Abstract
Formaldehyde (HCHO) is the main source of indoor air pollutant. HCHO sensors are therefore of paramount importance for timely detection in daily life. However, existing sensors do not meet the stringent performance targets, while deactivation due to sensing detection at room temperature, for example, at extremely low concentration of formaldehyde (especially lower than 0.08 ppm), is a widely unsolved problem. Herein, we present the Ag nanoparticles (Ag NPs) sensitized dispersed In2O3 nanograin via a low-fabrication-cost hydrothermal strategy, where the Ag NPs reduces the apparent activation energy for HCHO transporting into and out of the In2O3 nanoparticles, while low concentrations detection at low working temperature is realized. The pristine In2O3 exhibits a sluggish response (Ra/Rg = 4.14 to 10 ppm) with incomplete recovery to HCHO gas. After Ag functionalization, the 5%Ag-In2O3 sensor shows a dramatically enhanced response (135) with a short response time (102 s) and recovery time (157 s) to 1 ppm HCHO gas at 30 °C, which benefits from the Ag NPs that electronically and chemically sensitize the crystal In2O3 nanograin, greatly enhancing the selectivity and sensitivity.
Keywords: In2O3, Ag loading, HCHO detection, Gas sensors
Introduction
All kinds of hazardous volatile organic compounds (VOCs) gases in indoor and outdoor, such as HCHO, ethanol, acetone, benzene, methanol, and toluene, are routinely and daily emitted from agriculture and industrial processes, or released as vehicle exhaust emissions [1]. VOCs, such as HCHO, are harmful to human health and the environment when their concentrations are above a critical threshold, sometimes as low as parts-per-million (ppm) levels [2, 3]. For safety reasons, anything out of limits in HCHO storage systems, appliances, and vehicles, as well as the entire internal environment infrastructure, must be detected immediately [4–6]. The ever-increasing attention concerning indoor and outdoor air quality and workplace safety has brought out the steady development of the gas sensors market over the past few years, and therefore, gas sensors are expected to attain wider application [7–9]. Therefore, formaldehyde sensors will play a critical role on account of formaldehyde’s extensive carcinogenicity range in air [10, 11].
Metal oxide semiconductor based on chemiresistors, mainly including In2O3 [12–14], WO3 [15–17], SnO2 [18, 19], ZnO [20, 21] and LaFeO3 [22–24], is an outstanding technique for detecting VOCs, due to its unique advantages in terms of low cost, good sensitivity, fast response/recovery time, and a large number of gases detected [25]. However, traditional gas sensors based on metal oxide semiconductors usually have a high working temperature of 150–400 °C, which may decrease the sensor stability and life. In addition, high operating temperature leads to high power consumption, which is an important parameter for the new generation of battery-loaded wireless sensors [26, 27]. However, this can be reversed when the sensing materials are elaborately designed. A typical method used for lowering working temperature is the surface modification of the semiconductor metal oxide with noble metals such as Ag [28, 29], Pt [30], and Pd [31, 32] or different metal oxides [26]. By either chemical sensitization or electronic sensitization, one can modify the semiconductor surface with various metal promoters to achieve an effective room-temperature sensor material. Outstanding sensing performance is attributed not only to the sensitizer effect of noble metals but also to the synergistic effect of large surface area, appropriate particle size, and abundant mesoporous surface of nanostructure [15, 20, 23, 33].
In2O3 is an important n-type semiconductor with about 3.6 eV wide band gap and has been widely studied owing to its high catalytic activity and electronic properties [34, 35]. Unfortunately, the pure In2O3 as sensing material possessing simply poor selectivity and a high response can hardly be obtained at low temperatures, which restricts its further application. To further enhance its sensing properties, In2O3 has been modified by noble metals [36], metal ions [37], and carbon materials [38]. Composites of multi-phase semiconducting metal oxide nanostructures have also been frequently reported [39]. To date, few researches have been carried out on the gas-sensing properties of In2O3 sensor to HCHO. Wang et al. [29] reported that the Ag-loaded In2O3 hierarchical nanostructure sensors showed fast response (0.9 s), recovery (14 s), and high response (11.3) towards 20 ppm HCHO at 240 °C. Dong et al. [40] reported that the as-synthesized 3 wt%Ag-functionalized In2O3/ZnO samples exhibited high response of about 842.9 towards 2000 ppm HCHO at operating temperature of 300 °C. Currently, formaldehyde gas sensors have been reported to require higher operating temperatures. Zhang et al. [28] have reported the results of formaldehyde gas-sensing tests, which revealed that a sensor based on 6%-Ag/Ni5.0In exhibits ultra-high sensitivity (123.97) toward 100 ppm of formaldehyde at a lower operating temperature (160 °C). Wang et al. [33] reported that the graphene oxide in situ modified two-dimensional SnO2 nanosheets with in-plane mesopores was utilized as the sensing material and that the sensor response was larger than 2000 toward 100 ppm HCHO at 60 °C. The problem that formaldehyde gas sensors with high sensitivity and high selectivity to low concentration HCHO at room temperature have remains unsolved.
In this work, we report a high-response formaldehyde gas sensor that operates at room temperature, which is prepared with In2O3 nanograin sensitized by Ag nanoparticles. The comparative study of HCHO gas detection between pure and Ag-loaded In2O3 nanoparticles was investigated, and the influence of Ag loading on sensing performance was revealed. The results show that 5%Ag-In2O3 sensor exhibits an excellent response of 1670 to 5 ppm HCHO at 30 °C and a ultra-low detection concentration of 0.05 ppm (to which the response value is 3.85). Simultaneously, the 5%Ag-In2O3 sensor also presents superior selectivity and stability, all of which reaches the level of metal oxide sensors.
Methods
Sample Preparation
The pure In2O3 was synthesized through dissolving 6 mmol In(NO3)3.4.5H2O (99.99%, Aladdin), and 24 mmol urea (99%, Aladdin) in 45 mL of deionized water; the mixture was kept in a 50-mL polyethylene reaction pot at 140 °C for 16 h and then cooled to room temperature. The prepared sediment was washed with ethyl alcohol for three times, dried for 20 h at 70 °C, and calcined for 2 h at 600 °C in pure nitrogen flow with a heat rate of 5 °C min−1. The pure In2O3 was dissolved in deionized water being stirred for 20 min, and then AgNO3(99.8%, Sigma-Aldrich) was added to transparent solution. Under magnetic stirring, the freshly prepared NaBH4(98%, Aladdin) solution was drop by drop into the above mixture solution. After being stirred, the as-made sediments of Ag-loaded In2O3 were collected through centrifugation, washed with absolute ethanol for three times, and dried in air at 60 °C for 12 h. Finally, yellowish nanotructural In2O3 samples were obtained. To study the effect of the Ag loading ratio on the gas sensing response, various contrast composites with different Ag loading rates (1 wt%, 3 wt%, 5 wt%, and 7 wt%) were prepared and named 1%Ag-In2O3, 3%Ag-In2O3, 5%Ag-In2O3, and 7%Ag-In2O3, respectively.
