Abstract
Background
Calcitriol, the active metabolite of vitamin D, is an essential regulator in the hematopoiesis and immunity. However, knowledge revealing its influence on the immune and hematologic reconstitution after hematopoietic stem cell transplantation (HSCT) in clinical trials is very limited.
Objectives
The effects of calcitriol on short-term and long-term hematopoietic recovery, relapse-free survival (RFS) and overall survival (OS) in multiple myeloma, Hodgkin’s and non-Hodgkin’s lymphoma following autologous peripheral blood HSCT were assessed.
Methods
Eighty patients (age: 18–68 years) in complete remission were allocated 1:1 to two groups by balanced block randomization. Calcitriol 0.25 μg or placebo capsule was administered three times daily from transplantation to day 30. Absolute neutrophil count (ANC), absolute lymphocyte count (ALC), and platelet count (PC) were determined daily from transplantation to day 30. White blood cell count (WBC), PC, and hemoglobin concentration (HC) of days 180 and 365 were extracted from clinic files. A thorough examination for oral mucositis (OM) was completed daily during hospital stay. Adverse drug reactions (ADRs) as well as two-year RFS and OS were evaluated.
Results
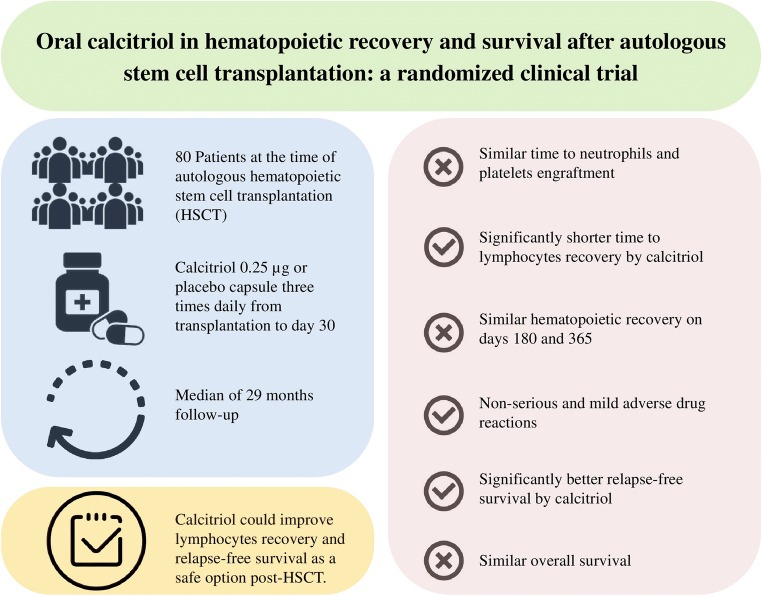
Median time to ANC engraftment (≥0.5 × 103/μl: 10.0 vs. 11.0 days; P = 0.98) and PC engraftment (≥20.0 × 103/μl: both 14.0 days; P = 0.58) was similar between groups. However, the median time to ALC recovery was significantly shorter in the calcitriol group (≥0.5 × 103/μl: 13.0 vs. 20.0 days; P < 0.001). Moreover, ALC recovery rates on day 15 (≥0.5 × 103/μl: 82.1% vs. 42.5%; P < 0.001) and on day 30 (≥1.0 × 103/μl: 91.7% vs. 57.5%; P = 0.001) was significantly higher with calcitriol. WBC, PC, and HC on days 180 and 365 were not significantly different between groups. None of the OM indices were modulated by calcitriol. All the ADRs were non-serious and mild, possibly or unlikely related to the intervention. In a median of 29 months follow-up, RFS was significantly better in the calcitriol group (77.0%, SE = 7.0% vs. 59.0%, SE = 8.0%; P = 0.03), albeit the OS was not affected (87.0%, SE = 5.0% vs. 92.0%, SE = 4.0%; P = 0.72).
Conclusion
Calcitriol could improve ALC recovery and RFS as a safe option post-HSCT.
Graphical abstract.
Oral calcitriol 0.25 µg three times daily from transplantation to day 30 improved lymphocytes recovery and two-year relapse-free survival as a safe option in 80 patients of autologous hematopoietic stem cell transplantation in comparison with placebo.
Keywords: Calcitriol, Engraftment, Immune reconstitution, Hematopoietic stem cell transplantation, Hodgkin, Lymphocyte, Lymphoma, Mucositis, Multiple myeloma, Survival, Vitamin D
Introduction
Autologous hematopoietic stem cell transplantation (HSCT) following high-dose chemotherapy improves survival in selected patients with Hodgkin’s lymphoma (HL), non-Hodgkin’s lymphoma (NHL), and multiple myeloma (MM). However, relapse is the most common cause of death after autologous HSCT [1]. Delayed hematopoietic recovery has been associated with higher rates of infections, relapse, and death in autologous HSCT [2–5]. Furthermore, early engraftment does not ensure normal lasting hematopoiesis, though information concerning predictive factors for long-term autologous hematopoietic function is limited [6, 7].
An essential regulator involved in the hematopoiesis is calcitriol, the active metabolite of vitamin D (VD) [8, 9]. Moreover, calcitriol plays key roles in both innate and adaptive immunity through VD receptors (VDRs). It stimulates the innate responses by inducing phagocytosis and potent antimicrobial peptides expression whereas suppresses the adaptive system, shifting from a pro-inflammatory towards a tolerogenic status [10–13]. Additionally, calcitriol inhibits proliferation, differentiation, and angiogenesis besides induces apoptosis in tumor cells [14]. However, findings of the human studies on immune functions of VD are conflicting and much of the evidence is at high risk of bias [15].
Sufficient levels of VD at the time of HSCT have been significantly related to better survivals [16–18]. Despite its central roles, VD deficiency (<20 ng/ml) has been reported in 30% to 70% of pediatrics and adults at the time of HSCT [16, 17, 19, 20]. If the 25-hydroxy-VD (25-OH-D) level < 30 ng/ml (most agreed threshold for immune functions) [21] is considered, the stated statistics will even grow more to 50% to 90% despite supplementation [16, 18, 19, 22, 23].
In the HSCT setting, VD has been mainly evaluated in observational studies and most of the data regarding its immunoregulatory functions originate from properties against GvHD [17, 24–26]. Knowledge revealing the influence of VD or calcitriol on the immune and hematologic reconstitution post-HSCT in clinical trials is very limited [27, 28]. We preferred calcitriol over VD for this trial due to interpatient differences in the transformation of VD to 25-OH-D and finally active 1,25-dihydroxy-VD (1,25-(OH)2-D) [29, 30], additional time needed for these conversions, and shorter half-life of the 1,25-(OH)2-D than 25-OH-D (4 h vs. 2–3 weeks) that makes it more feasible to manage toxicity [31].
This clinical trial was designed in order to assess the effects of oral calcitriol on the short-term and long-term hematopoietic recovery, relapse-free survival (RFS), and overall survival (OS) in HL, NHL, and MM patients following autologous peripheral blood HSCT.
Methods
Trial design and settings
A parallel, single-centered, patient ratio 1:1, placebo-controlled, balanced block randomized clinical trial was conducted at the adult bone marrow transplantation wards of Shariati Hospital. Ethics Committee of Tehran University of Medical Sciences approved this trial protocol and the ethics code of IR.TUMS.REC.1394.1368 was assigned. The trial was registered at IRCT.ir, numbers IRCT2015120816837N2 and IRCT2016052727435N2.
Participants
HL, NHL, and MM patients waiting for autologous peripheral blood HSCT were evaluated for eligibility. Patients from 18 to 68 years, in complete remission of the disease and bone marrow (NCCN guidelines) [32], with optimal kidney and liver functions [33], were included if declared consent. Those with baseline serum corrected total calcium >10.5 mg/dl, phosphorous >4.5 mg/dl, magnesium >2.5 mg/dl, nephrolithiasis in the past 5 years, calcitriol therapy in the previous 30 days, hypersensitivity or inability to take oral calcitriol were excluded.
