Abstract
Background
The lemongrass (LG) leaves could be a useful source of cellulose after its oil extraction, which is still either dumped or burned, not considered as a cost-effective approach. The synthesis of cellulose nanofibers (CNF) from LG waste has emerged as a beneficial alternative in the value-added applications. The non-toxicity, biodegradability, and biocompatibility of CNF have raised the interest in its manufacturing.
Method
In the present study, we have isolated and characterized CNFs using enzymatic hydrolysis. We also explored the cytotoxic properties of the final material. The obtained products were characterized using dynamic light scattering (DLS), fourier transform infrared spectroscopy (FT-IR), scanning electron microscopy (SEM), transmission electron microscopy (TEM), x-ray diffraction (XRD), and thermogravimetric/differential thermal gravimetric analysis (TG/DTG). The cytotoxicity of CNF was assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay against three different cancer cell lines NCIH460, PA1, and L132 cells.
Results
The FT-IR results showed that the resulting sample was of cellulose species, and CNF was found free from the non-cellulosic components like lignin and hemicellulose. The SEM micrographs of the cellulose showed a bundle like structure. The TEM micrographs of CNF showed diverse long fibers structure with 105.7 nm particle size analysed using DLS. The TGA analysis revealed that the thermal stability was slightly lower, compared to cellulose. Additionally, CNF did not show the cytotoxic effect at the tested concentrations (~10-1000 μg/ml) in any of the cell lines.
Conclusion
Overall, the results concluded that LG waste-derived CNF is a potential sustainable material and could be employed as a favourable reinforcing agent or nanocarriers in diverse areas, mainly in food and drug delivery sectors.
Graphical abstract
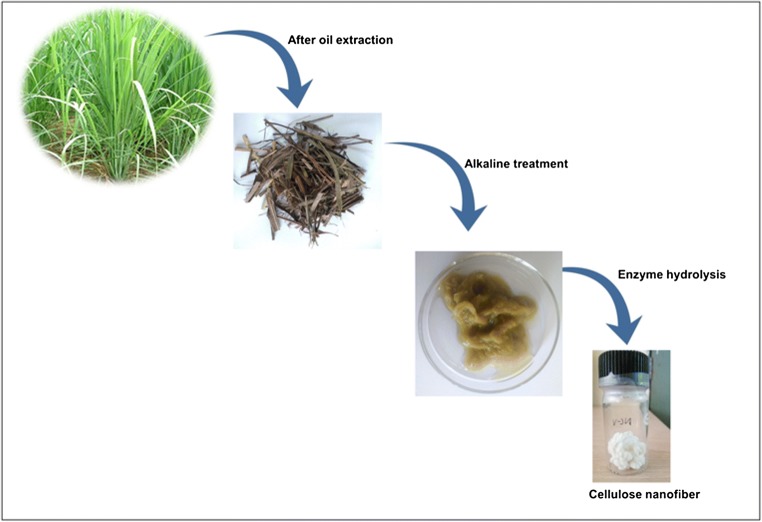
Systematic representation of the synthesis of the cellulose nanofibers: The lignocellulosic waste of lemongrass (after oil extraction) was pretreated for the isolation of raw cellulose, followed by enzyme hydrolysis for the synthesis of pure cellulose nanofibers.
Keywords: Cellulose nanofibers; Lemongrass; Lignocellulosic waste; Characteristics, enzymatic hydrolysis
Introduction
The environmental concern led to the evolution of novel biodegradable materials, which are considered to be an emerging research area in industry and academia [1]. Cellulose is the linear homopolymer produced up to 105-1010 tons annually. It is among the most abundant, eco-friendly, renewable, low-cost, and biodegradable polymers in the world, which can replace synthetic materials [2]. The production of biodegradable and innovative products like cellulose nanofibers (CNF) become an essential area of research due to its unique properties. CNF are generally prepared using strong acids, which is known as acid hydrolysis method [3, 4]. However, the acid hydrolysis method showed limitations like the release of chemicals and prolonged processing steps. To overcome such issues, researchers have used eco-friendly approaches like enzymatic hydrolysis for the synthesis of CNF [5, 6]. It allows for milder treatment conditions as it does not require solvents and acidic reagents [7]. Hence, it is considered as an environment-friendly, high yielding, and organic method that can improve CNF quality [8, 9]. So far, enzymes from Xanthomonas axonopodispvpv .citri [10], cellulases [11], Viscozyme® L [5] and endoglucanases [12] has been used for the synthesis of different types of cellulosic nanoparticles. The xylanase helps in the degradation of the hemicelluloses, breaking the polysaccharide β-1, 4-xylan into xylose. The cellulases cut the cellulose molecules into shorter polysaccharides by the hydrolysis of β-1, 4-D-glycosidic bonds of the glucose units. The product named viscozyme is a multi-enzyme complex of carbohydrases, including arabanase, cellulase, β-glucanase, hemicellulase, and xylanase. The isolation of CNF using enzymatic pretreatment in combination with mechanical treatment or chemical hydrolysis has also been achieved [8, 13, 14].
The CNF can be obtained from renewable agricultural, and biomass feedstocks, which could form a platform for sustainable products [4, 15–17]. The agricultural waste like lemongrass (LG, after its oil extraction) are usually burned; however, its proper utilization may help in resolving the waste disposal issue. Composting is a solution to this type of waste materials, but it is not a cost-effective approach. An alternative potential utilization is the manufacturing of the pulp and paper, animal feed, textiles, or as reinforcing material in composites [18, 19]. LG is an aromatic grass with known medicinal properties; it belongs to the Gramineae family [20]. The leaves of the grass are used for extracting LG oil, which has been reported to have different chemical compositions and used in different areas like cosmetics, perfumery and used in different herbal preparation due to its antimicrobial property [21]. The steam distillation of LG for the extraction of essential oil led to the production of approximately 30,000,000 tons per annum of lignocellulosic biomass or residues [22]. The importance and commercial value of the LG waste have not been reported so far. Although, many research articles have shown the synthesis of nanocrystals from LG waste using acid hydrolysis and further its use in various application [23]. However, isolation of the nanofibers from the LG waste using enzymatic hydrolysis has not been reported so far.