Characterization
The X-ray powder diffraction (XRD) of the prepared products was conducted on a D/max-2300 diffractometer (Rigaku Corporation; 35 kV) in a scanning range of 10–90° at a rate of 2°min−1 with Cu Kα1 radiation (l = 1.540 Å). X-ray photoelectron spectroscopy (XPS) was carried out on a K-Alpha+ spectrometer with Al Kα excitation (Thermo Fisher Scientific Co. Ltd; 1486.6 eV) to observe the chemical binding states of each element. The morphology of the samples was recorded by scanning electron microscopy (SEM, Thermo Fisher Scientific Co. Ltd.). The elemental composition was performed by SEM equipped with an energy dispersive X-ray spectroscopy (EDS) detector. Transition electron microscopy (TEM) and high-resolution transmission electron microscopy (HRTEM) of the size and crystallinity of the grain were performed by a JEM-2100 microscope (JEOL Co. Ltd.) operating at 200 kV. The N2 adsorption–desorption analysis of the obtained samples was collected on Beth equipment (Bestech Instrument Technology Co. Ltd.) at liquid nitrogen temperature.
Sensor Fabrication and Sensing Test
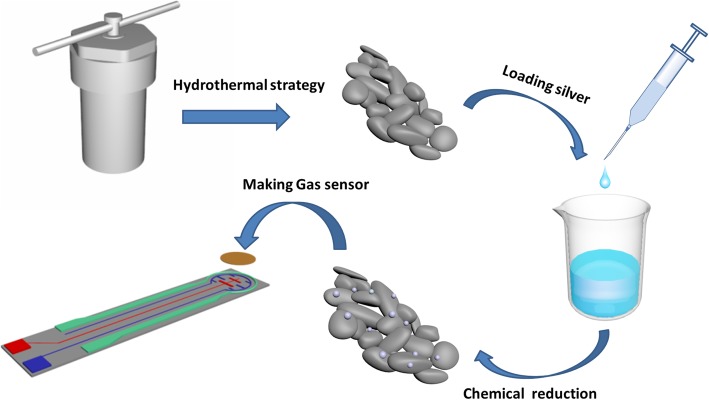
In the as-prepared of gas sensing materials (pure In2O3, 1%, 3%, 5%, and 7% Ag-loaded In2O3), 2 mg gas-sensing material samples were mixed with 2 mg printing oil in mortar, which was ground for 1 min in agate mortar for forming a uniform mash. The mash sensing materials were screen-printed with a mesh on the outer surface of substrate and dried at 60 °C for 10 min in a drying oven. The gas sensing material forming on the surface of substrate has a thickness of about 10 mm. Figure 1 presents the schematic diagram of the gas sensor. Finally, the devices were sintered at 400 °C for 2 h in an electric furnace to ensure its stability. Afterwards, the sensing properties were evaluated by HCRK-SD101 gas sensing analyzer (Wuhan HCRK Technology Co. Ltd.) at the relative humidity of 16 ± 10%. The prepared sensors were installed in the test chamber (2.7 L) and then injected with different concentrations of tested gas by a micro syringe. The response of the gas sensor can be defined as the ratio of the resistance value Ra to the resistance value Rg, where Ra and Rg refer to the resistance in air and target gas, respectively [41]. Response and recovery times refer to the time required to achieve 90% of the maximum sensing value during adsorption and desorption.
Fig. 1.
Schematic illustration of the preparation of Ag-functionalized In2O3 nanoparticles and screen printing
Results and Discussion
Morphology and Structure Characterization
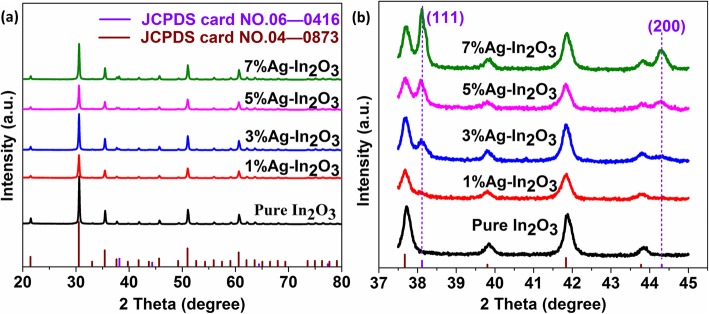
The crystal phase of the pure and Ag-loaded dispersed In2O3 were investigated utilizing XRD. XRD patterns of the pure and Ag-loaded dispersed In2O3 were shown in Fig. 2. It can be seen from Fig. 2a that the diffraction peaks of the In2O3 sample are similar according to the JCPDS card NO. 06-0416, which can be assigned to the cubic structure of In2O3. The diffraction peaks of In2O3 samples are located at 2y of 30.58, 35.46, 51.03, and 60.67, which are ascribed to the 222, 400, 440, and 622 planes, respectively. For Ag-loaded In2O3 samples, in Fig. 2b, the XRD curves corresponding to 1%Ag-In2O3, 3%Ag-In2O3, 5%Ag-In2O3, and 7%Ag-In2O3 are similar to those pure In2O3, indicating that the crystalline phase of In2O3 is barely influenced during the surface functionalization process. As the loading amount of Ag is increasing, the diffraction peaks of 200 and 111 agreeing with Ag (JCPDS card NO.04-0873) can be gradually detected by small bulges and continuously shift to larger angles. No impurity phase was examined from the XRD patterns, which further confirmed the prominent purity of the samples.
Fig. 2.
a XRD patterns of pure In2O3, 1%Ag-In2O3, 3%Ag-In2O3, 5%Ag-In2O3, and 7%Ag-In2O3 samples. b Corresponding high magnification of the 111 and 200 peaks of the samples
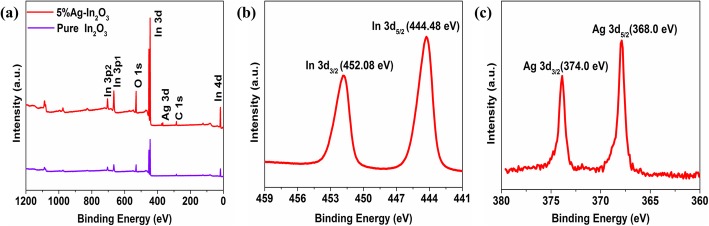
To further demonstrate the component and the chemical states of the as-synthesized samples in the surface region, XPS was presented. The full XPS spectra (Fig. 3a) reveal that the 5%Ag-In2O3 sample mainly contains In, O, Ag, and C elements. The presence of elemental C in the spectrum is due to the binding energy of C 1 s, which is usually used as an internal reference in the spectrum during XPS measurements. All XPS spectra were calibrated with a C1s peak of 284.8 eV, as shown in Fig. 3. The high-resolution In 3d XPS spectrum can be fitted with two strong peaks with binding energies at 452.08 eV (In 3d3/2) and 444.48 eV (In 3d5/2) in Fig. 3b. Compared with the reported In 3d5/2 (443.60 eV) signal of metallic indium, there is no metallic indium peak in our samples, demonstrating that the elemental indium exists only in the form of oxide and the major state is In3+. The high-resolution XPS spectra of the Ag peak is sketched, where the peak corresponding to metallic silver can be assigned to 374.0 eV (Ag 3d3/2) and 368.0 eV (Ag 3d5/2) in Fig. 3c, indicating that the Ag species loaded on the surface region are metallic silver.