Interventions
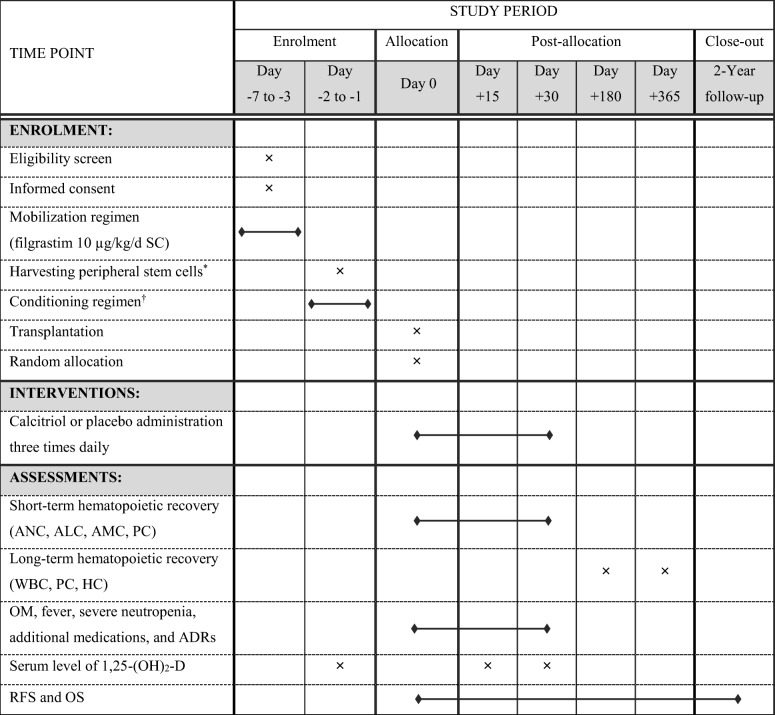
Participant timeline is demonstrated in Table 1. Mobilizing regimen, collection of the peripheral blood progenitor cells, and conditioning regimen were managed based on hospital protocols from day −7 through −1 pre-HSCT. Autologous peripheral blood HSCT was performed on day zero.
Table 1.
Participant timeline
*Collection was implemented if the circulating CD34+ cells count >20/μl achieved
†Hodgkin’s and non-Hodgkin’s lymphoma patients received the conditioning regimen of etoposide (600 mg/m2), cytarabine (1200 mg/m2), carboplatin (1500 mg/m2), and melphalan (140 mg/m2) on days −2 to −1. Multiple myeloma patients received the melphalan (200 mg/m2) conditioning regimen on day −1
Abbreviations: 1,25-(OH)2-D 1,25-dihydroxy-vitaminD, ADRs adverse drug reactions, ALC absolute lymphocyte count, AMC absolute monocyte count, ANC absolute neutrophil count, HC hemoglobin concentration, OM oral mucositis, OS overall survival, PC platelet count, RFS relapse-free survival, SC subcutaneous, WBC white blood cell count
Oral capsules of calcitriol 0.25 μg (Zahravi Pharmaceutical Co., Tehran, Iran) or placebo (Zahravi Pharmaceutical Co., Tehran, Iran) were administered three times daily from day 0 to 30.
Outcomes
Absolute neutrophil count (ANC), absolute lymphocyte count (ALC), absolute monocyte count (AMC), and platelet count (PC) were determined daily from day 0 until 30. Complete blood count on whole blood by the automated hematology analyzer (Sysmex KX-21 N, Sysmex Corp., Kobe, Japan) and differential test on the peripheral blood smears by the biological microscope (Olympus® CX31, Olympus Corp., Tokyo, Japan) were performed. White blood cell count (WBC), PC, and hemoglobin concentration (HC) of days 180 and 365 were extracted from clinic files.
A thorough examination for oral mucositis (OM) was completed daily during hospital stay with the help of mouth mirrors and a high-power lamp. In order to quantitatively analyze the 1,25-(OH)2-D, blood specimens (5 ml) were obtained in the morning prior to the administration of conditioning regimen (baseline), day 15 and 30. Samples were centrifuged for five minutes and serums were stored in microtubes at −80°C until enzyme immunoassay, by the kits of Immunodiagnostic Systems Ltd., Frankfurt am Main, Germany.
Pharmacologic doses of VD and its derivatives, uncontrolled intake of additional calcium (diet or supplementation), and dehydration were avoided during calcitriol/placebo phase. Serum electrolytes as well as kidney and liver profiles were monitored twice-weekly from day 0 to 30. Interventions were temporarily withheld if serum corrected total calcium >11.5 mg/dl or [calcium] × [phosphorous] >70 mg2/dl2 was noticed [34, 35]. Then, daily monitoring was continued until renormalization and re-administration of calcitriol/placebo.
Relapse was defined by the criteria in current NCCN clinical practice guidelines in oncology [32]. RFS was defined as the probability of survival without relapse from the date of transplantation to relapse, or last follow-up (live non-relapsed patients), or date of death (deceased non-relapsed patients). OS was defined as the probability of survival irrespective of disease status from the date of transplant to death or last follow-up.
Time to recovery of ANC, ALC, and PC were the primary outcomes. First of three consecutive days from the day of transplantation to reach the ANC ≥0.5 × 103/μl, ALC ≥0.5 × 103/μl, ALC ≥1.0 × 103/μl, and PC ≥20.0 × 103/μl (without platelet transfusion) were defined as the recovery days of the mentioned cells.
Secondary endpoints were as follow: cell counts and recovery rates on days 15, 30, 180, and 365; adverse drug reactions (ADRs) incidence, causality (WHO-UMC system) [36], severity (Hartwig-Siegel scale) [37], and seriousness (FDA criteria) [38]; OM incidence, duration, and severity (WHO scale) [39]; incidence and duration of fever (oral temperature ≥ 38.3°C); duration of severe neutropenia (ANC <0.5 × 103/μl); length of hospitalization (transplantation to discharge); anti-infective medicines (ATC classification: J) [40] in addition to usual care; total dose of hematopoietic supports; duration of total parenteral nutrition; RFS, and OS.
Sample size and power analysis
At a significance level of 0.05 (alpha = 5%), the sample size of 40 patients in each group for comparing time to recovery (by log-rank test) showed the statistical power of >80% to detect 25% differences by day 15.
Randomization
A computerized random number generator created the fixed balanced blocks of size 10 with randomization ratio of 1:1. An independent pharmacist with no clinical involvement in the trial dispensed the calcitriol and placebo in identical packaging sequentially numbered from 1 to 80 according to the allocation sequence. The allocation list was locked away in a safe deposit box until analysis. KR enrolled the participants and assigned them to interventions.
Blinding
The placebo capsules were identical to the calcitriol for color, shape, size, taste, and smell. The analysis of both was confirmed by high performance liquid chromatography.
The study data were independently monitored. Participants, healthcare providers (pharmacists, physicians, nurses responsible for drug administration and sampling, laboratory technicians responsible for smear slides examination and serum measurements), data collector (KR), outcome adjudicators (KR: determiner of hematopoietic recovery and mucositis score, MV: determiner of relapse), and data analyst (AS) were blinded to group assignments. A list of allocated numbers divided in two groups “A” and “B” was given by the independent pharmacist decoded as “calcitriol” and “placebo,” respectively, at the end of the analysis.
Statistical methods
The analysis was planned based on an intention-to-treat approach. Comparisons between the two groups were performed with independent samples t-test (mean of quantitative data), Mann-Whitney U test (median of not-normally distributed continuous data), and chi-square tests or Fisher’s exact test (qualitative data). Survival analyses were performed by Kaplan-Meier curve, and the log-rank test was used to compare survival rates between two groups.
Statistical significance was declared at P < 0.05. Data were analyzed by the software of IBM SPSS Statistics for Windows, Released 2017, Version 25.0, IBM Corp., Armonk, NY, USA.