There are numerous application of the cellulose nanofibers like food packaging [24], nanocomposites [25], water purification [26], tissue engineering, drug delivery [27, 28]. It also finds vast applications as energy storage devices [29]. Despite CNF potential applications in numerous areas, there are certain risks which need to ascertain else it could be harmful to human health [30]. The small size, high reactive behavior, chemical and physical characteristics of nanoparticles may be cytotoxic [31]. So far, various studies have analyzed the cytotoxicity profile of the CNF; for example, CNF isolated from cotton showed low cytotoxicity in human lung cells [32]. However, the need to explore new, readily available resources which may produce CNF with better physio-chemical properties is never-ending. Therefore, the present study aims to isolate the CNFs from the lignocellulosic waste of LG using enzymatic hydrolysis, along with ultrasonication. In this study, mild operating conditions of enzymatic hydrolysis have been explored to prepare CNF. The properties of the obtained CNF were characterized using structural and physicochemical, and morphological analysis. The toxicity assessment of the obtained CNF was performed in NCIH460, PA1, and L132 cell lines.
Materials and methods
Materials
The raw material of lemongrass (Cymbopogon flexuosus (family: Poaceae, Krishna variety) was collected during the months of May-Jun 2017 from the experimental farm of CSIR-Central Institute of Medicinal, and Aromatic Plants (CSIR-CIMAP), Lucknow and the voucher specimen was submitted in the herbarium with the assigned number 8862. The chemicals required for the extraction of fiber and preparation of CNF were sodium hydroxide (NaOH), sodium chlorite (NaClO2), citrate buffer, and deionized water. The Viscozyme® L enzyme, cellulolytic enzyme mixture (enzymatic activity >100 FBGU/G) were purchased from Sigma-Aldrich, India. All reagents were of analytical grade or higher.
Extraction of cellulose nanofibers and characterization
The cellulose was isolated from the lignocellulosic biomass of LG [17]. The lignocellulosic biomass was collected and washed with tap water, followed by distilled water and oven-dried. Further, the dried material was chopped into a uniform size. The fibers were exposed to a steam explosion with 2% NaOH (1:10 g/ml fiber: solution ratio) in an autoclave for 3 h. and kept under 15 psi pressure at a temperature of 121 °C to swell the cell walls and saturate the fibers. The digested material was removed from the autoclave and washed thoroughly with distilled water until neutral pH. Subsequently, the cellulose residues were further de-lignified with 1% acidified sodium chlorite solution (15 ml/g of fiber) at 70 °C for 3 h. The obtained LG-cellulose pulp turned white and rinsed with distilled water until neutral pH. Finally, the resultant pulp was then oven-dried and used for the synthesis of CNF.
The enzymatic hydrolysis method was performed as per the described method with few modifications [5]. The lignocellulosic fibers (100 mg) were added in 20 ml of 50 mM sodium-citrate buffer at pH 4.8; mixed with the help of magnetic stirrer for 1 h. to make a suspension. The defined concentration of enzyme was added to the cellulose suspension and stirred at 50 °C for 24 h. using a magnetic stirrer. The reaction was terminated by boiling the cellulose suspension for 10 min. Consequently, the sample was centrifuged at 10,000 rpm for 15 min. to remove excess enzyme, rinsed with a buffer solution of pH 7.4 and finally washed with deionized water, sonicated at 50% intensity using a probe-sonicator for 20 min. in a beaker which was moderately immersed in an ice bath. The final product was freeze-dried and stored for further characterization. The obtained samples were preserved at 4 °C for further characterization. The yield of the CNF obtained following each method was calculated using Eq. 1:
| 1 |
Morphology, yield, size and zeta potential analysis
The morphology of the cellulose was observed by light microscopy using a light microscope (Nikon Eclipse TS100) at 20X magnification. The samples were coated with gold using vacuum sputter coater. The selected images represent the surfaces of the cellulose fibers. The structure of CNF was obtained by transmission electron microscopy using (JEOL JEM 2100) with an accelerating voltage of 200 kV. The CNF was suspended in distilled water. A drop of the diluted suspension was deposited on the surface of carbon-coated copper grids. Then, the CNF was negatively stained with a 0.2% solution of uranyl acetate. Dynamic light scattering (DLS) technique (Mastersizer 2000, Malvern Instruments, UK) was employed for the size and zeta potential measurement. It is used to determine the hydrodynamic diameter of the CNF present in the colloidal suspension. Zeta potential measurement was used to determine the dispersion stability of the samples present in the colloidal suspension, by photon correlation spectroscopy at 25 °C. The movement of the particles, undergoing electrophoresis, was estimated by dynamic light scattering studies. The estimated electrophoretic movability was then converted to zeta potential.
Fourier-transformed infrared spectra analysis (FT-IR)
FT-IR spectra of the cellulose and CNF were recorded as per described method [33] on the Fourier transform infrared spectroscopy (FTIR) instrument (Perkin Elmer Spectrum BX) in the range of 4000-500 cm−1. The experiment was carried out using a KBr disk (ultrathin pellet) method. The samples were dried, ground, and pelletized using KBr (1:100 w/w). This experiment was carried out to confirm the presence of cellulose content from the source material.
X-ray diffraction (XRD)
X-ray diffraction (XRD) technique was used for measuring the crystallinity of the resulting cellulose and CNF [34]. The X-ray diffraction of cellulose and CNF were computed with X-ray diffractometer (Bruker D8 Advanced X-ray diffraction system) using CuK radiation (λ = 0.154056 nm) from 10 to 50° (2θ). The crystallinity index (%) was obtained using the Segal method [35] as shown in Eq. 2. The calculation was performed for more intense peaks in the XRD diffraction patterns at 2θ = 14.1° and 22° with respect to the less intense peaks at 2θ = 18.6°.
| 2 |
where Icr is the peak intensity corresponding to the maximum diffraction peak measured as the height of the crystalline region at 2θ = 14.1° or 2θ = 22°, this showed crystalline as well as amorphous regions and Iam is the peak intensity corresponding to the height of smaller diffraction peak at 2θ = 18.6°, represents the amorphous region.
Thermal analysis
The thermal stability of the resulting cellulose and CNF as a function of temperature was estimated by thermo-gravimetric analysis [36]. The derivative thermogravimetric (DTG) curve was prepared from the differential thermal gravimetric curves. The analysis was conducted using simultaneous thermal analyzer STA 8000 (PerkinElmer) from 35 to 500 °C. Pyrolysis was terminated at 700 °C with the heating rate of 10 °C/min under the flow of 20 ml/min of nitrogen gas.
