Introduction
Sentinel node (SN) biopsy is now routinely performed for breast cancer patients. It was first described 40 years ago by Ernest Gould et al. [1]. SN biopsy in breast cancer is performed to avoid the morbidity of axillary lymph node dissection (ALND). SLN (sentinel lymph node) procedure requires identification and resection of the first lymph nodes upon which the primary tumor drains. Subsequently SLN undergoes frozen section examination to see if node is involved or not. This predicts chance of metastases to other nodes and dictates the need for axillary node dissection and further adjuvant treatment. SLN has high sensitivity and specificity, but studies have demonstrated a failure rate of 5%. To have a good oncological outcome, false negative rates should be very low. False negative means we are leaving behind malignant node in axilla and assuming status of axilla to be negative for malignancy. A false negative result leads to the undertreatment of patients. The good outcome of SLN depends on surgeon’s expertise and knowledge of anatomy of lymphatic’s [2]. False negative SNs are because of technical failures, which may arise because of not removing and later examining true SN. The surgeon should be familiar with the anatomy of the lymphatic of breast to avoid this technical error. We present a rare presentation of multiple route–multiple SLN with reviews of different lymphatic routes, which a patient can present with the surgeon.
Case Report
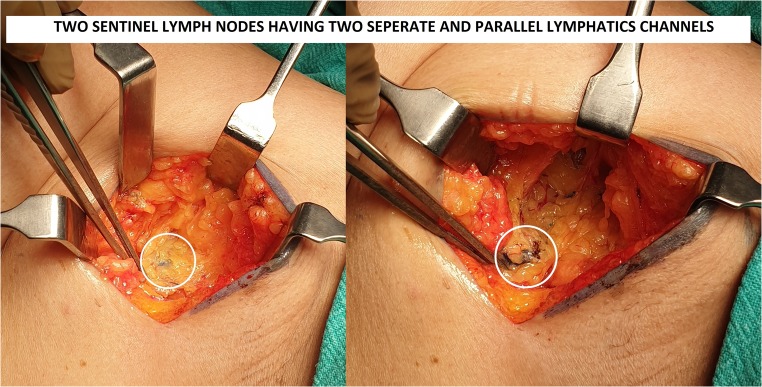
A 46-year-old female presented with 2 × 3 cm lump in the left breast with no axillary lymph nodes in the axilla (clinically or radiological). Patient was posted for breast conservative surgery after routine evaluation and anesthetic checkup. During surgery, we found two parallel lymphatic draining into two separate lymph nodes (Fig. 1). We traced lymphatic proximally and there was no communication between two lymphatic channels in its entire course. Both lymph nodes were labeled as SLN, removed and sent for frozen section examination (Fig. 2). Both lymph nodes came negative for metastasis in this case. Axillary dissection was not done in our patient and axillary incision was closed. Patient’s post-op recovery was uneventful and was discharged on third post-op day.
Fig. 1.
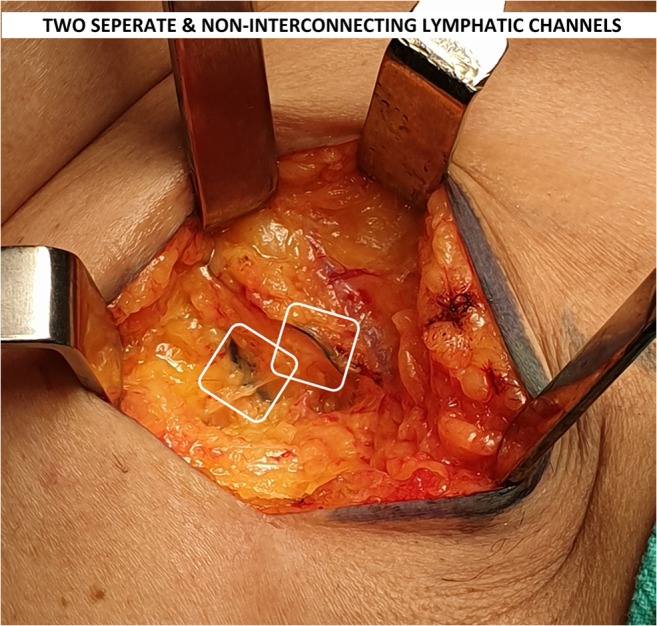
Lymphatic channels
Fig. 2.
