Abstract
Purpose
The role of oxidative stress in pathogenesis of diabetes is well established. In addition, an association between gut microbiota and type 2 diabetes mellitus (T2DM) is widely observed in previously published reports. This meta-analysis critically examines the association between gut microbiota, and oxidative stress in T2DM.
Methods
A systematic search for clinical trials was performed in PubMed, Web of Science and Scopus web databases up to 1 Jan 2019. Primary search terms include “microbiota”, “diabetes”, and “oxidative stress”. Study was conducted according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guideline. All clinical trials that compared the effects of probiotic supplementations with a control group using end points serum levels of fasting blood sugar (FBS), hemoglobin A1C (HbA1C) and oxidative stress biomarkers were included. Two independent researchers screened the data extracted from the relevant studies. The pooled standardized mean difference (SMD) was estimated using the random or fixed effects model. Heterogeneity among the studies was assessed using Q-test.
Results
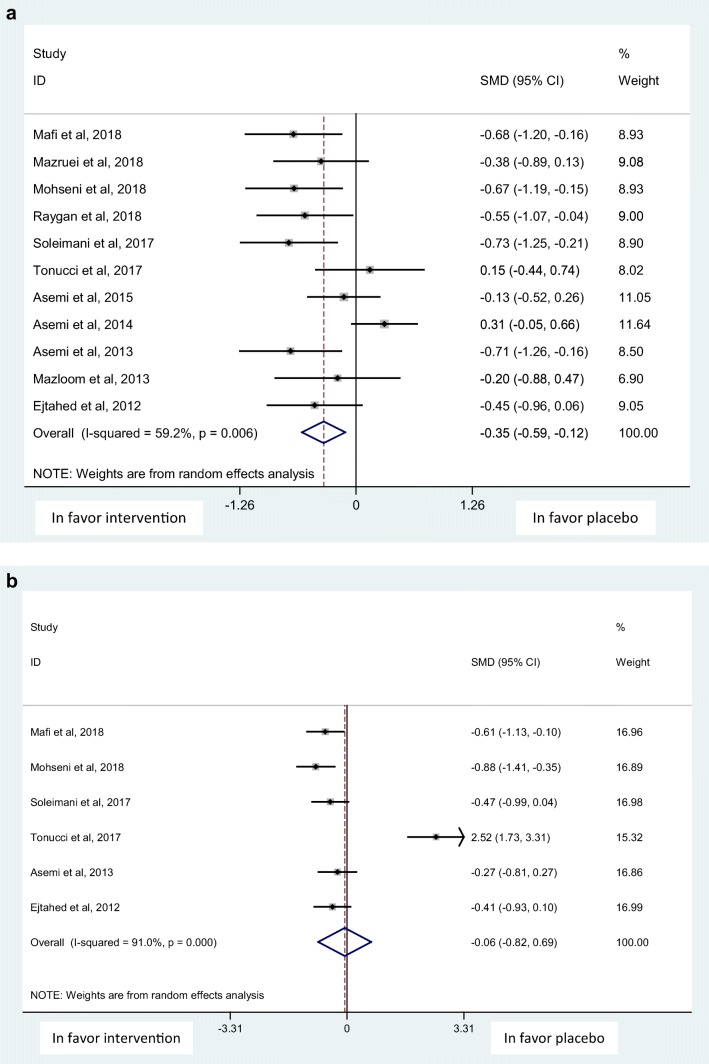
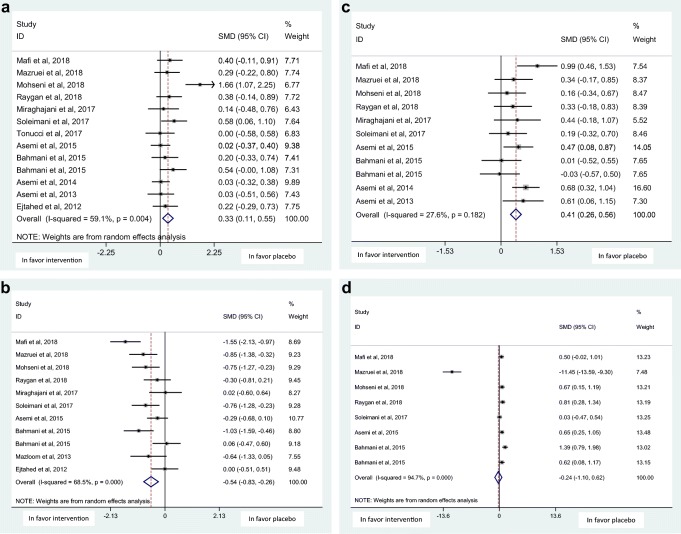
Overall, 13 randomized clinical trials (RCTs) involving 840 subjects with T2DM were included in the meta-analysis. The analysis showed that probiotics intake resulted in significant improvement in serum levels of FBS [SMD: -0.35, 95% CI: (−0.59, −0.12)], total antioxidant status (TAS) [SMD: 0.33, 95% CI: (0.11, 0.55)], total glutathione (GSH) [SMD: 0.41, 95% CI: (0.26, 0.56)] and malondialdehyde (MDA) [SMD: -0.54, 95% CI: (−0.83, −0.26)]. No significant improvement was found in HbA1C [SMD: -0.06, 95% CI:(−0.82, 0.69)], and nitric oxide (NO) [SMD:-0.24, 95% CI:(−1.10, 0.62)] levels.