Results
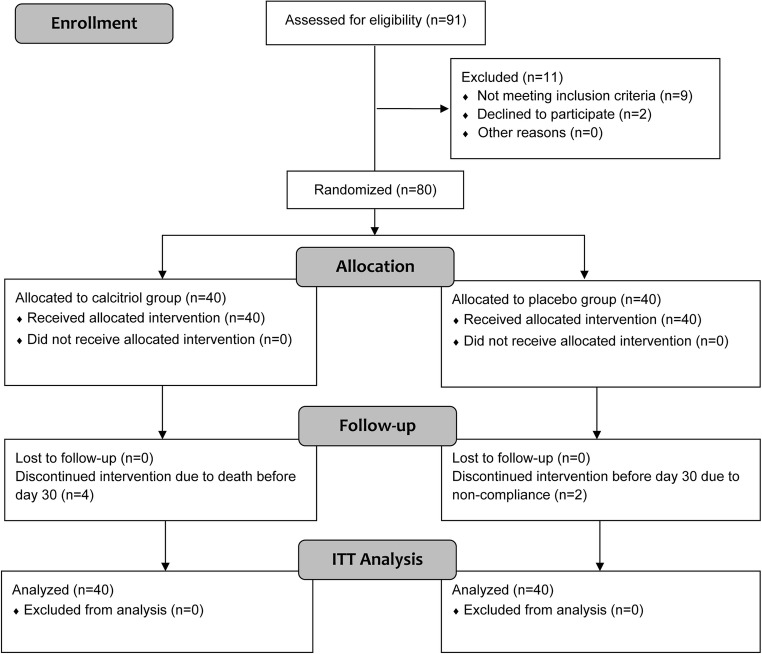
During the course of study, 91 patients of HL, NHL, and MM were referred to the hospital for autologous peripheral blood HSCT. From whom, nine patients did not meet the inclusion criteria (two MM partial remission, two hyperphosphatemia, two renal insufficiency, two history of nephrolithiasis, one hypersensitivity), and two of the eligible patients declined to participate. Ultimately, data of 80 patients were analyzed (Fig. 1). They were enrolled from May 2015 to March 2016 and followed from May 2015 to June 2018. Baseline demographic, clinical, and HSCT features of the two groups are summarized in Table 2.
Fig. 1.
Flow diagram of the participants from enrollment to intention-to-treat (ITT) analysis
Table 2.
Baseline demographic, clinical, and transplantation characteristics
| Calcitriol n = 40 |
Placebo n = 40 |
|
|---|---|---|
| Gender | ||
| Male | 25 (62.5%) | 29 (72.5%) |
| Female | 15 (37.5%) | 11 (27.5%) |
| Age (y) | ||
| Mean ± SD | 47.53 ± 13.49 | 46.93 ± 14.39 |
| Median (range) | 50.0 (21.0–65.0) | 50.0 (18.0–68.0) |
| Body mass index (kg/m2) | ||
| Mean ± SD | 26.75 ± 4.49 | 27.07 ± 4.25 |
| Background conditions | ||
| Diabetes | 1 (2.5%) | 2 (5.0%) |
| Hepatitis B | 1 (2.5%) | 1 (2.5%) |
| Cardiovascular disease | 2 (5.0%) | 1 (2.5%) |
| Thyroid disease | 0 (0.0%) | 1 (2.5%) |
| Hematologic malignancy | ||
| Multiple myeloma | 22 (55.0%) | 20 (50.0%) |
| Hodgkin lymphoma | 10 (25.0%) | 10 (25.0%) |
| Non-Hodgkin lymphoma | 8 (20.0%) | 10 (25.0%) |
| Conditioning regimen | ||
| MLP | 22 (55.0%) | 20 (50.0%) |
| VP16 + CYT + CBP + MLP | 18 (45.0%) | 20 (50.0%) |
| Cycles of chemotherapy before transplantation | ||
| Median (range) | 8.0 (2.0–24.0) | 8.0 (3.0–23.0) |
| Cycles of radiotherapy before transplantation | ||
| Median (range) | 0.0 (0.0–24.0) | 0.0 (0.0–65.0) |
| Time from diagnosis to transplantation (month) | ||
| Median (range) | 19.50 (7.0–108.0) | 19.50 (5.0–156.0) |
| Viability of the transplant product (%) | ||
| Mean ± SD | 94.41 ± 4.78 | 94.56 ± 4.94 |
| Volume of the transplant product (ml) | ||
| Median (range) | 390.0 (160.0–793.0) | 432.0 (300.0–953.0) |
| Dose of CD34+ cells (×106/kg) | ||
| Median (range) | 1.89 (0.21–22.50) | 1.38 (0.45–15.10) |
| Dose of TNC (×108/kg) | ||
| Median (range) | 13.06 (4.98–34.83) | 12.20 (3.88–34.72) |
| Dose of MNC (×108/kg) | ||
| Median (range) | 10.25 (5.09–19.30) | 9.81 (4.0–17.01) |
Abbreviations: CBP carboplatin, CYT cytarabine, MLP melphalan, MNC mononuclear cells, SD standard deviation, TNC total nucleated cells, VP16 etoposide
Short-term and long-term hematopoietic recovery
Median time to ANC and PC engraftment was similar between the two groups (Table 3). However, the median time to ALC ≥0.5 × 103/μl (13.0 vs. 20.0 days; P < 0.001) and to ALC ≥1.0 × 103/μl (20.0 vs. 28.0 days; P < 0.001) was significantly shorter in the calcitriol group.
Table 3.
Median time to the recovery of ANC, ALC, and PC in the calcitriol and placebo groups of HL, NHL, and MM after autologous HSCT
| Calcitriol | Placebo | P value* | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n† | Median | SE | 95% CI | n† | Median | SE | 95% CI | ||
| Time to ANC ≥0.5 × 103/μl (day) | 38 | 10.00 | 0.37 | 9.28 to 10.72 | 39 | 11.00 | 0.26 | 10.50 to 11.50 | 0.98 |
| Time to ALC ≥0.5 × 103/μl (day) | 34 | 13.00 | 0.51 | 12.00 to 14.00 | 33 | 20.00 | 1.89 | 16.29 to 23.71 | <0.001 |
| Time to ALC ≥1.0 × 103/μl (day) | 33 | 20.00 | 1.65 | 16.17 to 23.23 | 23 | 28.00 | 0.28 | 27.44 to 28.56 | <0.001 |
| Time to PC ≥20.0 × 103/μl (day) | 32 | 14.00 | 1.03 | 11.98 to 16.02 | 32 | 14.00 | 1.26 | 11.52 to 16.48 | 0.58 |
*Kaplan-Meier curve, log-rank test
†Number of patients who recovered within 30 days. The rest failed to recover or died before day 30
Abbreviations: ALC absolute lymphocyte count, ANC absolute neutrophil count, CI confidence interval, HL Hodgkin’s lymphoma, HSCT hematopoietic stem cell transplantation, MM multiple myeloma, NHL non-Hodgkin’s lymphoma, PC platelet count, SE standard error
Recovery rates of hematological parameters on days 15, 30, 180, and 365 are presented in Table 4. Among all, the recovery rates of ALC were significantly higher in the calcitriol group than the placebo to ≥0.5 × 103/μl on day 15 (82.1% vs. 42.5%; P < 0.001) and to ≥1.0 × 103/μl on day 30 (91.7% vs. 57.5%; P = 0.001).
Table 4.
