Abstract
Enantiomerically enriched indole-containing heterocycles play a vital role in bioscience, medicine, and chemistry. As one of the most attractive subtypes of indole alkaloids, highly substituted tetrahydro-γ-carbolines are the basic structural unit in many natural products and pharmaceuticals. However, the syntheses of tetrahydro-γ-carbolines with high functionalities from readily available reagents are significant challenging. In particular, the stereodivergent syntheses of tetrahydro-γ-carbolines containing multi-stereogenic centers remain quite difficult. Herein, we report an expedient and stereodivergent assembly of tetrahydro-γ-carbolines with remarkably high levels of stereoselective control in an efficient cascade process from aldimine esters and indolyl allylic carbonates via a synergistic Cu/Ir catalyst system. Control experiments-guided optimization of synergistic catalysts and mechanistic investigations reveal that a stereodivergent allylation reaction and a subsequent highly stereoselective iso-Pictet-Spengler cyclization are the key elements to success.
Subject terms: Asymmetric catalysis, Synthetic chemistry methodology
Tetrahydro-γ-carbolines are the basic structural unit in many natural products and pharmaceuticals. Here, the authors report a synergistic Cu/Ir system to assemble chiral tetrahydro-γ-carbolines via a stereodivergent allylation followed by a stereoselective iso-Pictet-Spengler cyclization.
Introduction
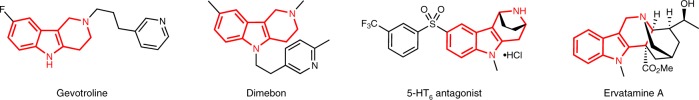
Catalytic asymmetric cascade reaction is recognized as one of the most efficient strategies for the synthesis of enantioenriched molecules1–3. The power of catalytic asymmetric cascade process is exceptionally intriguing in qualifying direct transformations of simple and achiral starting materials into complex and chiral compounds incorporating multi-stereogenic centers, as constructions of such frameworks are time-consuming process and normally require multiple synthetic steps. Accordingly, much attention has been paid to the development of such cascade methodologies, where the focus has been on the synthesis of biologically important and highly functionalized heterocycles4–9. The indole-containing heterocycles hold a vital role in modern organic chemistry owing to their widely distribution in nature10–14, and the tetrahydro-γ-carboline scaffolds are one of the most attractive subtypes of indole alkaloids since they have long been identified as structural cores in many bioactive natural products and pharmaceuticals15–21. Representative examples, such as gevotroline, dimebon, tubastatin A, (7S,10R)-epiminocyclohepta[b]indole and ervatamine A22–27, all have polycyclic tetrahydro-γ-carboline structures (Fig. 1).
Fig. 1.
Representative examples of bioactive compounds. Tetrahydro-γ-carboline scaffolds are structural cores in many natural products and pharmaceuticals.
The appealing skeletons and potential biological activities of tetrahydro-γ-carboline skeletons have received much attention among medicinal and synthetic chemists, and a number of approaches to chiral tetrahydro-γ-cabolines have been developed including the classical resolution28 and diastereoselective iso-Pictet-Spengler cyclization reactions29–31. Catalytic asymmetric synthesis of enantiomerically enriched tetrahydro-γ-carbolines was also realized recently. Pioneering study by Jacobsen and co-workers has been carried out on organo-catalyzed enantioselective iso-Pictet-Spengler cyclization reaction of isotryptamines and aldehydes32. Most recently, Shi and Deng, respectively, identified azomethine ylides as efficient reaction components in chiral Bronsted or chiral Lewis acid-catalyzed cycloadditions for the elegant construction of highly substituted tetrahydro-γ-carbolines with good to high diastereoselectivity and excellent enantioselectivity33–36. All those reported catalytic asymmetric cyclization methods have focused on the preparation of only one stereoisomer or its enantiomer, which was rendered by selecting switch between a pair of enantiomeric catalysts. However, no stereodivergent protocol has been developed so far for the construction of biologically active tetrahydro-γ-carboline derivatives bearing multiple stereogenic centers.
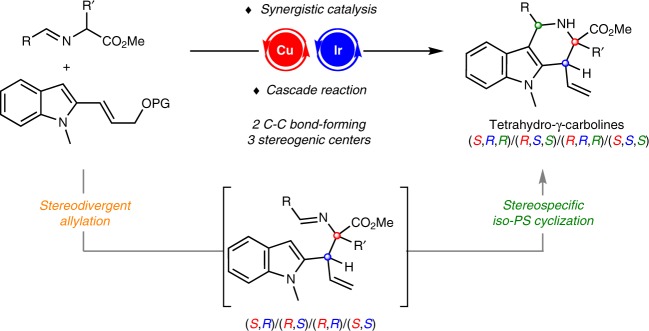
Stereodivergent synthesis37–50 has emerged as a powerful synthetic tool that allows access to stereoisomers of a product incorporating multiple stereogenic centers from the same set of substrates, which exactly caters for the ever-increasing drive in the pharmaceutical industry for molecular complexity in an effort to advance drug discovery and chemical-biology study51–56. Based on the utility of such strategy, we57 and others58 previously developed synergistic Cu/Ir catalyst systems for the stereodivergent access to α,α-disubstituted α-amino acids by the asymmetric allylation of aldimine esters with full control of the diastereoselectivity and enantioselectivity. The current design stemmed from the contemplation of the imino functional group retaining in the allylation products, which might serve as potential electrophilic moiety for a subsequent intramolecular nucleophilic cyclization to construct highly functionalized nitrogen-containing heterocycles. We envisioned that appropriate incorporation of indole moiety in allyl carbonate could facilitate a cascade allylation/iso-Pictet-Spengler cyclization, and therefore provides an expedient access to biologically important tetrahydro-γ-carboline derivatives with multiple stereocenters and high functionality. The cascade strategy that combines asymmetric allylation with subsequent intramolecular nucleophilic cyclization imparts three stereogenic centers to the overall two carbon–carbon bond-forming processes and constitutes an intriguing protocol to rapidly build complex molecular architectures from readily available reagents (Fig. 2).
Fig. 2.
Reaction design. Stereodivergent synthesis via synergistic catalytic asymmetric cascade allylation and iso-Pictet-Spengler cyclization reaction.
However, several challenging problems need to be considered in this reaction design: (1) the compatibility of indolyl-containing allyl carbonates in our previously developed Cu/Ir dual catalyst system, that is, no deterioration of the stereo-integrity in the initial allylation step; (2) the feasibility of the ensuing intramolecular cyclization under basic reaction condition, which is an essential pre-requisite for promoting the event of azomethine ylide-involved allylation process but inconsistent with the well-established acid-promoted Pictet-Spengler-type cyclization59,60; (3) the efficacy of the diastereoselectivity control of the second carbon–carbon bond-forming event in the ensuing iso-Pictet-Spengler cyclization with respect to how it is affected by the initially generated two contiguous stereogenic centers in the potential four sets of allylation stereoisomers.