Fig. 3.
a XPS spectrum of pure In2O3 and 5%Ag-In2O3 samples. b In 3d spectrum. c Ag 3d spectrum
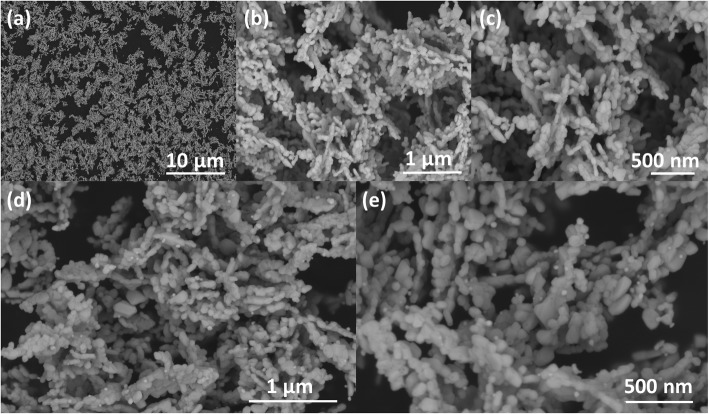
The morphology of the pure In2O3 and 5%Ag-In2O3 samples was preliminarily demonstrated in Fig. 4a–e by SEM analysis. All samples showed nanograin morphologies with diameters ranged from 20 to 50 nm and ranged from a few hundred nanometers to over 1 μm in lengths. For the pure In2O3 samples, from Fig. 4a–c, we can see that the surface of each nanograins is smooth. After functionalization processes, we can plainly see that the surface of the In2O3 nanograins is a little rough in Fig. 4d–e, and that the Ag NPs are distributed on the surface of In2O3 nanograins. The presented SEM images show that the loading of Ag has no significant effect on the morphology of In2O3.
Fig. 4.
SEM images of pure In2O3 (a, b, and c) and 5%Ag-In2O3 (d and e) samples
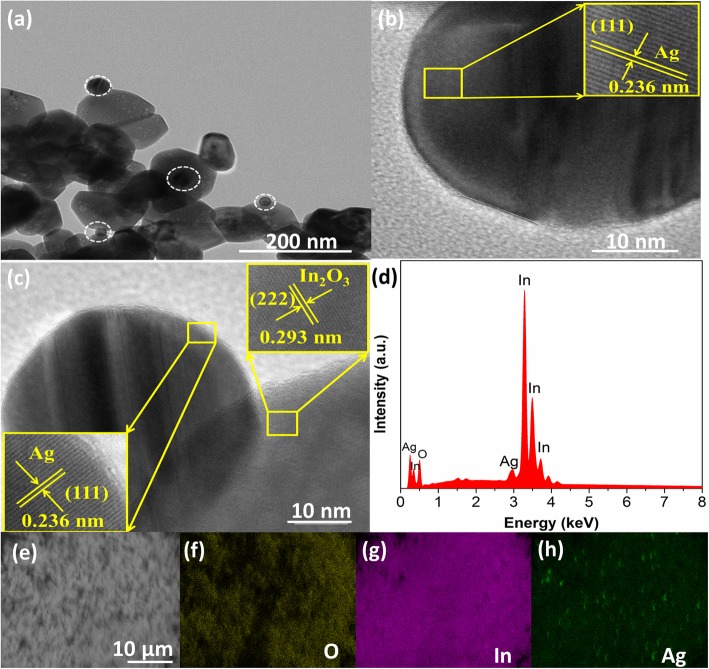
After Ag nanoparticles are decorated onto the dispersed In2O3 nanograins, the morphology and crystalline phases of 5%Ag-In2O3 samples are presented through the TEM images in Fig. 5. It can be seen that Ag NPs with a size from 30 nm to about 100 nm are well pinned on the surfaces of the dispersed In2O3 nanoparticle, which will be helpful for enhancing gas-sensing properties. In order to further observe the detailed microstructure of In2O3 and Ag NPs, high-resolution TEM images of the 5%Ag-In2O3 sample were obtained (Fig. 5b, c). The dispersed In2O3 are assembled into a single crystal in Fig. 5b and c. The high-resolution TEM images from Fig. 5c show that the lattice plane is 0.293 nm, corresponding to the (222) crystal plane of cubic In2O3, while the crystal spacing of 0.236 nm is in good agreement with the (111) spacing of Ag. In addition, the interface reveals the existence of strong electronic interaction between In2O3 nanostructures and Ag nanoparticles.
Fig. 5.
a TEM image of 5%Ag-In2O3 samples. b, c HRTEM images of 5%Ag-In2O3 samples. d EDS spectra pattern of 5%Ag-In2O3 samples. e–h The EDS mapping picture of O, In, and Ag elements of 5%Ag-In2O3 samples
The energy dispersive X-ray spectroscopy pattern (Fig. 5d) is eloquent proof of the existence of In, O, and a few Ag without any impurity elements. The atomic percentages of In, O, and Ag are 33.99%, 62.43%, and 3.59%, respectively. The atomic ratio of In and O is about 1:2, indicating that 5%Ag-In2O3 samples are the major phase component in selected region. To further determine the distribution of Ag, the 5%Ag-In2O3 samples were performed by the EDS. As observed from Fig. 5e–h, the Ag-loaded In2O3 sample was evenly distributed by elemental mappings of O, In, and Ag, respectively. The results show that there are obvious loads of Ag NPs onto the surface of dispersed In2O3 nanoparticle and dispersed In2O3 nanostructure does not accumulate with the decoration of Ag.
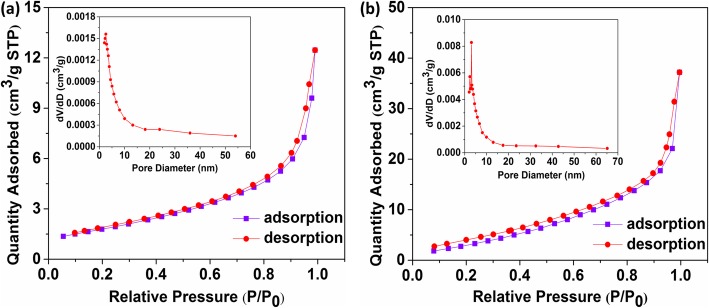
In order to obtain the porosity and the specific surface area of the pure In2O3 and 5%Ag-In2O3 samples, N2 adsorption-desorption experiment method was employed. On the basis of the current IUPAC classification, Fig. 6a and b show the classic type III isotherm to relative pressure (0.1 < P/P0 < 1.0) with a type H3 hysteresis loop, which consist of granular material and have no obvious saturated adsorption platform, indicating that the pore structure is very irregular. The pore volume and surface area of 5%Ag-In2O3 are 0.0650 cm3 g−1 and 14.4 m2 g−1 characterized with the Brunauer–Emmett–Teller (BET) method, both of which are larger than the pure In2O3 (6.5 m2g−1and 0.0204 cm3 g−1), demonstrating that the specific surface area gradually raises as being loaded certain content of Ag NPs. The pore-size distribution was measured by using the Barrett–Joyner–Halenda (BJH) way. One can see that the pore sizes of the pure In2O3 distributes in the range from 2 to 54 nm. For 5%Ag-In2O3 samples, the pore sizes are all distributed between 2 and 65 nm.