Hematopoietic recovery rates on days 15, 30, 180, and 365 in the calcitriol and placebo groups of HL, NHL, and MM after autologous HSCT
| Calcitriol n† (%) |
Placebo n† (%) |
Relative risk | 95% CI | P value* | |
|---|---|---|---|---|---|
| ANC-15 ≥ 0.5 × 103/μl | 34/39 (87.2) | 37/40 (92.5) | 0.94 | 0.81 to 1.09 | 0.48 |
| ANC-30 ≥ 2.0 × 103/μl | 25/36 (69.4) | 23/40 (57.5) | 1.21 | 0.86 to 1.70 | 0.28 |
| ALC-15 ≥ 0.5 × 103/μl | 32/39 (82.1) | 17/40 (42.5) | 1.93 | 1.31 to 2.85 | <0.001 |
| ALC-30 ≥ 1.0 × 103/μl | 33/36 (91.7) | 23/40 (57.5) | 1.59 | 1.20 to 2.12 | 0.001 |
| PC-15 ≥ 20.0 × 103/μl | 24/39 (61.5) | 25/40 (62.5) | 0.98 | 0.70 to 1.39 | 0.93 |
| PC-30 ≥ 100.0 × 103/μl | 20/36 (55.6) | 18/40 (45.0) | 1.23 | 0.79 to 1.94 | 0.36 |
| PC-180 ≥ 150.0 × 103/μl‡ | 19/32 (59.4) | 21/31 (67.7) | 0.88 | 0.60 to 1.28 | 0.49 |
| PC-365 ≥ 150.0 × 103/μl‡ | 20/28 (71.4) | 20/29 (69.0) | 1.04 | 0.74 to 1.45 | 0.84 |
| WBC-180 ≥ 4.0 × 103/μl‡ | 27/32 (84.4) | 26/31 (83.9) | 1.01 | 0.81 to 1.25 | >0.99 |
| WBC-365 ≥ 4.0 × 103/μl‡ | 26/28 (92.9) | 24/29 (82.8) | 1.12 | 0.92 to 1.36 | 0.42 |
| HC-180 recovery‡ | 17/32 (53.1) | 17/31 (54.8) | 0.97 | 0.61 to 1.53 | 0.89 |
| HC-365 recovery‡ | 17/28 (60.7) | 14/29 (48.3) | 1.26 | 0.78 to 2.03 | 0.35 |
*Chi-square tests or Fisher’s exact test (if appropriate)
†Fraction of live patients who recovered by defined days. The rest failed to recover or died before scheduled dates. Additionally laboratory results of days 180 and 365 were not available for all live patients
‡Normal values were defined as: WBC: 4.0–11.0 × 103/μl; PC: 150.0–450.0 × 103/μl; HC: males: 14.0–18.0 g/dl, females: 12.0–16.0 g/dl
Abbreviations: ALC-15 absolute lymphocyte count on day 15, ALC-30 absolute lymphocyte count on day 30, ANC-15 absolute neutrophil count on day 15, ANC-30 absolute neutrophil count on day 30, CI confidence interval, HC-180 hemoglobin concentration on day 180, HC-365 hemoglobin concentration on day 365, HL Hodgkin’s lymphoma, HSCT hematopoietic stem cell transplantation, MM multiple myeloma, NHL non-Hodgkin’s lymphoma, PC-15 platelet count on day 15, PC-30 platelet count on day 30, PC-180 platelet count on day 180, PC-365 platelet count on day 365, WBC-180 white blood cell count on day 180, WBC-365 white blood cell count on day 365
There was not a notable difference between the two groups regarding the infused CD34+ cells dose (Table 2). Nevertheless, statistical analysis with Poisson Regression was performed to evaluate the confounding effect of CD34+ cells dose on the ALC recovery of days 15 and 30. Calcitriol, independent of the CD34+ cells dose had a significant effect on ALC recovery of days 15 (P = 0.001) and 30 (P = 0.001).
Mean number of hematologic cells on days 15, 30, 180, and 365 were not statistically different between the groups (Table 5), with the exception of ALC mean for which the calcitriol group was significantly higher on day 15 (0.71 ± 0.44 × 103/μl vs. 0.43 ± 0.35 × 103/μl; P = 0.002) and day 30 (1.44 ± 0.69 × 103/μl vs. 0.94 ± 0.52 × 103/μl; P = 0.001).
Table 5.
Blood cell counts on days 15, 30, 180, and 365 in the calcitriol and placebo groups of HL, NHL, and MM after autologous HSCT
| Calcitriol | Placebo | Mean Difference | 95% CI | P value* | |||
|---|---|---|---|---|---|---|---|
| n† | Mean ± SD | n† | Mean ± SD | ||||
| ANC-15 (×103/μl) | 39 | 3.16 ± 3.32 | 40 | 2.78 ± 2.19 | 0.37 | −0.88 to 1.63 | 0.56 |
| ANC-30 (×103/μl) | 36 | 2.51 ± 1.38 | 40 | 2.18 ± 1.02 | 0.33 | −0.22 to 0.88 | 0.24 |
| ALC-15 (×103/μl) | 39 | 0.71 ± 0.44 | 40 | 0.43 ± 0.35 | 0.28 | 0.10 to 0.46 | 0.002 |
| ALC-30 (×103/μl) | 36 | 1.44 ± 0.69 | 40 | 0.94 ± 0.52 | 0.49 | 0.22 to 0.77 | 0.001 |
| AMC-15 (×103/μl) | 39 | 0.56 ± 0.58 | 40 | 0.57 ± 0.83 | −0.01 | −0.33 to 0.31 | 0.96 |
| AMC-30 (×103/μl) | 36 | 0.60 ± 0.39 | 40 | 0.65 ± 0.44 | −0.05 | −0.24 to 0.14 | 0.60 |
| PC-15 (×103/μl) | 39 | 33.95 ± 32.31 | 40 | 39.43 ± 43.75 | −5.48 | −22.74 to 11.79 | 0.53 |
| PC-30 (×103/μl) | 36 | 117.06 ± 90.95 | 40 | 114.13 ± 98.62 | 2.93 | −40.59 to 46.45 | 0.89 |
| PC-180 (×103/μl) | 32 | 161.13 ± 59.04 | 31 | 174.87 ± 71.00 | −13.75 | −46.60 to 19.11 | 0.41 |
| PC-365 (×103/μl) | 28 | 185.07 ± 67.50 | 29 | 183.38 ± 55.06 | 1.69 | −30.95 to 34.34 | 0.92 |
| WBC-180 (×103/μl) | 32 | 5.29 ± 1.63 | 31 | 5.35 ± 1.40 | −0.06 | −0.83 to 0.70 | 0.87 |
| WBC-365 (×103/μl) | 28 | 6.03 ± 1.97 | 29 | 5.39 ± 1.60 | 0.64 | −0.31 to 1.59 | 0.18 |
| HC-180 (g/dl) | 32 | 12.97 ± 1.81 | 31 | 13.31 ± 1.40 | −0.34 | −1.16 to 0.48 | 0.41 |
| HC-365 (g/dl) | 28 | 13.45 ± 1.81 | 29 | 13.31 ± 1.45 | 0.14 | −0.73 to 1.01 | 0.75 |
*Independent samples t-test
†Number of patients who were alive on scheduled dates. Laboratory results of days 180 and 365 were not available for all live patients
Abbreviations: ALC-15 absolute lymphocyte count on day 15, ALC-30 absolute lymphocyte count on day 30, AMC-15 absolute monocyte count on day 15, AMC-30 absolute monocyte count on day 30, ANC-15 absolute neutrophil count on day 15, ANC-30 absolute neutrophil count on day 30, CI confidence interval, HC-180 hemoglobin concentration on day 180, HC-365 hemoglobin concentration on day 365, HL Hodgkin’s lymphoma, HSCT hematopoietic stem cell transplantation, MM multiple myeloma, NHL non-Hodgkin’s lymphoma, PC-15 platelet count on day 15, PC-30 platelet count on day 30, PC-180 platelet count on day 180, PC-365 platelet count on day 365, SD standard deviation, WBC-180 white blood cell count on day 180, WBC-365: white blood cell count on day 365
Serum levels of 1,25-(OH)2-D
Mean baseline levels of 1,25-(OH)2-D in the calcitriol (n = 18) and placebo (n = 19) group subsamples were 38.02 ± 17.83 pg/ml and 32.65 ± 14.76 pg/ml, respectively. Mean levels elevated significantly (by repeated measure ANOVA, adjusted for baseline values, P = 0.005) with calcitriol than the placebo on day 15 (48.39 ± 19.78 pg/ml vs. 31.06 ± 22.92 pg/ml) and day 30 (59.51 ± 24.83 pg/ml vs. 39.60 ± 23.11 pg/ml).
Hospital profile and complications of HSCT
Medications and hematopoietic supports during hospital stay along with complications of the transplantation are listed in Table 6. Calcitriol group did not show significant differences when compared against placebo group regarding any of the evaluated issues.
Table 6.