Herein, we reported the development of stereodivergent assembly of tetrahydro-γ-carbolines via a synergistic Cu/Ir catalyst system to achieve a stereodivergent allylation of aldimine esters and indolyl allylic carbonates followed by a spontaneous intramolecular iso-Pictet-Spengler cyclization. The stereochemical outcome of the cascade process is remarkable in view of two sequential C–C bond-forming events along with three generated stereogenic centers. The current protocol enables a general process for the predictable preparation of four different stereoisomers of tetrahydro-γ-carbolines at will from readily available starting materials by using four available sets of catalyst permutations.
Results
Reaction development and optimization
We began with an investigation of the model reaction between glycine-derived aldimine ester 1a (serving as Cu-azomethine ylide61–63 precursor) and 2-indolyl allyl carbonate 2a (serving as Ir-π-ally64 precursor) in dichloromethane at room temperature under our previously established dual Cu/Ir catalyst system for the stereodivergent α-allylation of aldimine ester57. N,N-Diisopropylethylamine (DIPEA) was chosen as the base in order to suppress the undesired epimerization of the allylation products. With one set of previously utilized catalyst combination [Cu(I)/(S,Sp)-L1 + Ir(I)/(S,S,Sa)-L5], the designed cascade allylation/iso-Pictet-Spengler cyclization occurred smoothly and the desired tetrahydro-γ-carboline 3a was isolated in good yield albeit with a 1:1 diastereoselectivity and acceptable enantioselectivities (91% and 90% ee for the two inseparable diastereomers, respectively) (Table 1, entry 1). Similar stereoselectivity control (1:1 dr with 93% and 97% ee) was also observed when another set of diastereomeric catalyst combination [Cu(I)/(S,Sp)-L1 + Ir(I)/(R,R,Ra)-L5] was employed to promote this annulation (Table 1, entry 2). Considering two carbon–carbon bond-forming events accompanied by three generated stereogenic centers in this cascade allylation/iso-Pictet-Spengler process, we wondered whether the observed lower diastereoselectivity was caused by the coupling of Cu(I)-ylide and Ir(III)-π-allyl species or by the ensuing intramolecular iso-Pictet-Spengler cyclization. To investigate the stereoselective control of the allylation step, two control experiments catalyzed with the combination of [chiral Cu-complex + racemic Ir-complex] and [racemic Cu-complex + chiral Ir-complex] were performed, respectively. Employing the combined [Cu/(S,Sp)-L1 + Ir/rac-L5] complexes as the catalyst, the same two diastereomers were generated in good yields with similar diastereoselectivity (1:1 dr) and enantioselectivities (88 and 89% ee) (Table 1, entry 3). On the contrary, using the combined [Cu/rac-L1 + r/(S,S,Sa)-L5] complexes as the catalyst, 1.6:1 diastereoselective ratio was observed for the two cycloisomers with only 22 and 15% ee, respectively (Table 1, entry 4). The obtained cycloadducts were revealed as the same two sets of stereoisomers with the crude 1H NMR spectra analysis regardless of the different set of catalyst combination utilized in the above four experiments. In consideration of the similar enantioselectivity controls delivered by Cu(I)/(S,Sp)-L1 combined respectively with Ir(I)/(S,S,Sa)-L5, Ir(I)/(R,R,Ra)-L5 or Ir(I)/rac-L5, we surmised that the unsatisfied diastereoselectivity in this cascade process was probably caused by the inferior stereoselectivity control in the first allylation step, which was caused by the indistinctively facial recognition of the in situ-generated electrophilic indole-containing Ir-π-allyl intermediate coordinated with (S,S,Sa)-L5 or (R,R,Ra)-L5, and the ensuing intramolecular cyclization is highly stereoselective transformation. The indiscriminating facial selectivity of the in situ-generated nucleophilic rac-Cu(I)-ylide further deteriorated the whole stereochemical outcome and rationalizes the worse diastereoselectivity and enantioselectivity control associated with the catalyst combination of [Cu/rac-L1 + Ir/(S,S,Sa)-L5] (Table 1, entry 4). Therefore, in order to improve the diastereoselectivity, we further examined several chiral phosphoramidite ligands in Ir-complex combined with Cu(I)/(S,Sp)-L1 complex, as summarized in Table 1. The employment of the chiral ligand (Sa,R,R)-L6, a diastereoisomer of (Sa,S,S)-L5, delivered 2:7 diastereoselectivity and slightly enhanced enantioselectivities (92% and 99% ee) (Table 1, entry 5). The reactions with Ir-complex coordinated by ligand L7 or L8 bearing bulky chiral amine moieties afforded the cycloadducts with a slightly lower or reversed diastereoselectivity (Table 1, entries 6 and 7). To our delight, after screening THQPhos (R,Ra)-L9 and (S,Ra)-L10 developed by You’s group65 (Table 1, entries 8 and 9), we found that the combined [Cu(I)/(S,Sp)-L1 + Ir(I)/(R,Ra)-L9] complexes provided the promising results in terms of diastereoselectivity and enantioselectivity (>20:1 dr and 99% ee). Subsequently, a series of chiral Phosferrox ligands with various substituents on the oxazoline ring were further evaluated (Table 1, entries 8 and 10–12), and Cu(I)/L2-L4 complex combined with Ir(I)/(R,Ra)-L9 complex all exhibited high catalytic activities with excellent enantioselectivities but slightly lower diastereoselectivities. No reaction occurred when acyl protected cinnamyl alcohol was employed as the π-ally precursor, and tert-butyl carbonate was also proved to be unsuccessful in terms of yield and diastereoselectivity (Table 1, entries 13 and 14). Finally, two additional control experiments executed in the absence of either Cu(I)/L1 or Ir(I)/L9 are proved to be nonreactive, which verifies the superiority of synergistic catalysis in this cascade transformation (entries 15 and 16).
Table 1.
Optimization of reaction conditionsa.