Cell viability assessment
The cell viability was determined by using NCIH-460 (lung cancer), PA-1 (human teratocarcinoma), and L132 (normal embryonic tissue) cell lines as per the described method [34, 37]. The cell lines used in the study were procured from the National Centre for Cell Science (NCCS) Pune, India. PA-1 was grown in cultured flask supplemented with Minimal Essential medium (MEM) with heat-inactivated fetal bovine serum (10% v/v) and 100 U/ml of penicillin and 100 μg/mL of streptomycin; whereas NCIH-460 and L132 were grown in Roswell Park Memorial Institute −1640, and incubated at 37 °C with 5% CO2 for 24 h before the suitable treatment. The effect of CNF on cell viability was measured by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. CNF was prepared at a concentration of (10, 25, 50, 100, 500 and 1000 μg/ml) by suspending the solution in phosphate buffer saline followed by the sonication for 2 h. Podophyllotoxin (PDT) was used as a positive control to check cell cytotoxicity. All the suspended cells were seeded in 96-well tissue culture plates (2 × 106 cells/ well) in 200 μl of media and incubated at 37 °C in 5% CO2 atmosphere for 24 h. A blank sample (MEM without cells) and positive control (Podophyllotoxin), as it is considered to be effective antimitotic agent were also tested. Then, the supernatant was removed, and MTT solution (5 mg/ml) was prepared, and added into each well in dim light and incubated at 37 °C for 4 h. so that purple formazon crystals are formed. Afterward, the medium was removed out, and the addition of 100 μl of DMSO solubilized the obtained purple formazon crystals. The plate was allowed for incubation for 5 min. at room temperature to solubilize the purple formazon crystals completely. Then, the enzymatic reaction of yellow tetrazolium MTT to purple formazan was measured at 570 nm using EPOCH 2 microplate reader (Biotek instruments). The cell viability percentage was computed by Eq. 3.
| 3 |
where, Xexp is the absorbance value of the sample (CNF solution + cell); Xcontrol is the absorbance value of the blank (MEM without cells) sample, and Xpositive is the absorbance value of the positive control (PDT).
Statistical analysis
The experiments were performed in replicates (n = 3) and the data are expressed as average ± standard deviation. The effect of the CNF compared to control was performed using GraphPad InStat version 3.06, where p value <0.05 was considered significant.
Results and discussion
Cellulose nanofiber formation
The purpose of the steam explosion was to swell the cell walls, saturate the fibers and eliminates the non-cellulosic constituents like pectin, hemicellulose, and lignin from the cellulose pulp. Based on the partial removal of lignin and hemicellulose, it is the most effective pre-treatment for plant-based fibers. In this step, the space between fibers increases and the alkaline solution easily breaks the hydrogen bonds (mercerization), which led to increment in the amorphous domain. It also led to an increment in the specific surface area and the absorption capacity of cellulose [38]. The material changed from pale brown to dark brown. After that, bleaching treatments were executed to eliminate the lignin content from the lamellae region to isolate the fibers of cellulose [39]. The chromogen groups like conjugated carbonyls, double bonds, and their solution depict the brown colour of the lignocellulosic content, which go through ionization [40], along with hydrolysis of the other components like pectin, hemicellulose and starch [41, 42]. The bleaching treatment executed after alkaline treatment converts the colour to pale brown and ultimately off-whitish colour, as it helps in elimination of the tannins and lignin. In this step, the chlorine and chlorites immediately oxidized the lignin species; initiate the formation of carbonyl, hydroxyl, and carboxylic groups. These groups increased the solubility of lignin component in alkaline solution as well as purification of cellulose [43]. Figure 1 describes the final appearance of the white coloured cellulose, and cellulose nanofibers after enzymatic hydrolysis in dried form. After bleaching treatment, the cellulose fibers were found tough and stiff in morphology, making it more accessible for physical force. In the final step, enzyme treatment and ultra-sonication were applied to break the β-1,4- glycosidic linkage that linked the D-glucopyranoside in plant cellulose fibers [10] and sonicated to get the segment of nanofibers in dispersed form. Thus, nanofibers or bundles of nanofibers were obtained.
Fig. 1.
Schematic illustration of the separation process of cellulose nanofibers from the lignocellulosic waste of lemongrass
Morphological and yield
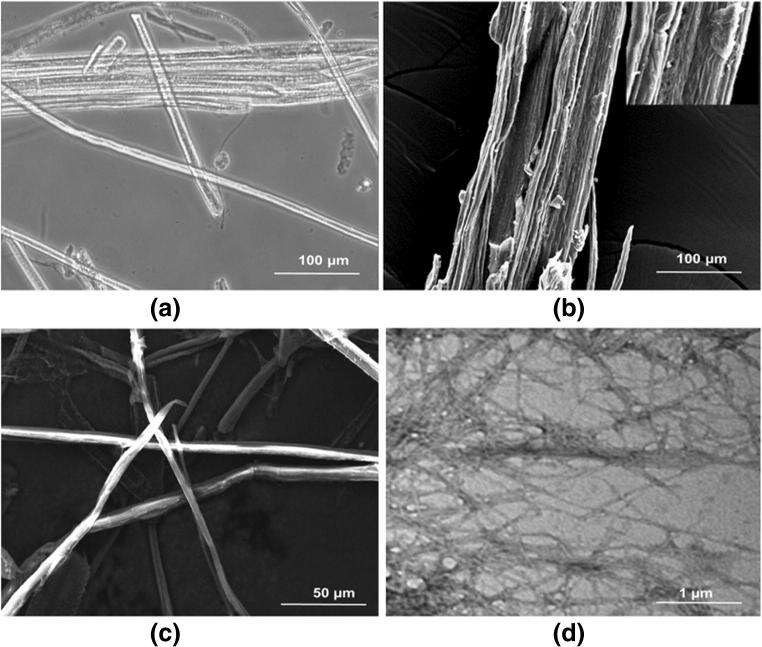
The morphological analysis is considered to be an essential parameter because the source of the CNF and treatment method has a significant impact on the dimensions and characteristics of the CNF. The microstructure of the cellulose exhibited smooth surfaces with small granules, forming a clear bundle-like structure due to the presence of non-cellulosic layer, which consists of lignin, hemicelluloses, pectin (Fig. 2a, b). The lignocellulosic fibers convert into a cluster of sub-micron wide and micrometer long fibers of cellulose, which may be due to hydrogen bonding between hydroxyl groups of cellulose [44]. Similar results were found to be reported for cellulose isolated from curaua and sugarcane bagasse [5] and lignocellulosic fibers [45]. It was found that the fibers were individualized to nano-dimension after enzymatic hydrolysis due to the successful removal of the non-cellulosic regions, which was showed by SEM (Fig. 2c) and further confirmed through TEM micrograph (Fig. 2d). The reduction in the width of CNF was obtained after enzyme hydrolysis due to the successful removal of non-cellulosic materials.
Fig. 2.