Fig. 6.
Nitrogen adsorption–desorption isotherms curves of pure In2O3 (a) and 5%Ag-In2O3 (b) samples. Insets are the corresponding pore size distribution curves obtained by the BJH method
Gas-Sensing Performance
The gas response upon exposure to 1 ppm HCHO was investigated by increasing the operating temperature of the sensor device to observe the relation between the operating temperature and gas response, and to determine the optimized operating temperature. The pure In2O3, 1%Ag-In2O3, 3%Ag-In2O3, 5%Ag-In2O3, and 7%Ag-In2O3 were continuously tested under the 5 ppm gaseous formaldehyde conditions at operating temperatures of 30–300 °C, respectively. The sensing responses of each gas sensor were measured at a fixed temperature, and the recorded values of the gas sensors are shown at room temperature in Fig. 7.
Fig. 7.
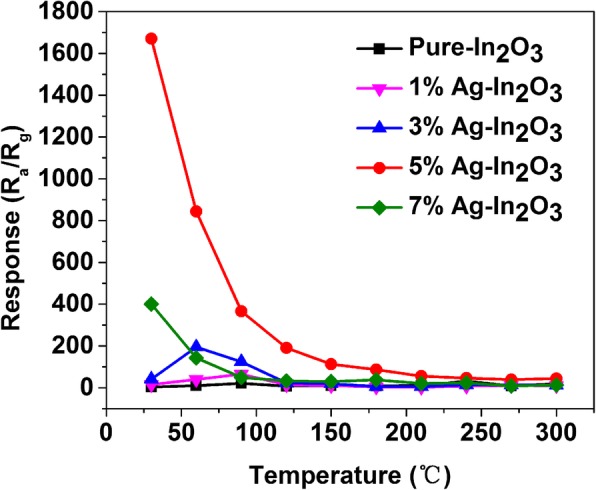
Responses of pure In2O3, 1%Ag-In2O3, 3%Ag-In2O3, 5%Ag-In2O3, and 7%Ag-In2O3 gas sensors to 5 ppm gaseous formaldehyde in the operating temperature range from 30 to 300 °C
It can be seen that the 5%Ag-In2O3 sensor has maximum response to formaldehyde gas at 30 °C, and the response is 1670. It tends to increase at lower operating temperatures (5%Ag-In2O3: 1670, 844, 366, 191, 113, 87, 56, 46.3, 39, and 44.2 at 30, 60, 90, 120, 150, 180, 210, 240, 270, and 300 °C; 7%Ag-In2O3: 400, 143, 49, 33.1, 29.3, 37.8, 20.3, 23.3, 8.66, and 12.8 at the same operating temperatures). The gas responses of the Ag-In2O3 sensors show higher values than pure In2O3 in all operating temperature ranges, which can fix the optimal operating temperature and the optimum HCHO response of the sensors. Among them, it can be clearly seen that 5%Ag-In2O3 sensor has the highest responses (1670) to 5 ppm HCHO at room temperature demonstrating the superior sensing properties of the sensor, which is higher than other sensors. The reason the higher gas response appears at room operating temperatures can be attributed to Ag NPs catalytic (spill-over effect) and electronic (generation of schottky barrier) sensitization. After loading the Ag NPs, the operating temperature is reduced and the formaldehyde sensitive response enhanced significantly. Nevertheless, with the loading of Ag NPs further enhancing, the response value decreases. This can be ascribed to the reduction in number of surface active sites of In2O3, which indicates that the excessive coverage of Ag NPs and the permeability of gas are affected, and then the catalytic action of Ag NPs is weaken, causing a decrease in adsorbed oxygen ions [28]. Compared with other previously reported gas sensors based on In2O3 sunflower, In2O3/ZnO nanocomposites, or In2O3 nanorods, our gas sensor shows notable gas response at room temperature [28, 29, 40].
To further confirm the selectivity of the synthesized gas sensors toward HCHO, the selectivity sensing performance of pure In2O3, 1%Ag-In2O3, 3%Ag-In2O3, 5%Ag-In2O3, and 7%Ag-In2O3 sensors was tested at room temperature toward 10 ppm of several volatile organic compounds, including benzene, toluene, xylene, methane, formaldehyde, acetone, ethanol, and ammonia, 5%Ag-In2O3 and 7%Ag-In2O3 toward this gases with a concentration of 1 ppm at room temperature. As shown in Fig. 8, the Ag-In2O3 sensors demonstrate superior selectivity to formaldehyde, whereas they have poor responses to other typical interference gases at the same temperature, especially the 5%Ag-In2O3 sensor. This indicates that the prepared sensor has quite excellent selectivity towards formaldehyde.
Fig. 8.
The gas response of pure In2O3, 1%Ag-In2O3, and 3%Ag-In2O3 (benzene, toluene, xylene, methane, formaldehyde, acetone, ethanol, and ammonia) with a concentration of 10 ppm at 30 °C, 5%Ag-In2O3 and 7%Ag-In2O3 toward this gases with a concentration of 1 ppm at 30 °C
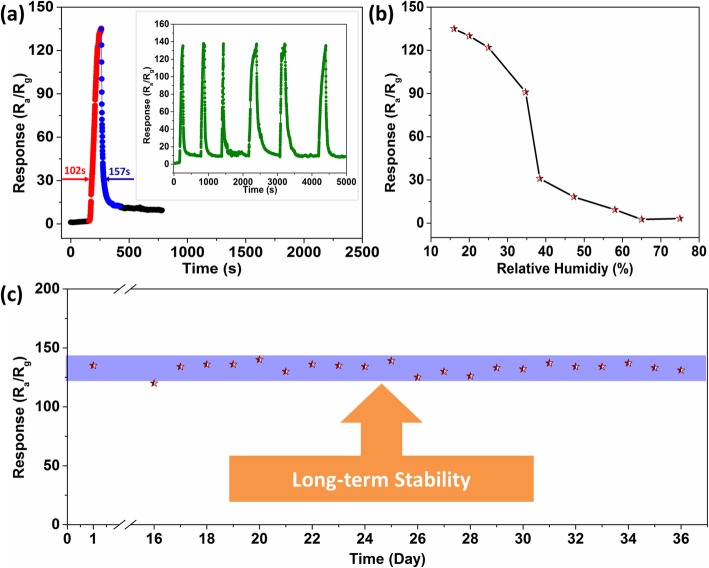
Furthermore, the stability of the 5%Ag-In2O3 sensor is shown in Fig. 9. The 5%Ag-In2O3 sensor was investigated toward 1 ppm HCHO for 6 cycles at room temperature (Fig. 9a), which demonstrates the excellent reproducibility to HCHO at room temperature. As demonstrated in Fig. 9c, the 36-day response test results show that the 5%Ag-In2O3 sensor not only possesses high gas-sensing performance but also excellent long-term stability. Meanwhile, the gas-sensing properties of 5%Ag-In2O3 sensor under different humidity conditions were investigated (Fig. 9b). Obviously, the sensor has not been significant affected on the sensing performance under a relative humidity range of 10−30%. Nevertheless, when the relative humidity range increases from 30 to 80%, the gas-sensing properties begin to reduce gradually.