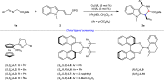 | ||||||
|---|---|---|---|---|---|---|
| Entry | L for Cu | L for Ir | PG | Yield (%)b | drc | ee (%)d |
| 1 | (S,Sp)-L1 | (Sa,S,S)-L5 | CO2Me | 95 | 1:1 | 91 (90) |
| 2 | (S,Sp)-L1 | (Ra,R,R)-L5 | CO2Me | 96 | 1:1 | 93 (97) |
| 3 | (S,Sp)-L1 | rac-L5 | CO2Me | 95 | 1:1 | 88 (89) |
| 4 | rac-L1 | (Sa,S,S)-L5 | CO2Me | 94 | 1.6:1 | 22 (15) |
| 5 | (S,Sp)-L1 | (Sa,R,R)-L6 | CO2Me | 92 | 2:7 | 92 (99) |
| 6 | (S,Sp)-L1 | (Sa,S,S)-L7 | CO2Me | 90 | 1:1 | 98 (98) |
| 7 | (S,Sp)-L1 | (Sa,S,S)-L8 | CO2Me | 94 | 3:2 | 99 (93) |
| 8 | (S,Sp)-L1 | (R,Ra)-L9 | CO2Me | 98 | >20:1 | 99 |
| 9 | (S,Sp)-L1 | (S,Ra)-L10 | CO2Me | 96 | 9:1 | 99 (63) |
| 10 | (S,Sp)-L2 | (R,Ra)-L9 | CO2Me | 95 | 5:1 | 99 (97) |
| 11 | (S,Sp)-L3 | (R,Ra)-L9 | CO2Me | 89 | 10:1 | 99 (89) |
| 12 | (S,Sp)-L4 | (R,Ra)-L9 | CO2Me | 90 | 9:1 | 99 (94) |
| 13 | (S,Sp)-L1 | (R,Ra)-L9 | Ac | n.r. | - | - |
| 14 | (S,Sp)-L1 | (R,Ra)-L9 | Boc | 30 | 12:1 | 99 |
| 15e | - | (R,Ra)-L9 | CO2Me | n.r. | - | - |
| 16f | (S,Sp)-L1 | - | CO2Me | n.r. | - | - |
aAll reactions were carried out with 0.30 mmol of 1a, 0.20 mmol of 2 and 0.40 mmol of DIPEA in 2 mL of CH2Cl2 at room temperature (see Supplementary Methods for the detailed preparation of Cu/L and Ir(I)/L complexes). Cu(I) = Cu(MeCN)4BF4, Ir(I) = [Ir(COD)Cl]2
bYields refer to the isolated products after chromatographic purification
cThe dr value was determined by the crude 1H NMR
dThe ee value was determined by HPLC analysis
eWithout Cu(I)/(S,Sp)-L1
fWithout Ir(I)/(R,Ra)-L9
Substrate scope
With the optimized reaction conditions in hand, we then decided to explore various aldimine esters to examine the generality of this cascade process with the combined [Cu(I)/(S,Sp)-L1 + Ir(I)/(R,Ra)-L9] complexes as the dual catalysts. The representative results are shown in Table 2. Various aldimine esters derived from glycinate bearing electron-deficient (para-Cl, ortho-Cl), electron-neutral, and electron-rich substituents (para-Me, meta-Me, ortho-Me) on the aromatic ring reacted smoothly with 2-indoly allylic carbonate 2a, affording the corresponding tetrahydro-γ-carbolines in high yields (78–99%), exclusive diastereoselectivity (>20:1 dr) and almost perfect enantioselectivities (99% ee) (Table 2, entries 1–6). The substitution pattern of the arene had no negative effect on the stereoselectivity of this cascade reaction, and ortho-chloro and ortho-methyl substituted aldimino ester 1b and 1f were well tolerated in this annulation delivering the desired tetrahydro-γ-carbolines 3b and 3f in good yields with excellent enantioselectivities (Table 2, entries 2 and 6). Encouraged by the excellent results for less sterically congested α-nonsubstituted aldimine esters derived from glycinate, we then investigated the performance of more challenging α-substituted aldimine ester in this annulation process, from which a N-quaternary stereogenic center can be generated along with two tertiary stereogenic centers in the heterocyclic rings. Switching the base from DIPEA to Cs2CO3 under otherwise similar reaction conditions, we were particularly delighted to find that a wide array of aldimine esters derived from (±)-alanine have proved to be viable ylide precursors, affording the corresponding cycloadducts in good to high yields with exclusive diastereoselectivities and excellent enantioselectivities, regardless of electronic properties and positions of the substituted groups on the arene ring (Table 2, entries 7–15). Furthermore, aldimine esters bearing heteroaromatic groups underwent this transformation successfully as fused 2-naphthylaldehyde derived aldimine ester, affording the desired cycloadducts in good to high yields with excellent stereoselective control (Table 2, entries 16–18). In addition, aldimine ester 1s bearing cinnamyl group was proven to be a viable substrate, affording satisfied yield and stereoselectivity (Table 2, entry 19). Importantly, less-reactive alkyl imino esters 1t derived from valeraldehyde was also tolerated in this reaction, resulting in the corresponding adduct 3t in good yield with excellent diastereoselectivity and enantioselectivity (Table 2, entry 20). Aldimine esters derived from other α-substituted amino esters have also been investigated. Under the above reaction conditions, satisfied yields and exclusive diastereoselectivities and excellent enantioselectivities were consistently obtained for (±)-2-aminobutyric acid, (±)-phenylalanine, and (±)-glutamic acid derived aldimine esters (Table 2, entries 22–24). Notably, (±)-homoserine-derived cyclic aldimine ester 1y also worked well in this cascade reaction (81% yield, >20:1 dr and 99% ee), affording the corresponding highly functionalized spiro heterocycle 3y incorporating both biologically important tetrahydro-γ-carboline15–21 and γ-butyrolactone66,67 moieties (Table 2, entry 25).
Table 2.
Substrate scope of aldimine estersa.
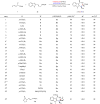 |
aAll reactions were carried out with 0.30 mmol of 1, 0.20 mmol of 2a in 2 mL of CH2Cl2 at room temperature for 6–10 h
bYields refer to the isolated products after chromatographic purification
cThe dr value was determined by the crude 1H NMR
dThe ee value was determined by HPLC analysis
eCs2CO3 was used as the base
ftert-Butyl aldimine ester was used
Next, we examined the generality of this asymmetric cascade allylation/iso-Pictet-Spengler cyclization process with respect to 2-indolyl allyl carbonates. The representative results are summarized in Table 3. Reactions of various 2-indolyl allyl carbonates containing N-methyl or N-benzyl group all provided the corresponding cycloadducts in good yields (80–96%) with high diastereoselectivities (12:1–>20:1) and excellent enantioselectivities (99% ee) (Table 3, entries 1–9). Meanwhile, the cascade process also proved to be almost unbiased toward either electron property (electron-deficient, -rich or -neutral) or substitution position (C-4, -5, -6, or -7) on the indole core, and the corresponding cycloadducts were obtained in good yields with excellent stereoselectivities. However, no reaction occurred when 2-indolyl allyl carbonate bearing methyl group at C3 position on the indole ring probably owing to the steric congestion. (E)-methyl (3-(1-methyl-1H-pyrrol-3-yl)allyl) carbonate, in which indole ring is replaced by pyrrole one, was also tested under the standard reaction conditions, and the reaction became messy and no desired product was observed.
Table 3.
Substrate scope of 2-indolyl alyl carbonatesa.