Conclusion
It seems that gut microbiota can exert beneficial effects in diabetic patients via altering oxidative stress’ biomarkers. The beneficial effect of gut microbiota however was modest on FBS and non-significant on HbA1C. These results need to be confirmed by conducting more reliable RCTs.
PROSPERO Registration number
CRD42019134905.
Graphical abstract.
Flow diagram of the study selection process.
Electronic supplementary material
The online version of this article (10.1007/s40199-019-00302-2) contains supplementary material, which is available to authorized users.
Keywords: Diabetes mellitus type 2, gut microbiota, oxidative stress
Introduction
The worldwide prevalence of type 2 diabetes mellitus (T2DM) and its complications has significantly increased over the recent decades. It has been estimated that the number of people living with diabetes will rise to 629 million by 2045 [1]. T2DM is an important metabolic disorder stemming from multifactorial sources including genetic liability, environmental factors, and behavioral changes [2, 3]. In recent years, the role of emerging risk factors such as inflammatory markers, gut microbiota, and oxidative stress in T2DM pathogenesis has been taken into account [4].
The human intestine harbors a vast group of microorganisms, known as “microbiota”, which contains trillions of different species of microorganisms such as bacteria, and yeasts [2, 5, 6]. Symbiotic interaction between gut microbiota and the host plays an important role in human health determinants such as intestinal integrity, protection against pathogens, immune system modulation, and digestion [7, 8]. The alteration in the composition of gut microbiota has been shown to influence the metabolism of glucose, lipid, and insulin action and induce metabolic syndrome, insulin resistance, and T2DM [9]. Recent findings have revealed that adequate intake of probiotics, as live microorganisms, and prebiotics, as non-digestible food ingredients, could bring beneficial effects on health by improving gut microbiota composition, preserving intestinal integrity, and promoting the growth of beneficial microorganisms in the intestine [10–12]. Furthermore, glucose biomarkers and insulin resistance could be improved by modulating gut microbiota [13, 14].
Oxidative stress, characterized by an imbalance between formation of free oxygen species and antioxidant defense system, can be modified by enzymatic or non-enzymatic antioxidants [15]. The beneficial effects of probiotics and antioxidants on glucose markers and insulin sensitivity in T2DM patients have been separately demonstrated in several systematic reviews [2, 16–18]. An antioxidant activity for various lactic acid bacteria species including Lactobacillus rhamnosus, Lactobacillus lactics, and Lactobacillus plantarum was reported in several studies [19, 20]. Other studies have indicated that probiotics-induced glycemic improvement could be attributed to the antioxidant property of probiotics [14, 20, 21]. In other words, probiotics may influence the glucose control, although partially, by the modulation of oxidative stress. However, there are some controversies around this concept [22]. Hence, in this study we systematically retrieved the available clinical trials to evaluate the concomitant effectiveness of probiotics on oxidative stress and T2DM.
Methods
Search strategy
This systematic review was registered a priori (CRD42019134905) and conducted by searching in PubMed, Web of Science and Scopus web databases for clinical trials documented by 1 Jan 2019. The used search terms were “microbiota”, “gut bacteria”, “intestinal microbes”, “diabetes”, and “oxidative stress” and their Medical Subject Headings (MeSH) terms limited in human. The search strategy is available as supplementary data. Whenever the data was inaccessible or incomplete, we sent at least 3 emails to corresponding authors.
The title and abstract of the papers were looked through by two researchers independently to exclude the duplicated articles. Moreover, hand searching was performed in reference lists of included studies for full-text retrieval. Possible disagreements were resolved by discussion and consensus. Within multiple publications from a single dataset, only the largest study was included. This study was approved by Ethics Committee of Vice-Chancellor in Research Affairs-Tehran University of Medical Sciences (IR.TUMS.VCR.REC.1397.1010).
Study selection
All clinical trials that met the following criteria were included: 1) conducted on T2DM subjects; 2) examined efficacy of probiotics or prebiotics on blood glucose or glycemic status concurrent oxidative stress biomarkers; and 3) compared probiotics or prebiotics with a placebo or other treatments. Studies that followed the above criteria, but conducted on a healthy population, children, pregnant women, or patients with type 1 DM were excluded. Other exclusion criteria were animal studies, in vitro studies, letters to the editor and theses. English was considered as the language restriction.
Data extraction
The following data were extracted; authors, year of publication, design of the study, participants’ characteristics (total number, age, sex), sample size of each groups (intervention or control), source of probiotic/prebiotic, dosage and duration of each treatment (intervention or placebo), the mean serum level of fasting blood sugar (FBS), hemoglobin A1C (HbA1C), and oxidative stress biomarkers. Main outcome measures included the net changes in serum levels of FBS, HbA1C and oxidative stress biomarkers after probiotic/prebiotic supplementation.
Data synthesis and analysis
This study was reported according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guideline [23].
Quality assessment and data extraction
The methodological quality of each included clinical trial was assessed using the Jadad scoring system [24]. In this scale, for each part addressed in the study (randomized, double-blinding, description of withdrawals and dropouts, generation of random numbers and allocation concealment), one point was considered, with possible total score of Jadad ranged between 0 and 5. Clinical trials with total score < 3 were considered as low quality.