Optical image of untreated cellulose fibers (a), Scanning electron microscopy images of untreated cellulose fibers (b) and enzyme treated cellulose nanofibers (c) and Transmission electron microscopy images of enzyme treated cellulose nanofibers (d)
TEM analysis was implemented to determine the structure of the resulting CNF. The TEM images strongly confirm the process of isolation of CNF using enzyme hydrolysis. It was also observed that the CNF had a web-like structure along with long entangled cellulosic segments. This type of interconnected network of nanofibers could have a reinforcing possibility for composite applications [46]. The TEM images clearly showed the homogeneity of the nanofibers [47]. Similar results were observed in CNF when isolated from cassava root bagasse and peelings [4]. Regarding the yield of the CNF, the enzymatic treatment led to higher yield, i.e., 57%, compared to cellulose (30%). This may be due to limited processing steps involved in enzymatic treatment and higher efficiency of the enzyme in breaking inter/intra cellulosic bonds.
Particle size and zeta potential measurement
The TEM micrographs also provide useful information regarding the size of the nanofibers. However, the reliability of the measurement is constrained by the small fraction of particles studied relative to the whole sample. Hence, the DLS method was employed to assess the particle size of the samples. The DLS data confirmed that the size of the CNF decreases significantly after enzyme hydrolysis (105.7 nm), compared to the cellulose (~ 0.001 mm) (Fig. 3a, b). The results are correlated with reported literature [12]. The zeta potential value of the CNF was found to −22.4 mV, showing moderate stability. Higher zeta potential suggests higher electrostatic repulsion between the fibers, providing enough surface charges to stabilize the suspension, explaining the good dispersion, but when the zeta potential value is low, the attraction between the fibers is high and dispersion will break and flocculate [48]. The CNF prepared from the banana peel enzymatic method were observed to have a zeta potential values of −25.7 mV [7]. CNF has been from soybean straw by using Optimash® VR enzyme with almost similar ranges of zeta potential values (−20.8 to −24.5 mV) [49]. On taking this into account, the resulting CNF exhibited moderate stability.
Fig. 3.
Particle size of (a) cellulose and (b) cellulose nanofibers measured using dynamic light scattering
Fourier transform infrared spectroscopy (FTIR) analysis
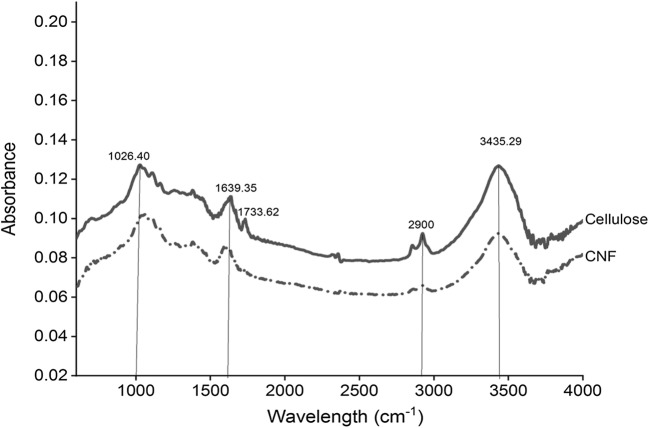
The cellulose showed the typical absorption peaks of the lignocellulosic components (Fig. 4). The broad absorption band in 3650-3000 cm−1 region attributed to O-H stretching vibrations from intra and intermolecular hydrogen bonding. It also describes the hydrophilic nature of cellulose and CNF [50]. The cellulose displayed a small band in the 2900 cm−1 region, denoting the symmetrical and asymmetrical stretching vibrations of the C-H bonds [51]. The small peak present at 2848 cm−1region confirms the presence of lignin in the cellulose. Several studies also showed vibrations around 2850 cm-1, which resemble –CH stretching of the waxes and lignin [39, 52]. The main constituents of hemicellulose are D-xylose and 4-o-methyl-D-glucouronic acid, and aldoses as arabinose, glucose, mannose, and galactose in different quantities that produce uronic acid after oxidation [53]. The shoulder peak associated with hemicelluloses occurs at 1768 cm−1 in cellulose is due to acetyl and uronic ester groups of hemicelluloses and the ester linkage of carboxylic group of ferulic and p-coumaric acids of lignin [54, 55]. The disappearance of a small band at 1768 cm−1region indicates that hemicellulose and lignin were removed from CNF by applied enzyme hydrolysis. The peak at ~1640 cm−1 in both the spectra attributed to the absorbed water into the structure of the cellulose fibers [56, 57]. These results showed that the cellulose species was not removed during the pretreatment and enzyme hydrolysis process. The peaks around 1100 cm−1and 1160 cm−1 present in both the samples, attributed to the ring C-C bending vibration and C-O-C glycoside ether bond of the β-1,4, −glycosidic bond between D-glucose units of cellulose [58, 59]. The peaks in ~1025-1038 cm−1 regions in both samples corresponds to the C-O-C glycosidic linkage of the cellulose constituents [56]. Based on the analysis, the results clearly showed that the molecular structure of the cellulose remains unchanged after enzyme hydrolysis.
Fig. 4.
Fourier transform infrared spectra of the (a) cellulose and (b) cellulose nanofibers (CNF)
X-ray diffraction (XRD) analysis
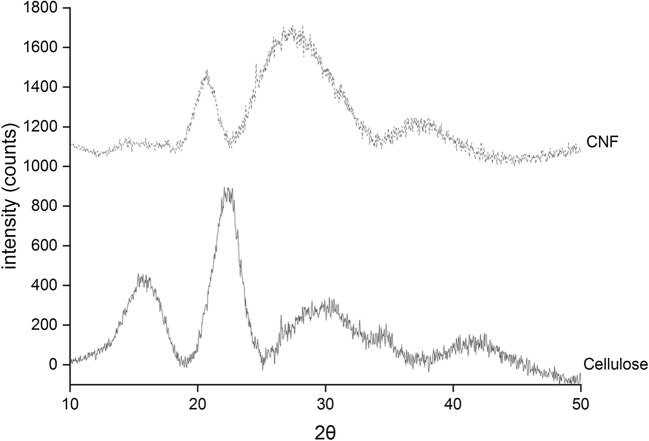
Cellulose composed of a crystalline structure along with hemicellulose and lignin components. The crystalline structure of the cellulose was due to the hydrogen bond interactions and Vander Waal forces between the molecules [60]. Cellulose obtained from lignocellulosic fibers was characterized by X-ray diffraction technique. The diffractogram (Fig. 5) of the cellulose shows sharp peaks at 2θ of 15°, 22.4° and broad peak at 2θ of 30°, demonstrating the crystalline behaviour of typical cellulose I with ordered regions [61, 62]. The XRD pattern of the resulting cellulose nanofibers show low and diffuse peaks at 15.2°, and a sharp peak at 20.8° and broad diffraction peak at 27°, corresponding to cellulose II with antiparallel structure [3]. Almost similar results were reported in the literature [63–65]. The crystallinity was found to decrease after enzymatic hydrolysis, i.e., 48.9%, compared to cellulose (66.51%). It may be possible that the surface of the fibers gets loosen after the sonication process, which results in loss of its crystalline behaviour [5]. The low degradation temperature was observed in TGA analysis due to low crystallinity of cellulose nanofibers. The results are correlating from TGA analysis.