| Entry | R | R' | PG | (1R,3S,4R)-3 | yield (%)b | drc | ee (%)d |
|---|---|---|---|---|---|---|---|
| 1 | H | 5-Me | Me | 3z | 92 | 11:1 | 99 |
| 2 | H | 5-Cl | Me | 3A | 96 | 14:1 | 99 |
| 3 | Me | 4-Me | Me | 3B | 85 | >20:1 | 99 |
| 4 | Me | 5-Me | Me | 3C | 84 | >20:1 | 99 |
| 5 | Me | 6-Me | Me | 3D | 88 | >20:1 | 99 |
| 6 | Me | 7-Me | Me | 3E | 95 | >20:1 | 99 |
| 7 | Me | 5-Cl | Me | 3F | 93 | >20:1 | 99 |
| 8 | Me | 5-Br | Me | 3G | 80 | >20:1 | 99 |
| 9 | Me | H | Bn | 3H | 95 | >20:1 | 99 |
aAll reactions were carried out with 0.30 mmol of 1, 0.20 mmol of 2 in 2 mL of CH2Cl2 at room temperature for 6–10 h
bYields refer to the isolated products after chromatographic purification
cThe dr value was determined by the 1H NMR
dThe ee value was determined by HPLC analysis
After investigating the generality of this asymmetric cascade allylation/iso-Pictet-Spengler cyclization with the set of [Cu(I)/(S,Sp)-L1 + Ir(I)/(R,Ra)-L9] catalyst, we further explored the performance of the other three sets of catalyst combinations with 2-indolyl allylic carbonate 2a under otherwise similar reaction conditions. Aldimine esters 1a, 1c, 1d, and 1g were chosen as the representative ylide precursors. As tabulated in Table 4, with the full four sets of [Cu/L1 + Ir/L9] combinations, in each case, four stereoisomeric cycloadducts containing three stereogenic centers were generated in good yields with high diastereoselectivity and excellent enantioselectivities via a ligand-controlled stereodivergent allylation followed by a spontaneous stereoselective intramolecular iso-Pictet-Spengler cyclization. The absolute configuration of cycloadduct 3g and its enantiomer using [Cu(I)/(R,Rp)-L1 + Ir(I)/(S,Sa)-L9] or [Cu(I)/(S,Sp)-L1 + Ir(I)/(R,Ra)-L9] as the catalyst combinations was unequivocally determined as (1S,3R,4S) and (1R,3S,4R) by X-ray diffraction analysis, respectively; the absolute configuration of cycloadduct 3c and its enantiomer using [Cu(I)/(S,Sp)-L1 + Ir(I)/(S,Sa)-L9] or [Cu(I)/(R,Rp)-L1 + Ir(I)/(R,Ra)-L9] as the catalyst combinations were unequivocally determined as (1S,3S,4S) and (1R,3R,4R) by X-ray diffraction analysis, respectively.
Table 4.
Representative examples of stereodivergent synthesis of four stereoisomers from reaction of 2-indolyl allyl carbonates with aldimine estersa.
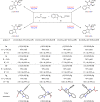 |
aAll reactions were carried out with 0.30 mmol of 1 and 0.20 mmol of 2a in 2 mL of CH2Cl2 at room temperature. Yields refer to the isolated products after chromatographic purification. The dr value was determined by the crude 1H NMR. The ee value was determined by HPLC analysis
Gram scale and synthetic applications
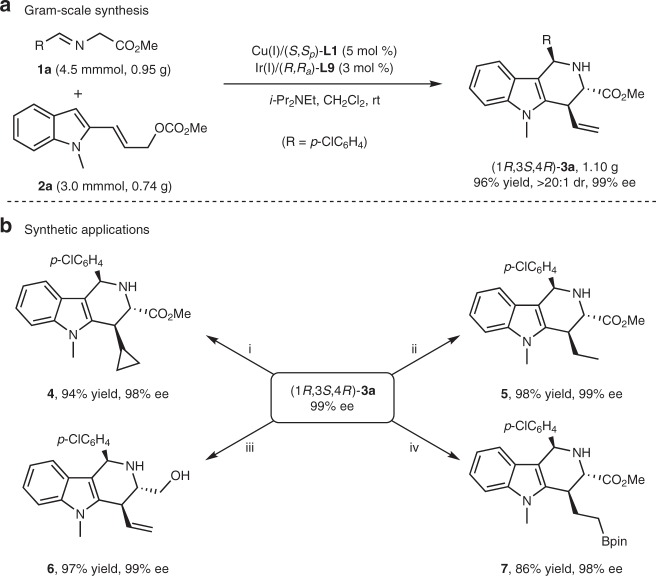
To highlight the synthetic utility of this asymmetric cascade reaction, a gram-scale synthesis of (1R,3S,4R)-3a was performed under the standard conditions with the combined [Cu(I)/(S,Sp)-L1 + Ir(I)/(R,Ra)-L9] complexes as the co-catalysts, and comparable yields were obtained without loss of diastereoselectivity and enantioselectivity as shown in Fig. 3a. The tetrahydro-γ-carboline derivatives obtained herein can be readily converted into synthetically useful compounds in a facile manner. For example, subjecting 3a to a Pd(II)-catalyzed cyclopropanation with diazomethane provided compound 4 in 94% yield and with maintained stereoselectivity. Exposure of 3a to usual hydrogenation conditions (H2, Pd/C in methanol) at room temperature afforded compound 5 in 98% yield without erosion of stereoselectivity. In addition, reduction of 3a with DIBAL-H furnished the amino-alcohol compound 6 in 97% yield without loss of enantioselectivity. Hydroboration of terminal alkene in 3a catalyzed by Ir/dppm complex afforded the borate 7 in 86% yield68. These elaborations clearly demonstrate the utility of the asymmetric cascade allylation/iso-Pictet-Spengler cyclization as an expedient method to synthesize various optically active tetrahydro-γ-carboline derivatives (Fig. 3b).
Fig. 3.
Gram-scale synthesis and derivatization of the cycloadduct 3a. a Gram-scale reaction. b Synthetic applications. i: Pd(OAc)2 (1 mol %), CH2N2, Et2O, −20 °C; ii: Pd/C (catal.), H2 (1 atm), MeOH, room temperature; iii: DIBAL-H, THF, −40 °C to rt; iv: [Ir(COD)Cl]2 (3 mol %), dppm (6 mol %), HBpin, CH2Cl2, rt.