Statistical analysis
All data from included studies were extracted as mean difference (MD) and standard deviation (SD) in both treatment and control groups. Heterogeneity of reported MD of the effect of probiotic/prebiotic supplementation was assessed by the Chi-square-based Q-test and was regarded to be statistically significant at P < 0.1. The degree of heterogeneity was estimated using I2 statistic [25]. In order to estimate the pooled effect of probiotics/prebiotics, the random effect method by DerSimonian and Laird was used to perform meta-analysis due to the existing heterogeneity between studies. The pooled standardized mean difference (SMD) and its 95% confidence interval (CI) was then presented in form of a forest plot. The SMD is an inverse of variance and presents a point estimation of the effect of the treatment. In addition, Egger’s test [26] and random-effect meta-regression were conducted to assess publication bias and the effect of influencing factors, respectively. The results of publication bias were presented schematically using a forest plot. All analyses were conducted with STATA software, version 11.0 (StataCorp, USA).
Results
Characteristics of included papers
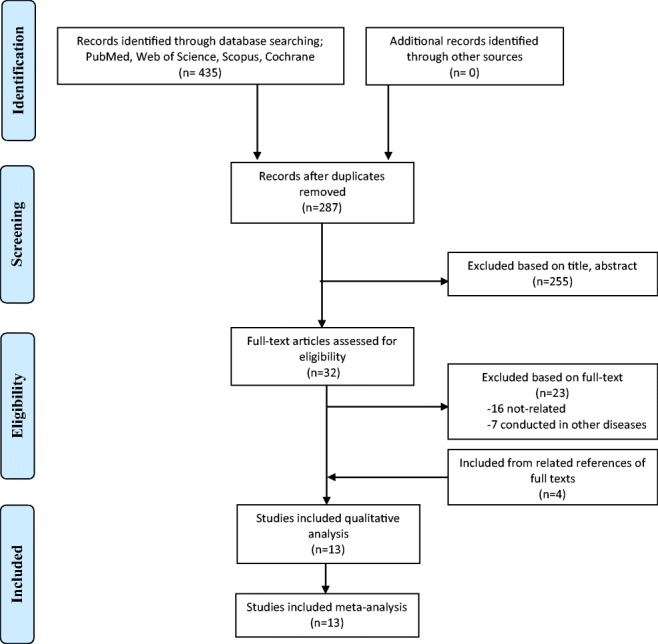
Our initial search yielded 435 articles. After screening abstracts and full texts, 13 randomized clinical trials (RCTs) were included which involved 840 subjects with T2DM [10, 14, 22, 27–36]. The number of the initial search results and included studies are shown in Fig. 1. Most of included studies were double-blind RCTs except two studies that were designed as single-blind RCT [22, 30]. Probiotics were supplemented from 6 to 12 weeks and intervention groups were mostly compared with a control group who consumed the same food without adding probiotics [14, 27–30, 32, 34]. Details of the extracted data are shown in Table 1.
Fig. 1.
Flow diagram of the study selection process
Table 1.
Characteristics of included studies in the meta-analysis
| Ref. | Design | Participants, age (yr), sex, case/control (n) | Intervention | Dose/ Duration |
Outcome measures | Jadad Score | |
|---|---|---|---|---|---|---|---|
| Case | Control | ||||||
| Ejtahed et al., 2012 [14] | DB-RCT | T2DM, 30–60, both, 30/30 |
Probiotic yogurt: L. acidophilus La5, Bifidobacterium lactis Bb12 |
Conventional yogurt | 300 g daily/ 6 w | Anthropometric, FBS, FSI, HbA1C, oxidative stress biomarkers | 5 |
| Asemi et al., 2013 [10] | Cross-over DB-RCT | T2DM, 35–70, both, 27/27 |
7 viable & freeze-dried strains: L. acidophilus, L. casei, L. rhamnosus, L. bulgaricus, Bifidobacterium breve, B. longum, Streptococcus thermophilus |
Placebo | One capsule daily/ 8 w | Anthropometric, FBS, FSI, HOMA-IR, HbA1C, oxidative stress biomarkers, hs-CRP, lipid profile | 4 |
| Mazloom et al., 2013 [22] | SB-RCT | T2DM, 25–65, both, 16/18 |
L. acidophilus, L. bulgaricus, L. bifidum, L. casei |
Placebo | One capsule twice daily/ 6 w | Anthropometric, FBS, FSI, HOMA-IR, oxidative stress biomarkers, hs-CRP, lipid profile, inflammatory markers | 3 |
| Asemi et al., 2014 [27] | Cross-over DB-RCT | T2DM, 35–70, both, 62/62 | Synbiotic food: probiotic viable & heat-resistant Lactobacillus sporogenes, inulin as prebiotic | Same food without probiotic bacteria & prebiotic inulin | 3 times daily/ 6 w | Anthropometric, FBS, FSI, HOMA-IR, HbA1C, oxidative stress biomarkers, hs-CRP, lipid profile | 4 |
| Asemi et al., 2016 [28] | Cross-over DB-RCT | T2DM, 35–70, both, 51/51 |
Synbiotic food: L. sporogenes, inulin, beta-carotene |
Same food without probiotic, inulin, and beta-carotene | 3 times daily/ 6 w | Anthropometric, FBS, FSI, HOMA-IR, oxidative stress biomarkers, hs-CRP, lipid profile, liver enzymes | 4 |
| Bahmani et al., 2016 [29] | DB-RCT | T2DM, 35–70, both, 27/27 |
Synbiotic bread: probiotic viable & heat-resistant L. sporogenes, inulin |
Control bread | 3 times daily/ 8 w | Anthropometric, oxidative stress biomarkers, liver enzymes | 4 |
| T2DM, 35–70, both, 27/27 |
Probiotic bread: probiotic viable & heat-resistant L. sporogenes |