Fig. 5.
X-ray diffraction pattern of cellulose and cellulose nanofibers (CNF)
Thermal characterization
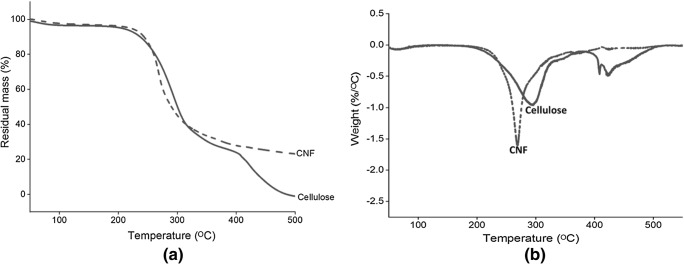
The TG and DTG curves of the cellulose and CNF are illustrated in Fig. 6. The thermograms of TGA showed an initial weight loss occurs at 50–100 °C in cellulose and CNF, which is mainly due to the evaporation and removal of the bound water molecules [66]. It usually decomposes at different temperature due to a difference in its chemical structure. The second dramatic weight loss occurred for both the samples between 200 and 300 °C, which is due depolymerisation of the hemicellulose and breakdown of glycosidic bonds of cellulose [67, 68]. The hemicellulose content has lower thermal stability, compared to lignin and cellulose [50]. The broad peak occurred between 250 and 450 °C due to lignin degradation, and 275 to 400 °C is contributed to cellulose degradation [42, 69]. The DTG curve of the cellulose shows decomposition peaks at 288 °C and 420 °C, showed the degradation of the hemicellulose and α-cellulose component of the fibers. For cellulose, the sudden reduction in weight occurred between 250 and 390 °C, due to the decomposition of the cellulose. The cellulose content was almost completely pyrolysed at 400 °C [70]. From the thermal analysis, it is clear that there is a shift in the degradation temperature from cellulose to CNF. The CNF showed little bit lower degradation temperature than the cellulose. The lower decomposition temperature for CNF was due to the decrease in the molecular weight and crystallinity of the resulting CNF [34, 36].
Fig. 6.
(a) Thermogravimetric and (b) Differential thermal gravimetric curve of cellulose and cellulose nanofibers (CNF)
Cellulose nanofiber cytotoxicity
Since nanofibers are considered to have potential as a reinforcing agent, biomedical purposes and other food-related applications. It became necessary to determine its toxicological characteristics. The CNF was found to be non-toxic. Three different cell lines NCIH 460 (lung-carcinoma cell line), PA-1 (human terato-carcinoma) and L132 (normal embryonic tissue) were exposed to different concentrations of CNF in the range of 10-1000 μg/ml for 24 h. The effect of CNF on cell viability was assessed using MTT assay [37]. The untreated cells were taken as a negative control, and podophyllotoxin was taken as a positive control. Podophyllotoxin was considered to be a known potential natural anticancer agent [71, 72]. In each cell line, the percentage of viable cells with or without CNF treatment was almost similar in contrast to the positive control. This result suggests the CNF produced by enzymatic treatment does not show cytotoxicity up to higher tested concentration i.e.1000 μg/ml in cancer as well as normal cell lines (Fig. 7). Similarly, in-vitro exposure from 0 to 100 μg/ml of cellulose nanofibers isolated from cotton were not found to be cytotoxic [31].
Fig. 7.
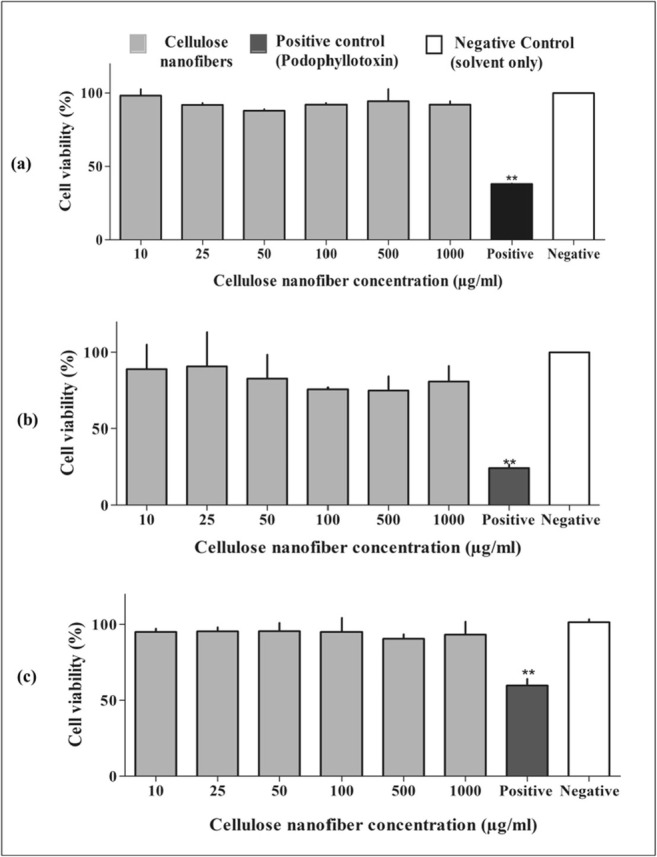
Effect of cellulose nanofibers(CNF) on (a) NCIH 460 (lung carcinoma) (b) PA-1 (human teratocarcinoma) and (c) L132 (normal embryonic tissue) cell viability after 24 h incubation at different concentrations (10-1000 μg/ml). The results are expressed as Mean ± standard deviation from independent experiments (n = 3). A p value (PDT) of <0.05 was considered to be significant compared to negative control
Conclusions
The study encourages the practice of using lignocellulosic waste of LG as an eco-friendly, biodegradable, renewable source for the production of CNFs, making it a profitable approach using enzymatic hydrolysis, which is an effective and milder process. The resulting CNFs showed nanofibers nanometric dimension, with the fibril-like structure, as compared to the raw cellulose. The enzymatic approach used in the study led to a higher yield of 57% CNF, along with moderate stability. The FT-IR analysis revealed the removal of non-cellulosic components. The XRD analysis showed the amorphous behavior of the synthesised CNFs.