Mechanism explanations and control experiments
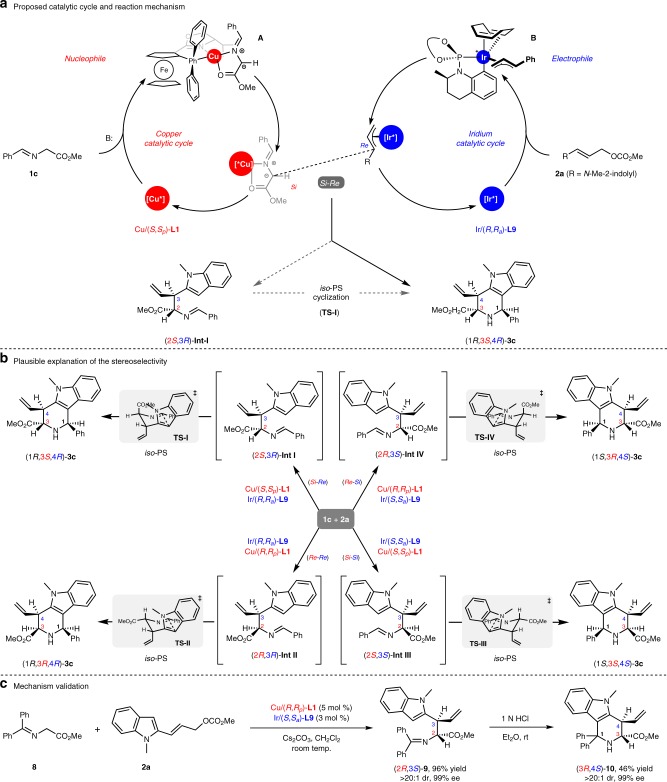
A full mechanism involved two catalytic cycles was proposed for this cascade allylation/iso-Pictet-Spengler cyclization with the set of catalyst combination of [Cu(I)/(S,Sp)-L1 + Ir(I)/(R,Ra)-L9]. As shown in Fig. 4a, the nucleophilic Cu(I)-azomethine ylide (A) coordinated by chiral ligand (S,Sp)-L1 was in situ formed under basic condition. The orientation of Cu(I)-ylide (A) was supported by the reported DFT calculations69, and its Re-face was blocked by the isopropyl group on the oxazoline ring. Meanwhile, the electrophilic Ir(III)-π-allyl species (B) was generated by 2-indolyl allyl carbonate (2a) and Ir/(R,Ra)-L9 complex through decarboxylative oxidative addition. The orientation of Ir(III)-π-allyl intermediate (B) was revealed by the reported X-ray structure of the related metallacyclic allyl iridium complex65, and its Si-face was shielded by the cyclometallated moiety and blocks the approach of the aforementioned Cu(I)-ylide A. Thus, the two catalytic cycles merge via a preferential nucleophilic addition of the Si-face of the Cu(I)-ylide species to the Re-face of the Ir(III)-π-allyl species (Si-Re), in which the two distinct metal complexes exert almost absolute stereoselective control over the two active intermediates, leads to the allylation intermediate (2S,3R)-Int-I and regenerates both catalysts. Based on the relative and absolute configuration of the final cycloadduct, it is believed that the ensuing intramolecular nucleophilic attack of the indole C3 position to the Si-face of imine moiety in highly stereoselective manner followed by deprotonation (iso-Pictet-Spengler cyclization) delivers tetrahydro-γ-carboline 3c with (1R,3S,4R)-configuration, in which the diastereoselectivity of intramolecular cyclization is harnessed likely through a highly ordered chair-like Zimmerman-Traxler transition state TS-I70. The highly stereoselective iso-Pictet-Spengler cyclization of allylation intermediates (2R,3R)-Int-II, (2S,3S)-Int-III, and (2R,3S)-Int-IV correlated with the other three set of catalyst combinations, could also be rationalized via similar Zimmerman-Traxler transition states TS-II-IV as shown in Fig. 4b, respectively. The electrophilicity of the imine moiety in the allylation intermediates might be further enhanced via a coordination of copper cation, which facilitates the ensuing intramolecular cyclization.
Fig. 4.
Proposed mechanism with experimental validation and rationalization of the stereoselectivity. a Proposed catalytic cycle. b Plausible explanation of the stereoselectivity. c Mechanism validation.
To gain insight into the mechanism and stereoselective control of this cascade reaction, control experiments were further performed to validate the key allylation intermediate. The attempt to directly isolate the allylation intermediate through interrupting the reaction at incomplete conversion failed, probably due to the intrinsically preferred intramolecular cyclization. Then, benzophenone derived ketimine ester 8 instead of aldimine ester was utilized in order to slow or suppress the ensuing intramolecular cyclization with the hope of enhancing the steric hindrance of the imine moiety to reduce its electrophilicity. To our delight, the reaction of ketimine ester 8 under standard reaction conditions with the combined [Cu(I)/(R,Rp)-L1 + Ir(I)/(S,Sa)-L9] complexes, did stall after the first C-C bond-forming step, and the branched allylation intermediate (2R,3S)-9 was accumulated in high yield with excellent stereoselectivity without further intramolecular cyclization to the tetrahydro-γ-carboline under the optimal reaction conditions. Notably, an acid-promoted intramolecular iso-Pictet-Spengler cyclization of the allylation intermediate (2R,3S)-9 occurred smoothly, and successfully delivered (3R,4S)-tetrahydro-γ-carboline 10 in moderate yield without erosion of the diastereoselectivity and enantioselectivity (Fig. 4c). The direct isolation of allylation intermediate (2R,3S)-9 and the proton-mediated further cyclization explicitly supported the proposed cascade reaction pathway incorporating sequential stereodivergent allylation and highly stereoselective intramolecular cyclization.
Discussion
In summary, we have successfully developed a synergistic Cu/Ir-catalyzed asymmetric cascade allylation/iso-Pictet-Spengler cyclization reaction with aldimine esters and indolyl allylic carbonates. Control experiments-guided development of a dual metallic catalytic system allows stereoselective control over in situ formed Cu(I)-ylide and Ir(III)-π-ally species independently. Consequently, the desired cascade reaction is established as the stereodivergent allylation followed by subsequent highly stereoselective iso-Pictet-Spengler cyclization while tolerating a wide range of variations including both the ylide precursors and the allyl precursors. The cascade process presented herein opens up a conceptually novel prospect for the stereoselective construction of highly functionalized tetrahydro-γ-carboline, a valuable core structure for drug discovery, up to four stereoisomers with predictable stereoselective control.
Methods
General reaction procedure
A flame dried Schlenk tube A was cooled to room temperature and filled with N2. To this flask were added [Ir(COD)Cl]2 (0.003 mmol, 1.5 mol %), (R,Ra)-Me-THQphos-L9 (0.006 mmol, 3.0 mol %), degassed THF (0.5 mL) and degassed n-propylamine (0.5 mL). The reaction mixture was heated at 50 °C for 30 min and then the volatile solvents were removed under vacuum to gain a pale yellow solid. Meanwhile, Cu(MeCN)4BF4 (0.01 mmol, 5 mol %) and (S,Sp)-iPr-Phosferrox-L1 (0.011 mmol, 5.5 mol %) were dissolved in 1.0 mL of CH2Cl2 in a Schlenk tube B, and stirred at room temperature for ~ 30 min. Indole-derived allylic carbonates (0.20 mmol), aldimine esters (0.30 mmol), base (0.40 mmol DIPEA for glycine-derived aldimine esters and 0.40 mmol Cs2CO3 for α-substituted aldimine esters) and CH2Cl2 (1.0 mL) were added into the Schlenk tube A and filled with N2. The Cu/L1 complex solution was then transferred from the Schlenk tube B to the Schlenk tube A via syringe. Finally, the reaction mixture was continuously stirred at room temperature under N2 atmosphere. Although the starting material was consumed (monitored by thin layer chromatography), the reaction mixture was quenched by adding 1 m HCl aqueous solution (2.0 mL) and stirring vigorously for 1 min. The organic layers were separated, and the aqueous layer was extracted with CH2Cl2 (5.0 mL × 2). The organic layer was combined, washed with saturated brine (10 mL) and dried over anhydrous Na2SO4. The organic solvent was removed by rotary evaporation to obtain a crude mixture, which was purified by flash column chromatography to give the pure product. The dr value was determined by 1H NMR spectrum of the product, and the enantiomeric excess was recorded by HPLC analysis in comparison with the racemic sample.
Supplementary information
Acknowledgements
This work was supported by NSFC (21525207, 21772147), China Postdoctoral Science Foundation funded project (2017M620331, BX20190253). The Program of Introducing Talents of Discipline to Universities of China (111 Program) is also appreciated. We thank Prof. Shu-Li You at Shanghai Institute of Organic Chemistry (SIOC) for generously providing chiral ligand THQPhos (R,Ra)-L9 and (S,Ra)-L10. We also thank Prof. Ding-Ce Yan at Huazhong University of Science and Technology for solving the crystal structures of (1S,3S,4S)-3c, (1R,3R,4R)-3c and (1R,3S,4R)-3g; and Prof. Heng-Jiang Cong at Wuhan University for solving the crystal structure of (1S,3R,4S)-3c.