||||||
| Miraghajani et al., 2017 [30] | Parallel SB-RCT | T2DM, 32–68, both, 20/20 |
Probiotic soy milk: L plantarum A7 |
Soy milk | 200 ml daily/8 w | Anthropometric, oxidative stress biomarkers | 4 |
| Soleimani et al. 2017 [31] | Parallel DB-RCT | DN (T1DM & T2DM), 36–75, both, 30/30 |
Capsule: L. acidophilus, L. casei, Bifidobacterium bifidum |
Placebo | One capsule daily/12 w | FBS, FSI, HOMA-IR, HbA1C, oxidative stress biomarkers, hs-CRP | 3 |
| Tonucci et al. 2017 [32] | DB-RCT | T2DM, 35–60, both, 23/22 | Fermented milk: L. acidophilus La5, Bifidobacterium animalis subsp. lactis Bb12 | Conventional fermented milk | 120 g daily/6 w | Anthropometric, body composition, lipid profile, inflammatory markers, oxidative stress biomarkers, FBS, HOMA-IR, HbA1C, fecal samples | 5 |
| Mafi et al. 2018 [33] | Parallel DB-RCT | DN (T1DM & T2DM), 45–85, NA, 30/30 |
Probiotic capsule: L. acidophilus, Bifidobacterium bifidum, L. reuteri, L. fementum |
Placebo | One capsule daily/12 w | FBS, HOMA-IR, HbA1C, lipid profile, hs-CRP, BUN, Cr, urine protein, inflammatory markers, oxidative stress biomarkers | 4 |
| Mazruei et al., 2018 [34] | Parallel DB-RCT | DN (T1DM & T2DM), 45–85, NA, 30/30 |
Probiotic honey: Bacillus coagulans T4 |
Honey | 25 g daily/ 12 w | FBS, HOMA-IR, lipid profile, hs-CRP, BUN, Cr, urine protein, inflammatory markers, oxidative stress biomarkers | 4 |
| Mohseni et al., 2018 [35] | Parallel DB-RCT | DF (T1DM & T2DM), 45–85, both, 30/30 |
Probiotic capsule: L. acidophilus, Bifidobacterium bifidum, L. casei, L. fementum |
Placebo | One capsule daily/12 w | FBS, HOMA-IR, lipid profile, hs-CRP, HbA1C, wound characteristics, inflammatory markers, oxidative stress biomarkers | 4 |
| Raygan et al., 2018 [36] | Parallel DB-RCT | T2DM, 40–85, NA, 30/30 |
Probiotic capsule: L. acidophilus, Bifidobacterium bifidum, L. casei, L. fementum |
Placebo | One capsule daily/12 w |
Anthropometric, FBS, HOMA-IR, lipid profile, hs-CRP, inflammatory markers, oxidative stress biomarkers |
5 |
Legend: SB-RCT: Single blind randomized clinical trial; DB-RCT: Double blind randomized clinical trial; DN: Diabetic nephropathy; DF: diabetic foot; W: Week; FBS: Fasting blood sugar; HOMA-IR: Homeostasis model of assessment-insulin resistance; HbA1C: Hemoglobin A1C; FSI: Fasting serum insulin; hs-CRP: High sensitivity C - reactive protein; BUN: blood urea nitrogen; Cr: Creatinine; NA: Unknown
Meta-analysis
Probiotics supplementation showed a significant effects on FBS, but was non-significant on HbA1C with the SMD and 95% CI [− 0.35 (−0.59, −0.12), (Q = 24.49, p = 0.006, I2% = 59.2)], and [−0.06 (−0.82, 0.69), (Q = 55.48, p < 0.001, I2% = 91.0)], respectively in a random-effect method (Fig. 2 a,b). Probiotic supplementation was also shown to exert a significant effect on the serum levels of oxidative stress biomarkers; total antioxidant status (TAS), and malondialdehyde (MDA) in a random-effect method and on total glutathione (GSH) using a fixed-effect method with the SMD and 95% CI [0.33 (0.11, 0.55), (Q = 29.34, p = 0.004, I2% = 59.1)], [−0.54 (−0.83,-0.26), (Q = 31.77, p < 0.001, I2% = 68.5)], and [0.41 (0.26, 0.56), (Q = 13.81, p = 0.182, I2% = 27.6], respectively (Fig. 3 a,b,c). The effect of probiotic supplementation on nitric oxide (NO) was however non-significant using a random-effect method; [−0.24 (−1.10, 0.62), (Q = 133.33, p < 0.001, I2% = 94.7], (Fig. 3 d).
Fig. 2.
a. Forest plot of probiotic supplementation’ effects on fasting blood sugar (FBS) b. Forest plot of probiotic supplementation’ effects on Hemoglobin A1C (HbA1C)
Fig. 3.
a. Forest plot of probiotic supplementation’ effects on total antioxidant status (TAS) b. Forest plot of probiotic supplementation’ effects on malondialdehyde (MDA) c. Forest plot of probiotic supplementation’ effects on total glutathione (GSH) d. Forest plot of probiotic supplementation’ effects on nitric oxide (NO)
Quality assessment
All included studies were categorized as high quality trials as assessed by the Jadad score, Table 1.