Additionally, CNF obtained after enzyme hydrolysis did not show any cytotoxic effect on the cell lines at a concentration range of 10-1000 μg/ml. Therefore, the enzymatic hydrolysis using a multi-component enzyme is a clean and milder process for the production of CNF. Hence the cellulose from the lignocellulosic waste of LG can be employed for the production of nanofibers, which may be useful in different applications such as a reinforcing agent in nanocomposites, water purification, material for microencapsulation, tissue engineering, drug delivery systems.
Acknowledgments
The authors are grateful to the Director, CSIR-Central Institute of Medicinal and Aromatic Plants, Lucknow for rendering essential facilities required for the experimental work. This work was supported by CSIR-Aroma Mission Project (HCP 007). The authors would also like to acknowledge Dr. Puja Khare, Senior Scientist, CSIR-CIMAP for initial assistance and providing laboratory space to begin this work and Dr. P.V. Ajayakumar, Chief Scientist and Dr. Pooja Singh, Technical assistant CSIR-CIMAP for FT-IR and SEM analysis and timely guidance. PK acknowledges University Grants Commission, New Delhi for financial assistance.
Abbreviations
- CNF
Cellulose nanofibers
- DLS
Dynamic light scattering
- FTIR
Fourier transform infrared spectroscopy
- SEM
Scanning electron microscopy
- TEM
Transmission electron microscopy
- XRD
X-ray diffraction
- TG/DTG
Thermogravimetric/Differential thermal gravimetric analysis
Compliance with ethical standards
Conflict of interest
The author(s) declared that the article has no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wróblewska-Krepsztul J, Rydzkowski T, Borowski G, Szczypiński M, Klepka T, Thakur VK. Recent progress in biodegradable polymers and nanocomposite-based packaging materials for sustainable environment. Int J Polym Anal Charact. 2018;23:383–395. [Google Scholar]
- 2.Brigham Christopher. Green Chemistry. 2018. Biopolymers; pp. 753–770. [Google Scholar]
- 3.Mandal A, Chakrabarty D. Isolation of nanocellulose from waste sugarcane bagasse (SCB) and its characterization. Carbohydr Polym. 2011;86:1291–1299. [Google Scholar]
- 4.Leite ALMP, Zanon CD, Menegalli FC. Isolation and characterization of cellulose nanofibers from cassava root bagasse and peelings. Carbohydr Polym. 2017;157:962–970. doi: 10.1016/j.carbpol.2016.10.048. [DOI] [PubMed] [Google Scholar]
- 5.de Campos A, Correa AC, Cannella D, de M Teixeira E, Marconcini JM, Dufresne A, et al. Obtaining nanofibers from curauá and sugarcane bagasse fibers using enzymatic hydrolysis followed by sonication. Cellulose. 2013;20:1491–1500. [Google Scholar]
- 6.Fattahi Meyabadi T, Dadashian F, Mir Mohamad Sadeghi G, Ebrahimi Zanjani Asl H. Spherical cellulose nanoparticles preparation from waste cotton using a green method. Powder Technol. 2014;261:232–240. [Google Scholar]
- 7.Tibolla H, Pelissari FM, Menegalli FC. Cellulose nanofibers produced from banana peel by chemical and enzymatic treatment. LWT Food Sci Technol. 2014;59:1311–1318. [Google Scholar]
- 8.Henriksson M, Henriksson G, Berglund LA, Lindström T. An environmentally friendly method for enzyme-assisted preparation of microfibrillated cellulose (MFC) nanofibers. Eur Polym J. 2007;43:3434–3441. [Google Scholar]
- 9.Yassin MA, Gad AAM, Ghanem AF, Abdel Rehim MH. Green synthesis of cellulose nanofibers using immobilized cellulase. Carbohydr Polym. 2019;205:255–260. doi: 10.1016/j.carbpol.2018.10.040. [DOI] [PubMed] [Google Scholar]
- 10.Mariño M, Lopes da Silva L, Durán N, Tasic L. Enhanced materials from nature: Nanocellulose from Citrus waste. Molecules. 2015;20:5908–5923. doi: 10.3390/molecules20045908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martelli-Tosi M, Masson MM, Silva NC, Esposto BS, Barros TT, Assis OBG, et al. Soybean straw nanocellulose produced by enzymatic or acid treatment as a reinforcing filler in soy protein isolate films. Carbohydr Polym. 2018;198:61–68. doi: 10.1016/j.carbpol.2018.06.053. [DOI] [PubMed] [Google Scholar]
- 12.Filson PB, Dawson-Andoh BE, Schwegler-Berry D. Enzymatic-mediated production of cellulose nanocrystals from recycled pulp. Green Chem. 2009;11:1808. [Google Scholar]
- 13.Siddiqui N, Mills RH, Gardner DJ, Bousfield D. Production and characterization of cellulose nanofibers from wood pulp. J Adhes Sci Technol. 2011;25:709–721. [Google Scholar]
- 14.Dhandapani Renuka, Sharma Suraj. ACS Symposium Series. Washington, DC: American Chemical Society; 2014. Environmentally Benign Pretreatments for Producing Microfibrillated Cellulose Fibers from Hemp; pp. 69–87. [Google Scholar]
- 15.Abdul Khalil HPS, Bhat AH, Ireana Yusra AF. Green composites from sustainable cellulose nanofibrils: a review. Carbohydr Polym. 2012;87:963–979. [Google Scholar]
- 16.Cherian BM, Pothan LA, Nguyen-Chung T, Mennig G, Kottaisamy M, Thomas S. A novel method for the synthesis of cellulose Nanofibril whiskers from Banana fibers and characterization. J Agric Food Chem. 2008;56:5617–5627. doi: 10.1021/jf8003674. [DOI] [PubMed] [Google Scholar]
- 17.Deepa B, Abraham E, Cordeiro N, Mozetic M, Mathew AP, Oksman K, et al. Utilization of various lignocellulosic biomass for the production of nanocellulose: a comparative study. Cellulose. 2015;22:1075–1090. [Google Scholar]
- 18.Bardet Raphael, Bras Julien. Handbook of Green Materials. 2014. Cellulose Nanofibers and Their Use in Paper Industry; pp. 207–232. [Google Scholar]
- 19.Saurabh CK, Mustapha A, Masri MM, Owolabi AF, Syakir MI, Dungani R, et al. Isolation and characterization of cellulose nanofibers from Gigantochloa scortechinii as a reinforcement material. J Nanomater. 2016;2016:1–8. [Google Scholar]
- 20.Akhila A. Essential oil-bearing grasses : the genus Cymbopogon. Boca Raton: CRC Press; 2009. [Google Scholar]
- 21.Sarma A, Sarma H, Sarma TC, Handique AK. Screening of essential oil obtained from inflorescence of lemongrass [Cymbopogon flexuosus (Nees ex Steud.) Wats] accessions. 2011.