Author contributions
C.J.W. conceived and directed the project. S.M.X. performed experiments and prepared the Supplementary Information. L.W., C.S. L.X. & H.Y.T. took part in the reaction development and synthesized several substrates. C.J.W. and S.M.X. co-wrote the paper. All authors analyzed the data, discussed the results and commented on the manuscript.
Data availability
Experimental procedures and characterization data are available within this article and its Supplementary Information. Data are also available from the corresponding author on request. The X-ray crystallographic coordinates for the structures of compounds (1S,3R,4S)-3g, (1R,3S,4R)-3g, (1S,3S,4S)-3c, and (1R,3R,4R)-3c reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers 1915538, 1915539, 1915540, and 1915541. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information is available for this paper at 10.1038/s41467-019-13529-z.
References
- 1.Xu, P. F. & Wang, W. Catalytic Cascade Reactions (Wiley-VCH, Weinheim, 2014).
- 2.Wang, Q. & Zhu, J. Multicomponent Domino Process: Rational Design and Serendipity (Wiley-VCH, Weinheim, 2014).
- 3.Tietze, L. F., Brasche, G. & Gericke, K. M. Domino Reactions in Organic Synthesis (Wiley-VCH, Weinheim, 2006).
- 4.Liu X, Zheng H, Xia Y, Lin L, Feng X. Asymmetric cycloaddition and cyclization reactions catalyzed by chiral N, N’- dioxide- metal complexes. Acc. Chem. Res. 2017;50:2621–2631. doi: 10.1021/acs.accounts.7b00377. [DOI] [PubMed] [Google Scholar]
- 5.Chauhan P, Mahajan S, Enders D. Achieving molecular complexity via stereoselective multiple domino reactions promoted by a secondary amine organocatalyst. Acc. Chem. Res. 2017;50:2809–2821. doi: 10.1021/acs.accounts.7b00406. [DOI] [PubMed] [Google Scholar]
- 6.Ohno H, Inuki S. Recent progress in palladium-catalyzed cascade cyclization for natural product synthesis. Synthesis. 2017;50:700–710. doi: 10.1055/s-0036-1589165. [DOI] [Google Scholar]
- 7.Wang Y, Lu H, Xu PF. Asymmetric catalytic cascade reactions for constructing diverse scaffolds and complex molecules. Acc. Chem. Res. 2015;48:1832–1844. doi: 10.1021/acs.accounts.5b00217. [DOI] [PubMed] [Google Scholar]
- 8.Yu J, Zhou Y, Chen DF, Gong LZ. Organocatalytic asymmetric synthesis of chiral nitrogenous heterocycles and natural products. Pure Appl. Chem. 2014;86:1217–1226. doi: 10.1515/pac-2013-1208. [DOI] [Google Scholar]
- 9.Chen JB, Jia YX. Recent progress in transition-metal-catalyzed enantioselective indole functionalizations. Org. Biomol. Chem. 2017;15:3550–3567. doi: 10.1039/C7OB00413C. [DOI] [PubMed] [Google Scholar]
- 10.Joule JA, Mills K. Heterocyclic Chemistry. Oxford: Blackwell Science; 2000. [Google Scholar]
- 11.Pelletier, S. W. Alkaloids: Chemical and Biological Perspectives (John Wiley & Sons, 1983).
- 12.Radwanski ER, Last RL. Tryptophan biosynthesis and metabolism: biochemical and molecular genetics. Plant Cell. 1995;7:921–934. doi: 10.1105/tpc.7.7.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vitaku E, Smith DT, Njardarson JT. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J. Med. Chem. 2014;57:10257–10274. doi: 10.1021/jm501100b. [DOI] [PubMed] [Google Scholar]
- 14.De Sá Alves FR, Barreiro EJ, Fraga CAM. From nature to drug discovery: the indole scaffold as a ‘privileged structure’. Mini-Rev. Med. Chem. 2009;9:782–793. doi: 10.2174/138955709788452649. [DOI] [PubMed] [Google Scholar]
- 15.Dai J, Dan W, Zhang Y, Wang J. Recent developments on synthesis and biological activities of γ-carboline. Eur. J. Med. Chem. 2018;157:447–461. doi: 10.1016/j.ejmech.2018.08.015. [DOI] [PubMed] [Google Scholar]
- 16.Harbert CA, Plattner JJ, Welch WM, Weissman A, Koe BK. Neuroleptic activity in 5-aryltetrahydro-γ-carbolines. J. Med. Chem. 1980;23:635–643. doi: 10.1021/jm00180a011. [DOI] [PubMed] [Google Scholar]
- 17.Khorana N, et al. Binding of Tetrahydrocarboline Derivatives at Human 5-HT5A Receptors. J. Med. Chem. 2003;46:3930–3937. doi: 10.1021/jm030080s. [DOI] [PubMed] [Google Scholar]
- 18.Bridoux A, Millet R, Pommery J, Pommery N, Henichart JP. Synthesis and biological activity of N-aroyl-tetrahydro-γ-carbolines. Bioorg. Med. Chem. Lett. 2010;18:3910–3913. doi: 10.1016/j.bmc.2010.04.034. [DOI] [PubMed] [Google Scholar]
- 19.Ivachtchenko AV, et al. Synthesis and biological activity of 5-styryl and 5-phenethyl-substituted 2,3,4,5-tetrahydro-1H-pyrido[4,3-b]indoles. Bioorg. Med. Chem. Lett. 2010;20:78–82. doi: 10.1016/j.bmcl.2009.11.037. [DOI] [PubMed] [Google Scholar]
- 20.Fretz H, et al. Identification of 2-(2-(1-Naphthoyl)-8-fluoro-3,4-dihydro-1H-pyrido[4,3-b]indol-5(2H)-yl)acetic acid (Setipiprant/ACT-129968), a potent, selective, and orally bioavailable chemoattractant receptor-homologous molecule expressed on Th2 cells (CRTH2) antagonist. J. Med. Chem. 2013;56:4899–4911. doi: 10.1021/jm400122f. [DOI] [PubMed] [Google Scholar]
- 21.Wang S, et al. Novel carboline derivatives as potent antifungal lead compounds: design, synthesis, and biological evaluation. ACS Med. Chem. Lett. 2014;5:506–511. doi: 10.1021/ml400492t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dooy RS, et al. Effect of dimebon on cognition, activities of daily living, behaviour, and global function in patients with mild-to-moderate alzheimer’s disease: a randomised, double-blind, placebo-controlled study. Lancet. 2008;372:207–215. doi: 10.1016/S0140-6736(08)61074-0. [DOI] [PubMed] [Google Scholar]
- 23.Ivachtchenko AV, et al. Synthesis and biological evaluation of novel γ-carboline analogues of Dimebon as potent 5-HT6 receptor antagonists. Bioorg. Med. Chem. Lett. 2009;19:3183–3187. doi: 10.1016/j.bmcl.2009.04.128. [DOI] [PubMed] [Google Scholar]