Meta-regression
The effect of influencing factors was analyzed using the random-effects meta-regression model. We found that factors such as age and sample size of participants, duration and mode of intervention have no effect on the heterogeneity (P > 0.05).
Publication bias
The result of Egger’s test did not show any publication bias among the studies investigated the effect of probiotics supplementation on glucose hemostasis concurrent oxidative stress biomarkers (z = 0.85, p = 0.396).
Discussion
The results of this meta-analysis suggest that probiotics supplementation has a significant beneficial yet modest effect on serum levels of FBS as well as oxidative -and antioxidative stress biomarkers. And, the overall effect was found to be non-significant on HbA1C and NO levels. As these findings were based on high quality RCTs, it can be concluded that the gut microbiome compositions potentially exert beneficial effects on patients with T2DM via altering oxidative stress biomarkers.
T2DM as one of the most prevalent health problems has imposed a major burden on communities and health systems. Proper management and care of patients with diabetes still remain as a challenge Variation in composition of microbiota among T2DM and healthy individuals has been confirmed in several intestinal microbiome analyses [37, 38]. Further, efficacy of probiotics supplementation on diabetes management was confirmed in animal studies [21]. Hence, in recent years, several clinical trials have evaluated the effect of probiotics supplementation on metabolic profiles in T2DM. In some meta-analyses on RCTs that assessed the effect of probiotics on FBS, a significant reduction in FBS level was found by 0.61, 0.62, 0.01 mmol/L, respectively [39–41]. Those findings are in line our result but are in contrast with the pooling data of RCTs in another meta-analysis, which suggested a non-significant reduction in FBS by 1.05 mmol/ L [42]. Furthermore, several meta-analyses that assessed the effects of probiotics on HbA1C in T2DM also reported a variation from non-significant effect as 0.06, −0.19 [37, 38], or 0.30% in our study to a significant reduction by −0.81%, and − 0.38% [40, 42]. In the clinical trials included in our meta-analysis was shown some controversial effects of probiotics supplementation on HbA1C. In one of the clinical trials, multispecies probiotic supplementation (L. acidophilus, L. casei, L. rhamnosus, L. bulgaricus, B. breve, B. longum, S. thermophiles-109 CFUs each) plus fructo-oligosacchaide was not associated with significant reduction in HbA1C after 8w intervention [10]. However, another trail showed that consuming fermented milk containing L. acidophilus La-5, and Bifidobacterium animals subsp. Lactis BB-12 (109 CFUs) for 6w resulted in a significant reduction in HbA1C [32]. Trials with a longer duration (12w) to evaluate the effect of probiotics on HbA1C also found controversial effects [31, 33, 35]. All participants in Soleimani et al. and Mafi et al. [31, 33] studies who suffered from diabetic nephropathy supplemented separately with different multispecies for 12w. The supplements containing L. acidophilus, L. casei, and Bifidobacterium bifidm-109 CFUs each [31] resulted in a significant reduction in HbA1C in contrast to the supplements containing L. acidophilus, L. reuters, L. fermentum, and Bifidobacterium bifidm − 109 CFUs each [33]. Nevertheless, although all of these observed effects were limited, it should be noted that even a modest improvement in FBS and HbA1C levels were shown to be beneficial in the management of diabetes by prevention or delay of diabetes complications. The apparent discrepancy between pooling effect of probiotics on FBS and HbA1c found in several studies [37, 38, 40–42] and also in our analysis can be related to the fact that HbA1C reflects the average level of blood glucose over 120 days. While the majority of our 13 included RCTs that meta-analyzed was conducted for 6-8w.
The mechanism of beneficial effect of probiotics on hyperglycemia is not fully known. One hypothesis is that probiotics improve glucose level in diabetic patients via inhibition of oxidative stress [41]. Only some studies attempted to evaluate the oxidative stress biomarkers to investigate its potential association with intestinal microbiota on glycemic control [10, 14, 22, 27–36]. The reported antioxidant activity in several previous animal studies was shown in particular strains of lactic acid bacteria such as B. lactis, L. acidophilus, L. plantarum and L. casei [43–45]. These bacteria could delay the development of hyperglycemia by inhibiting the production of lipid peroxidation and increasing total serum antioxidant capacity. Moreover, it was shown that probiotics alter the absorption of antioxidants [46]. The same species of lactic acid bacteria were used as probiotic or synbiotic regimens in the clinical trials analyzed in our study, but shown to have contradicting effects on oxidative stress biomarkers [10, 14, 22, 27, 30–33, 36]. After pooling data, we did not find a substantial beneficial effect with regard to glycemic control in the meta-analysis; however, a modest, but significant, alteration in the serum levels of oxidative -and antioxidative stress biomarkers was observed. The observed variation between our results and that of others could be partly attributed to difference in probiotic dosage, duration of treatment, and also the type of fermented food; dairy, bread, etc. The range of treatment duration in our meta-analysis was 6-12w, but it was 4-8w for Kasinska meta-analysis [42].