- 22.Kaur H, Dutt D, Tyagi CH. Optimization of soda pulping process of ligno-cellulosic residues of lemon and Sofia grasses produced after steam distillation. BioResources. 2011;6(1):103–120. [Google Scholar]
- 23.Mishra D, Yadav V, Khare P, Jyotshna, Das MR, Meena A, et al. Development of crystalline cellulosic Fibres for sustained release of drug. Curr Top Med Chem. 2016;16:2026–2035. doi: 10.2174/1568026616666160215160426. [DOI] [PubMed] [Google Scholar]
- 24.Azeredo HMC, Rosa MF, Mattoso LHC. Nanocellulose in bio-based food packaging applications. Ind Crop Prod. 2017;97:664–671. [Google Scholar]
- 25.Tibolla H, Pelissari FM, Martins JT, Lanzoni EM, Vicente AA, Menegalli FC, et al. Banana starch nanocomposite with cellulose nanofibers isolated from banana peel by enzymatic treatment: in vitro cytotoxicity assessment. Carbohydr Polym. 2019;207:169–179. doi: 10.1016/j.carbpol.2018.11.079. [DOI] [PubMed] [Google Scholar]
- 26.Ma H, Burger C, Hsiao BS, Chu B. Ultra-fine cellulose nanofibers: new nano-scale materials for water purification. J Mater Chem. 2011;21:7507. [Google Scholar]
- 27.Kapahi H, Khan N, Bhardwaj A, Mishra N. Implication of nanofibers in Oral drug delivery. Curr Pharm Des. 2015;21:2021–2036. doi: 10.2174/1381612821666150302153306. [DOI] [PubMed] [Google Scholar]
- 28.Prakash Menon M, Selvakumar R, Suresh Kumar P, Ramakrishna S. Extraction and modification of cellulose nanofibers derived from biomass for environmental application. RSC Adv. 2017;7:42750–42773. [Google Scholar]
- 29.Yang C, Chen C, Pan Y, Li S, Wang F, Li J, et al. Flexible highly specific capacitance aerogel electrodes based on cellulose nanofibers, carbon nanotubes and polyaniline. Electrochim Acta. 2015;182:264–271. [Google Scholar]
- 30.Jones CF, Grainger DW. In vitro assessments of nanomaterial toxicity. Adv Drug Deliv Rev. 2009;61:438–456. doi: 10.1016/j.addr.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pereira MM, Raposo NR, Brayner R, Teixeira EM, Oliveira V, Quintao CC, et al. Cytotoxicity and expression of genes involved in the cellular stress response and apoptosis in mammalian fibroblast exposed to cotton cellulose nanofibers. Nanotechnology. 2013;24:075103. doi: 10.1088/0957-4484/24/7/075103. [DOI] [PubMed] [Google Scholar]
- 32.Clift MJ, Foster EJ, Vanhecke D, Studer D, Wick P, Gehr P, et al. Investigating the interaction of cellulose nanofibers derived from cotton with a sophisticated 3D human lung cell Coculture. Biomacromolecules. 2011;12:3666–3673. doi: 10.1021/bm200865j. [DOI] [PubMed] [Google Scholar]
- 33.Ilyas RA, Sapuan SM, Ishak MR. Isolation and characterization of nanocrystalline cellulose from sugar palm fibres (Arenga Pinnata) Carbohydr Polym. 2018;181:1038–1051. doi: 10.1016/j.carbpol.2017.11.045. [DOI] [PubMed] [Google Scholar]
- 34.Gao H, Duan B, Lu A, Deng H, Du Y, Shi X, et al. Fabrication of cellulose nanofibers from waste brown algae and their potential application as milk thickeners. Food Hydrocoll. 2018;79:473–481. [Google Scholar]
- 35.Segal L, Creely JJ, Martin AE, Conrad CM. An empirical method for estimating the degree of crystallinity of native cellulose using the X-Ray diffractometer. Text Res J. 1959;29:786–794. [Google Scholar]
- 36.Sharma PR, Varma AJ. Functionalized celluloses and their nanoparticles: morphology, thermal properties, and solubility studies. Carbohydr Polym. 2014;104:135–142. doi: 10.1016/j.carbpol.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 37.Tibolla H, Pelissari FM, Martins JT, Vicente AA, Menegalli FC. Cellulose nanofibers produced from banana peel by chemical and mechanical treatments: characterization and cytotoxicity assessment. Food Hydrocoll. 2018;75:192–201. [Google Scholar]
- 38.Brodeur G, Yau E, Badal K, Collier J, Ramachandran KB, Ramakrishnan S. Chemical and physicochemical pretreatment of lignocellulosic biomass: a review. Enzyme Res. 2011;2011:787532. doi: 10.4061/2011/787532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.María Andrade-Mahecha M, Pelissari FM, Tapia-Blácido DR, Menegalli FC. Achira as a source of biodegradable materials: isolation and characterization of nanofibers. Carbohydr Polym. 2015;123:406–415. doi: 10.1016/j.carbpol.2015.01.027. [DOI] [PubMed] [Google Scholar]
- 40.Viikari L, Kantelinen A, Sundquist J, Linko M. Xylanases in bleaching: from an idea to the industry. FEMS Microbiol Rev. 1994;13:335–350. [Google Scholar]
- 41.Dufresne A, Cavaille JY, Vignon MR. Mechanical behavior of sheets prepared from sugar beet cellulose microfibrils. J Appl Polym Sci. 1997;64:1185–1194. [Google Scholar]
- 42.Pelissari FM, Sobral PJ d A, Menegalli FC. Isolation and characterization of cellulose nanofibers from banana peels. Cellulose. 2014;21:417–432. [Google Scholar]
- 43.Dufresne A, Vignon MR. Improvement of starch film performances using cellulose microfibrils. Macromolecules. 1998;31(8):2693–2696. [Google Scholar]
- 44.Jiang F, Hsieh YL. Chemically and mechanically isolated nanocellulose and their self-assembled structures. Carbohydr Polym. 2013;95:32–40. doi: 10.1016/j.carbpol.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 45.Abraham E, Deepa B, Pothan LA, Jacob M, Thomas S, Cvelbar U, et al. Extraction of nanocellulose fibrils from lignocellulosic fibres: a novel approach. Carbohydr Polym. 2011;86:1468–1475. [Google Scholar]
- 46.Bhatnagar A, Sain M. Processing of cellulose nanofiber-reinforced composites. J Reinf Plast Compos. 2005;24:1259–1268. [Google Scholar]
- 47.Nascimento P, Marim R, Carvalho G, Mali S. Nanocellulose Produced from Rice hulls and its effect on the properties of biodegradable starch films. Mater Res. 2016;19:167–174. [Google Scholar]
- 48.Pelissari FM. Do Amaral Sobral PJ, Menegalli FC. Isolation and characterization of cellulose nanofibers from banana peels. Cellulose. 2014;21:417–432. [Google Scholar]
- 49.Martelli-Tosi M, Torricillas MD, Martins MA, Assis OB, Tapia-Blácido DR. Using commercial enzymes to produce cellulose nanofibers from soybean straw. J Nanomater. 2016;2016:1–10. [Google Scholar]
- 50.Chirayil CJ, Joy J, Mathew L, Mozetic M, Koetz J, Thomas S. Isolation and characterization of cellulose nanofibrils from Helicteres isora plant. Ind Crop Prod. 2014;59:27–34. [Google Scholar]
- 51.Alemdar A, Sain M. Isolation and characterization of nanofibers from agricultural residues – wheat straw and soy hulls. Bioresour Technol. 2008;99:1664–1671. doi: 10.1016/j.biortech.2007.04.029. [DOI] [PubMed] [Google Scholar]
- 52.Zuluaga R, Putaux JL, Cruz J, Vélez J, Mondragon I, Gañán P. Cellulose microfibrils from banana rachis: effect of alkaline treatments on structural and morphological features. Carbohydr Polym. 2009;76:51–59. [Google Scholar]
- 53.Sun RC, Ren JL, Sun RC. Hemicelluloses. In: Cereal straw as a resource for sustainable biomaterials and biofuels; 2010. p. 73–130.