Fig. 9.
a The response–recovery of the 5%Ag-In2O3 toward 1 ppm HCHO gas for 6 cycles at 30 °C. b Responses of 5%Ag-In2O3 sensor toward 1 ppm of HCHO under different humidity conditions at 30 °C. c Long-term stability tests of 5%Ag-In2O3 sensor toward 1 ppm HCHO after continuous evaluation for 36 days at 30 °C
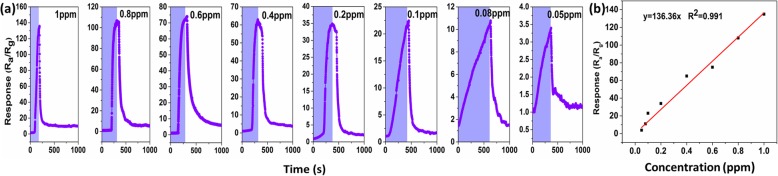
The real-time dynamic gas responses of the 5%Ag-In2O3 sensors toward HCHO at various concentrations at room temperature are presented in Fig. 10. The responses to 1, 0.8, 0.6, 0.4, 0.2, 0.1, 0.08, and 0.05 ppm formaldehyde were calculated to be Ra/Rg = 135, 108, 75, 65, 34, 23, 11, and 3.85, respectively. The sensitivity amplitude increases monotonically with gas concentration and is far from saturation until the gas concentration reaches 0.05 ppm, which is beneficial to the quantitative measurement of formaldehyde. Notably, the response is still as high as 3.85 when sensor is exposed to concentrations of formaldehyde as low as 0.05 ppm, indicating the ultra-low detection concentration of the sensor.
Fig. 10.
a, b Real-time response–recovery characteristic curve of the 5%Ag-In2O3 toward formaldehyde at different concentrations (1, 0.8, 0.6, 0.4, 0.2, 0.1, 0.08, 0.05 ppm) at 30 °C
Mechanism of the Gas Sensor
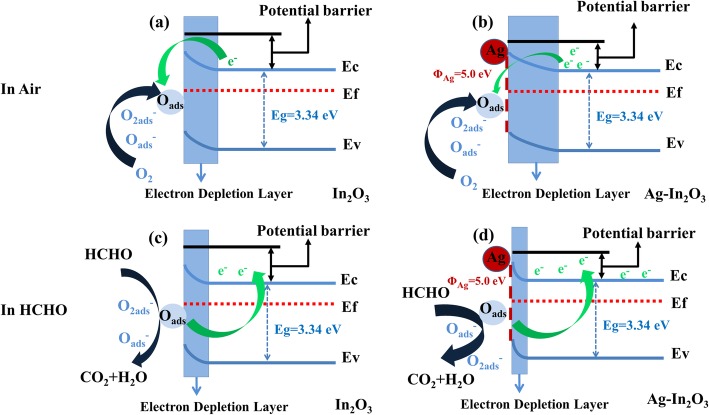
The In2O3 semiconductor is a chemical resistance–sensing material, and its electrical property changes mainly with the reaction of HCHO on the surface of In2O3. A HCHO sensing schematic diagram is shown in Fig. 11. When the sensor is exposed to air, an abundance of oxygen molecules in air will be absorbed onto the surface of the In2O3, and this oxygen captures electron from the material’s conductive band and converts them into more active chemical adsorbed oxygen, thereby creating a space charge area (depletion layer) that greatly increases initial resistance. The electron depletion layer has a great influence on the initial resistance of the sensor in the air. The major forms of chemisorbed oxygen species are O2− and O−, which can be described as Eqs. (1)-(3):
| 1 |
| 2 |
| 3 |
Fig. 11.
a–d HCHO sensing mechanism of schematic illustration for 5%Ag-In2O3 and pure In2O3, respectively
When the sensor is placed in an environment inflated with HCHO, the chemical adsorption oxygen reacts with HCHO, discharging electrons back to the conductive band, reducing the space charge area thickness, and thus decreasing the sensor resistance. The occurred reaction can be explained as followed in Eqs. (4) and (5):
| 4 |
| 5 |
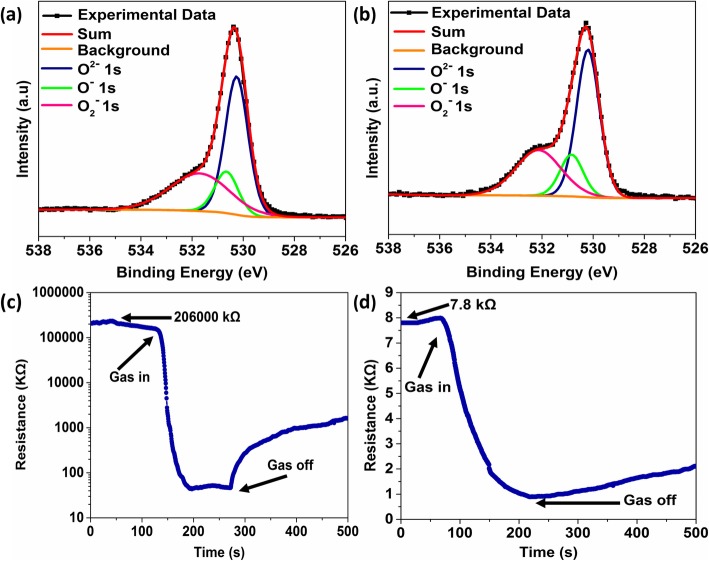
Obviously, the sensor performances based on 5%Ag-In2O3 are much higher than those of pure In2O3. This excellent response is ascribed to the electronic sensitization and chemical effect of Ag NPs. The Ag NPs have high availability for the catalytic activation of the dissociation of molecular oxygen, and the activated oxygen species created are then spilled onto the metal oxides surface and interact with the adsorption-desorption reactions of oxygen [42]. The chemisorbed oxygen plays a critical role in the gas sensing of sensors by regulating the reaction with tested gases [43]. Pure In2O3 and 5%Ag-In2O3 based on sensors were investigated by XPS to confirm the ratio of the chemisorbed oxygen in the samples. The O1 spectra of the pure In2O3 and 5%Ag- In2O3 (Fig. 12a, b and Table 1) show that the adsorbed oxygen content (2.46% of O− and 19.54% of O2−) of 5%Ag- In2O3 is higher than that of pure In2O3 (1.83% of O− and 16.05% of O2−), which is mainly due to the Ag Nps spill-over effect on metal oxide semiconductors [44]. Owing to the high conductivity and catalytic properties of Ag NPs [28, 42, 45, 46], Ag NPs on the surface of the metal oxides enhance the chemical activity of the chemisorbed oxygen species and spill the oxygen species over the substrate, which accelerates for gas sensing at low temperature.
