared by culturing the cells overnight in Luria broth (pH 7.4) at 37 °C under constant shaking conditions (150 rpm) throughout the study23,24.
Agents
Antibiotic kanamycin stock solution (50 mg/ml), Luria- Broth (LB) and agar from Himedia were used during the study. Enzymes such as Taq polymerase and restriction enzymes HindIII and NdeI were procured from Thermo Fishers. Isopropyl β-D-1-thiogalactopyranoside (IPTG) purchased from Sigma. Freund’s adjuvant procured for Sigma. Mouse cytokines kits: TNF-α was purchased from Krishgen Biosystems (Mumbai, India) and IL-6 and 10 from Diaclone, Besancon, France. Secondary anti-human and anti-mouse antibodies were purchased from Genei, Banglore, India.
Cell-culture
Caco-2 and RAW 264.7 cell lines (obtained from National Centre for Cell Science (NCCS), Pune, India) were grown at 37 °C in humidified incubator with 5% CO2. The culture medium, Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 5% fetal bovine serum, 50 U/ml of streptomycin and 100 U/ml of penicillin was changed every 2 days.
Animals and ethical clearance
Animal studies were approved by the Animal Ethics Committee of Panjab University (approval no. PU/45/99/CPCSE/IAEC/2018/226). Inbred Balb/c female mice (6–8 weeks old) were used in the study. All animals were housed in clean polypropylene cages and fed standard antibiotic-free diet and water. All the experimental protocols were approved by Institutional Animal Ethics Committee (IAEC), Panjab University, Chandigarh (India) and performed in accordance with the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India. Animals were handled and disposed according to the guidelines of the Animal Institutional Ethical Committee, Panjab University, Chandigarh (India).
In-silico studies and validating CdtB gene presence in Salmonella clinical isolates
In order to check the homology of the CdtB gene in various strains of Salmonella Typhi, gene sequence was subjected to BLASTn analysis (https://blast.ncbi.nlm.nih.gov/Blast.cgi). T cell and B cell immune epitopes of CdtB protein and T cell epitope-immunogenicity predictions were done using IEBD analysis resource (http://tools.iedb.org/main/)25. Further, the presence of CdtB gene was validated in S. Typhi Ty2 strain and 15 clinical isolates. To confirm the presence of this gene, PCR was run using forward 5′ TGGCATCGGACTGGGTAATG 3′ and reverse 5′ GTCTGTCTGGGCTGCGATTA 3′ primers at following conditions; Denaturation 95 °C for 30 seconds, annealing at 58 °C for 45 seconds and extension at 72 °C for 30 seconds.
Plasmid construction and transformation
Pet28a, which is a widely used expression vector for the production of recombinant protein, was used in the present study. Briefly, CdtB gene was amplified using forward 5′ TAAGCACATATGATGAAAAAACCTGTTTTTTT 3′ and reverse primer 5′ TGCTTAAAGCTTTTAACAGCTTCGTGCCAAAA3′ having sites for HindIII and NdeI restriction enzymes, respectively. Amplified CdtB gene and vector were digested using thermo fast digest HindIII and NdeI enzymes. After digestion, both vector and gene were gel purified and ligation was done using Takara T4 DNA ligase enzyme at 16 °C for 30 min. Transformation of recombinant DNA was done into E.coli BL21(DE3) host cells using CaCl2 method26. Transformed cells obtained on kanamycin-containing Luria-agar plates were checked for the presence of recombinant plasmid by extracting plasmid by alkaline lysis method. To confirm the integrity of the inserted DNA into the pET28a plasmid nucleotide sequencing analysis was also done. The vector was extracted from transformed BL21 (DE3) E.coli cells, purified using Qiagen gel purification kit and was sequenced using T7 promoter and terminator primers.
Expression and purification of the protein
For expression of CdtB protein, a single colony of transformed cells was inoculated in fresh antibiotic containing LB and incubated overnight at 37 °C under shaking condition. Next day 1% culture was inoculated in fresh antibiotic containing LB and allowed to grow at standard condition until O.D600 reaches to 0.4–0.6. 1 mM concentration of IPTG was used to induce protein expression.
His-tagged CdtB was purified from inclusion bodies by nickel-nitrilotriacetic acid (NTA)-agarose chromatography. Briefly, bacteria were harvested by centrifugation at 8000 rpm for 10 min, washed and suspended in binding buffer (20 mM Tris-Cl (pH 8), 5 mM imidazole, 500 mM NaCl). The suspension was sonicated for half an hour on ice and further centrifuged at 12000 rpm for 20 min to collect the inclusion bodies. The inclusion bodies were washed twice and suspended in 6 M urea. After incubation for an hour to dissolve the protein completely in urea, the suspension was centrifuged at 12000 g for 20 min. The obtained extract was applied to Ni-NTA column pre-equilibrated with binding buffer containing 6 M urea. The column was washed with the wash buffer (20 mM Tris-Cl (pH 8), 500 mM NaCl, 15 mM imidazole and 6 M urea). Bound protein was eluted with buffer containing 300 mM imidazole and further dialyzed against 10 mM Tris-Cl (pH 8) and 100 mM NaCl. The protein was concentrated using 10 kDa cut-off concentrators. The purity of the purified protein was checked using SDS-PAGE27.
In-vitro nuclease activity of purified CdtB protein
To assess the in-vitro nuclease activity of CdtB protein, a protocol of Pons et al.28 was followed with few modifications. 200 ng of purified human DNA (extracted using Qiagen DNA extraction kit) was incubated at 37 °C with three different concentration of CdtB protein (100 ng, 250 ng and 500 ng) for 40 min in digestion buffer (50 mM NaCl, 20 mM Tris-HCl (pH 7.5), 5 mM CaCl2, 5 mM MgCl2, 50 μg/mL BSA). The reaction was stopped by adding 10 mM of EDTA and digestion products were analyzed on 1% agarose gel. Another experiment set with above-mentioned concentrations of protein was also performed in which incubation time was exceeded to 4 h and products were analyzed on 2% agarose gel, in order to observe digested DNA fragments (if any). DNA incubated at 37 °C in absence of CdtB and presences of bovine DNase I (2 ng for 10 min) were taken as negative and positive controls, respectively. The densitometry of DNA bands to determine % band intensity was done using ImageJ software.
Evaluation of undesired effects of the toxin (if any) using various cell lines
Morphological alteration
To study the morphological alterations (if any) caused by CdtB protein in the eukaryotic cell, Caco-2 (human) and RAW 264.7 (mouse) cell lines were used. Briefly, 24 h semi-confluent cultures containing 4 × 104 cells/ml were examined after 1 day of incubation in the presence of the protein preparation of different concentrations (1, 10 and 100 μg/ml). The cells were examined microscopically for any morphological alteration caused by CdtB protein at different concentrations29.