Our study had some strengths and limitations. The main strength of this meta-analysis was the inclusion of all clinical trials that compared effect of probiotics intake with a control group. The second strength was that most of included clinical trials were randomized, which reduced the risk of bias. The main limitation was the low number of identified RCTs that met the inclusion criteria. As such, we were not able to compare the effects of prebiotics and prebiotics separately on the outcome measures. In addition, the lack of sufficient clinical trials on species and strains of probiotics made it not practical to perform subgroup analysis to assess the efficacy of a particular probiotic type, dosage, safety and treatment duration in our outcome measures. Due to low treatment duration should be caution in interpretation of pooling data of hbA1C. Other limitations were restricted search to English literature, and not assessing the gray literature and thesis. In addition, although we included all eligible articles based on our inclusion and exclusion criteria and didn’t have any missing data there is a possibility of selection bias due to the fact that most of the included studies were conducted by Iranian researchers. This fact reflects a major interest to this subject among Iranian scientists and clinicians.
Conclusion
In conclusion, our results revealed a beneficial effect of probiotics on reducing FBS, or oxidative stress biomarkers in T2DM patients without any significant improvement of glycemic status. Hence, probiotics might be considered as potential target for care and management of T2DM. However, larger well-designed and long duration RCTs are required before making a conclusive recommendation for subjects with T2DM. In addition, due to potential safety concerns in at-risk individuals such as elderly [47] careful selection criteria need to be considered in designing RCTs.
Electronic Supplementary Materials
(DOCX 13 kb)
Acknowledgements
This research has been supported by Tehran University of Medical Sciences & health services grant numbered 39856-192-03-97. All of authors thank the TUMS for its financial support.
Authors’ contributions
OTM and EAL did literature bibliography, reviewed data, wrote draft and conceived the paper. SHM and RBJ did literature bibliography, and drafted some parts of the paper. MQ reviewed data, conducted meta-analysis, and drafted the paper. BL conceived, supervised, and edited the paper. All authors read and approved the final manuscript.
Funding
This research has been supported by Tehran University of Medical Sciences & health services grant numbered 39856–192–03-97. The funder had no role in any part of study; design, data collection, analysis, interpretation or writing.
Compliance with ethical standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Footnotes
Edris Ardeshirlarijani and Ozra Tabatabaei-Malazy contributed as first author.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Edris Ardeshirlarijani, Email: ediardeshir@gmail.com.
Ozra Tabatabaei-Malazy, Email: tabatabaeiml@sina.tums.ac.ir.
Shahrzad Mohseni, Email: shmohseni58@gmail.com.
Mostafa Qorbani, Email: mqorbani1379@yahoo.com.
Bagher Larijani, Phone: (+98) 2188631296-7, Email: emrc@tums.ac.ir.
Reza Baradar Jalili, Email: reza.jalili@ubc.ca.
References
- 1.IDF diabetes atlas, 8th edition 2017. Available at: www.IDF.org/e-library/epidemiology-research/diabetes-atlas.html. Last available: 22 May 2019.
- 2.Gomes AC, Bueno AA, de Souza RG, Mota JF. Gut microbiota, probiotics and diabetes. Nutr J. 2014;13:60. doi: 10.1186/1475-2891-13-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu FB. Globalization of Diabetes: The role of diet, lifestyle, and genes. Diabetes Care. 2011;34:1249–1257. doi: 10.2337/dc11-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bordalo Tonucci L, Dos Santos KM. De Luces Fortes Ferreira CL, Ribeiro SM, De Oliveira LL, Martino HS. Gut microbiota and probiotics: Focus on diabetes mellitus. Crit Rev Food Sci Nutr. 2017;57:2296–2309. doi: 10.1080/10408398.2014.934438. [DOI] [PubMed] [Google Scholar]
- 5.Hansen AK, Hansen CH, Krych L, Nielsen DS. Impact of the gut microbiota on rodent models of human disease. World J Gastroenterol. 2014;20:17727–17736. doi: 10.3748/wjg.v20.i47.17727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jia W, Li H, Zhao L, Nicholson JK. Gut microbiota: a potential new territory for drug targeting. Nat Rev Drug Discov. 2008;7:123–129. doi: 10.1038/nrd2505. [DOI] [PubMed] [Google Scholar]
- 7.Thomas LV, Ockhuizen T, Suzuki K. Exploring the influence of the gut microbiota and probiotics on health: a symposium report. Br J Nutr. 2014;112(Suppl 1):S1–18. doi: 10.1017/S0007114514001275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quigley EM. Gut bacteria in health and disease. Gastroenterol Hepatol (N Y) 2013;9:560–569. [PMC free article] [PubMed] [Google Scholar]