Fig. 12.
XPS spectra of 5%Ag-In2O3 (a) and pure In2O3 (b) in the vicinity of O1s. Dynamic resistance transition characteristics of the 5%Ag-In2O3 (c) and pure In2O3 (d) toward 40 ppm of formaldehyde at 30 °C
Table 1.
The ratio of chemical component of 5%Ag-In2O3 and pure In2O3.
| Content | O2− | O− | O2− |
|---|---|---|---|
| 5%Ag-In2O3 | 78.00 | 2.46 | 19.54 |
| pure In2O3 | 82.13 | 1.83 | 16.05 |
Moreover, the Schottky junction can be formed at the interface between In2O3 and Ag due to the difference in band gap and work function [47, 48]. When the 5%Ag-In2O3 material is exposed to the atmosphere, compared with pure In2O3, the depletion region in 5%Ag-In2O3 composites is further broadened due to the presence of Schottky junction between Ag and In2O3 interface. The charged species such as O− and O2− adsorbed on the surface of In2O3 also contribute to electron depletion by capturing free electrons from the sensing materials [15] (Fig. 12a, b). The base resistance of 5%Ag-In2O3 was investigated to 206000 kΩ, far higher than the resistance (7.8 kΩ) of pure In2O3 (Fig. 12c, d), further demonstrating that the Ag NPs can remarkably enhance baseline resistance. When the 5%Ag-In2O3 material is exposed to HCHO in the sensing process, the Schottky junction forming at the interface between Ag and In2O3 produces more overflow electrons and donates it to the In2O3 matrix, resulting in efficient modulation of the depletion layer. Besides, with more oxygen substances adsorbed on the surface of Ag/In2O3, the redox reactions occurred between HCHO and chemical adsorbed oxygen are enhanced. The redundant electrons generated by these increased surface reactions result in a greater reduction in resistance of the 5%Ag-In2O3-based sensors in HCHO (Fig. 12c, d). Hence, 5%Ag-In2O3 sensor possesses superior sensing performance to HCHO.
Conclusion
In summary, we realized an ultra-high performance HCHO chemiresistor with Ag nanoparticles sensitized dispersed In2O3 semiconductor. The 5%Ag-In2O3 sensor demonstrates ultra-high response (135), short response time (102 s) and recovery time (157 s) to 1 ppm HCHO gas, and an ultra-low detection concentration (0.05 ppm) at room temperature. Compared with other HCHO sensors, the sensor has good reproducibility and strong responsivity at room temperature, and will have an excellent practical application prospect.
Acknowledgements
Not applicable.
Abbreviations
- Ag NPs
Ag nanoparticles
- EDS
Energy dispersive X-ray spectroscopy
- HCHO
Formaldehyde
- HRTEM
High-resolution transmission electron microscopy
- ppm
Parts-per-million
- Ra
Resistance in air
- Rg
Resistance in target gas
- SEM
Scanning electron microscopy
- TEM
Transition electron microscopy
- VOCs
Volatile organic compounds
- XPS
X-ray photoelectron spectroscopy
- XRD
X-ray powder diffraction
Authors’ Contributions
SZ and MC contributed to the conceptualization of the study. SZ contributed to the methodology of the study. SZ and YZ were assigned for the software in the study. QL and JH contributed to the validation of the study. HW contributed to the formal analysis of the study. HW and BL contributed to the investigation of the study. JZ and QL contributed to the resources of the study. SZ contributed to the data curation of the study. SZ contributed to the writing—original draft preparation of the study. SZ and MC contributed to the writing—review and editing of the study. JZ contributed to the visualization of the study. QL contributed to the supervision of the study. QL contributed to the project administration of the study. QL contributed to the funding acquisition of the study. All authors read and approved the final manuscript.
Funding
This work was supported by National Natural Science Foundation of China (no. 51562038), Yunnan Key Project of Natural Science Foundation of Yunnan (2018FY001(-011)), Yunnan Basic Applied Research Project (no. 2017FB086), and Yunnan Provincial Department of Education Science Research Fund Project (no. 2019Y0002).
Availability of Data and Materials
The datasets supporting the conclusions of this article are included within the article, and further information about the data and materials could be made available to the interested party under a motivated request addressed to the corresponding author.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shiqiang Zhou and Mingpeng Chen contributed equally to this work.
References
- 1.Bartzis J, Wolkoff P, Stranger M, Efthimiou G, Tolis EI, Maes F, Norgaard AW, Ventura G, Kalimeri KK, Goelen E, Fernandes O. On organic emissions testing from indoor consumer products’ use. J Hazard Mater. 2015;285:37–45. doi: 10.1016/j.jhazmat.2014.11.024. [DOI] [PubMed] [Google Scholar]
- 2.Sun X, Shao K, Wang T. Detection of volatile organic compounds (VOCs) from exhaled breath as noninvasive methods for cancer diagnosis. Anal Bioanal Chem. 2016;408:2759–2780. doi: 10.1007/s00216-015-9200-6. [DOI] [PubMed] [Google Scholar]
- 3.Bennett GF. Volatile organic compounds in the atmosphere. J Hazard Mater. 2008;151:285–287. doi: 10.1016/j.jhazmat.2007.10.014. [DOI] [Google Scholar]
- 4.Mirzaei A, Leonardi SG, Neri G. Detection of hazardous volatile organic compounds (VOCs) by metal oxide nanostructures-based gas sensors: a review. Ceram Int. 2016;42:15119–15141. doi: 10.1016/j.ceramint.2016.06.145. [DOI] [Google Scholar]
- 5.Tripathi KM, Kim T, Losic D, Tung TT. Recent advances in engineered graphene and composites for detection of volatile organic compounds (VOCs) and non-invasive diseases diagnosis. Carbon. 2016;110:97–129. doi: 10.1016/j.carbon.2016.08.040. [DOI] [Google Scholar]