MTT (3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide) assay
To measure the metabolic activity of cells (Caco-2 and Raw 264.7) in the presence of protein, MTT assay was done. Cells were harvested by trypsinization or scraping and the dilutions of the cells were made in culture medium up to 1 × 104 cells/ml and plated out in triplicate into wells of a microtiter plate. The plate was then supplemented with different concentrations of the CdtB protein (1, 10 and 100 μg/ml) and incubated under appropriate conditions for 24 h. 100 μl of MTT (3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide) solution was added and the plate was returned to cell culture incubator for 4 h. After 4 h, MTT solubilization buffer (100 μl) was added to each well and absorbance was recorded including the blank at 570 nm in a microtiter plate reader30.
Cell cycle analysis
Flow cytometry was done in order to investigate the effect of the protein on the cell cycle. Cells (Raw 264.7 and Caco-2) were seeded in 24-well plates at a density of 4 × 104 cells/ml and treated with different concentrations of protein (1, 10 and 100 μg/ml) for 24 h. Cells were centrifuged, washed twice with PBS, suspended in 500 μl cold ethanol (70%) and incubated on ice for 15 min. After fixation, cells were suspended in 300 μl propidium iodide (PI) solution (0.05 mg/ml PI; 0.02 mg/ml RNase; 0.3% Triton X; 1 mg/ml sodium citrate) and re-incubated for 1 h on ice. Cells were washed with PBS and analyzed29,31.
Evaluation of in-vivo immunogenicity of CdtB
Using sera of experimentally immunized mice
Potential of CdtB to act as an immunogen was checked in mice by injecting purified protein with an adjuvant. Primary dose (50 µg) of CdtB protein mixed with Freund’s complete adjuvant in 1:1 ratio was administered through intraperitoneal route to a group of 8 mice, followed by three booster doses of half the protein concentration (25 µg) mixed in 1:1 ratio with Freund’s incomplete adjuvant given at 14th, 21th and 28th day. In the control group, comprised of 4 mice, adjuvant mixed with PBS was injected. Sera collected from both, control and immunized group at day 17th, 23th, 30th were subjected to quantitative (indirect ELISA) as well as qualitative (by western blotting, only after the second booster) analysis to measure and detect anti-CdtB IgG antibodies, respectively.
Briefly, 10 μg/ml of CdtB protein, diluted in carbonate buffer pH 9.6 was coated in the wells of microtitre plates overnight at 4 °C. Plates were washed thrice after every step with PBS pH 7.2 and blocked with 5% skim milk overnight. Next day, 100 μl serum samples at different dilution (made in blocking buffer) starting from 1: 500 to 1: 256000 were added to each well and incubated for 1 h at 37 °C. After that, 100 μl of anti-human IgG- HRP conjugated secondary antibody (1: 20000) was added to each well and incubated for 1 h at the same conditions. On completion of the incubation period, a substrate (TMB) 50 μl was added and allowed to develop color for 10 min in dark. The reaction was stopped with 50 μl/well of 0.1 N H2SO4, and the plate was read at 450 nm with a microplate reader32,33.
Western blotting
To detect CdtB specific antibody in the immunized mice, CdtB protein was run on 12% Tris-glycine SDS-PAGE gel and protein bands were transferred onto PVDF membrane. The membrane was blocked with 5% skimmed milk made in PBS-Tween 20 (0.05%) for 2 h at 37 °C. Two sera samples (obtained after the second booster) at 1: 500 dilutions (in blocking buffer) were incubated with the membrane for 1 h at 37 °C. The membrane was washed every time with wash buffer (PBS-T, 0.05%) and then incubated with anti-human IgG-HRP conjugated antibody (1: 20000 dilution) for 1 h at 37 °C. After this, the membrane was developed with chromogenic TMB solution (in the dark) for 10–15 min and the reaction was stopped by washing with wash buffer32.
Establishment of S.Typhi Peritonitis mouse model
To establish the peritonitis model of S.Typhi (Ty2), the organism in 5% hog mucin was injected through intraperitoneal route33–35 at different doses (107, 108, 109 CFU) in mice (n = 3) to standardize the dose that causes death within 24 h in order to understand the effect of vaccination on the survivability of the animals. Control group (n = 3) was administered with PBS.
Active immunization
After checking the immunogenic potential of the protein, active immunization of two mice groups, comprised of 8 mice each was done. Immunization of mice was done in the same manner (at 1st, 14th, 21st and 28th day) as described in above section followed by challenge with a lethal dose (109 CFU) of S.Typhi via intra-peritoneal route. With one group, the survival of the animals was recorded for the next 30 days post challenge and another group used for bacterial load studies, histological examination and cytokines level estimation. In the control group, comprised of 4 mice, only adjuvant mixed with PBS was administered at above stated days followed by the bacterial challenge (served as unimmunized-infected control).
Determination of bacterial load
Bacterial burden in the spleen and liver of control (unimmunized-infected) group at 20 h and in the immunized-infected group at 20 h and 7th day post infection was checked. Mice were sacrificed by cervical dislocation and dissected under an aseptic condition to remove organs. Organs were suspended in sterile PBS (pH 7.2), homogenized, spread plated on MacConkey agar plates and kept at 37 °C for overnight incubation. The number of colony forming units was counted and expressed as log10 CFU.
Histoarchitectural studies
Liver and spleen specimens of unimmunized-uninfected, unimmunized-infected and immunized-infected groups removed aseptically (20 h and 7th day in case of immunized-infected post infection) were fixed in 10% buffered formalin, stained with hematoxylin-eosin and observed under the microscope for assessing cellular infiltration and aggregation36.
Estimation of cytokines
Pro-inflammatory (TNF-α and IL-6) and anti-inflammatory (IL-10) cytokines in serum and organs (liver and spleen) were measured in control (unimmunized-infected) and immunized-infected groups at 20 h and 7th day in case of immunized-infected post-infection using kits according to manufacturer’s instruction (TNF-α, Krishgen Biosystems, Mumbai, India37), IL-6 and 10, Diaclone, Besancon, Cedex, France38,39). Serum and organs were collected under aseptic conditions and processed further to measure cytokines levels33,40.
Statistical analysis
Experiments were conducted at least three times and data are expressed as mean ± SD. Statistical analysis was done using GraphPad Prism 8 software by evaluating significance of data using Student’s t-test and one way analysis of variance (ANOVA). Values less than 0.05 (p < 0.05) were considered statistically significant.
Results and Discussion
Salmonella Typhi and another emerging human restricted serovar S. Paratyphi A poses huge health challenges on developing nations. In addition to this, reports of MDR strains of S. Typhi have elevated the possibility of the recurrence of untreatable typhoid fever5,41. Currently, Ty21a and Vi-capsular polysaccharide-based vaccines (alone and conjugate; coupled to tetanus toxoid (TT) and HepatyrixTM) are available commercially8,13, but have some major limitations such as fail to provide complete protection against S.Typhi infection as Vi-antigen is not universally expressed by all the strains of S.Typhi42,43, inability to elicit an immune response in children below 2 years of age and local reactogenicity13,14. Clinical reports mainly from Asian countries, where typhoid fever is still a matter of concern, indicated around 55% and 52% efficacy of Vi-TT (tetanus toxoid) conjugate and Vi (polysaccharide) vaccines, respectively44,45; which has necessitated the need to look for other potent vaccine candidates against typhoid fever. A recent study has reported various new S.Typhi specific antigens46, one such novel antigen identified is CdtB, which constitutes an active motif of typhoid toxin and induces acute typhoid symptoms47. CdtB was selected for this study because the protein is unique as it is S.Typhi specific and is secreted post infection48.