- 9.Hartstra AV, Bouter KE, Backhed F, Nieuwdorp M. Insights into the role of the microbiome in obesity and type 2 diabetes. Diabetes Care. 2015;38:159–165. doi: 10.2337/dc14-0769. [DOI] [PubMed] [Google Scholar]
- 10.Asemi Z, Zare Z, Shakeri H, Sabihi SS, Esmaillzadeh A. Effect of multispecies probiotic supplements on metabolic profiles, hs-CRP, and oxidative stress in patients with type 2 diabetes. Ann Nutr Metab. 2013;63:1–9. doi: 10.1159/000349922. [DOI] [PubMed] [Google Scholar]
- 11.FAO and WHO. Report of a joint FAO/WHO working group on drafting guidelines for the evaluation of probiotics in food. Guidelines for evaluation of probiotics in food. London, Ontario, Canada. 2002. Available at: http://www.who.int/foodsafety/publications/fs_management/probiotics2/en/
- 12.Schrezenmeir J, de Vrese M. Probiotics, prebiotics, and synbiotics-approaching a definition. The Am J clin Nutr. 2001;73(2 Suppl):361s–364s. doi: 10.1093/ajcn/73.2.361s. [DOI] [PubMed] [Google Scholar]
- 13.Rajkumar H, Mahmood N, Kumar M, Varikuti SR, Challa HR, Myakala SP. Effect of probiotic (VSL# 3) and omega-3 on lipid profile, insulin sensitivity, inflammatory markers, and gut colonization in overweight adults: a randomized, controlled trial. Mediat Inflamm. 2014;2014:348959. doi: 10.1155/2014/348959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ejtahed HS, Mohtadi-Nia J, Homayouni-Rad A, Niafar M, Asghari-Jafarabadi M, Mofid V. Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition. 2012;28:539–543. doi: 10.1016/j.nut.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 15.Rahimi R, Nikfar S, Larijani B, Abdollahi M. A review on the role of antioxidants in the management of diabetes and its complications. Biomed Pharmacother. 2005;59:365–373. doi: 10.1016/j.biopha.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Tabatabaei-Malazy O, Nikfar S, Larijani B, Abdollahi M. Influence of ascorbic acid supplementation on type 2 diabetes mellitus in observational and randomized controlled trials; a systematic review with meta-analysis. J Pharm Pharm Sci. 2014;17:554–582. doi: 10.18433/J3ZG6R. [DOI] [PubMed] [Google Scholar]
- 17.Khodaeian M, Tabatabaei-Malazy O, Qorbani M, Farzadfar F, Amini P, Larijani B. Effect of vitamins C and E on insulin resistance in diabetes: a meta-analysis study. Eur J Clin Investig. 2015;45:1161–1174. doi: 10.1111/eci.12534. [DOI] [PubMed] [Google Scholar]
- 18.Tabatabaei-Malazy O, Larijani B, Abdollahi M. A systematic review of in vitro studies conducted on effect of herbal products on secretion of insulin from Langerhans islets. J Pharm Pharm Sci. 2012;15:447–466. doi: 10.18433/J32W29. [DOI] [PubMed] [Google Scholar]
- 19.Uskova MA, Kravchenko LV. Antioxidant properties of lactic acid bacteria--probiotic and yogurt strains. Vopr Pitan. 2009;78:18–23. [PubMed] [Google Scholar]
- 20.Mikelsaar M, Zilmer M. Lactobacillus fermentum ME-3- an antimicrobial and antioxidative probiotic. Microb Ecol Health Dis. 2009;21(1):1–27. doi: 10.1080/08910600902815561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yadav H, Jain S, Sinha PR. Oral administration of dahi containing probiotic Lactobacillus acidophilus and Lactobacillus casei delayed the progression of streptozotocin-induced diabetes in rats. J Dairy Res. 2008;75:189–195. doi: 10.1017/S0022029908003129. [DOI] [PubMed] [Google Scholar]
- 22.Mazloom Z, Yousefinejad A, Dabbaghmanesh MH. Effect of probiotics on lipid profile, glycemic control, insulin action, oxidative stress, and inflammatory markers in patients with type 2 diabetes: a clinical trial. Iran J Med Sci. 2013;38:38–43. [PMC free article] [PubMed] [Google Scholar]
- 23.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 25.Ioannidis JP. Interpretation of tests of heterogeneity and bias in meta-analysis. J Eval Clin Pract. 2008;14(5):951–957. doi: 10.1111/j.1365-2753.2008.00986.x. [DOI] [PubMed] [Google Scholar]
- 26.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asemi Z, Khorrami-Rad A, Alizadeh SA, Shakeri H, Esmaillzadeh A. Effects of synbiotic food consumption on metabolic status of diabetic patients: a double-blind randomized cross-over controlled clinical trial. Clin Nutr. 2014;33:198–203. doi: 10.1016/j.clnu.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 28.Asemi Z, Alizadeh SA, Ahmad K, Goli M, Esmaillzadeh A. Effects of beta-carotene fortified synbiotic food on metabolic control of patients with type 2 diabetes mellitus: A double-blind randomized cross-over controlled clinical trial. Clin Nutr. 2016;35:819–825. doi: 10.1016/j.clnu.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 29.Bahmani F, Tajadadi-Ebrahimi M, Kolahdooz F, Mazouchi M, Hadaegh H, Jamal AS, et al. The consumption of synbiotic bread containing lactobacillus sporogenes and inulin affects nitric oxide and malondialdehyde in patients with type 2 diabetes mellitus: randomized, double-blind, placebo-controlled trial. J Am Coll Nutr. 2016;35(6):506–513. doi: 10.1080/07315724.2015.1032443. [DOI] [PubMed] [Google Scholar]