Medications and hematopoietic supports during hospital stay as well as the complications of transplantation in the calcitriol and placebo groups of HL, NHL, and MM after autologous HSCT
| Calcitriol n = 40 |
Placebo n = 40 |
Effect size | 95% CI | P value | |
|---|---|---|---|---|---|
| Mean duration of hospitalization (day) | 16.31 ± 7.17 | 15.25 ± 3.50 | 1.06* | −1.48 to 3.59 | 0.41* |
| Mean number of anti-infectives in addition to usual care (n)§ | 2.63 ± 1.72 | 3.03 ± 1.25 | −0.40* | −1.07 to 0.27 | 0.24* |
| Mean total dose of filgrastim (mcg)§ | 4920.0 ± 2626.61 | 4867.50 ± 2443.35 | 52.50* | −1076.73 to 1181.73 | 0.93* |
| Mean total units of platelet (n)§ | 9.15 ± 10.77 | 10.35 ± 15.54 | −1.20* | −7.15 to 4.75 | 0.69* |
| Median total units of packed cell (n)§ | 0.0 (0.0–5.0) | 0.0 (0.0–8.0) | N/A | N/A | 0.70† |
| Median duration of TPN (day)¶ | 0.0 (0.0–8.0) | 0.0 (0.0–3.0) | N/A | N/A | 0.58† |
| Incidence of OM | 24 (60.0%) | 27 (67.5%) | 0.89‡ | 0.64 to 1.24 | 0.49‡ |
| Mean duration of OM (day) | 10.17 ± 5.20 | 7.85 ± 3.54 | 2.32* | −0.19 to 4.83 | 0.07* |
| Incidence of OM grade 3–4 | 4 (10.0%) | 7 (17.5%) | 0.57‡ | 0.18 to 1.80 | 0.33‡ |
| Mean duration of OM grade 3–4 (day) | 9.25 ± 6.08 | 5.67 ± 1.63 | 3.58* | −5.84 to 13.00 | 0.33* |
| OM severity (WHO scale) | |||||
| None | 16 (40.0%) | 13 (32.5%) | |||
| Grade 1 | 10 (25.0%) | 11 (27.5%) | N/A | N/A | 0.88‡ |
| Grade 2 | 10 (25.0%) | 9 (22.5%) | |||
| Grade 3 | 2 (5.0%) | 4 (10.0%) | |||
| Grade 4 | 2 (5.0%) | 3 (7.5%) | |||
| Incidence of fever | 35 (87.5%) | 37 (92.5%) | 0.95‡ | 0.82 to 1.10 | 0.71‡ |
| Mean duration of fever (day) | 3.54 ± 3.53 | 2.49 ± 2.23 | 1.06* | −0.32 to 2.44 | 0.13* |
| Mean duration of severe neutropenia (day) | 8.78 ± 3.81 | 8.35 ± 3.31 | 0.43* | −1.16 to 2.01 | 0.60* |
*Independent samples t-test with mean difference
†Independent samples Mann-Whitney U test
‡Chi-square tests or Fisher’s exact test (if appropriate) with relative risk
§All the post-HSCT patients received oral fluconazole (100 mg q.12.h), oral acyclovir (200 mg q.8.h), oral nystatin (20 drops q.3.h), and subcutaneous filgrastim (5–10 μg/kg/day based on the neutrophil response) in the hospital. Additionally, according to the hospital protocol, transfusion of platelet for PC <10.0 × 103/μl or higher if clinical bleeding, and packed cell for HC <7.0 g/dl was indicated
¶One patient in the calcitriol group and two patients in the placebo group received TPN in the hospital
Abbreviations: CI confidence interval, HL Hodgkin’s lymphoma, HSCT hematopoietic stem cell transplantation, MM multiple myeloma, N/A not applicable, NHL non-Hodgkin’s lymphoma, OM oral mucositis, TPN total parenteral nutrition
ADRs
Regarding the ADRs, there were no significant differences between the two groups. Hyperphosphatemia (>4.5 mg/dl) in 11/40 (27.5%) of the calcitriol and 12/40 (30.0%) of the placebo group (P = 0.80), and hypercalcemia (>10.5 mg/dl) in 1/40 (2.5%) of each group was detected.
Hyperphosphatemia in 8/40 (20.0%) of the calcitriol group was “possibly” and in 3/40 (7.5%) was “unlikely” related to the medicine. Additionally, calcitriol was “unlikely” responsible for 1/40 (2.5%) report of hypercalcemia.
All the reported ADRs in two groups were “non-serious” and “mild-level-1,” except for 1/40 (2.5%) case of the calcitriol and 3/40 (7.5%) patients of the placebo group recorded as “mild-level-2” due to temporarily holding the intervention for a few doses to successfully reduce the serum calcium and phosphorous and restart calcitriol/placebo.
RFS and OS
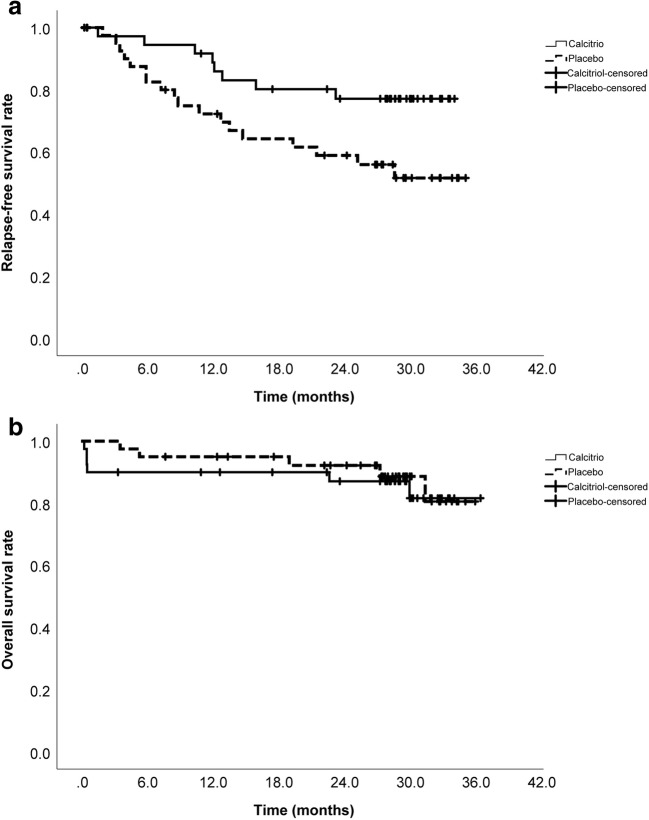
The median follow-up time of the survivors was 29.34 (3.34 to 36.46) months in the calcitriol and 29.05 (7.67 to 35.97) months in the placebo group. Relapse was diagnosed in 8/40 of the calcitriol (five MM, two NHL, one HL) and 18/40 of the placebo (nine MM, five NHL, four HL) group patients. Two-year RFS was significantly higher in the calcitriol than the placebo group (77.0%, SE = 7.0% vs. 59.0%, SE = 8.0%; P = 0.03; Fig. 2a).
Fig. 2.
Relapse-free survival (a) and overall survival (b) graphs in the calcitriol and placebo groups after a median of 29 months follow-up post-HSCT
Generally, 6/40 of the calcitriol and 5/40 of the placebo group patients died during the course of trial. Except 4/40 patients of the calcitriol group who deceased before discharge due to the HSCT complications (two of sepsis, one of intracranial hemorrhage, and one of heart failure), relapse of the primary hematologic malignancy was the cause of death in all others. Two-year OS was not significantly different between the calcitriol and placebo groups (87.0%, SE = 5.0% vs. 92.0%, SE = 4.0%; P = 0.72; Fig. 2b).
Discussion
In this randomized clinical trial, a one-month (day 0 to 30) course of three-times-daily calcitriol 0.25 μg versus placebo capsule was administered in HL, NHL, and MM adult patients following autologous peripheral blood HSCT. Short-term and long-term hematopoietic recovery, RFS, OS, OM, medications, and ADRs were evaluated.
Our findings suggest that calcitriol could accelerate the recovery time of ALC and enhance its recovery rates on days 15 and 30 after autologous HSCT. However, we did not find calcitriol improving the engraftment of ANC or PC. Recovery of ALC post-HSCT in relation to VD or calcitriol has not been studied before.
Hamidieh et al. [27] reported no significant difference in neutrophil or platelet engraftment time with once-daily calcitriol 0.25 μg vs. placebo capsules from conditioning initiation up to 14 days, in a double-blind randomized clinical trial on allogenic HSCT pediatrics. Caballero-Velázquez et al. [28] also found no significant differences amongst three adult groups (control, VD 1000 IU/d, VD 5000 IU/d orally from day −5 to +100) of their clinical trial in terms of ANC and PC engraftment after allogenic HSCT. Beebe et al. [18] stated equal time to neutrophils engraftment with pre-transplantation normal and low levels of 25-OH-D in pediatric allogenic HSCT.