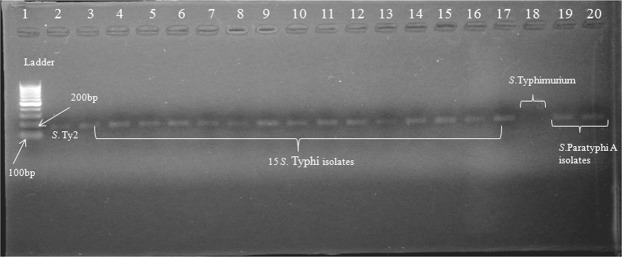
To provide a broad range of protection to the host against the pathogen, the foremost requirement of any vaccine candidate is that it should be prevalent and conserved throughout the strains of specific species. BLASTn analysis indicated conservation of CdtB gene with 100% and 99% sequence homology in all strains of S.Typhi and S. Paratyphi A, respectively. Further, CdtB shares no similarity with human as well as mouse genome, which is crucial to avoid autoimmunity in the host. Various B and T cell (MHC class 1 and II) binding epitopes (peptides) of CdtB protein were predicted against different human HLA alleles in order predict immunogenic nature of protein; additionally, these predicted peptides could be exploited in the generation of epitope-based vaccines against typhoidal Salmonella (Suppl. Tables S1–S4). However, as it is not necessary that every epitope binding to MHC will be recognized by T-cells and generates an effective immune response, hence prediction of peptide-MHC (class I MHC) complexes to elicit a good immune response was also done, which showed good score against various CdtB peptides, indicating a greater probability of peptide-MHC complexes to elicit an immune response (Suppl. Table S1). Validation of the in-silico prediction of presence of CdtB gene done using 15 clinical isolates (of S.Typhi) showed gene prevalence in all along with two isolates of S.Paratyphi A (Fig. 1). However, it was found to be absent in Salmonella Typhimurium (Fig. 1), thereby supporting the fact of human restricted nature of “typhoidal Salmonella” as typhoid toxin is encoded by both typhoidal serovars S. Typhi and S. Paratyphi while it is mostly lacked by non-typhoidal serovars49,50.
Figure 1.
CdtB gene presence was confirmed in standard strain Ty2, 15 clinical isolates of S. Typhi and two S. Paratyphi isolates. Lane 1: 100 bp ladder (100–1000 bp), 2: S.Typhi Ty2, 3–17: 15 clinical isolates of S. Typhi, 18: S. Typhimurium and 19–20: S. Paratyphi A isolates (Full-length gel is presented in Supplementary Fig. S1).
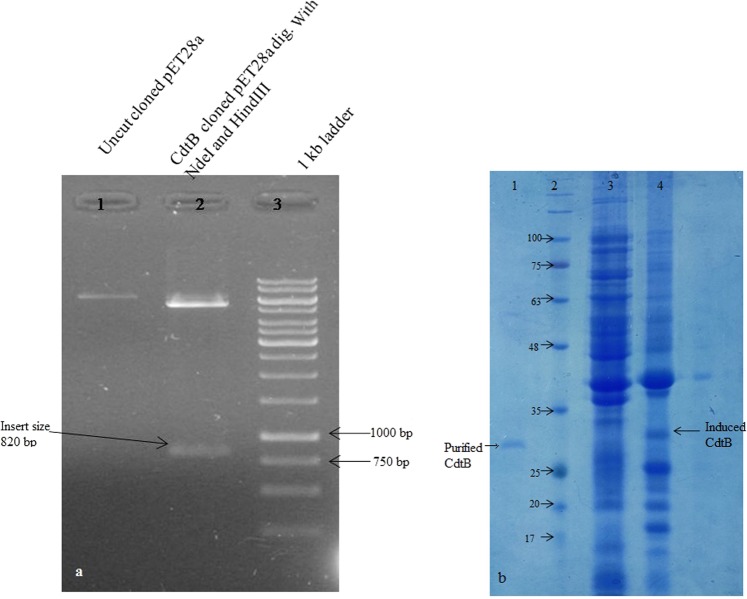
As CdtB protein has been reported to be expressed under in-vivo condition only (inside the infected cells post-infection)47, therefore, in order to have protein in abundance for further studies, its gene was cloned into pET28a vector. Construction of recombinant plasmid was confirmed by restriction mapping of the positive cloned plasmid with NdeI and HindIII enzymes, as shown in Fig. 2a. Furthermore, nucleotide analysis of positive clones indicated 100% sequence homology with the CdtB gene of S.Typhi (Accession no. NC_003198.1), which signified the successful insertion of CdtB gene into the vector (Suppl. Fig. S3). Optimization of the protein expression was carried at different temperatures (28 °C, 37 °C and 40 °C) to maximize its production using 1 mM IPTG (Fig. 2b). As the protein was expressed in the insoluble fraction (as inclusion bodies), therefore it was purified under denaturing conditions51 using Ni-NTA affinity chromatography. After purification refolding of denatured protein to soluble form was done and final post-refolding yield of protein obtained was about 10 mg/liter culture. The purity of the purified protein was determined by Coomassie-stained SDS-PAGE gel analysis, which showed a band of approximately 31 kDa (Fig. 2b).
Figure 2.
(a) Recombinant plasmid construction. Lane 1: Recombinant uncut plasmid, 2: Double digested (HindIII and NdeI) product of recombinant plasmid, 3: ladder (Full-length gel is presented in Supplementary Fig. S2a). (b) Showing the un-induced, induced BL21 cells at 37 °C and purified protein. Lane 1: Purified protein, 2: ladder (in kDa), 3: Uninduced cells and 4: Induced cells. (Uncropped, full-length gels are presented in Supplementary Fig. S2b).