- 24.Butler KV, et al. Rational design and simple chemistry yield a superior, neuroprotective HDAC6 inhibitor, tubastatin A. J. Am. Chem. Soc. 2010;132:10842–10846. doi: 10.1021/ja102758v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalin JH, Butler KV, Akimova T, Hancock WW, Kozikowski AP. Second-generation histone deacetylase 6 inhibitors enhance the immunosuppressive effects of Foxp3+ T-regulatory cells. J. Med. Chem. 2012;55:639–651. doi: 10.1021/jm200773h. [DOI] [PubMed] [Google Scholar]
- 26.Isherwood ML, et al. An efficient synthesis of (7S,10R)-2-bromo-5,6,7,8,9,10-hexahydro-7,10-epiminocyclohepta[b]indole: application in the preparation and structural confirmation of a potent 5-HT6 antagonist. Tetrahedron Asymmetry. 2012;23:1522–1527. doi: 10.1016/j.tetasy.2012.10.012. [DOI] [Google Scholar]
- 27.Zhang DB, et al. Ervatamines A-I, anti-inflammatory monoterpenoid indole alkaloids with diverse skeletons from Ervatamia hainanensis. J. Nat. Prod. 2015;78:1253–1261. doi: 10.1021/acs.jnatprod.5b00051. [DOI] [PubMed] [Google Scholar]
- 28.Vecchietti Vittorio, Clarke Geoffrey D., Colle Roberto, Giardina Giuseppe, Petrone Giuseppe, Sbacchi Massimo. (1S)-1-(Aminomethyl)-2-(arylacetyl)-1,2,3,4-tetrahydroisoquinoline and heterocycle-condensed tetrahydropyridine derivatives: members of a novel class of very potent .kappa. opioid analgesics. Journal of Medicinal Chemistry. 1991;34(8):2624–2633. doi: 10.1021/jm00112a042. [DOI] [PubMed] [Google Scholar]
- 29.García-Muñoz MJ, Foubelo F, Yus M. Stereoselective synthesis of 2-(2-aminoalkyl)- and 1,3-disubstituted tetrahydro-1H-pyrido[4,3-b]-benzofuran and indole derivatives. J. Org. Chem. 2016;81:10214–10226. doi: 10.1021/acs.joc.6b01047. [DOI] [PubMed] [Google Scholar]
- 30.Fandrick DR, et al. Highly diastereoselective zinc-catalyzed propargylation of tert-butanesulfinyl imines. Org. Lett. 2010;12:748–751. doi: 10.1021/ol9028258. [DOI] [PubMed] [Google Scholar]
- 31.Sheng YF, Li GQ, Kang Q, Zhang AJ, You SL. Asymmetric Friedel-Crafts reaction of 4,7-dihydroindoles with nitroolefins by chiral Brønsted acids under low catalyst loading. Chem. Eur. J. 2009;15:3351–3354. doi: 10.1002/chem.200900033. [DOI] [PubMed] [Google Scholar]
- 32.Lee Y, Klausen RS, Jacobsen EN. Thiourea-catalyzed enantioselective iso-Pictet-Spengler reactions. Org. Lett. 2011;13:5564–5567. doi: 10.1021/ol202300t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun XX, Zhang HH, Li GH, He YY, Shi F. Catalytic enantioselective and regioselective [3+3] cycloadditions using 2-indolylmethanols as 3 C building blocks. Chem. Eur. J. 2016;22:17526–17532. doi: 10.1002/chem.201603049. [DOI] [PubMed] [Google Scholar]
- 34.Liu YZ, Shang SJ, Zhu JY, Yang WL, Deng WP. Regioselective and stereoselective [3+3] annulation of ketones derived azomethine ylides with 2-indolylethylenes: direct access to highly substituted tetrahydro-γ-carbolines. Adv. Synth. Catal. 2018;360:2191–2203. doi: 10.1002/adsc.201800274. [DOI] [Google Scholar]
- 35.Zheng X, Yang WL, Liu YZ, Wu SX, Deng WP. Enantioselective synthesis of tropanes via [3+3] annulation of cyclic azomethine ylides with substituted 2-vinylindoles and 2-vinylpyrroles. Adv. Synth. Catal. 2018;360:2843–2853. doi: 10.1002/adsc.201800553. [DOI] [Google Scholar]
- 36.Yang WL, et al. Cu(I)-catalyzed chemoselective and stereoselective [3+3] cycloaddition of azomethine ylides with 2-indolylnitroethylenes: facile access to highly substituted tetrahydro-γ-carbolines. ACS Catal. 2016;6:5685–5690. doi: 10.1021/acscatal.6b01596. [DOI] [Google Scholar]
- 37.Krautwald S, Carreira EM. Stereodivergence in asymmetric catalysis. J. Am. Chem. Soc. 2017;139:5627–5639. doi: 10.1021/jacs.6b13340. [DOI] [PubMed] [Google Scholar]
- 38.Lin L, Feng X. Catalytic strategies for diastereodivergent synthesis. Chem. Eur. J. 2017;23:6464–6482. doi: 10.1002/chem.201604617. [DOI] [PubMed] [Google Scholar]
- 39.Krautwald S, Sarlah D, Schafroth MA, Carreira EM. Enantio- and diastereodivergent dual catalysis: α-allylation of branched aldehydes. Science. 2013;340:1065–1068. doi: 10.1126/science.1237068. [DOI] [PubMed] [Google Scholar]
- 40.Krautwald S, Schafroth MA, Sarlah D, Carreira EM. Stereodivergent α-allylation of linear aldehydes with dual iridium and amine catalysis. J. Am. Chem. Soc. 2014;136:3020–3023. doi: 10.1021/ja5003247. [DOI] [PubMed] [Google Scholar]
- 41.Sandmeier T, Krautwald S, Zipfel HF, Carreira EM. Stereodivergent dual catalytic α-allylation of protected α-amino- and α-hydroxyacetaldehydes. Angew. Chem. Int. Ed. 2015;54:14363–14367. doi: 10.1002/anie.201506933. [DOI] [PubMed] [Google Scholar]
- 42.Nasborg L, Halskov KS, Tur F, Monsted SMN, Jorgensen KA. Asymmetric γ-allylation of α,β-unsaturated aldehydes by combined organocatalysis and transition-metal catalysis. Angew. Chem. Int. Ed. 2015;54:10193–10197. doi: 10.1002/anie.201504749. [DOI] [PubMed] [Google Scholar]
- 43.Huo X, He R, Zhang X, Zhang W. An Ir/Zn dual catalysis for enantio- and diastereodivergent α-allylation of α-hydroxyketones. J. Am. Chem. Soc. 2016;138:11093–11096. doi: 10.1021/jacs.6b06156. [DOI] [PubMed] [Google Scholar]
- 44.Cruz FA, Dong VM. Stereodivergent coupling of aldehydes and alkynes via synergistic catalysis using Rh and Jacobsen’s amine. J. Am. Chem. Soc. 2017;139:1029–1032. doi: 10.1021/jacs.6b10680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiang X, Beiger JJ, Hartwig JF. Stereodivergent allylic substitutions with aryl acetic acid esters by synergistic iridium and Lewis base catalysis. J. Am. Chem. Soc. 2017;139:87–90. doi: 10.1021/jacs.6b11692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiang X, Boehm P, Hartwig JF. Stereodivergent allylation of azaaryl acetamides and acetates by synergistic iridium and copper catalysis. J. Am. Chem. Soc. 2018;140:1239–1242. doi: 10.1021/jacs.7b12824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaldre D, Klose I, Maulide N. Stereodivergent synthesis of 1,4-dicarbonyls by traceless charge-accelerated sulfonium rearrangement. Science. 2018;361:664–667. doi: 10.1126/science.aat5883. [DOI] [PubMed] [Google Scholar]