The first cells to engraft within few weeks after HSCT are monocytes, rapidly followed by granulocytes, platelets, and natural killer (NK) cells. B and T lymphocytes last on average 6 months to recover following autologous HSCT [41].
Currently, it is accepted that VD can boost the first line of innate defense and inhibit the post-developmental functional properties of adaptive immune cells, protecting from various autoimmune diseases and limiting graft rejection [11]. Calcitriol favors the monocytes differentiation and maturation into macrophages as well as neutrophils functions [42]. Contrary to the potent antiproliferative activity on B and T cells [8], the literature regarding the impact of calcitriol on NK cells has been varied and ranges from augmentation of proliferation and cytotoxicity to inhibition of these activities [43–47]. Indeed, chronic deficiency may also negatively regulate lymphocyte counts and functions [48, 49].
It has been suggested that VD stimulates the early developmental phases of innate immune recovery [16]. Cortes et al. [50] elucidated that treatment of human hematopoietic stem and progenitor cells with 1,25-(OH)2-D (10 μM) significantly elevated survival, proliferation, and activity of all lineage colony forming units via VDRs compared to control cells. Further, antagonism of 1,25-(OH)2-D synthesis or VDRs significantly reduced the percentage of lymphoid progenitors and hematopoietic stem cells. On the other hand, Weeres et al. [51] showed suppressive effects of 1,25-(OH)2-D (10 nM) on the numbers and functions of NK cells developed from hematopoietic stem cells in vitro, with no effects on mature NK cells. Addition of 1,25-(OH)2-D led to rapid commitment of hematopoietic progenitor cells to the myeloid lineage with significant monocyte development. Nonetheless, the AMC was similar between our groups on days 15 and 30.
An important caveat with in vitro data [50, 51] is that VD metabolism pathways are tightly regulated enzymatic processes that are difficult to model in vitro. In addition, the local concentrations of calcitriol in the bone marrow or secondary lymphoid tissues are not known. Hence, the influence of calcitriol on myelopoiesis and lymphopoiesis in human might be debatable. Although the contribution of VD within the immune system has been investigated in depth, its effect on stem cells and potentially on engraftment and immune reconstitution following HSCT is still far from being understood.
Higher RFS in the calcitriol group was another important outcome of this trial. In an observational study by Hansson et al. [16], following 123 children for up to 8 years after allogenic HSCT, RFS and OS were significantly better in VD-sufficient group at the time of transplantation than patients with insufficient levels. As well, Beebe et al. [18] indicated a significant lower one-year OS for children with deficient compared to normal pre-HSCT VD. Nevertheless, two other observations failed to prove significant association between higher pre-allogenic-HSCT 25-OH-D levels with better two/three-year survivals in adults [17, 25]. Besides, Caballero-Velázquez et al. [28] reported similar two-year progression-free survival and OS across their clinical trial arms.
Early ALC recovery has been found as a consistent predictor of improved survival after autologous HSCT regardless of exact mechanism of reconstitution. In Porrata et al. [2, 3, 5] investigations, ALC ≥0.5 × 103/μl on day 15 has been significantly linked with better progression-free survival and OS after autologous HSCT in HL, NHL, and MM. Similar outcomes were reflected by other researchers for reaching ALC ≥1.0 × 103/μl until day 30 [52–54]. In fact, early ALC recovery plays important antitumor roles against minimal residual disease progression as the innate immunity after autologous HSCT [5, 55]. That may suggest calcitriol early intervention profited the eradication of the residual disease by enhancing the innate lymphocytes and consequently resulted in better two-year RFS, albeit the OS was not affected.
Day-180 and day-365 WBC, PC, and HC recovery rates were not different by calcitriol from placebo in this trial. Possible factors affecting long-term hematopoietic recovery are rarely known. Hansson et al. [16] found significantly higher ANC up to three months, but similar ALC up to one year post-HSCT in baseline-VD-sufficient compared to deficient group. Hematopoietic effects of calcitriol post-HSCT might be time- and/or dose-dependent requiring longer courses and/or higher doses for more benefits.
In addition to immunomodulatory, anti-inflammatory, and antimicrobial properties [8, 10, 56], calcitriol exerts anti-oxidative characteristics [57]. These activities can fight against OM which significantly affects almost 75% of HSCT patients [58]. However, none of the OM indices were modulated by calcitriol in our trial, aligned with a similar trial in allogenic HSCT [27]. Perhaps, starting calcitriol before conditioning regimen could provide enough time for the inflammatory cascade of mucositis to be modulated.
Unexpectedly, the calcitriol group had a trend for longer duration of OM, the reasons for which are unclear. Since this result was only marginally towards statistical significance, and multiple comparisons were employed, the possibility of a type I error is more likely.
Calcitriol capsules were well tolerated and the recorded ADRs were confirmed previously in the literature [34, 35]. Although, hypercalcemia is the main concern with calcitriol therapy (>10% frequency) [35] and originates the majority of its ADRs [34], hyperphosphatemia was more frequent in our trial. Whereas Hamidieh et al. [27] detected neither hypercalcemia nor hyperphosphatemia with a lower dose of calcitriol in 14 children.
All the ADRs were “possibly” or even “unlikely” related to the calcitriol. Adverse reactions of the high-dose chemotherapy and multiple medicines administered post-HSCT weakened the causality relationship between the suspected ADRs and our intervention. However, all the imbalances were “non-serious” and similar between the two groups, consisting of “mild” transient episodes of hypercalcemia and/or hyperphosphatemia, which were managed effectively by temporary discontinuation of the treatment.
Heterogeneous population including HL, NHL, and MM might have affected our results; however, patients were equally distributed between the two groups regarding the hematologic malignancy, conditioning regimen, history of treatments prior to transplantation, HSCT product characteristics, age, and sex. In generalizability point of view, this heterogeneity suggests that administration of calcitriol and VD analogs can be implemented in all autologous HSCT patients.
Conclusion
Lineage specification during hematopoiesis is a complex process where stem and progenitor cells integrate external signals from cytokine receptors and surface proteins. Calcitriol plays key roles in immunity and hematopoiesis. Calcitriol 0.25 μg capsule three times daily, from day of transplantation up to one month, is an effective and safe option for early ALC recovery and better RFS after autologous HSCT. However, the OM and OS were not improved by calcitriol in our trial. The impact of different dosage forms and doses of calcitriol on the outcomes needs further exploration. In addition, calcitriol significantly reduces the apoptosis and increases the number and activity of CD34+ cells [50]. One might argue that initiation of calcitriol prior to the collection of CD34+ cells could be a beneficial action to take.
Acknowledgements
We greatly appreciate the participants without whom this investigational study would not be possible. We thank Ms. Ashraf Sadat Mousavi, Ms. Zahra Shahriari, BMT wards staff of Shariati Hospital, and Dr. Hamid Khoee (drug supply and randomization) for their kind assistances.