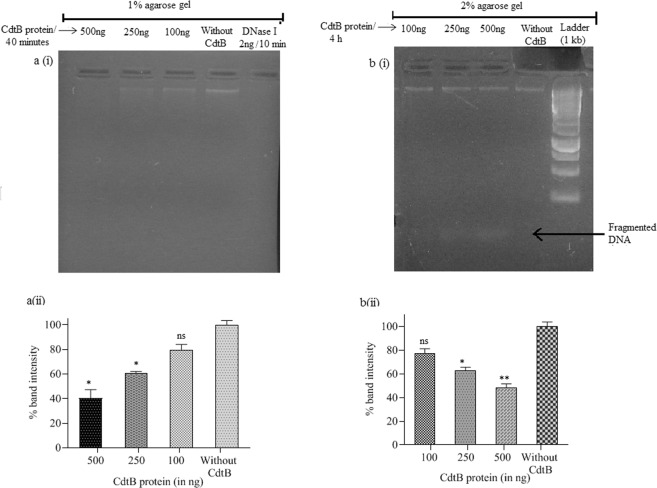
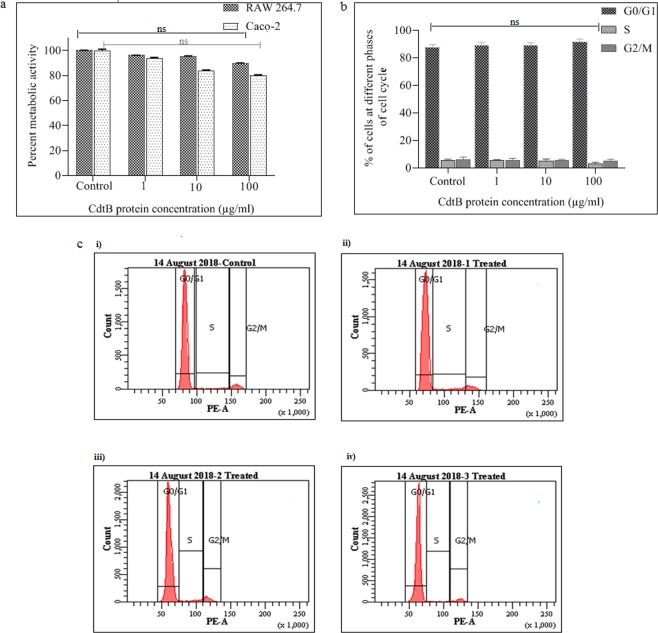
CdtB shares position-specific sequence homology to the members of DNase I protein family52, which supports its nuclease activity53,54. In-vitro assessment of DNase activity of CdtB protein showed a significant decrease in the band intensity of DNA on 1% agarose gel (Fig. 3a(i,ii)), however no fragments of digested DNA was observed. In case of positive control complete degradation of DNA occurred within 10 min. In order to observe fragments of digested DNA, if any, products were analyzed on 2% gel after exceeding incubation time to 4 h. In the later set of experiment, fragmented DNA was observed with decrease in intensity of DNA (Fig. 3b(i,ii)), thereby indicating protein’s in-vitro nuclease activity. Under in-vivo conditions, CdtB subunit executes its DNase like catalytic activity (in-vivo) only along with other subunits PltA and PltB, which facilitate its entry inside the cell29,49,55,56. However, there are other reports, which describe the intoxication of cells with recombinantly produced CdtB protein of other Gram negative pathogens57. Therefore, before evaluating its immunoprotective efficacy in experimental animals, cytotoxicity studies of the subunit was still carried out in order to rule out its undesired effect. The sensitivity studies of different cultured cells such as RAW 264.7(mouse) and Caco-2 (human) to CdtB protein were conducted at different protein concentrations (1, 10 and 100 μg/ml). No significant changes were observed in the morphology of all cell types (Suppl. Fig. S5) and no inhibition of metabolic activity were found in mouse cell lines compared to human cell line, where slight concentration-dependent inhibition of metabolic activity was recorded (Fig. 4a). Encouraged by these findings and keeping in view the relevance of animal model adopted for the study, cell cycle analysis was performed with RAW 264.7 cells. The results revealed that purified protein at all the used concentrations showed insignificant toxicity, as major population of cells was found at Go/G1 phase of the cell cycle same like control cells (Fig. 4b,c). Similar observations were obtained with Caco-2 cell lines and the results are provided in supplementary information (Suppl. S6). Thus the flow cytometric analysis further strengthened our former observations and was also found in agreement with the earlier findings, wherein it has been reported that all subunits of the toxin are required for intoxication of cells29,31,49.
Figure 3.
a(i) DNA run on 1% agarose gel after 40 min of incubation with different concentrations of CdtB protein and bovine DNase I (for 10 min) as positive control, a(ii) graph showing significant decrease in the % band intensity with increase in the concentration of protein, whereas DNase I (positive control) completely degraded the DNA within 10 min, b(i) DNA run on 2% agarose gel after 4 h of incubation with different concentrations of CdtB protein showing fragmented DNA and b(ii) graph showing significant decrease in the % band intensity with increase in the concentration of protein. The data are presented as mean ± SD. p value determined using one way ANOVA and t-test. *p < 0.05 and ns as non-significant as compared to control (without CdtB). (Full-length gels are presented in Supplementary Fig. S4).
Figure 4.
(a) Results of cell viability assay conducted on RAW 264.7 and Caco-2 cell lines. No significant metabolic inhibition was recorded in both cells. The data are presented as mean ± SD. p value determined using one-way ANOVA and t-test, ns as non-significant as compared to control. (b) Cell cycle analysis of RAW 264.7 cell lines. Purified protein at all the different concentrations (1, 10, and 100 µg/ml) showed no significant toxicity as the major population of the treated cells was found at Go/G1 phase of the cell cycle, similar to the one observed for control cells. The data are presented as mean ± SD. ns as non-significant as compared to control. (c) Representative figure of cell cycle analysis of RAW 264.7 cells, c (i) - control, (ii) 1 µg/ml, (iii) 10 µg/ml, (iv) 100 µg/ml.
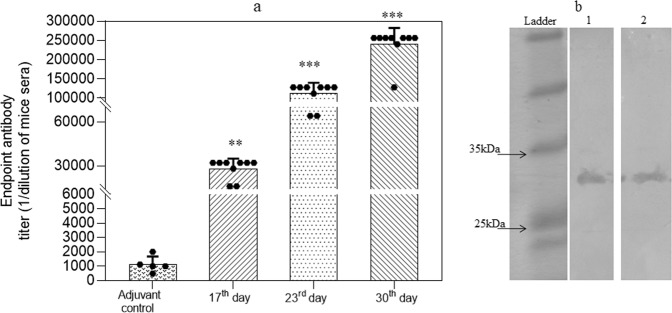
In reference to the humoral response, the significant titer of antibodies to typhoid toxin has been reported in convalescent typhoid fever patients46,58. In order to detect the antibody titer particularly against purified CdtB protein (anti-CdtB antibodies), mice were administered (intra-peritoneal) with CdtB protein along with Freund’s adjuvant to achieve the sustained release of protein; as it is considered as most effective for generating an immune response to diverse antigens59. CdtB protein generated antibody titer of >25000 (±7406 standard deviation) after first booster and >100000 (±29626 standard deviation) and >225000 (±45254 standard deviation) after the second and third booster, respectively, whereas in the case of control (adjuvant only) no considerable titer was recorded. A significant titer of IgG antibodies evoked after immunization with CdtB indicated its fairly good immunogenic potential in mouse (Fig. 5a). Western blot analysis of two serum samples done after the second booster further confirmed the presence of anti-CdtB antibodies in the sera (Fig. 5b), thereby indicating the seroreactivity of CdtB; thus signifying its application in the development of a point-of-care. During and after completion of immunization no ill-effect of immunization was observed as mice remained healthy, behaviorally active and organs were found completely sterile.
Figure 5.