Sentinel Lymph nodes
Discussion
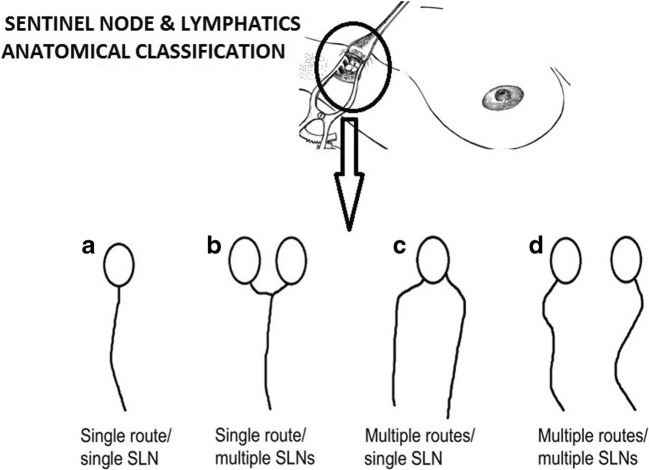
A false negative SLN biopsy is an inherent problem in the treatment of breast cancer. To avoid false negative SLN in the breast, it is important to understand the anatomy and number of lymphatic routes and SLNs. Yamamoto et al. has very nicely classified lymphatic routes and lymph nodes into four anatomical categories: (a) single route/single SLN, (b) single route/multiple SLN, (c) multiple routes/single SLN, and (d) multiple routes/multiple SLNs (Fig. 3). A chance of missing true SLN varies in different anatomical categories. Among four categories, highest chances of missing SLN are with category D (multiple routes/multiple SLNs). In all other three categories, if we find at least a single lymphatic channel, it will lead to SLN and chances of missing SLN are very low. But in category D, sometimes surgeon may find one lymphatic’s channel and remove SLN of that lymphatic’s and stop searching for other lymphatic’s. This leads to non-removal of true SLN. A surgeon’s lack of awareness of lymphatic anatomy may lead to missing true SLN. This missed true SLN may be positive for metastasis, leading to increase in local recurrence and poor oncological outcome. For best oncological outcome, it is very important to properly identify true SLN.
Fig. 3.
Anatomical classification
In addition to the knowledge of lymphatic anatomy, the chance of avoiding error in category D increases if we use both blue dye and indocyanine green [3]. In this technique, lymphatics are seen as shining structure over skin with the help of special probe. When we use indocyanine green in addition to blue dye, the chances of missing lymphatic and in end SLN decrease. But when we use only methylene blue, we may stop further dissection if we see one lymphatic duct. The false negative result can also be reduced by using radio colloid dye along with blue dye [4, 5]. Here, special probe is used to calculate radioactivity in axilla and the node is classified as hot (positive) or cold (negative) node. But the problem with Indian scenario is radio colloid and indocyanine dye is not available in many places. In our part of the world, blue dye alone is used in most of places to identify SLN. We want to stress with this paper, whatever technique a surgeon follows, always look for multiple lymphatic channels to avoid missing true SLN. This will reduce the false negative rate drastically. We follow strict surgical steps in our institute to prevent this error. While performing SLN technique in breast, before opening the clavipectoral fascia, we see lateral border of the pectoralis major muscle completely and make sure there are no multiple lymphatic’s channels. If multiple lymphatic channels are present, all lymphatic channels are traced distally till insertion onto SLN. All blue nodes are removed and examined.
Conclusion
Anatomical morphology of multiple lymphatic’s multiple SLNs should always be kept in mind. Lymphatic anatomy of breast knowledge is important to avoid false negative results in the treatment of breast cancer. High false negative rate leads to the undertreatment of patients, leading to a poor oncologic outcome.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gould EA, Winship T, Philbin PH, Hyland Kerr H. Observations on a “sentinel node” in cancer of the parotid. Cancer. 1960;13:77–78. doi: 10.1002/1097-0142(196001/02)13:1<77::AID-CNCR2820130114>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 2.Hill ADK, Tran KN, Akhurst T, Yeung H, Yeh SDJ, Rosen PP, Borgen PI, Cody HS., III Lessons learned from 500 cases of lymphatic mapping for breast cancer. Ann Surg. 1999;229:528–535. doi: 10.1097/00000658-199904000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo W, Zhang L, Ji J, Gao W, Liu J, Tong M. Breast cancer sentinel lymph node mapping using near-infrared guided indocyanine green in comparison with blue dye. Tumor Biol. 2014;35:3073–3078. doi: 10.1007/s13277-013-1399-2. [DOI] [PubMed] [Google Scholar]
- 4.Zengel B, et al. Sentinel lymph node biopsy in breast cancer: review on various methodological approaches. Tumori. 2013;99(2):149–153. doi: 10.1177/030089161309900205. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto S, Suga K, Maeda K, Maeda N, Yoshimura K, Oka M. Breast sentinel lymph node navigation with three-dimensional computed tomography–lymphography: a 12-year study. Breast Cancer. 2016;23:456–462. doi: 10.1007/s12282-015-0584-0. [DOI] [PubMed] [Google Scholar]