Funding information
This study was funded through an educational grant to the researchers from the Hematology-Oncology and Stem Cell Transplantation Research Center, Shariati Hospital, Tehran University of Medical Sciences, Tehran, Iran.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Relias V. Hematopoietic cell trasplantation. In: Zeind CS, Carvalho MG, editors. Applied therapeutics: the clinical use of drugs. 11. Philadelphia: Lippincott Williams & Wilkins, Wolters Kluwer; 2018. pp. 2102–2106. [Google Scholar]
- 2.Porrata LF, Gertz MA, Inwards DJ, Litzow MR, Lacy MQ, Tefferi A, et al. Early lymphocyte recovery predicts superior survival after autologous hematopoietic stem cell transplantation in multiple myeloma or non-Hodgkin lymphoma. Blood. 2001;98(3):579–585. doi: 10.1182/blood.V98.3.579. [DOI] [PubMed] [Google Scholar]
- 3.Porrata LF, Inwards DJ, Micallef IN, Ansell SM, Geyer SM, Markovic SN. Early lymphocyte recovery post-autologous haematopoietic stem cell transplantation is associated with better survival in Hodgkin's disease. Br J Haematol. 2002;117(3):629–633. doi: 10.1046/j.1365-2141.2002.03478.x. [DOI] [PubMed] [Google Scholar]
- 4.Gordan LN, Sugrue MW, Lynch JW, Williams KD, Khan SA, Moreb JS. Correlation of early lymphocyte recovery and progression-free survival after autologous stem-cell transplant in patients with Hodgkin's and non-Hodgkin's lymphoma. Bone Marrow Transplant. 2003;31(11):1009–1013. doi: 10.1038/sj.bmt.1704050. [DOI] [PubMed] [Google Scholar]
- 5.Porrata LF, Inwards DJ, Ansell SM, Micallef IN, Johnston PB, Gastineau DA, et al. Early lymphocyte recovery predicts superior survival after autologous stem cell transplantation in non-Hodgkin lymphoma: a prospective study. Biol Blood Marrow Transplant. 2008;14(7):807–816. doi: 10.1016/j.bbmt.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duggan P, Guo D, Luider J, Auer I, Klassen J, Chaudhry A, et al. Predictive factors for long-term engraftment of autologous blood stem cells. Bone Marrow Transplant. 2000;26(12):1299–1304. doi: 10.1038/sj.bmt.1702708. [DOI] [PubMed] [Google Scholar]
- 7.Amigo ML, del Cañizo MC, Caballero MD, Vazquez L, Corral M, Vidriales B, et al. Factors that influence long-term hematopoietic function following autologous stem cell transplantation. Bone Marrow Transplant. 1999;24(3):289–293. doi: 10.1038/sj.bmt.1701886. [DOI] [PubMed] [Google Scholar]
- 8.Studzinski GP, Harrison JS, Wang X, Sarkar S, Kalia V, Danilenko M. Vitamin D control of hematopoietic cell differentiation and leukemia. J Cell Biochem. 2015;116(8):1500–1512. doi: 10.1002/jcb.25104. [DOI] [PubMed] [Google Scholar]
- 9.Bunce CM, Brown G, Hewison M. Vitamin D and hematopoiesis. Trends Endocrinol Metab. 1997;8(6):245–251. doi: 10.1016/S1043-2760(97)00066-0. [DOI] [PubMed] [Google Scholar]
- 10.Ros-Soto Jose, Anthias Chloe, Madrigal Alejandro, Snowden John A. Vitamin D: is it important in haematopoietic stem cell transplantation? A review. Bone Marrow Transplantation. 2018;54(6):810–820. doi: 10.1038/s41409-018-0377-0. [DOI] [PubMed] [Google Scholar]
- 11.Bikle DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol. 2014;21(3):319–329. doi: 10.1016/j.chembiol.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bikle D. Nonclassic actions of vitamin D. J Clin Endocrinol Metab. 2009;94(1):26–34. doi: 10.1210/jc.2008-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borella E, Nesher G, Israeli E, Shoenfeld Y. Vitamin D: a new anti-infective agent? Ann N Y Acad Sci. 2014;1317(1):76–83. doi: 10.1111/nyas.12321. [DOI] [PubMed] [Google Scholar]
- 14.Trump DL, Deeb KK, Johnson CS. Vitamin D: considerations in the continued development as an agent for cancer prevention and therapy. Cancer J. 2010;16(1):1–9. doi: 10.1097/PPO.0b013e3181c51ee6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Damoiseaux J, Smolders J. The engagement between vitamin D and the immune system: is consolidation by a marriage to be expected? EBioMedicine. 2018;31:9–10. doi: 10.1016/j.ebiom.2018.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansson ME, Norlin A-C, Omazic B, Wikström A-C, Bergman P, Winiarski J, et al. Vitamin D levels affect outcome in pediatric hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2014;20(10):1537–1543. doi: 10.1016/j.bbmt.2014.05.030. [DOI] [PubMed] [Google Scholar]
- 17.von Bahr L, Blennow O, Alm J, Björklund A, Malmberg KJ, Mougiakakos D, et al. Increased incidence of chronic GvHD and CMV disease in patients with vitamin D deficiency before allogeneic stem cell transplantation. Bone Marrow Transplant. 2015;50(9):1217–1223. doi: 10.1038/bmt.2015.123. [DOI] [PubMed] [Google Scholar]
- 18.Beebe K, Magee K, McNulty A, Stahlecker J, Salzberg D, Miller H, et al. Vitamin D deficiency and outcomes in pediatric hematopoietic stem cell transplantation. Pediatr Blood Cancer. 2018;65(2):e26817. doi: 10.1002/pbc.26817. [DOI] [PubMed] [Google Scholar]
- 19.Wallace G, Jodele S, Howell J, Myers KC, Teusink A, Zhao X, et al. Vitamin D deficiency and survival in children after hematopoietic stem cell transplant. Biol Blood Marrow Transplant. 2015;21(9):1627–1631. doi: 10.1016/j.bbmt.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joseph RW, Alousi A, Konda B, Komanduri K, Neumann J, Trevino C, et al. High incidence of vitamin D deficiency in patients undergoing allogeneic stem cell transplantation. Am J Hematol. 2011;86(11):954–956. doi: 10.1002/ajh.22143. [DOI] [PubMed] [Google Scholar]
- 21.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 22.Urbain P, Ihorst G, Biesalski H-K, Bertz H. Course of serum 25-hydroxyvitamin D3 status and its influencing factors in adults undergoing allogeneic hematopoietic cell transplantation. Ann Hematol. 2012;91(5):759–766. doi: 10.1007/s00277-011-1365-2. [DOI] [PubMed] [Google Scholar]
- 23.Wallace G, Jodele S, Myers KC, Dandoy CE, El-Bietar J, Nelson A, et al. Vitamin D deficiency in pediatric hematopoietic stem cell transplantation patients despite both standard and aggressive supplementation. Biol Blood Marrow Transplant. 2016;22(7):1271–1274. doi: 10.1016/j.bbmt.2016.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenblatt J, Bissonnette A, Ahmad R, Wu Z, Vasir B, Stevenson K, et al. Immunomodulatory effects of vitamin D: implications for GvHD. Bone Marrow Transplant. 2010;45(9):1463–1468. doi: 10.1038/bmt.2009.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glotzbecker B, Ho VT, Aldridge J, Kim HT, Horowitz G, Ritz J, et al. Low levels of 25-hydroxyvitamin D before allogeneic hematopoietic SCT correlate with the development of chronic GvHD. Bone Marrow Transplant. 2013;48(4):593–597. doi: 10.1038/bmt.2012.177. [DOI] [PubMed] [Google Scholar]
- 26.Arain Abeer, Matthiesen Chance. Vitamin D deficiency and graft-versus-host disease in hematopoietic stem cell transplant population. Hematology/Oncology and Stem Cell Therapy. 2019;12(3):133–139. doi: 10.1016/j.hemonc.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Hamidieh AA, Sherafatmand M, Mansouri A, Hadjibabaie M, Ashouri A, Jahangard-Rafsanjani Z, et al. Calcitriol for oral mucositis prevention in patients with Fanconi anemia undergoing hematopoietic SCT: a double-blind, randomized, placebo-controlled trial. Am J Ther. 2016;23(6):e1700–e17e8. doi: 10.1097/MJT.0000000000000269. [DOI] [PubMed] [Google Scholar]
- 28.Caballero-Velazquez T., Montero I., Sanchez-Guijo F., Parody R., Saldana R., Valcarcel D., Lopez-Godino O., Ferra i Coll C., Cuesta M., Carrillo-Vico A., Sanchez-Abarca L. I., Lopez-Corral L., Marquez-Malaver F. J., Perez-Simon J. A. Immunomodulatory Effect of Vitamin D after Allogeneic Stem Cell Transplantation: Results of a Prospective Multicenter Clinical Trial. Clinical Cancer Research. 2016;22(23):5673–5681. doi: 10.1158/1078-0432.CCR-16-0238. [DOI] [PubMed] [Google Scholar]
- 29.Lips P. Relative value of 25(OH)D and 1,25(OH)2D measurements. J Bone Miner Res. 2007;22(11):1668–1671. doi: 10.1359/jbmr.070716. [DOI] [PubMed] [Google Scholar]
- 30.Kreutz M, Eissner G, Hahn J, Andreesen R, Drobnik W, Holler E. Variations in 1α,25-dihydroxyvitamin D3 and 25-hydroxyvitamin D3 serum levels during allogeneic bone marrow transplantation. Bone Marrow Transplant. 2004;33(8):871–873. doi: 10.1038/sj.bmt.1704448. [DOI] [PubMed] [Google Scholar]
- 31.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 32.NCCN guidelines. National Comprehensive Cancer Network, Plymouth Meeting, PA, USA. 2018. https://www.nccn.org/professionals/physician_gls/default.aspx. Accessed Jan 13 2019.