(a) Endpoint antibody titer measured after every booster at 17th, 23th and 30th day (n = 8). Black dots represent each mouse and bars represent mean value of all mice plus SD. The data are presented as mean ± SD. p value determined using one way ANOVA and t-test, *p < 0.05, **p < 0.01 and ***p < 0.001 as compared to adjuvant control. (b) Western blot of two serum samples of mice (sera dilution used 1:500) after the second booster showed evident blot, indicating the generation of anti-CdtB antibodies (Full-length image is presented in Supplementary Fig. S7).
After checking the in-vivo immunogenicity of CdtB protein, immunization studies were carried out. To monitor the immunoprotective efficacy of CdtB, peritonitis murine model was established. As S.Typhi causes self-limiting infection in mice with low bacterial load in organs and low dissemination, therefore, 5% hog mucin was used to increase the virulence of organisms as reported earlier33,60,61. Out of the three different doses given to mice, 109 CFU dose led to 100% mortality of mice within 24 hours of infection. Therefore, this dose was selected and used to carry out the efficacy studies of the protein against this challenge dose.
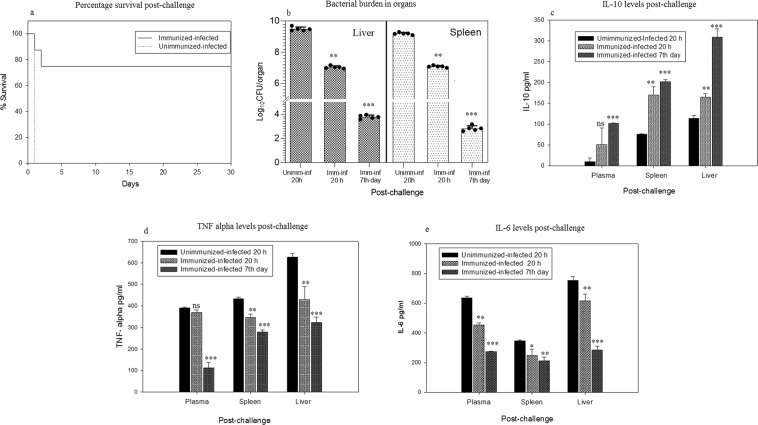
Mice survival recorded up to thirty days post-challenge showed 75% survival in the immunized-infected group, whereas in the control group (unimmunized-infected) all mice died within 24 h (Fig. 6a). Active immunization of mice followed by bacterial challenge not only increased the mice survival rate but also led to the significant decrease in the bacterial burden as evident by bacterial load studies (Fig. 6b). At 20 h post challenge, bacterial load was checked in the liver and spleen. In the liver, approximately 9 log and 7 log CFU was recorded in control (unimmunized-infected) and immunized-infected mice, respectively, indicating a significant decrease of two log folds in immunized mice. Similarly, bacterial count checked at 7th day in immunized-infected mice showed almost five log reduction. The same trend was observed in the case of spleen also, where a significant reduction in the bacterial burden was recorded in the immunized-infected group as compared to the control (unimmunized-infected, Fig. 6b).
Figure 6.
(a) Percentage survival of mice of the immunized-infected group post-challenge (n = 8), recorded up to 30 days. (b) Bacterial load detected in the spleen and liver of control (Unimm-Inf 20 h) and immunized-infected (Imm-Inf 20 h) mice at 20 h post-challenge and at 7th day in immunized-infected (Imm-Inf 7th day) showing significant log reduction. Black dots represent each mouse and bars represent mean value plus SD. (c) IL-10, anti-inflammatory cytokine measured in serum, spleen and liver of control (unimmunized-infected) and immunized-infected mice at 20 h post-infection and at 7th day in immunized-infected mice. (d,e) TNF-alpha and IL-6 levels measured in serum, spleen and liver of the control (unimmunized-infected) and immunized-infected mice at 20 h post-infection and at 7th day in immunized-infected mice, respectively. The data are presented as mean ± SD. p value determined using one way ANOVA and t-test, *p < 0.05, **p < 0.01 and ***p < 0.001 and ns as non-significant as compared to control (unimmunized-infected) mice.
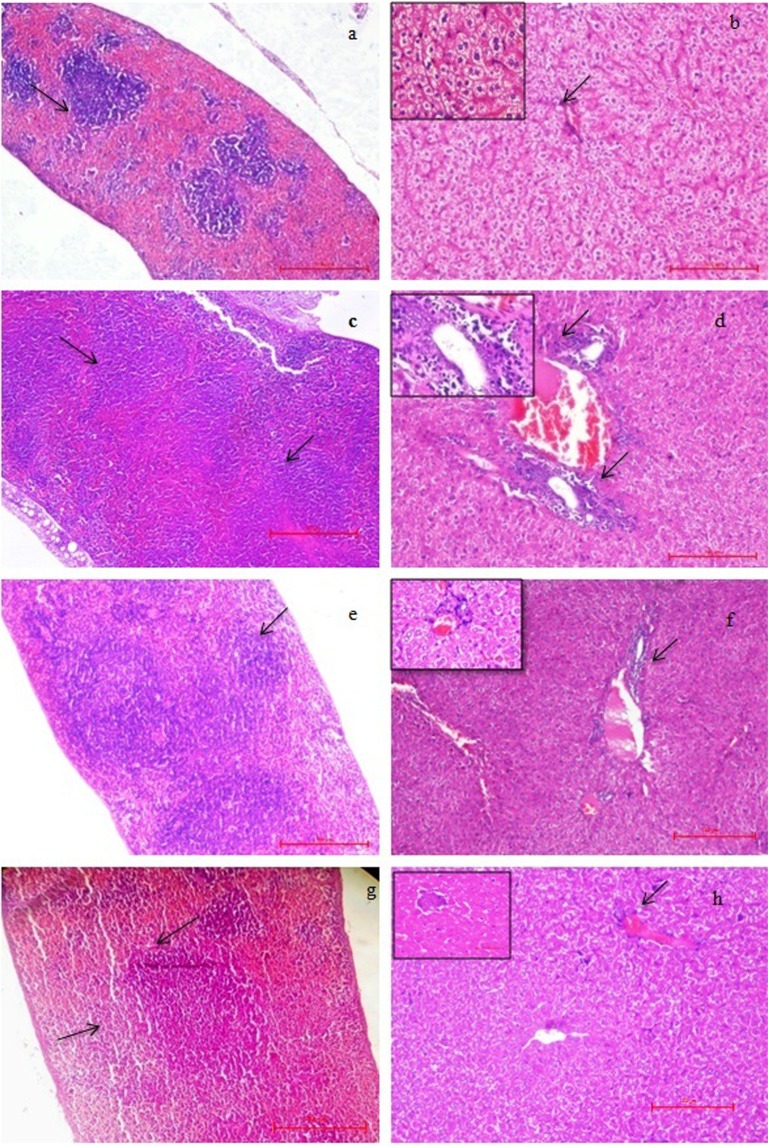
Tissue architecture studies and cytokines level estimation (pro and anti-inflammatory) serve as a good indicator to study the initiation of infection, inflammation and effectiveness of any treatment or vaccine37,62. Histology of spleen and liver of the control (unimmunized-infected) group showed signs of active infection 20 h post challenge. In the case of spleen, hyperplasia (increased in number) of lymphoid follicles and enlargement of size (hypertrophy) was observed with a reduction of red pulp (score 4, after giving challenge to unimmunized-infected mice, Fig. 7c) as compared to the normal spleen of unimmunized-uninfected mice where distinct follicles with clear red and white pulp could be seen, score 0 (Fig. 7a). However, the red and white pulp of the spleen of immunized-infected mice at 20 h (score 2) and 7th day (score 1) post-infection appeared to be restored (Fig. 7e,g). In context to the liver, portal triaditis was seen in control (unimmunized-infected) group post challenge compared to unimmunized-uninfected mice (score 0, Fig. 7b), which indicated inflammation as both portal tracts showed lymphocytic infiltrations (score 3, Fig. 7d). However, the immunized-infected group showed reduced inflammation with a clearance of portal tracts after 20 h (score 2) post-infection (Fig. 7f). Liver of immunized-infected mice at 7th day showed normal hepatic structure (score 1, Fig. 7h).