- 6.Xing R, Xu L, Song J, Zhou C, Li Q, Liu D, Wei Song H. Preparation and gas sensing properties of In2O3/Au nanorods for detection of volatile organic compounds in exhaled breath. Sci Rep. 2015;2015(5):10717–10731. doi: 10.1038/srep10717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng ZQ, Zhu LF, Wang B. In2O3 nanotower hydrogen gas sensors based on both schottky junction and thermoelectronic emission. Nanoscale Res Lett. 2015;10:293–307. doi: 10.1186/s11671-015-1002-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song L, Luo L, Xi Y, Song J, Wang Y, Yang L, Wang A, Chen Y, Han N, Wang F. Reduced graphene oxide-coated Si nanowires for highly sensitive and selective detection of indoor f ormaldehyde. Nanoscale Res Lett. 2019;14:97–106. doi: 10.1186/s11671-019-2921-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song L, Lukianov A, Butenko D, Li H, Zhang J, Feng M, Liu L, Chen D, Klyui NI. Facile synthesis of hierarchical tin oxide nanoflowers with ultra-high methanol gas sensing at low working temperature. Nanoscale Res Lett. 2019;14:84–95. doi: 10.1186/s11671-019-2911-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fine GF, Cavanagh LM, Afonja A, Binions R. Metal oxide semi-conductor gas sensors in environmental monitoring. Sensors (Basel) 2010;10:5469–5502. doi: 10.3390/s100605469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lai X, Wang D, Han N, Du J, Li J, Xing C, Chen Y, Li X. Ordered arrays of bead-chain-like In2O3 nanorods and their enhanced sensing performance for formaldehyde. Chem Mater. 2010;22:3033–3042. doi: 10.1021/cm100181c. [DOI] [Google Scholar]
- 12.Chen Y, Huang W, Sangwan VK, Wang B, Zeng L, Wang G, Huang Y, Lu Z, Bedzyk MJ, Hersam MC, Marks TJ, Facchetti A. Polymer doping enables a two-dimensional electron gas for high-performance homojunction oxide thin-film transistors. Adv Mater. 2019;31:1805082–1805090. doi: 10.1002/adma.201805082. [DOI] [PubMed] [Google Scholar]
- 13.Wang Z, Hou C, De Q, Gu F, Han D. One-step synthesis of Co-doped In2O3 nanorods for high response of formaldehyde sensor at low temperature. ACS Sens. 2018;3:468–475. doi: 10.1021/acssensors.7b00896. [DOI] [PubMed] [Google Scholar]
- 14.Li Z, Yan S, Zhang S, Wang J, Shen W, Wang Z, Fu YQ. Ultra-sensitive UV and H2S dual functional sensors based on porous In2O3 nanoparticles operated at room temperature. J. Alloys Compd. 2019;770:721–731. doi: 10.1016/j.jallcom.2018.08.188. [DOI] [Google Scholar]
- 15.Ma J, Ren Y, Zhou X, Liu L, Zhu Y, Cheng X, Xu P, Li X, Deng Y, Zhao D. Pt nanoparticles sensitized ordered mesoporous WO3 semiconductor: gas sensing performance and mechanism study. Adv Funct Mater. 2018;28:1705268–1705280. doi: 10.1002/adfm.201705268. [DOI] [Google Scholar]
- 16.Kamali Heidari E, Marzbanrad E, Zamani C, Raissi B. Nanocasting synthesis of ultrafine WO3 nanoparticles for gas sensing applications. Nanoscale Res Lett. 2009;5:370–373. doi: 10.1007/s11671-009-9490-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zou Y, Xi S, Bo T, Zhou X, Ma J, Yang X, Diao C, Deng Y. Mesoporous amorphous Al2O3/crystalline WO3 heterophase hybrids for electrocatalysis and gas sensing applications. J Mater Chem A. 2019;7:21874–21883. doi: 10.1039/C9TA08633A. [DOI] [Google Scholar]
- 18.Zha YF, Sun YP, Yin X, Yin GC, Wang XM, Jia FC, Liu B. Effect of surfactants on the microstructures of hierarchical SnO2 blooming nanoflowers and their gas-sensing properties. Nanoscale Res Lett. 2018;13:250–261. doi: 10.1186/s11671-018-2656-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wan W, Li Y, Ren X, Zhao Y, Gao F, Zhao H. 2D SnO2 nanosheets: synthesis, characterization, structures, and excellent sensing performance to ethylene glycol. Nanomaterials (Basel) 2018;8:112–122. doi: 10.3390/nano8020112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou X, Zou Y, Ma J, Cheng X, Li Y, Deng Y, Zhao D. Cementing mesoporous ZnO with silica for controllable and switchable gas sensing selectivity. Chem Mater. 2019;31:8112–81209. doi: 10.1021/acs.chemmater.9b02844. [DOI] [Google Scholar]
- 21.Cao Z, Wang Y, Li Z, Yu N. Hydrothermal synthesis of ZnO structures formed by high-aspect-ratio nanowires for acetone detection. Nanoscale Res Lett. 2016;11:347–353. doi: 10.1186/s11671-016-1563-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Rong Q, Zhao J, Zhang J, Zhu Z, Liu Q. Boron-doped graphene quantum dot/Ag–LaFeO3 p–p heterojunctions for sensitive and selective benzene detection. J Mater Chem A. 2018;6:12647–12653. doi: 10.1039/C8TA03425G. [DOI] [Google Scholar]
- 23.Chen M, Zhang Y, Zhang J, Li K, Lv T, Shen K, Zhu Z, Liu Q. Facile lotus-leaf-templated synthesis and enhanced xylene gas sensing properties of Ag-LaFeO3 nanoparticles. J Mater Chem C. 2018;6:6138–6145. doi: 10.1039/C8TC01402G. [DOI] [Google Scholar]
- 24.Rong Q, Zhang Y, Lv T, Shen K, Zi B, Zhu Z, Zhang J, Liu Q. Highly selective and sensitive methanol gas sensor based on molecular imprinted silver-doped LaFeO3 core-shell and cage structures. Nanotechnology. 2018;29:145503–145512. doi: 10.1088/1361-6528/aaabd0. [DOI] [PubMed] [Google Scholar]
- 25.Gurlo A. Nanosensors: towards morphological control of gas sensing activity. SnO2, In2O3, ZnO and WO3 case studies. Nanoscale. 2011;3:154–165. doi: 10.1039/C0NR00560F. [DOI] [PubMed] [Google Scholar]
- 26.Zhang J, Liu X, Neri G, Pinna N. Nanostructured materials for room-temperature gas sensors. Adv Mater. 2016;28:795–831. doi: 10.1002/adma.201503825. [DOI] [PubMed] [Google Scholar]
- 27.Li Z, Li H, Wu Z, Wang M, Luo J, Torun H, Hu P, Yang C, Grundmann M, Liu X, Fu Y. Advances in designs and mechanisms of semiconducting metal oxide nanostructures for high-precision gas sensors operated at room temperature. Mater Horiz. 2019;6:470–506. doi: 10.1039/C8MH01365A. [DOI] [Google Scholar]