- 54.Qua EH, Hornsby PR, Sharma HSS, Lyons G. Preparation and characterisation of cellulose nanofibres. J Mater Sci. 2011;46:6029–6045. [Google Scholar]
- 55.Lamaming J, Hashim R, Leh CP, Sulaiman O, Sugimoto T, Nasir M. Isolation and characterization of cellulose nanocrystals from parenchyma and vascular bundle of oil palm trunk (Elaeis guineensis) Carbohydr Polym. 2015;134:534–540. doi: 10.1016/j.carbpol.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 56.Mondragon G, Fernandes S, Retegi A, Peña C, Algar I, Eceiza A, et al. A common strategy to extracting cellulose nanoentities from different plants. Ind Crop Prod. 2014;55:140–148. [Google Scholar]
- 57.Li J, Zhang S, Gao B, Yang A, Wang Z, Xia Y, et al. Characteristics and deoxy-liquefaction of cellulose extracted from cotton stalk. Fuel. 2016;166:196–202. [Google Scholar]
- 58.Maiti S, Jayaramudu J, Das K, Reddy SM, Sadiku R, Ray SS, et al. Preparation and characterization of nano-cellulose with new shape from different precursor. Carbohydr Polym. 2013;98:562–567. doi: 10.1016/j.carbpol.2013.06.029. [DOI] [PubMed] [Google Scholar]
- 59.Robles E, Urruzola I, Labidi J, Serrano L. Surface-modified nano-cellulose as reinforcement in poly(lactic acid) to conform new composites. Ind Crop Prod. 2015;71:44–53. [Google Scholar]
- 60.Zhang YH, Lynd LR. Toward an aggregated understanding of enzymatic hydrolysis of cellulose: noncomplexed cellulase systems. Biotechnol Bioeng. 2004;88:797–824. doi: 10.1002/bit.20282. [DOI] [PubMed] [Google Scholar]
- 61.Klemm D, Heublein B, Fink HP, Bohn A. Cellulose: fascinating biopolymer and sustainable raw material. Angewandte Chemie international edition, vol. 44: John Wiley & Sons, Ltd.; 2005. p. 3358–93. [DOI] [PubMed]
- 62.Sofla MRK, Brown RJ, Tsuzuki T, Rainey TJ. A comparison of cellulose nanocrystals and cellulose nanofibres extracted from bagasse using acid and ball milling methods. Adv Nat Sci Nanosci Nanotechnol. 2016;7:035004. [Google Scholar]
- 63.Li S, Lu X, Xue Y, Lei J, Zheng T, Wang C. Fabrication of Polypyrrole/graphene oxide composite Nanosheets and their applications for Cr(VI) removal in aqueous solution. PLoS One. 2012;7:e43328. doi: 10.1371/journal.pone.0043328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Johar N, Ahmad I, Dufresne A. Extraction, preparation and characterization of cellulose fibres and nanocrystals from rice husk. Ind Crop Prod. 2012;37:93–99. [Google Scholar]
- 65.Ahmad N, Sultana S, Azam A, Sabir S, Khan MZ. Novel bio-nanocomposite materials for enhanced biodegradability and photocatalytic activity. New J Chem. 2017;41:10198–10207. [Google Scholar]
- 66.Soni B, Hassan EB, Mahmoud B. Chemical isolation and characterization of different cellulose nanofibers from cotton stalks. Carbohydr Polym. 2015;134:581–589. doi: 10.1016/j.carbpol.2015.08.031. [DOI] [PubMed] [Google Scholar]
- 67.Lamaming J, Hashim R, Sulaiman O, Leh CP, Sugimoto T, Nordin NA. Cellulose nanocrystals isolated from oil palm trunk. Carbohydr Polym. 2015;127:202–208. doi: 10.1016/j.carbpol.2015.03.043. [DOI] [PubMed] [Google Scholar]
- 68.Deepa B, Abraham E, Cherian BM, Bismarck A, Blaker JJ, Pothan LA, et al. Structure, morphology and thermal characteristics of banana nano fibers obtained by steam explosion. Bioresour Technol. 2011;102:1988–1997. doi: 10.1016/j.biortech.2010.09.030. [DOI] [PubMed] [Google Scholar]
- 69.Shebani AN, van Reenen AJ, Meincken M. The effect of wood extractives on the thermal stability of different wood species. Thermochim Acta. 2008;471:43–50. [Google Scholar]
- 70.Yang H, Yan R, Chen H, Lee DH, Zheng C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel. 2007;86:1781–1788. [Google Scholar]
- 71.Gordaliza M, Castro MA, del Corral JM, Feliciano AS. Antitumor properties of podophyllotoxin and related compounds. Curr Pharm Des. 2000;6:1811–1839. doi: 10.2174/1381612003398582. [DOI] [PubMed] [Google Scholar]
- 72.Ardalani H, Avan A, Ghayour-Mobarhan M. Podophyllotoxin: a novel potential natural anticancer agent. Avicenna J Phytomed. 2017;7:285–294. [PMC free article] [PubMed] [Google Scholar]