Figure 7.
Histopathology of the spleen (40X) and liver (100X). (a) Spleen of unimmunized-uninfected mice showed a normal structure with clearly distinct red pulp, white pulp and marginal zone, score 0. (b) Liver of unimmunized-uninfected showed the normal size of hepatocytes and normal portal tract indicated with an arrow, score 0 (inset 400X). (c) Spleen of the control (unimmunized-infected) mice, 20 h post-challenge showed an increased number of lymphoid follicles with the disappearance of red pulp and enlargement of size, score 4. (d) Liver from control (unimmunized-infected, 20 h) showed lymphocytic infiltration near portal tracts, indicated with arrows, score 3 (inset 400X). (e) Immunized-infected (20 h post-challenge) spleen showed rehabilitation of red and white pulp, score 2. (f) Immunized-infected liver (20 h)) showed less number of inflammatory cells near portal tracts (indicated with an arrow) with restoration of histological structure, score 2 (inset 400X). (g,h) spleen (score 1) and liver (score 1) of immunized-infected mice at 7th day showing restored histoarchitecture. (High quality image is provided in supplementary information, Fig. S8).
Cytokines play a key role in the development of disease and in providing immunity against the infection. Both the anti and pro-inflammatory cytokines are important in determining the protection mediated by the immune system against the specific pathogenic organisms40,63. Pro-inflammatory cytokines such as TNF-α, IL-6, Interferon-γ, etc., are signaling molecules secreted by the immune cells, which promote inflammation and play an important role in mediating the innate immune response23,40. TNF-α acts as a local inflammatory mediator and activates B and T cells to produced IL-6. On the other hand, anti-inflammatory cytokines (such as IL-10) are a series of immunoregulatory molecules that down-regulate inflammatory reactions64. Rise in the levels of anti-inflammatory cytokine IL-10 in sera as well as in tissue homogenates (liver and spleen) of immunized-infected group was detected post 20 h and 7th day of post- challenge, indicating the employment of immune cells to fight against the pathogen64 that was in contrast to the control (unimmunized-infected) group (Fig. 6c). Increased levels of pro-inflammatory cytokines such as TNF-α and IL-6 in control (unimmunized-infected) group indicated the active infection, which could be linked with the infiltration of lymphocytes in the liver, increase in number of lymphoid follicles in the spleen and heavy bacterial load in the organs; whereas in immunized-infected group significant decrease in the levels of TNF-α and IL-6 (at 20 h and 7th day post infection) signified the efficacy of CdtB as vaccine (Fig. 6d,e). These findings are in accordance with the earlier reports wherein cytokine-producing Th type cell-response has been documented to play a critical role in providing resistance to S.Typhi infection at the systemic level41. This study also indicates the involvement of Th cells to stimulate the B- cells for the production of antibodies as well as the cytokines influenced activation of macrophages residing in the reticuloendothelial system which are expected to play a critical role in clearing systemic S.Typhi infection. This study thus indicates that the protein is capable of eliciting the humoral as well as a cellular immune response against the infection.
It may be concluded from the study that successfully cloned and purified CdtB protein showed an immunoprotective response, as evident by the antibody titer and cellular immune response generated against the disease. Immunization with the protein conferred 75% protection against the lethal dose of S.Typhi with a reduction in bacterial burden, levels of pro-inflammatory cytokine and restoration of histoarchitecture. Thus, the proof of concept of this study may prove to be helpful in further developing it as a prophylactic option not only against S. Typhi but also against S. Paratyphi A, for which no vaccine is available commercially.
Supplementary information
Acknowledgements
This work was supported by the Department of Science and Technology (DST) for providing financial assistance to the research fellow (IF160272) to carry out this work. The authors also acknowledge Promotion of University Research and Scientific Excellence (PURSE) grant from the Department of Science and Technology, New Delhi, India for providing partial financial assistance.
Author contributions
P.R. conceived the idea and organized the study. R.T. performed all the experiments and wrote the manuscript. K.K.K. provided cell culture facility. P.P., N.K. and V.J. helped in protein purification and animal work, respectively. P.R. and R.C.S. critically reviewed and approved the manuscript.
Data availability
All the relevant data are within the manuscript and in the supplementary information.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Raman Chander Suri, Email: crsuri58@gmail.com.
Praveen Rishi, Email: rishipraveen@yahoo.com.
Supplementary information
is available for this paper at 10.1038/s41598-019-54690-1.
References
- 1.Pang T, Levine MM, Ivanoff B, Wain J, Finlay BB. Typhoid fever—important issues still remain. Trends Microbiol. 1998;6:131–133. doi: 10.1016/S0966-842X(98)01236-0. [DOI] [PubMed] [Google Scholar]
- 2.Crump JA, Mintz ED. Global trends in typhoid and paratyphoid fever. Clin Infect Dis. 2010;50:241–246. doi: 10.1086/649541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olsen, S. J., MacKinon, L. C., Goulding, J. S., Bean, N. H. & Slutsker, L. Surveillance for foodborne-disease outbreaks, United States, (1993–1997). [PubMed]
- 4.Siddiqui FJ, Rabbani F, Hasan R, Nizami SQ, Bhutta ZA. Typhoid fever in children: some epidemiological considerations from Karachi, Pakistan. Int J infectious dis. 2006;10:215–222. doi: 10.1016/j.ijid.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 5.Das JK, et al. Trends, associations, and antimicrobial resistance of Salmonella typhi and paratyphi in Pakistan. Am J Trop Med Hyg. 2018;99:48–54. doi: 10.4269/ajtmh.18-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crump JA, Sjölund-Karlsson M, Gordon MA, Parry CM. Epidemiology, clinical presentation, laboratory diagnosis, antimicrobial resistance, and antimicrobial management of invasive Salmonella infections. Clin Microbiol Rev. 2015;28:901–937. doi: 10.1128/CMR.00002-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.www.who.int/medicines/publications/global-priority-list-antibiotic-resistant-bacteria/en/.