- 30.Miraghajani M, Zaghian N, Mirlohi M, Feizi A, Ghiasvand R. The impact of probiotic soy milk consumption on oxidative stress among type 2 diabetic kidney disease patients: a randomized controlled clinical trial. J Ren Nutr. 2017;27(5):317–324. doi: 10.1053/j.jrn.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 31.Soleimani A, Zarrati Mojarrad M, Bahmani F, Taghizadeh M, Ramezani M, Tajabadi-Ebrahimi M, et al. Probiotic supplementation in diabetic hemodialysis patients has beneficial metabolic effects. Kidney Int. 2017;91(2):435–442. doi: 10.1016/j.kint.2016.09.040. [DOI] [PubMed] [Google Scholar]
- 32.Tonucci LB. Olbrich Dos Santos KM, Licursi de Oliveira L, Rocha Ribeiro SM, Duarte Martino HS. Clinical application of probiotics in type 2 diabetes mellitus: A randomized, double-blind, placebo-controlled study. Clin Nutr. 2017;36(1):85–92. doi: 10.1016/j.clnu.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 33.Mafi A, Namazi G, Soleimani A, Bahmani F, Aghadavod E, Asemi Z. Metabolic and genetic response to probiotics supplementation in patients with diabetic nephropathy: a randomized, double-blind, placebo-controlled trial. Food Funct. 2018;9(9):4763–4770. doi: 10.1039/c8fo00888d. [DOI] [PubMed] [Google Scholar]
- 34.Mazruei Arani N, Emam-Djomeh Z, Tavakolipour H, Sharafati-Chaleshtori R, Soleimani A, Asemi Z. The effects of probiotic honey consumption on metabolic status in patients with diabetic nephropathy: a randomized, double-blind, controlled trial. Probiotics Antimicrob Proteins. 2018. 10.1007/s12602-018-9468-x. [DOI] [PubMed]
- 35.Mohseni Sima, Bayani Masomeh, Bahmani Fereshteh, Tajabadi-Ebrahimi Maryam, Bayani Mohammad Ali, Jafari Parvaneh, Asemi Zatollah. The beneficial effects of probiotic administration on wound healing and metabolic status in patients with diabetic foot ulcer: A randomized, double-blind, placebo-controlled trial. Diabetes/Metabolism Research and Reviews. 2017;34(3):e2970. doi: 10.1002/dmrr.2970. [DOI] [PubMed] [Google Scholar]
- 36.Raygan F, Rezavandi Z, Bahmani F, Ostadmohammadi V, Mansournia MA, Tajabadi-Ebrahimi M, et al. The effects of probiotic supplementation on metabolic status in type 2 diabetic patients with coronary heart disease. Diabetol Metab Syndr. 2018;10:51. doi: 10.1186/s13098-018-0353-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Larsen N, Vogensen FK, van den Berg FW, Nielsen DS, Andreasen AS, Pedersen BK, et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One. 2010;5(2):e9085. doi: 10.1371/journal.pone.0009085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490(7418):55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 39.Li C, Li X, Han H, Cui H, Peng M, Wang G, et al. Effect of probiotics on metabolic profiles in type 2 diabetes mellitus: A meta-analysis of randomized, controlled trials. Medicine. 2016;95(26):e4088. doi: 10.1097/MD.0000000000004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu YM, Zhou F, Yuan Y, Xu YC. Effects of probiotics supplement in patients with type 2 diabetes mellitus: A meta-analysis of randomized trials. Med Clin (Barc) 2017;148(8):362–370. doi: 10.1016/j.medcli.2016.11.036. [DOI] [PubMed] [Google Scholar]
- 41.Yao K, Zeng L, He Q, Wang W, Lei J, Zou X. Effect of probiotics on glucose and lipid metabolism in type 2 diabetes mellitus: a meta-analysis of 12 randomized controlled trials. Med Sci Monit. 2017;23:3044–3053. doi: 10.12659/MSM.902600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kasinska MA, Drzewoski J. Effectiveness of probiotics in type 2 diabetes: a meta-analysis. Pol Arch Med Wewn. 2015;125(11):803–813. doi: 10.20452/pamw.3156. [DOI] [PubMed] [Google Scholar]
- 43.Rad AH, Abbasalizadeh S, Vazifekhah S, Abbasalizadeh F, Hassanalilou T, Bastani P, Ejtahed HS, Soroush AR, Javadi M, Mortazavian AM, Khalili L. The future of diabetes management by healthy probiotic microorganisms. Curr Diabetes Rev. 2017;13(6):582–589. doi: 10.2174/1573399812666161014112515. [DOI] [PubMed] [Google Scholar]
- 44.Noce Annalisa, Marrone Giulia, Di Daniele Francesca, Ottaviani Eleonora, Wilson Jones Georgia, Bernini Roberta, Romani Annalisa, Rovella Valentina. Impact of Gut Microbiota Composition on Onset and Progression of Chronic Non-Communicable Diseases. Nutrients. 2019;11(5):1073. doi: 10.3390/nu11051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khan IT, Nadeem M, Imran M, Ullah R, Ajmal M, Jaspal MH. Antioxidant properties of Milk and dairy products: a comprehensive review of the current knowledge. Lipids Health Dis. 2019;18(1):41. doi: 10.1186/s12944-019-0969-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chamari M, Djazayery A, Jalali M, Sadrzadeh Yeganeh H, Hosseini S, et al. The effect of daily consumption of probiotic and conventional yoghurt on some oxidative stress factors in plasma of young healthy women. ARYA Atheroscler J. 2008;4:175–179. [Google Scholar]
- 47.Sotoudegan F, Daniali M, Hassani S, Nikfar S, Abdollahi M. Reappraisal of probiotics' safety in human. Food Chem Toxicol. 2019;129:22–29. doi: 10.1016/j.fct.2019.04.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 13 kb)