- 33.Hamadani M, Craig M, Awan FT, Devine SM. How we approach patient evaluation for hematopoietic stem cell transplantation. Bone Marrow Transplant. 2010;45:1259. doi: 10.1038/bmt.2010.94. [DOI] [PubMed] [Google Scholar]
- 34.Calcitriol capsules 0.25 mcg capsule. DailyMed, National Library of Medicine, USA. 2018. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=bebefb2a-7365-48e2-9681-83ed4b41e26e. Accessed Jan 13 2019.
- 35.Calcitriol (systemic): drug information. In: Post T, editor. UpToDate. Waltham, MA, USA: UpToDate Inc., Wolters Kluwer; 2018.
- 36.The use of the WHO-UMC system for standardised case causality assessment. WHO–UMC, Uppsala, Sweden. 2000. https://who-umc.org/media/164200/who-umc-causality-assessment_new-logo.pdf. Accessed Jan 13 2019.
- 37.Hartwig SC, Siegel J, Schneider PJ. Preventability and severity assessment in reporting adverse drug reactions. Am J Hosp Pharm. 1992;49(9):2229–2232. [PubMed] [Google Scholar]
- 38.FDA. What is a serious adverse event? FDA, USA. 2016. http://www.fda.gov/Safety/MedWatch/HowToReport/ucm053087.htm. Accessed Jul 25 2019.
- 39.Bensinger W, Schubert M, Ang K, Brizel D, Brown E, Eilers J, et al. NCCN task force report: prevention and management of mucositis in cancer care. J Natl Compr Canc Netw. 2008;6:S1–21. doi: 10.6004/jnccn.2008.0001. [DOI] [PubMed] [Google Scholar]
- 40.Antiinfectives for systemic use. WHO, Oslo, Norway. 2018. http://www.whocc.no/atc_ddd_index/?code=J. Accessed Jan 13 2019.
- 41.Dudakov JA, Perales MA, van den Brink MRM. Immune reconstitution following hematopoietic cell transplantation. In: Forman SJ, Negrin RS, Antin JH, Appelbaum FR, editors. Thomas’ hematopoietic cell transplantation. 5. UK: Wiley; 2016. pp. 161–162. [Google Scholar]
- 42.Skrobot A, Demkow U, Wachowska M. Immunomodulatory role of vitamin D: a review. Current Trends in Immunity and Respiratory Infections. Springer; 2018. p. 13–23. [DOI] [PubMed]
- 43.Balogh G, de Boland AR, Boland R, Barja P. Effect of 1,25(OH)(2)-vitamin D(3) on the activation of natural killer cells: role of protein kinase C and extracellular calcium. Exp Mol Pathol. 1999;67(2):63–74. doi: 10.1006/exmp.1999.2264. [DOI] [PubMed] [Google Scholar]
- 44.Ravid A, Koren R, Maron L, Liberman UA. 1,25(OH)2D3 increases cytotoxicity and exocytosis in lymphokine-activated killer cells. Mol Cell Endocrinol. 1993;96(1–2):133–139. doi: 10.1016/0303-7207(93)90103-q. [DOI] [PubMed] [Google Scholar]
- 45.Ota K, Dambaeva S, Kim MW, Han AR, Fukui A, Gilman-Sachs A, et al. 1,25-Dihydroxy-vitamin D3 regulates NK-cell cytotoxicity, cytokine secretion, and degranulation in women with recurrent pregnancy losses. Eur J Immunol. 2015;45(11):3188–3199. doi: 10.1002/eji.201545541. [DOI] [PubMed] [Google Scholar]
- 46.Bochen F, Balensiefer B, Körner S, Bittenbring JT, Neumann F, Koch A, et al. Vitamin D deficiency in head and neck cancer patients–prevalence, prognostic value and impact on immune function. Oncoimmunology. 2018;7(9):e1476817. doi: 10.1080/2162402X.2018.1476817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lemire JM, Adams J, Sakai R, Jordan S. 1 alpha, 25-dihydroxyvitamin D3 suppresses proliferation and immunoglobulin production by normal human peripheral blood mononuclear cells. J Clin Invest. 1984;74(2):657–661. doi: 10.1172/JCI111465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Quesada JM, Serrano I, Borrego F, Martin A, Pena J, Solana R. Calcitriol effect on natural killer cells from hemodialyzed and normal subjects. Calcif Tissue Int. 1995;56(2):113–117. doi: 10.1007/BF00296341. [DOI] [PubMed] [Google Scholar]
- 49.Dogan M, Erol M, Cesur Y, Yuca SA, Doğan Ş. The effect of 25-hydroxyvitamin D3 on the immune system. J Pediatr Endocrinol Metab. 2009;22(10):929–936. doi: 10.1515/JPEM.2009.22.10.929. [DOI] [PubMed] [Google Scholar]
- 50.Cortes M, Chen Michael J, Stachura David L, Liu Sarah Y, Kwan W, Wright F, et al. Developmental vitamin D availability impacts hematopoietic stem cell production. Cell Rep. 2016;17(2):458–468. doi: 10.1016/j.celrep.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weeres MA, Robien K, Ahn Y-O, Neulen M-L, Bergerson R, Miller JS, et al. The effects of 1, 25-dihydroxyvitamin D3 on in vitro human NK cell development from hematopoietic stem cells. J Immunol. 2014;193(7):3456–3462. doi: 10.4049/jimmunol.1400698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hiwase DK, Hiwase S, Bailey M, Bollard G, Schwarer AP. Higher infused lymphocyte dose predicts higher lymphocyte recovery, which in turn, predicts superior overall survival following autologous hematopoietic stem cell transplantation for multiple myeloma. Biol Blood Marrow Transplant. 2008;14(1):116–124. doi: 10.1016/j.bbmt.2007.08.051. [DOI] [PubMed] [Google Scholar]
- 53.Kim H, Sohn H, Kim S, Kang H, Park S, Kim S, et al. Lymphocyte recovery as a positive predictor of prolonged survival after autologous peripheral blood stem cell transplantation in T-cell non-Hodgkin's lymphoma. Bone Marrow Transplant. 2004;34(1):43–49. doi: 10.1038/sj.bmt.1704530. [DOI] [PubMed] [Google Scholar]
- 54.Kim H, Sohn H, Kim S, Lee J, Kim W, Suh C. Early lymphocyte recovery predicts longer survival after autologous peripheral blood stem cell transplantation in multiple myeloma. Bone Marrow Transplant. 2006;37(11):1037–1042. doi: 10.1038/sj.bmt.1705373. [DOI] [PubMed] [Google Scholar]
- 55.Porrata LF, Litzow MR, Markovic SN. Immune reconstitution after autologous hematopoietic stem cell transplantation. Mayo Clin Proc. 2001;76(4):407–412. doi: 10.4065/76.4.407. [DOI] [PubMed] [Google Scholar]
- 56.Medrano M, Carrillo-Cruz E, Montero I, Perez-Simon JA. Vitamin D: effect on haematopoiesis and immune system and clinical applications. Int J Mol Sci. 2018;19(9):2663. doi: 10.3390/ijms19092663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou K, Chen D, Jin H, Wu K, Wang X, Xu H, et al. Effects of calcitriol on experimental spinal cord injury in rats. Spinal Cord. 2016;54(7):510–516. doi: 10.1038/sc.2015.217. [DOI] [PubMed] [Google Scholar]
- 58.Stiff P. Mucositis associated with stem cell transplantation: current status and innovative approaches to management. Bone Marrow Transplant. 2001;27(S2):S3–S11. doi: 10.1038/sj.bmt.1702863. [DOI] [PubMed] [Google Scholar]