- 8.MacLennan CA, Martin LB, Micoli F. Vaccines against invasive Salmonella disease: current status and future directions. Hum vacci immunother. 2014;10:1478–1493. doi: 10.4161/hv.29054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ivanoff B, Levine MM, Lambert PH. Vaccination against typhoid fever: present status. Bull World Health Organ. 1994;72:957–971. [PMC free article] [PubMed] [Google Scholar]
- 10.Klugman KP, et al. Protective efficacy of Vi-capsular polysaccharide vaccine against typhoid fever. Lancet. 1987;2:1165–1169. doi: 10.1016/S0140-6736(87)91316-X. [DOI] [PubMed] [Google Scholar]
- 11.Szu SC, Stone AL, Robins FD, Schmerrson R, Robbins JB. Vi-capsular polysaccharide protein conjugates for prevention of typhoid fever. J Exp Med. 1987;66:1510–1517. doi: 10.1084/jem.166.5.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh M, Ganguly NK, Kumar L, Vohra H. Protective efficacy and immunogenicity of Vi-porin conjugate against Salmonella typhi. Microbiol Immunol. 1999;43:535–542. doi: 10.1111/j.1348-0421.1999.tb02439.x. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization. Observed Rate of Vaccine Reactions – Typhoid Vaccine. 1–4 (2014).
- 14.Sur D, et al. A cluster-randomized effectiveness trial of Vi typhoid vaccine in India. N Engl J Med. 2009;361:335–344. doi: 10.1056/NEJMoa0807521. [DOI] [PubMed] [Google Scholar]
- 15.Haghjoo E, Galán JE. Salmonella typhi encodes a functional cytolethal distending toxin that is delivered into host cells by a bacterial-internalization pathway. Proc Natl Acad Sci USA. 2004;101:4614–4619. doi: 10.1073/pnas.0400932101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song J, et al. A mouse model for the human pathogen Salmonella typhi. Cell host & microbe. 2010;8:369–376. doi: 10.1016/j.chom.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chong A, Lee S, Yang YA, Song J. Focus: Infectious Diseases: The Role of Typhoid Toxin in Salmonella Typhi Virulence. Yale J Biol Med. 2017;90:283–290. [PMC free article] [PubMed] [Google Scholar]
- 18.Spanò S, Ugalde JE, Galán JE. Delivery of a Salmonella Typhi exotoxin from a host intracellular compartment. Cell host & microbe. 2008;3:30–38. doi: 10.1016/j.chom.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Chang SJ, Song J, Galán JE. Receptor-mediated sorting of typhoid toxin during its export from Salmonella Typhi-infected cells. Cell host & microbe. 2016;20:682–689. doi: 10.1016/j.chom.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ceelen LM, Decostere A, Ducatelle R, Haesebrouck F. Cytolethal distending toxin generates cell death by inducing a bottleneck in the cell cycle. Microbiological research. 2006;161:109–120. doi: 10.1016/j.micres.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Blazkova H, et al. Bacterial intoxication evokes cellular senescence with persistent DNA damage and cytokine signalling. J Cell Mol Med. 2010;14:357–367. doi: 10.1111/j.1582-4934.2009.00862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frisan T. Bacterial genotoxins: The long journey to the nucleus of mammalian cells. Biochim Biophys Acta -Biomembranes. 2016;1858:567–575. doi: 10.1016/j.bbamem.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 23.Chanana V, Majumdar S, Rishi P. Tumour necrosis factor α mediated apoptosis in murine macrophages by Salmonella enterica serovar Typhi under oxidative stress. FEMS Immuno. Med Microbiol. 2006;47:278–286. doi: 10.1111/j.1574-695X.2006.00090.x. [DOI] [PubMed] [Google Scholar]
- 24.Rishi P, Thakur R, Kaur UJ, Singh H, Bhasin KK. Potential of 2, 2′-dipyridyl diselane as an adjunct to antibiotics to manage cadmium-induced antibiotic resistance in Salmonella enterica serovar Typhi Ty2 strain. J Microbiol. 2017;55:737–744. doi: 10.1007/s12275-017-7040-0. [DOI] [PubMed] [Google Scholar]
- 25.Singh R, Capalash N, Sharma P. Immunoprotective potential of BamA, the outer membrane protein assembly factor, against MDR Acinetobacter baumannii. Sci Rep. 2017;7:12411. doi: 10.1038/s41598-017-12789-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sambrook, J. & Russell, D. W. Molecular Cloning: A Laboratory Manual, 3rd ed, Cold Spring Harbor Laboratory Press, New York (2001).
- 27.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 28.Pons BJ, et al. Cell transfection of purified cytolethal distending toxin B subunits allows comparing their nuclease activity while plasmid degradation assay does not. PloS ONE. 2019;14:e0214313. doi: 10.1371/journal.pone.0214313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wising C, et al. Toxicity and immunogenicity of purified Haemophilus ducreyi cytolethal distending toxin in a rabbit model. Microb pathog. 2002;33:49–62. doi: 10.1006/mpat.2002.0516. [DOI] [PubMed] [Google Scholar]
- 30.Van de Loosdrecht AA, Beelen RH, Ossenkoppele G, Broekhoven MG, Langenhuijsen MM. A tetrazolium-based colorimetric MTT assay to quantitate human monocyte mediated cytotoxicity against leukemic cells from cell lines and patients with acute myeloid leukemia. J Immunol Methods. 1994;174:311–320. doi: 10.1016/0022-1759(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 31.Sert V, et al. The bacterial cytolethal distending toxin (CDT) triggers a G2 cell cycle checkpoint in mammalian cells without preliminary induction of DNA strand breaks. Oncogene. 1999;18:6296–62304. doi: 10.1038/sj.onc.1203007. [DOI] [PubMed] [Google Scholar]
- 32.Sharma T, et al. Serodiagnostic evaluation of recombinant CdtB of S. Typhi as a potential candidate for acute typhoid. Immunol Res. 2018;66:503–512. doi: 10.1007/s12026-018-9009-4. [DOI] [PubMed] [Google Scholar]
- 33.Sood S, Rishi P, Dhawan V, Sharma S, Ganguly NK. Protection mediated by antibodies to iron-regulated outer-membrane proteins of S. typhi in a mouse peritonitis model. Mol Cell Bio. 2005;273:69–78. doi: 10.1007/s11010-005-7756-8. [DOI] [PubMed] [Google Scholar]
- 34.Muthukkumar S, Muthukkaruppan VR. Mechanism of protective immunity induced by porin lipopolysaccharide against murine salmonellosis. Infect Immun. 1993;61:3017–3025. doi: 10.1128/iai.61.7.3017-3025.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chibber S, Bhardwaj SB. Protection in a mouse peritonitis model mediated by iron-regulated outer-membrane protein of Salmonella typhi coupled to its Vi antigen. J med microbiol. 2004;53:705–709. doi: 10.1099/jmm.0.05378-0. [DOI] [PubMed] [Google Scholar]
- 36.Arora, S., Kaur, I. P., Chopra, K. & Rishi, P. Efficiency of double layered microencapsulated probiotic to modulate proinflammatory molecular markers for the management of alcoholic liver disease. Mediators inflamm. 2014 (2014). [DOI] [PMC free article] [PubMed]