- 28.Zhang X, Song D, Liu Q, Chen R, Hou J, Liu J, Zhang H, Yu J, Liu P, Wang J. Designed synthesis of Ag-functionalized Ni-doped In2O3 nanorods with enhanced formaldehyde gas sensing properties. J Mater Chem C. 2019;7:7219–7229. doi: 10.1039/C9TC00978G. [DOI] [Google Scholar]
- 29.Wang S, Xiao B, Yang T, Wang P, Xiao C, Li Z, Zhao R, Zhang M. Enhanced HCHO gas sensing properties by Ag-loaded sunflower-like In2O3 hierarchical nanostructures. J Mater Chem A. 2014;2:6598–6604. doi: 10.1039/C3TA15110G. [DOI] [Google Scholar]
- 30.Liu W, Xie Y, Chen T, Lu Q, Ur Rehman S, Zhu L. Rationally designed mesoporous In2O3 nanofibers functionalized Pt catalysts for high-performance acetone gas sensors. Sens. Actuators B. 2019;298:126871–126880. doi: 10.1016/j.snb.2019.126871. [DOI] [Google Scholar]
- 31.Liu B, Xu Y, Li K, Wang H, Gao L, Luo Y, Duan G. Pd-catalyzed reaction-producing intermediate S on a Pd/In2O3 surface: a key to achieve the enhanced CS2-sensing performances. ACS Appl Mater Interfaces. 2019;11:16838–16846. doi: 10.1021/acsami.9b01638. [DOI] [PubMed] [Google Scholar]
- 32.Wang Z, Li Z, Jiang T, Xu X, Wang C. Ultrasensitive hydrogen sensor based on Pd(0)-loaded SnO2 electrospun nanofibers at room temperature. ACS Appl Mater Interfaces. 2013;5:2013–2021. doi: 10.1021/am3028553. [DOI] [PubMed] [Google Scholar]
- 33.Wang D, Tian L, Li H, Wan K, Yu X, Wang P, Chen A, Wang X, Yang J. Mesoporous ultrathin SnO2 nanosheets in situ modified by graphene oxide for extraordinary formaldehyde detection at low temperatures. ACS Appl Mater Interfaces. 2019;11:12808–12818. doi: 10.1021/acsami.9b01465. [DOI] [PubMed] [Google Scholar]
- 34.Roso S, Degler D, Llobet E, Barsan N, Urakawa A. Temperature-dependent NO2 sensing mechanisms over indium oxide. ACS Sens. 2017;2:1272–1277. doi: 10.1021/acssensors.7b00504. [DOI] [PubMed] [Google Scholar]
- 35.Tao B, Zhang Y, Han D, Li Y, Yan Z. Synthesis of corundum-type In2O3 porous spheres and their photocatalytic properties. J Mater Chem A. 2014;2:5455–5461. doi: 10.1039/C4TA00139G. [DOI] [Google Scholar]
- 36.Xing R, Li Q, Xia L, Song J, Xu L, Zhang J, Xie Y, Song H. Au-modified three-dimensional In2O3 inverse opals: synthesis and improved performance for acetone sensing toward diagnosis of diabetes. Nanoscale. 2015;7:13051–13060. doi: 10.1039/C5NR02709H. [DOI] [PubMed] [Google Scholar]
- 37.Hu X, Tian L, Sun H, Wang B, Gao Y, Sun P, Liu F, Lu G. Highly enhanced NO2 sensing performances of Cu-doped In2O3 hierarchical flowers. Sens. Actuators B. 2015;221:297–304. doi: 10.1016/j.snb.2015.06.080. [DOI] [Google Scholar]
- 38.Gu F, Nie R, Han D, Wang Z. In2O3–graphene nanocomposite based gas sensor for selective detection of NO2 at room temperature. Sens. Actuators B: Chem. 2015;219:94–99. doi: 10.1016/j.snb.2015.04.119. [DOI] [Google Scholar]
- 39.Wang L, Gao J, Wu B, Kan K, Xu S, Xie Y, Li L, Shi K. Designed synthesis of In2O3 Beads@TiO2-In2O3 composite nanofibers for high performance NO2 sensor at room temperature. ACS Appl Mater Interfaces. 2015;7:27152–27159. doi: 10.1021/acsami.5b09496. [DOI] [PubMed] [Google Scholar]
- 40.Dong C, Liu X, Han B, Deng S, Xiao X, Wang Y. Nonaqueous synthesis of Ag-functionalized In2O3/ZnO nanocomposites for highly sensitive formaldehyde sensor. Sens. Actuators B: Chem. 2016;224:193–200. doi: 10.1016/j.snb.2015.09.107. [DOI] [Google Scholar]
- 41.Zhang Y, Liu Q, Zhang J, Zhu Q, Zhu Z. A highly sensitive and selective formaldehyde gas sensor using a molecular imprinting technique based on Ag–LaFeO3. J Mater Chem C. 2014;2:10067–10072. doi: 10.1039/C4TC01972E. [DOI] [Google Scholar]
- 42.Xu L, Xing R, Song J, Xu W, Song H. ZnO–SnO2 nanotubes surface engineered by Ag nanoparticles: synthesis, characterization, and highly enhanced HCHO gas sensing properties. J Mater Chem C. 2013;1:2174–2182. doi: 10.1039/c3tc00689a. [DOI] [Google Scholar]
- 43.Gao L, Ren F, Cheng Z, Zhang Y, Xiang Q, Xu J. Porous corundum-type In2O3 nanoflowers: controllable synthesis, enhanced ethanol-sensing properties and response mechanism. CrystEngComm. 2015;17:3268–3276. doi: 10.1039/C5CE00279F. [DOI] [Google Scholar]
- 44.Hubner M, Koziej D, Grunwaldt JD, Weimar U, Barsan N. An Au clusters related spill-over sensitization mechanism in SnO2-based gas sensors identified by operando HERFD-XAS, work function changes, DC resistance and catalytic conversion studies. Phys Chem Chem Phys. 2012;14:13249–13254. doi: 10.1039/c2cp41349c. [DOI] [PubMed] [Google Scholar]
- 45.Chen M, Wang H, Hu J, Zhang Y, Li K, Zhang D, Zhou S, Zhang J, Zhu Z, Liu Q. Near-room-temperature ethanol gas sensor based on mesoporous Ag/Zn–LaFeO3 nanocomposite. Adv Mater Interfaces. 2019;6:1801453–1801463. doi: 10.1002/admi.201801453. [DOI] [Google Scholar]
- 46.Yan S, Li Z, Li H, Wu Z, Wang J, Shen W, Fu YQ. Ultra-sensitive room-temperature H2S sensor using Ag–In2O3 nanorod composites. J Mater Sci. 2018;53:16331–16344. doi: 10.1007/s10853-018-2789-z. [DOI] [Google Scholar]
- 47.Prieto G, Zecevic J, Friedrich H, de Jong KP, de Jongh PE. Towards stable catalysts by controlling collective properties of supported metal nanoparticles. Nat Mater. 2013;12:34–39. doi: 10.1038/nmat3471. [DOI] [PubMed] [Google Scholar]
- 48.Xu Y, Cheng C, Du S, Yang J, Yu B, Luo J, Yin W, Li E, Dong S, Ye P, Duan X. Contacts between two- and three-dimensional materials: ohmic, schottky, and p-n heterojunctions. ACS Nano. 2016;10:4895–4919. doi: 10.1021/acsnano.6b01842. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article, and further information about the data and materials could be made available to the interested party under a motivated request addressed to the corresponding author.