- 48.Zheng H, et al. Stereodivergent synthesis of vicinal quaternary-quaternary stereocenters and bioactive hyperolactones. Nat. Commun. 2018;9:1968. doi: 10.1038/s41467-018-04123-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bruffaerts J, Pierrot D, Marek I. Efficient and stereodivergent synthesis of unsaturated acyclic fragments bearing contiguous stereogenic elements. Nat. Chem. 2018;10:1164–1170. doi: 10.1038/s41557-018-0123-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rastelli EJ, Bolinger AA, Coltart DM. Stereodivergent synthesis of β,γ-fused bicyclic γ-lactones via a multicomponent ring-expansion cascade. Chem. 2018;4:2228–2238. doi: 10.1016/j.chempr.2018.08.007. [DOI] [Google Scholar]
- 51.Blacker AJ, Williams MT. Pharmaceutical Process Development: Current Chemical and Engineering Challenges. Cambridge: Royal Society of Chemistry; 2011. [Google Scholar]
- 52.Jozwiak K, Lough WJ, Wainer IW. Drug Stereochemistry: Analytical Methods and Pharmacology. London: Informa; 2012. [Google Scholar]
- 53.Lovering F, Bikker J, Humblet C. Escape from flatland: increasing saturation as an approach to improving clinical success. J. Med. Chem. 2009;52:6752–6756. doi: 10.1021/jm901241e. [DOI] [PubMed] [Google Scholar]
- 54.Caille S, et al. Molecular complexity as a driver for chemical process innovation in the pharmaceutical industry. J. Org. Chem. 2019;84:4583–4603. doi: 10.1021/acs.joc.9b00735. [DOI] [PubMed] [Google Scholar]
- 55.Caldwell J. The importance of stereochemistry in drug action and disposition. J. Clin. Pharmacol. 1992;32:925–929. doi: 10.1002/j.1552-4604.1992.tb04640.x. [DOI] [PubMed] [Google Scholar]
- 56.Rentsch KM. The importance of stereoselective determination of drugs in the clinical laboratory. J. Biochem. Biophys. Methods. 2002;54:1–9. doi: 10.1016/S0165-022X(02)00124-0. [DOI] [PubMed] [Google Scholar]
- 57.Wei L, Zhu Q, Xu SM, Chang X, Wang CJ. Stereodivergent synthesis of α,α-disubstituted α-amino acids via synergistic Cu/Ir catalysis. J. Am. Chem. Soc. 2018;140:1508–1513. doi: 10.1021/jacs.7b12174. [DOI] [PubMed] [Google Scholar]
- 58.Huo X, Zhang J, Fu J, He R, Zhang W. Ir/Cu dual catalysis: enantio- and diastereodivergent access to α,α-disubstituted α-amino acids bearing vicinal stereocenters. J. Am. Chem. Soc. 2018;140:2080–2084. doi: 10.1021/jacs.8b00187. [DOI] [PubMed] [Google Scholar]
- 59.Pictet A, Spengler T. Über die bildung von isochinolin-derivaten durch einwirkung von methylal auf phenyl-äthylamin, phenyl-alanin und tyrosin. Ber. Dtsch. Chem. Ges. 1911;44:2030–2036. doi: 10.1002/cber.19110440309. [DOI] [Google Scholar]
- 60.Cox ED, Cook JM. The Pictet-Spengler condensation: a new direction for an old reaction. Chem. Rev. 1995;95:1797–1842. doi: 10.1021/cr00038a004. [DOI] [Google Scholar]
- 61.Tang S, Zhang X, Sun J, Niu D, Chruma JJ. 2-Azaallyl anions, 2-azaallyl cations, 2-azaallyl radicals, and azomethine ylides. Chem. Rev. 2018;118:10393–10457. doi: 10.1021/acs.chemrev.8b00349. [DOI] [PubMed] [Google Scholar]
- 62.Hashimoto T, Maruoka K. Recent advances of catalytic asymmetric 1,3-dipolar cycloadditions. Chem. Rev. 2015;115:5366–5412. doi: 10.1021/cr5007182. [DOI] [PubMed] [Google Scholar]
- 63.Fang X, Wang CJ. Catalytic asymmetric construction of spiropyrrolidines via 1,3-dipolar cycloaddition of azomethine ylides. Org. Biomol. Chem. 2018;16:2591–2601. doi: 10.1039/C7OB02686B. [DOI] [PubMed] [Google Scholar]
- 64.Cheng Q, et al. Iridium-catalyzed asymmetric allylic substitution reactions. Chem. Rev. 2019;119:1855–1969. doi: 10.1021/acs.chemrev.8b00506. [DOI] [PubMed] [Google Scholar]
- 65.Liu WB, Zheng C, Zhuo CX, Dai LX, You SL. Iridium-catalyzed allylic alkylation reaction with N-aryl phosphoramidite ligands: scope and mechanistic studies. J. Am. Chem. Soc. 2012;134:4812–4821. doi: 10.1021/ja210923k. [DOI] [PubMed] [Google Scholar]
- 66.Roach JS, et al. Characterization of a dispiroketal spirolide subclass from Alexandrium ostenfeldii. J. Nat. Prod. 2009;72:1237–1240. doi: 10.1021/np800795q. [DOI] [PubMed] [Google Scholar]
- 67.Huang S, et al. Wuweizidilactones A-F: novel highly oxygenated nortriterpenoids with unusual skeletons isolated from Schisandra chinensis. Chem. Eur. J. 2007;13:4816–4822. doi: 10.1002/chem.200700346. [DOI] [PubMed] [Google Scholar]
- 68.Yamamoto Y, Fujikawa R, Umemoto T, Miyaura N. Iridium-catalyzed hydroboration of alkenes with pinacolborane. Tetrahedron. 2004;60:10695–10700. doi: 10.1016/j.tet.2004.09.014. [DOI] [Google Scholar]
- 69.Yan X, et al. A highly enantio- and diastereoselective Cu-catalyzed 1,3-dipolar cycloaddition of azomethine ylides with nitroalkenes. Angew. Chem. Int. Ed. 2006;45:1979–1983. doi: 10.1002/anie.200503672. [DOI] [PubMed] [Google Scholar]
- 70.Zimmerman HE, Traxler MD. The stereochemistry of the Ivanov and Reformatsky reactions. J. Am. Chem. Soc. 1957;79:1920–1923. doi: 10.1021/ja01565a041. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Experimental procedures and characterization data are available within this article and its Supplementary Information. Data are also available from the corresponding author on request. The X-ray crystallographic coordinates for the structures of compounds (1S,3R,4S)-3g, (1R,3S,4R)-3g, (1S,3S,4S)-3c, and (1R,3R,4R)-3c reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers 1915538, 1915539, 1915540, and 1915541. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.