- 37.Patel NK, Bhutani KK. Pinostrobin and Cajanus lactone isolated from Cajanus cajan (L.) leaves inhibits TNF-α and IL-1β production: In vitro and in vivo experimentation. Phytomedicine. 2014;21:946–953. doi: 10.1016/j.phymed.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 38.Akgün J, et al. The role of alveolar epithelial type II-like cells in uptake of structurally different antigens and in polarisation of local immune responses. PloS ONE. 2015;10:e0124777. doi: 10.1371/journal.pone.0124777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carrión J, et al. UCP2 deficiency helps to restrict the pathogenesis of experimental cutaneous and visceral leishmaniosis in mice. PLoS Negl Trop Dis. 2013;7:e2077. doi: 10.1371/journal.pntd.0002077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sood S, Rishi P, Vohra H, Sharma S, Ganguly NK. Cellular immune response induced by Salmonella enterica serotype Typhi iron-regulated outer-membrane proteins at peripheral and mucosal levels. J Med Microbiol. 2005;54:815–821. doi: 10.1099/jmm.0.46042-0. [DOI] [PubMed] [Google Scholar]
- 41.Mirza SH, Beechmg NJ, Hart CA. Multi-drug resistant typhoid: a global problem. J Med Microbiol. 1996;44:317–319. doi: 10.1099/00222615-44-5-317. [DOI] [PubMed] [Google Scholar]
- 42.Baker S, et al. Detection of Vi-negative Salmonella enterica serovar typhi in the peripheral blood of patients with typhoid fever in the Faisalabad region of Pakistan. J Clin Microbiol. 2005;43:4418–4425. doi: 10.1128/JCM.43.9.4418-4425.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haque, A. Significance of Vi Negative Isolates of Salmonella enterica Serovar Typhi. In Infectious Diseases and Nanomedicine III. Springer, Singapore. 9–18 (2018). [DOI] [PubMed]
- 44.Bajracharya D, et al. 25 Years after Vi typhoid vaccine efficacy study, typhoid affects significant number of population in Nepal. PloS One. 2014;9:e77974. doi: 10.1371/journal.pone.0077974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jin C, et al. Efficacy and immunogenicity of a Vi-tetanus toxoid conjugate vaccine in the prevention of typhoid fever using a controlled human infection model of Salmonella Typhi: a randomised controlled, phase 2b trial. Lancet. 2017;390:2472–2480. doi: 10.1016/S0140-6736(17)32149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Charles RC, et al. Characterization of anti-Salmonella enterica serotype Typhi antibody responses in bacteremic Bangladeshi patients by an immunoaffinity proteomics-based technology. Clin Vaccine Immunol. 2011;17:1188–1195. doi: 10.1128/CVI.00104-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Song J, Gao X, Galán JE. Structure and function of the Salmonella Typhi chimaeric A2B5 typhoid toxin. Nature. 2013;499:350–354. doi: 10.1038/nature12377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mao X, DiRienzo JM. Functional studies of the recombinant subunits of a cytolethal distending holotoxin. Cell microbiol. 2002;4:245–255. doi: 10.1046/j.1462-5822.2002.00186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Galán JE. Typhoid toxin provides a window into typhoid fever and the biology of Salmonella Typhi. Proc Nati Acad Sci USA. 2016;113:6338–6344. doi: 10.1073/pnas.1606335113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rodriguez-Rivera LD, Bowen BM, den Bakker HC, Duhamel GE, Wiedmann M. Characterization of the cytolethal distending toxin (typhoid toxin) in non-typhoidal Salmonella serovars. Gut pathog. 2015;7:19. doi: 10.1186/s13099-015-0065-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thomson CA, Olson M, Jackson LM, Schrader JW. A simplified method for the efficient refolding and purification of recombinant human GM-CSF. PLoS One. 2012;7:e49891. doi: 10.1371/journal.pone.0049891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Elwell CA, Dreyfus LA. DNase I homologous residues in CdtB are critical for cytolethal distending toxin‐mediated cell cycle arrest. Mol Microbial. 2000;37:952–963. doi: 10.1046/j.1365-2958.2000.02070.x. [DOI] [PubMed] [Google Scholar]
- 53.Guidi R, et al. Chronic exposure to the cytolethal distending toxins of G ram‐negative bacteria promotes genomic instability and altered DNA damage response. Cell microbiol. 2013;15:98–113. doi: 10.1111/cmi.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bezine E, Vignard J, Mirey G. The cytolethal distending toxin effects on Mammalian cells: a DNA damage perspective. Cell. 2014;3:592–615. doi: 10.3390/cells3020592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lara-Tejero M, Galán JE. CdtA, CdtB, and CdtC form a tripartite complex that is required for cytolethal distending toxin activity. Infect Immun. 2001;69:4358–4365. doi: 10.1128/IAI.69.7.4358-4365.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lewis DA, et al. Characterization of Haemophilus ducreyi cdtA, cdtB, and cdtC mutants in in-vitro and in-vivo systems. Infect Immun. 2001;69:5626–5634. doi: 10.1128/IAI.69.9.5626-5634.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shenker BJ, Hoffmaster RH, McKay TL, Demuth DR. Expression of the cytolethal distending toxin (Cdt) operon in Actinobacillus actinomycetemcomitans: evidence that the CdtB protein is responsible for G2 arrest of the cell cycle in human T cells. J Immunol. 2000;165:2612–2618. doi: 10.4049/jimmunol.165.5.2612. [DOI] [PubMed] [Google Scholar]
- 58.Liang L, et al. Immune profiling with a Salmonella Typhi antigen microarray identifies new diagnostic biomarkers of human typhoid. Sci Rep. 2013;3:1043. doi: 10.1038/srep01043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jiao XD, Cheng S, Hu YH, Sun L. Comparative study of the effects of aluminum adjuvants and Freund’s incomplete adjuvant on the immune response to an Edwardsiella tarda major antigen. Vaccine. 2010;28:1832–1837. doi: 10.1016/j.vaccine.2009.11.083. [DOI] [PubMed] [Google Scholar]
- 60.Sharma P, et al. Mechanism of protection provided by active immunization with porins in mice challenged with Salmonella typhi. Jpn J Exp Med. 1990;60:247–252. [PubMed] [Google Scholar]
- 61.Derrien M, et al. Mucin-bacterial interactions in the human oral cavity and digestive tract. Gut Microbes. 2010;1:254–268. doi: 10.4161/gmic.1.4.12778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Al-Banna N, Raghupathy R, Albert MJ. Correlation of proinflammatory and anti-inflammatory cytokine levels with histopathological changes in an adult mouse lung model of Campylobacter jejuni infection. Clin Vaccine Immunol. 2008;15:1780–1787. doi: 10.1128/CVI.00193-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Henderson, B., Poole, S. & Wilson, M. Bacteria-cytokine interactions in health and disease. Portland Press, London, United Kingdom (1998).
- 64.Opal SM, Depalo VA. Anti-inflammatory cytokines. Chest. 2000;117:1162–1172. doi: 10.1378/chest.117.4.1162. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the relevant data are within the manuscript and in the supplementary information.